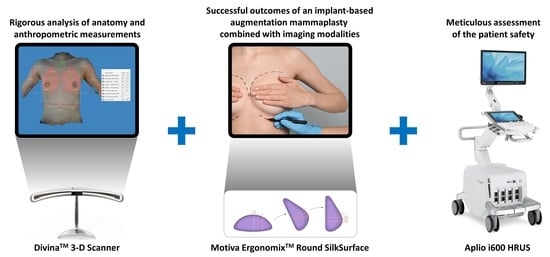
Feasibility of Imaging Modalities Combined with a Silicone Gel-Filled Breast Implant in Korean Women
Abstract
1. Introduction
2. Results and Discussion
2.1. Demographic and Clinical Characteristics of the Patients
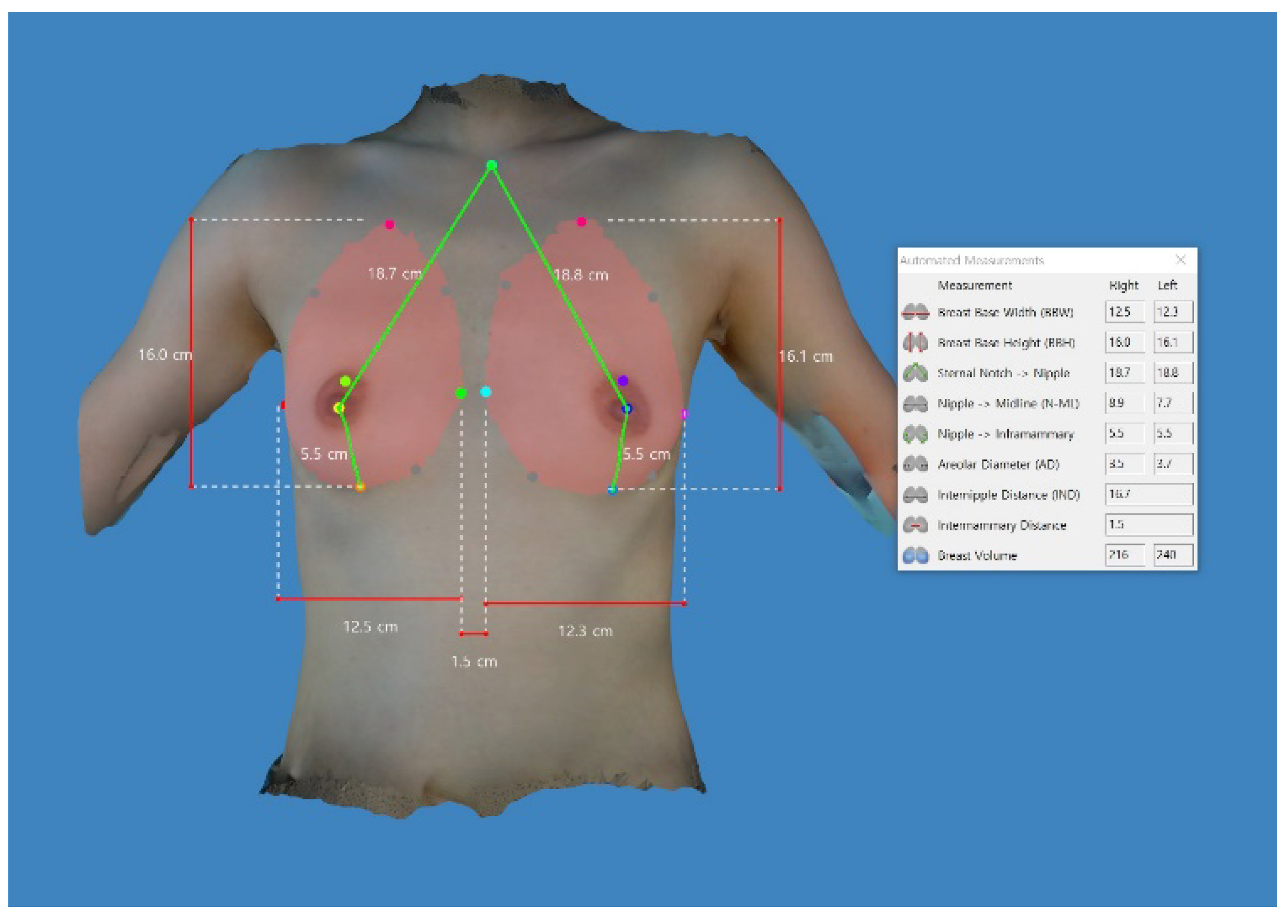
2.2. Differences in the Anthropometric Measurements between the Left and Right Side of the Breast
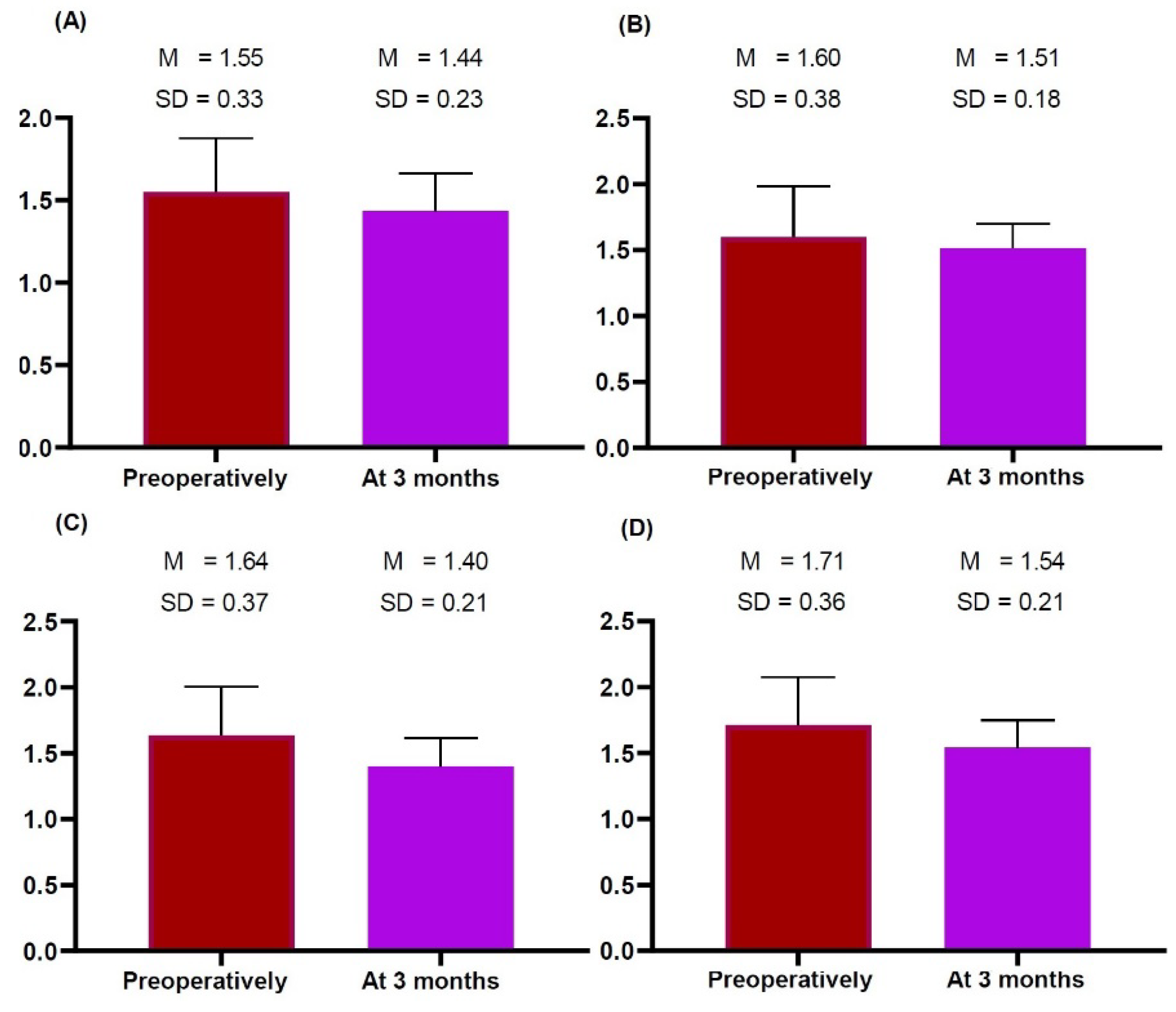
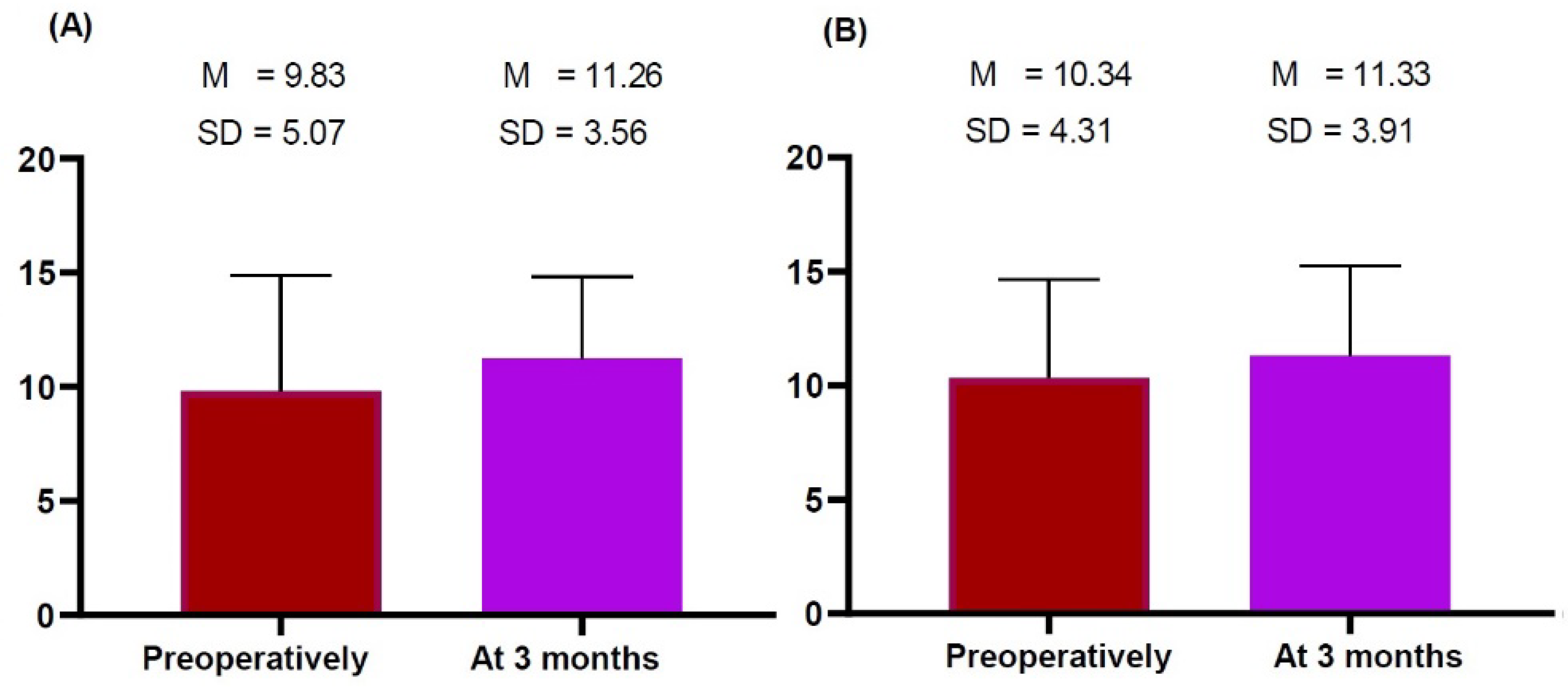
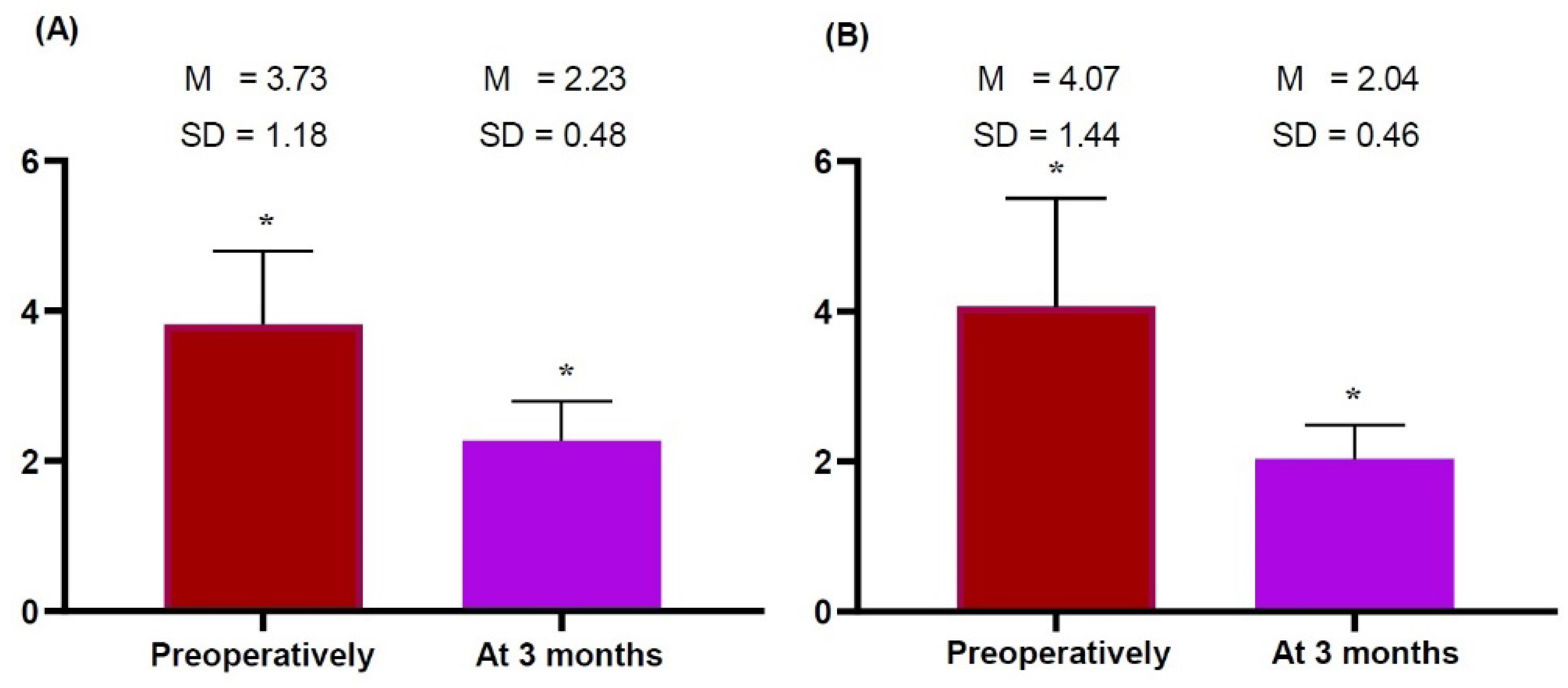
2.3. Time-Dependent Changes in the Thickness of the Dermis, Subcutaneous Tissue and Pectoralis Major Measured on HRUS
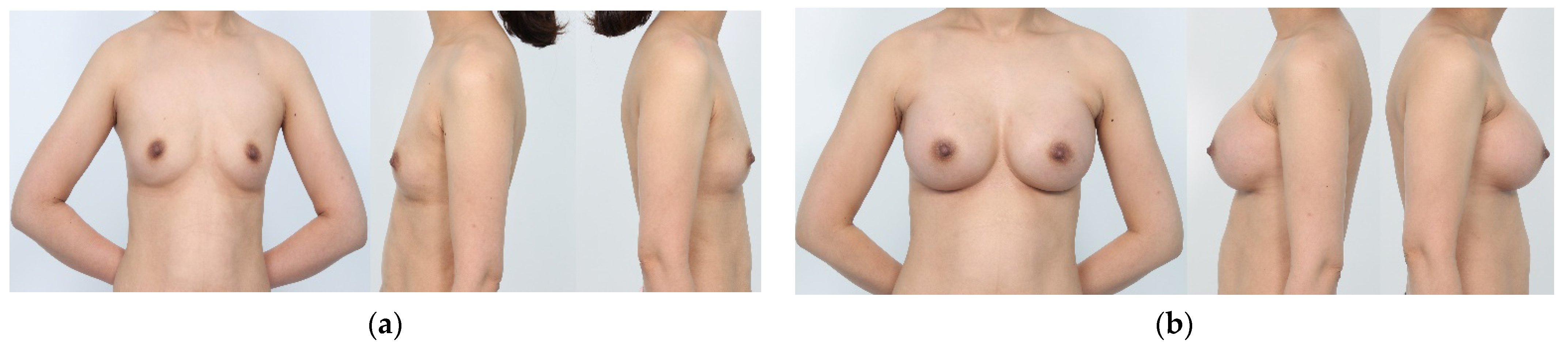
2.4. Aesthetic Outcomes
2.5. Safety Outcomes
3. Conclusions
4. Patients and Methods
4.1. Study Design
4.2. Combination of an Implant-Based Augmentation Mammaplasty with Imaging Modalities
4.3. Aessment Criteria
4.4. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Important Information for Women About Breast Augmentation with INAMED® Silicone-Filled Breast Implants. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf2/P020056d.pdf (accessed on 30 January 2023).
- Molitor, M.; Měšťák, O.; Kalinová, L.; Krajcová, A.; Měšťák, J. The history and safety of breast implants. Acta Chir. Plast. 2014, 56, 15–19. [Google Scholar]
- Park, A.J.; Black, R.J.; Sarhadi, N.S.; Chetty, U.; Watson, A.C. Silicone gel-filled breast implants and connective tissue diseases. Plast. Reconstr. Surg. 1998, 101, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Kessler, D.A. The basis of the FDA’s decision on breast implants. N. Engl. J. Med. 1992, 326, 1713–1715. [Google Scholar] [CrossRef]
- Women Should Be Aware of Ongoing Maintenance Requirements for Silicone Breast Implants. Available online: https://www.uclahealth.org/news/women-should-be-aware-of-ongoing-maintenance-requirements-for-silicone-breast-implants (accessed on 30 January 2023).
- Tanne, J.H. FDA approves silicone breast implants 14 years after their withdrawal. BMJ 2006, 333, 1139. [Google Scholar] [CrossRef]
- Rohrich, R.J.; Kaplan, J.; Dayan, E. Silicone Implant Illness: Science versus Myth? Plast. Reconstr. Surg. 2019, 144, 98–109. [Google Scholar] [CrossRef]
- Deva, A.K. A Perspective on the Never-Ending Cycle of Breast Implant Crises. Aesthet. Surg. J. 2019, 39, NP85–NP86. [Google Scholar] [CrossRef]
- McCrossan, S.; Martin, S.; Hill, C. Medical Tourism in Aesthetic Breast Surgery: A Systematic Review. Aesthet. Plast. Surg. 2021, 45, 1895–1909. [Google Scholar] [CrossRef]
- Birch, J.; Caulfield, R.; Ramakrishnan, V. The complications of ‘cosmetic tourism’—An avoidable burden on the NHS. J. Plast. Reconstr. Aesthet. Surg. 2007, 60, 1075–1077. [Google Scholar] [CrossRef] [PubMed]
- Connell, J. Medical tourism: Sea, sun, sand and surgery. Tour Manag. 2006, 27, 1093–1100. [Google Scholar] [CrossRef]
- Kim, S.; Arcodia, C.; Kim, I. Critical Success Factors of Medical Tourism: The Case of South Korea. Int. J. Environ. Res. Public Health 2019, 16, 4964. [Google Scholar] [CrossRef]
- Global Breast Implant Market, Size, Trends, and Forecasts Report 2022: A $2866.7 Million Market by 2027. Available online: https://www.prnewswire.com/news-releases/global-breast-implant-market-size-trends-and-forecasts-report-2022-a-2-866-7-million-market-by-2027--301746208.html (accessed on 16 February 2023).
- Sung, J.Y.; Jeong, J.P.; Moon, D.S.; Kim, M.S.; Kim, H.C.; Choi, W.S.; Song, K.Y.; Kim, H.J.; Lim, H.G.; Kim, J.H. Short-term Safety of Augmentation Mammaplasty Using the BellaGel Implants in Korean Women. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2566. [Google Scholar] [CrossRef]
- Roy, P.G.; Yan, Z.; Nigam, S.; Maheshwari, K. Aesthetic breast surgery: Putting in context-a narrative review. Gland Surg. 2021, 10, 2832–2846. [Google Scholar] [CrossRef]
- Thacoor, A.; van den Bosch, P.; Akhavani, M.A. Surgical Management of Cosmetic Surgery Tourism-Related Complications: Current Trends and Cost Analysis Study of the Financial Impact on the UK National Health Service (NHS). Aesthet. Surg. J. 2019, 39, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Groth, A.K.; Graf, R. Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) and the Textured Breast Implant Crisis. Aesthet. Plast. Surg. 2020, 44, 1–12. [Google Scholar] [CrossRef]
- Kim, J.H.; Paik, N.S.; Nam, S.Y.; Cho, Y.; Park, H.K. The Emerging Crisis of Stakeholders in Implant-based Augmentation Mammaplasty in Korea. J. Korean Med. Sci. 2020, 35, e103. [Google Scholar] [CrossRef]
- Kim, J.H. Association of the BellaGel® breast implant scandal with the poly implant prothèse fraud: A review of literatures. J. Surg. Open Access 2020, 7, 1–10. [Google Scholar]
- Kim, J.H. The manufacturer’s deliberate modification of the shell structure of the BellaGel® SmoothFine in violation of the regulatory requirement. J. Surg. Open Access 2021, 7, 1–5. [Google Scholar]
- Moon, D.S.; Choi, W.S.; Kim, H.C.; Jeong, J.P.; Sung, J.Y.; Kim, J.H. Short-term treatment outcomes and safety of two representative brands of the fifth-generation silicone gel-filled breast implants in Korea. J. Plast. Surg. Hand Surg. 2021, 55, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.E.; Bang, B.S.; Lee, E.K.; Sung, J.Y.; Song, K.Y.; Yoo, Y.B.; Park, D.W.; Kim, J.H. The Role of High Resolution Ultrasonography in Elucidating Features of the Breast Implants in Asymptomatic Patients After Implant-based Augmentation Mammaplasty. Aesthet. Plast. Surg. 2022, 46, 1135–1142. [Google Scholar] [CrossRef]
- Bang, B.S.; Jung, S.H.; Lee, E.K.; Sung, J.Y.; Song, K.Y.; Yoo, Y.B.; Park, D.W.; Sohn, J.E.; Kim, J.H. A Surgeon’s Empirical Perspectives on Use of High-resolution Ultrasound in Preoperatively Detecting a Rupture in the Context of Breast Implant Crisis in Korea. Aesthet. Plast. Surg. 2022, 46, 1668–1678. [Google Scholar] [CrossRef]
- Lee, S.; Jeong, J.P.; Sung, J.Y.; Choi, W.S.; Moon, D.S.; Kim, H.C.; Kim, J.H. High-Resolution Ultrasound-Assisted Assessment of Preliminary Short-term Safety Outcomes of an Implant-Based Augmentation Mammaplasty Using a Bioengineered, Cell-Friendly, Smooth-Surface Device in Korean Females. Aesthet. Surg. J. Open Forum 2021, 4, ojab046. [Google Scholar] [CrossRef]
- Nam, S.E.; Lee, S.; Cho, Y.; Kim, J.H. A Non-manufacturer-sponsored, Retrospective Study to Assess 2-year Safety Outcomes of the BellaGel® SmoothFine as Compared with Its Competitors in the Context of the First Korean Case of a Medical Device Fraud. PLoS ONE 2023, 18, e0259825. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, G.P.; Scheflan, M.; Spear, S.; Nava, M.B.; Hedén, P. Benefits and Limitations of Macrotextured Breast Implants and Consensus Recommendations for Optimizing Their Effectiveness. Aesthet. Surg. J. 2014, 34, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Adams, W.P., Jr. The process of breast augmentation: Four sequential steps for optimizing outcomes for patients. Plast. Reconstr. Surg. 2008, 122, 1892–1900. [Google Scholar] [CrossRef] [PubMed]
- Barr, S.; Bayat, A. Breast implant surface development: Perspectives on development and manufacture. Aesthet. Surg. J. 2011, 31, 56–67. [Google Scholar] [CrossRef]
- Maxwell, G.P.; Van Natta, B.W.; Bengtson, B.P.; Murphy, D.K. Ten-year results from the natrelle 410 anatomical form-stable silicone breast implant core study. Aesthet. Surg. J. 2015, 35, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Duteille, F.; Perrot, P.; Bacheley, M.H.; Bell, E.; Stewart, S. Ten-Year Safety Data for Eurosilicone’s Round and Anatomical Silicone Gel Breast Implants. Aesthet. Surg. J. Open Forum 2019, 1, ojz012. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.I.; Hammond, D.C. Clinical Results on Innovation in Breast Implant Design. Plast. Reconstr. Surg. 2018, 142, 31S–38S. [Google Scholar] [CrossRef]
- Danino, A.M.; Basmacioglu, P.; Saito, S.; Rocher, F.; Blanchet-Bardon, C.; Revol, M.; Servant, J.M. Comparison of the capsular response to the Biocell RTV and Mentor 1600 Siltex breast implant surface texturing: A scanning electron microscopic study. Plast. Reconstr. Surg. 2001, 108, 2047–2052. [Google Scholar] [CrossRef]
- Barnsley, G.P.; Sigurdson, L.J.; Barnsley, S.E. Textured surface breast implants in the prevention of capsular contracture among breast augmentation patients: A meta-analysis of randomized controlled trials. Plast. Reconstr. Surg. 2006, 117, 2182–2190. [Google Scholar] [CrossRef]
- Fagrell, D.; Berggren, A.; Tarpila, E. Capsular contracture around saline-filled fine textured and smooth mammary implants: A prospective 7.5-year follow-up. Plast. Reconstr. Surg. 2001, 108, 2108–2112. [Google Scholar] [CrossRef]
- Hakelius, L.; Ohlsén, L. Tendency to capsular contracture around smooth and textured gel-filled silicone mammary implants: A five-year follow-up. Plast. Reconstr. Surg. 1997, 100, 1566–1569. [Google Scholar] [CrossRef]
- Webb, L.H.; Aime, V.L.; Do, A.; Mossman, K.; Mahabir, R.C. Textured Breast Implants: A Closer Look at the Surface Debris Under the Microscope. Plast. Surg. 2017, 25, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, G.P.; Gabriel, A. The evolution of breast implants. Plast. Reconstr. Surg. 2014, 134, 12S–17S. [Google Scholar] [CrossRef]
- Barr, S.; Hill, E.; Bayat, A. Current implant surface technology: An examination of their nanostructure and their influence on fibroblast alignment and biocompatibility. Eplasty 2009, 9, e22. [Google Scholar] [PubMed]
- Calobrace, M.B.; Capizzi, P.J. The biology and evolution of cohesive gel and shaped implants. Plast. Reconstr. Surg. 2014, 134, 6S–11S. [Google Scholar] [CrossRef]
- Calobrace, M.B.; Schwartz, M.R.; Zeidler, K.R.; Pittman, T.A.; Cohen, R.; Stevens, W.G. Long-Term Safety of Textured and Smooth Breast Implants. Aesthet. Surg. J. 2017, 38, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Somogyi, R.B.; Brown, M.H. Outcomes in primary breast augmentation: A single surgeon’s review of 1539 consecutive cases. Plast. Reconstr. Surg. 2015, 135, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Adams, W.P., Jr.; Mallucci, P. Breast augmentation. Plast. Reconstr. Surg. 2012, 130, 597e–611e. [Google Scholar] [CrossRef]
- Mallucci, P. Discussion: Intraoperative Comparison of Anatomical versus Round Implants in Breast Augmentation: A Randomized Controlled Trial. Plast. Reconstr. Surg. 2017, 139, 599–600. [Google Scholar] [CrossRef] [PubMed]
- Adams, W.P., Jr.; Culbertson, E.J.; Deva, A.K.; Magnusson, M.R.; Layt, C.; Jewell, M.L.; Mallucci, P.; Hedén, P. Macrotextured Breast Implants with Defined Steps to Minimize Bacterial Contamination around the Device: Experience in 42,000 Implants. Plast. Reconstr. Surg. 2017, 140, 427–431. [Google Scholar] [CrossRef]
- Antony, A.K.; McCarthy, C.; Disa, J.J.; Mehrara, B.J. Bilateral implant breast reconstruction: Outcomes, predictors, and matched cohort analysis in 730 2-stage breast reconstructions over 10 years. Ann. Plast. Surg. 2014, 72, 625–630. [Google Scholar] [CrossRef]
- Shauly, O.; Gould, D.J.; Patel, K.M. Microtexture and the Cell/Biomaterial Interface: A Systematic Review and Meta-Analysis of Capsular Contracture and Prosthetic Breast Implants. Aesthet. Surg. J. 2019, 39, 603–614. [Google Scholar] [CrossRef]
- Hakelius, L.; Ohlsén, L. A clinical comparison of the tendency to capsular contracture between smooth and textured gel-filled silicone mammary implants. Plast. Reconstr. Surg. 1992, 90, 247–254. [Google Scholar] [CrossRef]
- Pollock, H. Breast capsular contracture: A retrospective study of textured versus smooth silicone implants. Plast. Reconstr. Surg. 1993, 91, 404–407. [Google Scholar] [CrossRef]
- Munhoz, A.M.; Clemens, M.W.; Nahabedian, M.Y. Breast Implant Surfaces and Their Impact on Current Practices: Where We Are Now and Where Are We Going? Plast. Reconstr. Surg. Glob. Open 2019, 7, e2466. [Google Scholar] [CrossRef]
- Atlan, M.; Nuti, G.; Wang, H.; Decker, S.; Perry, T. Breast implant surface texture impacts host tissue response. J. Mech. Behav. Biomed. Mater. 2018, 88, 377–385. [Google Scholar] [CrossRef]
- Kim, J.H.; Nam, S.E.; Sung, J.Y.; Song, K.Y.; Bang, B.S.; Lee, E.K. The Value of Capsule Thickness on Breast Ultrasound as an Indicator of the Severity of Capsular Contracture and Its Correlation with the Baker Classification. Aesthet. Plast. Surg. 2022, 46, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.E.; Bang, B.S.; Lee, E.K.; Sung, J.Y.; Song, K.Y.; Yoo, Y.B.; Park, D.W.; Kim, J.H. Use of High-resolution Ultrasound in Characterizing a Breast Implant and Detecting a Rupture of the Device Before Reoperation in Women Receiving Augmentation Mammaplasty. Plast. Reconstr. Surg. 2023; Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.; Kim, S.S.; Jeong, C.; Hwang, S.H.; Kim, T.S.; Park, J.H.; Song, Y.G.; Song, Y.K. Four-Year Interim Results of the Safety of Augmentation Mammaplasty Using the Motiva ErgonomixTM Round SilkSurface: A Multicenter, Retrospective Study. Aesthet. Plast. Surg. 2021, 45, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Tepper, O.M.; Karp, N.S.; Small, K.; Unger, J.; Rudolph, L.; Pritchard, A.; Choi, M. Three-dimensional imaging provides valuable clinical data to aid in unilateral tissue expander-implant breast reconstruction. Breast J. 2008, 14, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Longo, B.; Farcomeni, A.; Ferri, G.; Campanale, A.; Sorotos, M.; Santanelli, F. The BREAST-V: A unifying predictive formula for volume assessment in small, medium, and large breasts. Plast. Reconstr. Surg. 2013, 132, 1e–7e. [Google Scholar] [CrossRef]
- Jagsi, R.; Jiang, J.; Momoh, A.O.; Alderman, A.; Giordano, S.H.; Buchholz, T.A.; Kronowitz, S.J.; Smith, B.D. Trends and variation in use of breast reconstruction in patients with breast cancer undergoing mastectomy in the United States. J. Clin. Oncol. 2014, 32, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.W.; Cheng, Y.C.; Kurtay, M. A formula for surgical modifications of the breast. Plast. Reconstr. Surg. 2000, 106, 1342–1345. [Google Scholar] [CrossRef]
- Clemens, M.W.; Miranda, R.N. Coming of Age: Breast Implant-Associated Anaplastic Large Cell Lymphoma After 18 Years of Investigation. Clin. Plast. Surg. 2015, 42, 605–613. [Google Scholar] [CrossRef]
- Vorstenbosch, J.; McCarthy, C.M.; Shamsunder, M.G.; Polanco, T.O.; Dabic, S.; Wiser, I.; Matros, E.; Dayan, J.; Disa, J.J.; Pusic, A.L.; et al. Smooth versus Textured Implant Breast Reconstruction: Patient-Reported Outcomes and Complications. Plast. Reconstr. Surg. 2021, 148, 959–967. [Google Scholar] [CrossRef]
- McGuire, P.A.; Deva, A.K.; Glicksman, C.A.; Adams, W.P., Jr.; Haws, M.J. Management of Asymptomatic Patients With Textured Surface Breast Implants. Aesthet. Surg. J. Open Forum 2019, 1, ojz025. [Google Scholar] [CrossRef]
- Swanson, E. The Food and Drug Administration Bans Biocell Textured Breast Implants: Lessons for Plastic Surgeons. Ann. Plast. Surg. 2020, 84, 343–345. [Google Scholar] [CrossRef]
- Deva, A.K.; Cuss, A.; Magnusson, M.; Cooter, R. The “Game of Implants”: A Perspective on the Crisis-Prone History of Breast Implants. Aesthet. Surg. J. 2019, 39, S55–S65. [Google Scholar] [CrossRef]
- Clemens, M.W. Discussion: The Epidemiology of Breast Implant-Associated Anaplastic Large Cell Lymphoma in Australia and New Zealand Confirms the Highest Risk for Grade 4 Surface Breast Implants. Plast. Reconstr. Surg. 2019, 143, 1295–1297. [Google Scholar] [CrossRef] [PubMed]
- Weltz, T.K.; Larsen, A.; Hemmingsen, M.N.; Ørholt, M.; Rasmussen, L.E.; Andersen, P.S.; Sarmady, F.; Elberg, J.J.; Vester-Glowinski, P.V.; Herly, M. Breast Augmentation with Microtextured Anatomical Implants in 653 Women: Indications and Risk of Rotation. Plast. Reconstr. Surg. 2021, 147, 940e–947e. [Google Scholar] [CrossRef]
- Sforza, M.; Hammond, D.C.; Botti, G.; Hedén, P.; Chacón Quirós, M.; Munhoz, A.M.; Kinney, B.M.; Corduff, N. Expert Consensus on the Use of a New Bioengineered, Cell-Friendly, Smooth Surface Breast Implant. Aesthet. Surg. J. 2019, 39, S95–S102. [Google Scholar] [CrossRef] [PubMed]
- Sforza, M.; Zaccheddu, R.; Alleruzzo, A.; Seno, A.; Mileto, D.; Paganelli, A.; Sulaiman, H.; Payne, M.; Maurovich-Horvat, L. Preliminary 3-Year Evaluation of Experience with SilkSurface and VelvetSurface Motiva Silicone Breast Implants: A Single-Center Experience With 5813 Consecutive Breast Augmentation Cases. Aesthet. Surg. J. 2018, 38, S62–S73. [Google Scholar] [CrossRef] [PubMed]
- Kadin, M.E.; Epstein, A.L.; Adams, W.; Glicksman, C.; Sieber, D.; Hubbard, B.A.; Medeiros, L.J.; Clemens, M.W.; Miranda, R.N. Evidence that some breast implant associated anaplastic large cell lymphomas arise in the context of allergic inflammation. Blood 2017, 130, 4030. [Google Scholar]
- Leberfinger, A.N.; Behar, B.J.; Williams, N.C.; Rakszawski, K.L.; Potochny, J.D.; Mackay, D.R.; Ravnic, D.J. Breast Implant-Associated Anaplastic Large Cell Lymphoma: A Systematic Review. JAMA Surg. 2017, 152, 1161–1168. [Google Scholar] [CrossRef]
- Lista, F.; Tutino, R.; Khan, A.; Ahmad, J. Subglandular breast augmentation with textured, anatomic, cohesive silicone implants: A review of 440 consecutive patients. Plast. Reconstr. Surg. 2013, 132, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Song, K.Y.; Sung, J.Y.; Choi, W.S.; Lim, H.G.; Kim, J.H. An Ultrasound-Assisted Approach to an Early Detection of Complications of an Implant-Based Augmentation Mammaplasty using the BellaGel® SmoothFine: Preliminary 3-year Clinical Experience. J. Surg. Open Access 2021, 7, 1–12. [Google Scholar]
- Han, S.; Kim, R.; Kim, T.S.; Park, J.H.; Kim, S.S.; Jeong, C.; Lee, J.H. A Preliminary Retrospective Study to Assess the Short-Term Safety of Traditional Smooth or Microtextured Silicone Gel-Filled Breast Implants in Korea. Medicina 2021, 57, 1370. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.J.; Lee, H.; Park, B.H.; Song, Y.K.; Park, S.E.; Kim, R.; Lee, K.A. Efficacy of a 4-Week Nurse-Led Exercise Rehabilitation Program in Improving the Quality of Life in Women Receiving a Post-Mastectomy Reconstruction Using the Motiva ErgonomixTM Round SilkSurface. Int. J. Environ. Res. Public Health 2023, 20, 16. [Google Scholar] [CrossRef]
- Yoon, S.; Chang, J.H. Short-term Safety of a Silicone Gel-filled Breast Implant: A Manufacturer-sponsored, Retrospective Study. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2807. [Google Scholar] [CrossRef]
- Sforza, M.; Husein, R.; Atkinson, C.; Zaccheddu, R. Unraveling Factors Influencing Early Seroma Formation in Breast Augmentation Surgery. Aesthet. Surg. J. 2017, 37, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Berezovsky, A.B.; Pagkalos, V.A.; Krieger, Y.; Shoham, Y.; Perry, J.; Silberstein, E. Leaking seroma following breast augmentation: Technical fault or new complication? Eur. J. Plast. Surg. 2016, 39, 77–78. [Google Scholar] [CrossRef]
- Huemer, G.M.; Wenny, R.; Aitzetmüller, M.M.; Duscher, D. Motiva Ergonomix Round SilkSurface Silicone Breast Implants: Outcome Analysis of 100 Primary Breast Augmentations over 3 Years and Technical Considerations. Plast. Reconstr. Surg. 2018, 141, 831e–842e. [Google Scholar] [CrossRef] [PubMed]
- Quirós, M.C.; Bolaños, M.C.; Fassero, J.J. Six-Year Prospective Outcomes of Primary Breast Augmentation With Nano Surface Implants. Aesthet. Surg. J. 2019, 39, 495–508. [Google Scholar] [CrossRef]
- Montemurro, P.; Tay, V.K.S. Transitioning From Conventional Textured to Nanotextured Breast Implants: Our Early Experience and Modifications for Optimal Breast Augmentation Outcomes. Aesthet. Surg. J. 2021, 41, 189–195. [Google Scholar] [CrossRef]
- Munhoz, A.M.; Chala, L.; Melo, G.; Azevedo, M.N.A.; Tucunduva, T. Clinical and MRI Evaluation of Silicone Gel Implants with RFID-M Traceability System: A Prospective Controlled Cohort Study Related to Safety and Image Quality in MRI Follow-Up. Aesthetic Plast. Surg. 2021, 45, 2645–2655. [Google Scholar] [CrossRef]
- Botti, G.; Botti, C.; Ciancio, F. A Single Center’s Clinical Experience with Ergonomix Breast Implants. Aesthet. Surg. J. 2022, 42, NP312–NP318. [Google Scholar] [CrossRef]
- Kaplan, H.Y.; Rysin, R.; Zer, M.; Shachar, Y. A Single Surgeon’s experience with Motiva Ergonomix Round SilkSurface Silicone implants in breast reconstruction over a 5-year period. J. Plast. Reconstr. Aesthet. Surg. 2023; ahead of print. [Google Scholar] [CrossRef]
- Randquist, C.; Jaeger, M.; Stavrou, D. Six-Year Evaluation of Motiva Round and Ergonomix SmoothSilk Surface Silicone Breast Implants: A Two-Center, Two-Surgeon Outcome Analysis of 1053 Primary and Secondary Breast Augmentations and Augmentation Mastopexy. Aesthet. Surg. J. 2023, 43, 295–307. [Google Scholar] [CrossRef]
- Munhoz, A.M.; de Azevedo Marques Neto, A.; Maximiliano, J. Subfascial Ergonomic Axillary Hybrid (SEAH) Breast Augmentation: A Surgical Approach Combining the Advantages of Incision, Pocket, Silicone Gel, and Fat Grafting in Primary and Revision Breast Augmentation Surgery. Aesthet. Surg. J. 2021, 41, NP364–NP384. [Google Scholar] [CrossRef] [PubMed]
- Doloff, J.C.; Veiseh, O.; de Mezerville, R.; Sforza, M.; Perry, T.A.; Haupt, J.; Jamiel, M.; Chambers, C.; Nash, A.; Aghlara-Fotovat, S.; et al. The surface topography of silicone breast implants mediates the foreign body response in mice, rabbits and humans. Nat. Biomed. Eng. 2021, 5, 1115–1130. [Google Scholar] [CrossRef]
- Hamdi, M. Nano-Surface Implants: Indications and Limitations. Aesthet. Surg. J. 2021, 41, NP1141–NP1142. [Google Scholar] [CrossRef]
- Nava, M.B.; Catanuto, G.; De Vita, R.; Rancati, A.; Rocco, N. Comment on: Breast Implant Surfaces and Their Impact on Current Practices: Where Are We Now and Where Are We Going. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2639. [Google Scholar] [CrossRef]
- Niechajev, I.; Jurell, G.; Lohjelm, L. Prospective study comparing two brands of cohesive gel breast implants with anatomic shape: 5-year follow-up evaluation. Aesthet. Plast. Surg. 2007, 31, 697–710. [Google Scholar] [CrossRef]
- Marin, M.; Ellahi, R.; Vlase, S.; Bhatti, M.M. On the decay of exponential type for the solutions in a dipolar elastic body. J. Taibah Univ. Sci. 2020, 14, 534–540. [Google Scholar] [CrossRef]
- Scutaru, M.; Horatiu, T.-D.; Vlase, S.; Marin, M. Advanced HDPE with increased stiffness used for water supply networks. J. Optoelectron. Adv. M. 2015, 17, 484–488. [Google Scholar]
- Blount, A.L.; Martin, M.D.; Lineberry, K.D.; Kettaneh, N.; Alfonso, D.R. Capsular contracture rate in a low-risk population after primary augmentation mammaplasty. Aesthet. Surg. J. 2013, 33, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.; Peltoniemi, H.; Lilius, P.; Salmi, A. Povidone-iodine combined with antibiotic topical irrigation to reduce capsular contracture in cosmetic breast augmentation: A comparative study. Aesthet. Surg. J. 2013, 33, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Wixtrom, R.N.; Stutman, R.L.; Burke, R.M.; Mahoney, A.K.; Codner, M.A. Risk of breast implant bacterial contamination from endogenous breast flora, prevention with nipple shields, and implications for biofilm formation. Aesthet. Surg. J. 2012, 32, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Ajdic, D.; Zoghbi, Y.; Gerth, D.; Panthaki, Z.J.; Thaller, S. The Relationship of Bacterial Biofilms and Capsular Contracture in Breast Implants. Aesthet. Surg. J. 2016, 36, 297–309. [Google Scholar] [CrossRef]
- Hu, H.; Johani, K.; Almatroudi, A.; Vickery, K.; Natta, B.V.; Kadin, M.E.; Brody, G.; Clemens, M.; Cheah, C.Y.; Lade, S.; et al. Bacterial Biofilm Infection Detected in Breast Implant-Associated Anaplastic Large-Cell Lymphoma. Plast. Reconstr. Surg. 2016, 137, 1659–1669. [Google Scholar] [CrossRef]
- Hu, H.; Jacombs, A.; Vickery, K.; Merten, S.L.; Pennington, D.G.; Deva, A.K. Chronic biofilm infection in breast implants is associated with an increased T-cell lymphocytic infiltrate: Implications for breast implant-associated lymphoma. Plast. Reconstr. Surg. 2015, 135, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Thorne, C.H. An evidence-based approach to augmentation mammaplasty. Plast. Reconstr. Surg. 2010, 126, 2184–2188. [Google Scholar] [CrossRef]
- Lista, F.; Ahmad, J. Evidence-based medicine: Augmentation mammaplasty. Plast. Reconstr. Surg. 2013, 132, 1684–1696. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.R. Evidence-Based Medicine: Breast Augmentation. Plast. Reconstr. Surg. 2017, 140, 109e–119e. [Google Scholar] [CrossRef] [PubMed]
- Wiener, T.C. Relationship of incision choice to capsular contracture. Aesthet. Plast. Surg. 2008, 32, 303–306. [Google Scholar] [CrossRef]
- Li, S.; Chen, L.; Liu, W.; Mu, D.; Luan, J. Capsular Contracture Rate After Breast Augmentation with Periareolar Versus Other Two (Inframammary and Transaxillary) Incisions: A Meta-Analysis. Aesthet. Plast. Surg. 2018, 42, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Adams, W.P., Jr.; Conner, W.C.; Barton, F.E., Jr.; Rohrich, R.J. Optimizing breast pocket irrigation: An in vitro study and clinical implications. Plast. Reconstr. Surg. 2000, 105, 334–338. [Google Scholar] [CrossRef]
- Adams, W.P., Jr.; Conner, W.C.; Barton, F.E., Jr.; Rohrich, R.J. Optimizing breast-pocket irrigation: The post-betadine era. Plast. Reconstr. Surg. 2001, 107, 1596–1601. [Google Scholar] [CrossRef]
- Degnim, A.C.; Scow, J.S.; Hoskin, T.L.; Miller, J.P.; Loprinzi, M.; Boughey, J.C.; Jakub, J.W.; Throckmorton, A.; Patel, R.; Baddour, L.M. Randomized controlled trial to reduce bacterial colonization of surgical drains after breast and axillary operations. Ann. Surg. 2013, 258, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Ooi, A.S.; Song, D.H. Reducing infection risk in implant-based breast-reconstruction surgery: Challenges and solutions. Breast Cancer 2016, 8, 161–172. [Google Scholar]
- Schober, P.; Vetter, T.R. Survival Analysis and Interpretation of Time-to-Event Data: The Tortoise and the Hare. Anesth. Analg. 2018, 127, 792–798. [Google Scholar] [CrossRef] [PubMed]
| Variables | Values |
|---|---|
| Age (years old) | 33.79 ± 7.68 |
| Sex (male-to-female ratio) | 0:87 |
| BMI (kg/m2) | 20.38 ± 1.16 |
| FU period (days) | 183.14 ± 158.03 |
| Purpose of surgery | |
| Aesthetic augmentation mammaplasty | 86 (94.3%) |
| Type of incision | |
| Axillary incision | 74 (85.1%) |
| IMF incision | 7 (8.0%) |
| Peri-areolar incision | 6 (6.9%) |
| Volume of breast implant | |
| ≤245 | 9 (10.4%) |
| 250–295 | 26 (29.9%) |
| 300–345 | 23 (26.4%) |
| 350–395 | 16 (18.4%) |
| ≥400 | 13 (14.9%) |
| Variables | Values | t | p-Value | |
|---|---|---|---|---|
| Right | Left | |||
| Breast base width | 12.80 ± 1.06 | 12.97 ± 1.10 | −1.298 | 0.200 |
| Breast base height | 15.84 ± 1.22 | 15.94 ± 1.32 | −1.440 | 0.156 |
| Distance from the sternal notch to the nipple | 18.23 ± 1.42 | 18.13 ± 1.48 | 1.234 | 0.223 |
| Distance from the nipple to the midline | 9.26 ± 1.01 | 8.46 ± 0.86 | 4.841 | 0.000 * |
| Distance from the nipple to the inframammary fold | 5.48 ± 0.74 | 5.48 ± 0.90 | −0.025 | 0.980 |
| Breast volume | 189.67 ± 64.83 | 207.29 ± 66.57 | −2.896 | 0.006 * |
| Internipple distance | 17.84 ± 1.54 | Non-applicable | ||
| Intermammary distance | 2.10 ± 0.59 | Non-applicable | ||
| Variables | Values | t | p-Value | |
|---|---|---|---|---|
| Preoperatively | 3 Months Postoperatively | |||
| Skin | ||||
| Right superior | 1.55 ± 0.33 | 1.44 ± 0.23 | 1.252 | 0.233 |
| Right inferior | 1.60 ± 0.38 | 1.51 ± 0.28 | 0.863 | 0.404 |
| Left superior | 1.64 ± 0.37 | 1.40 ± 0.21 | 1.808 | 0.094 |
| Left inferior | 1.71 ± 0.36 | 1.54 ± 0.21 | 1.600 | 0.134 |
| Subcutaneous tissue | ||||
| Right | 9.83 ± 5.07 | 11.26 ± 3.56 | −1.609 | 0.128 |
| Left | 10.34 ± 4.31 | 11.33 ± 3.91 | −1.108 | 0.285 |
| Pectoralis major | ||||
| Right | 3.73 ± 1.18 | 2.23 ± 0.48 | 5.633 | 0.000 * |
| Left | 4.07 ± 1.44 | 2.04 ± 0.46 | 4.882 | 0.000 * |
| Variable | Value |
|---|---|
| Early seroma | 5 (5.7%) |
| Infection | 2 (2.3%) |
| Rippling | 2 (2.3%) |
| Hematoma | 1 (1.1%) |
| CC | 1 (1.1%) |
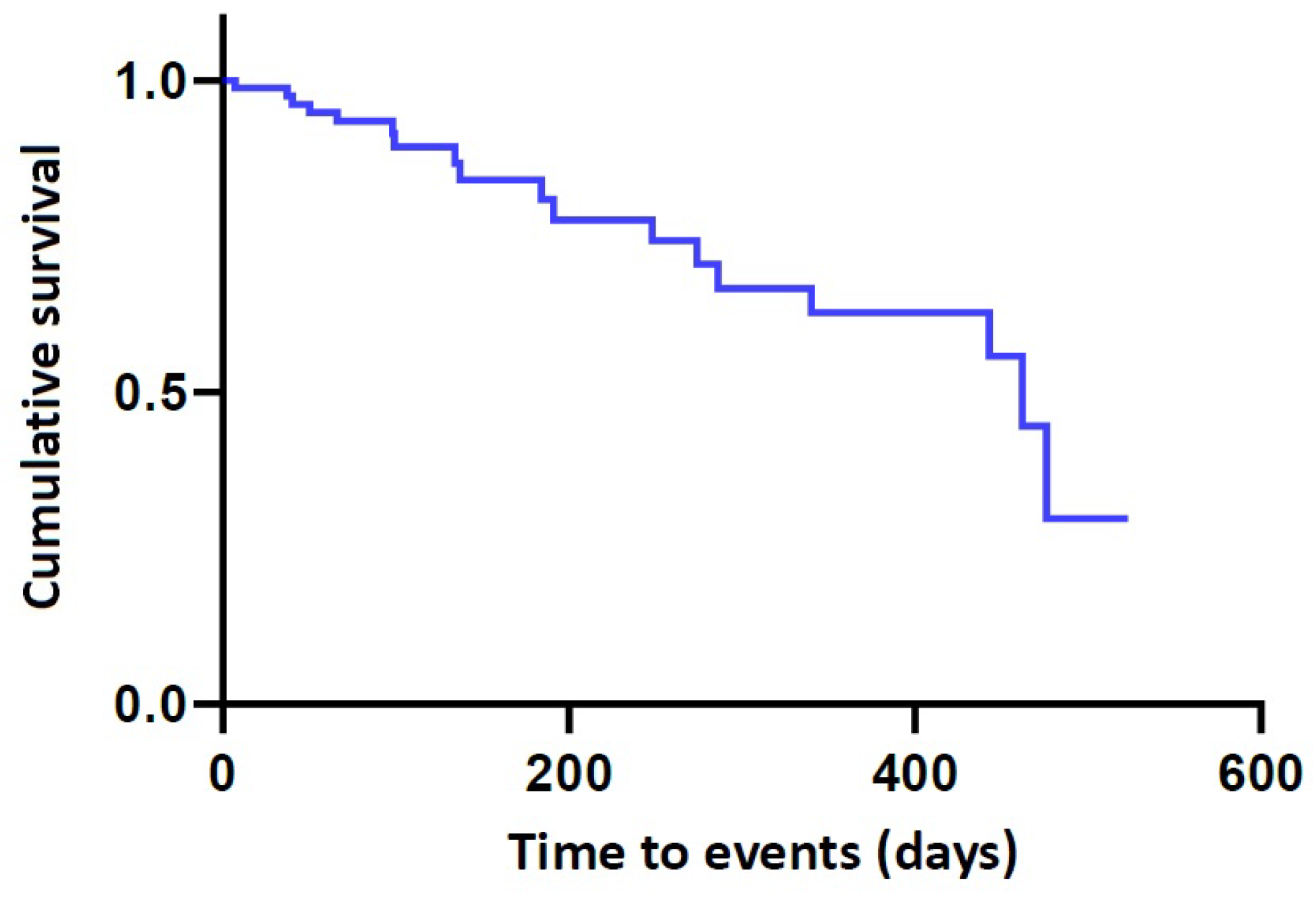
| N | n | Censored Value | Time-to-Events (months) | 95% CI |
|---|---|---|---|---|
| 87 | 18 | 69 (79.3%) | 386.68 ± 27.79 | 334.11–439.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, P.; Kang, J.K.; Hwang, S.H.; Lee, K.A. Feasibility of Imaging Modalities Combined with a Silicone Gel-Filled Breast Implant in Korean Women. Gels 2023, 9, 232. https://doi.org/10.3390/gels9030232
Hong P, Kang JK, Hwang SH, Lee KA. Feasibility of Imaging Modalities Combined with a Silicone Gel-Filled Breast Implant in Korean Women. Gels. 2023; 9(3):232. https://doi.org/10.3390/gels9030232
Chicago/Turabian StyleHong, Pa, Jae Kyoung Kang, Seung Hwan Hwang, and Kyung Ah Lee. 2023. "Feasibility of Imaging Modalities Combined with a Silicone Gel-Filled Breast Implant in Korean Women" Gels 9, no. 3: 232. https://doi.org/10.3390/gels9030232
APA StyleHong, P., Kang, J. K., Hwang, S. H., & Lee, K. A. (2023). Feasibility of Imaging Modalities Combined with a Silicone Gel-Filled Breast Implant in Korean Women. Gels, 9(3), 232. https://doi.org/10.3390/gels9030232