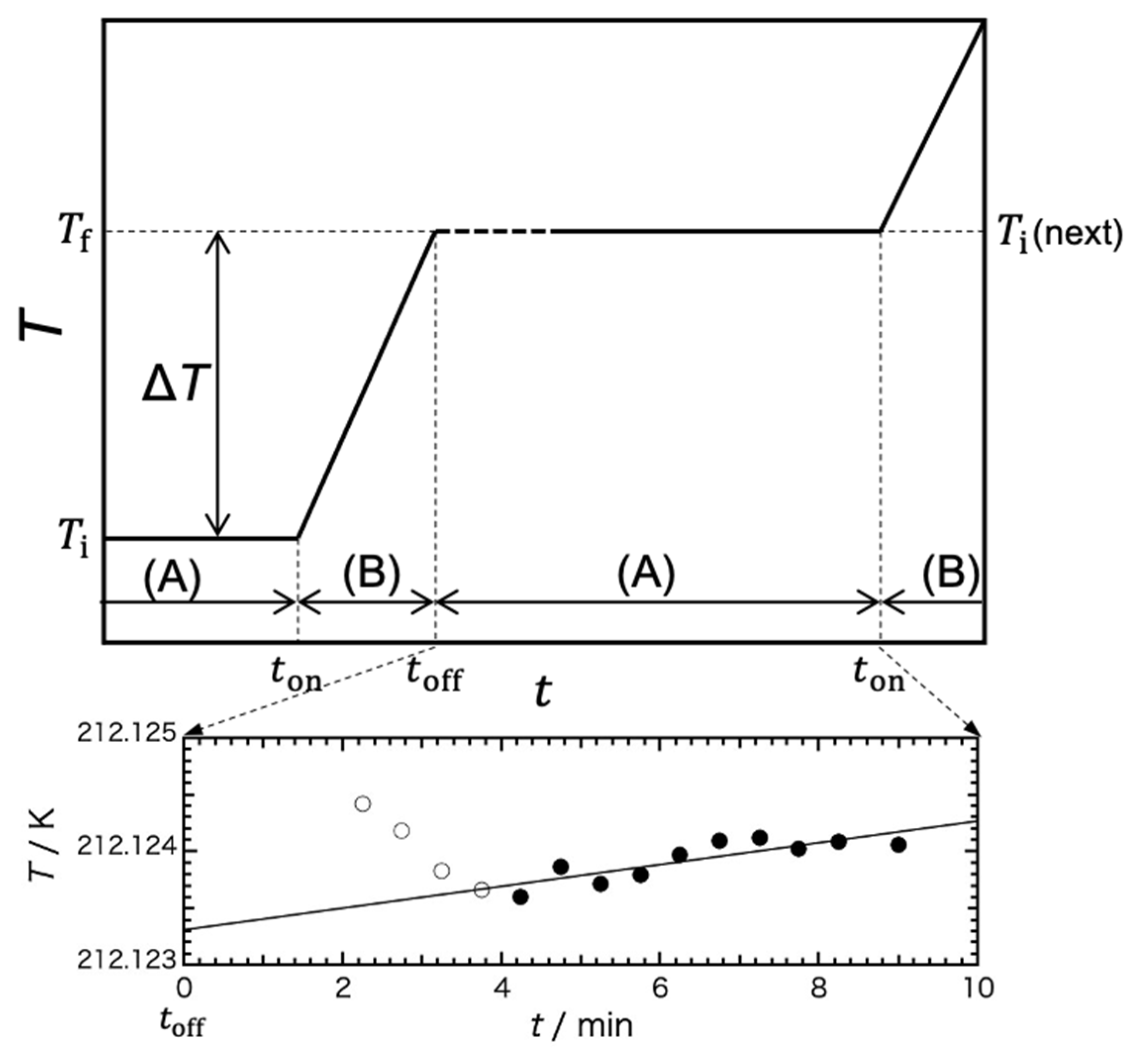
2.2. Adiabatic Calorimetry
Heat capacities of a Sephadex
® G25 gel (
h = 1.00) dependent on the rate of previous cooling are shown in
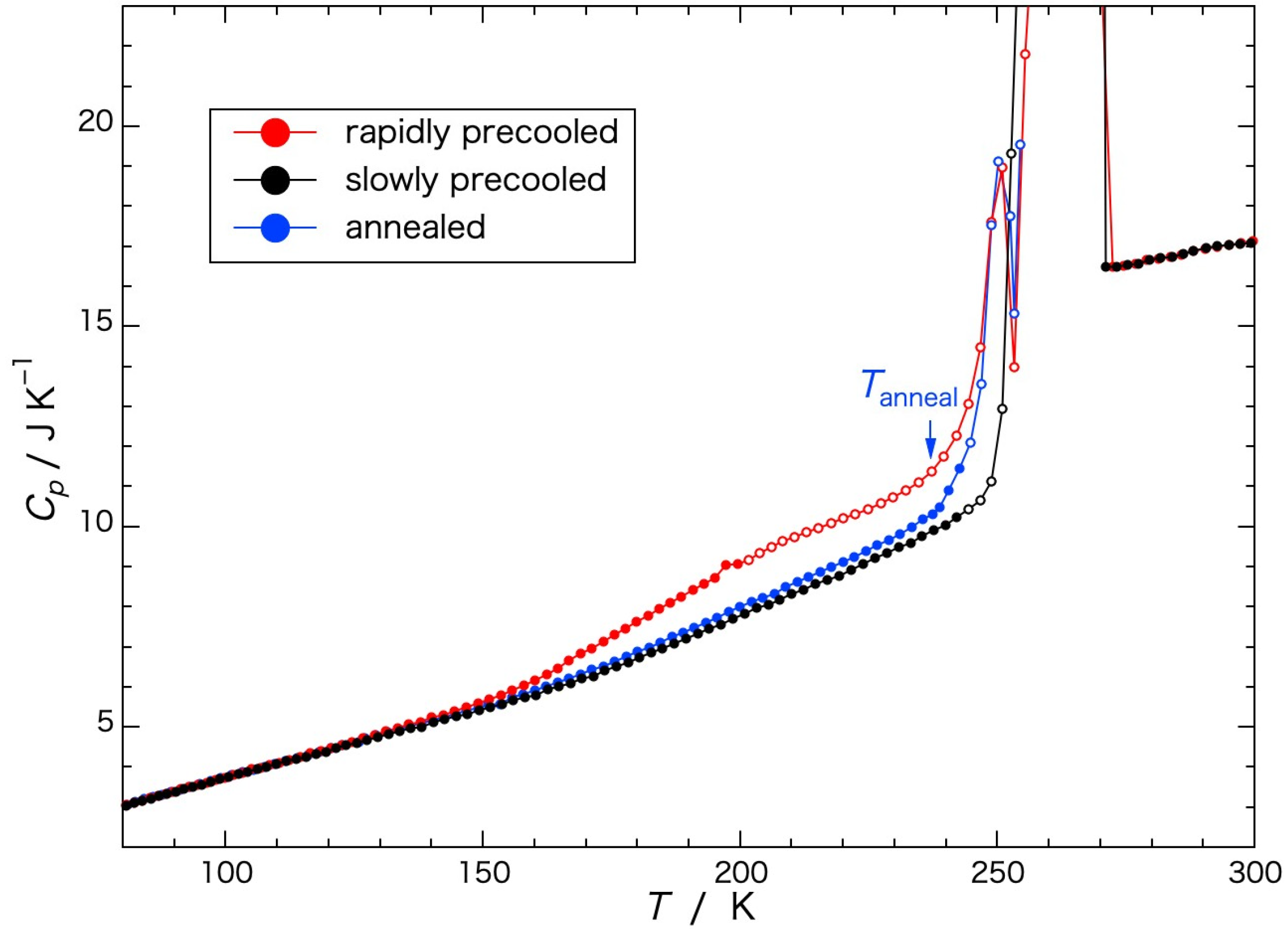
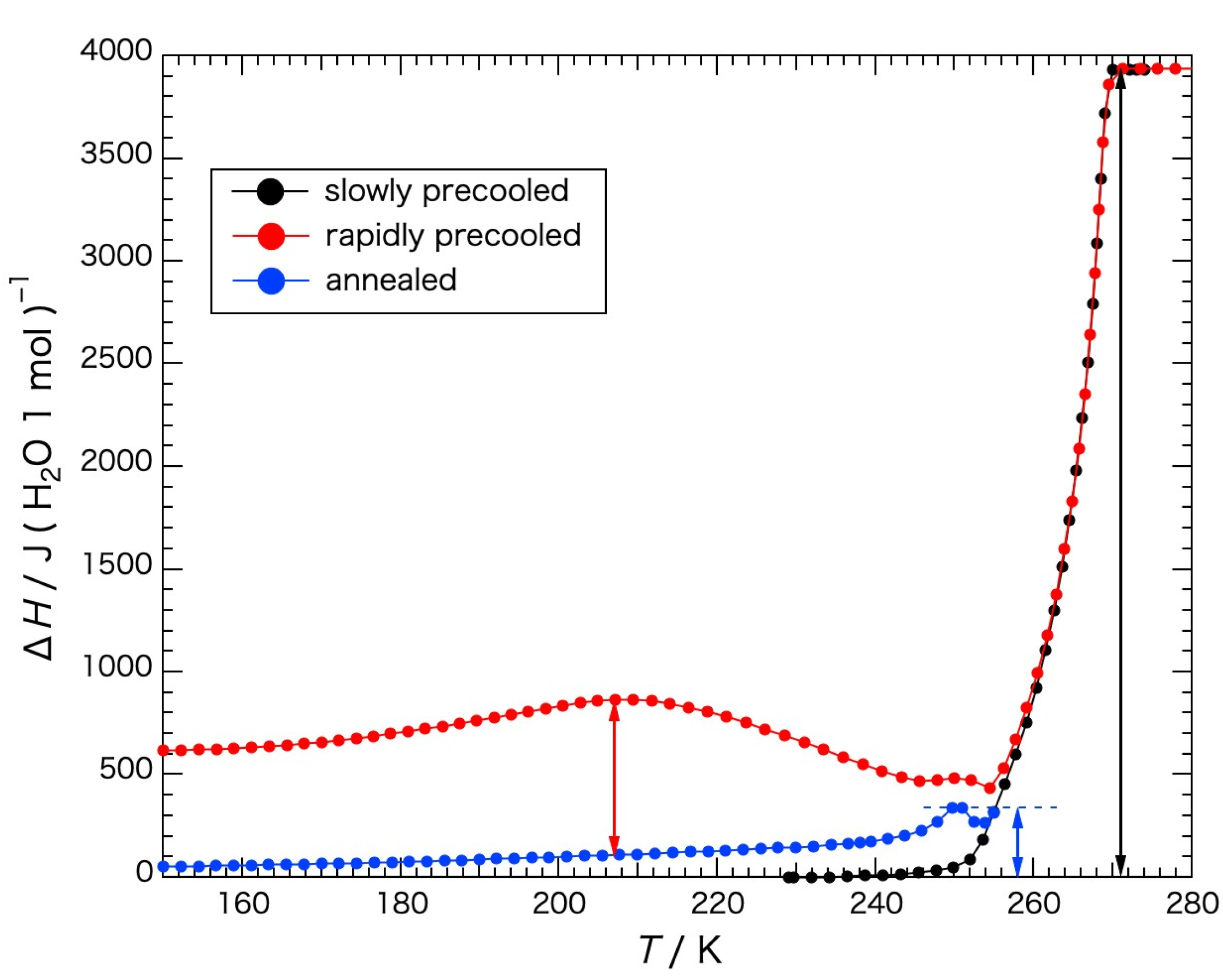
Figure 2, wherein the apparent heat capacities obtained when crystallization or melting was in progress and the equilibrium state was not reached are indicated by open circles. The heat capacities (
Cp) of the rapidly precooled Sephadex
® G25 gel (red circle) began to increase gradually in temperature from 150 K to 200 K, increasing steeply from around 240 K, and ice melting began at 245 K. Above the temperature of the initiation of ice melting, an exotherm due to ice crystallization appeared at 250–255 K. The endotherm due to ice melting finished at 271 K. In the case of the gel annealed at 237 K, the temperature below the initiation of the steep increase in the heat capacity, for 13 h (blue circle) followed by rapid cooling the heat capacity increased in a smooth curve from 80 K to the annealing temperature. Immediately above the annealing temperature, a steep increase occurred followed by the exotherm due to ice crystallization at 250–255 K, similar to the rapidly precooled gel. In the case of the gel precooled slowly (black circle), the heat capacity increased in a smooth curve until the temperature of ice melting, showing no exotherm in the temperature range from 80 K to 273 K.
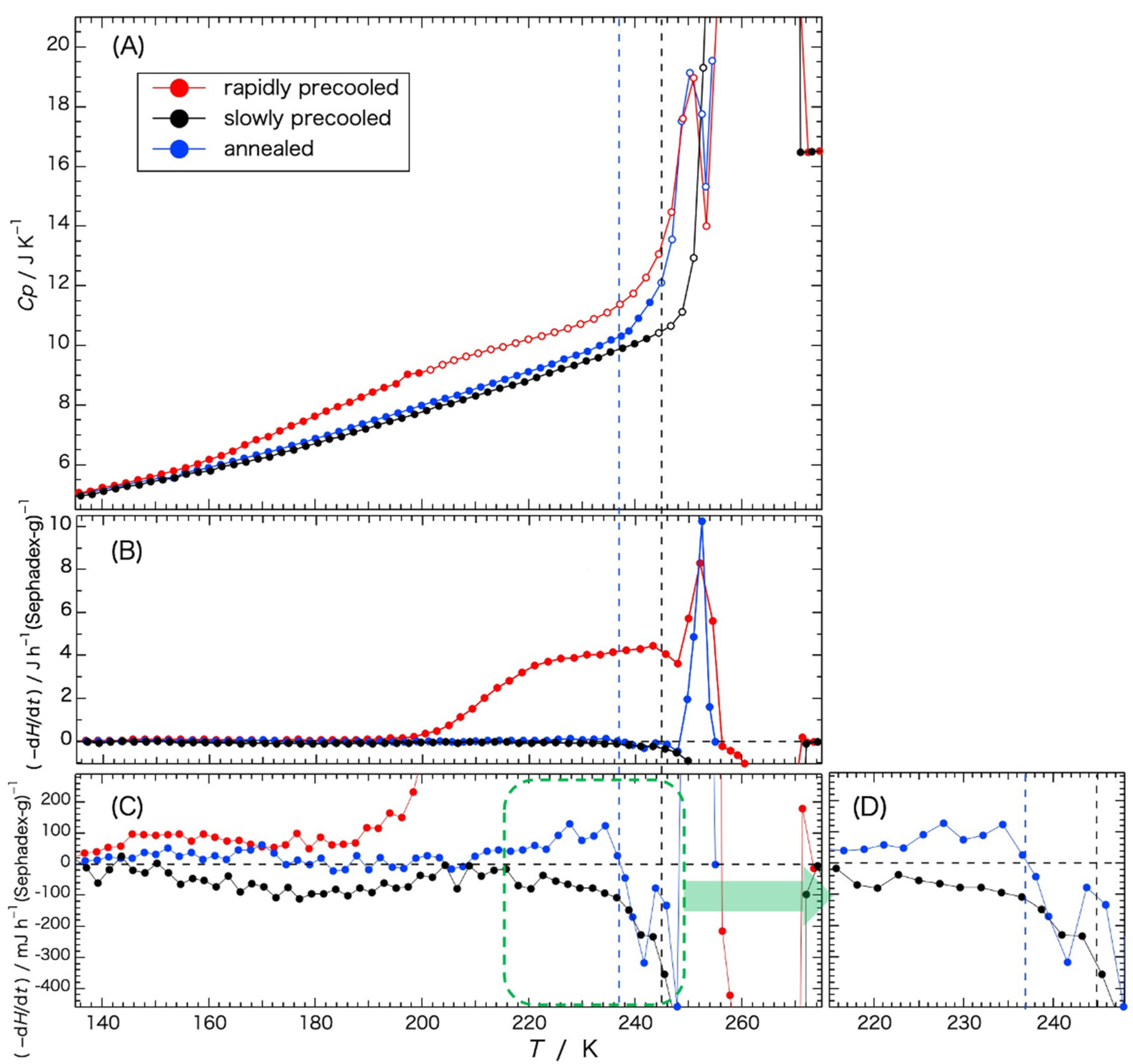
Temperature dependence of the rates of enthalpy change –(d
H/d
t) observed during the equilibration period in the heat-capacity measurement is shown in
Figure 3. The quantities were expressed by –(d
H/d
t) =
Cgross(d
T/d
t), where
Cgross is the gross heat capacity of the cell containing the sample. The rate (d
T/d
t) of the temperature increase was measured 5 min after each energy input. The time (5 min) was sufficiently longer than the thermal-equilibration time. In
Figure 3, data of the heat capacity shown in
Figure 2 are redisplayed for reference (
Figure 3A).
Figure 3C is a vertical enlargement of
Figure 3B.
Figure 3D is a lateral enlargement of the green dashed-line area in
Figure 3C.
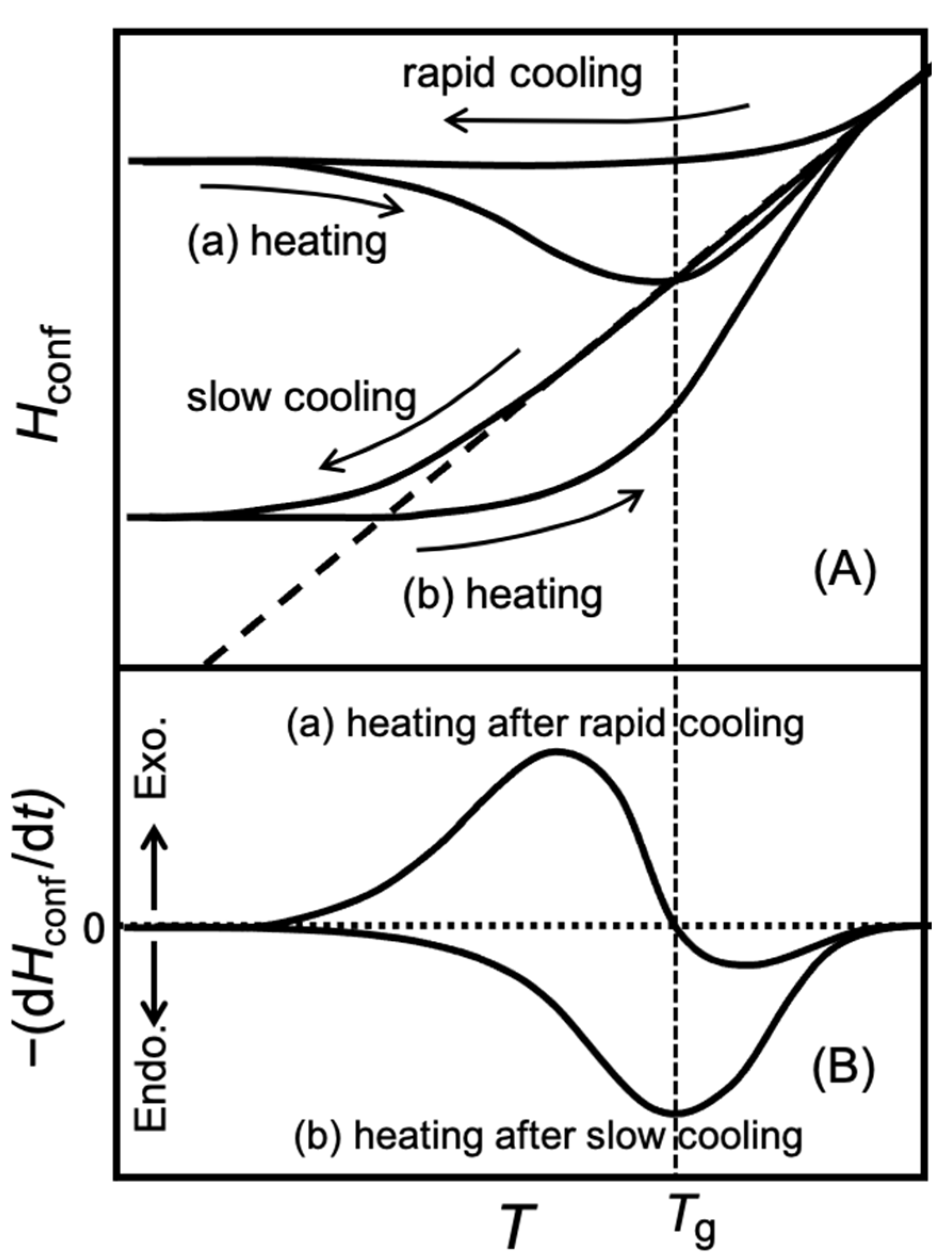
Rates of the enthalpy relaxation dependent on the rate of the previous cooling are shown in
Figure 3B,C. In the temperature range from 130 K to 190 K, the spontaneous enthalpy-relaxation rate (
Figure 3C) is shown with the pair of the gels precooled rapidly and indicated a behavior characteristic of slow glass transition, as explained in
Figure 4B. From the temperature of 200 K to 244 K, the rates of enthalpy change observed with the rapidly precooled gel (
Figure 3B) indicated a remarkable enthalpy-release effect, which corresponds to the exotherm observed in the temperature range from 210 K to 250 K in
Figure 1. This is due to the ice crystallization following the glass transition. It is true that the pattern of the spontaneous enthalpy-relaxation rate shown in
Figure 4B was for the sample showing no crystallization in the neighborhood of the glass-transition temperature. However, most of the water in the G25 gel precooled slowly had crystallized during cooling and hardly any enthalpy-release effect was observed (
Figure 3B), similar to the annealed gel precooled from the annealing temperature (237 K) that was already enthalpy-released during the annealing (see
Figure 4 for details). Taking these into consideration, i.e., spontaneous enthalpy release (130–190 K) and remarkable enthalpy release (200–244 K) observed with the rapidly precooled G25 gel, it can be concluded that the gradual increase in the heat capacity in the range from 150 K to 200 K in the rapidly precooled sample, shown in
Figure 3A, was a glass transition from a vitreous state to a supercooled liquid state, and that the exothermic effect observed in the broad temperature range from 200 K to 244 K in
Figure 3B was due to the ice crystallization proceeding gradually. The glass-transition temperature was estimated to be ca. 175 K.
The decrease in the exothermic effect at 245–248 K in the rapidly precooled sample (
Figure 3B) was caused by the overlapping of the enthalpy-absorption effect due to the melting of small ice crystals. Then, the rate of the enthalpy change showed an abrupt enthalpy release due to ice crystallization at 250–255 K, followed by the melting of the ice finishing at 271 K.
In the case of the annealed gel, the enthalpy-release effect observed with the rapidly precooled gel in the range from 200 K to 244 K in
Figure 3B disappeared. This means that the supercooled liquid water crystallized during the annealing treatment. The second remarkable enthalpy-release effect due to the ice crystallization at 250–255 K, observed with the rapidly precooled sample, remained also with the annealed gel. Looking at the enlarged figure (
Figure 3D), rates of the enthalpy relaxation indicated small positive values of the enthalpy-release effect from 210 K to the annealing temperature at 237 K, where the relaxation turned to negative values. Considering the enthalpy-relaxation behavior changing from a positive to a negative value and the succeeding exotherm due to ice crystallization, the steep increase in the heat capacity starting at 240–242 K (
Figure 3A) indicates a second (higher) glass transition. The heat capacity increased further because of the initiation of ice melting at 245 K (black dashed line in
Figure 3). The temperature of the second glass transition was estimated to be 240–242 K.
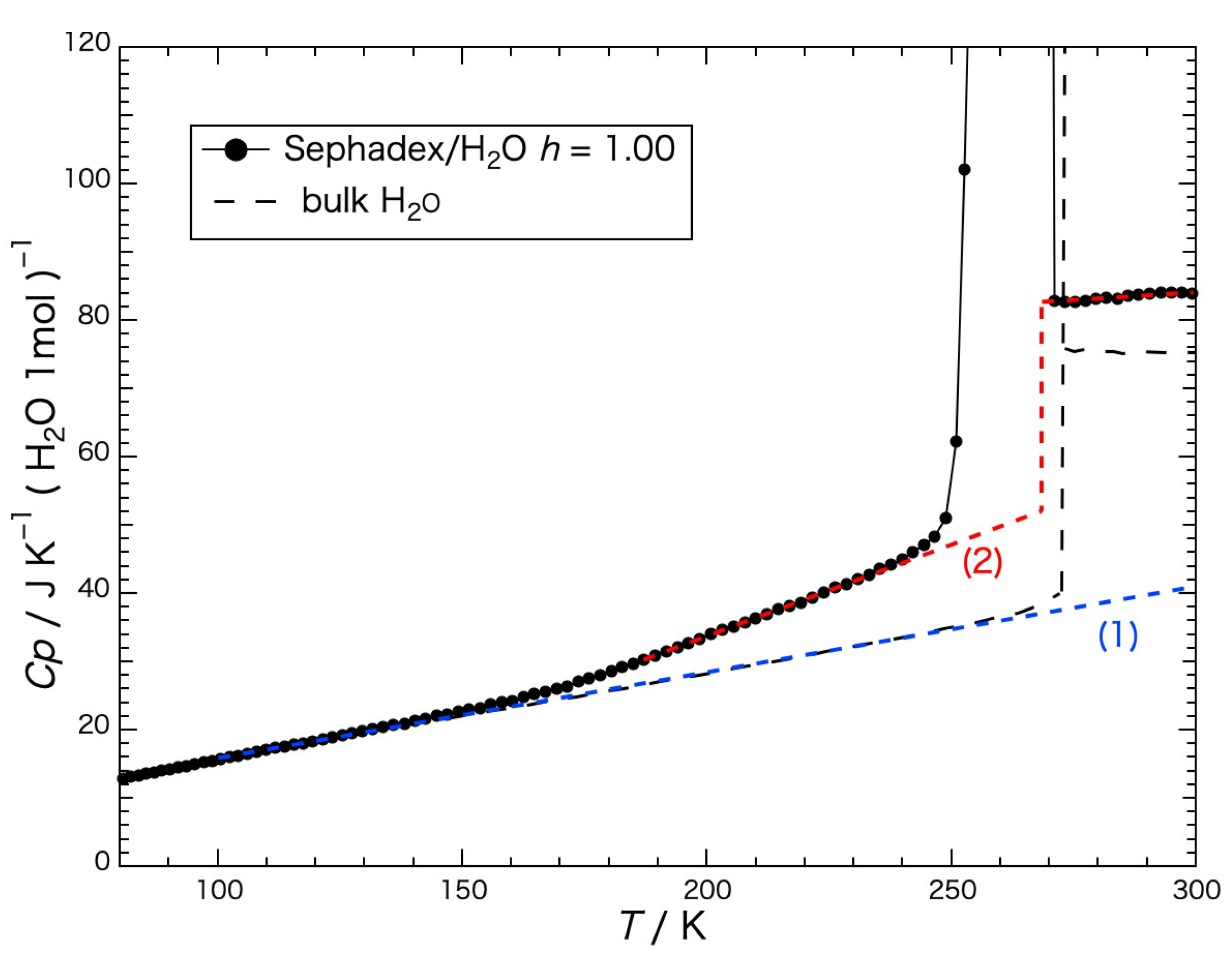
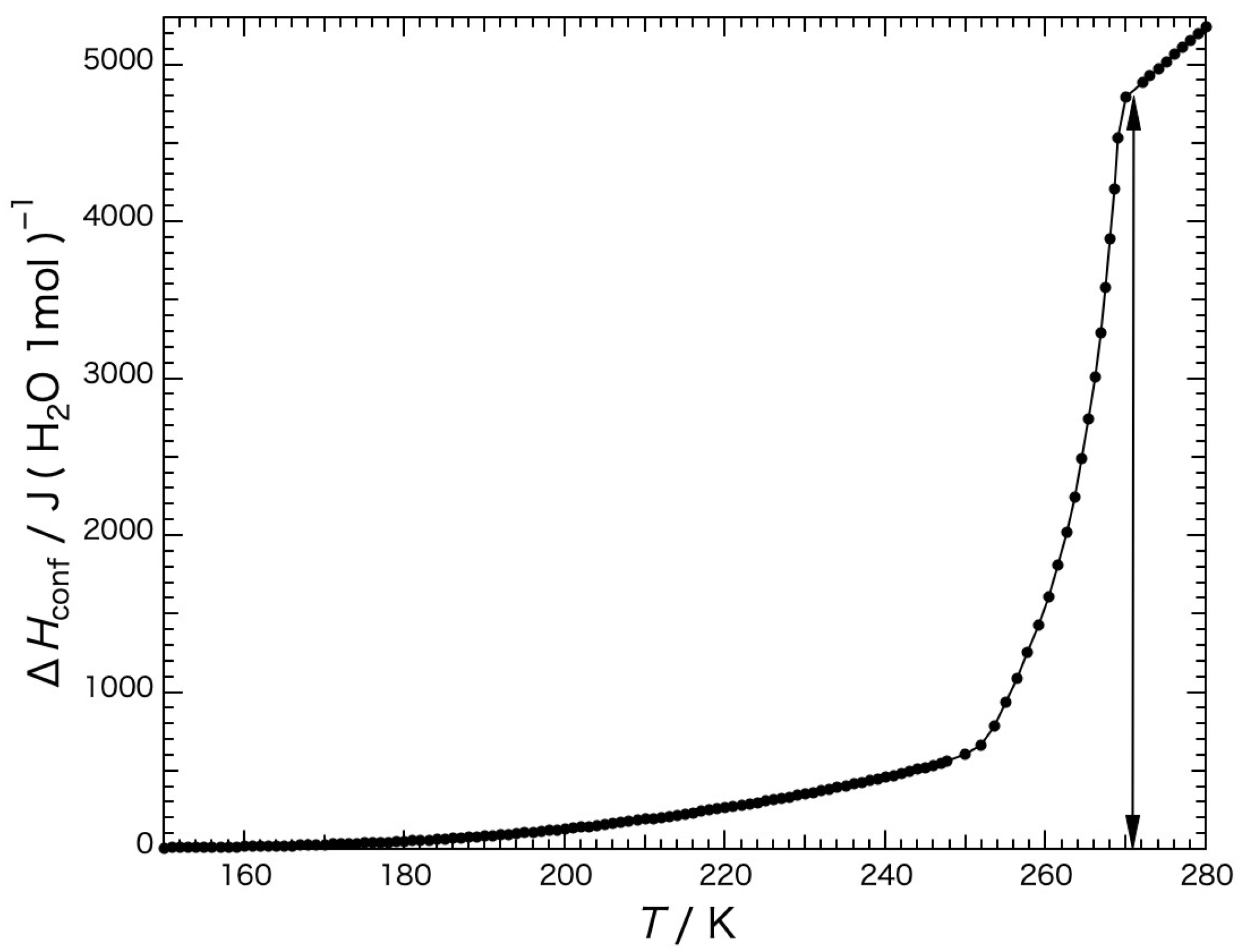
The heat capacity of the water per mole within the gel precooled slowly is shown in
Figure 5, and was obtained by subtracting the heat-capacity values of the dried gel from those of the gel containing water. The black dashed line in the figure indicates the heat capacity of the bulk H
2O (water or ice) [
18]. The melting temperature was 273.15 K. The blue dashed line (1) represents the baseline for determining the configurational enthalpy and the red dashed line (2) represents the baseline for determining the fusion enthalpy. The configurational enthalpy
Hconf is the enthalpy due to the spatial rearrangement of molecules. The heat capacity of the bulk ice, which mainly represents that of the vibrational motion without contribution of the rearrangement of the water molecules, increased with the temperature, and the motion of the configurational change occurred instantaneously at the melting temperature. Although the heat capacity of the water in the gel increased similarly to that of the bulk ice until reaching a temperature of ca. 150 K, it began to increase more than that of the bulk ice above the temperature, where the change in configuration gradually started and jumped up at around the melting temperatures as a first-order phase transition.
The temperature dependence of the configurational enthalpy of the water within the Sephadex
® G25 gel precooled slowly is shown in
Figure 6, where the enthalpy was obtained up to 248 K by the integration of the heat-capacity difference between that of the water within the gel and that of the bulk ice. The heat capacity of the bulk ice was approximated by a straight line connecting the
Cp values at 150 K and 230 K and extrapolated to higher temperatures (see the blue dashed line (1) in
Figure 5). In the first-order phase-transition-temperature region from 248 K to 270 K, the enthalpy was obtained by summing up the input energy corrected for the effect of slight heat leakage.
As shown in
Figure 6, it was found that the configurational enthalpy in the gel began to increase from 150 K, and that from 150 K to the temperature of the completion of ice melting (271 K) the increase in the enthalpy was 4.80 kJ (mol H
2O)
−1, which corresponds to ca. 80% of the configurational enthalpy, i.e., the fusion enthalpy of ice, 6.01 kJ mol
−1). One of the reasons for the configurational enthalpy of the water within the gel being small is because the change in configuration of the water within the gel was not as free just above the melting temperatures compared with that of the bulk water. As the heat capacity of the water within the gel was 11% larger above the melting temperatures than that of the bulk water, the rate of the configurational change of the water within the gel rapidly approached that of the bulk water.
For the determination of the fusion enthalpy (Δ
Hfus: the latent heat of the first-order phase transition) of the water within the gel, a baseline was drawn to the heat-capacity curve obtained with the slowly precooled gel (see the red dashed line (2) in
Figure 5). Below 230 K the heat capacity of the slowly precooled gel was used as a baseline. From 230 K to the melting temperature the data of the heat capacity from 180 K to 230 K were extrapolated to the melting temperature by approximating the data with a straight line, and above the melting temperature the data from 272 K to 290 K were extrapolated to the temperature range below the melting temperature by approximating the data with a straight line. The melting temperature was regarded as the temperature where the slope of the temperature dependency of the fusion enthalpy was steepest, 269 K. Corresponding to the temperature below and above the melting temperature the two baselines were switched. The fusion enthalpies dependent on the temperature were obtained in the same way as the determination of the configurational enthalpy and are shown in
Figure 7. In the case of the water in the gel precooled slowly, the fusion enthalpy, Δ
Hfus = 3.93 kJ mol
−1, corresponded to 82% of the configurational enthalpy (4.80 kJ (mol H
2O)
−1), which means that 82% of the configurational enthalpy was acquired by the fusion.
The enthalpy of ice crystallization during rewarming observed with the rapidly precooled and annealed gels corresponded to the excess configurational enthalpy on the basis of that observed with the gel precooled slowly. For the determination of the excess enthalpy of the water in both gels, it was necessary to obtain the heat-capacity data and to integrate the heat-capacity values from the baseline (the red dashed line (2) in
Figure 5) dependent on the temperature. Then, the excess enthalpy in the temperature range to the initiation temperature of crystallization was obtained by the integration. For the temperature range above the initiation of crystallization, where the heat-capacity values were not determined because a large exothermic effect due to crystallization occurred, the excess enthalpy leading to the calculation of the crystallization enthalpy was derived as follows.
Assuming the spontaneous temperature-drift rate (d
T/d
t) was constant during the single cycle of the stepwise heating from the beginning of heating to the end of the temperature measurement (Δ
t), Δ
T can be expressed by the equation Δ
T = (d
T/d
t) Δ
t. Then, exothermic heat (Δ
Hexo) can be written as −Δ
Hexo =
Cp gross Δ
T, where
Cp gross is the total heat capacity, including that of the sample cell. Then, the total enthalpy change, Δ
Hall, can be written as Δ
Hall =
E − Δ
Hexo, where
E is an applied electrical energy. Subtracting the contributions of the sample cell and Sephadex from the Δ
Hall, changing to the value per mole of water and reducing the contribution of the baseline value, the excess enthalpy was derived by the integration at each temperature of the measurement. The results are also shown in
Figure 7. Note that the calculations were carried out assuming the excess enthalpies above the melting temperature were the same for the gels irrespective of the cooling mode, as all the water in the gel was in the liquid state.
In the case of the annealed gel, glass transition/crystallization and melting were observed in the high subzero-temperature region. However, they overlapped each other. In the case of the gel precooled rapidly, two glass transitions, i.e., a glass transition/crystallization in the low subzero-temperature region and a glass transition/crystallization and melting in the high subzero-temperature region, were observed, although the glass transition/crystallization and the melting in the high subzero-temperature region overlapped and were difficult to differentiate from each other. With knowledge of the overlap, the enthalpy of the ice crystallization was tentatively obtained. The enthalpy of the crystallization in the low subzero-temperature region corresponded to the difference between the peak value of the fusion enthalpy of the water in the rapidly precooled gel at 207 K and the value of the water in the annealed gel at the same temperature, 0.76 kJ mol
−1, which is shown by the red two-directional arrow in
Figure 7. The enthalpy of crystallization in the high subzero-temperature region was obtained using the annealed gel. The difference between the apparent peak value of the fusion enthalpy of the water in the annealed gel and the baseline was 0.34 kJ mol
−1. These values correspond to 19% and 8.7% of the fusion enthalpy (3.90 kJ mol
−1), respectively.
Water in the Sephadex
® G25 gel was classified in terms of the ice-crystallization behavior, as shown in
Table 1. In the analysis, the value for the fusion enthalpy of ice, 6.01 kJ mol
−1, and the values in
Figure 5 and
Figure 6 were used. The ratio of the non-crystallizing water in the gel was assumed to be the same for the slowly precooled and rapidly precooled gels. Then, the non-crystallizing water corresponding to 18% (100−82%) of the total water within the gel could be regarded as the hydration water. Crystallizing water during heating (rewarming) corresponded to 23% of the total water within the gel.
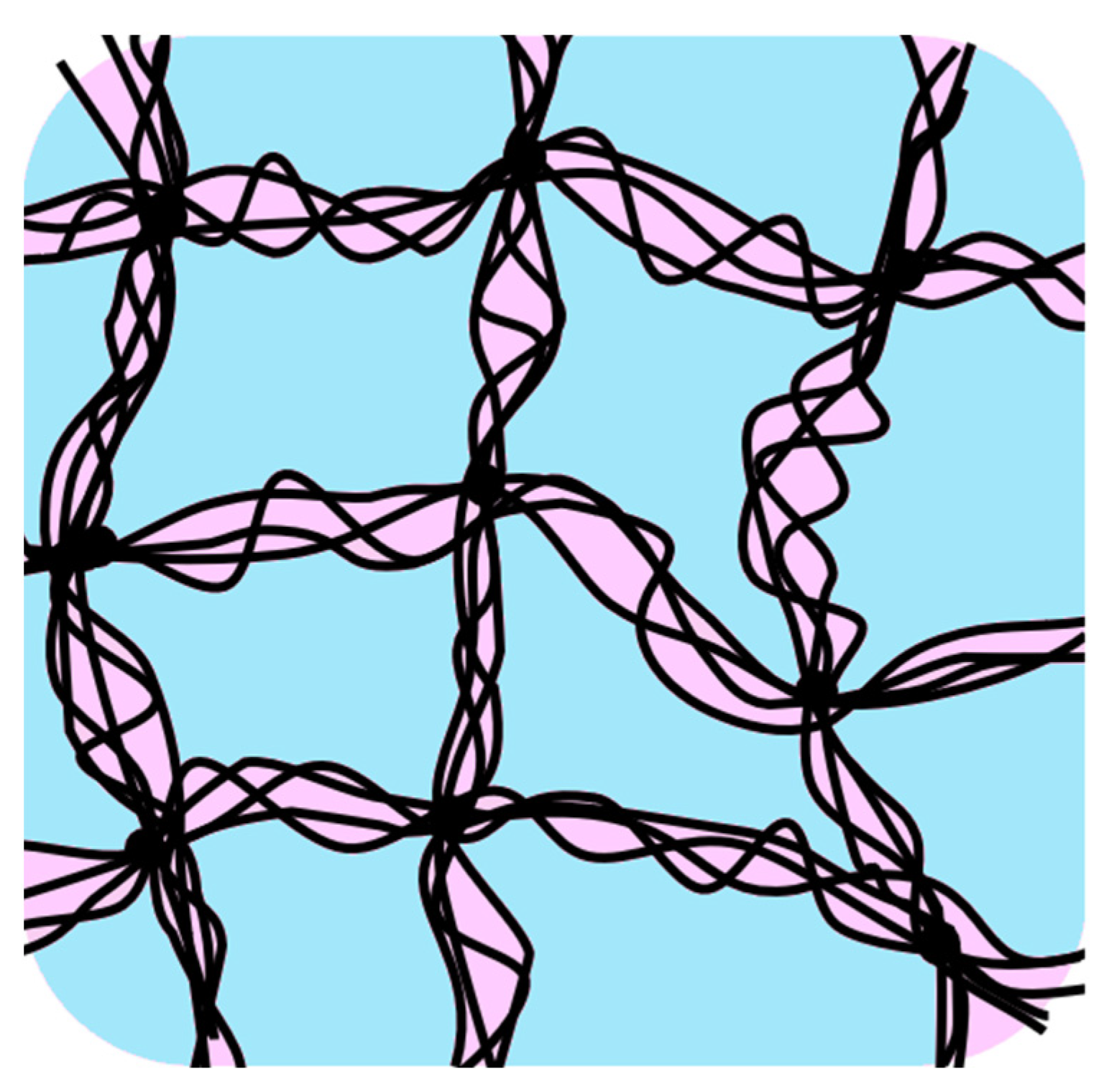
In
Figure 8, a schematic diagram of the network structure of Sephadex
® G25 containing water of mass ratio
h = 1.00 is shown. It is certain that the two glass transitions and ice crystallizations during rewarming observed in this study were caused by the different sites of the gel beads, as the glass-transition temperatures were very different from each other. Then, it can be considered that there were two types of molecular assemblies of water separated from each other. One was the water compartmentalized by the network structure made of crosslinked dextran (shown in blue) and the other was the water confined within the narrow spaces formed by the entangled dextran chains (shown in pink). These structures are shown in
Figure 8.
Ice crystallization during rewarming observed in the high subzero-temperature region was due to the water trapped by the polymer network at the time of freeze initiation during precooling and remained unfrozen in a glassy state. The higher-temperature glass transition, observed at 240–242 K, was confirmed in the present study by the thermal-relaxation behavior shown in
Figure 3C,D and the subsequent ice-crystallization exotherm. However, the temperature range of the glass transition was not as clearly overlapped with the endotherm due to the melting of small ice crystals and the exotherm due to the occurrence of ice crystallization triggered by the melting. The melting temperatures of small ice crystals are different depending on the crystal size, i.e., a smaller ice crystal will have a lower melting temperature. The complicated thermal behavior indicates that the ice crystals formed in the gel had a broad size distribution and that the water in the gel was vitrified when ice crystals of even the smallest size could not be formed.
Although both of the exotherms in the low subzero-temperature region (200–244 K) and in the higher-temperature region (250–255 K) indicating ice crystallization during rewarming were confirmed with the gel precooled rapidly, at 5 K min−1, it disappeared when precooled very slowly, at 20 mK min−1. This result suggests that there was a critical cooling rate for the appearance of the exotherm, which was determined by the competition between the rate of change in the polymer network at the time of freeze initiation during precooling and the rate of the diffusion of water molecules depending on the temperature.
The lower-temperature glass transition, observed at ca. 175 K, was probably due to the confined water within the narrow spaces formed by the entangled dextran chains, which was separated from the compartmentalized water and also from the confined water within the adjacent narrow spaces by the wall made of densely entangled dextran chains. Then, when crystallization was proceeding slowly at low temperatures during precooling, some of the confined water was trapped by the entangled dextran and turned into a glassy state, which crystallized during rewarming following the glass transition. As the water assemblies confined within the narrow spaces were small and separated from each other by the rigid wall made of the entangled dextran, they may have crystallized independently during rewarming in the broad temperature range at low subzero temperatures.
The anomalous ice-crystallization behavior during rewarming in polymer gels depends on the water content and the cooling rate as well as the compartment size, flexibility of the polymer chain, and continuity of the water phase between adjacent compartments that are inter-related via the density of crosslinks [
3]. The effect of the cooling rate was made clear in this study. The water content is the most important factor for the appearance of the ice crystallization during rewarming, as it affects the swelling of the polymer-network structure. The state of the water in the gel controlled by the preparation method and the storage time after the sample preparation is also important in order to obtain reproducible results with such a heterogeneous sample as polymer gels.