Injectable Methacrylated Gelatin Hydrogel for Safe Sodium Hypochlorite Delivery in Endodontics
Abstract
:1. Introduction
2. Results and Discussion
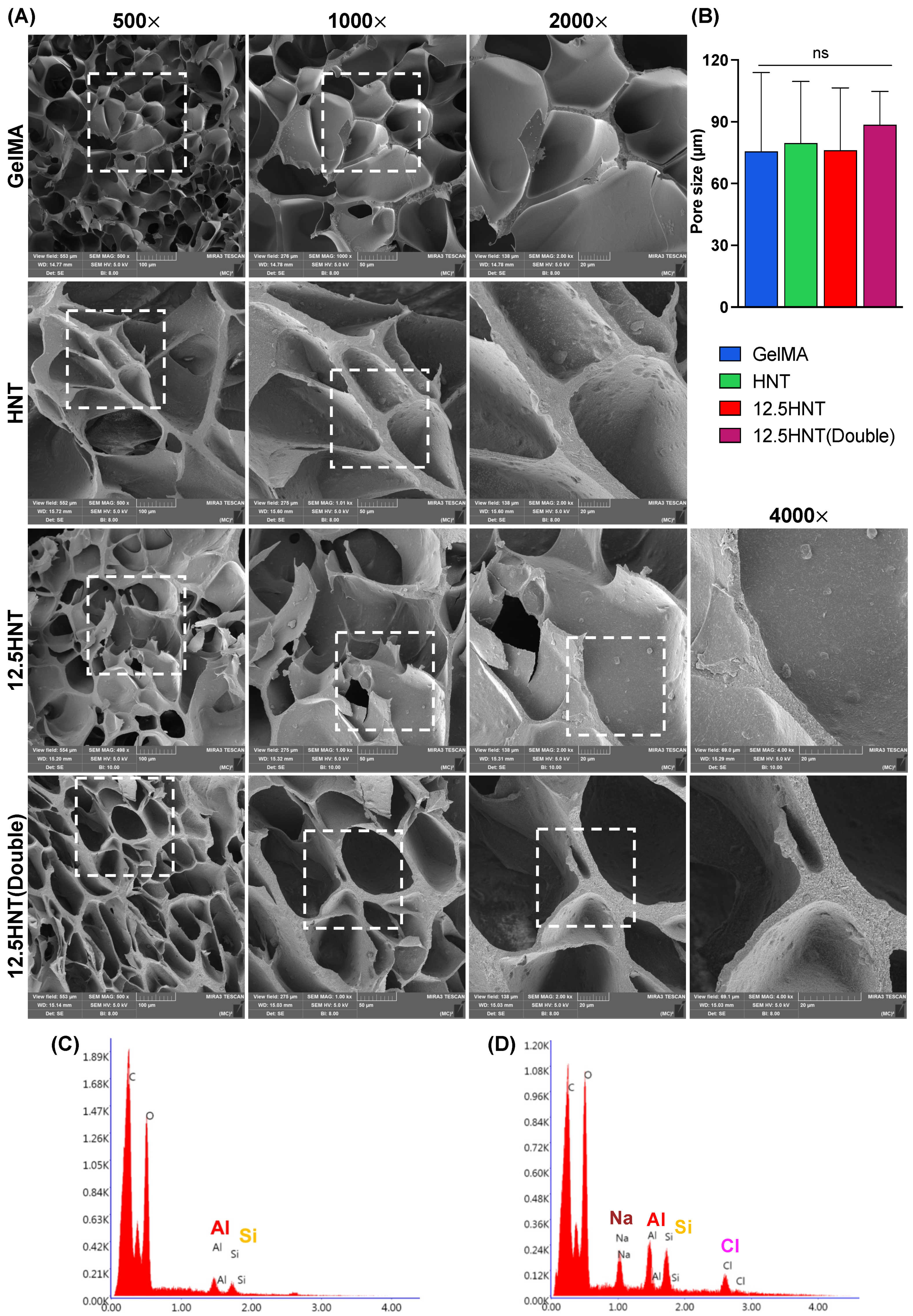
2.1. Chemo-Morphological Characterization
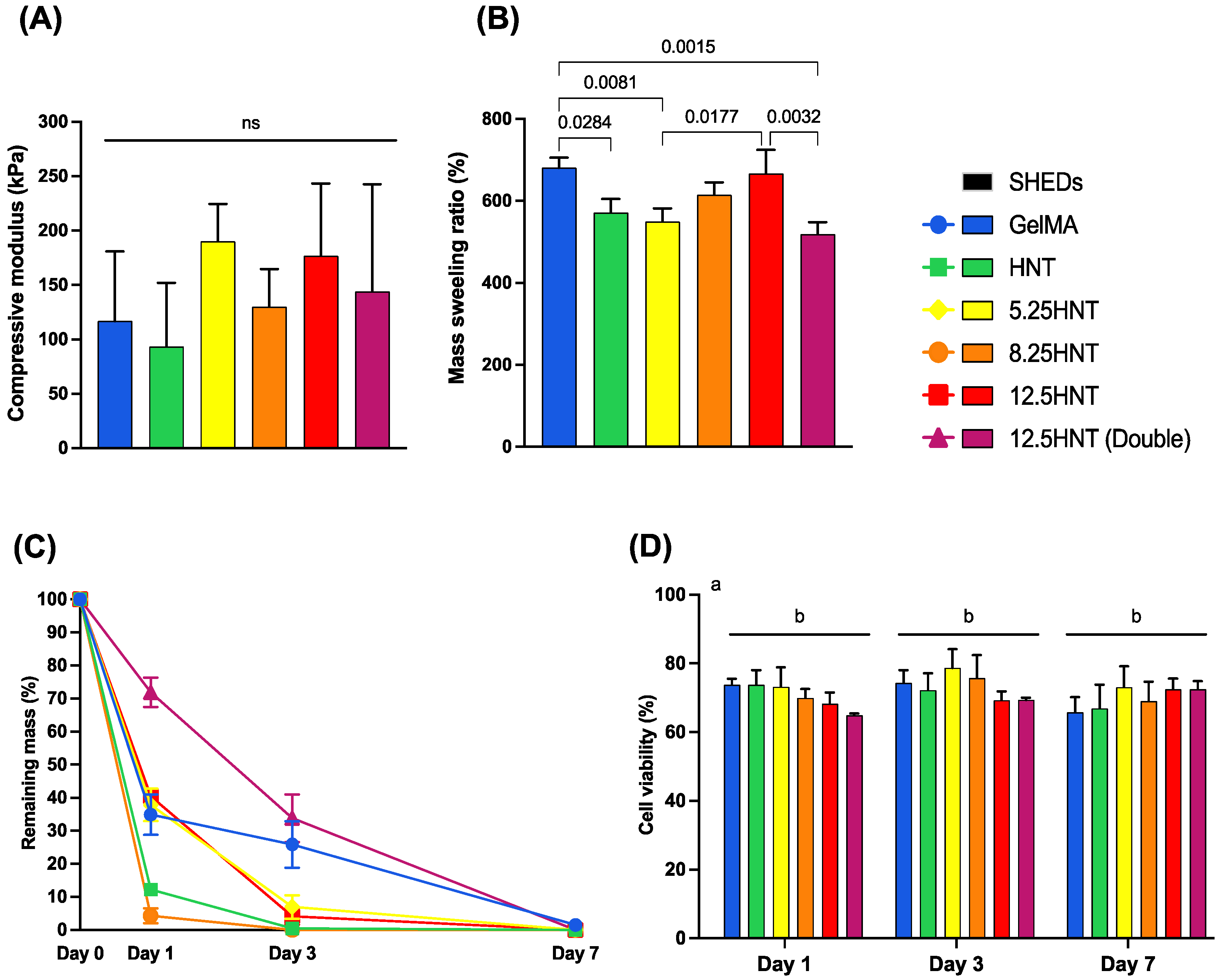
2.2. Mechanical Characterization
2.3. Swelling and Enzymatic Degradation
2.4. Cytocompatibility
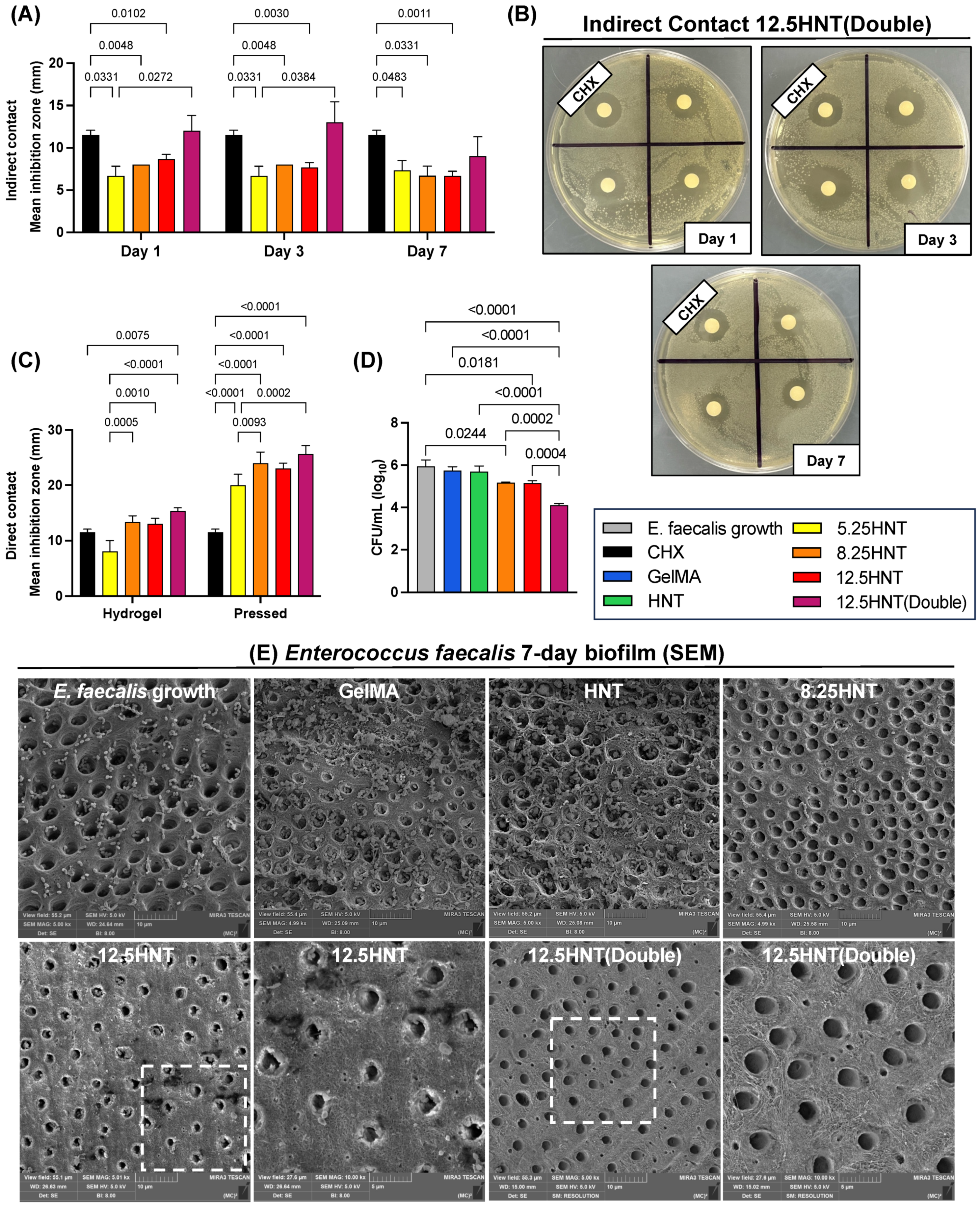
2.5. Antibacterial Properties
2.6. Antibiofilm Properties
2.7. Chlorine Test
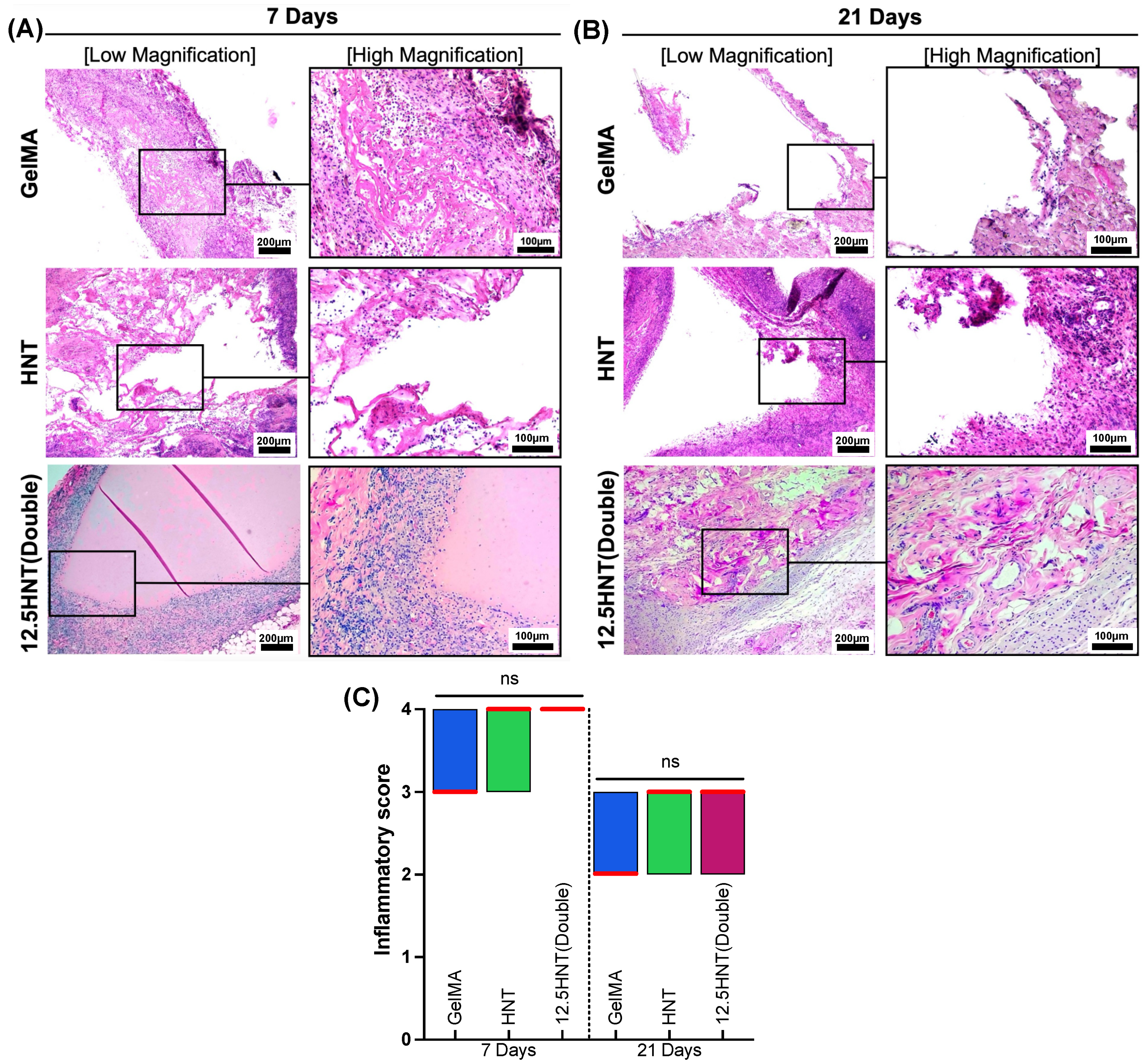
2.8. In Vivo Biocompatibility and Biodegradation
3. Conclusions
4. Materials and Methods
4.1. Chemical and Materials
4.2. NaOCl-HNT Loading
4.3. Gelatin Methacryloyl (GelMA)
4.4. Preparation of NaOCl-HNT-GelMA Hydrogels
4.5. Chemo-Morphological Characterization
4.6. Mechanical Characterization
4.7. Swelling and Enzymatic Degradation
4.8. Cytotoxicity
4.9. Antibacterial Properties
4.10. Antibiofilm Properties
4.11. Chlorine Test
4.12. In Vivo Biocompatibility and Biodegradation
4.13. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Shah, A.; Peacock, R.; Eliyas, S. Pulp therapy and root canal treatment techniques in immature permanent teeth: An update. Br. Dent. J. 2022, 232, 524–530. [Google Scholar] [CrossRef]
- Garcia-Godoy, F.; Murray, P.E. Recommendations for using regenerative endodontic procedures in permanent immature traumatized teeth. Dent. Traumatol. 2012, 28, 33–41. [Google Scholar] [CrossRef]
- Hameed, M.; Gul, M.; Ghafoor, R.; Badar, S. Management of Immature Necrotic Permanent Teeth with Regenerative Endodontic Procedures—A Review of Literature. J. Pak. Med. Assoc. 2019, 69, 1514–1520. [Google Scholar] [CrossRef]
- Tang, J.; Yi, W.; Yan, J.; Chen, Z.; Fan, H.; Zaldivar-Silva, D.; Agüero, L.; Wang, S. Highly absorbent bio-sponge based on carboxymethyl chitosan/poly-γ-glutamic acid/platelet-rich plasma for hemostasis and wound healing. Int. J. Biol. Macromol. 2023, 247, 125754. [Google Scholar] [CrossRef]
- Yang, L.; Wang, X.; Yu, Y.; Shang, L.; Xu, W.; Zhao, Y. Bio-inspired dual-adhesive particles from microfluidic electrospray for bone regeneration. Nano Res. 2023, 16, 5292–5299. [Google Scholar] [CrossRef]
- Duggal, M.; Tong, H.J.; Al-Ansary, M.; Twati, W.; Day, P.F.; Nazzal, H. Interventions for the endodontic management of non-vital traumatised immature permanent anterior teeth in children and adolescents: A systematic review of the evidence and guidelines of the European Academy of Paediatric Dentistry. Eur. Arch. Paediatr. Dent. 2017, 18, 139–151. [Google Scholar] [CrossRef]
- Kim, S.G.; Malek, M.; Sigurdsson, A.; Lin, L.M.; Kahler, B. Regenerative endodontics: A comprehensive review. Int. Endod. J. 2018, 51, 1367–1388. [Google Scholar] [CrossRef]
- Wei, X.; Yang, M.; Yue, L.; Huang, D.; Zhou, X.; Wang, X.; Zhang, Q.; Qiu, L.; Huang, Z.; Wang, H.; et al. Expert consensus on regenerative endodontic procedures. Int. J. Oral Sci. 2022, 14, 55. [Google Scholar] [CrossRef]
- Zehnder, M. Root canal irrigants. J. Endod. 2006, 32, 389–398. [Google Scholar] [CrossRef]
- Haapasalo, M.; Shen, Y.; Wang, Z.; Gao, Y. Irrigation in endodontics. Br. Dent. J. 2014, 216, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Clarke, P.; Jones, A.D.; Jarad, F.; Albadri, S. Technical outcome of root canal treatment on permanent teeth in children: A retrospective study. Eur. Arch. Paediatr. Dent. 2015, 16, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Wikstrom, A.; Brundin, M.; Lopes, M.F.; El Sayed, M.; Tsilingaridis, G. What is the best long-term treatment modality for immature permanent teeth with pulp necrosis and apical periodontitis? Eur. Arch. Paediatr. Dent. 2021, 22, 311–340. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Cui, W. Biomedical application of photo-crosslinked gelatin hydrogels. J. Leather Sci. Eng. 2021, 3, 3. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Bordini, E.A.F.; Ferreira, J.A.; Mei, L.; Dubey, N.; Fenno, J.C.; Piva, E.; Lund, R.G.; Schwendeman, A.; Bottino, M.C. Injectable MMP-Responsive Nanotube-Modified Gelatin Hydrogel for Dental Infection Ablation. ACS Appl. Mater. Interfaces 2020, 12, 16006–16017. [Google Scholar] [CrossRef]
- Vergaro, V.; Lvov, Y.M.; Leporatti, S. Halloysite clay nanotubes for resveratrol delivery to cancer cells. Macromol. Biosci. 2012, 12, 1265–1271. [Google Scholar] [CrossRef]
- Du, Y.; Lo, E.; Ali, S.; Khademhosseini, A. Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs. Proc. Natl. Acad. Sci. USA 2008, 105, 9522–9527. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Kirsch, M.; Birnstein, L.; Pepelanova, I.; Handke, W.; Rach, J.; Seltsam, A.; Scheper, T.; Lavrentieva, A. Gelatin-Methacryloyl (GelMA) Formulated with Human Platelet Lysate Supports Mesenchymal Stem Cell Proliferation and Differentiation and Enhances the Hydrogel’s Mechanical Properties. Bioengineering 2019, 6, 76. [Google Scholar] [CrossRef]
- Namazi, S.S.; Mahmoud, A.H.; Dal-Fabbro, R.; Han, Y.; Xu, J.; Sasaki, H.; Fenno, J.C.; Bottino, M.C. Multifunctional and biodegradable methacrylated gelatin/Aloe vera nanofibers for endodontic disinfection and immunomodulation. Biomater Adv. 2023, 150, 213427. [Google Scholar] [CrossRef]
- Maru, V.; Kb, A.; Madkaikar, M.; Devi, R.S.; Gada, A.; Bapat, S. Assessment of the Influence of Various Concentrations of Sodium Hypochlorite on Stem Cell Derived From Human Exfoliated Deciduous Teeth (SHED) Proliferation and Differentiation. Cureus 2022, 14, e33024. [Google Scholar] [CrossRef] [PubMed]
- Gomes, B.P.; Ferraz, C.C.; Vianna, M.E.; Berber, V.B.; Teixeira, F.B.; Souza-Filho, F.J. In vitro antimicrobial activity of several concentrations of sodium hypochlorite and chlorhexidine gluconate in the elimination of Enterococcus faecalis. Int. Endod. J. 2001, 34, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.N. On the causes of persistent apical periodontitis: A review. Int. Endod. J. 2006, 39, 249–281. [Google Scholar] [CrossRef] [PubMed]
- Luddin, N.; Ahmed, H.M. The antibacterial activity of sodium hypochlorite and chlorhexidine against Enterococcus faecalis: A review on agar diffusion and direct contact methods. J. Conserv. Dent. 2013, 16, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Jagannathan, N.; Neelakantan, P. Antibiofilm efficacy of photoactivated curcumin, triple and double antibiotic paste, 2% chlorhexidine and calcium hydroxide against Enterococcus fecalis in vitro. Sci. Rep. 2016, 6, 24797. [Google Scholar] [CrossRef]
- Neelakantan, P.; Romero, M.; Vera, J.; Daood, U.; Khan, A.U.; Yan, A.; Cheung, G.S.P. Biofilms in Endodontics-Current Status and Future Directions. Int. J. Mol. Sci. 2017, 18, 1748. [Google Scholar] [CrossRef]
- Albuquerque, M.T.; Valera, M.C.; Moreira, C.S.; Bresciani, E.; de Melo, R.M.; Bottino, M.C. Effects of ciprofloxacin-containing scaffolds on enterococcus faecalis biofilms. J. Endod. 2015, 41, 710–714. [Google Scholar] [CrossRef]
- Vianna, M.E.; Gomes, B.P.; Berber, V.B.; Zaia, A.A.; Ferraz, C.C.; de Souza-Filho, F.J. In vitro evaluation of the antimicrobial activity of chlorhexidine and sodium hypochlorite. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 97, 79–84. [Google Scholar] [CrossRef]
- Liu, S.; Zhai, H.; Fu, S.; Cui, C.; Xu, J.; Jiang, J.; Pan, P.; Zhang, B. Evaluation of the cytotoxic effects of sodium hypochlorite on human dental stem cells. Trop. J. Pharm. Res. 2019, 17, 2375–2380. [Google Scholar] [CrossRef]
- Essner, M.D.; Javed, A.; Eleazer, P.D. Effect of sodium hypochlorite on human pulp cells: An in vitro study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 112, 662–666. [Google Scholar] [CrossRef]
- Yang, L.; Fan, L.; Lin, X.; Yu, Y.; Zhao, Y. Pearl Powder Hybrid Bioactive Scaffolds from Microfluidic 3D Printing for Bone Regeneration. Adv. Sci. 2023, e2304190. [Google Scholar] [CrossRef]
- Bordini, E.A.F.; Ferreira, J.A.; Dubey, N.; Ribeiro, J.S.; de Souza Costa, C.A.; Soares, D.G.; Bottino, M.C. Injectable Multifunctional Drug Delivery System for Hard Tissue Regeneration under Inflammatory Microenvironments. ACS Appl. Bio Mater. 2021, 4, 6993–7006. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.H.; Han, Y.; Dal-Fabbro, R.; Daghrery, A.; Xu, J.; Kaigler, D.; Bhaduri, S.B.; Malda, J.; Bottino, M.C. Nanoscale beta-TCP-Laden GelMA/PCL Composite Membrane for Guided Bone Regeneration. ACS Appl. Mater. Interfaces 2023. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]
- Gu, L.; Li, T.; Song, X.; Yang, X.; Li, S.; Chen, L.; Liu, P.; Gong, X.; Chen, C.; Sun, L. Preparation and characterization of methacrylated gelatin/bacterial cellulose composite hydrogels for cartilage tissue engineering. Regen. Biomater. 2019, 7, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, X.; Shi, X.; Wang, Y. Injectable alendronate-functionalized GelMA hydrogels for mineralization and osteogenesis. RSC Adv. 2018, 8, 22764–22776. [Google Scholar] [CrossRef] [PubMed]
- ISO10993-5; Tests for Cytotoxicity—In Vitro Methods. ISO: Geneva, Switzerland, 2022. [CrossRef]
- Rosa, V.; Zhang, Z.; Grande, R.H.; Nör, J.E. Dental pulp tissue engineering in full-length human root canals. J. Dent. Res. 2013, 92, 970–975. [Google Scholar] [CrossRef]
- Cintra, L.T.; Benetti, F.; de Azevedo Queiroz, I.O.; de Araujo Lopes, J.M.; Penha de Oliveira, S.H.; Sivieri Araujo, G.; Gomes-Filho, J.E. Cytotoxicity, Biocompatibility, and Biomineralization of the New High-plasticity MTA Material. J. Endod. 2017, 43, 774–778. [Google Scholar] [CrossRef]
- Gomes-Filho, J.E.; de Azevedo Queiroz, I.O.; Watanabe, S.; da Silva Santos, L.M.; Lodi, C.S.; Okamoto, R.; Ervolino, E.; Dezan, E., Jr.; Cintra, L.T. Influence of diabetes mellitus on tissue response to MTA and its ability to stimulate mineralization. Dent. Traumatol. 2015, 31, 67–72. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dal-Fabbro, R.; Huang, Y.-C.; Toledo, P.T.A.; Capalbo, L.C.; Coleman, R.M.; Sasaki, H.; Fenno, J.C.; Bottino, M.C. Injectable Methacrylated Gelatin Hydrogel for Safe Sodium Hypochlorite Delivery in Endodontics. Gels 2023, 9, 897. https://doi.org/10.3390/gels9110897
Dal-Fabbro R, Huang Y-C, Toledo PTA, Capalbo LC, Coleman RM, Sasaki H, Fenno JC, Bottino MC. Injectable Methacrylated Gelatin Hydrogel for Safe Sodium Hypochlorite Delivery in Endodontics. Gels. 2023; 9(11):897. https://doi.org/10.3390/gels9110897
Chicago/Turabian StyleDal-Fabbro, Renan, Yu-Chi Huang, Priscila T. A. Toledo, Leticia C. Capalbo, Rhima M. Coleman, Hajime Sasaki, J. Christopher Fenno, and Marco C. Bottino. 2023. "Injectable Methacrylated Gelatin Hydrogel for Safe Sodium Hypochlorite Delivery in Endodontics" Gels 9, no. 11: 897. https://doi.org/10.3390/gels9110897
APA StyleDal-Fabbro, R., Huang, Y.-C., Toledo, P. T. A., Capalbo, L. C., Coleman, R. M., Sasaki, H., Fenno, J. C., & Bottino, M. C. (2023). Injectable Methacrylated Gelatin Hydrogel for Safe Sodium Hypochlorite Delivery in Endodontics. Gels, 9(11), 897. https://doi.org/10.3390/gels9110897










