Development of Scaffolds from Bio-Based Natural Materials for Tissue Regeneration Applications: A Review
Abstract
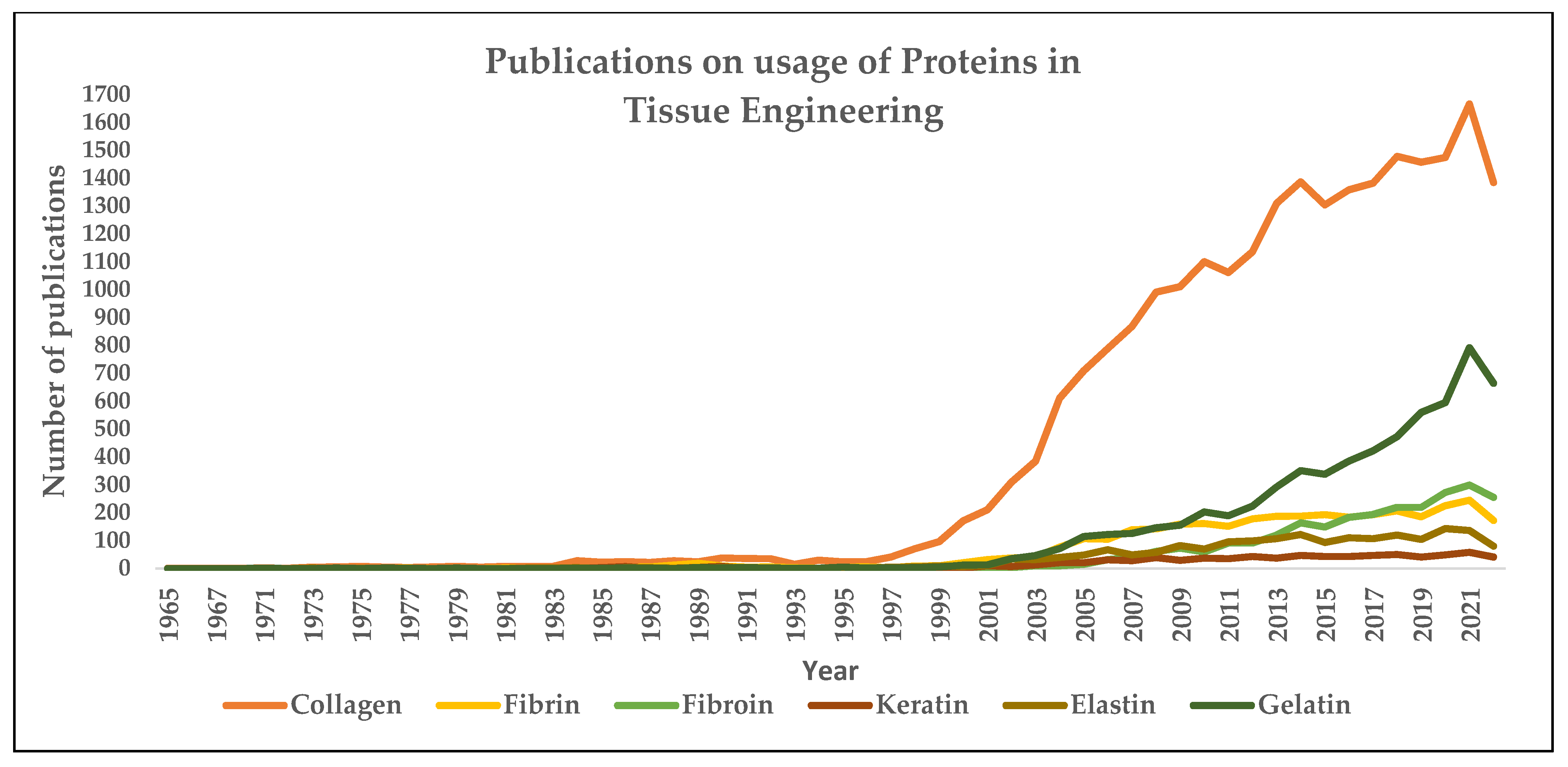
1. Introduction
2. Tissue Engineering
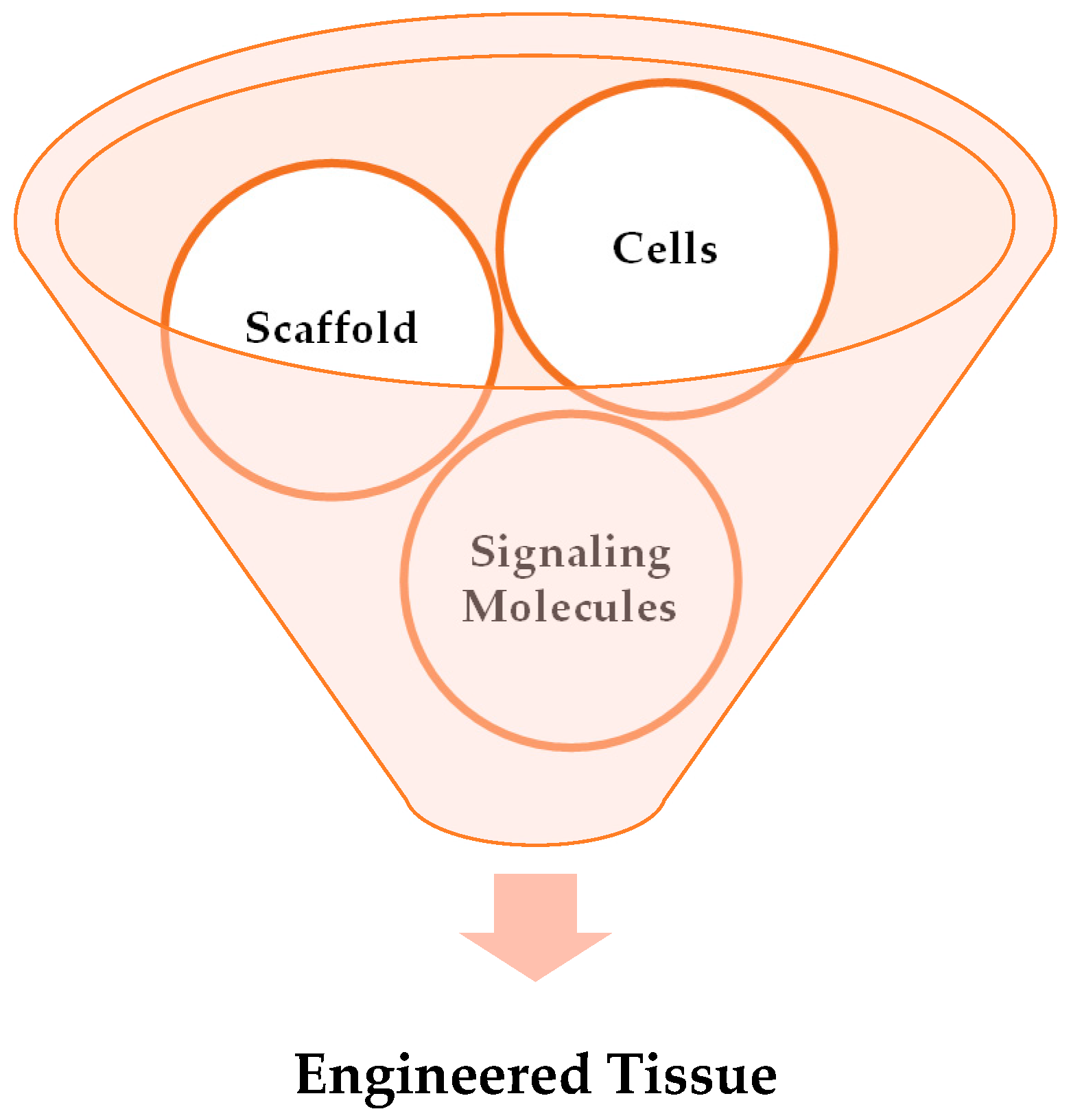
2.1. Key Elements of Tissue Engineering
2.1.1. Cells
2.1.2. Growth Factors
2.1.3. Scaffolds
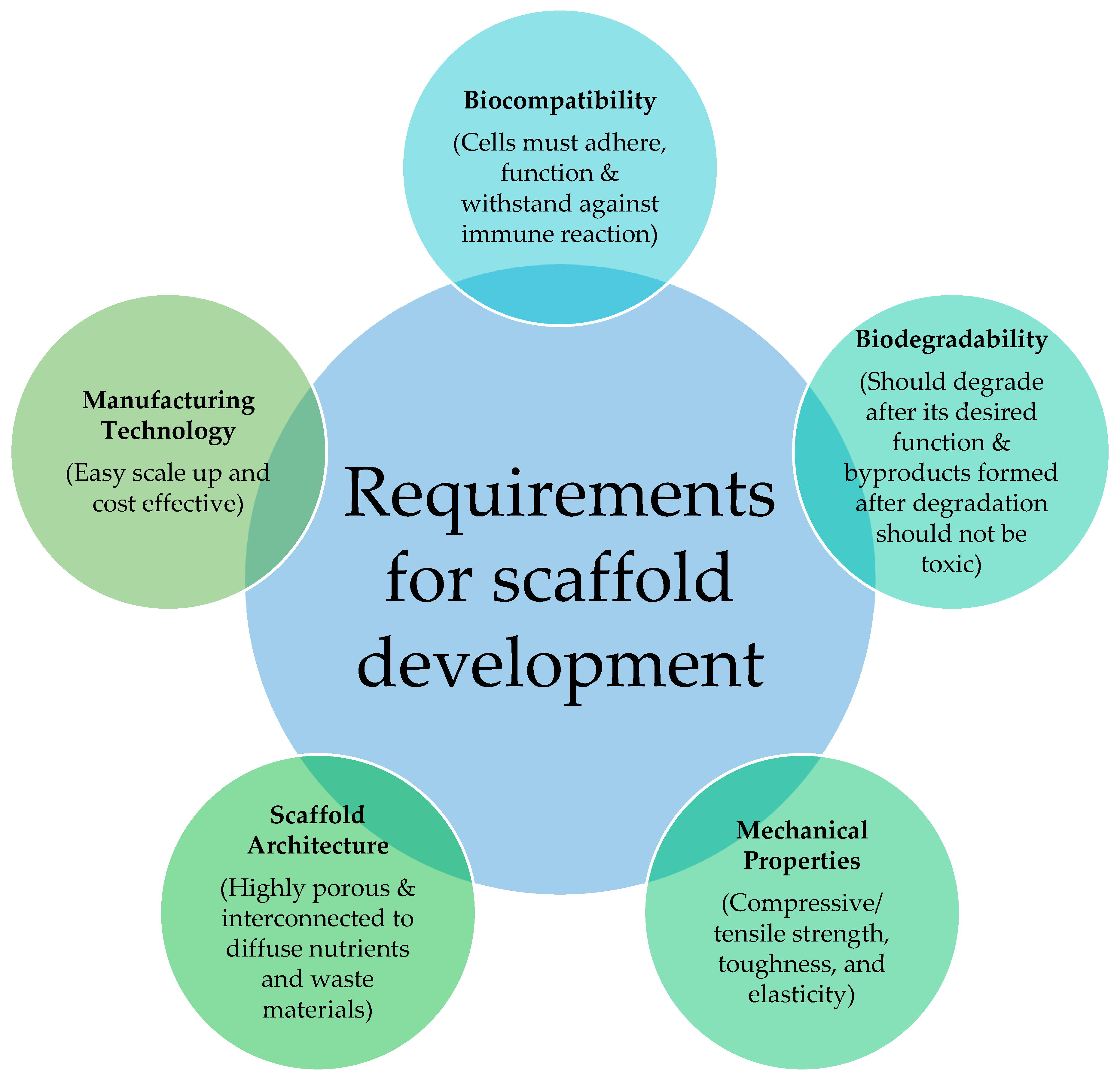
2.2. Requirements of Scaffold
2.2.1. Microarchitecture
2.2.2. Biodegradability
2.2.3. Biocompatibility
2.2.4. Bioactivity
2.2.5. Mechanical Properties
2.2.6. Manufacturing Technologies
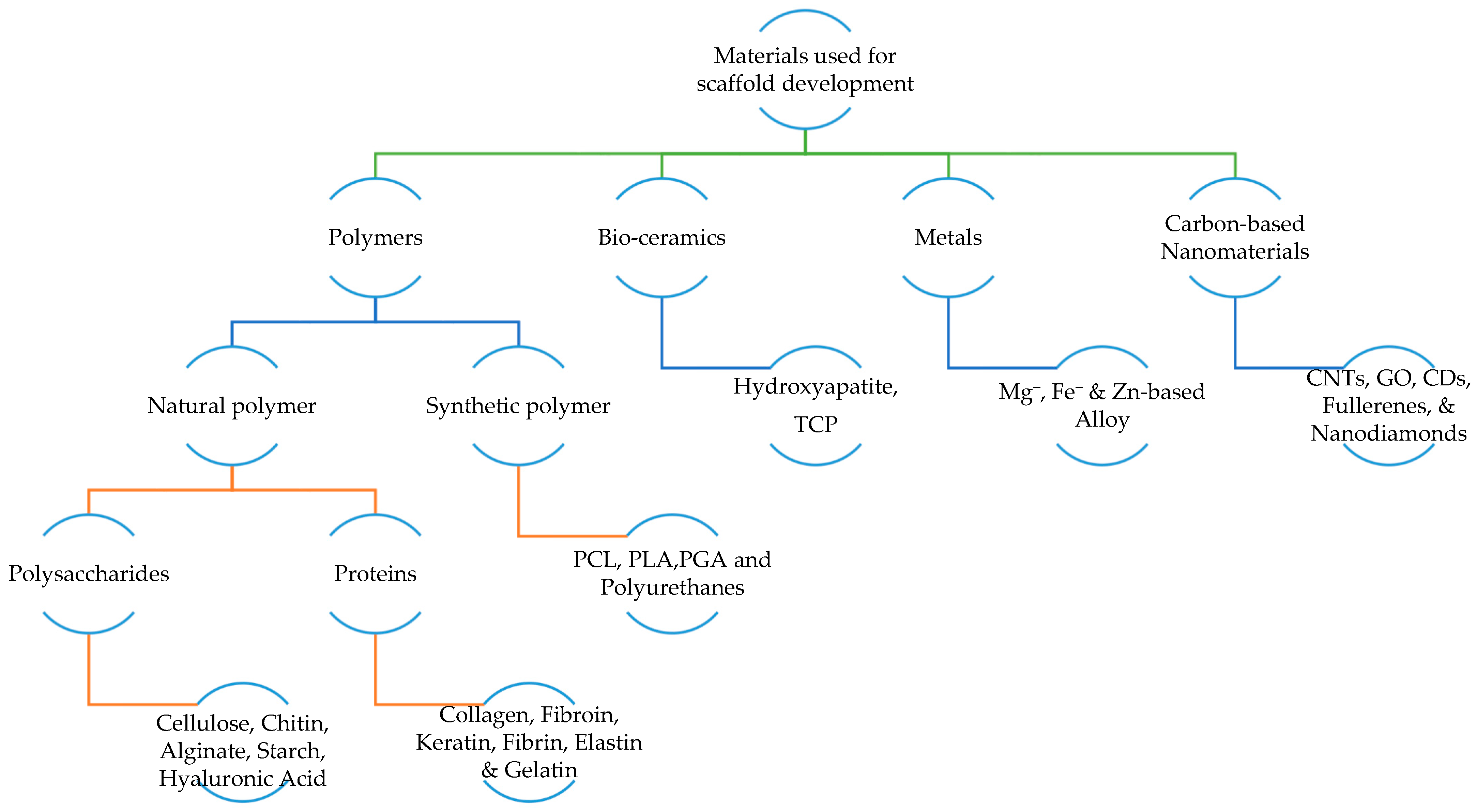
2.3. Materials Used for Developing Scaffold
2.3.1. Polymer
- Natural polymers: Biopolymers are toxic-free, highly biocompatible, easily adhere to cells, and improve proliferation and differentiation. Nevertheless, they have poor mechanical strength and are highly sensitive to elevated temperatures [46]. Biopolymers are also known as natural polymers. Natural polymers are materials that can be obtained from natural sources. They can be categorized into protein-based biomaterials (naturally occurring polymers in the human body such as collagen, fibrin, and elastin) and polysaccharides-based biomaterials (such as silk, chitosan, alginate, and gelatin). They exhibit similar characteristics to soft tissues, showing bioactivity, excellent cell adhesion and growth, and fulfilling biodegradability and biocompatibility. Moreover, they are also known for their wide availability, ecological safety, and modifiability to suit different applications. However, natural sources indicate the requirement of a purification step to avoid foreign immunological responses after implantation. In addition, natural polymers typically show poor physical and mechanical stability, limiting their applications in the load-bearing orthopaedic field [17,43].
- Synthetic polymers: In contrast to natural polymers, synthetic polymers have good mechanical properties. However, they also have a high risk of immune rejection, and toxic substances such as carbon dioxides are released during degradation, leading to cell damage [40]. Synthetic polymers serve as a more predictable biomaterial providing a wide range of mechanical and physical properties such as degradation rates. If they are synthesized under controlled conditions, they do not pose any immunological risks, and desired characteristics can be brought together. One common synthetic polymer used for BTE applications is aliphatic polyesters, including poly (ε-caprolactone) (PCL) and polylactide (PLA). PCL is a semi-crystalline, biodegradable, and non-toxic polyester that shows hydrophobicity and slow degradation rates of more than 24 months. These problems can be addressed by blending with other polymers or producing composites. In contrast, the porous PLA exhibits high biocompatibility, but shows slow degradation rates of 3–5 years. Thus, PLA is combined with hydroxyapatite (HAp) to improve its mechanical and physical strength [18,43].
2.3.2. Bio-Ceramics
2.3.3. Metals
2.3.4. Carbon-Based Nanomaterials
2.4. Common Natural Polymers Used in Tissue Regeneration Applications
2.4.1. Cellulose
2.4.2. Chitin and Chitosan
2.4.3. Alginate
2.4.4. Starch
2.4.5. Hyaluronic Acid
2.4.6. Guar Gum
2.4.7. Pullulan
2.4.8. Collagen
2.4.9. Fibroin
2.4.10. Keratin
2.4.11. Elastin
2.4.12. Fibrin
2.4.13. Gelatin
2.5. Scaffold Fabrication Techniques
2.5.1. Freeze Drying
2.5.2. Solvent Casting and Particle Leaching
2.5.3. Gas Foaming
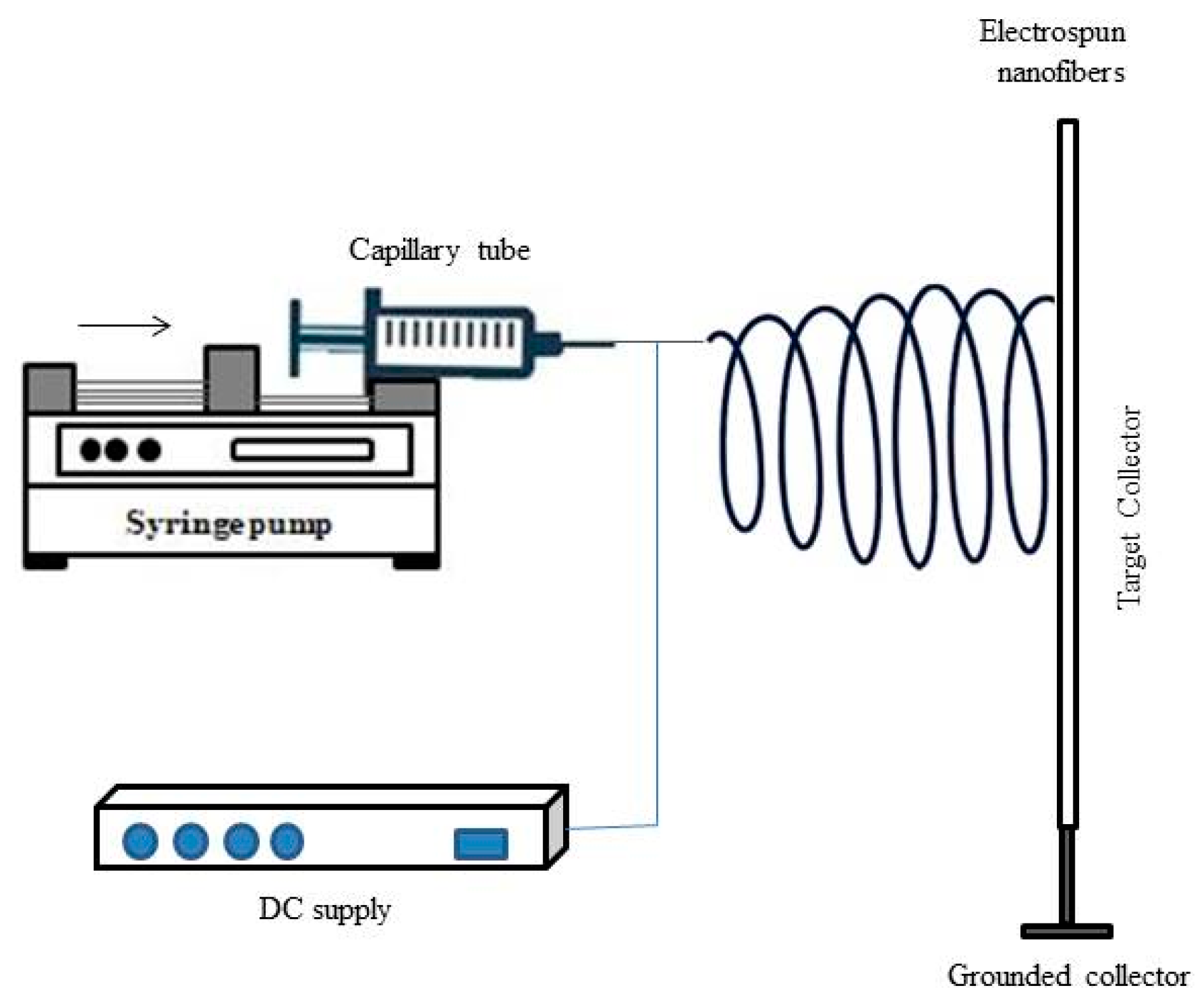
2.5.4. Electrospinning
2.5.5. Thermal-Induced Phase Separation Method
2.5.6. Stereolithography
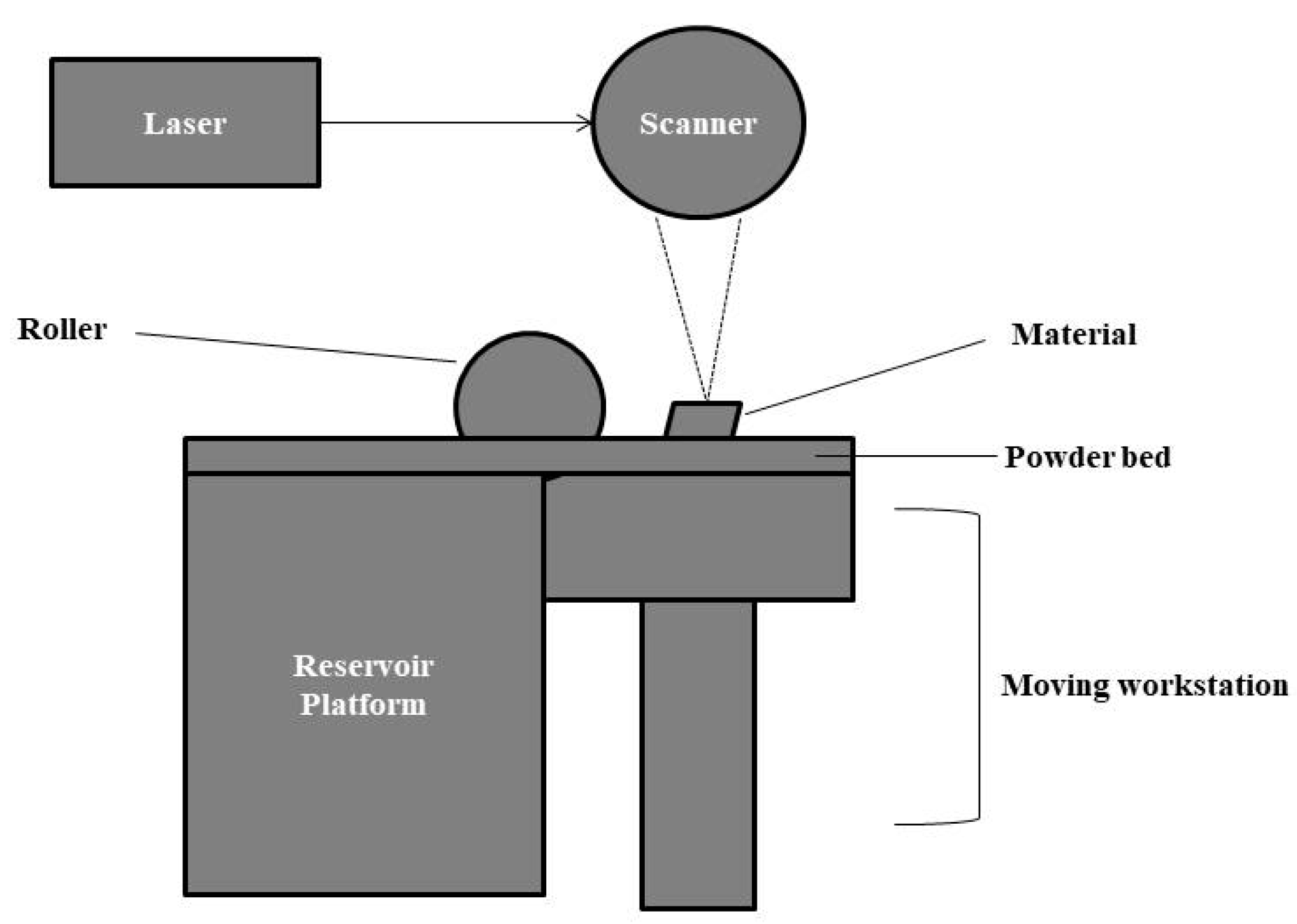
2.5.7. Selective Laser Sintering
2.5.8. Fused Deposition Model
2.5.9. Solvent-based Extrusion 3D Printing Method
2.5.10. Bioprinting Method
2.5.11. Aerosol Jet Printing
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 2019, 3429527. [Google Scholar] [CrossRef]
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of Scaffolds for Bone-Tissue Regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef]
- Lauritano, D.; Limongelli, L.; Moreo, G.; Favia, G.; Carinci, F. Nanomaterials for Periodontal Tissue Engineering: Chitosan-Based Scaffolds. A Systematic Review. Nanomaterials 2020, 10, 605. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.-L.; Kai, D.; Pasbakhsh, P.; Teow, S.-Y.; Lim, Y.-Y.; Pushpamalar, J. Electrospun cellulose acetate butyrate/polyethylene glycol (CAB/PEG) composite nanofibers: A potential scaffold for tissue engineering. Colloids Surf. B Biointerfaces 2020, 188, 110713. [Google Scholar] [CrossRef] [PubMed]
- Biswal, T. Biopolymers for tissue engineering applications: A review. Mater. Today: Proc. 2020, 41, 397–402. [Google Scholar] [CrossRef]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, 467–479. [Google Scholar] [CrossRef]
- Junior, A.L.; Pinheiro, C.C.G.; Fernandes, T.L.; Bueno, D.F. The use of human dental pulp stem cells for in vivo bone tissue engineering: A systematic review. J. Tissue Eng. 2018, 9, 2041731417752766. [Google Scholar] [CrossRef]
- Dehghan, M.; Mehrizi, M.K.; Nikukar, H. Modeling and optimizing a polycaprolactone/gelatin/polydimethylsiloxane nanofiber scaffold for tissue engineering: Using response surface methodology. J. Text. Inst. 2021, 112, 482–493. [Google Scholar] [CrossRef]
- Dutta, R.C.; Dey, M.; Dutta, A.K.; Basu, B. Competent processing techniques for scaffolds in tissue engineering. Biotechnol. Adv. 2017, 35, 240–250. [Google Scholar] [CrossRef]
- Mathew, A.; Augustine, R.; Kalarikal, N.; Thomas, S. Tissue Engineering: Principles, Recent Trends and the Future. In Nanomedicine and Tissue Engineering; Taylor & Francis: Abingdon, UK, 2016; pp. 31–82. [Google Scholar] [CrossRef]
- Castells-Sala, C.; Alemany-Ribes, M.; Fernández-Muiños, T.; Recha-Sancho, L.; López-Chicón, P.; Aloy-Reverté, C.; Caballero-Camino, J.; Márquez-Gil, A.; Semino, C.E. Current applications of tissue engineering in biomedicine. J. Biochips Tiss. Chips 2013, S2, 1. [Google Scholar] [CrossRef]
- Dufey, V.; Tacheny, A.; Art, M.; Becken, U.; De Longueville, F. Expansion of human bone marrow-derived mesenchymal stem cells in BioBLU 0.3 c single-use bioreactors. Appl. Note 2016, 305, 1–8. Available online: https://www.eppendorf.com/fileadmin/Community/Cell_Handling/Bioprocess/PDF/Application-Note_305_BioBLU-0.3c_DASbox_Expansion-of-Human-B.pdf (accessed on 29 December 2022).
- Ye, L.; Swingen, C.; Zhang, J. Induced Pluripotent Stem Cells and Their Potential for Basic and Clinical Sciences. Curr. Cardiol. Rev. 2013, 9, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Damjanov, I. Inflammation and Repair. In Pathology Secrets, 3rd ed.; Damjanov, I., Ed.; Mosby: Maryland Heights, MO, USA, 2009; pp. 19–37. [Google Scholar]
- Akter, F. Principles of Tissue Engineering. In Tissue Engineering Made Easy; Akter, F., Ed.; Academic Press: London, UK, 2016; pp. 3–16. [Google Scholar]
- Alaribe, F.N.; Manoto, S.L.; Motaung, S.C.K.M. Scaffolds from biomaterials: Advantages and limitations in bone and tissue engineering. Biologia 2016, 71, 353–366. [Google Scholar] [CrossRef]
- Perić Kačarević, Ž.; Rider, P.; Alkildani, S.; Retnasingh, S.; Pejakić, M.; Schnettler, R.; Gosau, M.; Smeets, R.; Jung, O.; Barbeck, M. An introduction to bone tissue engineering. Int. J. Artif. Organs 2019, 43, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Verma, V. Concepts of Tissue Engineering. In Animal Biotechnology, 2nd ed.; Academic Press: Cambridge, MA, US, 2020; pp. 295–307. [Google Scholar]
- Alonzo, M.; Primo, F.A.; Kumar, S.A.; Mudloff, J.A.; Dominguez, E.; Fregoso, G.; Ortiz, N.; Weiss, W.M.; Joddar, B. Bone tissue engineering techniques, advances, and scaffolds for treatment of bone defects. Curr. Opin. Biomed. Eng. 2020, 17, 100248. [Google Scholar] [CrossRef]
- Roberts, T.T.; Rosenbaum, A.J. Bone grafts, bone substitutes and orthobiologics: Bone grafts, bone substitutes and orthobiologics the bridge between basic science and clinical advancements in fracture healing. Organogenesis 2012, 8, 114–124. [Google Scholar] [CrossRef]
- Wong, Y.S.; Tay, C.Y.; Wen, F.; Venkatraman, S.S.; Tan, L.P. Engineered Polymeric Biomaterials for Tissue Engineering. Curr. Tissue Eng. 2012, 1, 41–53. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef]
- Gurumurthy, B.; Janorkar, A.V. Improvements in mechanical properties of collagen-based scaffolds for tissue engineering. Curr. Opin. Biomed. Eng. 2021, 17, 100253. [Google Scholar] [CrossRef]
- Pal, S. Mechanical Properties of Biological Materials. In Design of Artificial Human Joints & Organs; Springer: New York, NY, USA, 2014; pp. 23–40. [Google Scholar]
- Salati, M.A.; Khazai, J.; Tahmuri, A.M.; Samadi, A.; Taghizadeh, A.; Taghizadeh, M.; Zarrintaj, P.; Ramsey, J.D.; Habibzadeh, S.; Seidi, F.; et al. Agarose-Based Biomaterials: Opportunities and Challenges in Cartilage Tissue Engineering. Polymers 2020, 12, 1150. [Google Scholar] [CrossRef] [PubMed]
- Ogneva, I.V.; Lebedev, D.; Shenkman, B.S. Transversal Stiffness and Young’s Modulus of Single Fibers from Rat Soleus Muscle Probed by Atomic Force Microscopy. Biophys. J. 2010, 98, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.V.; Wang, Q.G.; Kuiper, N.J.; El Haj, A.J.; Thomas, C.R.; Zhang, Z. Biomechanical properties of single chondrocytes and chondrons determined by micromanipulation and finite-element modelling. J. R. Soc. Interface 2010, 7, 1723–1733. [Google Scholar] [CrossRef]
- Saha, K.; Keung, A.J.; Irwin, E.F.; Li, Y.; Little, L.; Schaffer, D.V.; Healy, K.E. Substrate Modulus Directs Neural Stem Cell Behavior. Biophys. J. 2008, 95, 4426–4438. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Novakofski, J.; Jenkins, W.; O’Brien, W. Young’s modulus measurements of soft tissues with application to elasticity imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 1996, 43, 191–194. [Google Scholar] [CrossRef]
- Spedden, E.; White, J.D.; Naumova, E.N.; Kaplan, D.L.; Staii, C. Elasticity Maps of Living Neurons Measured by Combined Fluorescence and Atomic Force Microscopy. Biophys. J. 2012, 103, 868–877. [Google Scholar] [CrossRef]
- Tran, T.; Hamid, Z.; Cheong, K. A Review of Mechanical Properties of Scaffold in Tissue Engineering: Aloe Vera Composites. J. Phys. Conf. Ser. 2018, 1082, 012080. [Google Scholar] [CrossRef]
- Collinsworth, A.M.; Zhang, S.; Kraus, W.E.; Truskey, G.A. Apparent elastic modulus and hysteresis of skeletal muscle cells throughout differentiation. Am. J. Physiol. Physiol. 2002, 283, C1219–C1227. [Google Scholar] [CrossRef]
- Nguyen-Truong, M.; Li, Y.; Wang, Z. Mechanical Considerations of Electrospun Scaffolds for Myocardial Tissue and Regenerative Engineering. Bioengineering 2020, 7, 122. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Fang, G.; Zhou, J. Additively Manufactured Scaffolds for Bone Tissue Engineering and the Prediction of their Mechanical Behavior: A Review. Materials 2017, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Kirsch, R.; Valente, K.; Perez, M.; Willerth, S. Physical and Mechanical Characterization of Fibrin-Based Bioprinted Constructs Containing Drug-Releasing Microspheres for Neural Tissue Engineering Applications. Processes 2021, 9, 1205. [Google Scholar] [CrossRef]
- Ezhilarasu, H.; Ramalingam, R.; Dhand, C.; Lakshminarayanan, R.; Sadiq, A.; Gandhimathi, C.; Ramakrishna, S.; Bay, B.H.; Venugopal, J.R.; Srinivasan, D.K. Biocompatible Aloe vera and Tetracycline Hydrochloride Loaded Hybrid Nanofibrous Scaffolds for Skin Tissue Engineering. Int. J. Mol. Sci. 2019, 20, 5174. [Google Scholar] [CrossRef]
- Place, E.S.; Evans, N.D.; Stevens, M.M. Complexity in biomaterials for tissue engineering. Nat. Mater. 2009, 8, 457–470. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Francois, E.L.; Yaszemski, M.J. Preclinical Bone Repair Models in Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Khan, F.; Tanaka, M. Designing Smart Biomaterials for Tissue Engineering. Int. J. Mol. Sci. 2018, 19, 17. [Google Scholar] [CrossRef]
- Sultana, N.; Hassan, M.I.; Lim, M.M. Scaffolding Biomaterials; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Asadi, N.; Del Bakhshayesh, A.R.; Davaran, S.; Akbarzadeh, A. Common biocompatible polymeric materials for tissue engineering and regenerative medicine. Mater. Chem. Phys. 2020, 242, 122528. [Google Scholar] [CrossRef]
- Gunathilake, T.M.S.U.; Ching, Y.C.; Ching, K.Y.; Chuah, C.H.; Abdullah, L.C. Biomedical and Microbiological Applications of Bio-Based Porous Materials: A Review. Polymers 2017, 9, 160. [Google Scholar] [CrossRef]
- Del Bakhshayesh, A.R.; Mostafavi, E.; Alizadeh, E.; Asadi, N.; Akbarzadeh, A.; Davaran, S. Fabrication of Three-Dimensional Scaffolds Based on Nano-biomimetic Collagen Hybrid Constructs for Skin Tissue Engineering. ACS Omega 2018, 3, 8605–8611. [Google Scholar] [CrossRef]
- Sabir, A.; Abbas, H.; Amini, A.Y.; Asmal, S. Characterization of duck egg shells and bioceramic materials in making denture applications. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1088, 012116. [Google Scholar] [CrossRef]
- Umeyama, R.; Yamawaki, T.; Liu, D.; Kanazawa, S.; Takato, T.; Hoshi, K.; Hikita, A. Optimization of culture duration of bone marrow cells before transplantation with a β-tricalcium phosphate/recombinant collagen peptide hybrid scaffold. Regen. Ther. 2020, 14, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Massimino, L.C.; Martins, V.D.C.A.; Vulcani, V.A.S.; De Oliveira, É.L.; Andreeta, M.B.; Bonagamba, T.J.; Klingbeil, M.F.G.; Mathor, M.B.; de Guzzi Plepis, A.M. Use of collagen and auricular cartilage in bioengineering: Scaffolds for tissue regeneration. Cell Tissue Bank. 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Barua, E.; Deoghare, A.B.; Deb, P.; Das Lala, S. Naturally derived biomaterials for development of composite bone scaffold: A review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 377, 012013. [Google Scholar] [CrossRef]
- Awasthi, S.; Pandey, S.K.; Arunan, E.; Srivastava, C. A review on hydroxyapatite coatings for the biomedical applications: Experimental and theoretical perspectives. J. Mater. Chem. B 2021, 9, 228–249. [Google Scholar] [CrossRef]
- Zouhair, S.; Sasso, E.D.; Tuladhar, S.R.; Fidalgo, C.; Vedovelli, L.; Filippi, A.; Borile, G.; Bagno, A.; Marchesan, M.; De Rossi, G.; et al. A Comprehensive Comparison of Bovine and Porcine Decellularized Pericardia: New Insights for Surgical Applications. Biomolecules 2020, 10, 371. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.-T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef]
- Murugiah, K.; Zakaria, M.I.; Suhaimi, H.; Caesarendra, W.; Sambudi, N.S. Synthesis and Characterisation of Hydroxyapatite (HAp) from Asiatic Hard Clam (Meretrix meretrix) and Blood Cockle Clam (Anadara granosa) Using Wet Precipitation Process. In Proceedings of the 2021 IEEE National Biomedical Engineering Conference (NBEC), Kuala Lumpur, Malaysia, 9–10 November 2021; pp. 1–6. [Google Scholar] [CrossRef]
- Mohd Pu’Ad, N.A.S.; Koshy, P.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Syntheses of hydroxyapatite from natural sources. Heliyon 2019, 5, e01588. [Google Scholar] [CrossRef]
- Karacan, I.; Ben-Nissan, B.; Sinutok, S. Marine-Based Calcium Phosphates from Hard Coral and Calcified Algae for Biomedical Applications. In Marine-Derived Biomaterials for Tissue Engineering Applications; Springer: Singapore, 2019; pp. 137–153. [Google Scholar]
- Carluccio, D.; Demir, A.G.; Bermingham, M.J.; Dargusch, M.S. Challenges and Opportunities in the Selective Laser Melting of Biodegradable Metals for Load-Bearing Bone Scaffold Applications. Met. Mater. Trans. A 2020, 51, 3311–3334. [Google Scholar] [CrossRef]
- Xie, Y.; Zhao, L.; Zhang, Z.; Wang, X.; Wang, R.; Cui, C. Fabrication and properties of porous Zn-Ag alloy scaffolds as biodegradable materials. Mater. Chem. Phys. 2018, 219, 433–443. [Google Scholar] [CrossRef]
- Ku, S.H.; Lee, M.; Park, C.B. Carbon-Based Nanomaterials for Tissue Engineering. Adv. Healthc. Mater. 2013, 2, 244–260. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Maleki, A.; de la Guardia, M.; Bani, M.S.; Chenab, K.K.; Pashazadeh-Panahi, P.; Baradaran, B.; Mokhtarzadeh, A.; Hamblin, M.R. Carbon based nanomaterials for tissue engineering of bone: Building new bone on small black scaffolds: A review. J. Adv. Res. 2019, 18, 185–201. [Google Scholar] [CrossRef]
- Girotti, A.; Gonzalez-Valdivieso, J.; Santos, M.; Martin, L.; Arias, F.J. Functional characterization of an enzymatically degradable multi-bioactive elastin-like recombinamer. Int. J. Biol. Macromol. 2020, 164, 1640–1648. [Google Scholar] [CrossRef]
- Wen, Q.; Mithieux, S.M.; Weiss, A.S. Elastin Biomaterials in Dermal Repair. Trends Biotechnol. 2020, 38, 280–291. [Google Scholar] [CrossRef]
- Gurumurthy, B.; Pal, P.; Griggs, J.A.; Janorkar, A.V. Optimization of collagen-elastin-like polypeptide-bioglass scaffold composition for osteogenic differentiation of adipose-derived stem cells. Materialia 2020, 9, 100572. [Google Scholar] [CrossRef]
- Martín-Del-Campo, M.; Fernández-Villa, D.; Cabrera-Rueda, G.; Rojo, L. Antibacterial Bio-Based Polymers for Cranio-Maxillofacial Regeneration Applications. Appl. Sci. 2020, 10, 8371. [Google Scholar] [CrossRef]
- Bedian, L.; Villalba-Rodríguez, A.M.; Hernández-Vargas, G.; Parra-Saldivar, R.; Iqbal, H.M. Bio-based materials with novel characteristics for tissue engineering applications—A review. Int. J. Biol. Macromol. 2017, 98, 837–846. [Google Scholar] [CrossRef]
- Diekjürgen, D.; Grainger, D.W. Polysaccharide matrices used in 3D in vitro cell culture systems. Biomaterials 2017, 141, 96–115. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Martinović, R.; Joksimović, D.; Petrenko, I.; Schiaparelli, S.; Wysokowski, M.; Tsurkan, D.; Stelling, A.L.; Springer, A.; Gelinsky, M.; et al. Conchixes: Organic scaffolds which resemble the size and shapes of mollusks shells, their isolation and potential multifunctional applications. Appl. Phys. A 2020, 126, 562. [Google Scholar] [CrossRef]
- Liu, W.; Du, H.; Zhang, M.; Liu, K.; Liu, H.; Xie, H.; Zhang, X.; Si, C. Bacterial Cellulose-Based Composite Scaffolds for Biomedical Applications: A Review. ACS Sustain. Chem. Eng. 2020, 8, 7536–7562. [Google Scholar] [CrossRef]
- Aki, D.; Ulag, S.; Unal, S.; Sengor, M.; Ekren, N.; Lin, C.-C.; Yılmazer, H.; Ustundag, C.B.; Kalaskar, D.M.; Gunduz, O. 3D printing of PVA/hexagonal boron nitride/bacterial cellulose composite scaffolds for bone tissue engineering. Mater. Des. 2020, 196, 109094. [Google Scholar] [CrossRef]
- Namkaew, J.; Laowpanitchakorn, P.; Sawaddee, N.; Jirajessada, S.; Honsawek, S.; Yodmuang, S. Carboxymethyl Cellulose Entrapped in a Poly(vinyl) Alcohol Network: Plant-Based Scaffolds for Cartilage Tissue Engineering. Molecules 2021, 26, 578. [Google Scholar] [CrossRef]
- Chen, P.-H.; Liao, H.-C.; Hsu, S.-H.; Chen, R.-S.; Wu, M.-C.; Yang, Y.-F.; Wu, C.-C.; Chen, M.-H.; Su, W.-F. A novel polyurethane/cellulose fibrous scaffold for cardiac tissue engineering. RSC Adv. 2015, 5, 6932–6939. [Google Scholar] [CrossRef]
- Zha, F.; Chen, W.; Hao, L.; Wu, C.; Lu, M.; Zhang, L.; Yu, D. Electrospun cellulose-based conductive polymer nanofibrous mats: Composite scaffolds and their influence on cell behavior with electrical stimulation for nerve tissue engineering. Soft Matter 2020, 16, 6591–6598. [Google Scholar] [CrossRef]
- Madub, K.; Goonoo, N.; Gimié, F.; Arsa, I.A.; Schönherr, H.; Bhaw-Luximon, A. Green seaweeds ulvan-cellulose scaffolds enhance in vitro cell growth and in vivo angiogenesis for skin tissue engineering. Carbohydr. Polym. 2021, 251, 117025. [Google Scholar] [CrossRef]
- Maharjan, B.; Park, J.; Kaliannagounder, V.K.; Awasthi, G.P.; Joshi, M.K.; Park, C.H.; Kim, C.S. Regenerated cellulose nanofiber reinforced chitosan hydrogel scaffolds for bone tissue engineering. Carbohydr. Polym. 2021, 251, 117023. [Google Scholar] [CrossRef]
- Pezeshki-Modaress, M.; Zandi, M.; Rajabi, S. Tailoring the gelatin/chitosan electrospun scaffold for application in skin tissue engineering: An in vitro study. Prog. Biomater. 2018, 7, 207–218. [Google Scholar] [CrossRef]
- Saderi, N.; Rajabi, M.; Akbari, B.; Firouzi, M.; Hassannejad, Z. Fabrication and characterization of gold nanoparticle-doped electrospun PCL/chitosan nanofibrous scaffolds for nerve tissue engineering. J. Mater. Sci. Mater. Med. 2018, 29, 134. [Google Scholar] [CrossRef]
- Sadeghianmaryan, A.; Naghieh, S.; Sardroud, H.A.; Yazdanpanah, Z.; Soltani, Y.A.; Sernaglia, J.; Chen, X. Extrusion-based printing of chitosan scaffolds and their in vitro characterization for cartilage tissue engineering. Int. J. Biol. Macromol. 2020, 164, 3179–3192. [Google Scholar] [CrossRef]
- Ahmadi, P.; Nazeri, N.; Derakhshan, M.A.; Ghanbari, H. Preparation and characterization of polyurethane/chitosan/CNT nanofibrous scaffold for cardiac tissue engineering. Int. J. Biol. Macromol. 2021, 180, 590–598. [Google Scholar] [CrossRef]
- Ghahremanzadeh, F.; Alihosseini, F.; Semnani, D. Investigation and comparison of new galactosylation methods on PCL/chitosan scaffolds for enhanced liver tissue engineering. Int. J. Biol. Macromol. 2021, 174, 278–288. [Google Scholar] [CrossRef]
- Dutta, S.D.; Hexiu, J.; Patel, D.K.; Ganguly, K.; Lim, K.-T. 3D-printed bioactive and biodegradable hydrogel scaffolds of alginate/gelatin/cellulose nanocrystals for tissue engineering. Int. J. Biol. Macromol. 2020, 167, 644–658. [Google Scholar] [CrossRef]
- Jadbabaei, S.; Kolahdoozan, M.; Naeimi, F.; Ebadi-Dehaghani, H. Preparation and characterization of sodium alginate–PVA polymeric scaffolds by electrospinning method for skin tissue engineering applications. RSC Adv. 2021, 11, 30674–30688. [Google Scholar] [CrossRef]
- Shirehjini, L.M.; Sharifi, F.; Shojaei, S.; Irani, S. Poly-caprolactone nanofibrous coated with sol-gel alginate/ mesenchymal stem cells for cartilage tissue engineering. J. Drug Deliv. Sci. Technol. 2022, 74, 103488. [Google Scholar] [CrossRef]
- Ghaderinejad, P.; Najmoddin, N.; Bagher, Z.; Saeed, M.; Karimi, S.; Simorgh, S.; Pezeshki-Modaress, M. An injectable anisotropic alginate hydrogel containing oriented fibers for nerve tissue engineering. Chem. Eng. J. 2021, 420, 130465. [Google Scholar] [CrossRef]
- Ahmadi, M.; Mehdikhani, M.; Varshosaz, J.; Farsaei, S.; Torabi, H. Pharmaceutical evaluation of atorvastatin-loaded nanostructured lipid carriers incorporated into the gelatin/hyaluronic acid/polycaprolactone scaffold for the skin tissue engineering. J. Biomater. Appl. 2021, 35, 958–977. [Google Scholar] [CrossRef] [PubMed]
- Entekhabi, E.; Nazarpak, M.H.; Moztarzadeh, F.; Sadeghi, A. Design and manufacture of neural tissue engineering scaffolds using hyaluronic acid and polycaprolactone nanofibers with controlled porosity. Mater. Sci. Eng. C 2016, 69, 380–387. [Google Scholar] [CrossRef]
- Mirab, F.; Eslamian, M.; Bagheri, R. Fabrication and characterization of a starch-based nanocomposite scaffold with highly porous and gradient structure for bone tissue engineering. Biomed. Phys. Eng. Express 2018, 4, 055021. [Google Scholar] [CrossRef]
- Yang, X.; Lu, Z.; Wu, H.; Li, W.; Zheng, L.; Zhao, J. Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Mater. Sci. Eng. C 2018, 83, 195–201. [Google Scholar] [CrossRef]
- Goodarzi, H.; Jadidi, K.; Pourmotabed, S.; Sharifi, E.; Aghamollaei, H. Preparation and in vitro characterization of cross-linked collagen–gelatin hydrogel using EDC/NHS for corneal tissue engineering applications. Int. J. Biol. Macromol. 2019, 126, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, X.-C.; Li, X.-Y.; Zhang, L.-L.; Jiang, F. A 3D porous microsphere with multistage structure and component based on bacterial cellulose and collagen for bone tissue engineering. Carbohydr. Polym. 2020, 236, 116043. [Google Scholar] [CrossRef]
- Vázquez, J.J.; Martínez, E.S.M. Collagen and elastin scaffold by electrospinning for skin tissue engineering applications. J. Mater. Res. 2019, 34, 2819–2827. [Google Scholar] [CrossRef]
- Tabatabaei, F.; Moharamzadeh, K.; Tayebi, L. Fibroblast encapsulation in gelatin methacryloyl (GelMA) versus collagen hydrogel as substrates for oral mucosa tissue engineering. J. Oral Biol. Craniofacial Res. 2020, 10, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Wu, S.; Kuss, M.; Jiang, X.; Sun, R.; Reid, P.; Qin, X.; Duan, B. 3D printing of silk fibroin-based hybrid scaffold treated with platelet rich plasma for bone tissue engineering. Bioact. Mater. 2019, 4, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Peifen, M.; Mengyun, L.; Jinglong, H.; Danqian, L.; Yan, T.; Liwei, X.; Han, Z.; Jianlong, D.; Lingyan, L.; Guanghui, Z.; et al. New skin tissue engineering scaffold with sulfated silk fibroin/chitosan/hydroxyapatite and its application. Biochem. Biophys. Res. Commun. 2023, 640, 117–124. [Google Scholar] [CrossRef]
- Hong, H.; Seo, Y.B.; Kim, D.Y.; Lee, J.S.; Lee, Y.J.; Lee, H.; Ajiteru, O.; Sultan, T.; Lee, O.J.; Kim, S.H.; et al. Digital light processing 3D printed silk fibroin hydrogel for cartilage tissue engineering. Biomaterials 2020, 232, 119679. [Google Scholar] [CrossRef]
- Forouzideh, N.; Nadri, S.; Fattahi, A.; Abdolahinia, E.D.; Habibizadeh, M.; Rostamizadeh, K.; Baradaran-Rafii, A.; Bakhshandeh, H. Epigallocatechin gallate loaded electrospun silk fibroin scaffold with anti-angiogenic properties for corneal tissue engineering. J. Drug Deliv. Sci. Technol. 2020, 56, 101498. [Google Scholar] [CrossRef]
- Li, T.; Song, X.; Weng, C.; Wang, X.; Wu, J.; Sun, L.; Gong, X.; Zeng, W.-N.; Yang, L.; Chen, C. Enzymatically crosslinked and mechanically tunable silk fibroin/pullulan hydrogels for mesenchymal stem cells delivery. Int. J. Biol. Macromol. 2018, 115, 300–307. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, H.; Liu, H.; Fan, Y. Double coating of graphene oxide–polypyrrole on silk fibroin scaffolds for neural tissue engineering. J. Bioact. Compat. Polym. 2020, 35, 216–227. [Google Scholar] [CrossRef]
- Sarrami, P.; Karbasi, S.; Farahbakhsh, Z.; Bigham, A.; Rafienia, M. Fabrication and characterization of novel polyhydroxybutyrate-keratin/nanohydroxyapatite electrospun fibers for bone tissue engineering applications. Int. J. Biol. Macromol. 2022, 220, 1368–1389. [Google Scholar] [CrossRef]
- Carvalho, C.R.; Costa, J.B.; Costa, L.; Silva-Correia, J.; Moay, Z.K.; Ng, K.W.; Reis, R.L.; Oliveira, J.M. Enhanced performance of chitosan/keratin membranes with potential application in peripheral nerve repair. Biomater. Sci. 2019, 7, 5451–5466. [Google Scholar] [CrossRef]
- Dou, J.; Wang, Y.; Jin, X.; Li, P.; Wang, L.; Yuan, J.; Shen, J. PCL/sulfonated keratin mats for vascular tissue engineering scaffold with potential of catalytic nitric oxide generation. Mater. Sci. Eng. C 2020, 107, 110246. [Google Scholar] [CrossRef]
- Ye, J.-P.; Gong, J.-S.; Su, C.; Liu, Y.-G.; Jiang, M.; Pan, H.; Li, R.-Y.; Geng, Y.; Xu, Z.-H.; Shi, J.-S. Fabrication and characterization of high molecular keratin based nanofibrous membranes for wound healing. Colloids Surf. B Biointerfaces 2020, 194, 111158. [Google Scholar] [CrossRef]
- Lv, X.; Li, Z.; Chen, S.; Xie, M.; Huang, J.; Peng, X.; Yang, R.; Wang, H.; Xu, Y.; Feng, C. Structural and functional evaluation of oxygenating keratin/silk fibroin scaffold and initial assessment of their potential for urethral tissue engineering. Biomaterials 2016, 84, 99–110. [Google Scholar] [CrossRef]
- Rosellini, E.; Madeddu, D.; Barbani, N.; Frati, C.; Graiani, G.; Falco, A.; Lagrasta, C.; Quaini, F.; Cascone, M.G. Development of Biomimetic Alginate/Gelatin/Elastin Sponges with Recognition Properties toward Bioactive Peptides for Cardiac Tissue Engineering. Biomimetics 2020, 5, 67. [Google Scholar] [CrossRef]
- Kazemi, T.; Mohammadpour, A.A.; Matin, M.M.; Mahdavi-Shahri, N.; Dehghani, H.; Riabi, S.H.K. Decellularized bovine aorta as a promising 3D elastin scaffold for vascular tissue engineering applications. Regen. Med. 2021, 16, 1037–1050. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Ku, H.-F.; Rajesh, R. Chitosan/γ-poly(glutamic acid) scaffolds with surface-modified albumin, elastin and poly- l -lysine for cartilage tissue engineering. Mater. Sci. Eng. C 2017, 78, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Smith, L.A.; Hu, J.; Ma, P.X. Biomimetic nanofibrous gelatin/apatite composite scaffolds for bone tissue engineering. Biomaterials 2009, 30, 2252–2258. [Google Scholar] [CrossRef]
- Han, F.; Dong, Y.; Su, Z.; Yin, R.; Song, A.; Li, S. Preparation, characteristics and assessment of a novel gelatin–chitosan sponge scaffold as skin tissue engineering material. Int. J. Pharm. 2014, 476, 124–133. [Google Scholar] [CrossRef]
- Semitela, Â.; Girão, A.F.; Fernandes, C.; Ramalho, G.; Bdikin, I.; Completo, A.; Marques, P.A. Electrospinning of bioactive polycaprolactone-gelatin nanofibres with increased pore size for cartilage tissue engineering applications. J. Biomater. Appl. 2020, 35, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Zeinali, K.; Khorasani, M.T.; Rashidi, A.; Joupari, M.D. Preparation and characterization of graphene oxide aerogel/gelatin as a hybrid scaffold for application in nerve tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 674–683. [Google Scholar] [CrossRef]
- Bonhome-Espinosa, A.B.; Campos, F.; Durand-Herrera, D.; Sánchez-López, J.D.; Schaub, S.; Durán, J.D.; Lopez-Lopez, M.T.; Carriel, V. In vitro characterization of a novel magnetic fibrin-agarose hydrogel for cartilage tissue engineering. J. Mech. Behav. Biomed. Mater. 2020, 104, 103619. [Google Scholar] [CrossRef]
- Rajalekshmi, R.; Shaji, A.K.; Joseph, R.; Bhatt, A. Scaffold for liver tissue engineering: Exploring the potential of fibrin incorporated alginate dialdehyde–gelatin hydrogel. Int. J. Biol. Macromol. 2021, 166, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Balagholi, S.; Kanavi, M.R.; Alizadeh, S.; Dabbaghi, R.; Karami, S.; Kheiri, B.; Daftarian, N. Effects of fibrin glue as a three-dimensional scaffold in cultivated adult human retinal pigment epithelial cells. J. Biomater. Appl. 2018, 33, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Hasanzadeh, E.; Ebrahimibarough, S.; Mirzaei, E.; Azami, M.; Tavangar, S.M.; Mahmoodi, N.; Basiri, A.; Ai, J. Preparation of fibrin gel scaffolds containing MWCNT/PU nanofibers for neural tissue engineering. J. Biomed. Mater. Res. Part A 2019, 107, 802–814. [Google Scholar] [CrossRef] [PubMed]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Berton, P.; Rogers, R.D. Advances in Functional Chitin Materials: A Review. ACS Sustain. Chem. Eng. 2019, 7, 6444–6457. [Google Scholar] [CrossRef]
- Nezhad-Mokhtari, P.; Akrami-Hasan-Kohal, M.; Ghorbani, M. An injectable chitosan-based hydrogel scaffold containing gold nanoparticles for tissue engineering applications. Int. J. Biol. Macromol. 2020, 154, 198–205. [Google Scholar] [CrossRef]
- Sahoo, D.R.; Biswal, T. Alginate and its application to tissue engineering. SN Appl. Sci. 2021, 3, 30. [Google Scholar] [CrossRef]
- Farokhi, M.; Shariatzadeh, F.J.; Solouk, A.; Mirzadeh, H. Alginate Based Scaffolds for Cartilage Tissue Engineering: A Review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 230–247. [Google Scholar] [CrossRef]
- Kaczmarek-Pawelska, A. Alginate-Based Hydrogels in Regenerative Medicine. In Alginate Recent Uses of This Natural Polymer; Pereira, L., Ed.; IntechOpen: London, UK, 2020. [Google Scholar]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 14055602, Cellulose, Microcrystalline. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cellulose_-microcrystalline (accessed on 15 January 2023).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 129662530, Chitosan. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/129662530 (accessed on 15 January 2023).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 131704328, Alginate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Alginate (accessed on 15 January 2023).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 24759, Hyaluronan. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Hyaluronan (accessed on 15 January 2023).
- Mastalska-Popławska, J.; Sikora, M.; Izak, P.; Góral, Z. Applications of starch and its derivatives in bioceramics. J. Biomater. Appl. 2019, 34, 12–24. [Google Scholar] [CrossRef]
- Waghmare, V.S.; Wadke, P.R.; Dyawanapelly, S.; Deshpande, A.; Jain, R.; Dandekar, P. Starch based nanofibrous scaffolds for wound healing applications. Bioact. Mater. 2018, 3, 255–266. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 51003661, Starch Soluble. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Starch-soluble (accessed on 15 January 2023).
- Lin, W.; Klein, J. Recent Progress in Cartilage Lubrication. Adv. Mater. 2021, 33, e2005513. [Google Scholar] [CrossRef] [PubMed]
- Dovedytis, M.; Liu, Z.J.; Bartlett, S. Hyaluronic acid and its biomedical applications: A review. Eng. Regen. 2020, 1, 102–113. [Google Scholar] [CrossRef]
- Zhai, P.; Peng, X.; Li, B.; Liu, Y.; Sun, H.; Li, X. The application of hyaluronic acid in bone regeneration. Int. J. Biol. Macromol. 2020, 151, 1224–1239. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, F.; Samani, S.M.; Tanideh, N.; Ahmadi, F. Hybrid Scaffolds of Hyaluronic Acid and Collagen Loaded with Prednisolone: An Interesting System for Osteoarthritis. Adv. Pharm. Bull. 2018, 8, 11–19. [Google Scholar] [CrossRef]
- Sieni, E.; Dettin, M.; De Robertis, M.; Bazzolo, B.; Conconi, M.T.; Zamuner, A.; Marino, R.; Keller, F.; Campana, L.G.; Signori, E. The Efficiency of Gene Electrotransfer in Breast-Cancer Cell Lines Cultured on a Novel Collagen-Free 3D Scaffold. Cancers 2020, 12, 1043. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; Liao, M.; Dai, L.; Tang, Y.; Zhang, H.; Coates, P.; Sefat, F.; Zheng, L.; Song, J.; et al. Aligned electrospun cellulose scaffolds coated with rhBMP-2 for both in vitro and in vivo bone tissue engineering. Carbohydr. Polym. 2019, 213, 27–38. [Google Scholar] [CrossRef]
- Hickey, R.J.; Pelling, A.E. Cellulose Biomaterials for Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7, 45. [Google Scholar] [CrossRef]
- Kuzmenko, V.; Karabulut, E.; Pernevik, E.; Enoksson, P.; Gatenholm, P. Tailor-made conductive inks from cellulose nanofibrils for 3D printing of neural guidelines. Carbohydr. Polym. 2018, 189, 22–30. [Google Scholar] [CrossRef]
- Luo, H.; Cha, R.; Li, J.; Hao, W.; Zhang, Y.; Zhou, F. Advances in tissue engineering of nanocellulose-based scaffolds: A review. Carbohydr. Polym. 2019, 224, 115144. [Google Scholar] [CrossRef]
- Khan, S.; Ul-Islam, M.; Ikram, M.; Islam, S.U.; Ullah, M.W.; Israr, M.; Jang, J.H.; Yoon, S.; Park, J.K. Preparation and structural characterization of surface modified microporous bacterial cellulose scaffolds: A potential material for skin regeneration applications in vitro and in vivo. Int. J. Biol. Macromol. 2018, 117, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zhang, T.; Song, Y.; Sun, W. Assessment of various crosslinking agents on collagen/chitosan scaffolds for myocardial tissue engineering. Biomed. Mater. 2020, 15, 045003. [Google Scholar] [CrossRef] [PubMed]
- Maged, A.; Abdelkhalek, A.A.; Mahmoud, A.A.; Salah, S.; Ammar, M.M.; Ghorab, M.M. Mesenchymal stem cells associated with chitosan scaffolds loaded with rosuvastatin to improve wound healing. Eur. J. Pharm. Sci. 2019, 127, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Shahruzzaman, M.; Biswas, S.; Sakib, N.; Rashid, T.U. Chitosan based bioactive materials in tissue engineering applications-A review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. BioMed Res. Int. 2015, 2015, 821279. [Google Scholar] [CrossRef] [PubMed]
- Shabunin, A.S.; Yudin, V.E.; Dobrovolskaya, I.P.; Zinovyev, E.V.; Zubov, V.; Ivan’kova, E.M.; Morganti, P. Composite Wound Dressing Based on Chitin/Chitosan Nanofibers: Processing and Biomedical Applications. Cosmetics 2019, 6, 16. [Google Scholar] [CrossRef]
- Sadeghi, A.; Moztarzadeh, F.; Mohandesi, J.A. Investigating the effect of chitosan on hydrophilicity and bioactivity of conductive electrospun composite scaffold for neural tissue engineering. Int. J. Biol. Macromol. 2019, 121, 625–632. [Google Scholar] [CrossRef]
- Niu, X.; Wei, Y.; Liu, Q.; Yang, B.; Ma, N.; Li, Z.; Zhao, L.; Chen, W.; Huang, D. Silver-loaded microspheres reinforced chitosan scaffolds for skin tissue engineering. Eur. Polym. J. 2020, 134, 109861. [Google Scholar] [CrossRef]
- Naghieh, S.; Sarker, M.; Abelseth, E.; Chen, X. Indirect 3D bioprinting and characterization of alginate scaffolds for potential nerve tissue engineering applications. J. Mech. Behav. Biomed. Mater. 2019, 93, 183–193. [Google Scholar] [CrossRef]
- Deepthi, S.; Jayakumar, R. Alginate nanobeads interspersed fibrin network as in situ forming hydrogel for soft tissue engineering. Bioact. Mater. 2018, 3, 194–200. [Google Scholar] [CrossRef]
- Prasadh, S.; Wong, R.C.W. Unraveling the mechanical strength of biomaterials used as a bone scaffold in oral and maxillofacial defects. Oral Sci. Int. 2018, 15, 48–55. [Google Scholar] [CrossRef]
- Hernández-González, A.C.; Téllez-Jurado, L.; Rodríguez-Lorenzo, L.M. Alginate hydrogels for bone tissue engineering, from injectables to bioprinting: A review. Carbohydr. Polym. 2019, 229, 115514. [Google Scholar] [CrossRef]
- Sun, J.; Tan, H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef]
- Huang, S.; Wang, C.; Xu, J.; Ma, L.; Gao, C. In situ assembly of fibrinogen/hyaluronic acid hydrogel via knob-hole interaction for 3D cellular engineering. Bioact. Mater. 2017, 2, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Pajorova, J.; Zikmundova, M.; Filova, E.; Mikes, P.; Jencova, V.; Kostakova, E.K.; Sinica, A. Nanofibrous Scaffolds for Skin Tissue Engineering and Wound Healing Based on Nature-Derived Polymers. In Current and Future Aspects of Nanomedicine; IntechOpen: London, UK, 2019; pp. 1–30. [Google Scholar]
- Monteiro, I.P.; Shukla, A.; Marques, A.P.; Reis, R.L.; Hammond, P.T. Spray-assisted layer-by-layer assembly on hyaluronic acid scaffolds for skin tissue engineering. J. Biomed. Mater. Res. Part A 2015, 103, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Chircov, C.; Grumezescu, A.M.; Bejenaru, L.E. Hyaluronic acid-based scaffolds for tissue engineering. Rom. J. Morphol. Embryol. 2018, 59, 71–76. [Google Scholar] [PubMed]
- Bejoy, J.; Wang, Z.; Bijonowski, B.; Yang, M.; Ma, T.; Sang, Q.-X.; Li, Y. Differential Effects of Heparin and Hyaluronic Acid on Neural Patterning of Human Induced Pluripotent Stem Cells. ACS Biomater. Sci. Eng. 2018, 4, 4354–4366. [Google Scholar] [CrossRef] [PubMed]
- Movahedi, M.; Asefnejad, A.; Rafienia, M.; Khorasani, M.T. Potential of novel electrospun core-shell structured polyurethane/starch (hyaluronic acid) nanofibers for skin tissue engineering: In vitro and in vivo evaluation. Int. J. Biol. Macromol. 2020, 146, 627–637. [Google Scholar] [CrossRef]
- Spearman, B.S.; Agrawal, N.K.; Rubiano, A.; Simmons, C.S.; Mobini, S.; Schmidt, C.E. Tunable methacrylated hyaluronic acid-based hydrogels as scaffolds for soft tissue engineering applications. J. Biomed. Mater. Res. Part A 2020, 108, 279–291. [Google Scholar] [CrossRef]
- Thompson, R.E.; Pardieck, J.; Smith, L.; Kenny, P.; Crawford, L.; Shoichet, M.; Sakiyama-Elbert, S. Effect of hyaluronic acid hydrogels containing astrocyte-derived extracellular matrix and/or V2a interneurons on histologic outcomes following spinal cord injury. Biomaterials 2018, 162, 208–223. [Google Scholar] [CrossRef]
- Roslan, M.R.; Nasir, N.F.M.; Cheng, E.M.; Amin, N.A.M. Tissue engineering scaffold based on starch: A review. In Proceedings of the 2016 International Conference on Electrical, Electronics, and Optimization Techniques (ICEEOT), Chennai, India, 3–5 March 2016; pp. 1857–1860. [Google Scholar]
- Espigares, I.; Elvira, C.; Mano, J.; Vázquez, B.; Román, J.S.; Reis, R.L. New partially degradable and bioactive acrylic bone cements based on starch blends and ceramic fillers. Biomaterials 2002, 23, 1883–1895. [Google Scholar] [CrossRef]
- Das, A.; Das, A.; Basu, A.; Datta, P.; Gupta, M.; Mukherjee, A. Newer guar gum ester/chicken feather keratin interact films for tissue engineering. Int. J. Biol. Macromol. 2021, 180, 339–354. [Google Scholar] [CrossRef]
- Long, N.S.W.; Ahmad, M.; Hairom, N.H.H. Tensile and Thermogravimetry Analysis of Pullulan/Cellulose Films Incorporated with Carica Papaya Seeds Extract. In Materials: Technology and Applications Series 1; Ahmad, M.B., Hairom, N.H.H.B., Othman, S.A.B., Eds.; Penerbit UTHM Universiti Tun Hussein Onn Malaysia: Parit Raja, Malaysia, 2019; pp. 1–20. [Google Scholar]
- Selvakumar, G.; Lonchin, S. Fabrication and characterization of collagen-oxidized pullulan scaffold for biomedical applications. Int. J. Biol. Macromol. 2020, 164, 1592–1599. [Google Scholar] [CrossRef]
- Cavelier, S. New Strategies for Bone Graft Materials. Master’s Thesis, McGill University, Montréal, QC, Canada, 2015. [Google Scholar]
- Zarei, M.; Samimi, A.; Khorram, M.; Abdi, M.M.; Golestaneh, S.I. Fabrication and characterization of conductive polypyrrole/chitosan/collagen electrospun nanofiber scaffold for tissue engineering application. Int. J. Biol. Macromol. 2021, 168, 175–186. [Google Scholar] [CrossRef]
- Grabska-Zielińska, S.; Sionkowska, A.; Carvalho, Â; Monteiro, F. Biomaterials with Potential Use in Bone Tissue Regeneration—Collagen/Chitosan/Silk Fibroin Scaffolds Cross-Linked by EDC/NHS. Materials 2021, 14, 1105. [Google Scholar] [CrossRef]
- Szychlinska, M.A.; Calabrese, G.; Ravalli, S.; Dolcimascolo, A.; Castrogiovanni, P.; Fabbi, C.; Puglisi, C.; Lauretta, G.; Di Rosa, M.; Castorina, A.; et al. Evaluation of a Cell-Free Collagen Type I-Based Scaffold for Articular Cartilage Regeneration in an Orthotopic Rat Model. Materials 2020, 13, 2369. [Google Scholar] [CrossRef]
- Jiang, J.P.; Liu, X.Y.; Zhao, F.; Zhu, X.; Li, X.-Y.; Niu, X.G.; Yao, Z.T.; Dai, C.; Xu, H.-Y.; Ma, K.; et al. Three-dimensional bioprinting collagen/silk fibroin scaffold combined with neural stem cells promotes nerve regeneration after spinal cord injury. Neural Regen. Res. 2020, 15, 959. [Google Scholar] [CrossRef]
- Bayrak, E.; Huri, P.Y. Engineering Musculoskeletal Tissue Interfaces. Front. Mater. 2018, 5, 24. [Google Scholar] [CrossRef]
- Ma, D.; Wang, Y.; Dai, W. Silk fibroin-based biomaterials for musculoskeletal tissue engineering. Mater. Sci. Eng. C 2018, 89, 456–469. [Google Scholar] [CrossRef]
- Hadisi, Z.; Bakhsheshi-Rad, H.R.; Walsh, T.; Dehghan, M.M.; Farzad-Mohajeri, S.; Gholami, H.; Diyanoush, A.; Pagan, E.; Akbari, M. In vitro and in vivo evaluation of silk fibroin-hardystonite-gentamicin nanofibrous scaffold for tissue engineering applications. Polym. Test. 2020, 91, 106698. [Google Scholar] [CrossRef]
- Zakeri-Siavashani, A.; Chamanara, M.; Nassireslami, E.; Shiri, M.; Hoseini-Ahmadabadi, M.; Paknejad, B. Three dimensional spongy fibroin scaffolds containing keratin/vanillin particles as an antibacterial skin tissue engineering scaffold. Int. J. Polym. Mater. Polym. Biomater. 2020, 71, 220–231. [Google Scholar] [CrossRef]
- Feroz, S.; Muhammad, N.; Ratnayake, J.; Dias, G. Keratin-Based materials for biomedical applications. Bioact. Mater. 2020, 5, 496–509. [Google Scholar] [CrossRef]
- Naderi, P.; Zarei, M.; Karbasi, S.; Salehi, H. Evaluation of the effects of keratin on physical, mechanical and biological properties of poly (3-hydroxybutyrate) electrospun scaffold: Potential application in bone tissue engineering. Eur. Polym. J. 2020, 124, 109502. [Google Scholar] [CrossRef]
- Rojas-Martínez, L.; Flores-Hernandez, C.; López-Marín, L.; Martinez-Hernandez, A.; Thorat, S.; Vasquez, C.R.; Del Rio-Castillo, A.; Velasco-Santos, C. 3D printing of PLA composites scaffolds reinforced with keratin and chitosan: Effect of geometry and structure. Eur. Polym. J. 2020, 141, 110088. [Google Scholar] [CrossRef]
- Wan, X.; Li, P.; Jin, X.; Su, F.; Shen, J.; Yuan, J. Poly(ε-caprolactone)/keratin/heparin/VEGF biocomposite mats for vascular tissue engineering. J. Biomed. Mater. Res. Part A 2020, 108, 292–300. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6913668, Collagen I, Alpha Chain (98–110). Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Collagen-I_-alpha-chain-_98-110 (accessed on 15 January 2023).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 446715, Keratan. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Keratan (accessed on 15 January 2023).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 439199, Fibrin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Fibrin (accessed on 15 January 2023).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 439221, Elastin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Elastin (accessed on 15 January 2023).
- Vazquez-Portalatin, N.; Alfonso-Garcia, A.; Liu, J.C.; Marcu, L.; Panitch, A. Physical, Biomechanical, and Optical Characterization of Collagen and Elastin Blend Hydrogels. Ann. Biomed. Eng. 2020, 48, 2924–2935. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L.; Mithieux, S.M.; Weiss, A.S. Fabricating Organized Elastin in Vascular Grafts. Trends Biotechnol. 2021, 39, 505–518. [Google Scholar] [CrossRef]
- Rodrigues, I.C.P.; Pereira, K.D.; Woigt, L.F.; Jardini, A.L.; Luchessi, A.D.; Lopes, É.S.N.; Webster, T.J.; Gabriel, L.P. A novel technique to produce tubular scaffolds based on collagen and elastin. Artif. Organs 2020, 45, E113–E122. [Google Scholar] [CrossRef]
- Dubey, A.P. Carbon Nanofiber and Polymer Conjugate. In Carbon Nanofibers: Fundamentals and Applications; Wiley-Scrivener: Hoboken, NJ, USA, 2021; pp. 75–98. [Google Scholar]
- Park, C.H.; Woo, K.M. Fibrin-Based Biomaterial Applications in Tissue Engineering and Regenerative Medicine. Adv. Exp. Med. Biol. 2018, 1064, 253–261. [Google Scholar] [CrossRef]
- Noori, A.; Ashrafi, S.J.; Vaez-Ghaemi, R.; Hatamian-Zaremi, A.; Webster, T.J. A review of fibrin and fibrin composites for bone tissue engineering. Int. J. Nanomed. 2017, 12, 4937–4961. [Google Scholar] [CrossRef]
- Purohit, S.D.; Singh, H.; Bhaskar, R.; Yadav, I.; Chou, C.-F.; Gupta, M.K.; Mishra, N.C. Gelatin—alginate—cerium oxide nanocomposite scaffold for bone regeneration. Mater. Sci. Eng. C 2020, 116, 111111. [Google Scholar] [CrossRef] [PubMed]
- Abedinia, A.; Nafchi, A.M.; Sharifi, M.; Ghalambor, P.; Oladzadabbasabadi, N.; Ariffin, F.; Huda, N. Poultry gelatin: Characteristics, developments, challenges, and future outlooks as a sustainable alternative for mammalian gelatin. Trends Food Sci. Technol. 2020, 104, 14–26. [Google Scholar] [CrossRef]
- Ashwin, B.; Abinaya, B.; Prasith, T.; Chandran, S.V.; Yadav, L.R.; Vairamani, M.; Patil, S.; Selvamurugan, N. 3D-poly (lactic acid) scaffolds coated with gelatin and mucic acid for bone tissue engineering. Int. J. Biol. Macromol. 2020, 162, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Yoshida, F.; Ueno, M.; Taguchi, M. Application of radiation crosslinking technique to development of gelatin scaffold for tissue engineering. Radiat. Phys. Chem. 2021, 180, 109287. [Google Scholar] [CrossRef]
- Singh, S.; Dutt, D.; Kaur, P.; Singh, H.; Mishra, N.C. Microfibrous paper scaffold for tissue engineering application. J. Biomater. Sci. Polym. Ed. 2020, 31, 1091–1106. [Google Scholar] [CrossRef]
- Goudarzi, Z.M.; Behzad, T.; Ghasemi-Mobarakeh, L.; Kharaziha, M. An investigation into influence of acetylated cellulose nanofibers on properties of PCL/Gelatin electrospun nanofibrous scaffold for soft tissue engineering. Polymer 2021, 213, 123313. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, X.; Chen, J.; Lin, K. The development of collagen based composite scaffolds for bone regeneration. Bioact. Mater. 2018, 3, 129–138. [Google Scholar] [CrossRef]
- Lim, Y.-S.; Ok, Y.-J.; Hwang, S.-Y.; Kwak, J.-Y.; Yoon, S. Marine Collagen as A Promising Biomaterial for Biomedical Applications. Mar. Drugs 2019, 17, 467. [Google Scholar] [CrossRef]
- Kim, H.; Jang, J.; Park, J.; Lee, K.P.; Lee, S.; Lee, D.M.; Kim, K.H.; Kim, H.K.; Cho, D.W. Shear-induced alignment of collagen fibrils using 3D cell printing for corneal stroma tissue engineering. Biofabrication 2019, 11, 3. [Google Scholar] [CrossRef]
- Nabavi, M.H.; Salehi, M.; Ehterami, A.; Bastami, F.; Semyari, H.; Tehranchi, M.; Semyari, H. A collagen-based hydrogel containing tacrolimus for bone tissue engineering. Drug Deliv. Transl. Res. 2020, 10, 108–121. [Google Scholar] [CrossRef]
- Chen, X.; Hao, W.; Li, X.; Xiao, Z.; Yao, Y.; Chu, Y.; Farkas, B.; Romano, I.; Brandi, F.; Dai, J. Functional Multichannel Poly(Propylene Fumarate)-Collagen Scaffold with Collagen-Binding Neurotrophic Factor 3 Promotes Neural Regeneration After Transected Spinal Cord Injury. Adv. Heal. Mater. 2018, 7, e1800315. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Q.; Li, H.; Wei, Q.; Zhao, X.; Chen, F. Elastin-like polypeptide modified silk fibroin porous scaffold promotes osteochondral repair. Bioact. Mater. 2021, 6, 589–601. [Google Scholar] [CrossRef]
- Gholipourmalekabadi, M.; Sapru, S.; Samadikuchaksaraei, A.; Reis, R.L.; Kaplan, D.L.; Kundu, S.C. Silk fibroin for skin injury repair: Where do things stand? Adv. Drug Deliv. Rev. 2020, 153, 28–53. [Google Scholar] [CrossRef] [PubMed]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Keirouz, A.; Zakharova, M.; Kwon, J.; Robert, C.; Koutsos, V.; Callanan, A.; Chen, X.; Fortunato, G.; Radacsi, N. High-throughput production of silk fibroin-based electrospun fibers as biomaterial for skin tissue engineering applications. Mater. Sci. Eng. C 2020, 112, 110939. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Lorentz, K.L.; Haskett, D.G.; Cunnane, E.M.; Ramaswamy, A.K.; Weinbaum, J.S.; Vorp, D.A.; Mandal, B.B. Bioresorbable silk grafts for small diameter vascular tissue engineering applications: In vitro and in vivo functional analysis. Acta Biomater. 2020, 105, 146–158. [Google Scholar] [CrossRef]
- Atrian, M.; Kharaziha, M.; Emadi, R.; Alihosseini, F. Silk-Laponite® fibrous membranes for bone tissue engineering. Appl. Clay Sci. 2019, 174, 90–99. [Google Scholar] [CrossRef]
- De Torre, I.G.; Alonso, M.; Rodriguez-Cabello, J.-C. Elastin-Based Materials: Promising Candidates for Cardiac Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 657. [Google Scholar] [CrossRef]
- Miranda-Nieves, D.; Chaikof, E.L. Collagen and Elastin Biomaterials for the Fabrication of Engineered Living Tissues. ACS Biomater. Sci. Eng. 2017, 3, 694–711. [Google Scholar] [CrossRef]
- Nguyen, T.-U.; Shojaee, M.; Bashur, C.; Kishore, V. Electrochemical fabrication of a biomimetic elastin-containing bi-layered scaffold for vascular tissue engineering. Biofabrication 2019, 11, 015007. [Google Scholar] [CrossRef]
- Wang, X.; Ali, M.S.; Lacerda, C.M.R. A Three-Dimensional Collagen-Elastin Scaffold for Heart Valve Tissue Engineering. Bioengineering 2018, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Singh, R.; Sarker, B.; Papageorgiou, D.G.; Juhasz-Bortuzzo, J.A.; Roether, J.A.; Cicha, I.; Kaschta, J.; Schubert, D.W.; Chrissafis, K.; et al. Hydrogel matrices based on elastin and alginate for tissue engineering applications. Int. J. Biol. Macromol. 2018, 114, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Khalili, S.; Khorasani, S.N.; Razavi, S.M.; Hashemibeni, B.; Tamayol, A. Nanofibrous Scaffolds with Biomimetic Composition for Skin Regeneration. Appl. Biochem. Biotechnol. 2019, 187, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Prabhakaran, M.P.; Ramakrishna, S. Strategies for regeneration of components of nervous system: Scaffolds, cells and biomolecules. Regen. Biomater. 2015, 2, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Afewerki, S.; Sheikhi, A.; Kannan, S.; Ahadian, S.; Khademhosseini, A. Gelatin-polysaccharide composite scaffolds for 3D cell culture and tissue engineering: Towards natural therapeutics. Bioeng. Transl. Med. 2019, 4, 96–115. [Google Scholar] [CrossRef] [PubMed]
- Tytgat, L.; Van Damme, L.; Van Hoorick, J.; Declercq, H.; Thienpont, H.; Ottevaere, H.; Blondeel, P.; Dubruel, P.; Van Vlierberghe, S. Additive manufacturing of photo-crosslinked gelatin scaffolds for adipose tissue engineering. Acta Biomater. 2019, 94, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Li, H.; Yu, K.; Xie, C.; Wang, P.; Zheng, Y.; Zhang, P.; Xiu, J.; Yang, Y.; He, Y.; et al. 3D printing of gelatin methacrylate-based nerve guidance conduits with multiple channels. Mater. Des. 2020, 192, 108757. [Google Scholar] [CrossRef]
- Conrad, B.; Han, L.-H.; Yang, F. Gelatin-Based Microribbon Hydrogels Accelerate Cartilage Formation by Mesenchymal Stem Cells in Three Dimensions. Tissue Eng. Part A 2018, 24, 1631–1640. [Google Scholar] [CrossRef]
- Celikkin, N.; Mastrogiacomo, S.; Jaroszewicz, J.; Walboomers, X.F.; Swieszkowski, W. Gelatin methacrylate scaffold for bone tissue engineering: The influence of polymer concentration. J. Biomed. Mater. Res. Part A 2018, 106, 201–209. [Google Scholar] [CrossRef]
- Rezaeeyazdi, M.; Colombani, T.; Memic, A.; Bencherif, S.A. Injectable Hyaluronic Acid-co-Gelatin Cryogels for Tissue-Engineering Applications. Materials 2018, 11, 1374. [Google Scholar] [CrossRef]
- Song, H.-H.G.; Rumma, R.T.; Ozaki, C.K.; Edelman, E.R.; Chen, C.S. Vascular Tissue Engineering: Progress, Challenges, and Clinical Promise. Cell Stem Cell 2018, 22, 340–354. [Google Scholar] [CrossRef] [PubMed]
- Abelseth, E.; Abelseth, L.; De la Vega, L.; Beyer, S.T.; Wadsworth, S.J.; Willerth, S.M. 3D Printing of Neural Tissues Derived from Human Induced Pluripotent Stem Cells Using a Fibrin-Based Bioink. ACS Biomater. Sci. Eng. 2019, 5, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, B.; Spitz, S.; Rothbauer, M.; Jordan, C.; Purtscher, M.; Zirath, H.; Schuller, P.; Eilenberger, C.; Ali, S.F.; Mühleder, S.; et al. Engineering of three-dimensional pre-vascular networks within fibrin hydrogel constructs by microfluidic control over reciprocal cell signaling. Biomicrofluidics 2018, 12, 042216. [Google Scholar] [CrossRef]
- Soleimannejad, M.; Ebrahimibarough, S.; Soleimani, M.; Nadri, S.; Tavangar, S.M.; Roohipoor, R.; Yazdankhah, M.; Bayat, N.; Riazi-Esfahani, M.; Ai, J. Fibrin gel as a scaffold for photoreceptor cells differentiation from conjunctiva mesenchymal stem cells in retina tissue engineering. Artif. Cells Nanomed. Biotechnol. 2018, 46, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, C. Fibrin Hydrogels for Endothelialized Liver Tissue Engineering with a Predesigned Vascular Network. Polymers 2018, 10, 1048. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Gu, H.; Mi, H.; Rao, C.; Fu, J.; Turng, L.-S. Fabrication of scaffolds in tissue engineering: A review. Front. Mech. Eng. 2018, 13, 107–119. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Touri, M.; Kabirian, F.; Saadati, M.; Ramakrishna, S.; Mozafari, M. Additive Manufacturing of Biomaterials—The Evolution of Rapid Prototyping. Adv. Eng. Mater. 2019, 21, 1800511. [Google Scholar] [CrossRef]
- Yuan, B.; Zhou, S.Y.; Chen, X.S. Rapid prototyping technology and its application in bone tissue engineering. J. Zhejiang Univ. Sci. B 2017, 18, 303–315. [Google Scholar] [CrossRef]
- Fereshteh, Z. Freeze-drying technologies for 3D scaffold engineering. In Functional 3D Tissue Engineering Scaffolds—Materials, Technologies and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 151–174. [Google Scholar]
- Brougham, C.M.; Levingstone, T.J.; Shen, N.; Cooney, G.M.; Jockenhoevel, S.; Flanagan, T.C.; O’Brien, F.J. Freeze-Drying as a Novel Biofabrication Method for Achieving a Controlled Microarchitecture within Large, Complex Natural Biomaterial Scaffolds. Adv. Heal. Mater. 2017, 6, 1700598. [Google Scholar] [CrossRef]
- Aghmiuni, A.I.; Keshel, S.H.; Sefat, F.; AkbarzadehKhiyavi, A. Fabrication of 3D hybrid scaffold by combination technique of electrospinning-like and freeze-drying to create mechanotransduction signals and mimic extracellular matrix function of skin. Mater. Sci. Eng. C 2021, 120, 111752. [Google Scholar] [CrossRef]
- Mikos, A.G.; Sarakinos, G.; Leite, S.M.; Vacant, J.P.; Langer, R. Laminated three-dimensional biodegradable foams for use in tissue engineering. Biomaterials 1993, 14, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Thadavirul, N.; Pavasant, P.; Supaphol, P. Development of polycaprolactone porous scaffolds by combining solvent casting, particulate leaching, and polymer leaching techniques for bone tissue engineering. J. Biomed. Mater. Res. Part A 2014, 102, 3379–3392. [Google Scholar] [CrossRef]
- Prasad, A.; Sankar, M.; Katiyar, V. State of Art on Solvent Casting Particulate Leaching Method for Orthopedic ScaffoldsFabrication. Mater. Today Proc. 2017, 4, 898–907. [Google Scholar] [CrossRef]
- Harris, L.D.; Kim, B.; Mooney, D.J. Open pore biodegradable matrices formed with gas foaming. J. Biomed. Mater. Res. 1998, 42, 396–402. [Google Scholar] [CrossRef]
- Kishan, A.P.; Cosgriff-Hernandez, E.M. Recent advancements in electrospinning design for tissue engineering applications: A review. J. Biomed. Mater. Res. Part A 2017, 105, 2892–2905. [Google Scholar] [CrossRef] [PubMed]
- Friend, D.F.L.; González, M.E.L.; Caraballo, M.M.; de Queiroz, A.A.A. Biological properties of electrospun cellulose scaffolds from biomass. J. Biomater. Sci. Polym. Ed. 2019, 30, 1399–1414. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.S.; Park, T.G. Biodegradable polymeric microcellular foams by modified thermally induced phase separation method. Biomaterials 1999, 20, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef]
- Kamboj, N.; Ressler, A.; Hussainova, I. Bioactive Ceramic Scaffolds for Bone Tissue Engineering by Powder Bed Selective Laser Processing: A Review. Materials 2021, 14, 5338. [Google Scholar] [CrossRef]
- Xia, X.; Xu, X.; Lin, C.; Yang, Y.; Zeng, L.; Zheng, Y.; Wu, X.; Li, W.; Xiao, L.; Qian, Q.; et al. Microalgal-Immobilized Biocomposite Scaffold Fabricated by Fused Deposition Modeling 3D Printing Technology for Dyes Removal. ES Mater. Manuf. 2020, 7, 40–50. [Google Scholar] [CrossRef]
- Zhang, B.; Cristescu, R.; Chrisey, D.B.; Narayan, R.J. Solvent-based Extrusion 3D Printing for the Fabrication of Tissue Engineering Scaffolds. Int. J. Bioprint. 2019, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, X.-F.; Gao, G.; Yonezawa, T.; Cui, X. 3D bioprinting and the current applications in tissue engineering. Biotechnol. J. 2017, 12, 8. [Google Scholar] [CrossRef]
- Ćatić, N.; Wells, L.; Al Nahas, K.; Smith, M.; Jing, Q.; Keyser, U.F.; Cama, J.; Kar-Narayan, S. Aerosol-jet printing facilitates the rapid prototyping of microfluidic devices with versatile geometries and precise channel functionalization. Appl. Mater. Today 2020, 19, 100618. [Google Scholar] [CrossRef]
- Salmoria, G.V.; Klauss, P.; Paggi, R.A.; Kanis, L.A.; Lago, A. Structure and mechanical properties of cellulose based scaffolds fabricated by selective laser sintering. Polym. Test. 2009, 28, 648–652. [Google Scholar] [CrossRef]
- Sharmila, G.; Muthukumaran, C.; Kirthika, S.; Keerthana, S.; Kumar, N.M.; Jeyanthi, J. Fabrication and characterization of Spinacia oleracea extract incorporated alginate/carboxymethyl cellulose microporous scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2020, 156, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Deepthi, S.; Viha, C.V.S.; Thitirat, C.; Furuike, T.; Tamura, H.; Jayakumar, R. Fabrication of Chitin/Poly(butylene succinate)/Chondroitin Sulfate Nanoparticles Ternary Composite Hydrogel Scaffold for Skin Tissue Engineering. Polymers 2014, 6, 2974–2984. [Google Scholar] [CrossRef]
- Entekhabi, E.; Nazarpak, M.H.; Shafieian, M.; Mohammadi, H.; Firouzi, M.; Hassannejad, Z. Fabrication and in vitro evaluation of 3D composite scaffold based on collagen/hyaluronic acid sponge and electrospun polycaprolactone nanofibers for peripheral nerve regeneration. J. Biomed. Mater. Res. Part A 2021, 109, 300–312. [Google Scholar] [CrossRef]
- Kitsara, M.; Joanne, P.; Boitard, S.E.; Ben Dhiab, I.; Poinard, B.; Menasché, P.; Gagnieu, C.; Forest, P.; Agbulut, O.; Chen, Y. Fabrication of cardiac patch by using electrospun collagen fibers. Microelectron. Eng. 2015, 144, 46–50. [Google Scholar] [CrossRef]
- Morris, V.B.; Nimbalkar, S.; Younesi, M.; McClellan, P.; Akkus, O. Mechanical Properties, Cytocompatibility and Manufacturability of Chitosan:PEGDA Hybrid-Gel Scaffolds by Stereolithography. Ann. Biomed. Eng. 2017, 45, 286–296. [Google Scholar] [CrossRef]
- Rastegar, A.; Mahmoodi, M.; Mirjalili, M.; Nasirizadeh, N. Platelet-Rich Fibrin-Loaded PCL/Chitosan Core-Shell fibers Scaffold for Enhanced Osteogenic Differentiation of Mesenchymal Stem Cells. Carbohydr. Polym. 2021, 269, 118351. [Google Scholar] [CrossRef] [PubMed]
- Zhong, N.; Dong, T.; Chen, Z.; Guo, Y.; Shao, Z.; Zhao, X. A novel 3D-printed silk fibroin-based scaffold facilitates tracheal epithelium proliferation in vitro. J. Biomater. Appl. 2019, 34, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yang, G.H.; Choi, C.H.; Cho, Y.S.; Kim, G. Gelatin/PVA scaffolds fabricated using a 3D-printing process employed with a low-temperature plate for hard tissue regeneration: Fabrication and characterizations. Int. J. Biol. Macromol. 2018, 120, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhao, X.; Du, C. Macroporous poly (l-lactic acid)/chitosan nanofibrous scaffolds through cloud point thermally induced phase separation for enhanced bone regeneration. Eur. Polym. J. 2018, 109, 303–316. [Google Scholar] [CrossRef]
- Beh, C.Y.; Cheng, E.M.; Nasir, N.F.M.; Majid, M.S.A.; Roslan, M.R.M.; You, K.Y.; Khor, S.F.; Ridzuan, M.J.M. Fabrication and characterization of three-dimensional porous cornstarch/n-HAp biocomposite scaffold. Bull. Mater. Sci. 2020, 43, 249. [Google Scholar] [CrossRef]
- Li, P.; Wang, Y.; Jin, X.; Dou, J.; Han, X.; Wan, X.; Yuan, J.; Shen, J. Fabrication of PCL/keratin composite scaffolds for vascular tissue engineering with catalytic generation of nitric oxide potential. J. Mater. Chem. B 2020, 8, 6092–6099. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, S.; Wang, L.; You, R.; Yan, S.; Zhang, Q.; Li, M. Bioactive silk fibroin scaffold with nanoarchitecture for wound healing. Compos. Part B Eng. 2021, 224, 109165. [Google Scholar] [CrossRef]
- Hejazi, F.; Ebrahimi, V.; Asgary, M.; Piryaei, A.; Fridoni, M.J.; Kermani, A.A.; Zare, F.; Abdollahifar, M.-A. Improved healing of critical-size femoral defect in osteoporosis rat models using 3D elastin/polycaprolactone/nHA scaffold in combination with mesenchymal stem cells. J. Mater. Sci. Mater. Med. 2021, 32, 27. [Google Scholar] [CrossRef]




| Tissue | Young’s Modulus | Reference |
|---|---|---|
| Bone | 1–20 GPa | [26,27] |
| Cardiac | 30–400 KPa | [27,28] |
| Cartilage | 10–20 KPa | [27,29] |
| Endothelium | 1–7 KPa | [27,30] |
| Liver | 0.3–0.8 KPa | [27,31] |
| Lung | 1–5 KPa | [27] |
| Nerve | 0.1–2 KPa | [32] |
| Skin | 4.6–20.0 MPa | [33] |
| Skeletal Muscle | 20–100 KPa | [34] |
| Natural Material, Application, and References | Cells | Assay | Result |
|---|---|---|---|
| Cellulose in Bone Tissue Engineering [69] | Human osteoblast cells | MTT assay | Significant increase in cell viability of scaffold with 0.5 weight% bacterial cellulose |
| Cellulose in Cartilage Tissue Engineering [70] | Chondrocytes | Presto BlueTM assay | Chondrocyte viability percentage in scaffold was found to be greater than 70% |
| Cellulose in Cardiac Tissue Engineering [71] | H9C2 rat cardiac myoblasts | MTT assay | Excellent biocompatibility in which scaffold exhibited cell proliferation and retention over the time frame of the study |
| Cellulose in Nerve Tissue Engineering [72] | Rat PC12 cells | Dojindo’s cell counting kit-8 (CCK-8) assay | An increase in cell viability was observed when the concentration of Poly(3-hexylthiophene)—an organic voltaic material, was up to 0.15M for the respective scaffold |
| Cellulose in Skin Tissue Engineering [73] | L929 mouse fibroblast | MTT assay | In vitro studies show low cell viability due to MTT assay, which is unreliable in calculating the number of cells settled inside the scaffold, but in vivo studies with Wistar rats revealed it is a promising material for diabetic wound healing |
| Chitosan in Bone Tissue Engineering [74] | MC3T3-E1 cells (Mouse calvaria pre-osteoblast) | Dojindo’s cell counting kit-8 (CCK-8) assay | Cell attachment, viability, and proliferation in regenerated cellulose nanofibers into chitosan hydrogel is more excellent than pure chitosan hydrogel |
| Chitosan in Skin Tissue Engineering [75] | Human dermal fibroblast | MTS assay | The presence of chitosan along with gelatin helps in the cellular behavior of substrates and enhances the proliferation rate of fibroblasts |
| Chitosan in Nerve Tissue Engineering [76] | Schwann cells | MTT assay | The rate of Schwann cell proliferation was increased after the introduction of gold nanoparticles |
| Chitosan in Cartilage Tissue Engineering [77] | ATDC5 (Chondrocytes) | Live/dead kit (Invitrogen) | The cells migrated toward the edges of the scaffold, and the cell population at the edges became higher. From this result, necessary modifications were carried out to develop smooth strands without any slope to encourage the cells to spread on the whole surface of scaffold |
| Chitosan in Cardiac Tissue Engineering [78] | H9C2 (Rat cardiac myoblast cells) and HUVEC (Human umbilical vein endothelial cells) | Alamar Blue assay | For HUVEC, more cell viability was seen in the scaffold combined with polyurethane, chitosan, and carbon nanotube than in the polyurethane scaffold and control, since polyurethane is hydrophobic and lacks enough surface of cell recognition sites, while chitosan is hydrophilic. H9C2 revealed that the developed scaffold is promising for infarcted myocardium |
| Chitosan in Liver Tissue Engineering [79] | HepG2 (Human hepatic carcinoma cells) | MTT assay | The P-value greater than 0.05 in all cases indicates that the scaffold is suitable for liver tissue engineering and in vivo tests |
| Alginate, Cellulose, and Gelatin in Bone Tissue Engineering [80] | hBMSC (Human bone marrow stromal cells) | WST-1 assay | No evidence of side effects after the cell seeding in the scaffold revealed its biocompatibility, and rapid bone regeneration was observed in the in vivo model three weeks after transplantation |
| Alginate in Skin Tissue Engineering [81] | Fibroblast L929 cell line | MTT assay | The resulting scaffold showed good cell adhesion based on cell concentration in the scaffold as well as the assessment of cell growth |
| Alginate in Cartilage Tissue Engineering [82] | AMSC (Mesenchymal stem cells derived from adipose tissue) | MTT assay | Alginate helps AMSC for chondrogenesis differentiation without any aid of exogenous differential agents |
| Alginate in Nerve Tissue Engineering [83] | Olfactory ecto-mesenchymal stem cells | Resazurin assay and live/dead viability assay | From the live/dead viability assay, hydrogels with 5 µm and 25 µm magnetic short fibers (MSF) and alginate ease neural-like cell proliferation. From the Resazurin assay, the cell proliferation is higher in MSF-containing hydrogel than in pure alginate |
| Hyaluronic Acid in Skin Tissue Engineering [84] | HDF (Human dermal fibroblast) | MTT assay | Enhanced cell proliferation was seen on the nanocomposite scaffold along with cell viability. Not only the cell proliferation but also the drug delivery was exhibited by the MTT assay |
| Hyaluronic Acid in Neural Tissue Engineering [85] | SH–SY5Y (Human neuroblastoma cell line) | MTT assay | The rate of SH–SY5Y proliferation is accelerated by the optimal amount of hyaluronic acid |
| Starch in Bone Tissue Engineering [86] | MG-63 (Human osteoblast cells) | MTT assay | Cell viability of all samples was found to be greater than 94%, which shows good cytocompatibility |
| Collagen in Cartilage Tissue Engineering [87] | Articular chondrocytes from new-born Sprague Dawley (SD) rats | Live/dead cell viability assay | The proportion of live cells in the collagen and sodium alginate scaffold was found to be greater than in the sodium alginate and agarose scaffold |
| Collagen in Corneal Tissue Engineering [88] | hBM-MSCs (Human bone marrow mesenchymal stem cells) | Cell Counting Kit-8 assay | Hydrogel combined with gelatin and collagen showed increased cell viability and proliferation with time than gelatin hydrogel |
| Collagen in Bone Tissue Engineering [89] | MC3T3-E1 cells from mice | Cell Counting Kit-8 assay | Collagen I proteins are relatively expressed at a higher level, promoting cell differentiation. The constructed porous microsphere had excellent biocompatibility and effectively enhanced cell adhesion, proliferation, and differentiation |
| Collagen in Skin Tissue Engineering [90] | ATCCR PCS-201-012 (Normal adult human dermal fibroblasts) and ATCCR PCS-200-011 (Normal primary human adult epidermal keratinocytes) | MTT assay | The scaffold made up of collagen and elastin promotes cell adhesion and proliferation |
| Collagen in Oral Mucosa Tissue Engineering [91] | Human primary oral fibroblast and keratinocyte cells | PrestoBlue assay | The comparative study revealed that the biological properties of the collagen-based hydrogel are superior to gelatin methacryloyl in terms of growth of oral fibroblast within the scaffold and epithelial cell differentiation and adhesion on the engineered substrate surface |
| Fibroin in Bone Tissue Engineering [92] | HADMSC (Human adipose-derived mesenchymal stem cells) | Live/dead assay and MTT assay | The MTT assay revealed that PRP (platelet-rich plasma)-treated composite scaffold showed a greater cell proliferation rate than untreated scaffold. The live/dead assay revealed that cells were active after day 14 on both PRP-treated and untreated scaffolds |
| Fibroin in Skin Tissue Engineering [93] | L929 cells | Cell Counting Kit-8 assay | In the total of 7 days, the cell proliferation rate was found to be lowest on day 3, and the cell proliferation rate increased significantly on days 5 and 7 |
| Fibroin in Cartilage Tissue Engineering [94] | Human chondrocyte | Cell Counting Kit-8 assay and Live/dead assay | The CCK-8 assay revealed that significant cell growth was noticed from 7–14 days. From the live/dead assay, the cell viability was detected from 5–14 days |
| Fibroin in Corneal Tissue Engineering [95] | The limbal cells (Isolated from corneal limbus) | MTT assay | Vigorous cell adhesion and proliferation were seen on the surface of the scaffold |
| Fibroin in Musculoskeletal System Tissue Engineering [96] | Bone marrow-derived mesenchymal stem cells from rabbit | Live/dead assay | Silk fibroin/pullulan hydrogels contain 90% live cells after seven days of culture, which explicitly shows its good cytocompatibility |
| Fibroin in Neural Tissue Engineering [97] | SH-SY5Y (Human neuroblastoma cell line) | Cell Counting Kit-8 assay | Silk fibroin scaffold showed good cell survival with an increased number over time |
| Keratin in Bone Tissue Engineering [98] | MG-63 cells | MTT assay | Hydroxyapatite-containing scaffold showed higher cell viability |
| Keratin in Nerve Tissue Engineering [99] | L929 mouse lung fibroblasts, human skin fibroblasts, human Schwann cells, and human pulmonary microvascular endothelial cells | MTS assay & Alamar Blue assay | Cells seeded on keratin combined chitosan membrane showed more significant cell adhesion and metabolic activity than plain chitosan membrane |
| Keratin in Vascular Tissue Engineering [100] | HUVEC (Human umbilical vein endothelial cells) and HUASMC (Human umbilical arterial smooth muscle cells) | MTT assay | The developed mat had good biocompatibility, including prolonged activated partial thromboplastin time (APTT), cytocompatibility, and lower platelet adhesion. Moreover, these mats could speed up the nitric oxide generation from the donor in the blood, which accelerates endothelial cell growth, reduces smooth muscle cell proliferation, and inhibits platelet adhesion |
| Keratin in Skin Tissue Engineering [101] | L929 cells from mouse fibroblast | MTT assay | In vitro studies revealed cell adhesion and proliferation, whereas in vivo studies revealed wound healing |
| Keratin in Urethral Tissue Engineering [102] | Smooth muscle cells from rabbit | Live/dead assay | Scaffold containing calcium peroxide (CPO) displayed greater cell viability (92%–94%) than scaffold without CPO (88%–93%) |
| Elastin in Cardiac Tissue Engineering [103] | Cardiac progenitor cells from rats | Dil Cell Labeling | Quantitative evaluation of Dil-labelled cells occupying a fractional area after 72 h of seeding in the scaffold confirms the cell viability by in vitro studies. From the detection of immunofluorescence in the myocardium, after ten days of implant, the cell viability by in vivo study is revealed |
| Elastin in Vascular Tissue Engineering [104] | hAd-MSCs (Human adipose-derived mesenchymal stem cells) | MTT assay | Cell viability and proliferation were confirmed by the MTT assay, and reverse transcription-polymerase chain reaction ensures cell differentiation |
| Elastin in Cartilage Tissue Engineering [105] | Chondrocytes (Cartilaginous tissues of the bovine knee from calves) | XTT (2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) | During implantation, the surface of the scaffold affected the cell, resulting in decreased cell activity. Then, better cell proliferation was seen after the surface modification with elastin and other materials |
| Gelatin in Bone Tissue Engineering [106] | MC3T3-E1 osteoblasts | Histology assay | The biocompatibility of the scaffold was determined by comparing it with Gelfoam. The cell number on the gelatin scaffold is significantly higher than on Gelfoam |
| Gelatin in Skin Tissue Engineering [107] | HSF (Human skin fibroblast) | MTT assay | Cells activity was not affected by the scaffold material, and it was well-suited for cell proliferation and adhesion |
| Gelatin in Cartilage Tissue Engineering [108] | Articular cartilage progenitor cell line | Resazurin assay | Not only the hydrophilic character of gelatin but also the presence of Arginylglycylaspartic acid (RGD)—a cell recognition domain, in its structure facilitates cell attachment |
| Gelatin in Nerve Tissue Engineering [109] | L929 cells from mouse fibroblast | MTT assay | Axons and neuronal dendrites formed on day 14 confirm cell differentiation along with cell viability and proliferation |
| Fibrin in Cartilage Tissue Engineering [110] | Human hyaline-derived chondrocytes | WST-1 assay | An increase in cellular metabolic activity with time, along with a decrease in the biomaterial volume |
| Fibrin in Liver Tissue Engineering [111] | HepG2 cell lines | MTT assay | From the MTT assay, the quantitative assessment of cell viability was found to be 86.75 ± 1.7% |
| Fibrin in Retinal Tissue Engineering [112] | ahRPE cells (Adult human retinal pigment epithelial cells) | MTT assay | Proper ahRPE cell encapsulation was done by a 84 mg/dL concentration of fibrin glue |
| Fibrin in Neural Tissue Engineering [113] | hEnSC (Human endometrial stem cells) | MTT assay | Novel hydrogel fabricated with fibrin, polyurethane, and multiwall carbon nanotube showed more significant cell viability and proliferation than fibrin |
| Polysaccharide | Advantages | Disadvantages | Applications |
|---|---|---|---|
| Cellulose [132,133,134,135,136] | Excellent bioactivity and biocompatibility, having high mechanical properties, depends on the chosen source | Non biodegradability | Bone, tendons, cartilage, cardiovascular, muscle, neural, and skin |
| Chitosan [137,138,139,140,141,142,143] | Easy digestion, biocompatibility, biodegradability, antibacterial activity, and hemostatic activity | Low mechanical resistance, stiff and brittle | Bone, cartilage, skin, cardiac, muscle, liver, and nervous tissue engineering |
| Alginate [144,145,146,147,148] | Bioactivity, biocompatibility, biodegradability, non-immunogenicity, and non-antigenicity | Toughness, limited strength, and difficulty in controlled gelation | Bone, cartilage, Skin, and neural regeneration |
| Hyaluronic acid [149,150,151,152,153,154,155,156] | Bioactivity, biocompatibility, biodegradability, and easy chemical modification | Rapid degradation and poor mechanical properties | Skin and neural regeneration |
| Starch [124,157,158] | Biocompatibility, biodegradability, cheap, pertinent porosity | Low mechanical strength, high water uptake, difficult to process, and unstable in long-term application | Bone cement in bone defects and dental cavities |
| Protein and References | Advantages | Disadvantages | Applications |
|---|---|---|---|
| Collagen [46,150,191,192,193,194,195] | Bioactive, biocompatible, biodegradable, poorly immunogenic, and mimics ECM | Poor mechanical properties | Bone, skin, dental, cornea, vascular, and cartilage regeneration |
| Fibroin [196,197,198,199,200,201] | Bioactivity, biocompatibility, biodegradability, low immunogenic, good mechanical properties, high tensile strength, excellent structural integrity, water-based processing, and cheap | Weak and brittle as a scaffold | Bone, skin, vascular, cartilage, tendon, hepatic, cornea, and neural regeneration, and musculoskeletal tissue engineering |
| Keratin [100,171,174] | Biocompatibility, biodegradability, mechanical durability | Poor mechanical properties and brittle | Skin, bone, and nerve regeneration, urinary tract and vascular tissue engineering |
| Elastin [202,203,204,205,206,207,208] | Bioactivity, biocompatibility, good biomechanical and biophysical properties | Difficult in sourcing, water-insoluble, difficult to manipulate in vitro, risk of contamination and inflammation | Cartilage, skin, tendon, and cardiovascular regeneration |
| Gelatin [209,210,211,212,213,214] | Bioactive, biocompatible, biodegradable, ECM mimicking, low immunogenic, water-soluble, and cheap | Poor mechanical properties, low solubility in concentrated aqueous media, and speed enzymatic degradation, | Bone, skin, cartilage, adipose, and neural regeneration |
| Fibrin [215,216,217,218,219] | Biocompatible, biodegradable, low immunogenic, and mimics ECM | Risk of contamination, expensive, poor mechanical properties, and rapid degradation rate | Cartilage, liver, retina, vascular, and neural regeneration |
| S.No | Techniques | Advantages | Disadvantages |
|---|---|---|---|
| 1. | Freeze drying | Capability to do away with high temperatures, applicable in a variety of purposes, and pore size can be manageable to be controlled by changing the freeze-drying method | Long time consumption, high energy consumption, usage of cytotoxic solvents, and irregular pore size |
| 2. | Solvent casting and practical leaching | Cheap and high porosity | Usage of toxic solvents |
| 3. | Gas foaming | Absence of caustic solvents | Poor interconnectivity, low reproducibility, and structural uniformity |
| 4. | Electrospinning | Porosity, control over morphology, and usage of simple equipment | Limited control of pore structure, use of toxic solvents, and many variables involved in the process |
| 5. | Thermal-induced phase separation | Fast, controllable, scalable, and formation of intrinsically interconnected pores | Only used for thermoplastic |
| 6. | Stereolithography | High resolution, fast processing, and smoother surface | Expensive, high temperature, and toxic uncured resin |
| 7. | Selective laser sintering | Fast processing, high resolution, and no support is needed during manufacturing | High temperature, rough surface finish |
| 8. | Fused deposition model | No requirement for solvents and good mechanical properties | Filament requirement and high temperature |
| 9. | Solvent-based extrusion 3D printing method | Applicable to precise control of micron-level scaffold structure, suitable for ceramic and metals too | Temperature extrusion |
| 10. | Bioprinting method | Cheap and higher accuracy | Depends on cell existence |
| Natural Polymer Used and Reference | Fabrication Technique | Cell Type | Applications |
|---|---|---|---|
| Cellulose and starch [240] | Selective laser sintering | - | Drug delivery and tissue engineering |
| Alginate [241] | Freeze drying | MG-63 human osteosarcoma cell line | Bone tissue engineering |
| Chitin and chondroitin sulphate [242] | Freeze drying | Human dermal fibroblast | Skin tissue engineering |
| Hyaluronic acid and collagen [243] | Electrospinning | Schwann cells | Nerve regeneration |
| Collagen [244] | Electrospinning followed by cross-linking | H9C2 cell line from embryonic rat heart tissue | Cardiac cell therapy |
| Chitosan [245] | Stereolithography | Human mesenchymal stem cells | Cartilage tissue engineering |
| Fibrin and chitosan [246] | Electrospinning | MG-63 cell line | Bone tissue engineering |
| Silk fibroin [247] | 3D printing | Human bronchial epithelial cell line (BEAS-2B) | Tracheal epithelial regeneration |
| Gelatin [248] | 3D printing | MG-63 cell line | Hard tissue regeneration |
| Chitosan [249] | Thermal-induced phase separation | Mouse bone marrow stromal cells | Bone tissue engineering |
| Corn starch [250] | Solvent casting and particulate leaching | - | Bone tissue engineering |
| Keratin [251] | Electrospinning | Human umbilical vein endothelial cells | Vascular tissue engineering |
| Silk fibroin [252] | Freeze drying | Human umbilical vein endothelial cells | Skin tissue engineering |
| Elastin [253] | Electrospinning | MG-63 osteosarcoma cell line | Bone tissue engineering |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krishani, M.; Shin, W.Y.; Suhaimi, H.; Sambudi, N.S. Development of Scaffolds from Bio-Based Natural Materials for Tissue Regeneration Applications: A Review. Gels 2023, 9, 100. https://doi.org/10.3390/gels9020100
Krishani M, Shin WY, Suhaimi H, Sambudi NS. Development of Scaffolds from Bio-Based Natural Materials for Tissue Regeneration Applications: A Review. Gels. 2023; 9(2):100. https://doi.org/10.3390/gels9020100
Chicago/Turabian StyleKrishani, Murugiah, Wong Yen Shin, Hazwani Suhaimi, and Nonni Soraya Sambudi. 2023. "Development of Scaffolds from Bio-Based Natural Materials for Tissue Regeneration Applications: A Review" Gels 9, no. 2: 100. https://doi.org/10.3390/gels9020100
APA StyleKrishani, M., Shin, W. Y., Suhaimi, H., & Sambudi, N. S. (2023). Development of Scaffolds from Bio-Based Natural Materials for Tissue Regeneration Applications: A Review. Gels, 9(2), 100. https://doi.org/10.3390/gels9020100







