Biopolymers for Tissue Engineering: Crosslinking, Printing Techniques, and Applications
Abstract
:1. Introduction
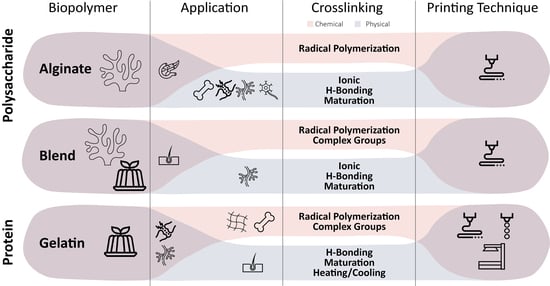
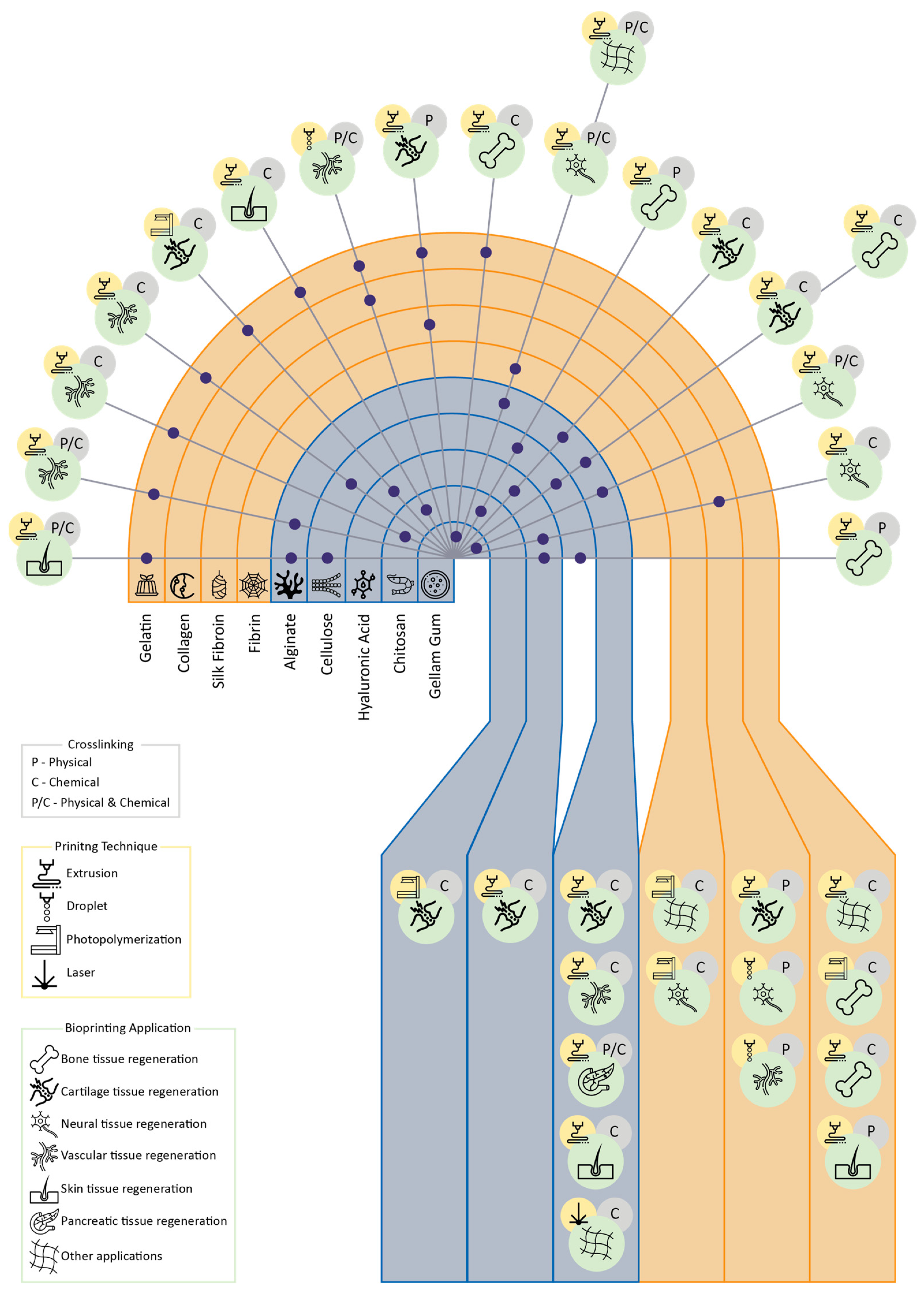
2. Biopolymers for TE-Formulated Bioinks
2.1. Proteins
2.1.1. Collagen
2.1.2. Gelatin
2.1.3. Silk Fibroin
2.1.4. Fibrin
2.1.5. Other Proteins
2.2. Polysaccharides
2.2.1. Alginate
2.2.2. Chitosan
2.2.3. Hyaluronic Acid
2.2.4. Cellulose
2.2.5. Gellan Gum
2.2.6. Other Polysaccharides
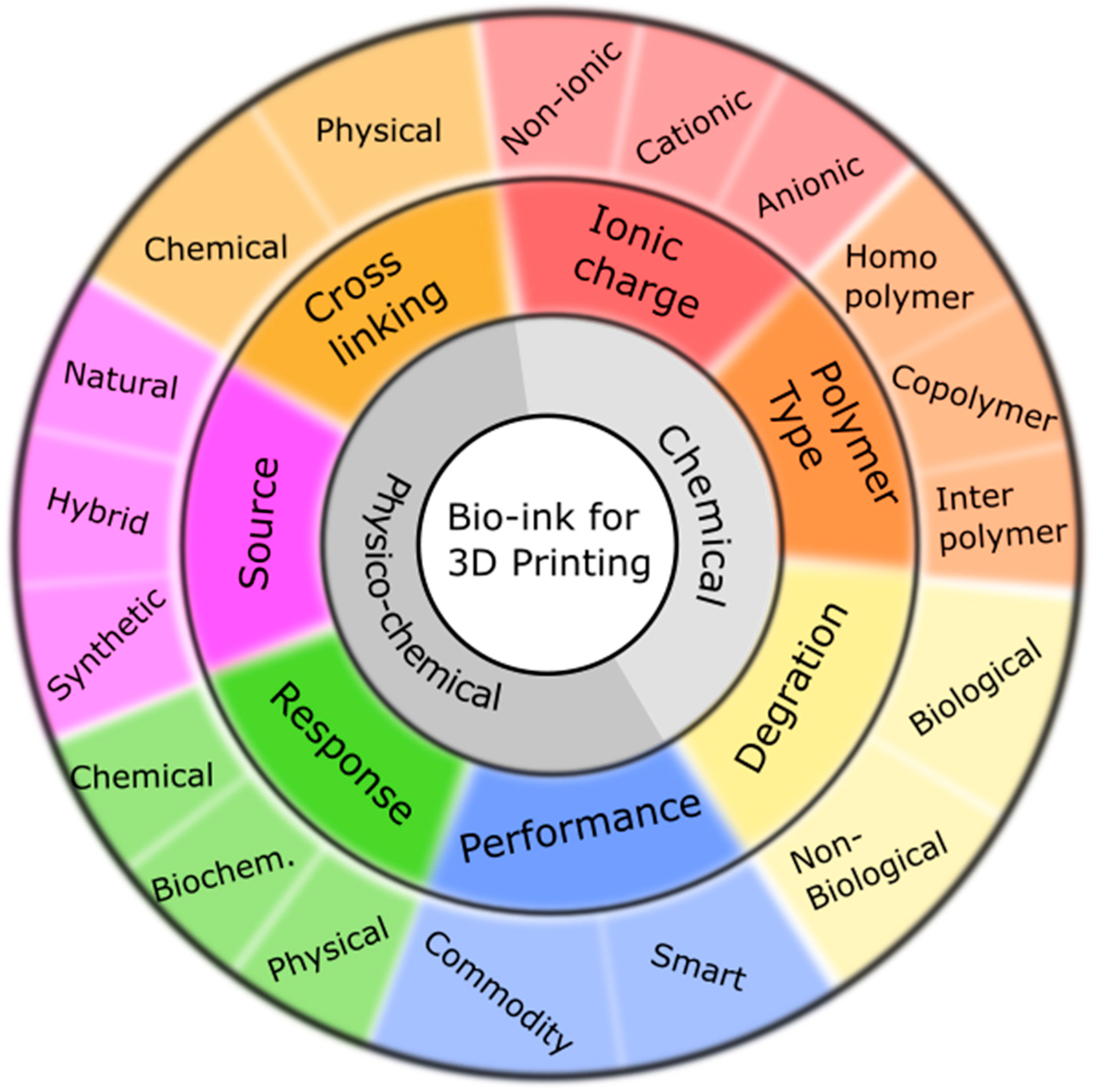
3. Biopolymers Requirements for Bioprinting
4. Crosslinking of Biopolymers
4.1. Physical Crosslinking
4.1.1. Crystallization
4.1.2. Stereocomplex Formation
4.1.3. Heating/Cooling
4.1.4. Hydrogen Bonding
4.1.5. Ionic Interaction
4.1.6. Hydrophobicity
4.1.7. Maturation
4.2. Chemical Crosslinking
4.2.1. Complementary Groups
- Aldehyde, dihydrazide, and Schiff’s base
- Thiol-ene Michael addition
- Condensation
4.2.2. Radical Polymerization
4.2.3. Enzymatic
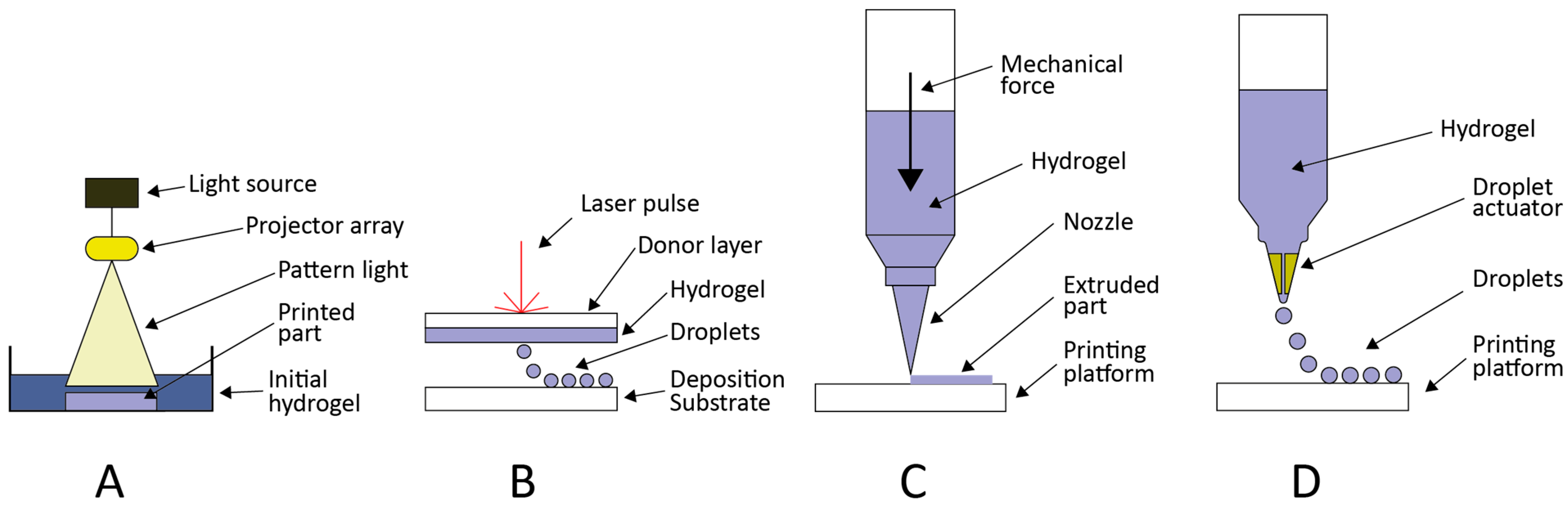
5. Printing Techniques for Biopolymers
5.1. Photopolymerization-Based
5.2. Laser-Based
5.3. Extrusion-Based
5.4. Droplet-Based
6. Discussion
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Viola, J.; Lal, B.; Grad, O. The Emergence of Tissue Engineering as a Research Field; The National Science Foundation Inc.: Alexandria, VA, USA, 2004. [Google Scholar]
- Badylak, S.F.; Tullius, R.; Kokini, K.; Shelbourne, K.D.; Klootwyk, T.; Voytik, S.L.; Kraine, M.R.; Simmons, C. The Use of Xenogeneic Small Intestinal Submucosa as a Biomaterial for Achille’s Tendon Repair in a Dog Model. J. Biomed. Mater. Res. 1995, 29, 977–985. [Google Scholar] [CrossRef]
- Guyette, J.P.; Charest, J.M.; Mills, R.W.; Jank, B.J.; Moser, P.T.; Gilpin, S.E.; Gershlak, J.R.; Okamoto, T.; Gonzalez, G.; Milan, D.J.; et al. Bioengineering Human Myocardium on Native Extracellular Matrix. Circ. Res. 2016, 118, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Jongpaiboonkit, L.; King, W.J.; Lyons, G.E.; Paguirigan, A.L.; Warrick, J.W.; Beebe, D.J.; Murphy, W.L. An Adaptable Hydrogel Array Format for 3-Dimensional Cell Culture and Analysis. Biomaterials 2008, 29, 3346–3356. [Google Scholar] [CrossRef] [PubMed]
- Rane, A.A.; Chuang, J.S.; Shah, A.; Hu, D.P.; Dalton, N.D.; Gu, Y.; Peterson, K.L.; Omens, J.H.; Christman, K.L. Increased Infarct Wall Thickness by a Bio-Inert Material Is Insufficient to Prevent Negative Left Ventricular Remodeling after Myocardial Infarction. PLoS ONE 2011, 6, e21571. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, A.M.; Varkey, M.; Gorkun, A.; Clouse, C.; Xu, L.; Chou, Z.; Murphy, S.V.; Molnar, J.; Lee, S.J.; Yoo, J.J.; et al. Bioprinted Skin Recapitulates Normal Collagen Remodeling in Full-Thickness Wounds. Tissue Eng. Part A 2020, 26, 512–526. [Google Scholar] [CrossRef]
- Yang, H.; Sun, L.; Pang, Y.; Hu, D.; Xu, H.; Mao, S.; Peng, W.; Wang, Y.; Xu, Y.; Zheng, Y.-C.; et al. Three-Dimensional Bioprinted Hepatorganoids Prolong Survival of Mice with Liver Failure. Gut 2021, 70, 567–574. [Google Scholar] [CrossRef]
- Knowlton, S.; Anand, S.; Shah, T.; Tasoglu, S. Bioprinting for Neural Tissue Engineering. Trends Neurosci. 2018, 41, 31–46. [Google Scholar] [CrossRef]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D Bioprinting Technology for Tissue/Organ Regenerative Engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, Y.B.; Ahn, S.H.; Lee, J.-S.; Jang, C.H.; Yoon, H.; Chun, W.; Kim, G.H. A New Approach for Fabricating Collagen/ECM-Based Bioinks Using Preosteoblasts and Human Adipose Stem Cells. Adv. Healthc. Mater. 2015, 4, 1359–1368. [Google Scholar] [CrossRef]
- Loo, Y.; Lakshmanan, A.; Ni, M.; Toh, L.L.; Wang, S.; Hauser, C.A.E. Peptide Bioink: Self-Assembling Nanofibrous Scaffolds for Three-Dimensional Organotypic Cultures. Nano Lett. 2015, 15, 6919–6925. [Google Scholar] [CrossRef]
- Grenier, J.; Duval, H.; Barou, F.; Lv, P.; David, B.; Letourneur, D. Mechanisms of Pore Formation in Hydrogel Scaffolds Textured by Freeze-Drying. Acta Biomater. 2019, 94, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and Bioactive Porous Polymer/Inorganic Composite Scaffolds for Bone Tissue Engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Sparkes, J.; Holland, C. The Rheological Properties of Native Sericin. Acta Biomater. 2018, 69, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Cadano, J.R.; Jose, M.; Lubi, A.G.; Maling, J.N.; Moraga, J.S.; Shi, Q.Y.; Vegafria, H.M.; VinceCruz-Abeledo, C.C. A Comparative Study on the Raw Chitin and Chitosan Yields of Common Bio-Waste from Philippine Seafood. Environ. Sci. Pollut. Res. 2021, 28, 11954–11961. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A. Hydrogels in Medicine and Pharmacy; CRC Press: Boca Raton, FL, USA, 1986; Volume 1. [Google Scholar]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Negrescu, A.-M.; Cimpean, A. The State of the Art and Prospects for Osteoimmunomodulatory Biomaterials. Materials 2021, 14, 1357. [Google Scholar] [CrossRef]
- Rana, D.; Desai, N.; Salave, S.; Karunakaran, B.; Giri, J.; Benival, D.; Gorantla, S.; Kommineni, N. Collagen-Based Hydrogels for the Eye: A Comprehensive Review. Gels 2023, 9, 643. [Google Scholar] [CrossRef]
- Teixeira, M.C.; Lameirinhas, N.S.; Carvalho, J.P.F.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. A Guide to Polysaccharide-Based Hydrogel Bioinks for 3D Bioprinting Applications. Int. J. Mol. Sci. 2022, 23, 6564. [Google Scholar] [CrossRef]
- Cheng, L.; Yao, B.; Hu, T.; Cui, X.; Shu, X.; Tang, S.; Wang, R.; Wang, Y.; Liu, Y.; Song, W.; et al. Properties of an Alginate-Gelatin-Based Bioink and Its Potential Impact on Cell Migration, Proliferation, and Differentiation. Int. J. Biol. Macromol. 2019, 135, 1107–1113. [Google Scholar] [CrossRef]
- Beketov, E.E.; Isaeva, E.V.; Yakovleva, N.D.; Demyashkin, G.A.; Arguchinskaya, N.V.; Kisel, A.A.; Lagoda, T.S.; Malakhov, E.P.; Kharlov, V.I.; Osidak, E.O.; et al. Bioprinting of Cartilage with Bioink Based on High-Concentration Collagen and Chondrocytes. Int. J. Mol. Sci. 2021, 22, 11351. [Google Scholar] [CrossRef]
- Costa, J.B.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L. Fast Setting Silk Fibroin Bioink for Bioprinting of Patient-Specific Memory-Shape Implants. Adv. Healthc. Mater. 2017, 6, 1701021. [Google Scholar] [CrossRef]
- Hong, S.; Sycks, D.; Chan, H.F.; Lin, S.; Lopez, G.P.; Guilak, F.; Leong, K.W.; Zhao, X. 3D Printing of Highly Stretchable and Tough Hydrogels into Complex, Cellularized Structures. Adv. Mater. 2015, 27, 4035–4040. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, S.; Liu, Y.; Yao, B.; Hu, T.; Shi, H.; Xie, J.; Fu, X. Tuning Alginate-Gelatin Bioink Properties by Varying Solvent and Their Impact on Stem Cell Behavior. Sci. Rep. 2018, 8, 8020. [Google Scholar] [CrossRef]
- Osidak, E.O.; Karalkin, P.A.; Osidak, M.S.; Parfenov, V.A.; Sivogrivov, D.E.; Pereira, F.D.A.S.; Gryadunova, A.A.; Koudan, E.V.; Khesuani, Y.D.; Kasyanov, V.A.; et al. Viscoll Collagen Solution as a Novel Bioink for Direct 3D Bioprinting. J. Mater. Sci. Mater. Med. 2019, 30, 31. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Smits, I.P.M.; De La Vega, L.; Lee, C.; Willerth, S.M. 3D Bioprinting Pluripotent Stem Cell Derived Neural Tissues Using a Novel Fibrin Bioink Containing Drug Releasing Microspheres. Front. Bioeng. Biotechnol. 2020, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, C. Fibrin Hydrogels for Endothelialized Liver Tissue Engineering with a Predesigned Vascular Network. Polymers 2018, 10, 1048. [Google Scholar] [CrossRef]
- Yan, W.-C.; Davoodi, P.; Vijayavenkataraman, S.; Tian, Y.; Ng, W.C.; Fuh, J.Y.H.; Robinson, K.S.; Wang, C.-H. 3D Bioprinting of Skin Tissue: From Pre-Processing to Final Product Evaluation. Adv. Drug Deliv. Rev. 2018, 132, 270–295. [Google Scholar] [CrossRef]
- Zheng, Z.; Wu, J.; Liu, M.; Wang, H.; Li, C.; Rodriguez, M.J.; Li, G.; Wang, X.; Kaplan, D.L. 3D Bioprinting of Self-Standing Silk-Based Bioink. Adv. Healthc. Mater. 2018, 7, 1701026. [Google Scholar] [CrossRef]
- Lan, X.; Liang, Y.; Erkut, E.J.N.; Kunze, M.; Mulet-Sierra, A.; Gong, T.; Osswald, M.; Ansari, K.; Seikaly, H.; Boluk, Y.; et al. Bioprinting of Human Nasoseptal Chondrocytes-laden Collagen Hydrogel for Cartilage Tissue Engineering. FASEB J. 2021, 35, 21191. [Google Scholar] [CrossRef]
- Lee, Y.-B.; Polio, S.; Lee, W.; Dai, G.; Menon, L.; Carroll, R.S.; Yoo, S.-S. Bio-Printing of Collagen and VEGF-Releasing Fibrin Gel Scaffolds for Neural Stem Cell Culture. Exp. Neurol. 2010, 223, 645–652. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Hsu, S. Double-Network Polyurethane-Gelatin Hydrogel with Tunable Modulus for High-Resolution 3D Bioprinting. ACS Appl. Mater. Interfaces 2019, 11, 32746–32757. [Google Scholar] [CrossRef] [PubMed]
- Anada, T.; Pan, C.-C.; Stahl, A.; Mori, S.; Fukuda, J.; Suzuki, O.; Yang, Y. Vascularized Bone-Mimetic Hydrogel Constructs by 3D Bioprinting to Promote Osteogenesis and Angiogenesis. Int. J. Mol. Sci. 2019, 20, 1096. [Google Scholar] [CrossRef] [PubMed]
- Leucht, A.; Volz, A.-C.; Rogal, J.; Borchers, K.; Kluger, P.J. Advanced Gelatin-Based Vascularization Bioinks for Extrusion-Based Bioprinting of Vascularized Bone Equivalents. Sci. Rep. 2020, 10, 5330. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Yeon, Y.K.; Lee, J.M.; Chao, J.R.; Lee, Y.J.; Seo, Y.B.; Sultan, M.T.; Lee, O.J.; Lee, J.S.; Yoon, S.; et al. Precisely Printable and Biocompatible Silk Fibroin Bioink for Digital Light Processing 3D Printing. Nat. Commun. 2018, 9, 1620. [Google Scholar] [CrossRef]
- Ajiteru, O.; Sultan, M.T.; Lee, Y.J.; Seo, Y.B.; Hong, H.; Lee, J.S.; Lee, H.; Suh, Y.J.; Ju, H.W.; Lee, O.J.; et al. A 3D Printable Electroconductive Biocomposite Bioink Based on Silk Fibroin-Conjugated Graphene Oxide. Nano Lett. 2020, 20, 6873–6883. [Google Scholar] [CrossRef]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical Applications of Collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.-J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-Dimensional Printing of Complex Biological Structures by Freeform Reversible Embedding of Suspended Hydrogels. Sci. Adv. 2015, 1, 1500758. [Google Scholar] [CrossRef]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D Bioprinting of Collagen to Rebuild Components of the Human Heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef]
- Roth, E.A.; Xu, T.; Das, M.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet Printing for High-Throughput Cell Patterning. Biomaterials 2004, 25, 3707–3715. [Google Scholar] [CrossRef]
- Xu, F.; Moon, S.; Emre, A.E.; Lien, C.; Turali, E.S.; Demirci, U. Cell Bioprinting as a Potential High-Throughput Method for Fabricating Cell-Based Biosensors (CBBs). In Proceedings of the 2009 IEEE Sensors, Christchurch, New Zealand, 25–28 October 2009; IEEE: Piscataway, NJ, USA, 2009; pp. 387–391. [Google Scholar]
- Stepanovska, J.; Supova, M.; Hanzalek, K.; Broz, A.; Matejka, R. Collagen Bioinks for Bioprinting: A Systematic Review of Hydrogel Properties, Bioprinting Parameters, Protocols, and Bioprinted Structure Characteristics. Biomedicines 2021, 9, 1137. [Google Scholar] [CrossRef]
- Lee, V.; Singh, G.; Trasatti, J.P.; Bjornsson, C.; Xu, X.; Tran, T.N.; Yoo, S.-S.; Dai, G.; Karande, P. Design and Fabrication of Human Skin by Three-Dimensional Bioprinting. Tissue Eng. Part C Methods 2014, 20, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; O’Brien, P.J. Structure-Activity Relationships for Hepatocyte Toxicity and Electrophilic Reactivity of α, β-Unsaturated Esters, Acrylates and Methacrylates. J. Appl. Toxicol. 2008, 28, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- LoPachin, R.M.; Gavin, T. Molecular Mechanisms of Aldehyde Toxicity: A Chemical Perspective. Chem. Res. Toxicol. 2014, 27, 1081–1091. [Google Scholar] [CrossRef]
- Liu, C.Z.; Xia, Z.D.; Han, Z.W.; Hulley, P.A.; Triffitt, J.T.; Czernuszka, J.T. Novel 3D Collagen Scaffolds Fabricated by Indirect Printing Technique for Tissue Engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 85B, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D Bioprinting: An Overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef]
- Yoon, H.; Lee, J.-S.; Yim, H.; Kim, G.; Chun, W. Development of Cell-Laden 3D Scaffolds for Efficient Engineered Skin Substitutes by Collagen Gelation. RSC Adv. 2016, 6, 21439–21447. [Google Scholar] [CrossRef]
- Albanna, M.; Binder, K.W.; Murphy, S.V.; Kim, J.; Qasem, S.A.; Zhao, W.; Tan, J.; El-Amin, I.B.; Dice, D.D.; Marco, J.; et al. In Situ Bioprinting of Autologous Skin Cells Accelerates Wound Healing of Extensive Excisional Full-Thickness Wounds. Sci. Rep. 2019, 9, 1856. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Kim, G. Collagen/Bioceramic-Based Composite Bioink to Fabricate a Porous 3D HASCs-Laden Structure for Bone Tissue Regeneration. Biofabrication 2019, 12, 015007. [Google Scholar] [CrossRef]
- Harrington, W.F.; Von Hippel, P.H. The Structure of Collagen And Gelatin. Adv. Protein Chem. 1962, 16, 1–138. [Google Scholar]
- Liu, D.; Nikoo, M.; Boran, G.; Zhou, P.; Regenstein, J.M. Collagen and Gelatin. Annu. Rev. Food Sci. Technol. 2015, 6, 527–557. [Google Scholar] [CrossRef]
- Singh, S.; Prakash, C.; Ramakrishna, S. Additive Manufacturing; World Scientific: Singapore, 2020; ISBN 978-981-12-2481-2. [Google Scholar]
- Li, D.; Sun, H.; Jiang, L.; Zhang, K.; Liu, W.; Zhu, Y.; Fangteng, J.; Shi, C.; Zhao, L.; Sun, H.; et al. Enhanced Biocompatibility of PLGA Nanofibers with Gelatin/Nano-Hydroxyapatite Bone Biomimetics Incorporation. ACS Appl. Mater. Interfaces 2014, 6, 9402–9410. [Google Scholar] [CrossRef] [PubMed]
- Pezeshki-Modaress, M.; Zandi, M.; Mirzadeh, H. Fabrication of Gelatin/Chitosan Nanofibrous Scaffold: Process Optimization and Empirical Modeling. Polym. Int. 2015, 64, 571–580. [Google Scholar] [CrossRef]
- Wu, S.; Deng, L.; Hsia, H.; Xu, K.; He, Y.; Huang, Q.; Peng, Y.; Zhou, Z.; Peng, C. Evaluation of Gelatin-Hyaluronic Acid Composite Hydrogels for Accelerating Wound Healing. J. Biomater. Appl. 2017, 31, 1380–1390. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.B.; Moriarty, R.A.; Kamalitdinov, T.; Etheridge, J.M.; Fisher, J.P. Collagen Hydrogel Scaffold Promotes Mesenchymal Stem Cell and Endothelial Cell Coculture for Bone Tissue Engineering. J. Biomed. Mater. Res. A 2017, 105, 1123–1131. [Google Scholar] [CrossRef]
- Claaßen, C.; Claaßen, M.H.; Truffault, V.; Sewald, L.; Tovar, G.E.M.; Borchers, K.; Southan, A. Quantification of Substitution of Gelatin Methacryloyl: Best Practice and Current Pitfalls. Biomacromolecules 2018, 19, 42–52. [Google Scholar] [CrossRef]
- Hoch, E.; Hirth, T.; Tovar, G.E.M.; Borchers, K. Chemical Tailoring of Gelatin to Adjust Its Chemical and Physical Properties for Functional Bioprinting. J. Mater. Chem. B 2013, 1, 5675. [Google Scholar] [CrossRef]
- Yin, J.; Yan, M.; Wang, Y.; Fu, J.; Suo, H. 3D Bioprinting of Low-Concentration Cell-Laden Gelatin Methacrylate (GelMA) Bioinks with a Two-Step Cross-Linking Strategy. ACS Appl. Mater. Interfaces 2018, 10, 6849–6857. [Google Scholar] [CrossRef]
- Kara Özenler, A.; Distler, T.; Tihminlioglu, F.; Boccaccini, A.R. Fish Scale Containing Alginate Dialdehyde-Gelatin Bioink for Bone Tissue Engineering. Biofabrication 2023, 15, 025012. [Google Scholar] [CrossRef]
- Semba, J.A.; Mieloch, A.A.; Tomaszewska, E.; Cywoniuk, P.; Rybka, J.D. Formulation and Evaluation of a Bioink Composed of Alginate, Gelatin, and Nanocellulose for Meniscal Tissue Engineering. Int. J. Bioprinting 2022, 9, 621. [Google Scholar] [CrossRef]
- Shi, W.; Fang, F.; Kong, Y.; Greer, S.E.; Kuss, M.; Liu, B.; Xue, W.; Jiang, X.; Lovell, P.; Mohs, A.M.; et al. Dynamic Hyaluronic Acid Hydrogel with Covalent Linked Gelatin as an Anti-Oxidative Bioink for Cartilage Tissue Engineering. Biofabrication 2022, 14, 014107. [Google Scholar] [CrossRef]
- Song, W.; Muthana, M.; Mukherjee, J.; Falconer, R.J.; Biggs, C.A.; Zhao, X. Magnetic-Silk Core–Shell Nanoparticles as Potential Carriers for Targeted Delivery of Curcumin into Human Breast Cancer Cells. ACS Biomater. Sci. Eng. 2017, 3, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, Y.; Shao, H.; Hu, X. Hybrid Silk Fibers Dry-Spun from Regenerated Silk Fibroin/Graphene Oxide Aqueous Solutions. ACS Appl. Mater. Interfaces 2016, 8, 3349–3358. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Kaplan, D.L.; Omenetto, F.G. Silk Materials—A Road to Sustainable High Technology. Adv. Mater. 2012, 24, 2824–2837. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Marelli, B.; Yang, M.; An, B.; Onses, M.S.; Rogers, J.A.; Kaplan, D.L.; Omenetto, F.G. Inkjet Printing of Regenerated Silk Fibroin: From Printable Forms to Printable Functions. Adv. Mater. 2015, 27, 4273–4279. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.J.; Lee, J.M.; Kim, J.H.; Kim, J.; Kweon, H.; Jo, Y.Y.; Park, C.H. Biodegradation Behavior of Silk Fibroin Membranes in Repairing Tympanic Membrane Perforations. J. Biomed. Mater. Res. A 2012, 100A, 2018–2026. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, O.J.; Lee, M.C.; Moon, B.M.; Ju, H.W.; Lee, J.M.; Kim, J.-H.; Kim, D.W.; Park, C.H. Fabrication of 3D Porous Silk Scaffolds by Particulate (Salt/Sucrose) Leaching for Bone Tissue Reconstruction. Int. J. Biol. Macromol. 2015, 78, 215–223. [Google Scholar] [CrossRef]
- Park, W.H.; Kim, M.H. Chemically Cross-Linked Silk Fibroin Hydrogel with Enhanced Elastic Properties, Biodegradability, and Biocompatibility. Int. J. Nanomed. 2016, 11, 2967. [Google Scholar] [CrossRef]
- Yang, J.; Li, Z.; Li, S.; Zhang, Q.; Zhou, X.; He, C. Tunable Metacrylated Silk Fibroin-Based Hybrid Bioinks for the Bioprinting of Tissue Engineering Scaffolds. Biomater. Sci. 2023, 11, 1895–1909. [Google Scholar] [CrossRef]
- Mohammadpour, Z.; Kharaziha, M.; Zarrabi, A. 3D-Printing of Silk Nanofibrils Reinforced Alginate for Soft Tissue Engineering. Pharmaceutics 2023, 15, 763. [Google Scholar] [CrossRef]
- Rodriguez, M.J.; Brown, J.; Giordano, J.; Lin, S.J.; Omenetto, F.G.; Kaplan, D.L. Silk Based Bioinks for Soft Tissue Reconstruction Using 3-Dimensional (3D) Printing with in Vitro and in Vivo Assessments. Biomaterials 2017, 117, 105–115. [Google Scholar] [CrossRef]
- Singh, Y.P.; Bandyopadhyay, A.; Mandal, B.B. 3D Bioprinting Using Cross-Linker-Free Silk–Gelatin Bioink for Cartilage Tissue Engineering. ACS Appl. Mater. Interfaces 2019, 11, 33684–33696. [Google Scholar] [CrossRef] [PubMed]
- Undas, A.; Ariëns, R.A.S. Fibrin Clot Structure and Function. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 88–99. [Google Scholar] [CrossRef] [PubMed]
- de Melo, B.A.G.; Jodat, Y.A.; Cruz, E.M.; Benincasa, J.C.; Shin, S.R.; Porcionatto, M.A. Strategies to Use Fibrinogen as Bioink for 3D Bioprinting Fibrin-Based Soft and Hard Tissues. Acta Biomater. 2020, 117, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Budharaju, H.; Sundaramurthi, D.; Sethuraman, S. Efficient Dual Crosslinking of Protein–in–Polysaccharide Bioink for Biofabrication of Cardiac Tissue Constructs. Biomater. Adv. 2023, 152, 213486. [Google Scholar] [CrossRef]
- Kotlarz, M.; Ferreira, A.M.; Gentile, P.; Russell, S.J.; Dalgarno, K. Droplet-Based Bioprinting Enables the Fabrication of Cell–Hydrogel–Microfibre Composite Tissue Precursors. Bio-Des. Manuf. 2022, 5, 512–528. [Google Scholar] [CrossRef]
- de Melo, B.A.G.; Jodat, Y.A.; Mehrotra, S.; Calabrese, M.A.; Kamperman, T.; Mandal, B.B.; Santana, M.H.A.; Alsberg, E.; Leijten, J.; Shin, S.R. 3D Printed Cartilage-Like Tissue Constructs with Spatially Controlled Mechanical Properties. Adv. Funct. Mater. 2019, 29, 1906330. [Google Scholar] [CrossRef]
- Anil Kumar, S.; Alonzo, M.; Allen, S.C.; Abelseth, L.; Thakur, V.; Akimoto, J.; Ito, Y.; Willerth, S.M.; Suggs, L.; Chattopadhyay, M.; et al. A Visible Light-Cross-Linkable, Fibrin–Gelatin-Based Bioprinted Construct with Human Cardiomyocytes and Fibroblasts. ACS Biomater. Sci. Eng. 2019, 5, 4551–4563. [Google Scholar] [CrossRef]
- Rutz, A.L.; Hyland, K.E.; Jakus, A.E.; Burghardt, W.R.; Shah, R.N. A Multimaterial Bioink Method for 3D Printing Tunable, Cell-Compatible Hydrogels. Adv. Mater. 2015, 27, 1607–1614. [Google Scholar] [CrossRef]
- de la Vega, L.; Gómez, D.A.R.; Abelseth, E.; Abelseth, L.; Allisson da Silva, V.; Willerth, S. 3D Bioprinting Human Induced Pluripotent Stem Cell-Derived Neural Tissues Using a Novel Lab-on-a-Printer Technology. Appl. Sci. 2018, 8, 2414. [Google Scholar] [CrossRef]
- Keane, T.J.; Swinehart, I.T.; Badylak, S.F. Methods of Tissue Decellularization Used for Preparation of Biologic Scaffolds and in Vivo Relevance. Methods 2015, 84, 25–34. [Google Scholar] [CrossRef]
- Dzobo, K.; Motaung, K.S.C.M.; Adesida, A. Recent Trends in Decellularized Extracellular Matrix Bioinks for 3D Printing: An Updated Review. Int. J. Mol. Sci. 2019, 20, 4628. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Singh, N.K.; Kim, J.J.; Kim, H.; Kim, B.S.; Park, J.Y.; Jang, J.; Cho, D.-W. Directed Differential Behaviors of Multipotent Adult Stem Cells from Decellularized Tissue/Organ Extracellular Matrix Bioinks. Biomaterials 2019, 224, 119496. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Jang, J.; Ha, D.-H.; Won Kim, S.; Rhie, J.-W.; Shim, J.-H.; Kim, D.-H.; Cho, D.-W. Printing Three-Dimensional Tissue Analogues with Decellularized Extracellular Matrix Bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef]
- Kim, B.S.; Kwon, Y.W.; Kong, J.-S.; Park, G.T.; Gao, G.; Han, W.; Kim, M.-B.; Lee, H.; Kim, J.H.; Cho, D.-W. 3D Cell Printing of in Vitro Stabilized Skin Model and in Vivo Pre-Vascularized Skin Patch Using Tissue-Specific Extracellular Matrix Bioink: A Step towards Advanced Skin Tissue Engineering. Biomaterials 2018, 168, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, A.M.; Chou, Z.; Gillispie, G.; Lee, S.J.; Yoo, J.J.; Soker, S.; Atala, A. Decellularized Skin Extracellular Matrix (DsECM) Improves the Physical and Biological Properties of Fibrinogen Hydrogel for Skin Bioprinting Applications. Nanomaterials 2020, 10, 1484. [Google Scholar] [CrossRef]
- Jang, J.; Kim, T.G.; Kim, B.S.; Kim, S.-W.; Kwon, S.-M.; Cho, D.-W. Tailoring Mechanical Properties of Decellularized Extracellular Matrix Bioink by Vitamin B2-Induced Photo-Crosslinking. Acta Biomater. 2016, 33, 88–95. [Google Scholar] [CrossRef]
- Jang, J.; Park, H.-J.; Kim, S.-W.; Kim, H.; Park, J.Y.; Na, S.J.; Kim, H.J.; Park, M.N.; Choi, S.H.; Park, S.H.; et al. 3D Printed Complex Tissue Construct Using Stem Cell-Laden Decellularized Extracellular Matrix Bioinks for Cardiac Repair. Biomaterials 2017, 112, 264–274. [Google Scholar] [CrossRef]
- Hiller, T.; Berg, J.; Elomaa, L.; Röhrs, V.; Ullah, I.; Schaar, K.; Dietrich, A.-C.; Al-Zeer, M.; Kurtz, A.; Hocke, A.; et al. Generation of a 3D Liver Model Comprising Human Extracellular Matrix in an Alginate/Gelatin-Based Bioink by Extrusion Bioprinting for Infection and Transduction Studies. Int. J. Mol. Sci. 2018, 19, 3129. [Google Scholar] [CrossRef]
- Nerger, B.A.; Brun, P.-T.; Nelson, C.M. Microextrusion Printing Cell-Laden Networks of Type I Collagen with Patterned Fiber Alignment and Geometry. Soft Matter 2019, 15, 5728–5738. [Google Scholar] [CrossRef]
- Duarte, A.C.; Costa, E.C.; Filipe, H.A.L.; Saraiva, S.M.; Jacinto, T.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P. Animal-Derived Products in Science and Current Alternatives. Biomater. Adv. 2023, 151, 213428. [Google Scholar] [CrossRef]
- Li, Y.; Cao, X.; Deng, W.; Yu, Q.; Sun, C.; Ma, P.; Shao, F.; Yusif, M.M.; Ge, Z.; Wang, K.; et al. 3D Printable Sodium Alginate-Matrigel (SA-MA) Hydrogel Facilitated Ectomesenchymal Stem Cells (EMSCs) Neuron Differentiation. J. Biomater. Appl. 2021, 35, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Piou, M.; Darling, E.; Cormier, D.; Sun, J.; Wan, J. Bio-Printing Cell-Laden Matrigel–Agarose Constructs. J. Biomater. Appl. 2016, 31, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.E.; Hamid, Q.; Wang, C.; Chang, R.; Emami, K.; Wu, H.; Sun, W. Bioprinting Cell-Laden Matrigel for Radioprotection Study of Liver by pro-Drug Conversion in a Dual-Tissue Microfluidic Chip. Biofabrication 2011, 3, 034112. [Google Scholar] [CrossRef] [PubMed]
- Maity, M.; Hasnain, M.S.; Nayak, A.K.; Aminabhavi, T.M. Biomedical Applications of Polysaccharides. In Tailor-Made Polysaccharides in Biomedical Applications; Nayak, A., Hasnain, M.S.T., Eds.; Elsevier: Boston, MA, USA, 2020; pp. 1–34. [Google Scholar]
- Souza, P.R.; de Oliveira, A.C.; Vilsinski, B.H.; Kipper, M.J.; Martins, A.F. Polysaccharide-Based Materials Created by Physical Processes: From Preparation to Biomedical Applications. Pharmaceutics 2021, 13, 621. [Google Scholar] [CrossRef]
- Chaabouni, E.; Gassara, F.; Brar, S.K. Biopolymers Synthesis and Application. In Biotransformation of Waste Biomass into High Value Biochemicals; Brar, S.K., Dhillon, G.S., Soccol, C.R., Eds.; Springer: New York, NY, USA, 2014; pp. 415–443. [Google Scholar]
- Stanton, M.M.; Samitier, J.; Sánchez, S. Bioprinting of 3D Hydrogels. Lab Chip 2015, 15, 3111–3115. [Google Scholar] [CrossRef]
- Abdelhak, M. A Review: Application of Biopolymers in the Pharmaceutical Formulation. J. Adv. Biopharm. Pharmacovigil. 2019, 1, 11. [Google Scholar] [CrossRef]
- Rowley, J.A.; Madlambayan, G.; Mooney, D.J. Alginate Hydrogels as Synthetic Extracellular Matrix Materials. Biomaterials 1999, 20, 45–53. [Google Scholar] [CrossRef]
- Benwood, C.; Chrenek, J.; Kirsch, R.L.; Masri, N.Z.; Richards, H.; Teetzen, K.; Willerth, S.M. Natural Biomaterials and Their Use as Bioinks for Printing Tissues. Bioengineering 2021, 8, 27. [Google Scholar] [CrossRef]
- López-Marcial, G.R.; Zeng, A.Y.; Osuna, C.; Dennis, J.; García, J.M.; O’Connell, G.D. Agarose-Based Hydrogels as Suitable Bioprinting Materials for Tissue Engineering. ACS Biomater. Sci. Eng. 2018, 4, 3610–3616. [Google Scholar] [CrossRef]
- Kreimendahl, F.; Köpf, M.; Thiebes, A.L.; Duarte Campos, D.F.; Blaeser, A.; Schmitz-Rode, T.; Apel, C.; Jockenhoevel, S.; Fischer, H. Three-Dimensional Printing and Angiogenesis: Tailored Agarose-Type I Collagen Blends Comprise Three-Dimensional Printability and Angiogenesis Potential for Tissue-Engineered Substitutes. Tissue Eng. Part C Methods 2017, 23, 604–615. [Google Scholar] [CrossRef]
- Morris, V.B.; Nimbalkar, S.; Younesi, M.; McClellan, P.; Akkus, O. Mechanical Properties, Cytocompatibility and Manufacturability of Chitosan:PEGDA Hybrid-Gel Scaffolds by Stereolithography. Ann. Biomed. Eng. 2017, 45, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Mihajlovic, M.; Rikkers, M.; Mihajlovic, M.; Viola, M.; Schuiringa, G.; Ilochonwu, B.C.; Masereeuw, R.; Vonk, L.; Malda, J.; Ito, K.; et al. Viscoelastic Chondroitin Sulfate and Hyaluronic Acid Double-Network Hydrogels with Reversible Cross-Links. Biomacromolecules 2022, 23, 1350–1365. [Google Scholar] [CrossRef] [PubMed]
- Piola, B.; Sabbatini, M.; Gino, S.; Invernizzi, M.; Renò, F. 3D Bioprinting of Gelatin–Xanthan Gum Composite Hydrogels for Growth of Human Skin Cells. Int. J. Mol. Sci. 2022, 23, 539. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Zheng, B.; Shen, H.; Qi, M.; Shang, Y.; Wu, C.; Zhu, R.; Cheng, L.; Wang, Q. Strength-Tunable Printing of Xanthan Gum Hydrogel via Enzymatic Polymerization and Amide Bioconjugation. Chem. Commun. 2020, 56, 3457–3460. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Mondal, P.; Chatterjee, K. Digital Light Processing-Based 3D Bioprinting of κ-Carrageenan Hydrogels for Engineering Cell-Loaded Tissue Scaffolds. Carbohydr. Polym. 2022, 290, 119508. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Hu, Y.; Chen, C.; Mao, H.; Wang, L.; Zhang, S.; Huang, X. 3D Bioprintable Methacrylated Carrageenan/Sodium Alginate Dual Network Hydrogel for Vascular Tissue Engineering Scaffolding. Int. J. Polym. Mater. Polym. Biomater. 2023, 72, 550–560. [Google Scholar] [CrossRef]
- Tavakoli, S.; Kharaziha, M.; Kermanpur, A.; Mokhtari, H. Sprayable and Injectable Visible-Light Kappa-Carrageenan Hydrogel for in-Situ Soft Tissue Engineering. Int. J. Biol. Macromol. 2019, 138, 590–601. [Google Scholar] [CrossRef]
- Hu, S.; Martinez-Garcia, F.D.; Moeun, B.N.; Burgess, J.K.; Harmsen, M.C.; Hoesli, C.; de Vos, P. An Immune Regulatory 3D-Printed Alginate-Pectin Construct for Immunoisolation of Insulin Producing β-Cells. Mater. Sci. Eng. C 2021, 123, 112009. [Google Scholar] [CrossRef]
- Johnson, D.L.; Ziemba, R.M.; Shebesta, J.H.; Lipscomb, J.C.; Wang, Y.; Wu, Y.; O’Connell, K.D.; Kaltchev, M.G.; van Groningen, A.; Chen, J.; et al. Design of Pectin-Based Bioink Containing Bioactive Agent-Loaded Microspheres for Bioprinting. Biomed. Phys. Eng. Express 2019, 5, 067004. [Google Scholar] [CrossRef]
- Koch, L.; Kuhn, S.; Sorg, H.; Gruene, M.; Schlie, S.; Gaebel, R.; Polchow, B.; Reimers, K.; Stoelting, S.; Ma, N.; et al. Laser Printing of Skin Cells and Human Stem Cells. Tissue Eng. Part C Methods 2010, 16, 847–854. [Google Scholar] [CrossRef]
- Farokhi, M.; Jonidi Shariatzadeh, F.; Solouk, A.; Mirzadeh, H. Alginate Based Scaffolds for Cartilage Tissue Engineering: A Review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 230–247. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, T.; Tenorio, A.J.; Campbell, K.T.; Silva, E.A.; Leach, J.K. Alginate-Based Bioinks for 3D Bioprinting and Fabrication of Anatomically Accurate Bone Grafts. Tissue Eng. Part A 2021, 27, 1168–1181. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Steiner, D.; Lingens, L.; Fischer, L.; Köhn, K.; Detsch, R.; Boccaccini, A.R.; Fey, T.; Greil, P.; Weis, C.; Beier, J.P.; et al. Encapsulation of Mesenchymal Stem Cells Improves Vascularization of Alginate-Based Scaffolds. Tissue Eng. Part A 2018, 24, 1320–1331. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Fernandez, T.; Sikorski, P.; Leach, J.K. Bio-Instructive Materials for Musculoskeletal Regeneration. Acta Biomater. 2019, 96, 20–34. [Google Scholar] [CrossRef]
- Axpe, E.; Oyen, M. Applications of Alginate-Based Bioinks in 3D Bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef]
- Leonardo, M.; Prajatelistia, E.; Judawisastra, H. Alginate-Based Bioink for Organoid 3D Bioprinting: A Review. Bioprinting 2022, 28, e00246. [Google Scholar] [CrossRef]
- Singh, R.; Shitiz, K.; Singh, A. Chitin and Chitosan: Biopolymers for Wound Management. Int. Wound J. 2017, 14, 1276–1289. [Google Scholar] [CrossRef]
- Sahranavard, M.; Zamanian, A.; Ghorbani, F.; Shahrezaee, M.H. A Critical Review on Three Dimensional-Printed Chitosan Hydrogels for Development of Tissue Engineering. Bioprinting 2020, 17, e00063. [Google Scholar] [CrossRef]
- Sun, Y.; You, Y.; Jiang, W.; Wu, Q.; Wang, B.; Dai, K. Generating Ready-to-Implant Anisotropic Menisci by 3D-Bioprinting Protein-Releasing Cell-Laden Hydrogel-Polymer Composite Scaffold. Appl. Mater. Today 2020, 18, 100469. [Google Scholar] [CrossRef]
- Cruz, G.J.F.; Rimaycuna, J.; Alfaro, R.; Tripul, V.S.; Solis, R.L.; Gómez, M.M.; Paraguay-Delgado, F.; Solis, J.L. Antimicrobial Activity of Chitosan Composites against Bacterial Strains Isolated from Goat Meat and Cheese. J. Phys. Conf. Ser. 2019, 1173, 012005. [Google Scholar] [CrossRef]
- Manouchehri, S.; Bagheri, B.; Rad, S.H.; Nezhad, M.N.; Kim, Y.C.; Park, O.O.; Farokhi, M.; Jouyandeh, M.; Ganjali, M.R.; Yazdi, M.K.; et al. Electroactive Bio-Epoxy Incorporated Chitosan-Oligoaniline as an Advanced Hydrogel Coating for Neural Interfaces. Prog. Org. Coat. 2019, 131, 389–396. [Google Scholar] [CrossRef]
- Mohebbi, S.; Nezhad, M.N.; Zarrintaj, P.; Jafari, S.H.; Gholizadeh, S.S.; Saeb, M.R.; Mozafari, M. Chitosan in Biomedical Engineering: A Critical Review. Curr. Stem Cell Res. Ther. 2019, 14, 93–116. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, B.; Zarrintaj, P.; Samadi, A.; Zarrintaj, R.; Ganjali, M.R.; Saeb, M.R.; Mozafari, M.; Park, O.O.; Kim, Y.C. Tissue Engineering with Electrospun Electro-Responsive Chitosan-Aniline Oligomer/Polyvinyl Alcohol. Int. J. Biol. Macromol. 2020, 147, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Mahmodi, G.; Zarrintaj, P.; Taghizadeh, A.; Taghizadeh, M.; Manouchehri, S.; Dangwal, S.; Ronte, A.; Ganjali, M.R.; Ramsey, J.D.; Kim, S.-J.; et al. From Microporous to Mesoporous Mineral Frameworks: An Alliance between Zeolite and Chitosan. Carbohydr. Res. 2020, 489, 107930. [Google Scholar] [CrossRef]
- Mora Boza, A.; Wlodarczyk-Biegun, M.K.; Del Campo, A.; Vázquez-Lasal, B.; San Román, J. Chitosan-Based Inks: 3D Printing and Bioprinting Strategies to Improve Shape Fidelity, Mechanical Properties, and Biocompatibility of 3D Scaffolds. Biomecánica 2019, 27, 7–16. [Google Scholar] [CrossRef]
- Tarassoli, S.P.; Jessop, Z.M.; Kyle, S.; Whitaker, I.S. Candidate Bioinks for 3D Bioprinting Soft Tissue. In 3D Bioprinting for Reconstructive Surgery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 145–172. [Google Scholar]
- Agarwal, T.; Chiesa, I.; Costantini, M.; Lopamarda, A.; Tirelli, M.C.; Borra, O.P.; Varshapally, S.V.S.; Kumar, Y.A.V.; Koteswara Reddy, G.; De Maria, C.; et al. Chitosan and Its Derivatives in 3D/4D (Bio) Printing for Tissue Engineering and Drug Delivery Applications. Int. J. Biol. Macromol. 2023, 246, 125669. [Google Scholar] [CrossRef]
- Lazaridou, M.; Bikiaris, D.N.; Lamprou, D.A. 3D Bioprinted Chitosan-Based Hydrogel Scaffolds in Tissue Engineering and Localised Drug Delivery. Pharmaceutics 2022, 14, 1978. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, M.; Du, W.; Zhao, J.; Ling, G.; Zhang, P. Chitosan-Based High-Strength Supramolecular Hydrogels for 3D Bioprinting. Int. J. Biol. Macromol. 2022, 219, 545–557. [Google Scholar] [CrossRef]
- Garg, H.G.; Hales, C.A. Chemistry and Biology of Hyaluronan; Elsevier: Amsterdam, The Netherlands, 2004; ISBN 9780080443829. [Google Scholar]
- Shim, J.-H.; Kim, J.Y.; Park, M.; Park, J.; Cho, D.-W. Development of a Hybrid Scaffold with Synthetic Biomaterials and Hydrogel Using Solid Freeform Fabrication Technology. Biofabrication 2011, 3, 034102. [Google Scholar] [CrossRef]
- Zhai, X.; Ruan, C.; Ma, Y.; Cheng, D.; Wu, M.; Liu, W.; Zhao, X.; Pan, H.; Lu, W.W. 3D-Bioprinted Osteoblast-Laden Nanocomposite Hydrogel Constructs with Induced Microenvironments Promote Cell Viability, Differentiation, and Osteogenesis Both In Vitro and In Vivo. Adv. Sci. 2018, 5, 1700550. [Google Scholar] [CrossRef] [PubMed]
- Ehsanipour, A.; Nguyen, T.; Aboufadel, T.; Sathialingam, M.; Cox, P.; Xiao, W.; Walthers, C.M.; Seidlits, S.K. Injectable, Hyaluronic Acid-Based Scaffolds with Macroporous Architecture for Gene Delivery. Cell Mol. Bioeng. 2019, 12, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Nusgens, B.-V. Hyaluronic Acid and Extracellular Matrix: A Primitive Molecule? Ann. Dermatol. Venereol. 2010, 137, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Petta, D.; D’Amora, U.; Ambrosio, L.; Grijpma, D.W.; Eglin, D.; D’Este, M. Hyaluronic Acid as a Bioink for Extrusion-Based 3D Printing. Biofabrication 2020, 12, 032001. [Google Scholar] [CrossRef]
- Sekar, M.P.; Suresh, S.; Zennifer, A.; Sethuraman, S.; Sundaramurthi, D. Hyaluronic Acid as Bioink and Hydrogel Scaffolds for Tissue Engineering Applications. ACS Biomater. Sci. Eng. 2023, 9, 3134–3159. [Google Scholar] [CrossRef]
- Bringmann, M.; Landrein, B.; Schudoma, C.; Hamant, O.; Hauser, M.-T.; Persson, S. Cracking the Elusive Alignment Hypothesis: The Microtubule–Cellulose Synthase Nexus Unraveled. Trends Plant Sci. 2012, 17, 666–674. [Google Scholar] [CrossRef]
- Novotna, K.; Havelka, P.; Sopuch, T.; Kolarova, K.; Vosmanska, V.; Lisa, V.; Svorcik, V.; Bacakova, L. Cellulose-Based Materials as Scaffolds for Tissue Engineering. Cellulose 2013, 20, 2263–2278. [Google Scholar] [CrossRef]
- Salmoria, G.V.; Klauss, P.; Paggi, R.A.; Kanis, L.A.; Lago, A. Structure and Mechanical Properties of Cellulose Based Scaffolds Fabricated by Selective Laser Sintering. Polym. Test. 2009, 28, 648–652. [Google Scholar] [CrossRef]
- Curvello, R.; Raghuwanshi, V.S.; Garnier, G. Engineering Nanocellulose Hydrogels for Biomedical Applications. Adv. Colloid. Interface Sci. 2019, 267, 47–61. [Google Scholar] [CrossRef]
- Piras, C.C.; Fernández-Prieto, S.; De Borggraeve, W.M. Nanocellulosic Materials as Bioinks for 3D Bioprinting. Biomater. Sci. 2017, 5, 1988–1992. [Google Scholar] [CrossRef]
- Wan Jusoh, W.N.L.; Sajab, M.S.; Mohamed Abdul, P.; Kaco, H. Recent Advances in 3D Bioprinting: A Review of Cellulose-Based Biomaterials Ink. Polymers 2022, 14, 2260. [Google Scholar] [CrossRef]
- Jovic, T.H.; Nicholson, T.; Arora, H.; Nelson, K.; Doak, S.H.; Whitaker, I.S. A Comparative Analysis of Pulp-Derived Nanocelluloses for 3D Bioprinting Facial Cartilages. Carbohydr. Polym. 2023, 321, 121261. [Google Scholar] [CrossRef]
- Jovic, T.H.; Kungwengwe, G.; Mills, A.C.; Whitaker, I.S. Plant-Derived Biomaterials: A Review of 3D Bioprinting and Biomedical Applications. Front. Mech. Eng. 2019, 5, 19. [Google Scholar] [CrossRef]
- Pitton, M.; Fiorati, A.; Buscemi, S.; Melone, L.; Farè, S.; Contessi Negrini, N. 3D Bioprinting of Pectin-Cellulose Nanofibers Multicomponent Bioinks. Front. Bioeng. Biotechnol. 2021, 9, 1186. [Google Scholar] [CrossRef]
- Tortorella, S.; Inzalaco, G.; Dapporto, F.; Maturi, M.; Sambri, L.; Vetri Buratti, V.; Chiariello, M.; Comes Franchini, M.; Locatelli, E. Biocompatible Pectin-Based Hybrid Hydrogels for Tissue Engineering Applications. New J. Chem. 2021, 45, 22386–22395. [Google Scholar] [CrossRef]
- Muthukrishnan, L. Imminent Antimicrobial Bioink Deploying Cellulose, Alginate, EPS and Synthetic Polymers for 3D Bioprinting of Tissue Constructs. Carbohydr. Polym. 2021, 260, 117774. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, F.B.; Dolderer, V.; Nellinger, S.; Schmidt, F.F.; Kluger, P.J. Gellan Gum Is a Suitable Biomaterial for Manual and Bioprinted Setup of Long-Term Stable, Functional 3D-Adipose Tissue Models. Gels 2022, 8, 420. [Google Scholar] [CrossRef] [PubMed]
- Milivojevic, M.; Pajic-Lijakovic, I.; Bugarski, B.; Nayak, A.K.; Hasnain, M.S. Gellan Gum in Drug Delivery Applications. In Natural Polysaccharides in Drug Delivery and Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 145–186. [Google Scholar]
- Morris, E.R.; Nishinari, K.; Rinaudo, M. Gelation of Gellan—A Review. Food Hydrocoll. 2012, 28, 373–411. [Google Scholar] [CrossRef]
- Bonifacio, M.A.; Cometa, S.; Cochis, A.; Scalzone, A.; Gentile, P.; Scalia, A.C.; Rimondini, L.; Mastrorilli, P.; De Giglio, E. A Bioprintable Gellan Gum/Lignin Hydrogel: A Smart and Sustainable Route for Cartilage Regeneration. Int. J. Biol. Macromol. 2022, 216, 336–346. [Google Scholar] [CrossRef]
- Li, Z.; Li, S.; Yang, J.; Ha, Y.; Zhang, Q.; Zhou, X.; He, C. 3D Bioprinted Gelatin/Gellan Gum-Based Scaffold with Double-Crosslinking Network for Vascularized Bone Regeneration. Carbohydr. Polym. 2022, 290, 119469. [Google Scholar] [CrossRef]
- Cernencu, A.I.; Ioniță, M. The Current State of the Art in Gellan-Based Printing Inks in Tissue Engineering. Carbohydr. Polym. 2023, 309, 120676. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Rao, K.M.; Han, S.S. Application of Xanthan Gum as Polysaccharide in Tissue Engineering: A Review. Carbohydr. Polym. 2018, 180, 128–144. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi Nagahara, M.H.; Caiado Decarli, M.; Inforçatti Neto, P.; Lopes da Silva, J.V.; Moraes, Â.M. Crosslinked Alginate-xanthan Gum Blends as Effective Hydrogels for 3D Bioprinting of Biological Tissues. J. Appl. Polym. Sci. 2022, 139, 52612. [Google Scholar] [CrossRef]
- Garcia-Cruz, M.R.; Postma, A.; Frith, J.E.; Meagher, L. Printability and Bio-Functionality of a Shear Thinning Methacrylated Xanthan–Gelatin Composite Bioink. Biofabrication 2021, 13, 035023. [Google Scholar] [CrossRef]
- Sun, G.; Mao, J.J. Engineering Dextran-Based Scaffolds for Drug Delivery and Tissue Repair. Nanomedicine 2012, 7, 1771–1784. [Google Scholar] [CrossRef]
- Turner, P.R.; Murray, E.; McAdam, C.J.; McConnell, M.A.; Cabral, J.D. Peptide Chitosan/Dextran Core/Shell Vascularized 3D Constructs for Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 32328–32339. [Google Scholar] [CrossRef]
- Musilová, L.; Achbergerová, E.; Vítková, L.; Kolařík, R.; Martínková, M.; Minařík, A.; Mráček, A.; Humpolíček, P.; Pecha, J. Cross-Linked Gelatine by Modified Dextran as a Potential Bioink Prepared by a Simple and Non-Toxic Process. Polymers 2022, 14, 391. [Google Scholar] [CrossRef]
- Du, Z.; Li, N.; Hua, Y.; Shi, Y.; Bao, C.; Zhang, H.; Yang, Y.; Lin, Q.; Zhu, L. Physiological PH-Dependent Gelation for 3D Printing Based on the Phase Separation of Gelatin and Oxidized Dextran. Chem. Commun. 2017, 53, 13023–13026. [Google Scholar] [CrossRef]
- Bean, S.R.; Zhu, L.; Smith, B.M.; Wilson, J.D.; Ioerger, B.P.; Tilley, M. Starch and Protein Chemistry and Functional Properties. In Sorghum and Millets; Elsevier: Amsterdam, The Netherlands, 2019; pp. 131–170. [Google Scholar]
- Gopinath, V.; Saravanan, S.; Al-Maleki, A.R.; Ramesh, M.; Vadivelu, J. A Review of Natural Polysaccharides for Drug Delivery Applications: Special Focus on Cellulose, Starch and Glycogen. Biomed. Pharmacother. 2018, 107, 96–108. [Google Scholar] [CrossRef]
- Zhuang, P.; Greenberg, Z.; He, M. Biologically Enhanced Starch Bio-Ink for Promoting 3D Cell Growth. Adv. Mater. Technol. 2021, 6, 2100551. [Google Scholar] [CrossRef]
- Butler, H.M.; Naseri, E.; MacDonald, D.S.; Andrew Tasker, R.; Ahmadi, A. Optimization of Starch- and Chitosan-Based Bio-Inks for 3D Bioprinting of Scaffolds for Neural Cell Growth. Materialia 2020, 12, 100737. [Google Scholar] [CrossRef]
- Marques, D.M.C.; Silva, J.C.; Serro, A.P.; Cabral, J.M.S.; Sanjuan-Alberte, P.; Ferreira, F.C. 3D Bioprinting of Novel κ-Carrageenan Bioinks: An Algae-Derived Polysaccharide. Bioengineering 2022, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tan, Y.J.; Li, L. A Strategy for Strong Interface Bonding by 3D Bioprinting of Oppositely Charged κ-Carrageenan and Gelatin Hydrogels. Carbohydr. Polym. 2018, 198, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Lee, Y.W.; Jung, W.-K.; Oh, J.; Nam, S.Y. Enhanced Rheological Behaviors of Alginate Hydrogels with Carrageenan for Extrusion-Based Bioprinting. J. Mech. Behav. Biomed. Mater. 2019, 98, 187–194. [Google Scholar] [CrossRef]
- Boonlai, W.; Tantishaiyakul, V.; Hirun, N. Characterization of Κ-carrageenan/Methylcellulose/Cellulose Nanocrystal Hydrogels for 3D Bioprinting. Polym. Int. 2022, 71, 181–191. [Google Scholar] [CrossRef]
- Lim, W.; Kim, G.J.; Kim, H.W.; Lee, J.; Zhang, X.; Kang, M.G.; Seo, J.W.; Cha, J.M.; Park, H.J.; Lee, M.-Y.; et al. Kappa-Carrageenan-Based Dual Crosslinkable Bioink for Extrusion Type Bioprinting. Polymers 2020, 12, 2377. [Google Scholar] [CrossRef] [PubMed]
- Mugnaini, G.; Resta, C.; Poggi, G.; Bonini, M. Photopolymerizable Pullulan: Synthesis, Self-Assembly and Inkjet Printing. J. Colloid. Interface Sci. 2021, 592, 430–439. [Google Scholar] [CrossRef]
- Koffi, K.L.; Yapo, B.M.; Besson, V. Extraction and Characterization of Gelling Pectin from the Peel of Poncirus Trifoliata Fruit. Agric. Sci. 2013, 4, 614–619. [Google Scholar] [CrossRef]
- Cui, X.; Li, J.; Hartanto, Y.; Durham, M.; Tang, J.; Zhang, H.; Hooper, G.; Lim, K.; Woodfield, T. Advances in Extrusion 3D Bioprinting: A Focus on Multicomponent Hydrogel-Based Bioinks. Adv. Healthc. Mater. 2020, 9, 1901648. [Google Scholar] [CrossRef]
- Methacanon, P.; Krongsin, J.; Gamonpilas, C. Pomelo (Citrus Maxima) Pectin: Effects of Extraction Parameters and Its Properties. Food Hydrocoll. 2014, 35, 383–391. [Google Scholar] [CrossRef]
- Stealey, S.; Guo, X.; Ren, L.; Bryant, E.; Kaltchev, M.; Chen, J.; Kumpaty, S.; Hua, X.; Zhang, W. Stability Improvement and Characterization of Bioprinted Pectin-Based Scaffold. J. Appl. Biomater. Funct. Mater. 2019, 17, 228080001880710. [Google Scholar] [CrossRef] [PubMed]
- Banks, A.; Guo, X.; Chen, J.; Kumpaty, S.; Zhang, W. Novel Bioprinting Method Using a Pectin Based Bioink. Technol. Health Care 2017, 25, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Andriotis, E.G.; Eleftheriadis, G.K.; Karavasili, C.; Fatouros, D.G. Development of Bio-Active Patches Based on Pectin for the Treatment of Ulcers and Wounds Using 3D-Bioprinting Technology. Pharmaceutics 2020, 12, 56. [Google Scholar] [CrossRef]
- Lapomarda, A.; Cerqueni, G.; Geven, M.A.; Chiesa, I.; De Acutis, A.; De Blasi, M.; Montemurro, F.; De Maria, C.; Mattioli-Belmonte, M.; Vozzi, G. Physicochemical Characterization of Pectin-Gelatin Biomaterial Formulations for 3D Bioprinting. Macromol. Biosci. 2021, 21, 2100168. [Google Scholar] [CrossRef] [PubMed]
- Lapomarda, A.; Pulidori, E.; Cerqueni, G.; Chiesa, I.; De Blasi, M.; Geven, M.A.; Montemurro, F.; Duce, C.; Mattioli-Belmonte, M.; Tiné, M.R.; et al. Pectin as Rheology Modifier of a Gelatin-Based Biomaterial Ink. Materials 2021, 14, 3109. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Abelseth, E.; de la Vega, L.; Willerth, S.M. Bioprinting a Novel Glioblastoma Tumor Model Using a Fibrin-Based Bioink for Drug Screening. Mater. Today Chem. 2019, 12, 78–84. [Google Scholar] [CrossRef]
- Kim, S.D.; Jin, S.; Kim, S.; Son, D.; Shin, M. Tyramine-Functionalized Alginate-Collagen Hybrid Hydrogel Inks for 3D-Bioprinting. Polymers 2022, 14, 3173. [Google Scholar] [CrossRef]
- Stratesteffen, H.; Köpf, M.; Kreimendahl, F.; Blaeser, A.; Jockenhoevel, S.; Fischer, H. GelMA-Collagen Blends Enable Drop-on-Demand 3D Printablility and Promote Angiogenesis. Biofabrication 2017, 9, 045002. [Google Scholar] [CrossRef]
- Trucco, D.; Sharma, A.; Manferdini, C.; Gabusi, E.; Petretta, M.; Desando, G.; Ricotti, L.; Chakraborty, J.; Ghosh, S.; Lisignoli, G. Modeling and Fabrication of Silk Fibroin–Gelatin-Based Constructs Using Extrusion-Based Three-Dimensional Bioprinting. ACS Biomater. Sci. Eng. 2021, 7, 3306–3320. [Google Scholar] [CrossRef]
- Restan Perez, M.; Sharma, R.; Masri, N.Z.; Willerth, S.M. 3D Bioprinting Mesenchymal Stem Cell-Derived Neural Tissues Using a Fibrin-Based Bioink. Biomolecules 2021, 11, 1250. [Google Scholar] [CrossRef]
- Abelseth, E.; Abelseth, L.; De la Vega, L.; Beyer, S.T.; Wadsworth, S.J.; Willerth, S.M. 3D Printing of Neural Tissues Derived from Human Induced Pluripotent Stem Cells Using a Fibrin-Based Bioink. ACS Biomater. Sci. Eng. 2019, 5, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, R.; Kasoju, N.; Raju, R.; Geevarghese, R.; Gauthaman, A.; Bhatt, A. Exploring the Potential of Alginate-Gelatin-Diethylaminoethyl Cellulose-Fibrinogen Based Bioink for 3D Bioprinting of Skin Tissue Constructs. Carbohydr. Polym. Technol. Appl. 2022, 3, 100184. [Google Scholar] [CrossRef]
- Im, S.; Choe, G.; Seok, J.M.; Yeo, S.J.; Lee, J.H.; Kim, W.D.; Lee, J.Y.; Park, S.A. An Osteogenic Bioink Composed of Alginate, Cellulose Nanofibrils, and Polydopamine Nanoparticles for 3D Bioprinting and Bone Tissue Engineering. Int. J. Biol. Macromol. 2022, 205, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, Q.; Dong, W.; Liu, S.; Zhang, H.; Gu, Y. Alginate/Gelatin-Based Hydrogel with Soy Protein/Peptide Powder for 3D Printing Tissue-Engineering Scaffolds to Promote Angiogenesis. Macromol. Biosci. 2022, 22, 2100413. [Google Scholar] [CrossRef] [PubMed]
- Thanh, T.N.; Laowattanatham, N.; Ratanavaraporn, J.; Sereemaspun, A.; Yodmuang, S. Hyaluronic Acid Crosslinked with Alginate Hydrogel: A Versatile and Biocompatible Bioink Platform for Tissue Engineering. Eur. Polym. J. 2022, 166, 111027. [Google Scholar] [CrossRef]
- Maturavongsadit, P.; Narayanan, L.K.; Chansoria, P.; Shirwaiker, R.; Benhabbour, S.R. Cell-Laden Nanocellulose/Chitosan-Based Bioinks for 3D Bioprinting and Enhanced Osteogenic Cell Differentiation. ACS Appl. Bio Mater. 2021, 4, 2342–2353. [Google Scholar] [CrossRef]
- Ngo, T.B.; Spearman, B.S.; Hlavac, N.; Schmidt, C.E. Three-Dimensional Bioprinted Hyaluronic Acid Hydrogel Test Beds for Assessing Neural Cell Responses to Competitive Growth Stimuli. ACS Biomater. Sci. Eng. 2020, 6, 6819–6830. [Google Scholar] [CrossRef]
- Janarthanan, G.; Shin, H.S.; Kim, I.-G.; Ji, P.; Chung, E.-J.; Lee, C.; Noh, I. Self-Crosslinking Hyaluronic Acid–Carboxymethylcellulose Hydrogel Enhances Multilayered 3D-Printed Construct Shape Integrity and Mechanical Stability for Soft Tissue Engineering. Biofabrication 2020, 12, 045026. [Google Scholar] [CrossRef]
- Shopperly, L.K.; Spinnen, J.; Krüger, J.; Endres, M.; Sittinger, M.; Lam, T.; Kloke, L.; Dehne, T. Blends of Gelatin and Hyaluronic Acid Stratified by Stereolithographic Bioprinting Approximate Cartilaginous Matrix Gradients. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 2310–2322. [Google Scholar] [CrossRef]
- Ma, N.; Cheung, D.Y.; Butcher, J.T. Incorporating Nanocrystalline Cellulose into a Multifunctional Hydrogel for Heart Valve Tissue Engineering Applications. J. Biomed. Mater. Res. A 2022, 110, 76–91. [Google Scholar] [CrossRef]
- Lan, X.; Ma, Z.; Szojka, A.R.A.; Kunze, M.; Mulet-Sierra, A.; Vyhlidal, M.J.; Boluk, Y.; Adesida, A.B. TEMPO-Oxidized Cellulose Nanofiber-Alginate Hydrogel as a Bioink for Human Meniscus Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 766399. [Google Scholar] [CrossRef]
- Kapr, J.; Petersilie, L.; Distler, T.; Lauria, I.; Bendt, F.; Sauter, C.M.; Boccaccini, A.R.; Rose, C.R.; Fritsche, E. Human Induced Pluripotent Stem Cell-Derived Neural Progenitor Cells Produce Distinct Neural 3D In Vitro Models Depending on Alginate/Gellan Gum/Laminin Hydrogel Blend Properties. Adv. Healthc. Mater. 2021, 10, 2100131. [Google Scholar] [CrossRef]
- Gulrez, S.K.; Al-Assaf, S.; Phillips, G.O. Hydrogels: Methods of Preparation, Characterisation and Applications. In Progress in Molecular and Environmental Bioengineering—From Analysis and Modeling to Technology Applications; InTech: Lahore, Pakistan, 2011. [Google Scholar]
- van Kampen, K.A.; Scheuring, R.G.; Terpstra, M.L.; Levato, R.; Groll, J.; Malda, J.; Mota, C.; Moroni, L. Biofabrication: From Additive Manufacturing to Bioprinting. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Varghese, S.A.; Rangappa, S.M.; Siengchin, S.; Parameswaranpillai, J. Natural Polymers and the Hydrogels Prepared from Them. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 17–47. ISBN 9780128164211. [Google Scholar]
- Akhtar, M.F.; Hanif, M.; Ranjha, N.M. Methods of Synthesis of Hydrogels … A Review. Saudi Pharm. J. 2016, 24, 554–559. [Google Scholar] [CrossRef]
- GhavamiNejad, A.; Ashammakhi, N.; Wu, X.Y.; Khademhosseini, A. Crosslinking Strategies for 3D Bioprinting of Polymeric Hydrogels. Small 2020, 16, 2002931. [Google Scholar] [CrossRef]
- Hennink, W.E.; van Nostrum, C.F. Novel Crosslinking Methods to Design Hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, Processing and Application of Hydrogels: A Review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Slager, J.; Domb, A.J. Biopolymer Stereocomplexes. Adv. Drug Deliv. Rev. 2003, 55, 549–583. [Google Scholar] [CrossRef]
- Varaprasad, K.; Raghavendra, G.M.; Jayaramudu, T.; Yallapu, M.M.; Sadiku, R. A Mini Review on Hydrogels Classification and Recent Developments in Miscellaneous Applications. Mater. Sci. Eng. C 2017, 79, 958–971. [Google Scholar] [CrossRef]
- Lee, M.; Rizzo, R.; Surman, F.; Zenobi-Wong, M. Guiding Lights: Tissue Bioprinting Using Photoactivated Materials. Chem. Rev. 2020, 120, 10950–11027. [Google Scholar] [CrossRef]
- Elomaa, L.; Yang, Y.P. Additive Manufacturing of Vascular Grafts and Vascularized Tissue Constructs. Tissue Eng. Part B Rev. 2017, 23, 436–450. [Google Scholar] [CrossRef]
- Gómez-Blanco, J.C.; Galván-Chacón, V.; Patrocinio, D.; Matamoros, M.; Sánchez-Ortega, Á.J.; Marcos, A.C.; Duarte-León, M.; Marinaro, F.; Pagador, J.B.; Sánchez-Margallo, F.M. Improving Cell Viability and Velocity in μ-Extrusion Bioprinting with a Novel Pre-Incubator Bioprinter and a Standard FDM 3D Printing Nozzle. Materials 2021, 14, 3100. [Google Scholar] [CrossRef] [PubMed]
- Barron, J.A.; Krizman, D.B.; Ringeisen, B.R. Laser Printing of Single Cells: Statistical Analysis, Cell Viability, and Stress. Ann. Biomed. Eng. 2005, 33, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Dou, C.; Perez, V.; Qu, J.; Tsin, A.; Xu, B.; Li, J. A State-of-the-Art Review of Laser-Assisted Bioprinting and Its Future Research Trends. ChemBioEng Rev. 2021, 8, 517–534. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Fan, X.; Zhou, H. Recent Advances in Bioprinting Techniques: Approaches, Applications and Future Prospects. J. Transl. Med. 2016, 14, 271. [Google Scholar] [CrossRef]
- Fu, Z.; Angeline, V.; Sun, W. Evaluation of Printing Parameters on 3D Extrusion Printing of Pluronic Hydrogels and Machine Learning Guided Parameter Recommendation. Int. J. Bioprinting 1970, 7, 434. [Google Scholar] [CrossRef]
- Malekpour, A.; Chen, X. Printability and Cell Viability in Extrusion-Based Bioprinting from Experimental, Computational, and Machine Learning Views. J. Funct. Biomater. 2022, 13, 40. [Google Scholar] [CrossRef]
- Erben, A.; Hörning, M.; Hartmann, B.; Becke, T.; Eisler, S.A.; Southan, A.; Cranz, S.; Hayden, O.; Kneidinger, N.; Königshoff, M.; et al. Precision 3D-Printed Cell Scaffolds Mimicking Native Tissue Composition and Mechanics. Adv. Healthc. Mater. 2020, 9, 2000918. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, Q.; Huang, G.; Shen, M.; Jian, Z.; Thoraval, M.-J.; Lian, Q.; Zhang, X.; Xu, F. Improved Resolution and Fidelity of Droplet-Based Bioprinting by Upward Ejection. ACS Biomater. Sci. Eng. 2019, 5, 4112–4121. [Google Scholar] [CrossRef]
- Nguyen, A.K.; Goering, P.L.; Elespuru, R.K.; Sarkar Das, S.; Narayan, R.J. The Photoinitiator Lithium Phenyl (2,4,6-Trimethylbenzoyl) Phosphinate with Exposure to 405 Nm Light Is Cytotoxic to Mammalian Cells but Not Mutagenic in Bacterial Reverse Mutation Assays. Polymers 2020, 12, 1489. [Google Scholar] [CrossRef]
- Boularaoui, S.; Al Hussein, G.; Khan, K.A.; Christoforou, N.; Stefanini, C. An Overview of Extrusion-Based Bioprinting with a Focus on Induced Shear Stress and Its Effect on Cell Viability. Bioprinting 2020, 20, e00093. [Google Scholar] [CrossRef]
- Yu, C.; Ma, X.; Zhu, W.; Wang, P.; Miller, K.L.; Stupin, J.; Koroleva-Maharajh, A.; Hairabedian, A.; Chen, S. Scanningless and Continuous 3D Bioprinting of Human Tissues with Decellularized Extracellular Matrix. Biomaterials 2019, 194, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Beheshtizadeh, N.; Lotfibakhshaiesh, N.; Pazhouhnia, Z.; Hoseinpour, M.; Nafari, M. A Review of 3D Bio-Printing for Bone and Skin Tissue Engineering: A Commercial Approach. J. Mater. Sci. 2020, 55, 3729–3749. [Google Scholar] [CrossRef]
- Galván-Chacón, V.P.; Zampouka, A.; Hesse, B.; Bohner, M.; Habibovic, P.; Barata, D. Bone-on-a-Chip: A Microscale 3D Biomimetic Model to Study Bone Regeneration. Adv. Eng. Mater. 2022, 24, 2101467. [Google Scholar] [CrossRef]
- Marino, A.; Filippeschi, C.; Genchi, G.G.; Mattoli, V.; Mazzolai, B.; Ciofani, G. The Osteoprint: A Bioinspired Two-Photon Polymerized 3-D Structure for the Enhancement of Bone-like Cell Differentiation. Acta Biomater. 2014, 10, 4304–4313. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Koo, S.; Finnegan, M.A.; Loskill, P.; Huebsch, N.; Marks, N.C.; Conklin, B.R.; Grigoropoulos, C.P.; Healy, K.E. Three-Dimensional Filamentous Human Diseased Cardiac Tissue Model. Biomaterials 2014, 35, 1367–1377. [Google Scholar] [CrossRef]
- Marino, A.; Tricinci, O.; Battaglini, M.; Filippeschi, C.; Mattoli, V.; Sinibaldi, E.; Ciofani, G. A 3D Real-Scale, Biomimetic, and Biohybrid Model of the Blood-Brain Barrier Fabricated through Two-Photon Lithography. Small 2018, 14, 1702959. [Google Scholar] [CrossRef]
- Skylar-Scott, M.A.; Liu, M.-C.; Wu, Y.; Dixit, A.; Yanik, M.F. Guided Homing of Cells in Multi-Photon Microfabricated Bioscaffolds. Adv. Healthc. Mater. 2016, 5, 1233–1243. [Google Scholar] [CrossRef]
- Rayner, S.G.; Howard, C.C.; Mandrycky, C.J.; Stamenkovic, S.; Himmelfarb, J.; Shih, A.Y.; Zheng, Y. Multiphoton-Guided Creation of Complex Organ-Specific Microvasculature. Adv. Healthc. Mater. 2021, 10, 2100031. [Google Scholar] [CrossRef]
- Tromayer, M.; Gruber, P.; Markovic, M.; Rosspeintner, A.; Vauthey, E.; Redl, H.; Ovsianikov, A.; Liska, R. A Biocompatible Macromolecular Two-Photon Initiator Based on Hyaluronan. Polym. Chem. 2017, 8, 451–460. [Google Scholar] [CrossRef]
- Fu, Z.; Naghieh, S.; Xu, C.; Wang, C.; Sun, W.; Chen, X. Printability in Extrusion Bioprinting. Biofabrication 2021, 13, 033001. [Google Scholar] [CrossRef]
- Michael, S.; Sorg, H.; Peck, C.-T.; Koch, L.; Deiwick, A.; Chichkov, B.; Vogt, P.M.; Reimers, K. Tissue Engineered Skin Substitutes Created by Laser-Assisted Bioprinting Form Skin-Like Structures in the Dorsal Skin Fold Chamber in Mice. PLoS ONE 2013, 8, e57741. [Google Scholar] [CrossRef] [PubMed]
- Domingos, M.; Intranuovo, F.; Russo, T.; De Santis, R.; Gloria, A.; Ambrosio, L.; Ciurana, J.; Bartolo, P. The First Systematic Analysis of 3D Rapid Prototyped Poly(ε-Caprolactone) Scaffolds Manufactured through BioCell Printing: The Effect of Pore Size and Geometry on Compressive Mechanical Behaviour and in Vitro HMSC Viability. Biofabrication 2013, 5, 045004. [Google Scholar] [CrossRef] [PubMed]
- Łabowska, M.B.; Cierluk, K.; Jankowska, A.M.; Kulbacka, J.; Detyna, J.; Michalak, I. A Review on the Adaption of Alginate-Gelatin Hydrogels for 3D Cultures and Bioprinting. Materials 2021, 14, 858. [Google Scholar] [CrossRef] [PubMed]
- Roche, C.D.; Sharma, P.; Ashton, A.W.; Jackson, C.; Xue, M.; Gentile, C. Printability, Durability, Contractility and Vascular Network Formation in 3D Bioprinted Cardiac Endothelial Cells Using Alginate-Gelatin Hydrogels. Front. Bioeng. Biotechnol. 2021, 9, 636257. [Google Scholar] [CrossRef]
- Singh, N.K.; Han, W.; Nam, S.A.; Kim, J.W.; Kim, J.Y.; Kim, Y.K.; Cho, D.-W. Three-Dimensional Cell-Printing of Advanced Renal Tubular Tissue Analogue. Biomaterials 2020, 232, 119734. [Google Scholar] [CrossRef]
- Gudapati, H.; Dey, M.; Ozbolat, I. A Comprehensive Review on Droplet-Based Bioprinting: Past, Present and Future. Biomaterials 2016, 102, 20–42. [Google Scholar] [CrossRef]
- Yanez, M.; Rincon, J.; Dones, A.; De Maria, C.; Gonzales, R.; Boland, T. In Vivo Assessment of Printed Microvasculature in a Bilayer Skin Graft to Treat Full-Thickness Wounds. Tissue Eng. Part A 2015, 21, 224–233. [Google Scholar] [CrossRef]
- Cui, X.; Boland, T. Human Microvasculature Fabrication Using Thermal Inkjet Printing Technology. Biomaterials 2009, 30, 6221–6227. [Google Scholar] [CrossRef]
| Bioink Compounds 1 | Cells 2 | Printing Techniques | Applications | Reference |
|---|---|---|---|---|
| Col | HCCs | Extrusion | Cartilage tissue regeneration | [31] |
| Col + VEGF | NSCs | Droplet | Neural tissue regeneration | [32] |
| Gel + PU | MSCs | Extrusion | Tissue regeneration | [33] |
| Gel + GelMA + OCP | HUVECs | SLA | Bone regeneration | [34] |
| Gel + GelMA | Endothelial and ASCs | Extrusion | Bone regeneration | [35] |
| SF + GMA | NIH/3T3 and chondrocytes | DLP | Heart, vessel, brain, trachea, and ear regeneration | [36] |
| SF_GMA + GO | Neuro2a | DLP | Neural tissue engineering | [37] |
| Bioink Compounds 1 | Cells 2 | Printing Techniques | Applications | Reference |
|---|---|---|---|---|
| Alg + Aga | FBCs | Extrusion | Cartilage tissue engineering | [105] |
| Col + Fg | HUVECs and HDFs | Droplet | Vascular tissue regeneration | [106] |
| CS + PEGDA | hMSCs | SLA | Cartilage tissue engineering | [107] |
| HA + ChS | MSCs | Extrusion | Cartilage tissue engineering | [108] |
| Gel + XG | Fibroblasts and keratinocytes | Extrusion | Skin regeneration | [109] |
| XG + GOx + Glu + NHS | NIH/3T3 | Extrusion | Soft tissue engineering | [110] |
| MA-κ-CA | NIH/3T3 | DLP | Soft tissue engineering | [111] |
| MA-κ-CA + Alg | HUVECs | Extrusion | Vascular tissue regeneration | [112] |
| MA-κ-CA | HeLa and Fibroblasts | Extrusion | Soft tissue engineering | [113] |
| Pc + Plu + Alg | MIN6 | Extrusion | Pancreatic tissue engineering | [114] |
| Pc + Plu | mBMSCs | Extrusion | Vascular tissue regeneration | [115] |
| Alg | NIH/3T3 and hMSCs | Laser | Skin regeneration | [116] |
| Alg + Fg | AC16 | Extrusion | Myocardial regeneration | [78] |
| Bioink Compounds 1 | Cells 2 | Printing Techniques | Applications | Reference |
|---|---|---|---|---|
| F + Alg + G | U87MG | Extrusion | Tissue construct | [186] |
| Col + Alg + TH | Fibroblasts | Extrusion | Vascular tissue regeneration | [187] |
| Col + GelMA | HUVECs and hMSCs | Droplet | Vascular tissue regeneration | [188] |
| SF + Gel | hMSCs | Extrusion | Cartilage tissue engineering | [189] |
| F + Alg + G | ASCs | Extrusion | Neural tissue engineering | [190] |
| F + G + Alg | hiPSCs | Extrusion | Neural tissue engineering | [191] |
| Alg + Gel + DEAE-C + Fg | HPFs and keratinocytes | Extrusion | Skin tissue engineering | [192] |
| Alg + TOCNF + PDA-NPs | Osteoblasts | Extrusion | Bone tissue regeneration | [193] |
| Alg + Gel + Soy | HUVECs | Extrusion | Vascular tissue regeneration | [194] |
| aAlg + aHA | MSCs | Extrusion | Cartilage tissue engineering | [195] |
| tCS + GHEC + CNCs | MC3T3-E1 | Extrusion | Bone tissue regeneration | [196] |
| sCS + aDex + GelMA | hBMSCs and HUVECs | Extrusion | Vascular tissue regeneration | [165] |
| HAMA + Col | PC-12 | Neural tissue engineering | [197] | |
| HA + CMC | MC3T3 | Extrusion | Bone tissue regeneration | [198] |
| HAMA + GelMA | CCs | Extrusion | Cartilage tissue engineering | [199] |
| TOCNF + GelMA | ASCs | Extrusion | Vascular tissue engineering | [200] |
| TOCNF + Alg | hMF | Extrusion | Cartilage tissue engineering | [201] |
| GGMA + GelMA + DFO-Eth | HUVECs and hBMSCs | Extrusion | Bone regeneration | [159] |
| GG + Alg + LM | hiPSCs | Extrusion | 3D neuromodeling | [202] |
| aDex + sCS + GelMA | hBMSCs and HUVECs | Extrusion | Skin regeneration | [165] |
| Physical Crosslinking | Biopolymers | Schema | References |
|---|---|---|---|
| Crystallization | Chitosan/PVA Gelatin/PVA Strach/PVA Dextran | 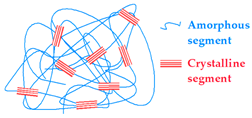 | [203,205,206,207,208] |
| Stereo complexation | Dextran | 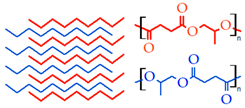 | [179,203,205,207,208,210] |
| Heating/Cooling | Gellan Gum Gelatin Carrageenan | 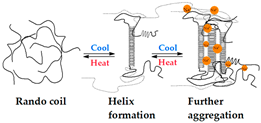 | [203,207,210] |
| Hydrogen bonding | Gelatin/Agar Starch/Carboxymethyl Cellulose Hyaluronic Acid/Methyl Cellulose |  | [203,205,206,207,208] |
| Ionic interaction | Alginate Chitosan Carrageenan | 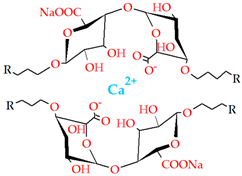 | [203,205,207,208,209] |
| Hydrophobicity | Chitosan Dextran Pullulan Carboxymethyl curdlan | 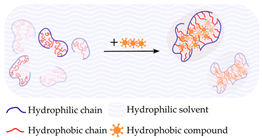 | [205,208] |
| Maturation | Alginate Chitosan Carrageenan Arabic gum |  | [203,205] |
| Chemical Crosslinking | Biopolymers | Schema | Reference |
|---|---|---|---|
| Complementary Groups | |||
| 1. Aldehyde, Dihydrazide, and Schiff’s base | Hyaluronic acid-based Dextran Chitosan Alginate | 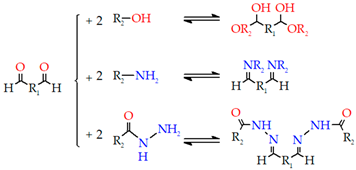 | [210] |
| 2. Thiol-ene Michael addition | Hyaluronic acid-based Dextran Chitosan Alginate | 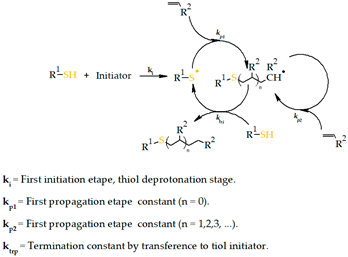 | [210] |
| 3. Condensation | Hyaluronic acid-based Dextran Chitosan Alginate | 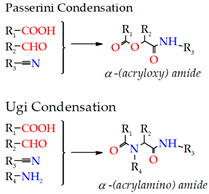 | [205,207,208,211] |
| Radical polymerization | Hyaluronic acid Dextran Chitosan | 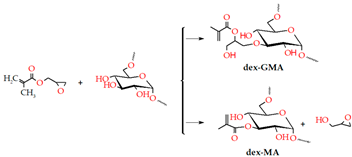 | [205,207,208,209,211] |
| Enzymatic | Gellan gum Polypeptides Hyaluronic acid Dextran Cellulose Alginate | 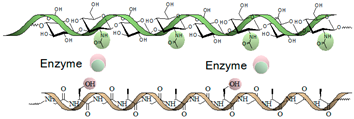 | [180,204,205,206,210,211] |
| Bioprinting Technique | Parameters | Reference |
|---|---|---|
| Photopolymerization | Wavelength Light/laser power Exposure time Repetition rate Pulse width Environmental temperature | [212,213,214] |
| Laser-based | Wavelength Laser intensity/power Pulse rate/frequency Laser fluence Hydrogel viscosity Thickness of the absorbing layer Thickness of the bioink layer Travel distance Printing velocity | [215,216,217] |
| Extrusion | Printing pressure Printing velocity Nozzle height Flow rate Printing temperature Hydrogel viscosity Extrusion gauge Environmental temperature | [217,218,219] |
| Droplet | Pressure Droplet rate Droplet volume Hydrogel viscosity Kinetic momentum Printing speed Printing time Voltage pulse (amplitude, rise and fall times, dwell time, echo time, and frequency) Substrate hydrophobicity/hydrophilicity | [212,220,221] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patrocinio, D.; Galván-Chacón, V.; Gómez-Blanco, J.C.; Miguel, S.P.; Loureiro, J.; Ribeiro, M.P.; Coutinho, P.; Pagador, J.B.; Sanchez-Margallo, F.M. Biopolymers for Tissue Engineering: Crosslinking, Printing Techniques, and Applications. Gels 2023, 9, 890. https://doi.org/10.3390/gels9110890
Patrocinio D, Galván-Chacón V, Gómez-Blanco JC, Miguel SP, Loureiro J, Ribeiro MP, Coutinho P, Pagador JB, Sanchez-Margallo FM. Biopolymers for Tissue Engineering: Crosslinking, Printing Techniques, and Applications. Gels. 2023; 9(11):890. https://doi.org/10.3390/gels9110890
Chicago/Turabian StylePatrocinio, David, Victor Galván-Chacón, J. Carlos Gómez-Blanco, Sonia P. Miguel, Jorge Loureiro, Maximiano P. Ribeiro, Paula Coutinho, J. Blas Pagador, and Francisco M. Sanchez-Margallo. 2023. "Biopolymers for Tissue Engineering: Crosslinking, Printing Techniques, and Applications" Gels 9, no. 11: 890. https://doi.org/10.3390/gels9110890
APA StylePatrocinio, D., Galván-Chacón, V., Gómez-Blanco, J. C., Miguel, S. P., Loureiro, J., Ribeiro, M. P., Coutinho, P., Pagador, J. B., & Sanchez-Margallo, F. M. (2023). Biopolymers for Tissue Engineering: Crosslinking, Printing Techniques, and Applications. Gels, 9(11), 890. https://doi.org/10.3390/gels9110890