Cross-Linking of Oxidized Hydroxypropyl Cellulose in Paper: Influence of Molecular Weight and Polymer Distribution on Paper Wet Strength Development
Abstract
1. Introduction
2. Results and Discussion
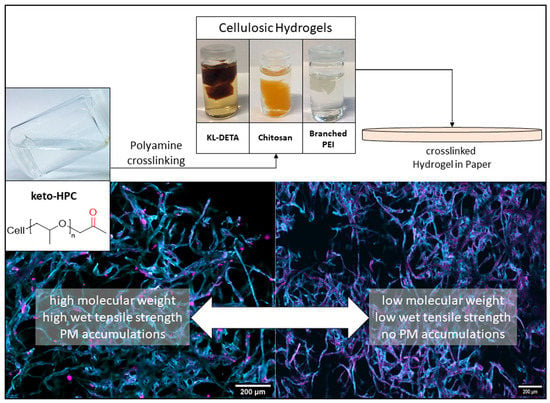
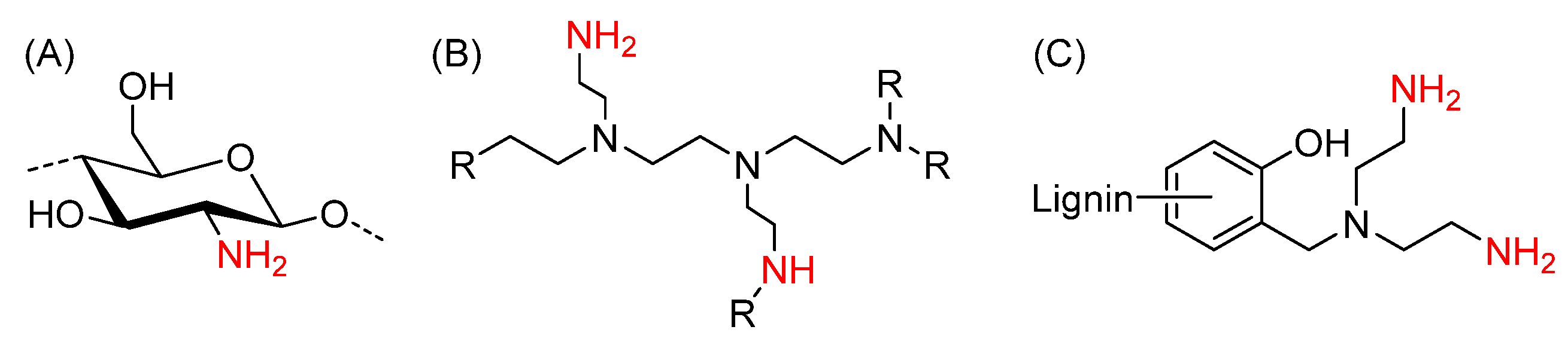
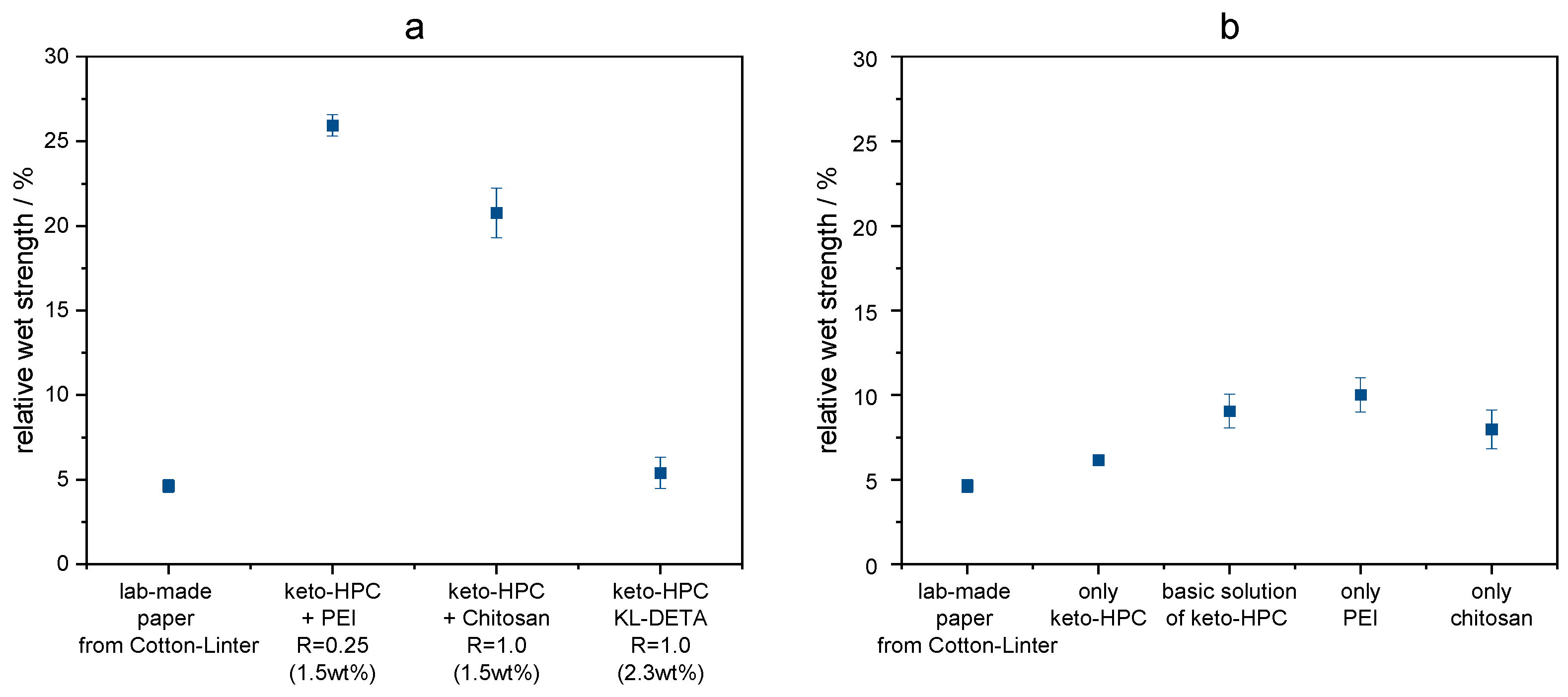
2.1. Hydrogels Made from Keto-HPC Cross-Linked with Polyamines
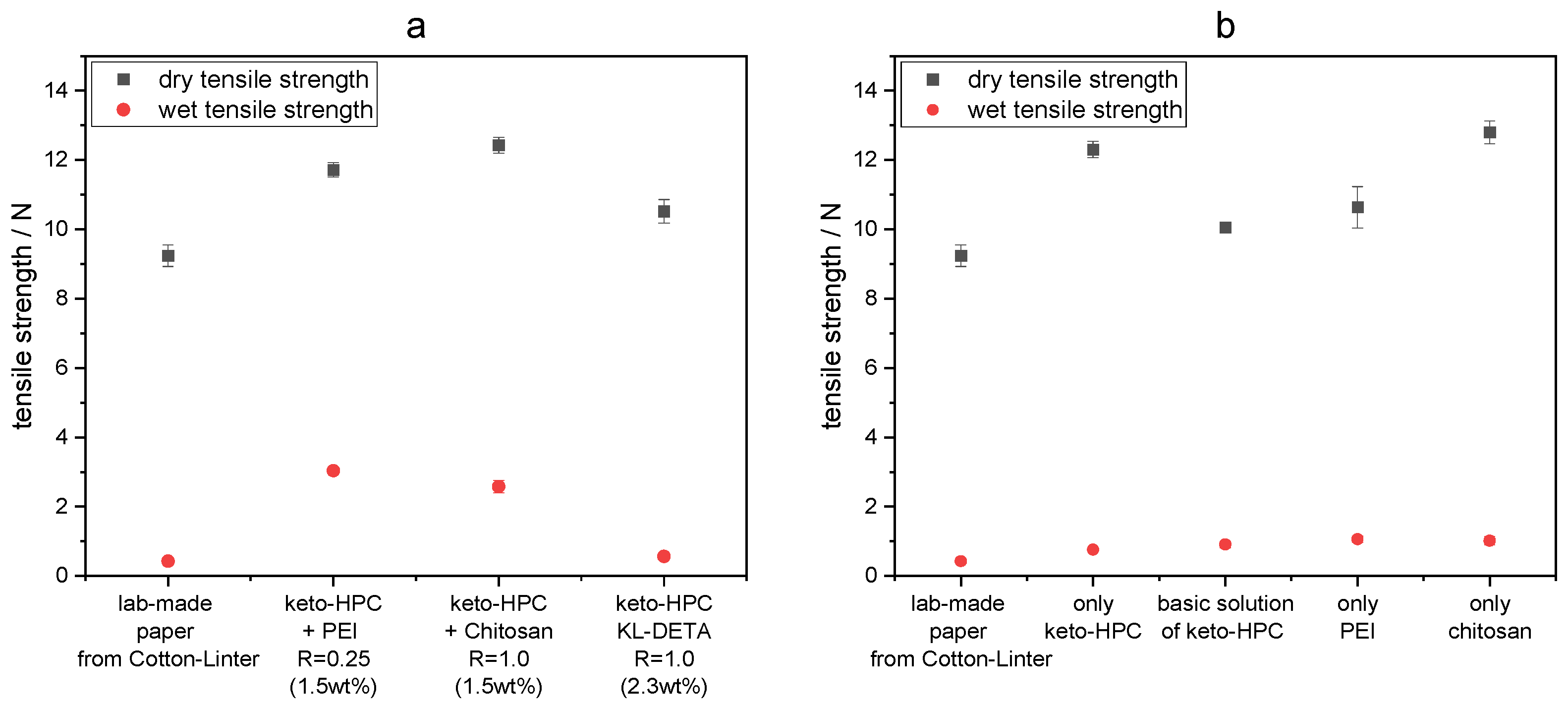
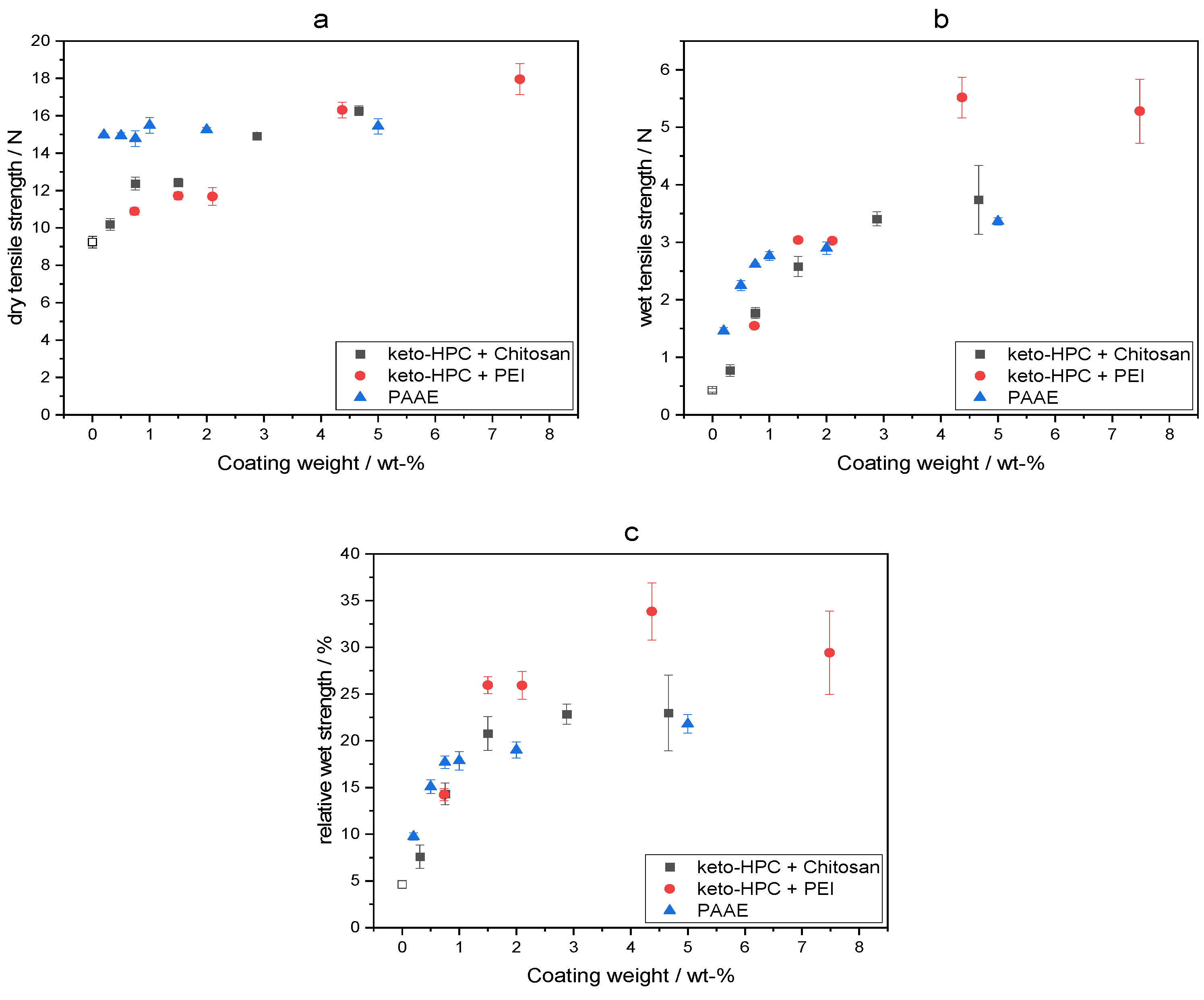
2.2. Cross-Linking of Keto-HPC with High-Molecular-Weight Polyamine in Paper and Impact on Wet Tensile Strength
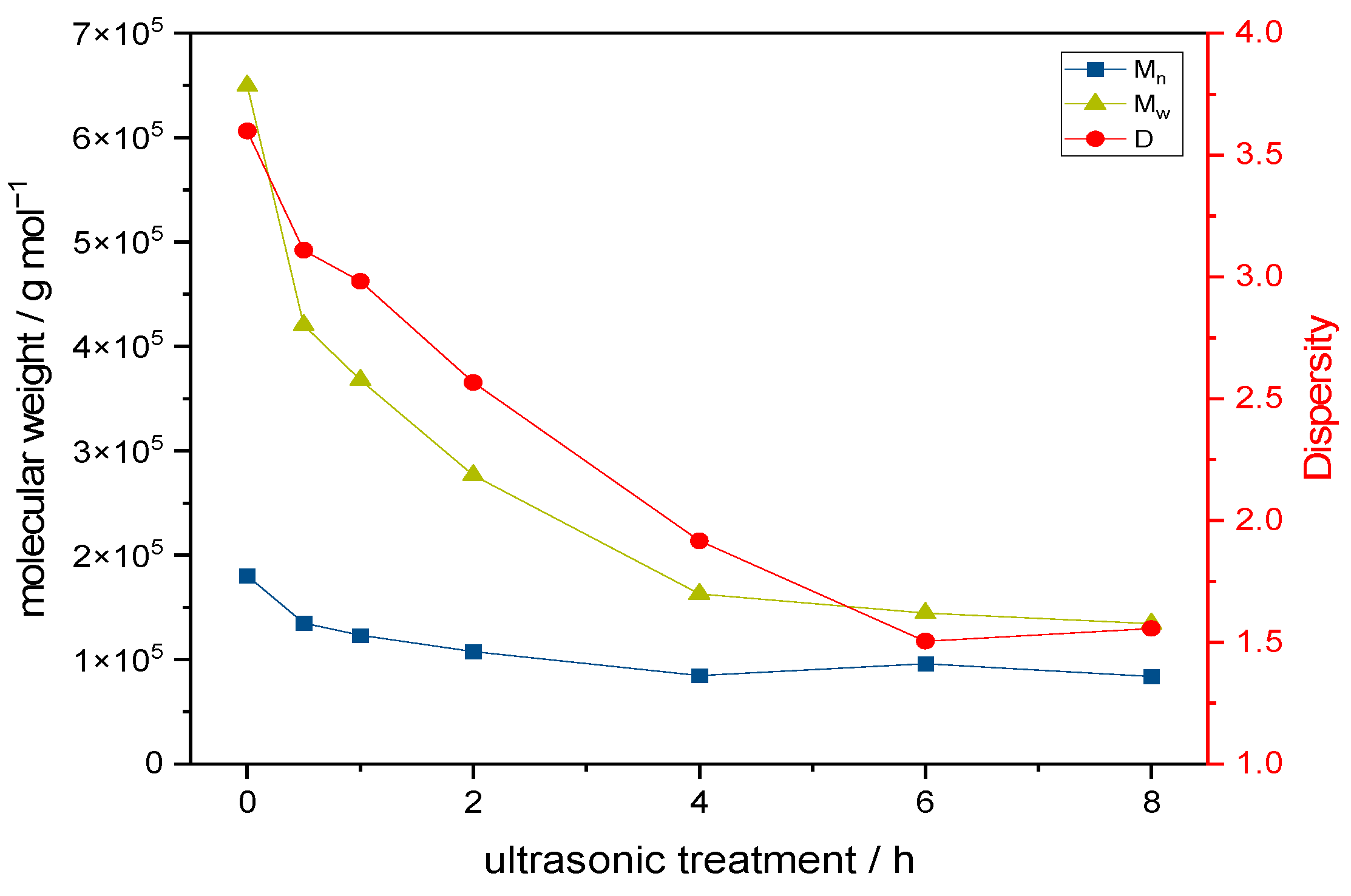
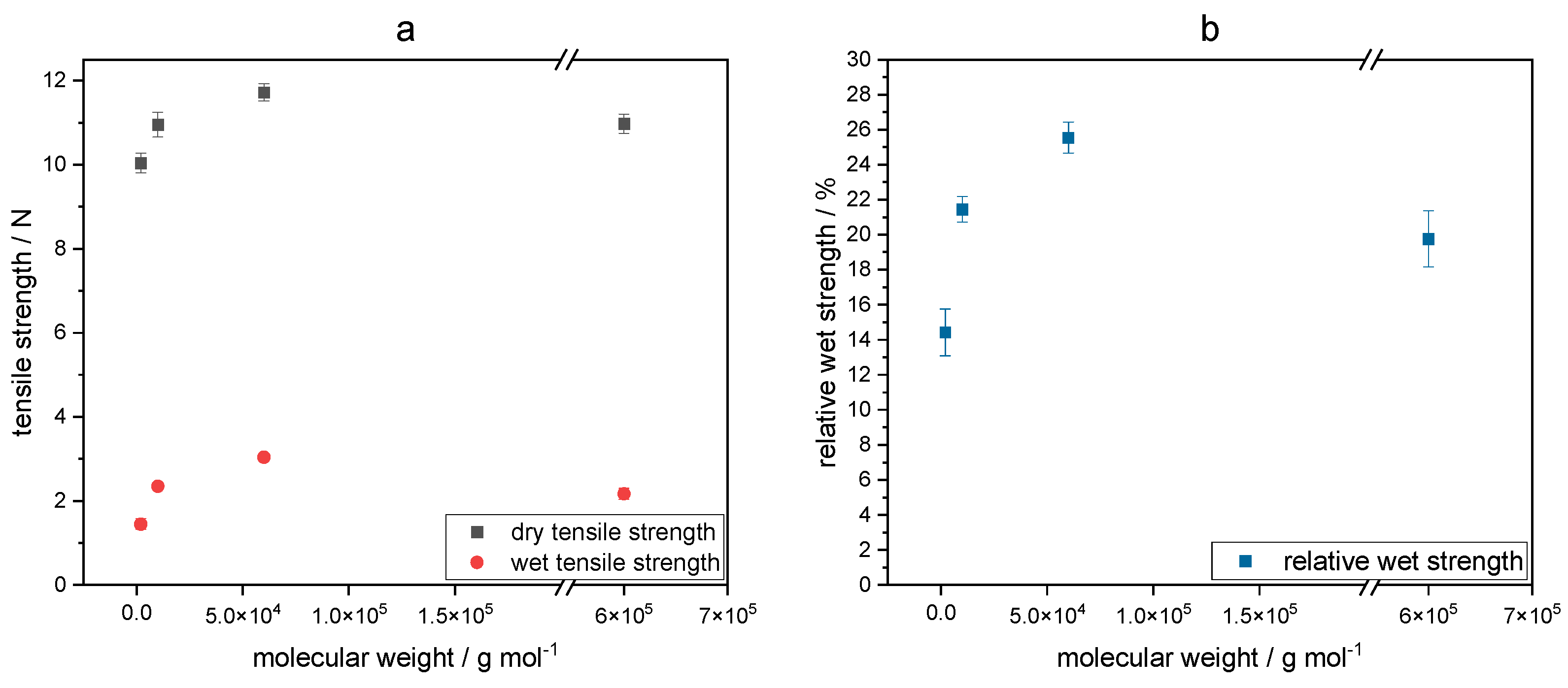
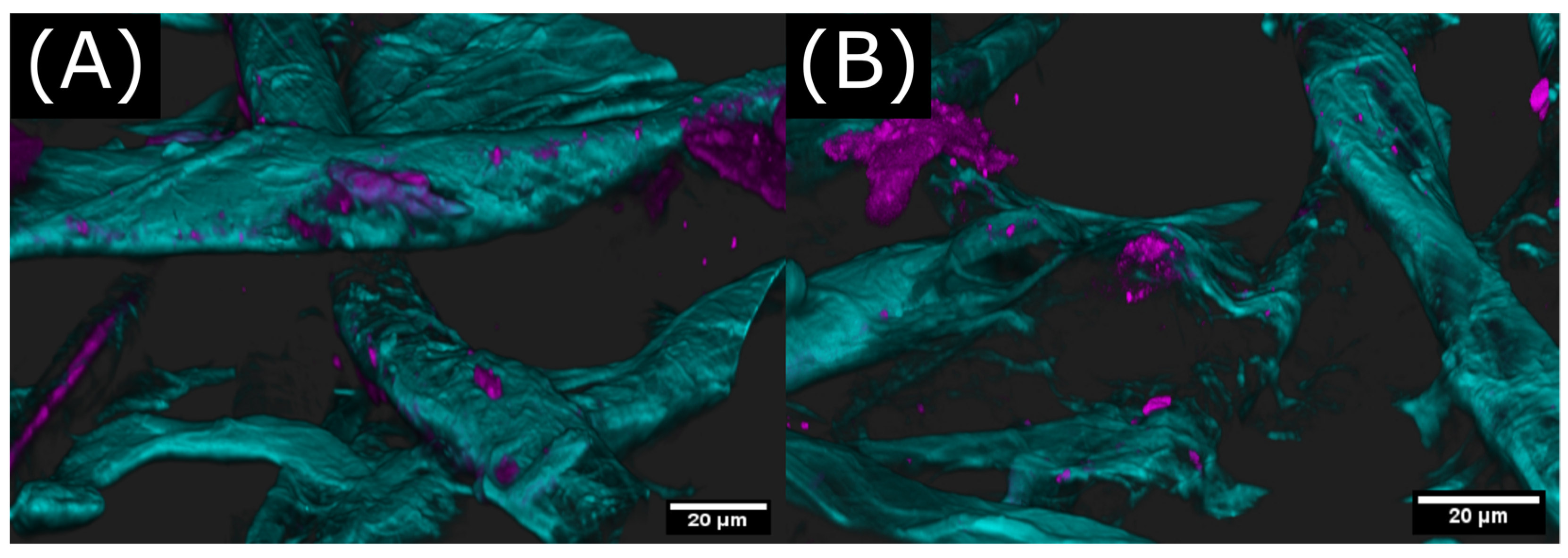
2.3. Polymer Distribution of Cross-Linked Keto-HPC in Paper
3. Conclusions
4. Materials and Methods
4.1. Materials
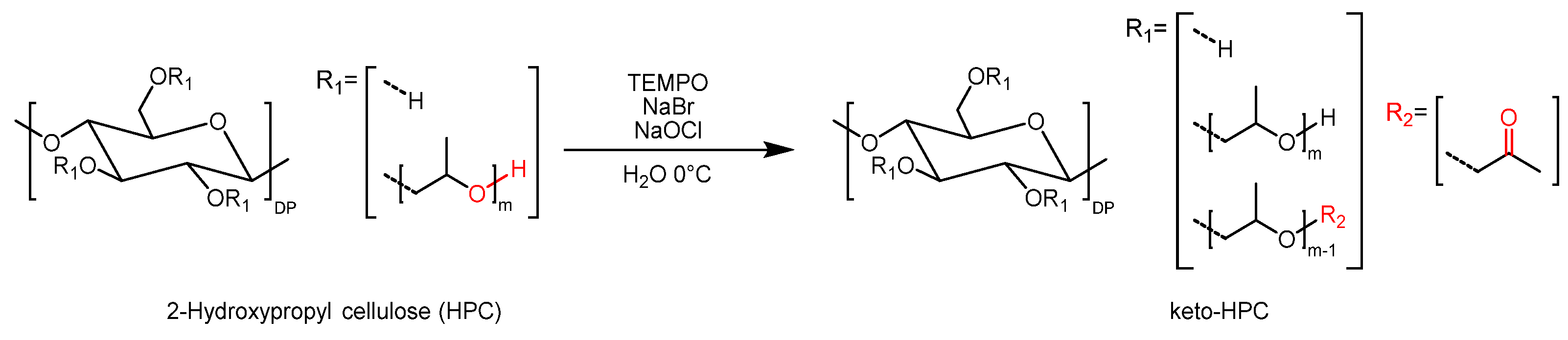
4.2. Synthesis of Keto-HPC with DOx 1.5 via TEMPO-Mediated Oxidation
4.3. Hydrogel Formation Made from Keto-HPC Cross-Linked with Branched PEI
4.4. Hydrogel Formation Made from Keto-HPC Cross-Linked with KL-DETA
4.5. Hydrogel Formation Made from Keto-HPC Cross-linked with Chitosan
4.6. Production of Lab-Made Paper
4.7. Application of Keto-HPC and Amine Cross-Linker to Paper via Size Press—General Procedure
4.8. Polymer Degradation of Keto-HPC via Ultrasonic Treatment
4.9. SEC Analysis of Degraded Keto-HPC
4.10. Fluorescent Labeling of Keto-HPC and Lab-Made Paper Samples
4.11. Tensile Measurements
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vartiainen, J.; Vähä-Nissi, M.; Harlin, A. Biopolymer Films and Coatings in Packaging Applications—A Review of Recent Developments. MSA 2014, 5, 708–718. [Google Scholar] [CrossRef]
- Khwaldia, K.; Arab-Tehrany, E.; Desobry, S. Biopolymer Coatings on Paper Packaging Materials. Compr. Rev. Food Sci. Food Saf. 2010, 9, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Marsh, K.; Bugusu, B. Food packaging—Roles, materials, and environmental issues. J. Food Sci. 2007, 72, R39–R55. [Google Scholar] [CrossRef] [PubMed]
- Hamm, U. Wet strength resins in hygienic paper production. Pap.-Z. Fur Die Erzeug. Von Holzst. Zellst. Pap. Und Pappe 1999, 51, 118–122. [Google Scholar]
- Lindström, T.; Wågberg, L.; Larsson, T. On the Nature of Joint Strength in Paper—A Review of Dry and Wet Strength Resins used in Paper Manufacturing. In 13th Fundamental Research Symposium; The Pulp and Paper Fundamental Research Society: Cambridge, UK, 2005. [Google Scholar]
- Hubbe, M. Bonding between cellulosic fibers in the absence and presence of dry-strength agents—A review. BioResources 2006, 1, 281–318. [Google Scholar] [CrossRef]
- Hirn, U.; Schennach, R. Comprehensive analysis of individual pulp fiber bonds quantifies the mechanisms of fiber bonding in paper. Sci. Rep. 2015, 5, 10503. [Google Scholar] [CrossRef]
- Pelton, R. On the Design of Polymers for Increased Paper Dry Strength—A Review. Appita Technol. Innov. Manuf. Environ. 2004, 57, 181–190. [Google Scholar]
- Bottger, J.; Krause, T. Investigation into wet strength development in papers by means of urea and melamine formaldehyde condensates. Papier 1980, 34, 214–219. [Google Scholar]
- Espy, H. The mechanism of wet-strength development in paper: A review. Tappi J. 1995, 78, 90–99. [Google Scholar]
- Yang, D.; Stimpson, T.C.; Soucy, J.; Esser, A.; Pelton, R.H. Increasing wet adhesion between cellulose surfaces with polyvinylamine. Cellulose 2019, 26, 341–353. [Google Scholar] [CrossRef]
- Wågberg, L.; Björklund, M. On the mechanism behind wet strength development in paper containing wet strength resins. Nord. Pulp Pap. Res. J. 1992, 8, 53–58. [Google Scholar] [CrossRef]
- Keim, G. Cationic Thermosetting Polyamide-Epichlorhydrin Resins and Process of Making the Same. U.S. Patent 2,926,154, 23 February 1960. [Google Scholar]
- Bodén, L.; Lundgren, M.; Stensiö, K.-E.; Gorzynski, M. Determination of 1,3-dichloro-2-propanol and 3-chloro-1,2-propanediol in papers treated with polyamidoamine-epichlorohydrin wet strength resins by gas chromatography-mass spectrometry using selective ion monitoring. J. Chromatogr. A 1997, 788, 195–203. [Google Scholar] [CrossRef]
- Bates, R.; Branton, H.J.; Hardman, D.; Robinson, G. Dehalogenation of Polyamine Neutral Curing Wet Strength Resins. U.S. Patent 5,972,691, 26 October 1999. [Google Scholar]
- Hardman, D.J.; Huxley, M.; Bull, A.T.; Slater, J.H.; Bates, R. Generation of environmentally enhanced products: Clean technology for paper chemicals. J. Chem. Technol. Biotechnol. 1997, 70, 60–66. [Google Scholar] [CrossRef]
- Schäfer, J.-L.; Schölch, S.; Prucker, O.; Brandstetter, T.; Rühe, J.; Stockert, A.R.V.; Meckel, T.; Biesalski, M. Accessibility of fiber surface sites for polymeric additives determines dry and wet tensile strength of paper sheets. Cellulose 2021, 28, 5775–5791. [Google Scholar] [CrossRef]
- Saito, T. A novel method to improve wet strength of paper. Tappi J. 2005, 4, 3–8. [Google Scholar]
- Saito, T.; Isogai, A. Wet Strength Improvement of TEMPO-Oxidized Cellulose Sheets Prepared with Cationic Polymers. Ind. Eng. Chem. Res. 2007, 46, 773–780. [Google Scholar] [CrossRef]
- Heinze, T.; Koschella, A. Solvents Applied in the Field of Cellulose Chemistry—A Mini Review. Polímeros Ciência Tecnol. 2005, 15, 84–90. [Google Scholar] [CrossRef]
- Nau, M.; Seelinger, D.; Biesalski, M. Functional surface coatings from tailor-made long-chain hydroxypropyl cellulose ester nanoparticles. Cellulose 2018, 25, 5769–5780. [Google Scholar] [CrossRef]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Filipič, M.; Jose Frutos, M.; Galtier, P.; Gott, D.; et al. Re-evaluation of celluloses E 460(i), E 460(ii), E 461, E 462, E 463, E 464, E 465, E 466, E 468 and E 469 as food additives. EFSA J. 2018, 16, e05047. [Google Scholar]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gundert-Remy, U.; Kuhnle, G.G.; et al. Safety of low-substituted hydroxypropyl cellulose (L-HPC) to be used as a food additive in food supplements in tablet form. EFSA J. 2018, 16, e05062. [Google Scholar]
- Ho, F.; Koehler, R.R.; Ward, G.A. Determination of molar substitution and degree of substitution of hydroxypropyl cellulose by nuclear magnetic resonance spectrometry. Anal. Chem. 1972, 44, 178–181. [Google Scholar] [CrossRef]
- Seelinger, D.; Trosien, S.; Nau, M.; Biesalski, M. Tailored oxidation of hydroxypropyl cellulose under mild conditions for the generation of wet strength agents for paper. Carbohydr. Polym. 2021, 254, 117458. [Google Scholar] [CrossRef] [PubMed]
- Burhani, D.; Septevani, A.A.; Setiawan, R.; Djannah, L.M.; Putra, M.A.; Kusumah, S.S.; Sondari, D. Self-Assembled Behavior of Ultralightweight Aerogel from a Mixture of CNC/CNF from Oil Palm Empty Fruit Bunches. Available online: https://www.mdpi.com/2073-4360/13/16/2649 (accessed on 14 February 2023).
- Bagheri-Khoulenjani, S.; Taghizadeh, S.M.; Mirzadeh, H. An investigation on the short-term biodegradability of chitosan with various molecular weights and degrees of deacetylation. Carbohydr. Polym. 2009, 78, 773–778. [Google Scholar] [CrossRef]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef]
- Goy, R.C.; Britto, D.D.; Assis, O.B.G. A Review of the Antimicrobial Activity of Chitosan. Polímeros Ciência Tecnol. 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Belle, J.; Odermatt, J. Initial wet web strength of paper. Cellulose 2016, 23, 2249–2272. [Google Scholar] [CrossRef]
- Yu, O.; Kim, K.H. Lignin to Materials: A Focused Review on Recent Novel Lignin Applications. Appl. Sci. 2020, 10, 4626. [Google Scholar] [CrossRef]
- Zieglowski, M.; Trosien, S.; Rohrer, J.; Mehlhase, S.; Weber, S.; Bartels, K.; Siegert, G.; Trellenkamp, T.; Albe, K.; Biesalski, M. Reactivity of Isocyanate-Functionalized Lignins: A Key Factor for the Preparation of Lignin-Based Polyurethanes. Front. Chem. 2019, 7, 562. [Google Scholar] [CrossRef]
- Gleede, T.; Reisman, L.; Rieger, E.; Mbarushimana, P.C.; Rupar, P.A.; Wurm, F.R. Aziridines and azetidines: Building blocks for polyamines by anionic and cationic ring-opening polymerization. Polym. Chem. 2019, 10, 3257–3283. [Google Scholar] [CrossRef]
- Wang, C.; Chen, C.; Ren, H.; Yang, Y.; Dai, H. Polyethyleneimine Addition for Control of Dissolved and Colloidal Substances: Effects on Wet-End Chemistry. BioResources 2016, 11, 9756–9770. [Google Scholar] [CrossRef]
- Li, X.; Pelton, R. Enhancing Wet Cellulose Adhesion with Proteins. Ind. Eng. Chem. Res. 2005, 44, 7398–7404. [Google Scholar] [CrossRef]
- Chen, J.; Frazier, C.E.; Edgar, K.J. In situ forming hydrogels based on oxidized hydroxypropyl cellulose and Jeffamines. Cellulose 2021, 28, 11367–11380. [Google Scholar] [CrossRef]
- Weidner, J. Wet Strength in Paper and Paperboard. Tappi J. 1965, 29, 159. [Google Scholar]
- Chen, Z.; Zhang, H.; Song, Z.; Qian, X. Combination of Glyoxal and Chitosan as the Crosslinking System to Improve Paper Wet Strength. BioResources 2013, 8, 6087–6096. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, H.; Song, Z.; Qian, X. Preparation and Application of Maleic Anhydride-Acylated Chitosan for Wet Strength Improvement of Paper. BioResources 2013, 8, 3901–3911. [Google Scholar] [CrossRef]
- Sun, S.; An, Q.; Li, X.; Qian, L.; He, B.; Xiao, H. Synergistic effects of chitosan-guanidine complexes on enhancing antimicrobial activity and wet-strength of paper. Bioresour. Technol. 2010, 101, 5693–5700. [Google Scholar] [CrossRef]
- Yano, T.; Ohtani, H.; Tsuge, S. Determination of wet strength resin in paper by pyrolysis-gas chromatography. Tappi J. 1991, 74, 197–201. [Google Scholar]
- Goodwin, D.J.; Picout, D.R.; Ross-Murphy, S.B.; Holland, S.J.; Martini, L.G.; Lawrence, M.J. Ultrasonic degradation for molecular weight reduction of pharmaceutical cellulose ethers. Carbohydr. Polym. 2011, 83, 843–851. [Google Scholar] [CrossRef]
- von Harpe, A.; Petersen, H.; Li, Y.; Kissel, T. Characterization of commercially avaiable and synthesized polyethylenimines for gene delivery. J. Control. Release 2000, 69, 309–322. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Miyamoto, R.; Zhang, X.; Hirai, T. Rhodamine-based fluorescent thermometer exhibiting selective emission enhancement at a specific temperature range. Org. Lett. 2007, 9, 3921–3924. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seelinger, D.; Biesalski, M. Cross-Linking of Oxidized Hydroxypropyl Cellulose in Paper: Influence of Molecular Weight and Polymer Distribution on Paper Wet Strength Development. Gels 2023, 9, 206. https://doi.org/10.3390/gels9030206
Seelinger D, Biesalski M. Cross-Linking of Oxidized Hydroxypropyl Cellulose in Paper: Influence of Molecular Weight and Polymer Distribution on Paper Wet Strength Development. Gels. 2023; 9(3):206. https://doi.org/10.3390/gels9030206
Chicago/Turabian StyleSeelinger, David, and Markus Biesalski. 2023. "Cross-Linking of Oxidized Hydroxypropyl Cellulose in Paper: Influence of Molecular Weight and Polymer Distribution on Paper Wet Strength Development" Gels 9, no. 3: 206. https://doi.org/10.3390/gels9030206
APA StyleSeelinger, D., & Biesalski, M. (2023). Cross-Linking of Oxidized Hydroxypropyl Cellulose in Paper: Influence of Molecular Weight and Polymer Distribution on Paper Wet Strength Development. Gels, 9(3), 206. https://doi.org/10.3390/gels9030206