Permeability-Enhanced Liposomal Emulgel Formulation of 5-Fluorouracil for the Treatment of Skin Cancer
Abstract
1. Introduction
2. Results and Discussion
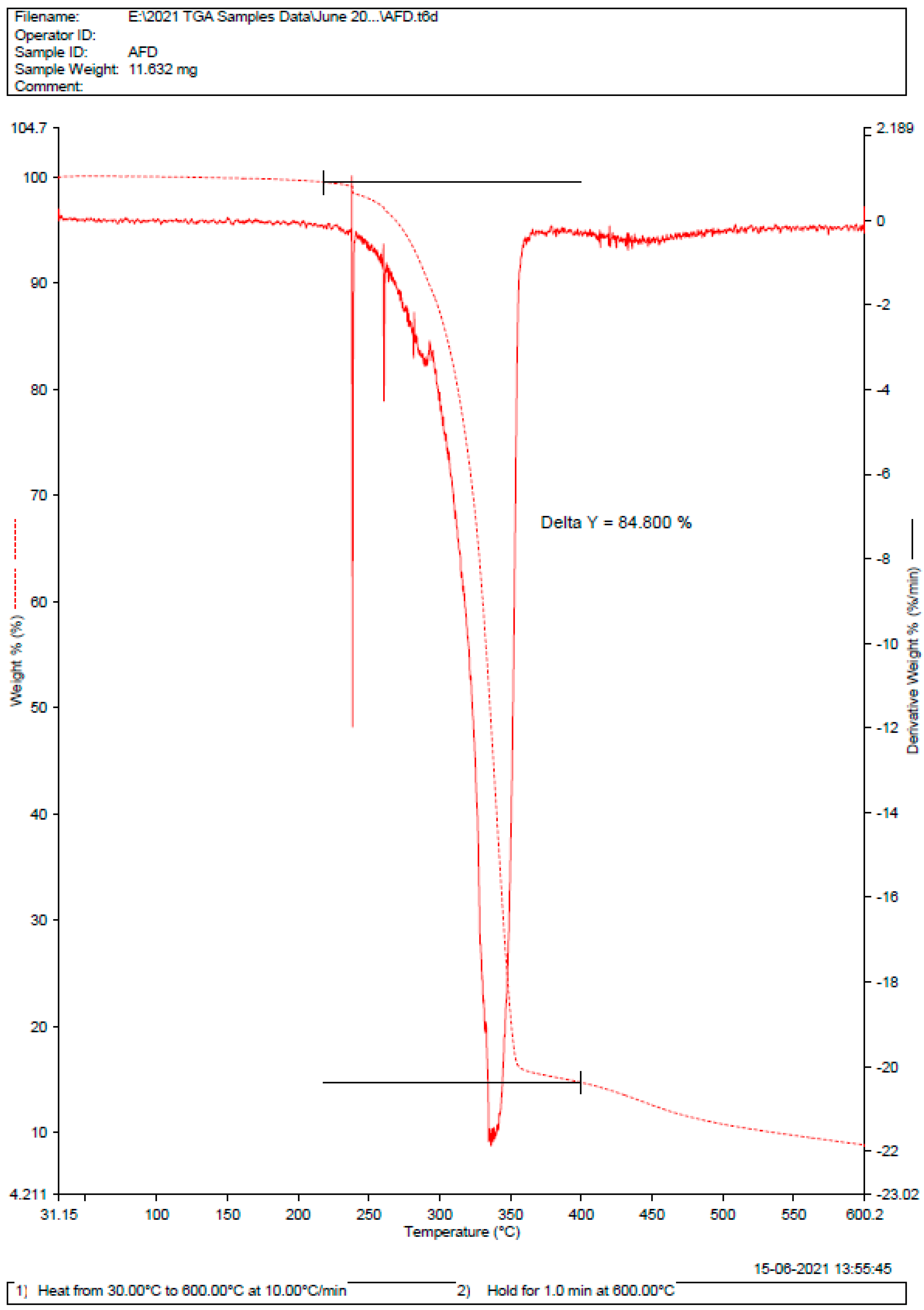
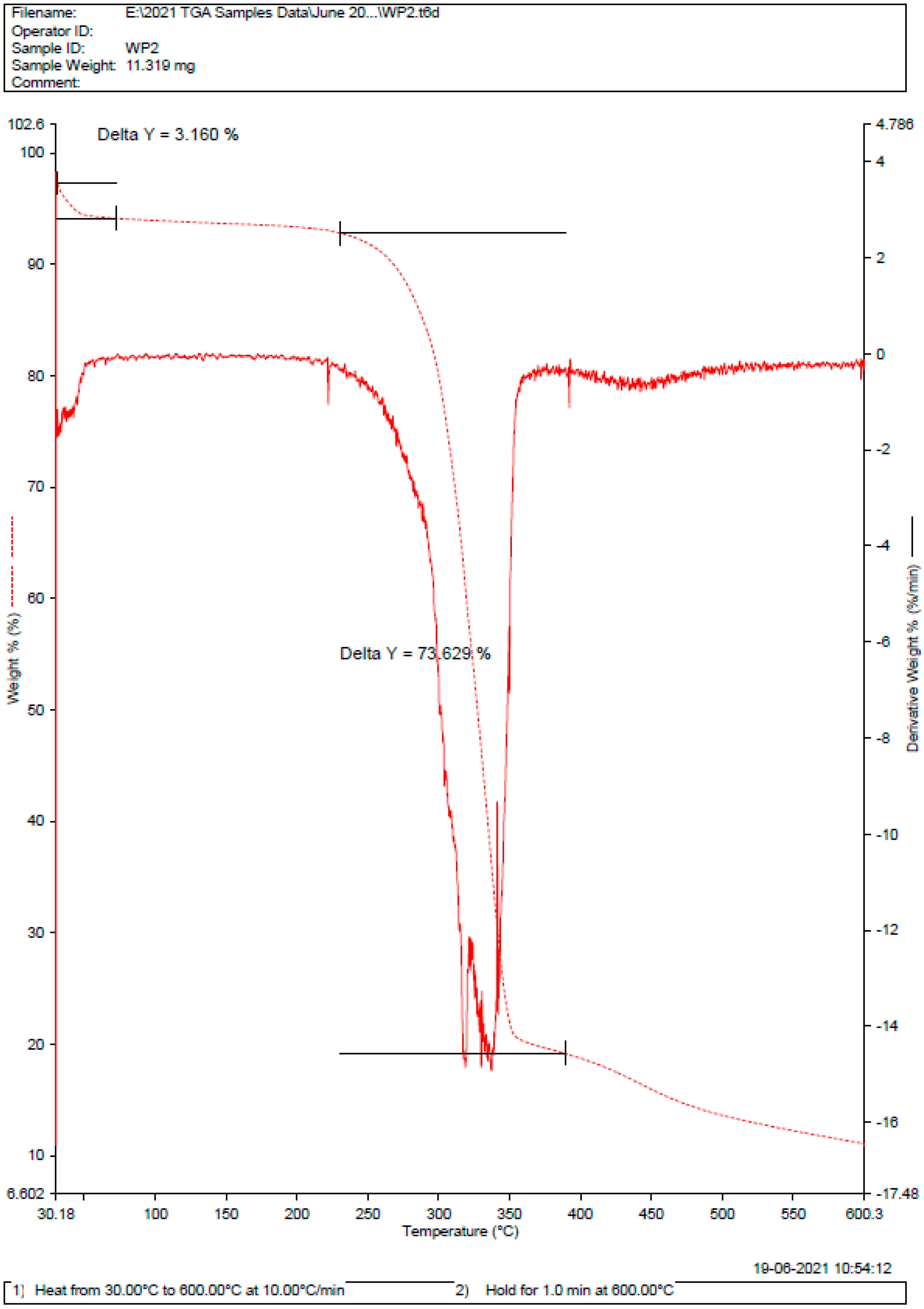
2.1. Pre-Formulation Studies
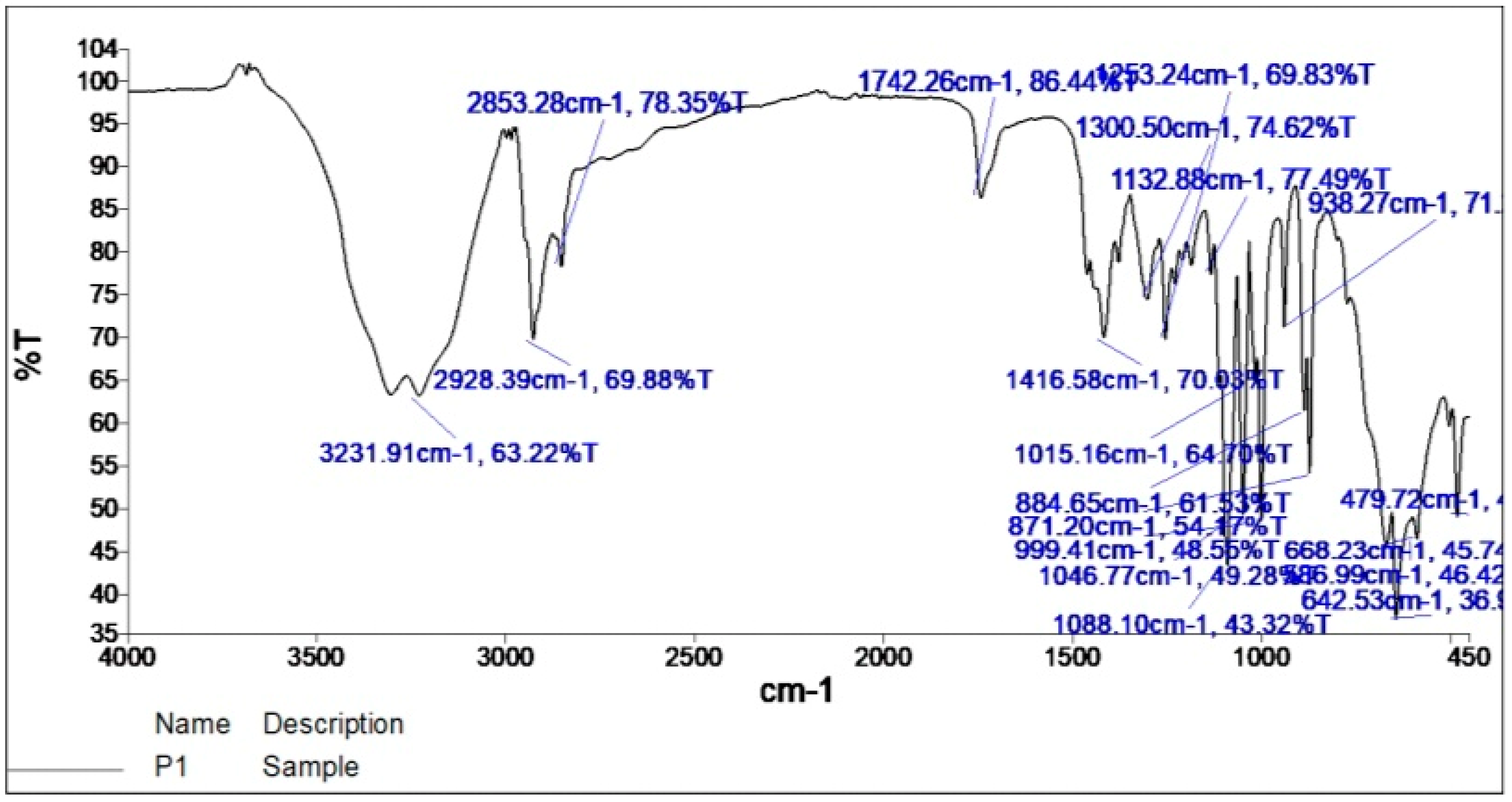
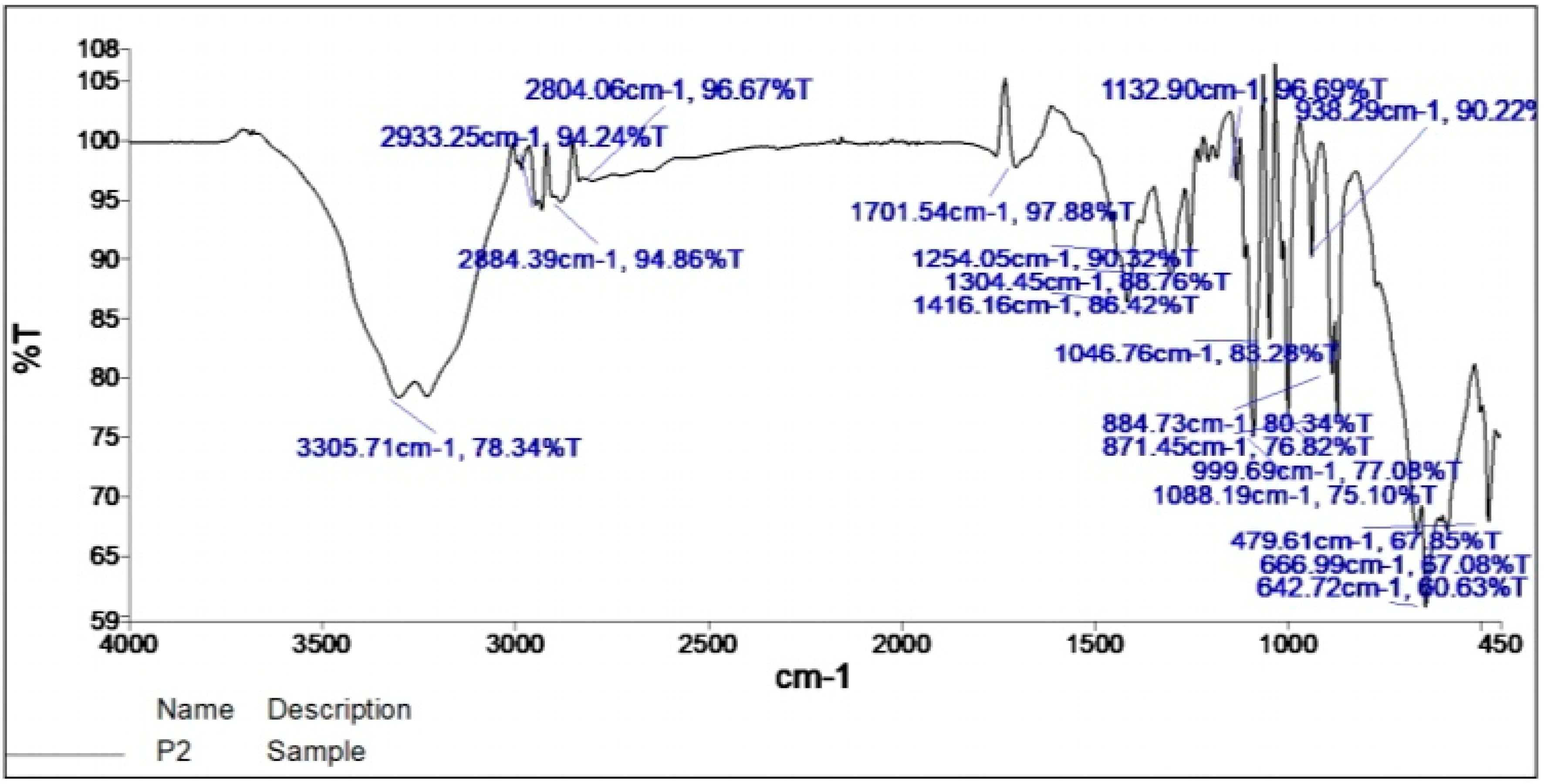
2.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.3. Differential Scanning Calorimetry (DSC)
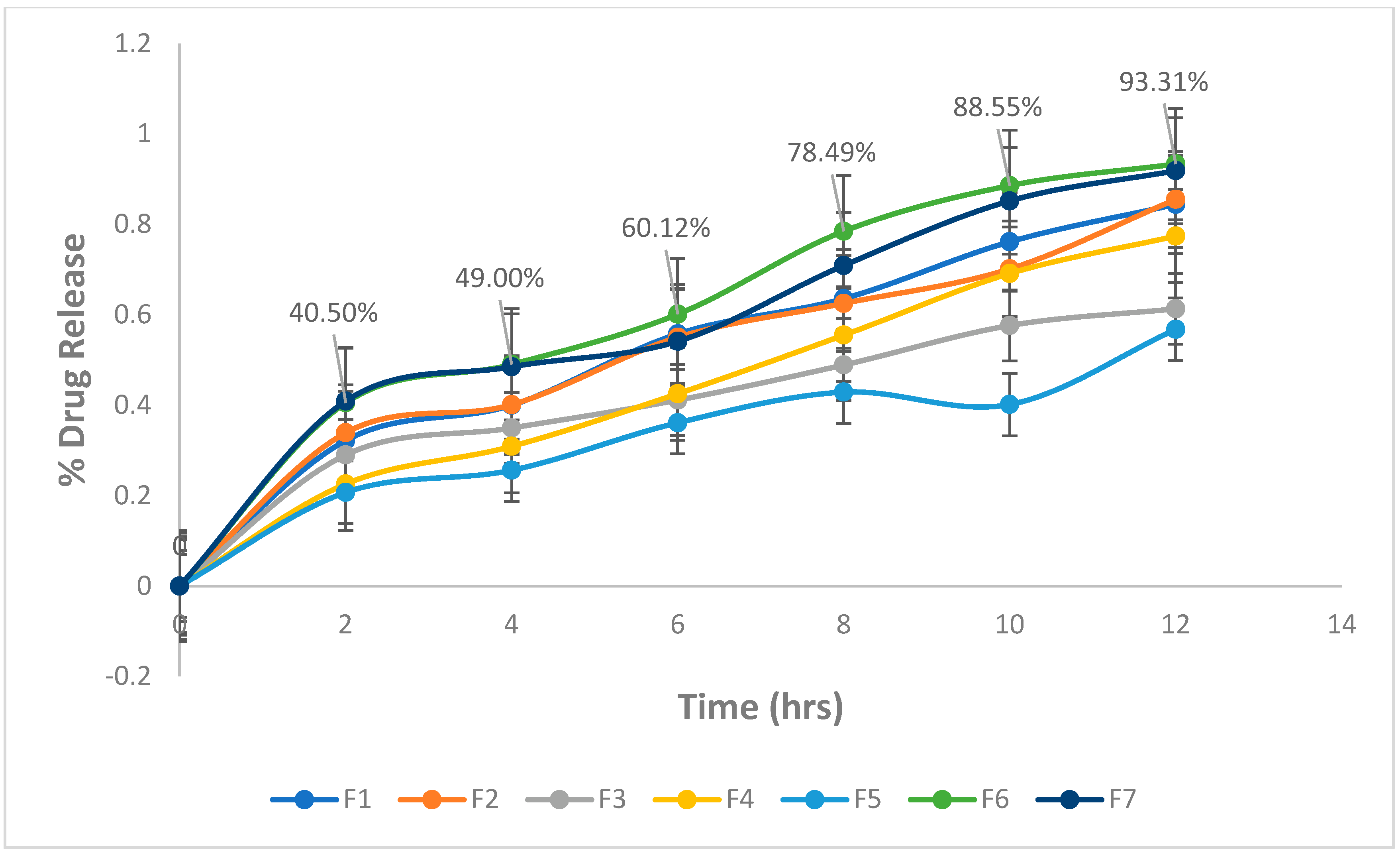
2.4. In-Vitro Drug Release Study
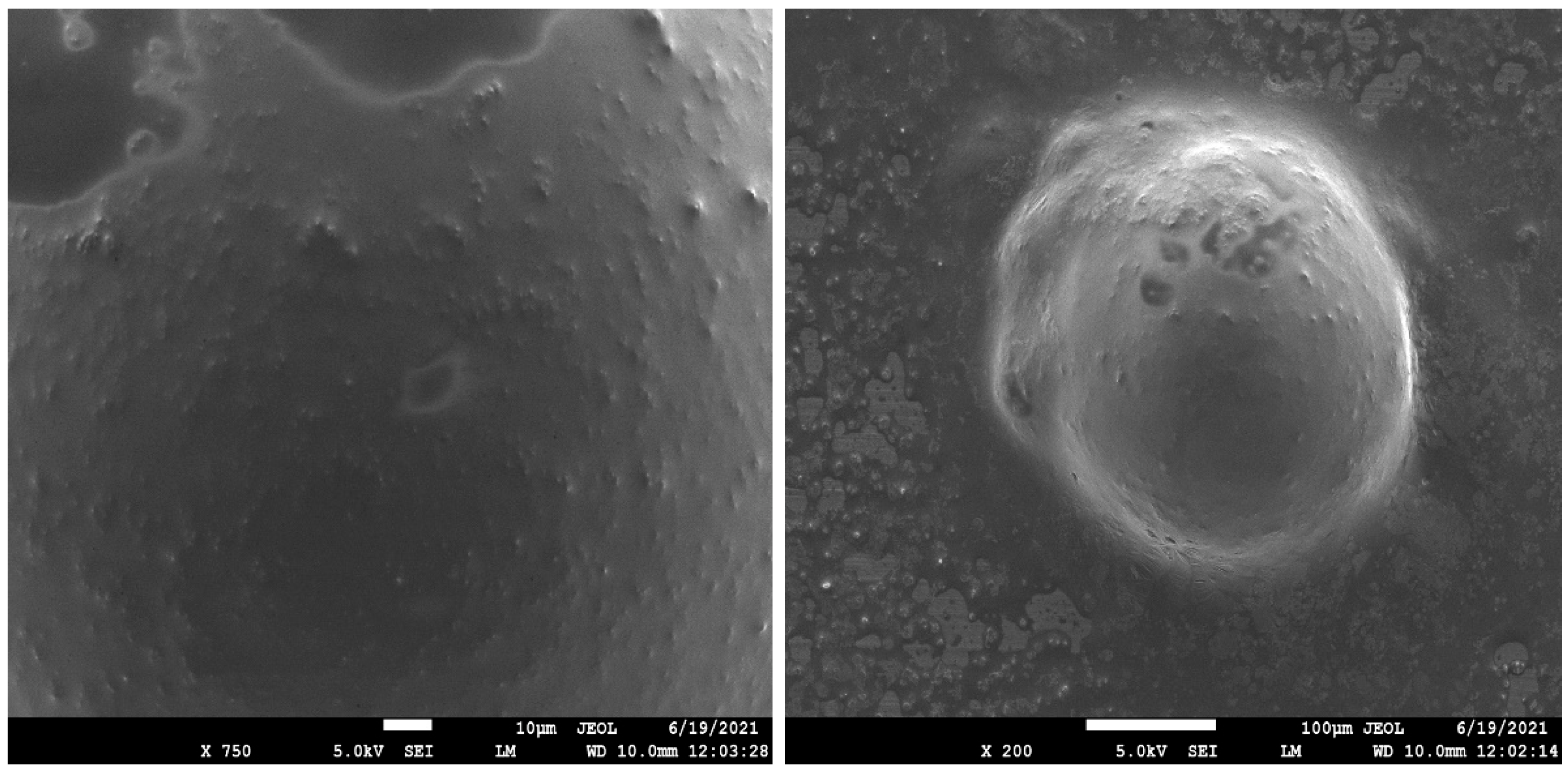
2.5. Scanning Electronic Microscopy (SEM) [39,40]
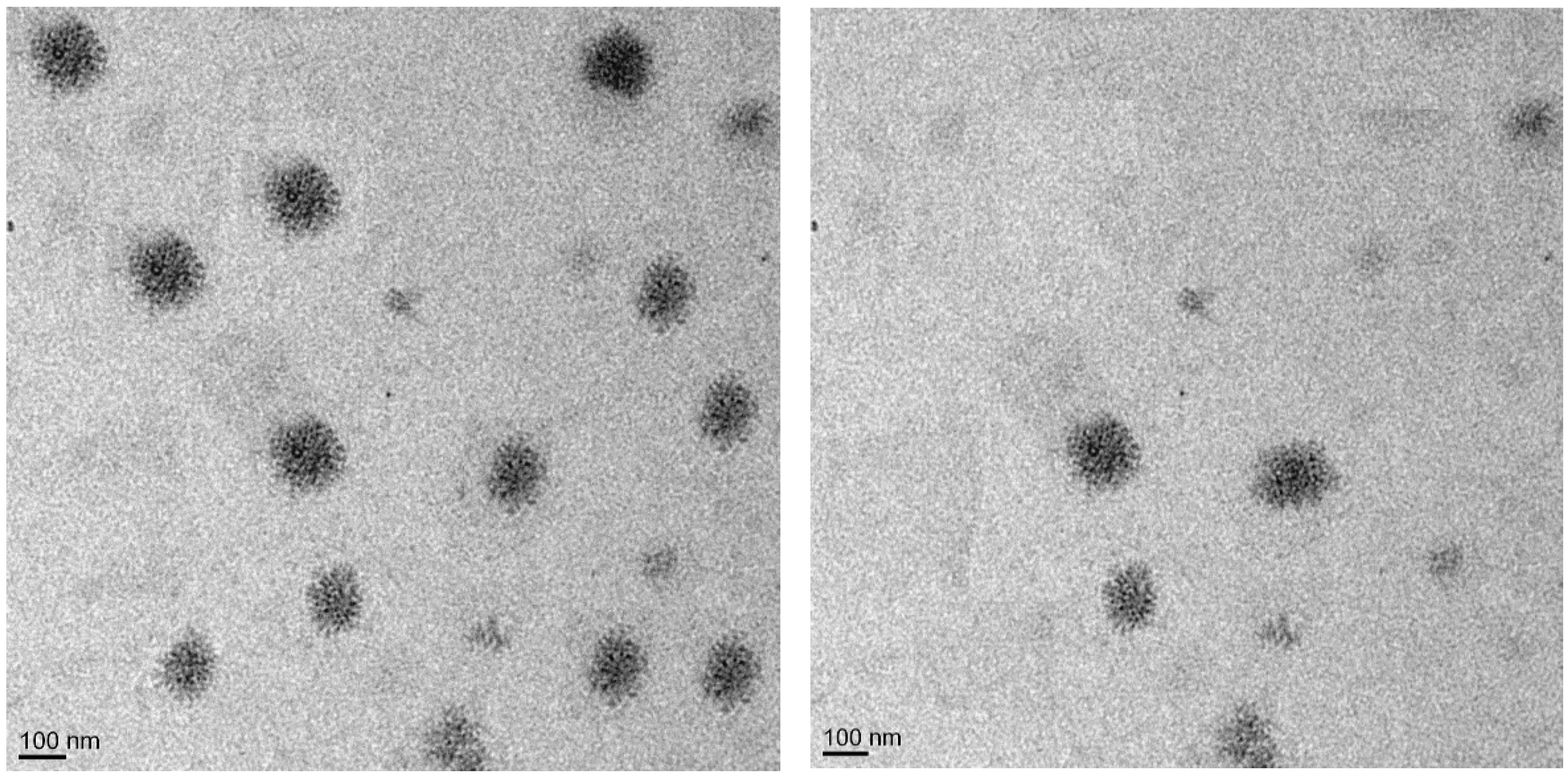
2.6. Transmission Electron Microscopy (TEM) [40,41]
2.7. Cytotoxicity Study: [43,44]
3. Conclusions
4. Materials and Methods
4.1. Drugs and Chemicals
4.2. Drug-Excipient Compatibility Studies
4.3. Fourier Transform Infrared Spectroscopy (FTIR) [50]
4.4. Diffraction and Scattering Techniques (DSC)
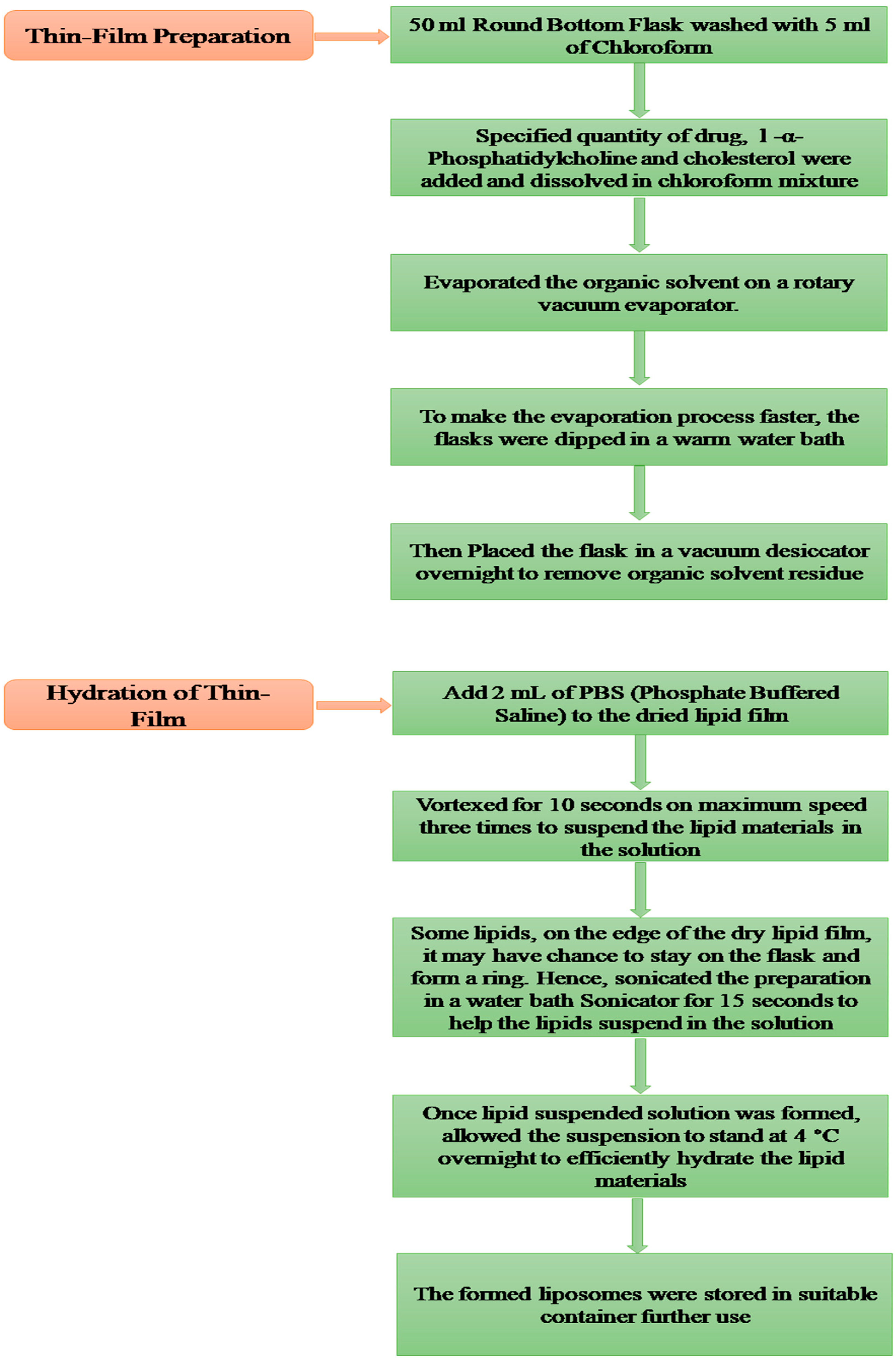
4.5. Thin Film Hydration Method for the Preparation of Liposomes
4.6. Preparation of 5FU Liposomal Emulgel
4.7. Post-Formulation Studies
4.7.1. In-Vitro Drug Release Study [54]
4.7.2. Negative Stain Transmission Electron Microscopy (TEM) [56]
4.7.3. Scanning Electron Microscopy (SEM)
4.7.4. Cytotoxicity Study [42,43]
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lomas, A.; Leonardi-Bee, J.; Bath-Hextall, F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br. J. Dermatol. 2012, 166, 1069–1080. [Google Scholar] [CrossRef]
- McKnight, G.; Shah, J.; Hargest, R. Physiology of the skin. Surgery 2022, 40, 8–12. [Google Scholar] [CrossRef]
- Hartshorne, S.; Manga, P. Chapter 6—Dermatological Aspects of Albinism. In Albinism in Africa; Kromberg, J., Manga, P., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 121–134. [Google Scholar]
- Sahu, P.; Kashaw, S.K.; Sau, S.; Kushwah, V.; Jain, S.; Agrawal, R.K.; Iyer, A.K. pH Responsive 5-Fluorouracil Loaded Biocompatible Nanogels for Topical Chemotherapy of Aggressive Melanoma. Colloids Surf. B Biointerfaces 2019, 174, 232–245. [Google Scholar] [CrossRef]
- Chadawar, V.; Shaji, J. Microsponge delivery system. Curr. Drug Deliv. 2007, 4, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Pandit, J.; Sultana, Y.; Sultana, S.; Ali, A.; Aqil, M.; Chauhan, M. Novel carbopol-based transfersomal gel of 5-fluorouracil for skin cancer treatment: In vitro characterization and in vivo study. Drug Deliv. 2015, 22, 795–802. [Google Scholar] [CrossRef]
- Al Sabbagh, C.; Seguin, J.; Agapova, E.; Kramerich, D.; Boudy, V.; Mignet, N. Thermosensitive hydrogels for local delivery of 5-fluorouracil as neoadjuvant or adjuvant therapy in colorectal cancer. Eur. J. Pharm. Biopharm. 2020, 157, 154–164. [Google Scholar] [CrossRef]
- Rai, S.; Pandey, V.; Rai, G. Transfersomes as versatile and flexible nano-vesicular carriers in skin cancer therapy: The state of the art. Nano Rev. Exp. 2017, 8, 1325708. [Google Scholar] [CrossRef]
- Carita, A.C.; Eloy, J.O.; Chorilli, M.; Lee, R.J.; Leonardi, G.R. Recent Advances and Perspectives in Liposomes for Cutaneous Drug Delivery. Curr. Med. Chem. 2018, 25, 606–635. [Google Scholar] [CrossRef]
- Abdullah, S.; El Hadad, S.; Aldahlawi, A. The development of a novel oral 5-Fluorouracil in-situ gelling nanosuspension to potentiate the anticancer activity against colorectal cancer cells. Int. J. Pharm. 2022, 613, 121406. [Google Scholar] [CrossRef]
- Pandey, M.; Choudhury, H.; Gorain, B.; Tiong, S.Q.; Wong, G.Y.S.; Chan, K.X.; They, X.; Chieu, W.S. Site-Specific Vesicular Drug Delivery System for Skin Cancer: A Novel Approach for Targeting. Gels 2021, 7, 218. [Google Scholar] [CrossRef]
- De Santis, S.; Diociaiuti, M.; Cametti, C.; Masci, G. Hyaluronic acid and alginate covalent nanogels by template cross-linking in polyion complex micelle nanoreactors. Carbohydr. Polym. 2014, 101, 96–103. [Google Scholar] [CrossRef]
- Sultana, S.S.; Parveen, P.; Rekha, M.S.; Deepthi, K.; Sowjanya, C.; Devi, A.S. Emulgel- A novel surrogate approach for transdermal drug delivery system. Indo Am. J. Pharm. Res. 2014, 4, 5250–5265. [Google Scholar]
- Talat, M.; Zaman, M.; Khan, R.; Jamshaid, M.; Akhtar, M.; Mirza, A.Z. Emulgel: An effective drug delivery system. Drug Dev. Ind. Pharm. 2021, 47, 1193–1199. [Google Scholar] [CrossRef]
- Tanaji, D.N. Emulgel: A comprehensive review for topical delivery of hydrophobic drugs. Asian J. Pharm. (AJP) 2018, 12, S382. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Essential oils and their constituents as skin penetration enhancer for transdermal drug delivery: A review. J. Pharm. Pharmacol. 2015, 67, 473–485. [Google Scholar] [CrossRef]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol. Ther. 2020, 206, 107447. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F. Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Campanholi, K.D.S.S.; Junior, R.C.D.S.; Jaski, J.M.; Silva, J.B.D.; Oliveira, M.C.; Santos, R.S.D.; Pozza, M.S.D.S.; Castro-Hoshino, L.V.; Baesso, M.L.; Cardozo-Filho, L.; et al. Thermo and Photoresponsive Emulgel Loaded with Copaifera reticulata Ducke and Chlorophylls: Rheological, Mechanical, Photodynamic and Drug Delivery Properties in Human Skin. Pharmaceutics 2022, 14, 2798. [Google Scholar] [CrossRef]
- Donthi, M.R.; Munnangi, S.R.; Krishna, K.V.; Saha, R.N.; Singhvi, G.; Dubey, S.K. Nanoemulgel: A Novel Nano Carrier as a Tool for Topical Drug Delivery. Pharmaceutics 2023, 15, 164. [Google Scholar] [CrossRef]
- Hawash, M.; Jaradat, N.; Eid, A.M.; Abubaker, A.; Mufleh, O.; Al-Hroub, Q.; Sobuh, S. Synthesis of novel isoxazole-carboxamide derivatives as promising agents for melanoma and targeted nano-emulgel conjugate for improved cellular permeability. BMC Chem. 2022, 16, 47. [Google Scholar] [CrossRef]
- Sindi, A.M.; Rizg, W.Y.; Khan, M.K.; Alkhalidi, H.M.; Alharbi, W.S.; Sabei, F.Y.; Alfayez, E.; Alkharobi, H.; Korayem, M.; Majrashi, M.; et al. Tailoring and optimization of a honey-based nanoemulgel loaded with an itraconazole-thyme oil nanoemulsion for oral candidiasis. Drug Deliv. 2023, 30, 2173337. [Google Scholar] [CrossRef] [PubMed]
- Madawi, E.A.; Al Jayoush, A.R.; Rawas-Qalaji, M.; Thu, H.E.; Khan, S.; Sohail, M.; Mahmood, A.; Hussain, Z. Polymeric Nanoparticles as Tunable Nanocarriers for Targeted Delivery of Drugs to Skin Tissues for Treatment of Topical Skin Diseases. Pharmaceutics 2023, 15, 657. [Google Scholar] [CrossRef]
- Manian, M.; Jain, P.; Vora, D.; Banga, A.K. Formulation and Evaluation of the In Vitro Performance of Topical Dermatological Products Containing Diclofenac Sodium. Pharmaceutics 2022, 14, 1892. [Google Scholar] [CrossRef] [PubMed]
- Zari, A.T.; Zari, T.A.; Hakeem, K.R. Anticancer Properties of Eugenol: A Review. Molecules 2021, 26, 7407. [Google Scholar] [CrossRef]
- Batiha, G.E.; Alkazmi, L.M.; Wasef, L.G.; Beshbishy, A.M.; Nadwa, E.H.; Rashwan, E.K. Syzygium aromaticum L. (Myrtaceae): Traditional Uses, Bioactive Chemical Constituents, Pharmacological and Toxicological Activities. Biomolecules 2020, 10, 202. [Google Scholar] [CrossRef]
- Abiri, R.; Atabaki, N.; Sanusi, R.; Malik, S.; Abiri, R.; Safa, P.; Shukor, N.A.A.; Abdul-Hamid, H. New insights into the biological properties of eucalyptus-derived essential oil: A promising green anti-cancer drug. Food Rev. Int. 2022, 38 (Suppl. 1), 598–633. [Google Scholar] [CrossRef]
- Shetty, S.; Jose, J.; Kumar, L.; Charyulu, R.N. Novel ethosomal gel of clove oil for the treatment of cutaneous candidiasis. J. Cosmet. Dermatol. 2019, 18, 862–869. [Google Scholar] [CrossRef]
- Amra, K.; Momin, M.; Desai, N.; Khan, F. Therapeutic benefits of natural oils along with permeation enhancing activity. Int. J. Dermatol. 2022, 61, 484–507. [Google Scholar] [CrossRef]
- Roy, A.; Nishchaya, K.; Rai, V.K. Nanoemulsion-based dosage forms for the transdermal drug delivery applications: A review of recent advances. Expert Opin. Drug Deliv. 2022, 19, 303–319. [Google Scholar] [CrossRef]
- Pawar, K.R.; Babu, R.J. Polymeric and lipid-based materials for topical nanoparticle delivery systems. Crit. Rev. Ther. Drug Carr. Syst. 2010, 27, 419–459. [Google Scholar] [CrossRef]
- Udofot, O.; Affram, K.; Israel, B.; Agyare, E. Cytotoxicity of 5-fluorouracil-loaded pH-sensitive liposomal nanoparticles in colorectal cancer cell lines. Integr. Cancer Sci. Ther. 2015, 2, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Chountoulesi, M.; Naziris, N.; Mavromoustakos, T.; Demetzos, C. A Differential Scanning Calorimetry (DSC) Experimental Protocol for Evaluating the Modified Thermotropic Behavior of Liposomes with Incorporated Guest Molecules. Methods Mol. Biol. 2021, 2207, 299–312. [Google Scholar] [CrossRef]
- Igisu, M.; Yokoyama, T.; Ueno, Y.; Nakashima, S.; Shimojima, M.; Ohta, H.; Maruyama, S. Changes of aliphatic C-H bonds in cyanobacteria during experimental thermal maturation in the presence or absence of silica as evaluated by FTIR microspectroscopy. Geobiology 2018, 16, 412–428. [Google Scholar] [CrossRef] [PubMed]
- Samy, M.; Abd El-Alim, S.H.; Rabia, A.E.G.; Amin, A.; Ayoub, M.M.H. Formulation, characterization and in vitro release study of 5-fluorouracil loaded chitosan nanoparticles. Int. J. Biol. Macromol. 2020, 156, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Velez-Saboyá, C.S.; Guzmán-Sepúlveda, J.R.; Ruiz-Suárez, J.C. Phase transitions of liposomes: When light meets heat. J. Phys. Condens. Matter Inst. Phys. J. 2022, 34, 124002. [Google Scholar] [CrossRef]
- Mohammed, A.M.; Osman, S.K.; Saleh, K.I.; Samy, A.M. In Vitro Release of 5-Fluorouracil and Methotrexate from Different Thermosensitive Chitosan Hydrogel Systems. AAPS PharmSciTech 2020, 21, 131. [Google Scholar] [CrossRef]
- Sopyan, I.; InsanSunan, K.; Gozali, D. A Review: A Novel of Efforts to Enhance Liposome Stability as Drug Delivery Approach. Syst. Rev. Pharm. 2020, 11, 555–562. [Google Scholar] [CrossRef]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef]
- Fan, Y.; Marioli, M.; Zhang, K. Analytical characterization of liposomes and other lipid nanoparticles for drug delivery. J. Pharm. Biomed. Anal. 2021, 192, 113642. [Google Scholar] [CrossRef]
- Katharotiya, K.; Shinde, G.; Katharotiya, D.; Shelke, S.; Patel, R.; Kulkarni, D.; Panzade, P. Development, evaluation and biodistribution of stealth liposomes of 5-fluorouracil for effective treatment of breast cancer. J. Liposome Res. 2022, 32, 146–158. [Google Scholar] [CrossRef]
- El Maghraby, G.M.; Arafa, M.F. Liposomes for Enhanced Cellular Uptake of Anticancer Agents. Curr. Drug Deliv. 2020, 17, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Al-Asmari, A.K.; Ullah, Z.; Al Balowi, A.; Islam, M. In vitro determination of the efficacy of scorpion venoms as anti-cancer agents against colorectal cancer cells: A nano-liposomal delivery approach. Int. J. Nanomed. 2017, 12, 559–574. [Google Scholar] [CrossRef] [PubMed]
- Ewert de Oliveira, B.; Junqueira Amorim, O.H.; Lima, L.L.; Rezende, R.A.; Mestnik, N.C.; Bagatin, E.; Leonardi, G.R. 5-Fluorouracil, innovative drug delivery systems to enhance bioavailability for topical use. J. Drug Deliv. Sci. Technol. 2021, 61, 102155. [Google Scholar] [CrossRef]
- Horváth, A.; Pandur, E.; Sipos, K.; Micalizzi, G.; Mondello, L.; Böszörményi, A.; Birinyi, P.; Horváth, G. Anti-inflammatory effects of lavender and eucalyptus essential oils on the in vitro cell culture model of bladder pain syndrome using T24 cells. BMC Complement. Med. Ther. 2022, 22, 119. [Google Scholar] [CrossRef]
- Prashar, A.; Locke, I.C.; Evans, C.S. Cytotoxicity of clove (Syzygium aromaticum) oil and its major components to human skin cells. Cell Prolif. 2006, 39, 241–248. [Google Scholar] [CrossRef]
- Crisóstomo, L.C.C.F.; Carvalho, G.S.G.; Leal, L.K.A.M.; de Araújo, T.G.; Nogueira, K.A.B.; da Silva, D.A.; de Oliveira Silva Ribeiro, F.; Petrilli, R.; Eloy, J.O. Sorbitan Monolaurate-Containing Liposomes Enhance Skin Cancer Cell Cytotoxicity and in Association with Microneedling Increase the Skin Penetration of 5-Fluorouracil. AAPS PharmSciTech 2022, 23, 212. [Google Scholar] [CrossRef]
- Tadić, V.M.; Žugić, A.; Martinović, M.; Stanković, M.; Maksimović, S.; Frank, A.; Nešić, I. Enhanced Skin Performance of Emulgel vs. Cream as Systems for Topical Delivery of Herbal Actives (Immortelle Extract and Hemp Oil). Pharmaceutics 2021, 13, 1919. [Google Scholar] [CrossRef]
- Sainy, J.; Atneriya, U.; Kori, J.L.; Maheshwari, R. Development of an Aloe vera-based Emulgel for the Topical Delivery of Desoximetasone. Turk. J. Pharm. Sci. 2021, 18, 465–475. [Google Scholar] [CrossRef]
- Patel, G.; Yadav, B.K.N. Formulation, Characterization and In vitro Cytotoxicity of 5-Fluorouracil Loaded Polymeric Electrospun Nanofibers for the Treatment of Skin Cancer. Recent Pat. Nanotechnol. 2019, 13, 114–128. [Google Scholar] [CrossRef]
- Karthika, C.; Sureshkumar, R.; Sajini, D.V.; Ashraf, G.M.; Rahman, M.H. 5-fluorouracil and curcumin with pectin coating as a treatment regimen for titanium dioxide with dimethylhydrazine-induced colon cancer model. Environ. Sci. Pollut. Res. Int. 2022, 29, 63202–63215. [Google Scholar] [CrossRef]
- Arafat, M.; Fouladian, P.; Wignall, A.; Song, Y.; Parikh, A.; Albrecht, H.; Prestidge, C.A.; Garg, S.; Blencowe, A. Development and In Vitro Evaluation of 5-Fluorouracil-Eluting Stents for the Treatment of Colorectal Cancer and Cancer-Related Obstruction. Pharmaceutics 2020, 13, 17. [Google Scholar] [CrossRef]
- Vanpariya, F.; Shiroya, M.; Malaviya, M. Emulgel: A Review. Int. J. Sci. Res 2021, 10, 847. [Google Scholar] [CrossRef]
- Zhang, L.; Tong, H.; Garewal, M.; Ren, G. Optimized negative-staining electron microscopy for lipoprotein studies. Biochim. Biophys. Acta 2013, 1830, 2150–2159. [Google Scholar] [CrossRef] [PubMed]
- Power, E.A.; Fernandez-Torres, J.; Zhang, L.; Yaun, R.; Lucien, F.; Daniels, D.J. Chorioallantoic membrane (CAM) assay to study treatment effects in diffuse intrinsic pontine glioma. PLoS ONE 2022, 17, e0263822. [Google Scholar] [CrossRef] [PubMed]
- Alvi, I.A.; Madan, J.; Kaushik, D.; Sardana, S.; Pandey, R.S.; Ali, A. Comparative study of transfersomes, liposomes, and niosomes for topical delivery of 5-fluorouracil to skin cancer cells: Preparation, characterization, in-vitro release, and cytotoxicity analysis. Anti Cancer Drugs 2011, 22, 774–782. [Google Scholar] [CrossRef]

| Ingredients | %w/w | ||||||
|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | |
| 5-fluorouracil | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| HPMC | 0.05 | 2.0 | 0.05 | 2.0 | 0.05 | 0.2 | 0.05 |
| Triethanolamine | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| Light liquid paraffin | 3.5 | 2.65 | 3.5 | 2.65 | 3.5 | 2.65 | 3.5 |
| Tween 20 | 0.5 | 0.3 | 0.3 | 0.3 | 0.5 | 0.5 | 0.5 |
| Propylene glycol | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 |
| Sodium benzoate | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Ethanol | 1.55 | 1.55 | 1.55 | 1.55 | 1.55 | 1.55 | 1.55 |
| Eucalyptus oil | 1 | 5 | - | - | - | 5 | 1 |
| Clove oil | - | - | 1 | 5 | - | 1 | 5 |
| Purified water (q.s) | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pachauri, A.; Chitme, H.; Visht, S.; Chidrawar, V.; Mohammed, N.; Abdel-Wahab, B.A.; Khateeb, M.M.; Habeeb, M.S.; Orabi, M.A.A.; Bakir, M.B. Permeability-Enhanced Liposomal Emulgel Formulation of 5-Fluorouracil for the Treatment of Skin Cancer. Gels 2023, 9, 209. https://doi.org/10.3390/gels9030209
Pachauri A, Chitme H, Visht S, Chidrawar V, Mohammed N, Abdel-Wahab BA, Khateeb MM, Habeeb MS, Orabi MAA, Bakir MB. Permeability-Enhanced Liposomal Emulgel Formulation of 5-Fluorouracil for the Treatment of Skin Cancer. Gels. 2023; 9(3):209. https://doi.org/10.3390/gels9030209
Chicago/Turabian StylePachauri, Ankur, Havagiray Chitme, Sharad Visht, Vijay Chidrawar, Nawaj Mohammed, Basel A. Abdel-Wahab, Masood Medleri Khateeb, Mohammed Shafiuddin Habeeb, Mohamed A. A. Orabi, and Marwa B. Bakir. 2023. "Permeability-Enhanced Liposomal Emulgel Formulation of 5-Fluorouracil for the Treatment of Skin Cancer" Gels 9, no. 3: 209. https://doi.org/10.3390/gels9030209
APA StylePachauri, A., Chitme, H., Visht, S., Chidrawar, V., Mohammed, N., Abdel-Wahab, B. A., Khateeb, M. M., Habeeb, M. S., Orabi, M. A. A., & Bakir, M. B. (2023). Permeability-Enhanced Liposomal Emulgel Formulation of 5-Fluorouracil for the Treatment of Skin Cancer. Gels, 9(3), 209. https://doi.org/10.3390/gels9030209








