Simple Complexity: Incorporating Bioinspired Delivery Machinery within Self-Assembled Peptide Biogels
Abstract
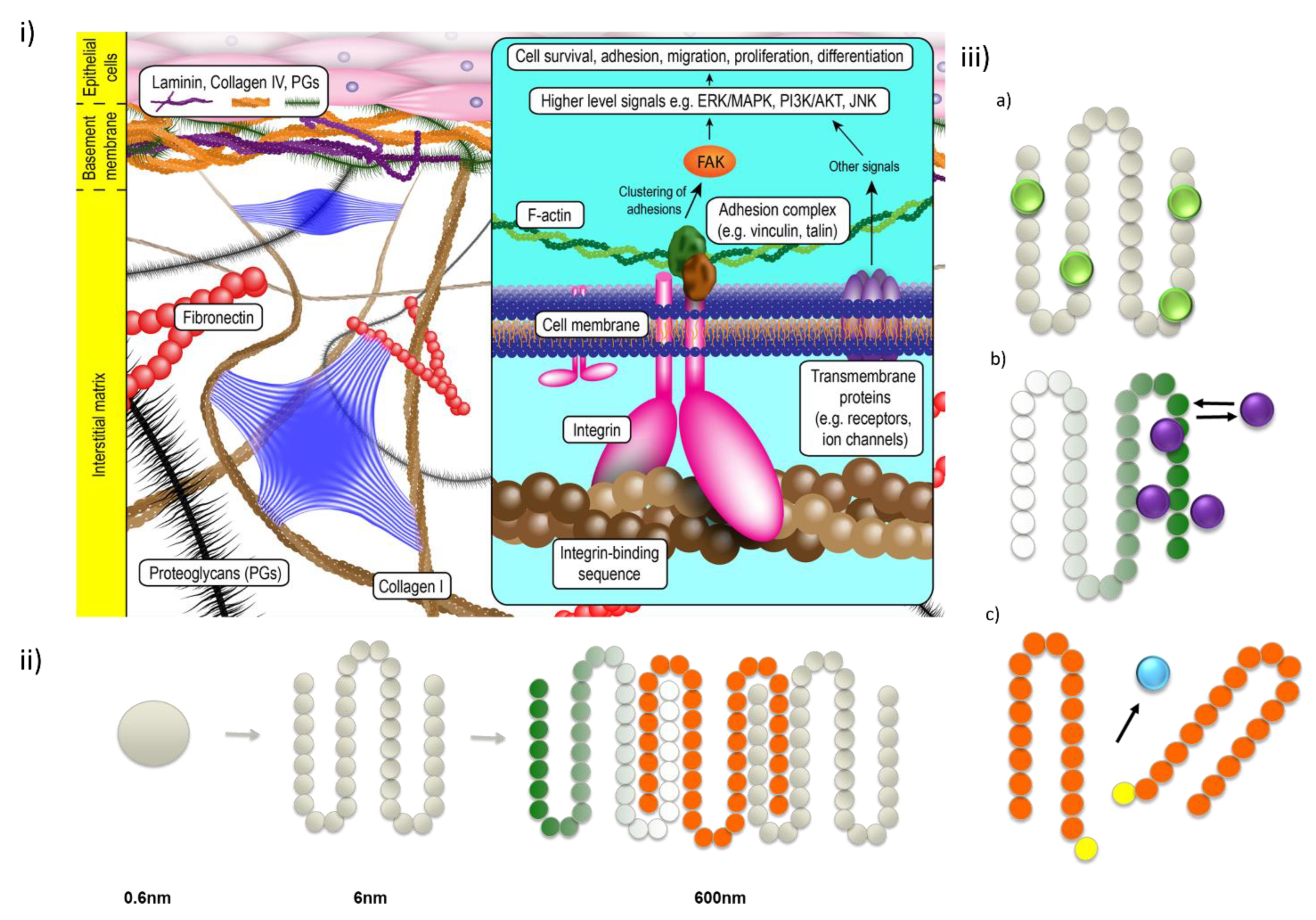
1. Introduction
2. Advantages of SAPs as Delivery Tools
3. Classification of Self-Assembling Peptides
3.1. Amphiphilic Linear Peptides
3.2. Cyclic Peptides
3.3. N-Terminally Protected Peptides
3.4. Hybrid Peptide Assemblies
4. Delivery of Bioactive from SAPs
4.1. Anticancer Drug Delivery
4.2. Cardiovascular Delivery
4.3. Bone Delivery
4.4. Preipheral Nervous System Delivery
4.5. Delivery to the Brain
4.6. Ocular Delivery
4.7. Pancreatic (Beta-Cell Replacement) Delivery
4.8. Immunogenic Delivery
| SAP | Delivered Molecule(s) | Secondary Structure | Physical Form | Factor Triggering Self-Assembly | Payload | Ref. |
|---|---|---|---|---|---|---|
| Gene Delivery | ||||||
| FF | SiRNA | β-turn and antiparallel β-sheet | nanoparticle | electron attractive forces | breast cancer | [133] |
| GGGAAAKRK | SiRNA | β-sheet | aqueous dispersion | probe sonication | CNS pathologies | [134] |
| IKVAV | lentiviral | β-sheet | Hydrogel | pH-driven | CNS | [50] |
| KKALLHAALAHLLALAHHLLALLKKA | lentiviral | α-helical, coiled-coil | spherical particles | pH-driven | HCT116 cells | [135] |
| RRRR | pDNA | - | Ordered aggregates | aggregation-induced | HeLa cells, HepG2 cells, NIH 3T3 cells, 293T cells, stem cell R1 | [136] |
| AAAAAAK | SiRNA | - | Nanotube | pH-driven | U87MG, U251MG, and T98G cells | [137] |
| Vaccine delivery | ||||||
| GDFDFDYDX-ss-ERGD (X=E, S or K) | Tumor vaccine | β-sheet | Hydrogel | GSH | C57BL/mice | [129] |
5. Novel Genetic Therapies
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Sis, M.J.; Webber, M.J. Drug delivery with designed peptide assemblies. Trends Pharmacol. Sci. 2019, 40, 747–762. [Google Scholar] [CrossRef] [PubMed]
- Mandal, D.; Nasrolahi Shirazi, A.; Parang, K. Self-assembly of peptides to nanostructures. Org. Biomol. Chem. 2014, 12, 3544–3561. [Google Scholar] [CrossRef]
- Delfi, M.; Sartorius, R.; Ashrafizadeh, M.; Sharifi, E.; Zhang, Y.; De Berardinis, P.; Zarrabi, A.; Varma, R.S.; Tay, F.R.; Smith, B.R.; et al. Self-assembled peptide and protein nanostructures for anti-cancer therapy: Targeted delivery, stimuli-responsive devices and immunotherapy. Nano Today 2021, 38, 101119. [Google Scholar] [CrossRef]
- Blake, C.; Massey, O.; Boyd-Moss, M.; Firipis, K.; Rifai, A.; Franks, S.; Quigley, A.; Kapsa, R.; Nisbet, D.R.; Williams, R.J. Replace and repair: Biomimetic bioprinting for effective muscle engineering. APL Bioeng. 2021, 5, 031502. [Google Scholar] [CrossRef] [PubMed]
- Boyd-Moss, M.; Firipis, K.; Quigley, A.; Rifai, A.; Cichocki, A.; Whitty, S.; Ngan, C.; Dekiwadia, C.; Long, B.; Nisbet, D.R. Hybrid Self-Assembling Peptide/Gelatin Methacrylate (GelMA) Bioink Blend for Improved Bioprintability and Primary Myoblast Response. Adv. NanoBiomed Res. 2022, 2, 2100106. [Google Scholar] [CrossRef]
- Urello, M.; Hsu, W.H.; Christie, R.J. Peptides as a material platform for gene delivery: Emerging concepts and converging technologies. Acta Biomater. 2020, 117, 40–59. [Google Scholar] [CrossRef]
- Cheetham, A.G.; Lin, Y.A.; Lin, R.; Cui, H. Molecular design and synthesis of self-assembling camptothecin drug amphiphiles. Acta Pharmacol. Sin. 2017, 38, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zoneff, E.R.; Thomas, J.W.; Hong, N.; Tan, L.L.; McGillivray, D.J.; Perriman, A.W.; Law, K.C.L.; Thompson, L.H.; Moriarty, N.; et al. Hydrogel oxygen reservoirs increase functional integration of neural stem cell grafts by meeting metabolic demands. Nat. Commun. 2023, 14, 457. [Google Scholar] [CrossRef]
- Eskandari, S.; Guerin, T.; Toth, I.; Stephenson, R.J. Recent advances in self-assembled peptides: Implications for targeted drug delivery and vaccine engineering. Adv. Drug Deliv. Rev. 2017, 110–111, 169–187. [Google Scholar] [CrossRef]
- Bruggeman, K.; Zhang, M.; Malagutti, N.; Soltani Dehnavi, S.; Williams, R.; Tricoli, A.; Nisbet, D. Using UV-Responsive Nanoparticles to Provide In Situ Control of Growth Factor Delivery and a More Constant Release Profile from a Hydrogel Environment. ACS Appl. Mater. Interfaces 2022, 14, 12068–12076. [Google Scholar] [CrossRef]
- Li, R.; Boyd-Moss, M.; Long, B.; Martel, A.; Parnell, A.; Dennison, A.J.C.; Barrow, C.J.; Nisbet, D.R.; Williams, R.J. Facile Control over the Supramolecular Ordering of Self-assembled Peptide Scaffolds by Simultaneous Assembly with a Polysacharride. Sci. Rep. 2017, 7, 4797. [Google Scholar] [CrossRef] [PubMed]
- Soltani Dehnavi, S.; Eivazi Zadeh, Z.; Harvey, A.R.; Voelcker, N.H.; Parish, C.L.; Williams, R.J.; Elnathan, R.; Nisbet, D.R.J.A.M. Changing fate: Reprogramming cells via engineered nanoscale delivery materials. Adv. Mater. 2022, 34, 2108757. [Google Scholar] [CrossRef]
- Chakroun, R.W.; Wang, F.; Lin, R.; Wang, Y.; Su, H.; Pompa, D.; Cui, H. Fine-Tuning the Linear Release Rate of Paclitaxel-Bearing Supramolecular Filament Hydrogels through Molecular Engineering. ACS Nano 2019, 13, 7780–7790. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-hydrogel: A hybrid biomaterial system for localized drug delivery. Ann. Biomed. Eng. 2016, 44, 2049–2061. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Zhu, F.; Li, B.; Zhao, L.; Liang, H.; Yan, Y.; Tan, H. Folate-conjugated and pH-triggered doxorubicin and paclitaxel co-delivery micellar system for targeted anticancer drug delivery. Mater. Chem. Front. 2018, 2, 1529–1538. [Google Scholar] [CrossRef]
- Bruggeman, K.F.; Wang, Y.; Maclean, F.L.; Parish, C.L.; Williams, R.J.; Nisbet, D.R. Temporally controlled growth factor delivery from a self-assembling peptide hydrogel and electrospun nanofibre composite scaffold. Nanoscale 2017, 9, 13661–13669. [Google Scholar] [CrossRef]
- Wang, Y.; Bruggeman, K.F.; Franks, S.; Gautam, V.; Hodgetts, S.I.; Harvey, A.R.; Williams, R.J.; Nisbet, D.R. Is Viral Vector Gene Delivery More Effective Using Biomaterials? Adv. Healthc. Mater. 2021, 10, e2001238. [Google Scholar] [CrossRef]
- Al Balushi, N.; Boyd-Moss, M.; Samarasinghe, R.M.; Rifai, A.; Franks, S.J.; Firipis, K.; Long, B.M.; Darby, I.A.; Nisbet, D.R.; Pouniotis, D.; et al. Self-Assembled Peptide Habitats to Model Tumor Metastasis. Gels 2022, 8, 332. [Google Scholar] [CrossRef] [PubMed]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart hydrogels in tissue engineering and regenerative medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef]
- Li, R.; McRae, N.L.; McCulloch, D.R.; Boyd-Moss, M.; Barrow, C.J.; Nisbet, D.R.; Stupka, N.; Williams, R.J. Large and Small Assembly: Combining Functional Macromolecules with Small Peptides to Control the Morphology of Skeletal Muscle Progenitor Cells. Biomacromolecules 2018, 19, 825–837. [Google Scholar] [CrossRef]
- Li, R.; Pavuluri, S.; Bruggeman, K.; Long, B.M.; Parnell, A.J.; Martel, A.; Parnell, S.R.; Pfeffer, F.M.; Dennison, A.J.; Nicholas, K.R.; et al. Coassembled nanostructured bioscaffold reduces the expression of proinflammatory cytokines to induce apoptosis in epithelial cancer cells. Nanomedicine 2016, 12, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Maclean, F.L.; Ims, G.M.; Horne, M.K.; Williams, R.J.; Nisbet, D.R.J.A.M. A Programmed Anti-Inflammatory Nanoscaffold (PAIN) as a 3D Tool to Understand the Brain Injury Response. Adv. Mater. 2018, 30, 1805209. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Pillay, V.; Modi, G.; Choonara, Y.E.; du Toit, L.C.; Naidoo, D. Self-assembling peptides: Implications for patenting in drug delivery and tissue engineering. Recent Pat. Drug Deliv. Formul. 2011, 5, 24–51. [Google Scholar] [CrossRef] [PubMed]
- Gholami, A.; Mousavi, S.M.; Hashemi, S.A.; Ghasemi, Y.; Chiang, W.H.; Parvin, N. Current trends in chemical modifications of magnetic nanoparticles for targeted drug delivery in cancer chemotherapy. Drug Metab. Rev. 2020, 52, 205–224. [Google Scholar] [PubMed]
- Karavasili, C.; Fatouros, D.G. Self-assembling peptides as vectors for local drug delivery and tissue engineering applications. Adv. Drug Deliv. Rev. 2021, 174, 387–405. [Google Scholar] [CrossRef]
- Hartgerink Jeffrey, D.; Beniash, E.; Stupp Samuel, I. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science 2001, 294, 1684–1688. [Google Scholar] [CrossRef]
- Gelain, F.; Luo, Z.; Zhang, S. Self-assembling peptide EAK16 and RADA16 nanofiber scaffold hydrogel. Chem. Rev. 2020, 120, 13434–13460. [Google Scholar] [CrossRef]
- Sangji, M.H.; Sai, H.; Chin, S.M.; Lee, S.R.; Sasselli, I.R.; Palmer, L.C.; Stupp, S.I. Supramolecular Interactions and Morphology of Self-Assembling Peptide Amphiphile Nanostructures. Nano Lett. 2021, 21, 6146–6155. [Google Scholar] [CrossRef]
- Zhang, S.; Lockshin, C.; Herbert, A.; Winter, E.; Rich, A. Zuotin, a putative Z-DNA binding protein in Saccharomyces cerevisiae. EMBO J. 1992, 11, 3787–3796. [Google Scholar] [CrossRef]
- Zhang, S. Discovery and design of self-assembling peptides. Interface Focus 2017, 7, 20170028. [Google Scholar] [CrossRef]
- Moore, A.N.; Hartgerink, J.D. Self-Assembling Multidomain Peptide Nanofibers for Delivery of Bioactive Molecules and Tissue Regeneration. Acc. Chem. Res. 2017, 50, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Yanlian, Y.; Ulung, K.; Xiumei, W.; Horii, A.; Yokoi, H.; Shuguang, Z. Designer self-assembling peptide nanomaterials. Nano Today 2009, 4, 193–210. [Google Scholar] [CrossRef]
- Brea, R.J.; Reiriz, C.; Granja, J.R. Towards functional bionanomaterials based on self-assembling cyclic peptide nanotubes. Chem. Soc. Rev. 2010, 39, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, A.; Deyle, K.; Heinis, C. Cyclic peptide therapeutics: Past, present and future. Curr. Opin. Chem. Biol. 2017, 38, 24–29. [Google Scholar] [CrossRef]
- Manchineella, S.; Govindaraju, T. Molecular self-assembly of cyclic dipeptide derivatives and their applications. ChemPlusChem 2017, 82, 88–106. [Google Scholar] [CrossRef]
- Xing, R.; Zou, Q.; Yan, X. Peptide-based supramolecular colloids. Acta Phys. Chim. Sin. 2020, 36, 1909048. [Google Scholar]
- Tao, K.; Chen, Y.; Orr, A.A.; Tian, Z.; Makam, P.; Gilead, S.; Si, M.; Rencus-Lazar, S.; Qu, S.; Zhang, M.; et al. Enhanced Fluorescence for Bioassembly by Environment-Switching Doping of Metal Ions. Adv. Funct. Mater. 2020, 30, 1909614. [Google Scholar] [CrossRef]
- Teixidó, M.; Zurita, E.; Malakoutikhah, M.; Tarragó, T.; Giralt, E. Diketopiperazines as a Tool for the Study of Transport across the Blood−Brain Barrier (BBB) and Their Potential Use as BBB-Shuttles. J. Am. Chem. Soc. 2007, 129, 11802–11813. [Google Scholar] [CrossRef]
- Li, R.; Horgan, C.C.; Long, B.; Rodriguez, A.L.; Mather, L.; Barrow, C.J.; Nisbet, D.R.; Williams, R.J. Tuning the mechanical and morphological properties of self-assembled peptide hydrogels via control over the gelation mechanism through regulation of ionic strength and the rate of pH change. RSC Adv. 2015, 5, 301–307. [Google Scholar] [CrossRef]
- Fleming, S.; Ulijn, R.V. Design of nanostructures based on aromatic peptide amphiphiles. Chem. Soc. Rev. 2014, 43, 8150–8177. [Google Scholar] [CrossRef]
- Williams, R.J.; Hall, T.E.; Glattauer, V.; White, J.; Pasic, P.J.; Sorensen, A.B.; Waddington, L.; McLean, K.M.; Currie, P.D.; Hartley, P.G. The in vivo performance of an enzyme-assisted self-assembled peptide/protein hydrogel. Biomaterials 2011, 32, 5304–5310. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xu, B. A simple visual assay based on small molecule hydrogels for detecting inhibitors of enzymes. Chem. Commun. 2004, 2004, 2424–2425. [Google Scholar] [CrossRef]
- Mahler, A.; Reches, M.; Rechter, M.; Cohen, S.; Gazit, E. Rigid, self-assembled hydrogel composed of a modified aromatic dipeptide. Adv. Mater. 2006, 18, 1365–1370. [Google Scholar] [CrossRef]
- Toledano, S.; Williams, R.J.; Jayawarna, V.; Ulijn, R.V. Enzyme-triggered self-assembly of peptide hydrogels via reversed hydrolysis. J. Am. Chem. Soc. 2006, 128, 1070–1071. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Williams, R.J.; Tang, C.; Coppo, P.; Collins, R.F.; Turner, M.L.; Saiani, A.; Ulijn, R.V. Fmoc-diphenylalanine self assembles to a hydrogel via a novel architecture based on π–π Interlocked β-sheets. Adv. Mater. 2008, 20, 37–41. [Google Scholar] [CrossRef]
- Xu, H.; Das, A.K.; Horie, M.; Shaik, M.S.; Smith, A.M.; Luo, Y.; Lu, X.; Collins, R.; Liem, S.Y.; Song, A.; et al. An investigation of the conductivity of peptide nanotube networks prepared by enzyme-triggered self-assembly. Nanoscale 2010, 2, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ge, S.; Song, Z.; Zhao, A.; Zhao, L.; Hu, Z.; Cai, D.; Zhang, Z.; Peng, L.; Lu, D.; et al. A novel self-assembled epitope peptide nanoemulsion vaccine targeting nasal mucosal epithelial cell for reinvigorating CD8+ T cell immune activity and inhibiting tumor progression. Int. J. Biol. Macromol. 2021, 183, 1891–1902. [Google Scholar] [CrossRef]
- Zhou, M.; Smith, A.M.; Das, A.K.; Hodson, N.W.; Collins, R.F.; Ulijn, R.V.; Gough, J.E. Self-assembled peptide-based hydrogels as scaffolds for anchorage-dependent cells. Biomaterials 2009, 30, 2523–2530. [Google Scholar] [CrossRef] [PubMed]
- Horgan, C.C.; Rodriguez, A.L.; Li, R.; Bruggeman, K.F.; Stupka, N.; Raynes, J.K.; Day, L.; White, J.W.; Williams, R.J.; Nisbet, D.R. Characterisation of minimalist co-assembled fluorenylmethyloxycarbonyl self-assembling peptide systems for presentation of multiple bioactive peptides. Acta Biomater. 2016, 38, 11–22. [Google Scholar] [CrossRef]
- Rodriguez, A.L.; Wang, T.-Y.; Bruggeman, K.F.; Li, R.; Williams, R.J.; Parish, C.L.; Nisbet, D.R. Tailoring minimalist self-assembling peptides for localized viral vector gene delivery. Nano Res. 2016, 9, 674–684. [Google Scholar] [CrossRef]
- Rodriguez, A.L.; Wang, T.Y.; Bruggeman, K.F.; Horgan, C.C.; Li, R.; Williams, R.J.; Parish, C.L.; Nisbet, D.R. In vivo assessment of grafted cortical neural progenitor cells and host response to functionalized self-assembling peptide hydrogels and the implications for tissue repair. J. Mater. Chem. B 2014, 2, 7771–7778. [Google Scholar] [CrossRef] [PubMed]
- Aye, S.S.; Li, R.; Boyd-Moss, M.; Long, B.; Pavuluri, S.; Bruggeman, K.; Wang, Y.; Barrow, C.R.; Nisbet, D.R.; Williams, R.J. Scaffolds Formed via the Non-Equilibrium Supramolecular Assembly of the Synergistic ECM Peptides RGD and PHSRN Demonstrate Improved Cell Attachment in 3D. Polymers 2018, 10, 690. [Google Scholar] [CrossRef]
- Modepalli, V.N.; Rodriguez, A.L.; Li, R.; Pavuluri, S.; Nicholas, K.R.; Barrow, C.J.; Nisbet, D.R.; Williams, R.J. In vitro response to functionalized self-assembled peptide scaffolds for three-dimensional cell culture. Biopolymers 2014, 102, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shen, G.; Yan, X. Bio-inspired short peptide self-assembly: From particles to functional materials. Particuology 2021, 64, 14–34. [Google Scholar] [CrossRef]
- Buck, J.; Grossen, P.; Cullis, P.R.; Huwyler, J.; Witzigmann, D. Lipid-Based DNA Therapeutics: Hallmarks of Non-Viral Gene Delivery. ACS Nano 2019, 13, 3754–3782. [Google Scholar] [CrossRef] [PubMed]
- Saher, O.; Rocha, C.S.J.; Zaghloul, E.M.; Wiklander, O.P.B.; Zamolo, S.; Heitz, M.; Ezzat, K.; Gupta, D.; Reymond, J.L.; Zain, R.; et al. Novel peptide-dendrimer/lipid/oligonucleotide ternary complexes for efficient cellular uptake and improved splice-switching activity. Eur. J. Pharm. Biopharm. 2018, 132, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-J.; Park, J.-E.; Hu, X.; Albert, S.K.; Park, S.-J. Peptide-Driven Shape Control of Low-Dimensional DNA Nanostructures. ACS Nano 2020, 14, 2276–2284. [Google Scholar] [CrossRef]
- Boyd-Moss, M.; Firipis, K.; O’Connell, C.D.; Rifai, A.; Quigley, A.; Boer, G.; Long, B.M.; Nisbet, D.R.; Williams, R.J. Shining a light on the hidden structure of gelatin methacryloyl bioinks using small-angle X-ray scattering (SAXS). Mater. Chem. Front. 2021, 5, 8025–8036. [Google Scholar] [CrossRef]
- Firipis, K.; Boyd-Moss, M.; Long, B.; Dekiwadia, C.; Hoskin, W.; Pirogova, E.; Nisbet, D.R.; Kapsa, R.M.I.; Quigley, A.F.; Williams, R.J. Tuneable hybrid hydrogels via complementary self-assembly of a bioactive peptide with a robust polysaccharide. ACS Biomater. Sci. Eng. 2021, 7, 3340–3350. [Google Scholar] [CrossRef]
- Firipis, K.; Footner, E.; Boyd-Moss, M.; Dekiwadia, C.; Nisbet, D.; Kapsa, R.M.I.; Pirogova, E.; Williams, R.J.; Quigley, A. Biodesigned bioinks for 3D printing via divalent crosslinking of self-assembled peptide-polysaccharide hybrids. Mater. Today Adv. 2022, 14, 100243. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, H.; Huang, Y.; Lian, B.; Ma, C.; Han, L.; Chen, Y.; Wu, S.; Li, N.; Zhang, W.; et al. A Self-Assembling Amphiphilic Peptide Dendrimer-Based Drug Delivery System for Cancer Therapy. Pharmaceutics 2021, 13, 1092. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.T.; Lim, M.; Jung, K.; Li, M.; Dong, H.; Dube, N.; Xu, T. Designing sub-20 nm self-assembled nanocarriers for small molecule delivery: Interplay among structural geometry, assembly energetics, and cargo release kinetics. J. Control. Release 2021, 329, 538–551. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Liu, X.; Zhou, B.; Wang, G.; Guan, X.; Xu, Y.; Zhang, J.; Hong, Z.; Cao, J.; Sun, X.; et al. Tumor acidic microenvironment-induced drug release of RGD peptide nanoparticles for cellular uptake and cancer therapy. Colloids Surf. B Biointerfaces 2021, 202, 111673. [Google Scholar] [CrossRef]
- Baek, K.; Noblett, A.D.; Ren, P.; Suggs, L.J. Self-assembled nucleo-tripeptide hydrogels provide local and sustained doxorubicin release. Biomater. Sci. 2020, 8, 3130–3137. [Google Scholar] [CrossRef]
- Karavasili, C.; Panteris, E.; Vizirianakis, I.S.; Koutsopoulos, S.; Fatouros, D.G. Chemotherapeutic Delivery from a Self-Assembling Peptide Nanofiber Hydrogel for the Management of Glioblastoma. Pharm. Res. 2018, 35, 166. [Google Scholar] [CrossRef] [PubMed]
- Veloso, S.R.S.; Jervis, P.J.; Silva, J.F.G.; Hilliou, L.; Moura, C.; Pereira, D.M.; Coutinho, P.J.G.; Martins, J.A.; Castanheira, E.M.S.; Ferreira, P.M.T. Supramolecular ultra-short carboxybenzyl-protected dehydropeptide-based hydrogels for drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 122, 111869. [Google Scholar] [CrossRef]
- Karavasili, C.; Andreadis, D.A.; Katsamenis, O.L.; Panteris, E.; Anastasiadou, P.; Kakazanis, Z.; Zoumpourlis, V.; Markopoulou, C.K.; Koutsopoulos, S.; Vizirianakis, I.S.; et al. Synergistic Antitumor Potency of a Self-Assembling Peptide Hydrogel for the Local Co-delivery of Doxorubicin and Curcumin in the Treatment of Head and Neck Cancer. Mol. Pharm. 2019, 16, 2326–2341. [Google Scholar] [CrossRef]
- Shi, J.; Liu, S.; Yu, Y.; He, C.; Tan, L.; Shen, Y.M. RGD peptide-decorated micelles assembled from polymer-paclitaxel conjugates towards gastric cancer therapy. Colloids Surf. B Biointerfaces 2019, 180, 58–67. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, S.; Sun, L.; Huang, Y.; Lenaghan, S.C.; Zhang, M. Doxorubicin-loaded cyclic peptide nanotube bundles overcome chemoresistance in breast cancer cells. J. Biomed. Nanotechnol. 2014, 10, 445–454. [Google Scholar] [CrossRef]
- Michiue, H.; Kitamatsu, M.; Fukunaga, A.; Tsuboi, N.; Fujimura, A.; Matsushita, H.; Igawa, K.; Kasai, T.; Kondo, N.; Matsui, H.; et al. Self-assembling A6K peptide nanotubes as a mercaptoundecahydrododecaborate (BSH) delivery system for boron neutron capture therapy (BNCT). J. Control. Release 2021, 330, 788–796. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, Y.; Mu, G.; Yang, L.; Wang, W.; Liu, J.; Liu, J. A peptide–drug hydrogel to enhance the anti-cancer activity of chlorambucil. Biomater. Sci. 2020, 8, 5638–5646. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Nazeer, N.; Bissessur, R.; Ahmed, M. Diatoms embedded, self-assembled carriers for dual delivery of chemotherapeutics in cancer cell lines. Int. J. Pharm. 2020, 573, 118887. [Google Scholar] [CrossRef]
- Burgess, K.A.; Frati, C.; Meade, K.; Gao, J.; Castillo Diaz, L.; Madeddu, D.; Graiani, G.; Cavalli, S.; Miller, A.F.; Oceandy, D.; et al. Functionalised peptide hydrogel for the delivery of cardiac progenitor cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111539. [Google Scholar] [CrossRef]
- Lin, Y.D.; Luo, C.Y.; Hu, Y.N.; Yeh, M.L.; Hsueh, Y.C.; Chang, M.Y.; Tsai, D.C.; Wang, J.N.; Tang, M.J.; Wei, E.I.H.; et al. Instructive Nanofiber Scaffolds with VEGF Create a Microenvironment for Arteriogenesis and Cardiac Repair. Sci. Transl. Med. 2012, 4, 146ra109. [Google Scholar] [CrossRef]
- Guo, H.D.; Cui, G.H.; Yang, J.J.; Wang, C.; Zhu, J.; Zhang, L.S.; Jiang, J.; Shao, S.J. Sustained delivery of VEGF from designer self-assembling peptides improves cardiac function after myocardial infarction. Biochem. Biophys. Res. Commun. 2012, 424, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Phipps, M.C.; Monte, F.; Mehta, M.; Kim, H.K. Intraosseous delivery of bone morphogenic protein-2 using a self-assembling peptide hydrogel. Biomacromolecules 2016, 17, 2329–2336. [Google Scholar] [CrossRef]
- Xing, J.Z.; Lu, L.; Unsworth, L.D.; Major, P.W.; Doschak, M.R.; Kaipatur, N.R. RANKL release from self-assembling nanofiber hydrogels for inducing osteoclastogenesis in vitro. Acta Biomater. 2017, 49, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Huang, B.J.; Kaltz, S.R.; Sur, S.; Newcomb, C.J.; Stock, S.R.; Shah, R.N.; Stupp, S.I. Bone regeneration with low dose BMP-2 amplified by biomimetic supramolecular nanofibers within collagen scaffolds. Biomaterials 2013, 34, 452–459. [Google Scholar] [CrossRef]
- Bessa, P.C.; Machado, R.; Nürnberger, S.; Dopler, D.; Banerjee, A.; Cunha, A.M.; Rodríguez-Cabello, J.C.; Redl, H.; van Griensven, M.; Reis, R.L.; et al. Thermoresponsive self-assembled elastin-based nanoparticles for delivery of BMPs. J. Control. Release 2010, 142, 312–318. [Google Scholar] [CrossRef]
- Xiao, Z.; Yao, Y.; Wang, Z.; Tian, Q.; Wang, J.; Gu, L.; Li, B.; Zheng, Q.; Wu, Y. Local Delivery of Taxol From FGL-Functionalized Self-Assembling Peptide Nanofiber Scaffold Promotes Recovery After Spinal Cord Injury. Front. Cell Dev. Biol. 2020, 8, 820. [Google Scholar] [CrossRef]
- Luo, J.; Shi, X.; Li, L.; Tan, Z.; Feng, F.; Li, J.; Pang, M.; Wang, X.; He, L. An injectable and self-healing hydrogel with controlled release of curcumin to repair spinal cord injury. Bioact. Mater. 2021, 6, 4816–4829. [Google Scholar] [CrossRef] [PubMed]
- Raspa, A.; Carminati, L.; Pugliese, R.; Fontana, F.; Gelain, F. Self-assembling peptide hydrogels for the stabilization and sustained release of active Chondroitinase ABC in vitro and in spinal cord injuries. J. Control. Release 2021, 330, 1208–1219. [Google Scholar] [CrossRef]
- Hassannejad, Z.; Zadegan, S.A.; Vaccaro, A.R.; Rahimi-Movaghar, V.; Sabzevari, O. Biofunctionalized peptide-based hydrogel as an injectable scaffold for BDNF delivery can improve regeneration after spinal cord injury. Injury 2019, 50, 278–285. [Google Scholar] [CrossRef]
- Lindsey, S.; Piatt, J.H.; Worthington, P.; Sonmez, C.; Satheye, S.; Schneider, J.P.; Pochan, D.J.; Langhans, S.A. Beta Hairpin Peptide Hydrogels as an Injectable Solid Vehicle for Neurotrophic Growth Factor Delivery. Biomacromolecules 2015, 16, 2672–2683. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Takenaka, T.; Toh, K.; Wu, S.; Nishihara, H.; Kano, M.R.; Ino, Y.; Nomoto, T.; Matsumoto, Y.; Koyama, H.; et al. Cyclic RGD-linked polymeric micelles for targeted delivery of platinum anticancer drugs to glioblastoma through the blood–brain tumor barrier. ACS Nano 2013, 7, 8583–8592. [Google Scholar] [CrossRef] [PubMed]
- Quader, S.; Liu, X.; Chen, Y.; Mi, P.; Chida, T.; Ishii, T.; Miura, Y.; Nishiyama, N.; Cabral, H.; Kataoka, K. cRGD peptide-installed epirubicin-loaded polymeric micelles for effective targeted therapy against brain tumors. J. Control. Release 2017, 258, 56–66. [Google Scholar] [CrossRef]
- Kulhari, H.; Telukutla, S.R.; Pooja, D.; Shukla, R.; Sistla, R.; Bansal, V.; Adams, D.J. Peptide grafted and self-assembled poly (γ-glutamic acid)-phenylalanine nanoparticles targeting camptothecin to glioma. Nanomedicine 2017, 12, 1661–1674. [Google Scholar] [CrossRef]
- Dragojevic, S.; Mackey, R.; Raucher, D. Evaluation of elastin-like polypeptides for tumor targeted delivery of doxorubicin to glioblastoma. Molecules 2019, 24, 3242. [Google Scholar] [CrossRef]
- Karavasili, C.; Komnenou, A.; Katsamenis, O.L.; Charalampidou, G.; Kofidou, E.; Andreadis, D.; Koutsopoulos, S.; Fatouros, D.G. Self-Assembling Peptide Nanofiber Hydrogels for Controlled Ocular Delivery of Timolol Maleate. ACS Biomater. Sci. Eng. 2017, 3, 3386–3394. [Google Scholar] [CrossRef]
- Taka, E.; Karavasili, C.; Bouropoulos, N.; Moschakis, T.; Andreadis, D.D.D.; Zacharis, C.K.K.; Fatouros, D.G.G. Ocular co-Delivery of Timolol and Brimonidine from a Self-Assembling Peptide Hydrogel for the Treatment of Glaucoma: In Vitro and Ex Vivo Evaluation. Pharmaceuticals 2020, 13, 126. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, Z.; Yu, J.; Chen, H.; Li, X. Self-assembly of a ibuprofen-peptide conjugate to suppress ocular inflammation. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 185–193. [Google Scholar] [CrossRef]
- Liu, H.; Bi, X.; Wu, Y.; Pan, M.; Ma, X.; Mo, L.; Wang, J.; Li, X. Cationic self-assembled peptide-based molecular hydrogels for extended ocular drug delivery. Acta Biomater. 2021, 131, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, H.; Yu, A.; Lin, D.; Bao, Z.; Wang, Y.; Li, X. Bioinspired self-assembly supramolecular hydrogel for ocular drug delivery. Chin. Chem. Lett. 2021, 32, 3936–3939. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, L.; Cheng, J.; Lu, Y.; Liu, J.J.I.J.o.N. Sustained release of hepatocyte growth factor by cationic self-assembling peptide/heparin hybrid hydrogel improves β-cell survival and function through modulating inflammatory response. Int. J. Nanomed. 2016, 11, 4875. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Sur, S.; Newcomb, C.J.; Appelt, E.A.; Stupp, S.I.J.A.b. Self-assembling glucagon-like peptide 1-mimetic peptide amphiphiles for enhanced activity and proliferation of insulin-secreting cells. Acta Biomater 2012, 8, 1685–1692. [Google Scholar] [CrossRef]
- DeSantis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Breast cancer statistics. CA Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef]
- Agazzi, M.L.; Herrera, S.E.; Cortez, M.L.; Marmisolle, W.A.; Azzaroni, O. Self-assembled peptide dendrigraft supraparticles with potential application in pH/enzyme-triggered multistage drug release. Colloids Surf. B Biointerfaces 2020, 190, 110895. [Google Scholar] [CrossRef]
- Galeano Niño, J.L.; Pageon, S.V.; Tay, S.S.; Colakoglu, F.; Kempe, D.; Hywood, J.; Mazalo, J.K.; Cremasco, J.; Govendir, M.A.; Dagley, L.F.; et al. Cytotoxic T cells swarm by homotypic chemokine signalling. eLife 2020, 9, e56554. [Google Scholar] [CrossRef]
- Duan, X.; He, C.; Kron, S.J.; Lin, W. Nanoparticle formulations of cisplatin for cancer therapy. WIREs Nanomed. Nanobiotechnol. 2016, 8, 776–791. [Google Scholar] [CrossRef]
- Cahill, T.J.; Kharbanda, R.K. Heart failure after myocardial infarction in the era of primary percutaneous coronary intervention: Mechanisms, incidence and identification of patients at risk. World J. Cardiol. 2017, 9, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.S.; Ambrosy, A.P.; Velazquez, E.J. Adverse Remodeling and Reverse Remodeling After Myocardial Infarction. Curr. Cardiol. Rep. 2017, 19, 71. [Google Scholar] [CrossRef]
- French, K.M.; Somasuntharam, I.; Davis, M.E. Self-assembling peptide-based delivery of therapeutics for myocardial infarction. Adv. Drug Deliv. Rev. 2016, 96, 40–53. [Google Scholar] [CrossRef]
- Hogrebe, N.J.; Reinhardt, J.W.; Tram, N.K.; Debski, A.C.; Agarwal, G.; Reilly, M.A.; Gooch, K.J. Independent control of matrix adhesiveness and stiffness within a 3D self-assembling peptide hydrogel. Acta Biomater. 2018, 70, 110–119. [Google Scholar] [CrossRef]
- Savi, M.; Bocchi, L.; Rossi, S.; Frati, C.; Graiani, G.; Lagrasta, C.; Miragoli, M.; Di Pasquale, E.; Stirparo, G.G.; Mastrototaro, G.; et al. Antiarrhythmic effect of growth factor-supplemented cardiac progenitor cells in chronic infarcted heart. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H1622–H1648. [Google Scholar] [CrossRef] [PubMed]
- Webber, M.J.; Appel, E.A.; Meijer, E.W.; Langer, R. Supramolecular biomaterials. Nat. Mater. 2016, 15, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Imagama, S.; Kobayashi, K.; Ito, K.; Tsushima, M.; Morozumi, M.; Tanaka, S.; Machino, M.; Ota, K.; Nishida, K.; et al. Effects of a self-assembling peptide as a scaffold on bone formation in a defect. PLoS ONE 2018, 13, e0190833. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, J.; Naruse, K.; Nagai, Y.; Kan, S.; Nakamura, N.; Hata, M.; Omi, M.; Hayashi, T.; Kawai, T.; Matsubara, T. Efficacy of a self-assembling peptide hydrogel, SPG-178-gel, for bone regeneration and three-dimensional osteogenic induction of dental pulp stem cells. Tissue Eng. Part A 2017, 23, 1394–1402. [Google Scholar] [CrossRef]
- Shah, M.; Peterson, C.; Yilmaz, E.; Halalmeh, D.R.; Moisi, M. Current advancements in the management of spinal cord injury: A comprehensive review of literature. Surg. Neurol. Int. 2020, 11, 2. [Google Scholar] [CrossRef]
- Wiseman, T.M.; Baron-Heeris, D.; Houwers, I.G.; Keenan, R.; Williams, R.J.; Nisbet, D.R.; Harvey, A.R.; Hodgetts, S.I. Peptide hydrogel scaffold for mesenchymal precursor cells implanted to injured adult rat spinal cord. Tissue Eng. Part A 2021, 27, 993–1007. [Google Scholar] [CrossRef]
- Yin, W.; Li, X.; Zhao, Y.N.; Tan, J.; Wu, S.Y.; Cao, Y.D.; Li, J.; Zhu, H.C.; Liu, W.D.; Tang, G.H.; et al. Taxol-modified collagen scaffold implantation promotes functional recovery after long-distance spinal cord complete transection in canines. Biomater. Sci. 2018, 6, 1099–1108. [Google Scholar] [CrossRef]
- Rosenzweig, E.S.; Salegio, E.A.; Liang, J.J.; Weber, J.L.; Weinholtz, C.A.; Brock, J.H.; Moseanko, R.; Hawbecker, S.; Pender, R.; Cruzen, C.L.; et al. Chondroitinase improves anatomical and functional outcomes after primate spinal cord injury. Nat. Neurosci. 2019, 22, 1269–1275. [Google Scholar] [CrossRef]
- Muir, E.; De Winter, F.; Verhaagen, J.; Fawcett, J. Recent advances in the therapeutic uses of chondroitinase ABC. Exp. Neurol. 2019, 321, 113032. [Google Scholar] [CrossRef] [PubMed]
- Duro-Castano, A.; Moreira Leite, D.; Forth, J.; Deng, Y.; Matias, D.; Noble Jesus, C.; Battaglia, G. Designing peptide nanoparticles for efficient brain delivery. Adv. Drug Deliv. Rev. 2020, 160, 52–77. [Google Scholar] [CrossRef] [PubMed]
- Spicer, C.D.; Jumeaux, C.; Gupta, B.; Stevens, M.M. Peptide and protein nanoparticle conjugates: Versatile platforms for biomedical applications. Chem. Soc. Rev. 2018, 47, 3574–3620. [Google Scholar] [CrossRef] [PubMed]
- Samaridou, E.; Alonso, M.J. Nose-to-brain peptide delivery–The potential of nanotechnology. Biorg. Med. Chem. 2018, 26, 2888–2905. [Google Scholar] [CrossRef]
- Kawamura, W.; Miura, Y.; Kokuryo, D.; Toh, K.; Yamada, N.; Nomoto, T.; Matsumoto, Y.; Sueyoshi, D.; Liu, X.; Aoki, I.; et al. Density-tunable conjugation of cyclic RGD ligands with polyion complex vesicles for the neovascular imaging of orthotopic glioblastomas. Sci. Technol. Adv. Mater. 2015, 16, 035004. [Google Scholar] [CrossRef]
- Zhu, X.; Jin, K.; Huang, Y.; Pang, Z. Brain drug delivery by adsorption-mediated transcytosis. In Brain Targeted Drug Delivery System; Elsevier: Amsterdam, The Netherlands, 2019; pp. 159–183. [Google Scholar]
- Opačak-Bernardi, T.; Ryu, J.S.; Raucher, D. Effects of cell penetrating Notch inhibitory peptide conjugated to elastin-like polypeptide on glioblastoma cells. J. Drug Target. 2017, 25, 523–531. [Google Scholar] [CrossRef]
- Wang, Y.; He, X.; Bruggeman, K.F.; Gayen, B.; Tricoli, A.; Lee, W.M.; Williams, R.J.; Nisbet, D.R. Peptide programmed hydrogels as safe sanctuary microenvironments for cell transplantation. Adv. Funct. Mater. 2020, 30, 1900390. [Google Scholar] [CrossRef]
- Hunt, C.P.; Penna, V.; Gantner, C.W.; Moriarty, N.; Wang, Y.; Franks, S.; Ermine, C.M.; de Luzy, I.R.; Pavan, C.; Long, B.M.; et al. Tissue programmed hydrogels functionalized with GDNF improve human neural grafts in Parkinson’s disease. Adv. Funct. Mater. 2021, 31, 2105301. [Google Scholar] [CrossRef]
- Penna, V.; Moriarty, N.; Wang, Y.; Law, K.C.; Gantner, C.W.; Williams, R.J.; Nisbet, D.R.; Parish, C.L. Extracellular matrix biomimetic hydrogels, encapsulated with stromal Cell-Derived Factor 1, improve the composition of foetal tissue grafts in a rodent model of Parkinson’s disease. Int. J. Mol. Sci. 2022, 23, 4646. [Google Scholar] [CrossRef]
- Cooper, R.C.; Yang, H. Hydrogel-based ocular drug delivery systems: Emerging fabrication strategies, applications, and bench-to-bedside manufacturing considerations. J. Control. Release 2019, 306, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Subrizi, A.; Del Amo, E.M.; Korzhikov-Vlakh, V.; Tennikova, T.; Ruponen, M.; Urtti, A. Design principles of ocular drug delivery systems: Importance of drug payload, release rate, and material properties. Drug Discov. Today 2019, 24, 1446–1457. [Google Scholar] [CrossRef]
- Li, X.; Meng, Q.; Zhang, L. The fate of allogeneic pancreatic islets following intraportal transplantation: Challenges and solutions. J. Immunol. Res. 2018, 2018, 2424586. [Google Scholar] [CrossRef] [PubMed]
- Pepper, A.R.; Gala-Lopez, B.; Ziff, O.; Shapiro, A.J. Current status of clinical islet transplantation. World J. Transplant. 2013, 3, 48. [Google Scholar] [CrossRef]
- Thomas, F.; Wu, J.; Contreras, J.L.; Smyth, C.; Bilbao, G.; He, J.; Thomas, J.J.S. A tripartite anoikis-like mechanism causes early isolated islet apoptosis. Surgery 2001, 130, 333–338. [Google Scholar] [CrossRef]
- Baral, A.; Roy, S.; Dehsorkhi, A.; Hamley, I.W.; Mohapatra, S.; Ghosh, S.; Banerjee, A. Assembly of an Injectable Noncytotoxic Peptide-Based Hydrogelator for Sustained Release of Drugs. Langmuir 2014, 30, 929–936. [Google Scholar] [CrossRef]
- Castelletto, V.; Hamley, I.W.; Stain, C.; Connon, C. Slow-Release RGD-Peptide Hydrogel Monoliths. Langmuir 2012, 28, 12575–12580. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ren, C.; Shang, Y.; Yang, C.; Guo, Q.; Chu, L.; Liu, J. Co-assembled Supramolecular Nanofibers With Tunable Surface Properties for Efficient Vaccine Delivery. Front. Chem. 2020, 8, 500. [Google Scholar] [CrossRef]
- Nevagi, R.J.; Dai, W.; Khalil, Z.G.; Hussein, W.M.; Capon, R.J.; Skwarczynski, M.; Toth, I. Self-assembly of trimethyl chitosan and poly(anionic amino acid)-peptide antigen conjugate to produce a potent self-adjuvanting nanovaccine delivery system. Bioorganic Med. Chem. 2019, 27, 3082–3088. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, H.; Wang, F.; Zhang, X. Amphiphilic self-assembly peptides: Rational strategies to design and delivery for drugs in biomedical applications. Colloids Surf. B Biointerfaces 2021, 208, 112040. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Kramer, J.; Smith, J.; Allen, B.; Leeper, C.; Li, X.L.; Morton, L.; Gallazzi, F.; Ulery, B.D. Vaccine Adjuvant Incorporation Strategy Dictates Peptide Amphiphile Micelle Immunostimulatory Capacity. AAPS J. 2018, 20, 73. [Google Scholar] [CrossRef]
- Bozdoğan, B.; Akbal, Ö.; Çelik, E.; Türk, M.; Denkbaş, E.B. Novel layer-by-layer self-assembled peptide nanocarriers for siRNA delivery. RSC Adv. 2017, 7, 47592–47601. [Google Scholar] [CrossRef]
- Mazza, M.; Hadjidemetriou, M.; de Lazaro, I.; Bussy, C.; Kostarelos, K. Peptide nanofiber complexes with siRNA for deep brain gene silencing by stereotactic neurosurgery. ACS Nano 2015, 9, 1137–1149. [Google Scholar] [CrossRef]
- Vermeer, L.S.; Hamon, L.; Schirer, A.; Schoup, M.; Cosette, J.; Majdoul, S.; Pastre, D.; Stockholm, D.; Holic, N.; Hellwig, P.; et al. Vectofusin-1, a potent peptidic enhancer of viral gene transfer forms pH-dependent alpha-helical nanofibrils, concentrating viral particles. Acta Biomater. 2017, 64, 259–268. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, T.; Jin, S.; Xue, X.; Yang, X.; Gong, N.; Zhang, J.; Wang, P.C.; Tian, J.-H.; Xing, J.; et al. Virus-Inspired Self-Assembled Nanofibers with Aggregation-Induced Emission for Highly Efficient and Visible Gene Delivery. ACS Appl. Mater. Interfaces 2017, 9, 4425–4432. [Google Scholar] [CrossRef]
- Yoshida, D.; Kim, K.; Takumi, I.; Yamaguchi, F.; Adachi, K.; Teramoto, A. A transfection method for short interfering RNA with the lipid-like self-assembling nanotube, A6K. Med. Mol. Morphol. 2013, 46, 86–91. [Google Scholar] [CrossRef]
- Lehto, T.; Ezzat, K.; Wood, M.J.A.; El Andaloussi, S. Peptides for nucleic acid delivery. Adv. Drug Deliv. Rev. 2016, 106, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Thapa, R.K.; Sullivan, M.O. Gene delivery by peptide-assisted transport. Curr. Opin. Biomed. Eng. 2018, 7, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Cembran, A.; Bruggeman, K.F.; Williams, R.J.; Parish, C.L.; Nisbet, D.R. Biomimetic materials and their utility in modeling the 3-dimensional neural environment. Iscience 2020, 23, 100788. [Google Scholar] [CrossRef]
- Ni, R.; Feng, R.; Chau, Y. Synthetic approaches for nucleic acid delivery: Choosing the right carriers. Life 2019, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Mumcuoglu, D.; Sardan Ekiz, M.; Gunay, G.; Tekinay, T.; Tekinay, A.B.; Guler, M.O. Cellular internalization of therapeutic oligonucleotides by peptide amphiphile nanofibers and nanospheres. ACS Appl. Mater. Interfaces 2016, 8, 11280–11287. [Google Scholar] [CrossRef]
- Cao, M.; Wang, Y.; Zhao, W.; Qi, R.; Han, Y.; Wu, R.; Wang, Y.; Xu, H. Peptide-induced DNA condensation into virus-mimicking nanostructures. ACS Appl. Mater. Interfaces 2018, 10, 24349–24360. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Barz, M.; Schmid, F. Complex formation between polyelectrolytes and oppositely charged oligoelectrolytes. J. Chem. Phys. 2016, 144, 164902. [Google Scholar] [CrossRef]
- Vasiliu, T.; Cojocaru, C.; Rotaru, A.; Pricope, G.; Pinteala, M.; Clima, L. Optimization of Polyplex Formation between DNA Oligonucleotide and Poly(L-Lysine): Experimental Study and Modeling Approach. Int. J. Mol. Sci. 2017, 18, 1291. [Google Scholar] [CrossRef]
- Guan, X.; Chang, Y.; Sun, J.; Song, J.; Xie, Y. Engineered Hsp protein Nanocages for siRNA delivery. Macromol. Biosci. 2018, 18, 1800013. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.G.; Ryu, Y.C.; Hwang, B.H. Synergistic gene delivery by self-assembled nanocomplexes using fusion peptide and calcium phosphate. J. Control. Release 2021, 338, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Angioletti-Uberti, S.; Battaglia, G. On the design of precision nanomedicines. Sci. Adv. 2020, 6, eaat0919. [Google Scholar] [CrossRef]
- Curk, T.; Brackley, C.A.; Farrell, J.D.; Xing, Z.; Joshi, D.; Direito, S.; Bren, U.; Angioletti-Uberti, S.; Dobnikar, J.; Eiser, E.; et al. Computational design of probes to detect bacterial genomes by multivalent binding. Proc. Natl. Acad. Sci. USA 2020, 117, 8719–8726. [Google Scholar] [CrossRef]
| SAP | Delivered Molecule(s) | Secondary Structure | Physical Form | Factor Triggering Self-Assembly | Payload | Ref. |
|---|---|---|---|---|---|---|
| Anticancer drug delivery | ||||||
| AmPDKK2/AmPDKK2K4 | doxorubicin | β-sheet | nanoparticle | hydrophobic and hydrophilic interactions | breast cancer MCF-7R cells | [61] |
| GGVVVRGDR | paclitaxel | β-sheet | Hydrogel | Ion | U87 cancer cells | [13] |
| EVEALEKKVAALEC KVQALEKKVEALEHGW | Doxorubicin, apomorphine, rapamycin, tamoxifen, dexamethasone, paclitaxel | Worm-like Micelles | Spheres | hydrophobic interactions/geometric packing | - | [62] |
| LLLLLLKKKGRGDS | doxorubicin | β-sheet and random coil | nanoparticles | pH-driven | HepG2 cell | [63] |
| Adenine acetic acid-FFF | doxorubicin | Random structure | Hydrogel | pH-driven | 4T1 cancer cells and tumor-bearing BALB/c mice | [64] |
| RADA16-I | Doxorubicin, curcumin | β-sheets | Hydrogel | Ion | Glioblastoma | [65] |
| Cbz-FF | Doxorubicin, curcumin | β-sheets | Hydrogel | pH-driven | - | [66] |
| RADA16-I | doxorubicin/curcumin | β-sheets | Hydrogel | Ion | head and neck cancer HSC-3 cells and HSC-3 tumor bearing SCID mice | [67] |
| RGD-PEG-SS-PTX | paclitaxel | - | Micelles | amphiphilic interactions | Human gastric carcinoma SGC7901 cells, mouse xenograft model of gastric tumor | [68] |
| PEG-QAEAQACA | doxorubicin | β-sheet | Nanotube | pH-driven | Human breast cancer MCF-7/ADR cells | [69] |
| AAAAAAK | Boron neutron | - | Nanotube | pH-driven | human U87 delta EGFR glioma cells | [70] |
| Chlorambucil -FFFK-cyclen | Chlorambucil | β-sheet | Hydrogel | heating-cooling | A549, Hela, MCF-7 cancer cells | [71] |
| GRVGPLGK | Doxorubicin/ paclitaxel/ curcumin | - | Hydrogel | ions | Hela, HT1080 cells | [72] |
| Cardiovascular | ||||||
| RGDSP-FEFEFKFK | rat cardiac progenitor cells | anti-parallel β-sheets | Hydrogel | pH-driven | cardiac injury rats | [73] |
| RADA 16-II | vascular endothelial growth factor | β-sheets | Hydrogel | ion | myocardial infarction rat, myocardial infarction pig | [74] |
| LRKKLGKA-RADA 16-I | vascular endothelial growth factor | β-sheets | Hydrogel | ion | infarcted myocardium rats | [75] |
| Musculoskeletal | ||||||
| RADA16-I | bone morphogenic protein-2 | β-sheets | Hydrogel | ion | bone marrow stromal cell | [76] |
| RADA16-I | NF-kB ligand protein | β-sheets | Hydrogel | ion | Mouse macrophage cell line RAW 264.7 cells | [77] |
| Palmitoyl-AAAAGGGLRKKLGKA | Bone morphogenetic protein-2 | β-sheets | Hydrogel | pH-driven | critical-size femoral defect rat | [78] |
| (VPAVG)220 | bone morphogenetic protein-2 | - | nanoparticles | temperature | C2C12 cells | [79] |
| Peripheral Nervous System | ||||||
| RADA16-I-FGL | Taxol | β-sheets | Hydrogel | ion | Spin cord injury rats | [80] |
| Fmoc-IKVAV/Fmoc-chitosan | curcumin | β-sheets | Hydrogel | pH-driven | Laminectomy rats | [81] |
| FAQ (FAQRVPPGGGLDLKLDLKLDLK), CK (CGGLKLKLKLKLKLKGGC) | Chondroitinase ABC | β-sheet | Hydrogel | electrolytes and pH driven | Murine Neural Stem Cells, spin cord injury rats | [82] |
| IKVAV-PA | brain-derived neurotrophic factor | β-helices | Ion | ion | spinal cord injury rats | [83] |
| Central Nervous System | ||||||
| MAX8 | Nerve growth factor, brain-derived neurotrophic factor | β-hairpin | Hydrogel | ion | Growth factor delivery | [84] |
| Cyclic RGD linked polymeric micelles | (1,2-diaminocyclohexane) platinum (II) | - | Micelles | ion | orthotopic mouse model of U87MG human glioblastoma | [85] |
| Cyclic RGD linked polymeric micelles | epirubicin | - | Micelles | ion | glioblastoma | [86] |
| cRGDfK-conjugated γ-PGA | camptothecin | - | nanoparticles | hydrophobic and hydrophilic interactions | U87MG human glioblastoma cells | [87] |
| SynB1-VPGXG | doxorubicin | - | Conjugate | temperature | D54, GBM6, U251-MG glioblastoma cell lines | [88] |
| Ocular Delivery | ||||||
| RADA 16-I/(IEIK)3I | timolol maleate | β-sheets | Hydrogel | ion | Rabbit eyes | [89] |
| RADA16-I | Timolol and Brimonidine | β-sheets | Hydrogel | ion | Glaucoma | [90] |
| ibuprofen– hydroxybenzoic acid –GFFY | ibuprofen | β-sheets | Hydrogel | Heat-cooling process | RAW264.7 macrophages, rabbit eyes, eye disorders | [91] |
| 2-naphthylacetic acid (Nap)-FFKK | ---- | β-sheet | Hydrogel | ion | Rabbit eyes | [92] |
| Nap-KKFKLKL | dexamethasone sodium phosphate | α-helical | Hydrogel | noncovalent interaction | retinal architecture and eyesight functions of experimental autoimmune uveitis rat model | [93] |
| Pancreatic Delivery | ||||||
| KLD12 | Hepatocyte growth factor | β-sheet | Hydrogel | Ion | INS-1 beta-cell line | [94] |
| HSEDTFTSD | - | α-helical | Hydrogel | Ion | RINm5f cell line | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Zhou, Q.-L.; Tai, M.-R.; Ashton-Mourney, K.; Harty, M.I.; Rifai, A.; Parish, C.L.; Nisbet, D.R.; Zhong, S.-Y.; Williams, R.J. Simple Complexity: Incorporating Bioinspired Delivery Machinery within Self-Assembled Peptide Biogels. Gels 2023, 9, 199. https://doi.org/10.3390/gels9030199
Li R, Zhou Q-L, Tai M-R, Ashton-Mourney K, Harty MI, Rifai A, Parish CL, Nisbet DR, Zhong S-Y, Williams RJ. Simple Complexity: Incorporating Bioinspired Delivery Machinery within Self-Assembled Peptide Biogels. Gels. 2023; 9(3):199. https://doi.org/10.3390/gels9030199
Chicago/Turabian StyleLi, Rui, Qing-Ling Zhou, Min-Rui Tai, Kathryn Ashton-Mourney, Mathew I. Harty, Aaqil Rifai, Clare L. Parish, David R. Nisbet, Sai-Yi Zhong, and Richard J. Williams. 2023. "Simple Complexity: Incorporating Bioinspired Delivery Machinery within Self-Assembled Peptide Biogels" Gels 9, no. 3: 199. https://doi.org/10.3390/gels9030199
APA StyleLi, R., Zhou, Q.-L., Tai, M.-R., Ashton-Mourney, K., Harty, M. I., Rifai, A., Parish, C. L., Nisbet, D. R., Zhong, S.-Y., & Williams, R. J. (2023). Simple Complexity: Incorporating Bioinspired Delivery Machinery within Self-Assembled Peptide Biogels. Gels, 9(3), 199. https://doi.org/10.3390/gels9030199












