Abstract
Polyester fibers are widely employed in a multitude of sectors and applications from the technical textiles to everyday life thanks to their durability, strength, and flexibility. Despite these advantages, polyester lacks in dyeability, adhesion of coating, hydrophilicity, and it is characterized by a low wettability respect to natural fibers. On this regard, beyond the harmful hydrophobic textile finishings of polyester fabrics containing fluorine-compounds, and in order to avoid pre-treatments, such as laser irradiation to improve their surface properties, research is moving towards the development of fluorine-free and safer coatings. In this work, the (3-glycidyloxypropyl)trimethoxysilane (GPTMS) and various long alkyl-chain alkoxysilanes were employed for the fabrication in the presence of a catalyst of a water-based superhydrophobic finishing for polyester fabrics with a simple sol-gel, non-fluorinated, sustainable approach and the dip-pad-dry-cure method. The finished polyester fabrics surface properties were investigated by static and dynamic water repellency tests. Additionally, the resistance to common water-based liquids, abrasion resistance, moisture adsorption, and air permeability measurements were performed. Scanning electron microscopy was employed to examine the micro- and nano-morphology of the functionalized polyester fabrics surfaces. The obtained superhydrophobic finishings displayed high water-based stain resistance as well as good hydrophobicity after different cycles of abrasion.
1. Introduction
Polyester fiber textiles are popular among customers because of their durability, strength, flexibility, and resilience to climatic conditions, making them perfect for long-term usage in outdoor applications. Furthermore, the greatest benefit of polyester fibers is that they can be mass-produced in massive quantities at a low cost, resulting in lower costs for the customer as well [1,2,3]. On the contrary, polyester fabrics do have certain issues, such as a lack of breathability especially after some finishing treatments [4,5]. As a consequence, it does not absorb sweat from a person’s skin, retaining perspiration and heat adjacent to the skin, making people feel sticky and clammy, causing discomfort and bacterial growth. As a result, water repellent treatment for polyester fabrics is often required [6,7,8]. Furthermore, this can widen the breadth of polyester fabrics usage, increase its economic worth, fulfill the rising market request for more performant textiles, and improve their versatility.
In order to increase the coating durability, it is crucial to increase the coating adhesion capability of polyester textiles themselves. In some investigations, polyester fibers were altered by UV irradiation, plasma pre-treatment or chemical hydrolysis to add functional groups to the surface, increasing the polymer’s hydrophilicity before the application of chemicals and functionalization [9,10].
As a matter of fact, fluorine and silicon finishing compounds are the most often used finishing agents [11,12]. In the past, despite their remarkable water repellent qualities, the most common industrial water-repellents contained perfluorooctanoic acid and perfluorooctane sulfonate, which were hard to breakdown and presented the risk of significant harmfulness and bioaccumulation [13,14,15]. For these reasons, their usage has been restricted, making critical the development of water repellents free of fluorine compounds. Nevertheless, there are a wide range of fluorine-containing formulations that can be utilized for improve the textile water repellency. Highly hydrophobic coatings are created from fluoroalkyl silane-based compounds and polytetrafluoroethylene-based ones [16,17,18].
Although opportunely coated fabrics can generate a water repellent surface with lower surface tension on the exterior of polyester fibers as in the case of silicone and polysiloxane water repellent agents, featuring the advantages of good biocompatibility and inexpensive raw material costs, the washing resistance is still insufficient [19,20].
Long-chain alkane compounds represent one of the existing water repellent finishing techniques able to introduce on cotton fabrics good water repellent properties. Unfortunately, due to the lack of functional groups able to bind to cotton fibers, they lead to the formation of finishings characterized by poor washing resistance, negative impact on hand feel, and poor air permeability [21,22].
One commonly explored strategy for producing superhydrophobic textiles is first to increase surface roughness by functionalizing the surface with proper inorganic precursors and nanoparticles via the sol-gel technique and then to reduce surface energy by attaching a hydrophobic groups [23]. Furthermore, the use of modified sols with suitable hydrophobic substituents may result in superhydrophobic and water-repellent films obtained in a single step [24,25,26,27,28]. Due to its adaptability, sustainability, versatility and simplicity, sol-gel chemistry is frequently used to produce functional and protective coatings for a wide range of industrial and applicative sectors, also allowing to obtain surfaces with improved mechanical strength, chemical stability, and thermal resistance [29,30,31,32].
Herein, in this study, a facile and eco-friendly sol-gel approach, to obtain superhydrophobic and breathable polyester fabrics, is presented; the chosen sol-gel reaction pathway is based on the hydrolysis and condensation of (3-glycidyloxypropyl)trimethoxysilane (GPTMS), crosslinked to different long alkyl-chain alkoxysilanes. The treated fabrics exhibited excellent superhydrophobicity useful for the assessment of their potential contribution to sustainable water repellent and fluorine-free textile coatings for a wide range of sectors, as shown in Figure 1 (i.e., from technical textiles and protective workwear to automotive, from buildings to biomedical applications, from furniture to high fashion industries).

Figure 1.
Potential application sectors of the purposed smart and sustainable non-fluorinated sol-gel approach to obtain superhydrophobic polyester fabrics.
In particular, (3-glycidyloxypropyl)trimethoxysilane (G) was co-condensed with various non-fluoro substances, such as triethoxy(ethyl)silane (C2), triethoxy(octyl)silane (C8), and hexadecyltrimethoxysilane (C16), in the presence of HCl and 1-methylimidazole as catalysts for the GPTMS epoxy ring opening, to prepare nanohybrid cross-linked polyalkylsiloxanes coatings that were applied on polyester surfaces by the dip-pad-dry-cure deposition approach.
The features of the finished polyester fabrics were additionally tested and examined by different chemical, physical, and mechanical techniques. In particular, the static and dynamic water repellency, resistance to conventional water-based liquids, abrasion resistance, moisture absorption, and air permeability of the coated polyester fabrics were analyzed, and the micro and nano-morphology of the functionalized fabrics surfaces were observed by scanning electron microscopy (SEM). Finally, a possible mechanism of the super hydrophobic behavior of the obtained micro- nano-structured hybrid coating was evaluated and proposed.
2. Results and Discussion
2.1. Water Repellent Nanohybrid Cross-Linked Polyalkylsiloxanes Sol-Gel Coating
As for the cotton fiber substrates, water repellent finishing of polyester fabrics is often conducted using physical or chemical procedures that involve lowering surface energy and modifying the roughness and surface morphology of the fabrics specimens [33,34,35]. Unfortunately, although the many advantageous qualities of polyester, it has some disadvantages, such as poor dyeability, adhesion of coating, hydrophilicity, and low wettability in comparison to natural fibers, e.g., cotton, wool, and silk, due to its non-polar and hydrophobic character, which negatively affects the washing fastness of deposited finishings [36,37].
In this study, in order to avoid surface pre-treatments, e.g., laser irradiation, the polyester samples (PL) were treated with the silane precursor (3-glycidyloxypropyl)trimethoxysilane as a crosslinker to encourage the formation of a tridimensional hybrid functional organic-inorganic network that displayed adhesive electrostatic and covalent interactions with the treated substrate. The development of these chemical contacts between the alkoxysilane precursors and fibers, however, can be aided by the presence of functional groups (hydroxyl and/or carboxyl) at the polyester polymeric ends [38]. Therefore, the establishment of covalent and electrostatic chemical connections between fibers and nanohybrid segments can be aided by these groups on the polyester surface (Figure 2a,b). In particular, a two-step pathway was performed in order to achieve the super hydrophobic coatings for polyester fabrics.
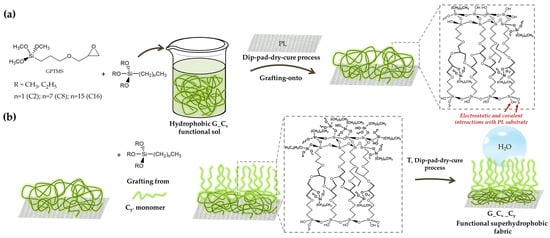
Figure 2.
Description of the double surface treatment of polyester fabrics by “grafting to” or “grafting from” covalent functionalization techniques, involving condensation reactions between the substrate and the GPTMS/alkoxysilane xerogel (a), and subsequent linkage of the selected C2, C8 and C16 alkyl(trialkoxy)silanes (b).
In a first step, the bifunctional (3-glycidyloxypropyl)trimethoxysilane and one of the C2, C8, or C16 alkyl(trialkoxy)silanes, each characterized by an alkyl chain of a different length, were combined by a “grafting onto” approach to create the functional 3D polymeric matrix (Figure 2a) through a dip-pad-dry-cure procedure.
According to earlier reports [39,40], the functional sol-gel solution was developed by a subsequent hydrolysis and condensation reaction, which results in the dissolution of pre-condensed polymeric hybrid polymers or colloidal particles. The nanohybrid sol-gel will be converted into a nanostructured xerogel after being applied to polyester fabrics and after receiving extra heat treatment (at high temperatures).
Five functional treated polyester specimens were produced by performing a second dip-pad-dry-cure step and using selected C2, C8, or C16 alkyl(trialkoxy)silanes, separately, in order to create more efficient hydrophobic finished polyester fabrics (Figure 2b). As a matter of fact, the in-situ graft density, structure, and molar ratio of the obtained brush polymer shells may be better controlled by a "grafting from" procedure. Moreover, this application technique leads to brush polymers that are not sterically constrained by the overall incoming functional long alkyl chains, thus leading to a notable decrease in surface energy and to the desired increase in hydrophobicity of the coated surfaces [41]. Additionally, it has been observed that the use of a double-layer deposition method can improve the properties, morphological features, and surface roughness of various developed films and coatings on textiles, thus resulting in improved mechanical and hydrophobic performances [40,42,43].
In this regard, WCA analysis was employed to study the static hydrophobicity of the functionalized polyester specimens. Moreover, spray tests were performed to determine their dynamic water repellence.
2.1.1. Aqueous Liquid Repellency Tests (Water/Alcohol Solution)
Using a reagent test, created in accordance with AATCC test method 193-2007, the contact angles of some aqueous liquids (characterized by various surface tensions) were measured to determine the degree of anti-wettability or repellency of the fabrics [44]. The water-rating method WRA, also known as the aqueous-liquid repellency test, is based on the application of 20 µL droplets of an isopropyl alcohol solution at various concentrations on fabrics (see Table 1). The following solution with a larger percentage of isopropanol is administered if the solution’s drops do not moisten the fabric within 10 s. The solution featuring the highest isopropanol content that doesn’t wet the fabric within 10 s is given the rating number.

Table 1.
Aqueous liquid repellency data for water/alcohol solution standard test liquids.
Table 1 summarizes aqueous repellency grade numbers, together with water/alcohol solution compositions and with related surface tension values.
To be considered successful, the fabrics must be capable to reject the solution for 10 s (Figure 3). This technique uses solutions whose surface tensions are lower than those of water, resulting as a more rigorous static test than conventional water-drop techniques.
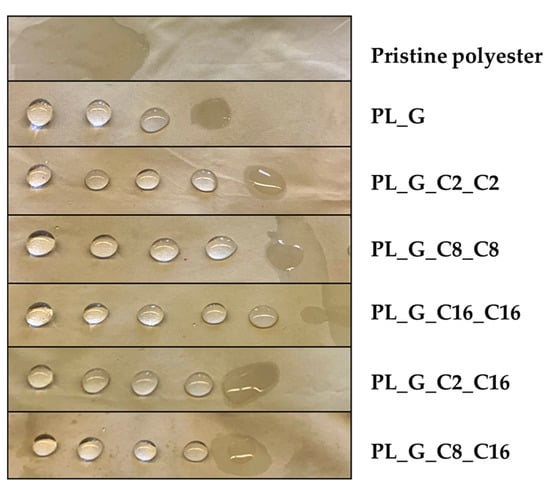
Figure 3.
Static wettability test of the pristine and alkoxysilane modified polyester fabrics.
2.1.2. Sessile Drop Method
The sessile drop method is frequently used to directly measure the contact angle in order to test the wettability of specified coatings by distilled water [45]. In this regard, the hydrophobicity of the treated polyester fabric was examined and related to the length of the hydrocarbon chains of the functional alkyl(trialkoxy)silane being varied from 2 to 8 to 16 methylene groups. From an operational point of view, a deionized water droplet (about 5 µL) was gently deposited onto the surface in a normal procedure, and the photographs were taken with the help of a digital camera. To increase the measurement accuracy, all the water contact-angle values here provided were derived as averages of five measurements done on separate spots of the sample surface. The obtained results of the contact angle measurements reported in Figure 4 show that all the modified samples are strongly hydrophobic.
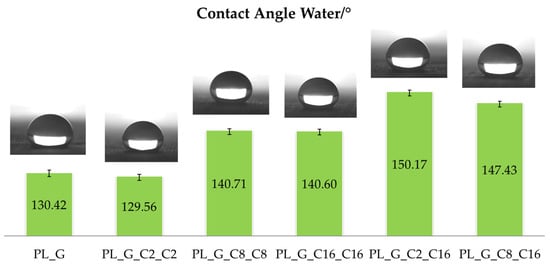
Figure 4.
WCA value of different drops on coated polyester fabric and representative images captured with a video camera of typical static water drop tests for the various functionalized samples.
Furthermore, in the case of fabrics modified by combining short and long hydrocarbon chains, high WCA values, as those achieved for the samples PL_G_C2_C16 and PL_G_C8_C16, are obtained. In fact, by creating an asymmetric nanostructured hyperbranched polymeric coating with both long and short chain C2 or C8 and C16 co-monomers, it was able to replicate a brush effect that increased roughness while simultaneously reducing surface wettability [46]. The drop on the finished polyester fabric has a definite spherical form, as illustrated in Figure 4, and the best WCA is 150.17° for the specimen PL_G_C2_C16. However, after the impact on the untreated polyester surface, the liquid drop spreads out and is stretched quickly on the surface, still revealing good hydrophilic properties.
Moreover, in order to compare the reported results with other published data, different examples of hydrophobic treatments for polyester fabrics and the relative WCA values are given in Table 2.

Table 2.
Comparison of hydrophobic polyester treatments and achieved WCA values.
2.1.3. Spray Test
The dynamic wettability of the treated polyester fabric was further investigated using spray testing (AATCC 22-2005) [53]. Spray testing measures the amount of moisture left on a fabric after being sprayed with water (Figure 5). Table 3 lists the water-stain characteristics (in ISO standard ratings) for various wetting degrees. The uncoated fabric’s wettability rating was 0, meaning it had no water repellency. As shown by the results, increasing the number of the alkyl chain improved the fabrics’ ability to repel water. After coating the sample PL_G, the functionalized fabrics wettability value climbed up to 50 and persisted at the same level also for the sample PL_G_C2_C2. Meanwhile, by increasing the number of the alkyl chain, the textiles’ ability to repel water could be improved, as demonstrated by the obtained results. In fact, spray testing revealed that the samples of treated polyester fabric PL_ G_C8_C8 and PL_G_C16_C16 had a rating number corresponding to 100.
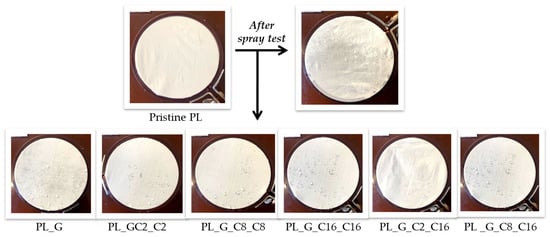
Figure 5.
Spray test results for the dynamic wettability measurements of pristine and functionalized polyester fabrics.

Table 3.
Wettability values definite in the spray tests’ ATTCC 22 standards.
Only a few droplets of the employed water volume (250 mL) that had been in touch with the treated textile samples remained quickly dispersed after that. The results show that polyester fabrics have high hydrophobicity and a low surface energy.
In contrast, the polyester fabrics exhibit greater surface energy when coated with GPTMS-based sol without the further addition of functional alkylsilane precursors. A water droplet placed upon the untreated polyester fabric spreads rapidly as well.
2.1.4. Self-Cleaning Behavior of the Fabric
The self-cleaning property of coated fabric (sample PL_G_C16_C16) is demonstrated in Figure 6. In particular, a certain amount of wet soil powder, used as model contaminants, was located on the functionalized specimen surface and then water was dripped from the impurity particles onto the fabrics surface as shown in Figure 6a. Rolling water droplets easily removed micron-sized contaminating particles from the fabrics, demonstrating the lotus effect of the functionalized polyester fabrics [54].
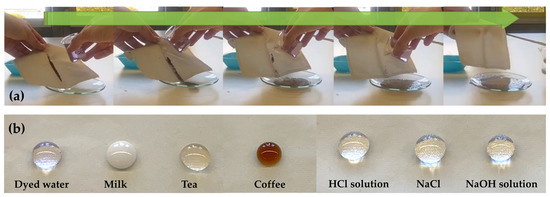
Figure 6.
Self-cleaning illustration of coated polyester fabrics (A4 sample) by using wet soil as contaminant powder (a); a variety of common water-based liquids standing on the finished polyester fabrics (b).
Additionally, as revealed in Figure 6b, some droplets of common liquids (i.e., dyed water, milk, tea, coffee, acid by HCl at pH = 1, alkali by NaOH at pH = 14) and salt solutions (NaCl, pH = 7) are able to assume the shape of a ball and remain on the surface of the polyester textiles treated with the different water repellent coatings, clearly revealing that they have got good stain resistance features to common water-based liquids.
2.2. Micro-Nano/Morphology Analysis by Scanning Electron Microscopy (SEM)
The micro-morphology of the water repellent fiber surface was observed by scanning electron microscopy. The surface morphology of the polyester fabric fibers, before and after water repellent coatings application, was studied after the gold coating of samples. The surface appearance of the fibers was detected and analyzed in order to study the relationship between the surface micro-morphology and water repellence features [55]. Figure 7 shows the micromorphology of pristine and treated polyester fabric surface after finishing.
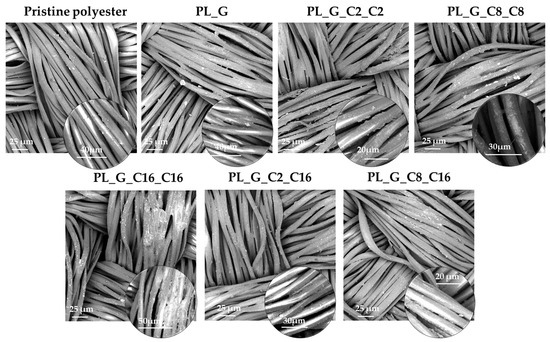
Figure 7.
SEM images of untreated and functionalized polyester fabrics with the polyalkylsiloxane nanohybrid sols.
The results exhibited the rougher surface of the functionalized fabrics. The insets in SEM images of Figure 7 show at high magnification that the coatings accumulated on the fabric’s surface in a uniform manner. However, after the modification of polyalkilsiloxane-coated fabric, the surface morphology becomes inhomogeneous. As shown in Figure 7, the surface of the untreated polyester fibers is relatively smooth at low magnification with a uniformly distribution of the coating. Meanwhile, it can be clearly observed that there are many tiny grooves and many little protrusions on the surface of the treated polyester fabric fibers at high magnification which provide a nano-scaled roughness, supporting the superhydrophobic behavior of the treated fabrics [56].
A thin film is therefore covering the surface of polyester fibers consisting in the long alkyl-chain polysiloxanes grafted onto the GPTMS-based 3D matrix. In comparison with traditional fluoro-based materials, the developed functional and fluorine-free coating has higher water repellency, which can successfully reduce the surface energy of the polyester fabric specimen and improve the hydrophobicity of the fabric [57].
Microparticle aggregates coated the polyester fibers, thus significantly influencing the development of coated polyester fabrics with excellent hydrophobicity and water repellency.
2.3. Functionalized Fabric Performances
2.3.1. Abrasion Resistance
Figure 8 illustrates the behavior of different nanohybrid coatings on polyester abrasion resistance, as evaluated through the Martindale method [58]. In all the experiments, some rises in the abrasion resistance of the treated specimens compared to pristine ones are evident.
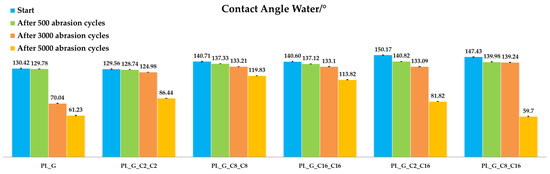
Figure 8.
Static contact angle water measurements of the functionalized polyester fabrics before the abrasion tests and after 500, 3000 and 5000 cycles of abrasion.
As the number of Martindale cycles increased up to 5000 cycles (end-point of the test because the control fabrics shows some defects), the wettability of the specimens gradually decreased. While this had no valuable effect on the general sample resistance, a more comprehensive analysis of the abrasion resistance behavior of treated textile supports displays that the appearance of the fabrics remains almost the same. In this regard, the quantity of catalyst is playing a key role. In fact, for polyester, the best behavior was achieved for the sample PL_G_C8_C8, showing a limited decrease of the static contact angle water even after 5000 cycles of abrasion.
2.3.2. Moisture-Adsorption Analysis
Moisture content has a crucial role in both cost and quality in a wide range of products, coatings and finishings. Compared to other synthetic fibers, polyester has a greater ability to avoid moisture absorption; this is one of the reasons why some types of polyester often tend to dry quickly [59,60]. Despite that, the presence of moisture also affects polyester fabrics, leading to harmful breakdown processes because the macromolecular chains hydrolyze, significantly reducing their molecular weight and material integrity [61,62]. However, it would be fair to specify that the permeability of a fabric largely depends more on its coating and its finish rather than only on the fiber with which it is made [63,64]. As a result, effective surface modification has a significant impact on how well materials absorb moisture. The moisture absorption characteristic of the developed coatings is reported in Table 4. As shown, the untreated polyester sample has a water absorption of 1.26%. Compared to the raw pristine fabric, from the data shown in the Table 4, it can be seen that the polyester fabric after finishing with the developed coatings presents a lower percentage of humidity, and this demonstrates once more how significantly the water repellency of polyester materials is improved.

Table 4.
Data for standard test of moisture adsorption of the different pristine and coated polyester samples.
The WVT values of the polyester fabric samples, treated with GPTMS, are lower than those of the original untreated samples. This water absorption was the same when using pure GPTMS-sol, since the modified GPTMS-based coating may still be hydrophilic. These GPTMS-sols must be changed with stronger hydrophobic components addition, such as hexadecyltriethoxysilane (a trialkoxysilane characterized by 16 carbon chain), to significantly alter their reaction to water absorption. The addition of the alkyl(trialkoxy)silane monomers therefore intensifies this decrease in proportion to the lengthening of the alkyl(trialkoxy)chain.
2.3.3. Air Permeability Test
Figure 9 summarizes the results of testing the breathability of coated polyester fabric and pristine polyester fabric in terms of air permeability. One of a fabric’s most crucial properties, primarily intended for technological or smart textile applications, is air permeability, which is closely related to porosity [65]. Finishings can influence this behavior because it is apparent that non-coated fabrics exhibit higher permeability than treated fabrics because of their low thicknesses and high porosity [66]. Here, it is clear that the air permeability of fabrics is slightly reduced by treating fabrics with hydrophobic alkyl(trialkoxy) silane formulations. For the PL_G_C16_C16 and PL_G_C8_C16 samples, the air permeability of polyester fabrics was not significantly impacted by the coating, indicating good overall breathability of the textiles substrates and making it appropriate for use in a variety of industrial-related areas.
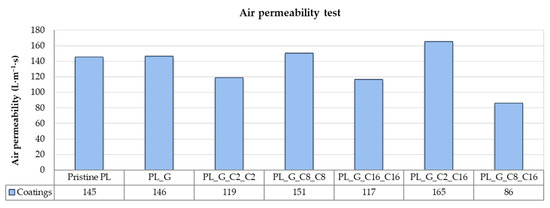
Figure 9.
Air permeability results for the different obtained uncoated and coated samples.
3. Conclusions
The treatment of polyester fabrics, using first a hybrid sol prepared by hydrolysis and condensation of GPTMS under acidic condition and in the presence of 1-methylimidazole as catalyst and long chain alkoxysilanes, and then hydrolyzed long chain alkoxysilanes, imparts a super-hydrophobic behavior to the coated polyester textiles. The results of the wettability test, as evidenced by the overall experimental data, led us to conclude that the addition of long alkyl-chain alkoxysilanes resulted in rougher surfaces, which decreased the surface free energy of the functionalized fabrics. As a matter of fact, the better alignment of the different long alkyl chains on the coated polyester surface and the increase in the textiles surface roughness are two explanations for the better featured water repellency. Furthermore, the treatment of the polyester fabrics causes a limited reduction in moisture adsorption and air permeability. Moreover, the treated polyester fabrics retain most of the hydrophobic properties even after 5000 abrasion cycles. The proposed two-step coatings system highlights that the use of fluorine-containing compounds is not strictly necessary and it can be easily replicated and extended towards a more sustainable preparation of transparent water-repellent functionalized surfaces over a large area. Last but not least, it may also offer a convenient alternative to traditional methods for the production of hydrophobic and superhydrophobic textile fabrics. Furthermore, in the case of polyester surfaces, this method has been shown to be an efficient and stable way to obtain functional coated fabrics, even in the absence of conventional polyester chemical and physical pre-treatments.
The obtained good water repellency, shown by the developed coatings, led us to conclude that this environmentally friendly, cost-effective, and relatively simple synthetic procedure may have positive effects on the production of a wide variety of textile fabrics featuring extremely high hydrophobicity and water-based anti-stain performance.
4. Materials and Methods
4.1. Materials
A commercial plain weave PET fabric (multifilament) provided by TEXOVERSUM School of Textiles (Reutlingen University, Reutlingen, Germany) was used for the experiments. The fabric has mass per unit area of 65.5 g/sqm, a thickness of 0.1 mm and a yarn fine of 5.1 tex (tex = g/km) (the average filament diameter is 11 µm). The warp and weft density are 50/cm and 75/cm respectively. Deionized water was employed in all the experiments. Highest purity level (3-glycidyloxypropyl)trimethoxysilane (G), hexadecyltrimethoxysilane (C16), triethoxy(octyl)silane (C8) and triethoxy(ethyl)silane (C2) were purchased from Merck Co. (Darmstadt, Germany) and employed without further purification. Hydrochloric acid (HCl 37% wt.) and 1-methylimidazole were used as sol-gel and GPTMS epoxy ring opening catalysts respectively. Ethanol (96% vol.) and isopropanol were provided from Sigma Aldrich.
4.2. Nanohybrid Functional Sols Synthesis
Three distinct alkoxysilanes (C2, C8, and C16) with progressively longer hydrocarbon chains were separately employed and combined with an equimolar amount of the G precursor (1 mol) to create the desired functional final sol-gel solution. Ethanol was gradually added to the prepared mixture while being stirred at room temperature. The hydrolysis-condensation reaction was induced by adding dropwise HCl (1.5 mol) and 1-methylimidazole (5% mol/mol to G, i.e., 0.05 mol). The final mixture was vigorously agitated for 24 h at room temperature.
4.3. Fabrication of Superhydrophobic Fabric by Dip-Pad-Dry-Cure Method
The dip-pad-dry-cure procedure was used to impregnate 15 × 15 cm squares of polyester fabrics, as shown in Figure 10.
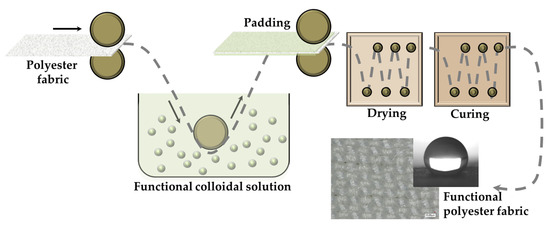
Figure 10.
Dip-pad-dry-cure process for the functionalization of polyester fabrics with the opportune functional nanohybrid sol-gel coatings.
The polyester fabric specimens were first submerged in the functional sols for 5 min at room temperature and padded using an automatic padder (basic two roller lab-padder of Mathis, Oberhasli, Switzerland) at a nip pressure of 2 kg/cm2 to achieve about 70% of wet gain.
After drying at 80 °C for a duration of 6 min, the step was repeated a total of three times and the padded fabrics were finally put in oven and cured at 130 °C for 6 min, in a convection oven. Based on an initial study, the best curing conditions (temperature and time) for polyester fabric were selected in order to avoid the thermal degradation of polymers and to achieve the siloxane cross-linking degree typical for robust xerogel coating. Additionally, polyester fabrics were immersed for 5 min in an alkyl(trialkoxy)silane-based ethanol solution (1.0 g, 30 mL), resulting in the deposition of the double-treated specimens (Figure 11).
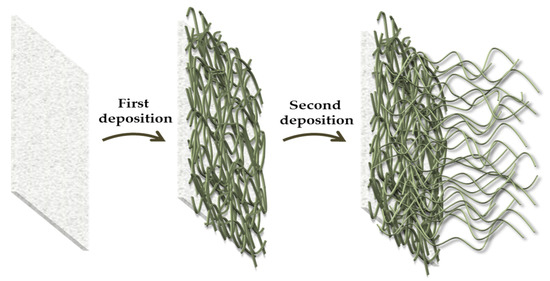
Figure 11.
Schematization of the double surface treatment of polyester fabrics.
Finally, the specimens were dried in the oven until constant weight at 130 °C for approximately 5 min. The fabrics were then climatized for 24 h in a typical climate chamber and subsequently weighted.
The total dry-solid add-on was measured through the weight gain (A wt.%), by weighing every sample before (W1) and after being impregnated with the functional formulation and after being subjected to an additional thermal treatment (W2) and calculated using Equation (1).
The collected add-on% data with the respective code are listed in Table 5 as well as each sample code (where PL was the chosen polyester textile support, G was the GPTMS precursor and C2, C8 and C16 the different long-alkyl chain alkoxysilanes).

Table 5.
Sample codes (showing coatings compositions), total add on values and sample appearance of the polyester fabrics treated with the different functional nanohybrid sol-gel coatings.
4.4. Characterizations
Aqueous liquid repellency: AATCC test method 193-2007 was used to develop a test reagent for the water/alcohol solution tests.
The static water contact angles (WCA) were measured using the sessile drop technique (in accordance with the international standard ASTM D7334) using a 5 µL water droplet at room temperature. The average of ten readings was used to create one representative WCA.
The spray testing was carried out to assess the dynamic wettability of the treated specimens using the AATCC Test Method 22-2005, appropriate to any textile material substrate. For the spray testing, three fabric samples 150 mm × 150 mm in size were needed to produce one representative standard. A sample of polyester was sprayed with 250 mL of distilled water that has been poured into the tester’s funnel at a 45° angle. The sample was knocked three times before being taken out. The degree of the wetting was evaluated using the water repellency rating (WRR). By evaluating the wetted sample’s appearance with the wetted standard polyester sample, the measurement was elaborated. A higher value indicates greater hydrophobicity.
A scanning electron microscope (SEM, SU-70, Hitachi, Chiyoda, Tokyo, Japan) was used to study the morphology fiber surface. In particular, the pristine and treated polyester fabrics were observed at 2.0 kV and a magnification of 1000 and 4000 for the insets. Prior to testing, gold was sputter-coated onto each sample.
Several water solutions, such as of coffee, milk, tea, methylene blue dyed water, pH = 1 acid (HCl), pH = 14 alkali (NaOH), and salt solution (NaCl), were separately applied to the G_C16_C16 modified polyester fibers in order to assess the wetting behavior. The self-cleaning properties of the functionalized polyester fibers were evaluated by applying soil to their surface and washing it with blue-dyed water.
All of the polyester fabric samples’ ability to carry moisture was assessed using the KERN DBS moisture meter (KERN & SOHN GmbH-TYPE DBS60-3). The thermogravimetry approach was used to assess the moisture-transfer capabilities of each polyester fabric specimen. Each substrate was weighed before and after drying in this procedure to ascertain the variation in moisture. The radiation in the KERN DBS moisture meter passes through the specimen and was converted to thermal energy, which causes the sample to heat up from the inside out. The sample does reflect some radiation, but it does so more strongly in dark samples than in light ones. Since bright samples reflect more thermal radiation than dark ones, they need to be dried at a higher temperature. For this reason, the analysis was performed using a drying temperature of 130 °C and a moisture measurement protocol (the drying method was TIME, drying temperature: 130 °C, unit denoting the result: M/W). Equation (2) was employed to compute the hygroscopicity ratio, which served as a scale for assessing the hygroscopicity of the different polyester specimens. In the following Equation (2), MB represents the weight of the conditioned specimen and MA the starting weight of pristine sample.
In agreement with ASTM D737-96 standard test procedure, the air permeability of the samples was assessed using a device (FX3300, Tex Test AG, Schwerzenbach, Switzerland) and 125 Pa of air pressure.
Author Contributions
Conceptualization, M.R.P., T.T. and S.S.; methodology, T.T. and M.R.P.; validation, S.S.; investigation, M.R.P., T.T., S.S. and G.R.; resources, M.R.P.; data curation, M.R.P., T.L., T.T., A.V., S.S. and G.R.; writing—original draft preparation, M.R.P., T.T., S.S. and G.R.; writing—review and editing, M.R.P., T.T. and S.S.; supervision, M.R.P. and T.T. All authors have read and agreed to the published version of the manuscript.
Funding
The research is supported by THALASSA—“TecHnology And materials for safe Low consumption And low life cycle cost veSSels And crafts” project. PNR 2014–2020. Area of specialization “BLUE GROWTH”, CUP ARS01_00293.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
All authors wish to thank S. Romeo, G. Napoli and F. Giordano for technical and informatic assistance in all instrumentation set-up and subsequent data fitting. This work was also conducted within the frame-work of the doctoral programs of S.S., as financed by Confindustria (Noxosorkem Group S.r.l.) and CNR, and of G.R., as financed by PON-MUR “Ricerca e Innovazione 2014–2020” RESTART project; MUR, Confindustria and CNR are gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, S.; Zhang, S.; Galluzzi, M.; Li, F.; Zhang, X.; Yang, X.; Liu, X.; Cai, X.; Zhu, X.; Du, B.; et al. Insight into multifunctional polyester fabrics finished by one-step eco-friendly strategy. Chem. Eng. J. 2019, 358, 634–642. [Google Scholar] [CrossRef]
- Chen, H.-L.; Burns, L.D. Environmental Analysis of Textile Products. Cloth. Text. Res. J. 2006, 24, 248–261. [Google Scholar] [CrossRef]
- Leonas, K.K. The Use of Recycled Fibers in Fashion and Home Products. In Textiles and Clothing Sustainability: Textile Science and Clothing Technology.; Muthu, S.S., Ed.; Springer: Singapore, 2017; pp. 55–77. ISBN 978-981-10-2146-6. [Google Scholar]
- Ke, W.H.; Hsia, C.F.; Chen, Y.J.; Huang, M.H. Synthesis of Ultrasmall Cu2O Nanocubes and Octahedra with Tunable Sizes for Facet-Dependent Optical Property Examination. Small 2016, 12, 3530–3534. [Google Scholar] [CrossRef]
- Zahid, M.; Papadopoulou, E.L.; Athanassiou, A.; Bayer, I.S. Strain-responsive mercerized conductive cotton fabrics based on PEDOT:PSS/graphene. Mater. Des. 2017, 135, 213–222. [Google Scholar] [CrossRef]
- Chaudhari, S.S.; Chitnis, R.S.; Ramkrishnan, R. Waterproof breathable active sports wear fabrics. Man-Made Text. India 2004, 5, 166–171. [Google Scholar]
- Yang, L.; Liu, H.; Ding, S.; Wu, J.; Zhang, Y.; Wang, Z.; Wei, L.; Tian, M.; Tao, G. Superabsorbent Fibers for Comfortable Disposable Medical Protective Clothing. Adv. Fiber Mater. 2020, 2, 140–149. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Joshi, R.; Chughtai, A.A.; Macintyre, C.R. Graphene Modified Multifunctional Personal Protective Clothing. Adv. Mater. Interfaces 2019, 6, 1900622. [Google Scholar] [CrossRef]
- Attar, R.M.S.; Alshareef, M.; Snari, R.M.; Alaysuy, O.; Aldawsari, A.M.; Abu-Melha, S.; El-Metwaly, N.M. Development of novel photoluminescent fibers from recycled polyester waste using plasma-assisted dyeing toward ultraviolet sensing and protective textiles. J. Mater. Res. Technol. 2022, 21, 1630–1642. [Google Scholar] [CrossRef]
- Adeakin, O.A.S.; Popoola, A.V.; Ajekwene, K.K. Effect of Solvent Pretreatment of Polyester Fiber on its Dye-Uptake Based on the Concept of Solubility Parameters. Fibers Polym. 2022, 23, 3118–3125. [Google Scholar] [CrossRef]
- Soto, D.; Ugur, A.; Farnham, T.A.; Gleason, K.K.; Varanasi, K.K. Short-Fluorinated iCVD Coatings for Nonwetting Fabrics. Adv. Funct. Mater. 2018, 28, 1707355. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.; Park, C.H. Superhydrophobic Textiles: Review of Theoretical Definitions, Fabrication and Functional Evaluation. J. Eng. Fiber. Fabr. 2015, 10, 1–18. [Google Scholar] [CrossRef]
- Xu, L.; Xie, K.; Liu, Y.; Zhang, C. Stable super-hydrophobic and comfort PDMS-coated polyester fabric. e-Polymers 2021, 21, 654–661. [Google Scholar] [CrossRef]
- Wang, D.M. Environmental protection in clothing industry. In Sustainable Development; World Scientific: Singapore, 2015; pp. 729–735. ISBN 978-981-4749-90-9. [Google Scholar]
- Rahmatinejad, J.; Khoddami, A.; Mazrouei-Sebdani, Z.; Avinc, O. Polyester hydrophobicity enhancement via UV-Ozone irradiation, chemical pre-treatment and fluorocarbon finishing combination. Prog. Org. Coatings 2016, 101, 51–58. [Google Scholar] [CrossRef]
- Ramesh, S.; Khan, S.; Park, Y.; Ford, E.; Menegatti, S.; Genzer, J. Self-healing and repair of fabrics: A comprehensive review of the application toolkit. Mater. Today 2022, 54, 90–109. [Google Scholar] [CrossRef]
- Periyasamy, A.P.; Venkataraman, M.; Kremenakova, D.; Militky, J.; Zhou, Y. Progress in Sol-Gel Technology for the Coatings of Fabrics. Materials 2020, 13, 1838. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.-C.; Zhang, G.; Xu, L.; Sun, W.-J.; Zhong, G.-J.; Lei, J.; Yan, D.-X.; Li, Z.-M. Robustly Superhydrophobic Conductive Textile for Efficient Electromagnetic Interference Shielding. ACS Appl. Mater. Interfaces 2019, 11, 1680–1688. [Google Scholar] [CrossRef]
- Dastjerdi, R.; Montazer, M.; Shahsavan, S. A new method to stabilize nanoparticles on textile surfaces. Colloids Surfaces A Physicochem. Eng. Asp. 2009, 345, 202–210. [Google Scholar] [CrossRef]
- Chruściel, J.J. Modifications of Textile Materials with Functional Silanes, Liquid Silicone Softeners, and Silicone Rubbers—A Review. Polymers 2022, 14, 4382. [Google Scholar] [CrossRef]
- Liu, X.; Zou, X.; Ge, Z.; Zhang, W.; Luo, Y. Novel waterborne polyurethanes containing long-chain alkanes: Their synthesis and application to water repellency. RSC Adv. 2019, 9, 31357–31369. [Google Scholar] [CrossRef]
- Yu, C.; Shi, K.; Ning, J.; Zheng, Z.; Yu, H.; Yang, Z.; Liu, J. Preparation and Application of Fluorine-Free Finishing Agent with Excellent Water Repellency for Cotton Fabric. Polymers 2021, 13, 2980. [Google Scholar] [CrossRef]
- Sfameni, S.; Hadhri, M.; Rando, G.; Drommi, D.; Rosace, G.; Trovato, V.; Plutino, M.R. Inorganic Finishing for Textile Fabrics: Recent Advances in Wear-Resistant, UV Protection and Antimicrobial Treatments. Inorganics 2023, 11, 19. [Google Scholar] [CrossRef]
- Ielo, I.; Giacobello, F.; Sfameni, S.; Rando, G.; Galletta, M.; Trovato, V.; Rosace, G.; Plutino, M.R. Nanostructured surface finishing and coatings: Functional properties and applications. Materials 2021, 14, 2733. [Google Scholar] [CrossRef]
- Trovato, V.; Sfameni, S.; Rando, G.; Rosace, G.; Libertino, S.; Ferri, A.; Plutino, M.R. A Review of Stimuli-Responsive Smart Materials for Wearable Technology in Healthcare: Retrospective, Perspective, and Prospective. Molecules 2022, 27, 5709. [Google Scholar] [CrossRef] [PubMed]
- Ielo, I.; Giacobello, F.; Castellano, A.; Sfameni, S.; Rando, G.; Plutino, M.R. Development of Antibacterial and Antifouling Innovative and Eco-Sustainable Sol–Gel Based Materials: From Marine Areas Protection to Healthcare Applications. Gels 2022, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Sfameni, S.; Rando, G.; Marchetta, A.; Scolaro, C.; Cappello, S.; Urzì, C.; Visco, A.; Plutino, M.R. Development of Eco-Friendly Hydrophobic and Fouling-Release Coatings for Blue-Growth Environmental Applications: Synthesis, Mechanical Characterization and Biological Activity. Gels 2022, 8, 528. [Google Scholar] [CrossRef] [PubMed]
- Sfameni, S.; Rando, G.; Galletta, M.; Ielo, I.; Brucale, M.; De Leo, F.; Cardiano, P.; Cappello, S.; Visco, A.; Trovato, V.; et al. Design and Development of Fluorinated and Biocide-Free Sol–Gel Based Hybrid Functional Coatings for Anti-Biofouling/Foul-Release Activity. Gels 2022, 8, 538. [Google Scholar] [CrossRef]
- Rando, G.; Sfameni, S.; Galletta, M.; Drommi, D.; Cappello, S.; Plutino, M.R. Functional Nanohybrids and Nanocomposites Development for the Removal of Environmental Pollutants and Bioremediation. Molecules 2022, 27, 4856. [Google Scholar] [CrossRef]
- Sfameni, S.; Del Tedesco, A.; Rando, G.; Truant, F.; Visco, A.; Plutino, M.R. Waterborne Eco-Sustainable Sol–Gel Coatings Based on Phytic Acid Intercalated Graphene Oxide for Corrosion Protection of Metallic Surfaces. Int. J. Mol. Sci. 2022, 23, 12021. [Google Scholar] [CrossRef]
- Puoci, F.; Saturnino, C.; Trovato, V.; Iacopetta, D.; Piperopoulos, E.; Triolo, C.; Bonomo, M.G.; Drommi, D.; Parisi, O.I.; Milone, C.; et al. Sol-gel treatment of textiles for the entrapping of an antioxidant/anti-inflammatory molecule: Functional coating morphological characterization and drug release evaluation. Appl. Sci. 2020, 10, 2287. [Google Scholar] [CrossRef]
- Libertino, S.; Plutino, M.R.; Rosace, G. Design and development of wearable sensing nanomaterials for smart textiles. AIP Conf. Proc. 2018, 1990, 020016. [Google Scholar] [CrossRef]
- Youn, S.; Hee Park, C. Development of breathable Janus superhydrophobic polyester fabrics using alkaline hydrolysis and blade coating. Text. Res. J. 2018, 89, 959–974. [Google Scholar] [CrossRef]
- Li, S.; Huang, J.; Chen, Z.; Chen, G.; Lai, Y. A review on special wettability textiles: Theoretical models, fabrication technologies and multifunctional applications. J. Mater. Chem. A 2017, 5, 31–55. [Google Scholar] [CrossRef]
- Ferrero, F.; Periolatto, M. Modification of surface energy and wetting of textile fibers. In Wetting Wettability; IntechOpen: London, UK, 2015; pp. 139–168. [Google Scholar]
- Hu, Y.; Wang, Y.; Zhang, X.; Qian, J.; Xing, X.; Wang, X. Regenerated cationic dyeable polyester deriving from poly(ethylene terephthalate) waste. Polym. Degrad. Stab. 2020, 179, 109261. [Google Scholar] [CrossRef]
- Kamel, M.M.; El Zawahry, M.M.; Helmy, H.; Eid, M.A. Improvements in the dyeability of polyester fabrics by atmospheric pressure oxygen plasma treatment. J. Text. Inst. 2011, 102, 220–231. [Google Scholar] [CrossRef]
- Trovato, V.; Mezzi, A.; Brucale, M.; Abdeh, H.; Drommi, D.; Rosace, G.; Plutino, M.R. Sol-Gel Assisted Immobilization of Alizarin Red S on Polyester Fabrics for Developing Stimuli-Responsive Wearable Sensors. Polymers 2022, 14, 2788. [Google Scholar] [CrossRef]
- Plutino, M.R.; Guido, E.; Colleoni, C.; Rosace, G. Effect of GPTMS functionalization on the improvement of the pH-sensitive methyl red photostability. Sensors Actuators B 2017, 238, 281–291. [Google Scholar] [CrossRef]
- Sfameni, S.; Lawnick, T.; Rando, G.; Visco, A.; Textor, T.; Plutino, M.R. Functional Silane-Based Nanohybrid Materials for the Development of Hydrophobic and Water-Based Stain Resistant Cotton Fabrics Coatings. Nanomaterials 2022, 12, 3404. [Google Scholar] [CrossRef]
- Radhakrishnan, B.; Ranjan, R.; Brittain, W.J. Surface initiated polymerizations from silica nanoparticles. Soft Matter 2006, 2, 386–396. [Google Scholar] [CrossRef]
- Koo, K.; Park, Y. Characteristics of double-layer coated fabrics with and without phase change materials and nano-particles. Fibers Polym. 2014, 15, 1641–1647. [Google Scholar] [CrossRef]
- Pan, H.; Wang, W.; Shen, Q.; Pan, Y.; Song, L.; Hu, Y.; Lu, Y. Fabrication of flame retardant coating on cotton fabric by alternate assembly of exfoliated layered double hydroxides and alginate. RSC Adv. 2016, 6, 111950–111958. [Google Scholar] [CrossRef]
- Kramar, A.D.; Obradović, B.M.; Vesel, A.; Kuraica, M.M.; Kostić, M.M. Preparation of Hydrophobic Viscose Fabric Using Nitrogen DBD and Copper Ions Sorption. Plasma Process. Polym. 2015, 12, 1095–1103. [Google Scholar] [CrossRef]
- Liu, Y.; Fuentes, C.A.; Willem Van Vuure, A.; Zhang, D.; Seveno, D. Wettability of a hybrid PTFE/Kevlar fabrics at micro-, meso-, and macroscales. Appl. Surf. Sci. 2022, 604, 154613. [Google Scholar] [CrossRef]
- Wang, Z.; Zuilhof, H. Antifouling Properties of Fluoropolymer Brushes toward Organic Polymers: The Influence of Composition, Thickness, Brush Architecture, and Annealing. Langmuir 2016, 32, 6571–6581. [Google Scholar] [CrossRef]
- Achagri, G.; Essamlali, Y.; Amadine, O.; Majdoub, M.; Chakir, A.; Zahouily, M. Surface modification of highly hydrophobic polyester fabric coated with octadecylamine-functionalized graphene nanosheets. RSC Adv. 2020, 10, 24941–24950. [Google Scholar] [CrossRef] [PubMed]
- Abeywardena, M.R.; Yashomala, M.A.D.H.; Elkaduwe, R.K.W.H.M.K.; Karunaratne, D.G.G.P.; Pitawala, H.M.T.G.A.; Rajapakse, R.M.G.; Manipura, A.; Mantilaka, M.M.M.G.P.G. Fabrication of water-repellent polyester textile via dip-coating of in-situ surface-modified superhydrophobic calcium carbonate from dolomite. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127397. [Google Scholar] [CrossRef]
- Mousaa, I.M.; Elhady, M.A.; El-Sayyad, G.S.; Attia, R.M. Development of a highly hydrophobic and antimicrobial surface for polyester fabrics treated with (vinyl acetate versatic ester/paraffin wax) blend containing sodium chloride using electron beam irradiation. Prog. Org. Coatings 2023, 174, 107230. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, W.; Ma, T.; Zhang, C.; Kuang, J.; Wang, R. Anti-UV and hydrophobic dual-functional coating fabrication for flame retardant polyester fabrics by surface-initiated PET RAFT technique. Eur. Polym. J. 2022, 173, 111275. [Google Scholar] [CrossRef]
- Narakaew, S.; Au-Pree, S.; Baison, W.; Thungprasert, S.; Wattalo, I.; Jaipor, P.; Promanan, T.; Sukdee, S.; Santakij, P.; Chanogkun, C.; et al. The finished polyester fabric with hot NH4OH pretreatment and mixed ZnO-Zn(OH)2 nanoparticles for hydrophobic property. J. Met. Mater. Miner. 2022, 32, 109–117. [Google Scholar] [CrossRef]
- Katiyar, P.; Mishra, S.; Srivastava, A.; Prasad, N.E. Preparation of TiO2–SiO2 Hybrid Nanosols Coated Flame-Retardant Polyester Fabric Possessing Dual Contradictory Characteristics of Superhydrophobicity and Self Cleaning Ability. J. Nanosci. Nanotechnol. 2020, 20, 1780–1789. [Google Scholar] [CrossRef]
- Yun, C.; Islam, M.I.; LeHew, M.; Kim, J. Assessment of environmental and economic impacts made by the reduced laundering of self-cleaning fabrics. Fibers Polym. 2016, 17, 1296–1304. [Google Scholar] [CrossRef]
- Ren, G.; Song, Y.; Li, X.; Wang, B.; Zhou, Y.; Wang, Y.; Ge, B.; Zhu, X. A simple way to an ultra-robust superhydrophobic fabric with mechanical stability, UV durability, and UV shielding property. J. Colloid Interface Sci. 2018, 522, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Chen, M.; Zong, Y.; Li, Z. Modification of fabric via co-grafted with fluorine-free carbene polymer and its hydrophobicity. Polymer 2022, 247, 124802. [Google Scholar] [CrossRef]
- Guo, F.; Fang, K.; Song, Y.; Fu, R.; Li, H.; Zhang, C. Optimizing a fabricating program for wearable super-hydrophobic cotton by clean production technology of plasma and reducing chemical consumption. J. Clean. Prod. 2020, 276, 124233. [Google Scholar] [CrossRef]
- Leroux, F.; Campagne, C.; Perwuelz, A.; Gengembre, L. Fluorocarbon nano-coating of polyester fabrics by atmospheric air plasma with aerosol. Appl. Surf. Sci. 2008, 254, 3902–3908. [Google Scholar] [CrossRef]
- Brzeziński, S.; Kowalczyk, D.; Borak, B.; Jasiorski, M.; Tracz, A. Applying the sol-gel method to the deposition of nanocoats on textiles to improve their abrasion resistance. J. Appl. Polym. Sci. 2012, 125, 3058–3067. [Google Scholar] [CrossRef]
- Dhanapal, V.K.; Dhanakodi, R. Influence of Moisture Management Properties on Socks Made from Recycled Polyester, Virgin Cotton and its Blends. Fibres Text. East. Eur. 2020, 4, 76–81. [Google Scholar]
- Belliveau, R.G.; DeJong, S.A.; Boltin, N.D.; Lu, Z.; Cassidy, B.M.; Morgan, S.L.; Myrick, M.L. Mid-infrared emissivity of nylon, cotton, acrylic, and polyester fabrics as a function of moisture content. Text. Res. J. 2019, 90, 1431–1445. [Google Scholar] [CrossRef]
- Bonartsev, A.P.; Boskhomodgiev, A.P.; Iordanskii, A.L.; Bonartseva, G.A.; Rebrov, A.V.; Makhina, T.K.; Myshkina, V.L.; Yakovlev, S.A.; Filatova, E.A.; Ivanov, E.A.; et al. Hydrolytic Degradation of Poly(3-hydroxybutyrate), Polylactide and their Derivatives: Kinetics, Crystallinity, and Surface Morphology. Mol. Cryst. Liq. Cryst. 2012, 556, 288–300. [Google Scholar] [CrossRef]
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Hydrolytic degradation of biodegradable polyesters under simulated environmental conditions. J. Appl. Polym. Sci. 2015, 132, 42189. [Google Scholar] [CrossRef]
- Karim, N.; Afroj, S.; Lloyd, K.; Oaten, L.C.; Andreeva, D.V.; Carr, C.; Farmery, A.D.; Kim, I.-D.; Novoselov, K.S. Sustainable Personal Protective Clothing for Healthcare Applications: A Review. ACS Nano 2020, 14, 12313–12340. [Google Scholar] [CrossRef]
- Zhu, F.L.; Feng, Q.Q. Recent advances in textile materials for personal radiative thermal management in indoor and outdoor environments. Int. J. Therm. Sci. 2021, 165, 106899. [Google Scholar] [CrossRef]
- Castano, L.M.; Flatau, A.B. Smart fabric sensors and e-textile technologies: A review. Smart Mater. Struct. 2014, 23, 53001. [Google Scholar] [CrossRef]
- Ullah, H.M.K.; Lejeune, J.; Cayla, A.; Monceaux, M.; Campagne, C.; Devaux, É. A review of noteworthy/major innovations in wearable clothing for thermal and moisture management from material to fabric structure. Text. Res. J. 2021, 92, 3351–3386. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





