Advanced Hydrogel-Based Strategies for Enhanced Bone and Cartilage Regeneration: A Comprehensive Review
Abstract
:1. Introduction
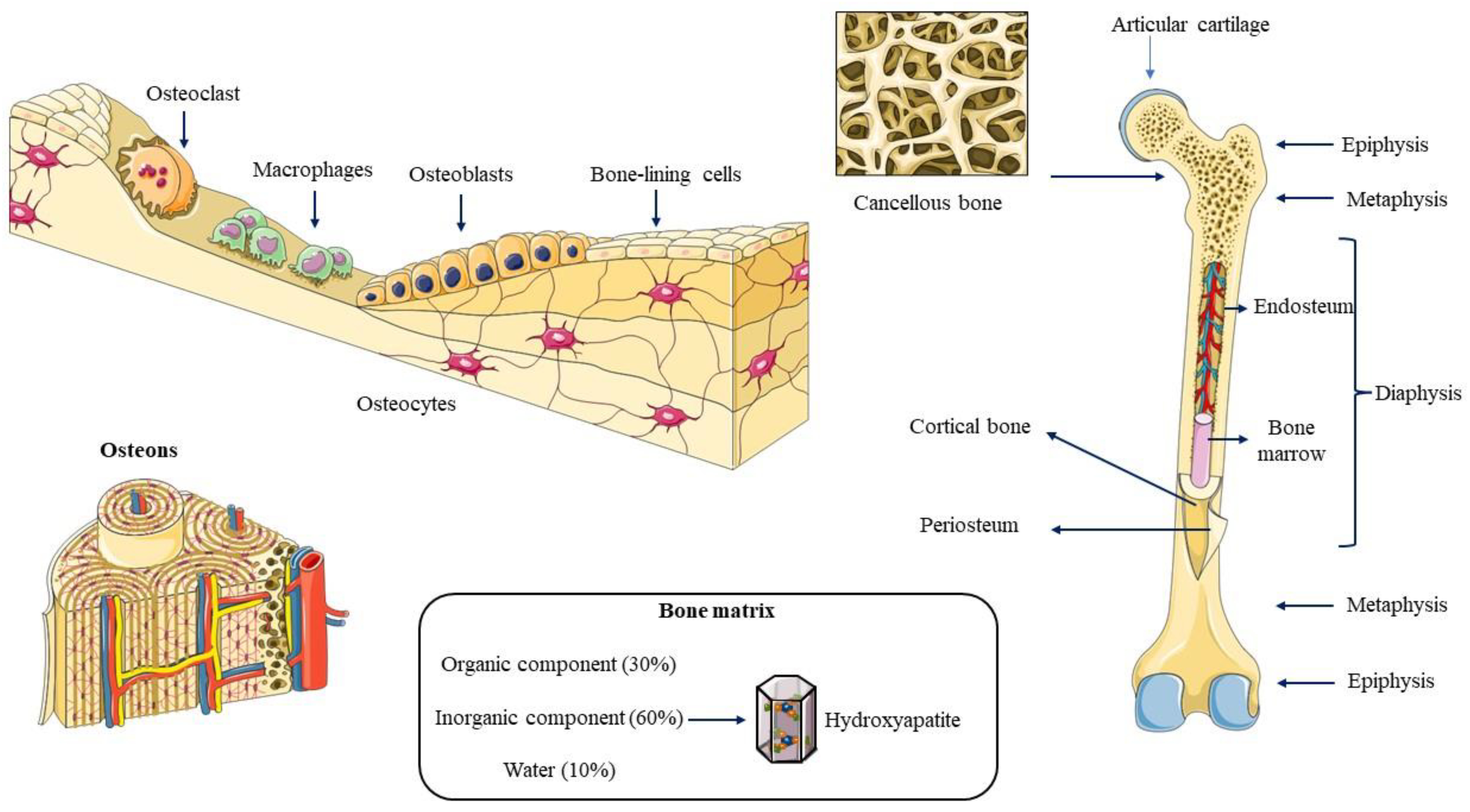
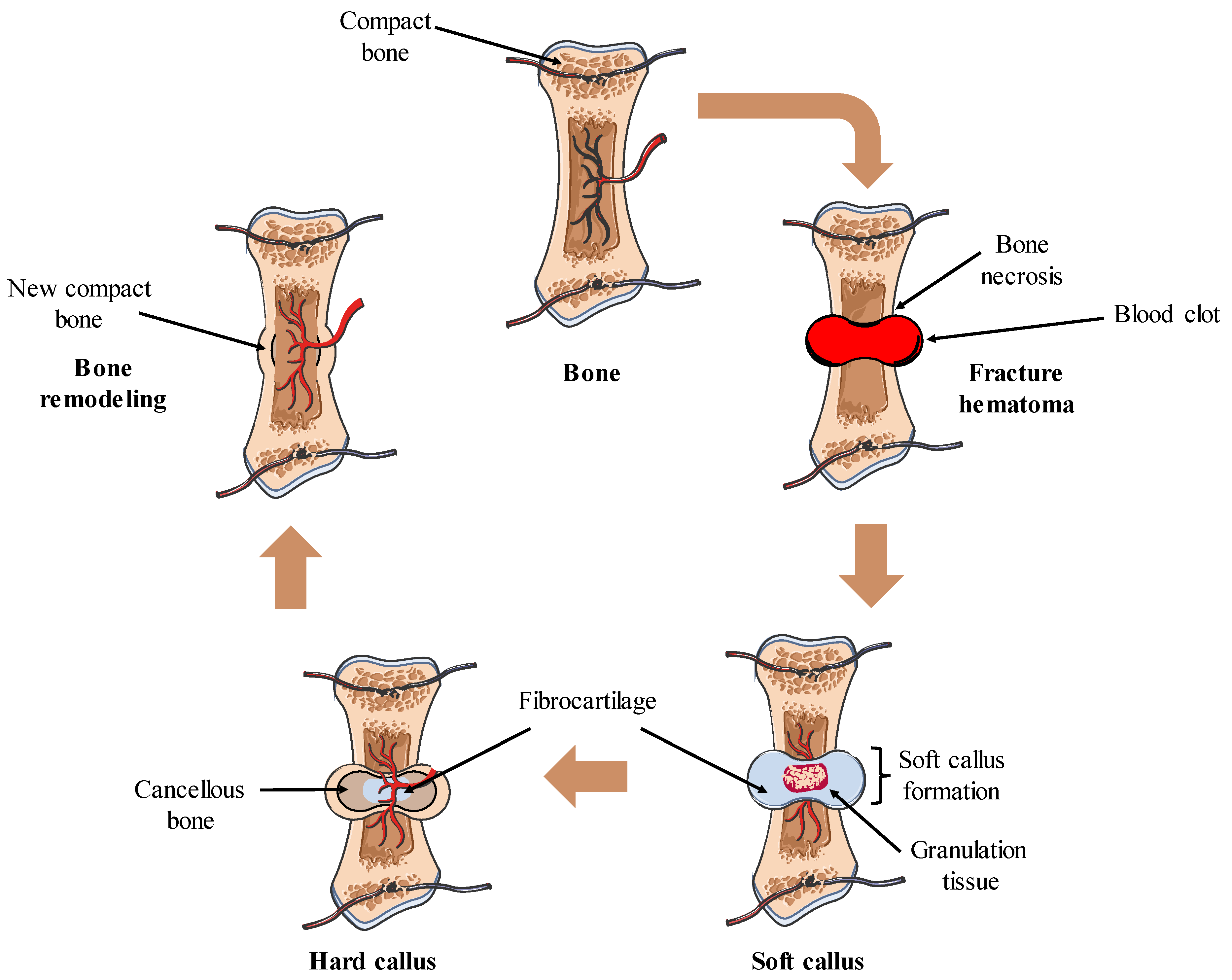
2. Bone Biology
3. Cartilage Biology
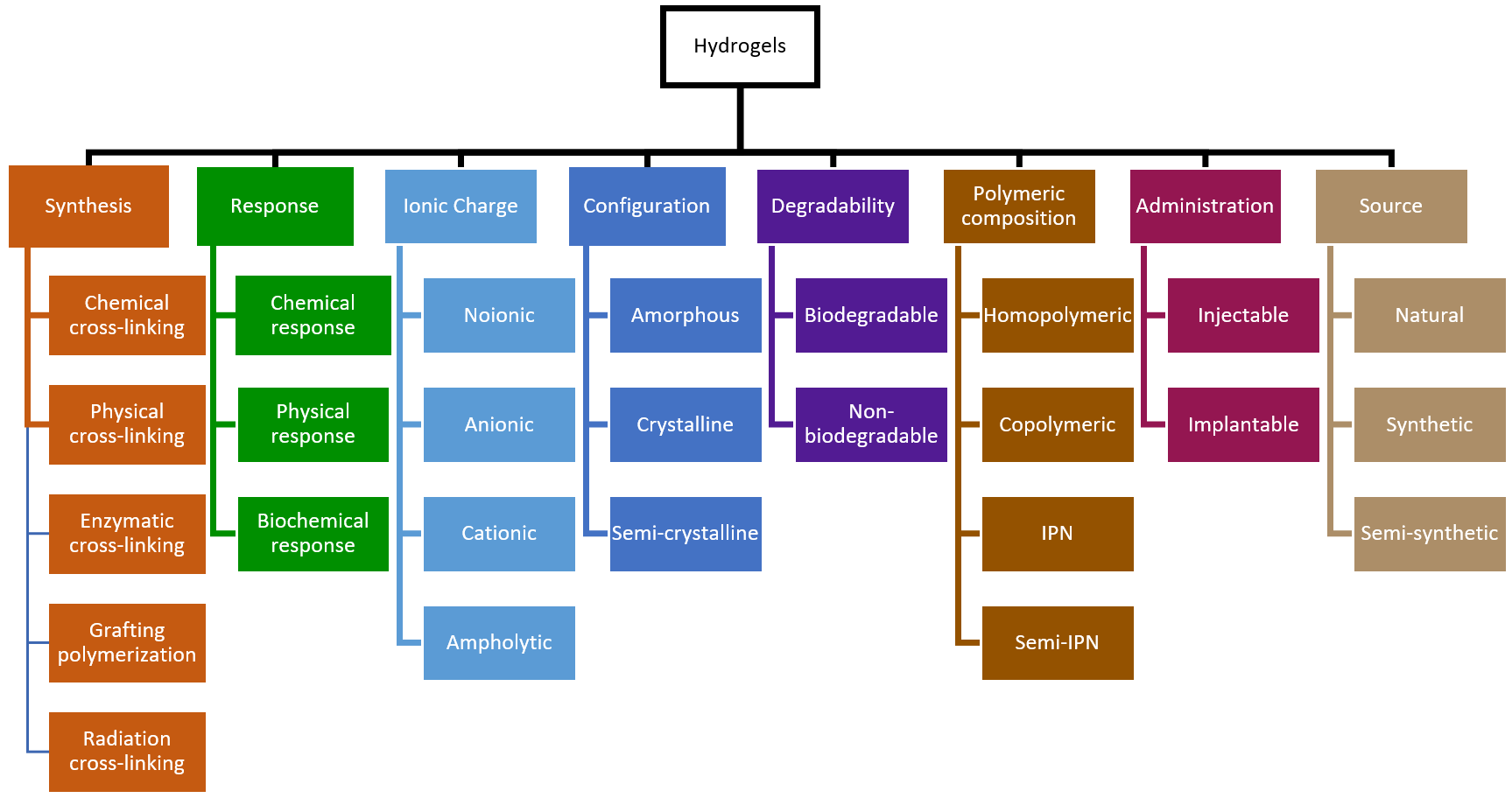
4. Hydrogels: Concept, Synthesis, and Biomedical Applications
5. Hydrogels for TERM of Bone and Cartilage
5.1. Hydrogels in Bone Tissue Regeneration
5.1.1. Bioactive Molecules-Loaded Hydrogels
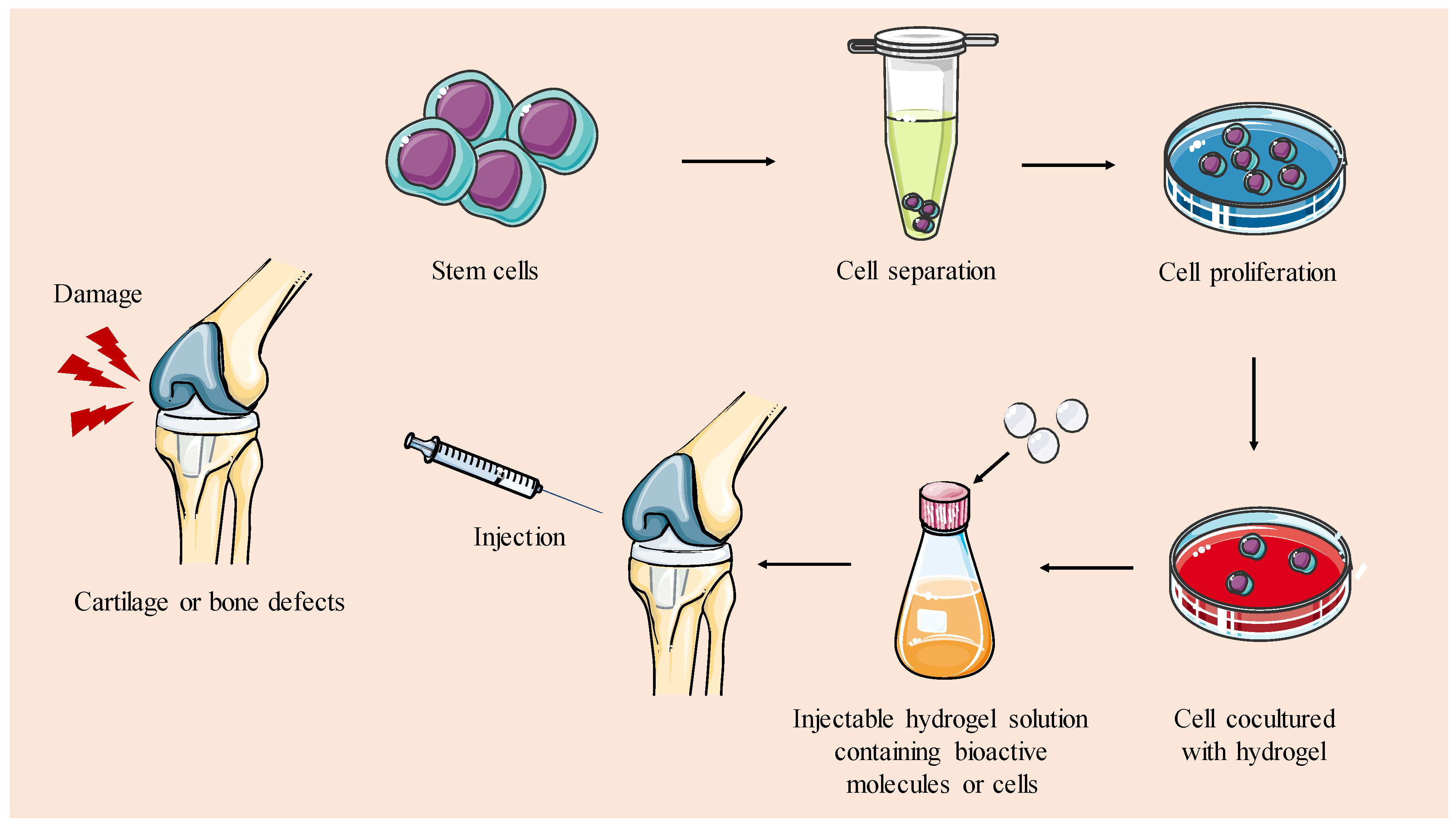
5.1.2. Cells-Loaded Hydrogels
| Polymer | Biological Factor | Mechanism of Gelation | Application | Year | References |
|---|---|---|---|---|---|
| PEG | BMP-2 | Chemical crosslinking | Murine non-healing radial bone defect | 2014 | [145] |
| Chitosan/β-glycerol phosphate disodium salt | SDF-1α | Chemical crosslinking | Critical-sized calvarial defects in rats | 2017 | [146] |
| Calcium alginate | Ultrashort peptide nanofibers | Physical crosslinking | Rebuild osteogenic immune microenvironments | 2024 | [147] |
| Chitosan/graphene oxide/hydroxyethyl cellulose/β-glycerol phosphate | Atsttrin | Physical crosslinking | Bone regeneration in diabetic mice model | 2023 | [149] |
| Vancomycin/D-Ala-D-Ala/acrylamide | OGP | Physical crosslinking | Infected bone fracture | 2023 | [150] |
| Glycol-based dendronized chitosan | GIT1 plasmids | Physical crosslinking | Bone defects | 2023 | [151] |
| Hydroxypropylmethylcellulose | Biphasic calcium phosphate | Chemical crosslinking | New bone formation | 2009/12 | [154,155] |
| Agarose and agarose–collagen | β-TCP | Chemical crosslinking | Osteogenic differentiation of hMSCs | 2018 | [156] |
| HA | CaP NPs and strontium ranelate | Chemical crosslinking | Osteoporosis | 2022 | [157] |
| Poloxamer 407/HA | BMSCs | Chemical crosslinking | Osteoporosis | 2023 | [159] |
| Gelatin-hydroxyphenyl propionic acid | TMSCs | Chemical crosslinking | Postmenopausal osteoporosis | 2018 | [160] |
5.2. Hydrogels in Cartilage Regeneration
5.2.1. Cell-Free Hydrogels
| Hydrogel | Core Material | Preparation | Application | Year | References |
|---|---|---|---|---|---|
| HA | Rapamycin-liposome microspheres | Physical crosslinking | Osteoarthritis | 2022 | [162] |
| Methacrylate gelatin hydrogel microspheres | Diclofenac sodium | Physical crosslinking | Osteoarthritis | 2021 | [163] |
| Marine collagen | Enzymatic crosslinking | Cartilage regeneration | 2020 | [164] | |
| Sodium alginate and gelatin | KGN/TGF-β3 | Double crosslinking | Cartilage regeneration | 2020 | [165,166] |
| PEGDA/ECM | Honokiol | Physical crosslinking | Osteochondral defect repair | 2020 | [167] |
| PEG-GelMA-HA | DPSCs | Physical crosslinking | Chondrogenic differentiation of DPSCs | 2014 | [169] |
| Carrageenan | MSCs | Physical crosslinking | 3D bioprinting | 2016 | [170] |
| Chitosan glycerol phosphate/starch | ASCs | Physical crosslinking | Cartilage tissue engineering | 2010 | [171,172] |
| HSMSSA | Chondrocytes | Di-self-crosslinking | Cartilage repair fille | 2020 | [173] |
5.2.2. Cell-Loaded Hydrogels
6. Conclusions and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, N.; Yang, J.; Xie, Y.; Du, X.; Chen, H.; Hong, Z.; Chen, L. Bone function, dysfunction and its role in diseases including critical illness. Int. J. Biol. Sci. 2019, 15, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Florencio-Silva, R.; da Silva Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed. Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Clynes, M.A.; Harvey, N.C.; Curtis, E.M.; Fuggle, N.R.; Dennison, E.M.; Cooper, C. The epidemiology of osteoporosis. Br. Med. Bull. 2020, 133, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.-M.; Bisignano, C.; James, S.L.; Abady, G.G.; Abedi, A.; Abu-Gharbieh, E.; Alhassan, R.K.; Alipour, V.; Arabloo, J.; Asaad, M.; et al. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet Health Longev. 2021, 2, e580–e592. [Google Scholar] [CrossRef] [PubMed]
- Litwic, A.; Edwards, M.H.; Dennison, E.M.; Cooper, C. Epidemiology and burden of osteoarthritis. Br. Med. Bull. 2013, 105, 185–199. [Google Scholar] [CrossRef]
- Audigé, L.; Griffin, D.; Bhandari, M.; Kellam, J.; Rüedi, T.P. Path Analysis of Factors for Delayed Healing and Nonunion in 416 Operatively Treated Tibial Shaft Fractures. Clin. Orthop. Relat. Res. 2005, 438, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Zheng, F.; Zhao, W.; Zhang, Y.; Yuan, J.; Zhang, B.; Li, L. Prevalence and influencing factors of nonunion in patients with tibial fracture: Systematic review and meta-analysis. J. Orthop. Surg. Res. 2020, 15, 377. [Google Scholar] [CrossRef]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M. Bone tissue regeneration: Biology, strategies and interface studies. Prog. Biomater. 2019, 8, 223–237. [Google Scholar] [CrossRef]
- Fernandez De Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.-M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef]
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Fathi-Achachelouei, M.; Knopf-Marques, H.; da Silva, C.E.R.; Barthès, J.; Bat, E.; Tezcaner, A.; Vrana, N.E. Use of Nanoparticles in Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2019, 7, 113. [Google Scholar] [CrossRef]
- Perez, J.R.; Kouroupis, D.; Li, D.J.; Best, T.M.; Kaplan, L.; Correa, D. Tissue Engineering and Cell-Based Therapies for Fractures and Bone Defects. Front. Bioeng. Biotechnol. 2018, 6, 105. [Google Scholar] [CrossRef]
- Teng, S.; Lee, E.; Wang, P.; Shin, D.; Kim, H. Three-layered membranes of collagen/hydroxyapatite and chitosan for guided bone regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 87B, 132–138. [Google Scholar] [CrossRef]
- Hu, S.; Zhou, Y.; Zhao, Y.; Xu, Y.; Zhang, F.; Gu, N.; Ma, J.; Reynolds, M.A.; Xia, Y.; Xu, H.H. Enhanced bone regeneration and visual monitoring via superparamagnetic iron oxide nanoparticle scaffold in rats. J. Tissue Eng. Regen. Med. 2018, 12, e2085–e2098. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Zhang, X.; Ramakrishna, S.; He, L.; Wu, G. Reactive blends based on polyhydroxyalkanoates: Preparation and biomedical application. Mater. Sci. Eng. C 2017, 70, 1107–1119. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Cao, D.; Ding, J. Recent advances in regenerative biomaterials. Regen. Biomater. 2022, 9, rbac098. [Google Scholar] [CrossRef]
- Fraile-Martínez, O.; García-Montero, C.; Coca, A.; Álvarez-Mon, M.A.; Monserrat, J.; Gómez-Lahoz, A.M.; Coca, S.; Álvarez-Mon, M.; Acero, J.; Bujan, J.; et al. Applications of Polymeric Composites in Bone Tissue Engineering and Jawbone Regeneration. Polymers 2021, 13, 3429. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Téllez, D.A.; Téllez-Jurado, L.; Rodríguez-Lorenzo, L.M. Hydrogels for Cartilage Regeneration, from Polysaccharides to Hybrids. Polymers 2017, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.; He, H.; Li, B.; Hou, T. Hydrogel as a Biomaterial for Bone Tissue Engineering: A Review. Nanomaterials 2020, 10, 1511. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hsu, S.-H. Synthesis and Biomedical Applications of Self-healing Hydrogels. Front. Chem. 2018, 6, 449. [Google Scholar] [CrossRef]
- Tayler, I.M.; Stowers, R.S. Engineering hydrogels for personalized disease modeling and regenerative medicine. Acta Biomater. 2021, 132, 4–22. [Google Scholar] [CrossRef]
- Galán-Olleros, M.; Marco, J.; Oteo, D.; Cristóbal-Bilbao, R.; Manrique, E.; García-Maroto, R.; Marco, F.; Cebrián-Parra, J.L. Orthopedic Surgical Treatment and Perioperative Complications in Multiple Myeloma Bone Disease: Analysis of a Series (2009–2018). Ann. Surg. Oncol. 2021, 28, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Tetteh, E.S.; Bajaj, S.; Ghodadra, N.S.; Cole, B.J. The Basic Science and Surgical Treatment Options for Articular Cartilage Injuries of the Knee. J. Orthop. Sports Phys. Ther. 2012, 42, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Karsenty, G.; Ferron, M. The contribution of bone to whole-organism physiology. Nature 2012, 481, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Nahian, A.; Davis, D.D. Histology, Osteoprogenitor Cells. In StatPearls—NCBI Bookshelf; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Weng, Y.; Wang, H.; Wu, D.; Xu, S.; Chen, X.; Huang, J.; Feng, Y.; Li, L.; Wang, Z. A novel lineage of osteoprogenitor cells with dual epithelial and mesenchymal properties govern maxillofacial bone homeostasis and regeneration after MSFL. Cell Res. 2022, 32, 814–830. [Google Scholar] [CrossRef]
- Capulli, M.; Paone, R.; Rucci, N. Osteoblast and osteocyte: Games without frontiers. Arch. Biochem. Biophys. 2014, 561, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Long, F. Building strong bones: Molecular regulation of the osteoblast lineage. Nat. Rev. Mol. Cell. Biol. 2012, 13, 27–38. [Google Scholar] [CrossRef]
- Rochefort, G.Y.; Pallu, S.; Benhamou, C.L. Osteocyte: The unrecognized side of bone tissue. Osteoporos. Int. 2010, 21, 1457–1469. [Google Scholar] [CrossRef] [PubMed]
- Franz-Odendaal, T.A.; Hall, B.K.; Witten, P.E. Buried alive: How osteoblasts become osteocytes. Dev. Dyn. 2006, 235, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Tsukasaki, M.; Takayanagi, H. Osteoclast biology in the single-cell era. Inflamm. Regen. 2022, 42, 27. [Google Scholar] [CrossRef] [PubMed]
- Jacome-Galarza, C.E.; Percin, G.I.; Muller, J.T.; Mass, E.; Lazarov, T.; Eitler, J.; Rauner, M.; Yadav, V.K.; Crozet, L.; Bohm, M.; et al. Developmental origin, functional maintenance and genetic rescue of osteoclasts. Nature 2019, 568, 541–545. [Google Scholar] [CrossRef]
- De Leon-Oliva, D.; Barrena-Blázquez, S.; Jiménez-Álvarez, L.; Fraile-Martinez, O.; García-Montero, C.; López-González, L.; Torres-Carranza, D.; García-Puente, L.M.; Carranza, S.T.; Álvarez-Mon, M.Á.; et al. The RANK–RANKL–OPG System: A Multifaceted Regulator of Homeostasis, Immunity, and Cancer. Medicina 2023, 59, 1752. [Google Scholar] [CrossRef]
- Wein, M.N. Bone Lining Cells: Normal Physiology and Role in Response to Anabolic Osteoporosis Treatments. Curr. Mol. Biol. Rep. 2017, 3, 79–84. [Google Scholar] [CrossRef]
- Sroga, G.E.; Vashishth, D. Effects of Bone Matrix Proteins on Fracture and Fragility in Osteoporosis. Curr. Osteoporos. Rep. 2012, 10, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Du, T.; Niu, X.; Fan, Y. Biomechanics and mechanobiology of the bone matrix. Bone Res. 2022, 10, 59. [Google Scholar] [CrossRef]
- Feng, X. Chemical and Biochemical Basis of Cell-Bone Matrix Interaction in Health and Disease. Curr. Chem. Biol. 2009, 3, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Patil, S.; Gao, Y.-G.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 757. [Google Scholar] [CrossRef]
- Huang, S.; Jin, M.; Su, N.; Chen, L. New insights on the reparative cells in bone regeneration and repair. Biol. Rev. 2021, 96, 357–375. [Google Scholar] [CrossRef] [PubMed]
- Löffler, J.; Noom, A.; Ellinghaus, A.; Dienelt, A.; Kempa, S.; Duda, G.N. A comprehensive molecular profiling approach reveals metabolic alterations that steer bone tissue regeneration. Commun. Biol. 2023, 6, 327. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Partridge, N.C. Physiological Bone Remodeling: Systemic Regulation and Growth Factor Involvement. Physiology 2016, 31, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Dallas, S.L.; Prideaux, M.; Bonewald, L.F. The Osteocyte: An Endocrine Cell … and More. Endocr. Rev. 2013, 34, 658–690. [Google Scholar] [CrossRef] [PubMed]
- Kenkre, J.S.; Bassett, J.H.D. The bone remodelling cycle. Ann. Clin. Biochem. 2018, 55, 308–327. [Google Scholar] [CrossRef] [PubMed]
- Duda, G.N.; Geissler, S.; Checa, S.; Tsitsilonis, S.; Petersen, A.; Schmidt-Bleek, K. The decisive early phase of bone regeneration. Nat. Rev. Rheumatol. 2023, 19, 78–95. [Google Scholar] [CrossRef] [PubMed]
- Salhotra, A.; Shah, H.N.; Levi, B.; Longaker, M.T. Mechanisms of bone development and repair. Nat. Rev. Mol. Cell Biol. 2020, 21, 696–711. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef]
- Perez, R.A.; Seo, S.-J.; Won, J.-E.; Lee, E.-J.; Jang, J.-H.; Knowles, J.C.; Kim, H.-W. Therapeutically relevant aspects in bone repair and regeneration. Mater. Today 2015, 18, 573–589. [Google Scholar] [CrossRef]
- Wachsmuth, L.; Söder, S.; Fan, Z.; Finger, F.; Aigner, T. Immunolocalization of matrix proteins in different human cartilage subtypes. Histol. Histopathol. 2006, 21, 477–485. [Google Scholar] [CrossRef]
- Vincent, T.L.; McClurg, O.; Troeberg, L. The Extracellular Matrix of Articular Cartilage Controls the Bioavailability of Pericellular Matrix-Bound Growth Factors to Drive Tissue Homeostasis and Repair. Int. J. Mol. Sci. 2022, 23, 6003. [Google Scholar] [CrossRef]
- Krishnan, Y.; Grodzinsky, A.J. Cartilage Diseases. Matrix Biol. 2018, 71–72, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Rikkers, M.; Korpershoek, J.V.; Levato, R.; Malda, J.; Vonk, L.A. The clinical potential of articular cartilage-derived progenitor cells: A systematic review. npj Regen. Med. 2022, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Degen, K.E.; Gourdie, R.G. Embryonic wound healing: A primer for engineering novel therapies for tissue repair. Birth Defects Res. Part C Embryo Today Rev. 2012, 96, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Roseti, L.; Desando, G.; Cavallo, C.; Petretta, M.; Grigolo, B. Articular Cartilage Regeneration in Osteoarthritis. Cells 2019, 8, 1305. [Google Scholar] [CrossRef]
- Burleigh, A.; Chanalaris, A.; Gardiner, M.D.; Driscoll, C.; Boruc, O.; Saklatvala, J.; Vincent, T.L. Joint immobilization prevents murine osteoarthritis and reveals the highly mechanosensitive nature of protease expression in vivo. Arthritis Rheum. 2012, 64, 2278–2288. [Google Scholar] [CrossRef] [PubMed]
- Huey, D.J.; Hu, J.C.; Athanasiou, K.A. Unlike Bone, Cartilage Regeneration Remains Elusive. Science 2012, 338, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shah, K.M.; Luo, J. Strategies for Articular Cartilage Repair and Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 770655. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; De Leon-Oliva, D.; Boaru, D.L.; Fraile-Martinez, O.; García-Montero, C.; Diaz, R.; Coca, S.; Barrena-Blázquez, S.; Bujan, J.; García-Honduvilla, N.; et al. Unraveling the New Perspectives on Antimicrobial Hydrogels: State-of-the-Art and Translational Applications. Gels 2023, 9, 617. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef] [PubMed]
- Varaprasad, K.; Raghavendra, G.M.; Jayaramudu, T.; Yallapu, M.M.; Sadiku, R. A mini review on hydrogels classification and recent developments in miscellaneous applications. Mater. Sci. Eng. C 2017, 79, 958–971. [Google Scholar] [CrossRef]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef] [PubMed]
- Kapusta, O.; Jarosz, A.; Stadnik, K.; Giannakoudakis, D.A.; Barczyński, B.; Barczak, M. Antimicrobial Natural Hydrogels in Biomedicine: Properties, Applications, and Challenges—A Concise Review. Int. J. Mol. Sci. 2023, 24, 2191. [Google Scholar] [CrossRef]
- Cifuentes, A.; Gómez-Gil, V.; Ortega, M.A.; Asúnsolo, Á.; Coca, S.; Román, J.S.; Álvarez-Mon, M.; Buján, J.; García-Honduvilla, N. Chitosan hydrogels functionalized with either unfractionated heparin or bemiparin improve diabetic wound healing. Biomed. Pharmacother. 2020, 129, 110498. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of PVA and PVP in medical and pharmaceutical applications: Perspectives and challenges. Biotechnol. Adv. 2019, 37, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Madduma-Bandarage, U.S.K.; Madihally, S.V. Synthetic hydrogels: Synthesis, novel trends, and applications. J. Appl. Polym. Sci. 2021, 138, 50376. [Google Scholar] [CrossRef]
- Ahmad, Z.; Salman, S.; Khan, S.A.; Amin, A.; Rahman, Z.U.; Al-Ghamdi, Y.O.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Versatility of Hydrogels: From Synthetic Strategies, Classification, and Properties to Biomedical Applications. Gels 2022, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Singhal, R.; Gupta, K. A Review: Tailor-made Hydrogel Structures (Classifications and Synthesis Parameters). Polym.-Plast. Technol. Eng. 2016, 55, 54–70. [Google Scholar] [CrossRef]
- Rebers, L.; Reichsöllner, R.; Regett, S.; Tovar, G.E.M.; Borchers, K.; Baudis, S.; Southan, A. Differentiation of physical and chemical cross-linking in gelatin methacryloyl hydrogels. Sci. Rep. 2021, 11, 3256. [Google Scholar] [CrossRef] [PubMed]
- Parhi, R. Cross-Linked Hydrogel for Pharmaceutical Applications: A Review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Gadhave, R.V.; Mahanwar, P.A.; Gadekar, P.T. Effect of glutaraldehyde on thermal and mechanical properties of starch and polyvinyl alcohol blends. Des. Monomers Polym. 2019, 22, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, K.; Jiao, T.; Xing, R.; Hong, W.; Zhang, L.; Zhang, Q.; Peng, Q. Preparation of graphene oxide-polymer composite hydrogels via thiol-ene photopolymerization as efficient dye adsorbents for wastewater treatment. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 668–676. [Google Scholar] [CrossRef]
- Smith, L.J.; Taimoory, S.M.; Tam, R.Y.; Baker, A.E.G.; Mohammad, N.B.; Trant, J.F.; Shoichet, M.S. Diels–Alder Click-Cross-Linked Hydrogels with Increased Reactivity Enable 3D Cell Encapsulation. Biomacromolecules 2018, 19, 926–935. [Google Scholar] [CrossRef]
- Maitra, J.; Shukla, V.K. Cross-linking in Hydrogels—A Review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar]
- Elsayed, M.M. Hydrogel Preparation Technologies: Relevance Kinetics, Thermodynamics and Scaling up Aspects. J. Polym. Environ. 2019, 27, 871–891. [Google Scholar] [CrossRef]
- Nasution, H.; Harahap, H.; Dalimunthe, N.F.; Ginting, M.H.S.; Jaafar, M.; Tan, O.O.H.; Aruan, H.K.; Herfananda, A.L. Hydrogel and Effects of Crosslinking Agent on Cellulose-Based Hydrogels: A Review. Gels 2022, 8, 568. [Google Scholar] [CrossRef] [PubMed]
- Naranjo-Alcazar, R.; Bendix, S.; Groth, T.; Ferrer, G.G. Research Progress in Enzymatically Cross-Linked Hydrogels as Injectable Systems for Bioprinting and Tissue Engineering. Gels 2023, 9, 230. [Google Scholar] [CrossRef]
- Badali, E.; Hosseini, M.; Mohajer, M.; Hassanzadeh, S.; Saghati, S.; Hilborn, J.; Khanmohammadi, M. Enzymatic Crosslinked Hydrogels for Biomedical Application. Polym. Sci. Ser. A 2021, 63, S1–S22. [Google Scholar] [CrossRef]
- Song, W.; Ko, J.; Choi, Y.H.; Hwang, N.S. Recent advancements in enzyme-mediated crosslinkable hydrogels: In vivo-mimicking strategies. APL Bioeng. 2021, 5, 021502. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.; Martins, M.C.L.; Gomes, P. Grafting Techniques towards Production of Peptide-Tethered Hydrogels, a Novel Class of Materials with Biomedical Interest. Gels 2015, 1, 194–218. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.G.; Sayed, A.Z.; El-Wahab, H.A.; Sayah, M.M. Synthesis of a hydrogel by grafting of acrylamide-co-sodium methacrylate onto chitosan for effective adsorption of Fuchsin basic dye. Int. J. Biol. Macromol. 2020, 159, 422–432. [Google Scholar] [CrossRef]
- Chung, Y.-C.; Bae, J.C.; Choi, J.W.; Chun, B.C. The preparation of hydrogel-like polyurethane using the graft polymerization of N,N-dimethylaminoethyl methacrylate and acrylic acid. Polym. Bull. 2019, 76, 6371–6386. [Google Scholar] [CrossRef]
- Xu, J.; Abetz, V. Synthesis of a Degradable Hydrogel Based on a Graft Copolymer with Unexpected Thermoresponsiveness. Macromol. Chem. Phys. 2022, 223, 2200058. [Google Scholar] [CrossRef]
- Yang, J.; Rao, L.; Wang, Y.; Zhao, Y.; Liu, D.; Wang, Z.; Fu, L.; Wang, Y.; Yang, X.; Li, Y.; et al. Recent Advances in Smart Hydrogels Prepared by Ionizing Radiation Technology for Biomedical Applications. Polymers 2022, 14, 4377. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.A.; Jeong, J.-O.; Park, S.H. State-of-the-Art Irradiation Technology for Polymeric Hydrogel Fabrication and Application in Drug Release System. Front. Mater. 2021, 8, 769436. [Google Scholar] [CrossRef]
- Călina, I.; Demeter, M.; Scărișoreanu, A.; Sătulu, V.; Mitu, B. One Step e-Beam Radiation Cross-Linking of Quaternary Hydrogels Dressings Based on Chitosan-Poly(Vinyl-Pyrrolidone)-Poly(Ethylene Glycol)-Poly(Acrylic Acid). Int. J. Mol. Sci. 2020, 21, 9236. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Hasnine, S.M.M.; Ahmed, T.; Sultana, S.; Bhuiyan, M.A.Q.; Manir, M.S.; Ullah, N.; Sen, S.K.; Hossain, M.N.; Hossain, M.S.; et al. Radiation crosslinked polyvinyl alcohol/polyvinyl pyrrolidone/acrylic acid hydrogels: Swelling, crosslinking and dye adsorption study. Iran. Polym. J. 2021, 30, 1101–1116. [Google Scholar] [CrossRef]
- Jeong, J.-O.; Park, J.-S.; Kim, E.J.; Jeong, S.-I.; Lee, J.Y.; Lim, Y.-M. Preparation of Radiation Cross-Linked Poly(Acrylic Acid) Hydrogel Containing Metronidazole with Enhanced Antibacterial Activity. Int. J. Mol. Sci. 2020, 21, 187. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Kumar Singh, S.; Dhyani, A.; Juyal, D. Hydrogel: Preparation, Characterization and Applications. Pharma Innov. J. 2017, 6, 25–32. [Google Scholar]
- Dragan, E.S. Design and applications of interpenetrating polymer network hydrogels. A review. Chem. Eng. J. 2014, 243, 572–590. [Google Scholar] [CrossRef]
- Crosby, C.O.; Stern, B.; Kalkunte, N.; Pedahzur, S.; Ramesh, S.; Zoldan, J. Interpenetrating polymer network hydrogels as bioactive scaffolds for tissue engineering. Rev. Chem. Eng. 2022, 38, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Gugoasa, A.I.; Racovita, S.; Vasiliu, S.; Popa, M. Semi-Interpenetrating Polymer Networks Based on Hydroxy-Ethyl Methacrylate and Poly(4-vinylpyridine)/Polybetaines, as Supports for Sorption and Release of Tetracycline. Polymers 2023, 15, 490. [Google Scholar] [CrossRef]
- Dhand, A.P.; Galarraga, J.H.; Burdick, J.A. Enhancing Biopolymer Hydrogel Functionality through Interpenetrating Networks. Trends Biotechnol. 2021, 39, 519–538. [Google Scholar] [CrossRef]
- Han, F.; Wang, J.; Ding, L.; Hu, Y.; Li, W.; Yuan, Z.; Guo, Q.; Zhu, C.; Yu, L.; Wang, H.; et al. Tissue Engineering and Regenerative Medicine: Achievements, Future, and Sustainability in Asia. Front. Bioeng. Biotechnol. 2020, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.M.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions—A 21st Century Perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, J.; Athanasiou, K.A. The Role of Tissue Engineering in Articular Cartilage Repair and Regeneration. Crit. Rev. Biomed. Eng. 2009, 37, 1–57. [Google Scholar] [CrossRef]
- Li, X.; Sun, Q.; Li, Q.; Kawazoe, N.; Chen, G. Functional Hydrogels with Tunable Structures and Properties for Tissue Engineering Applications. Front. Chem. 2018, 6, 499. [Google Scholar] [CrossRef]
- Nie, J.; Pei, B.; Wang, Z.; Hu, Q. Construction of ordered structure in polysaccharide hydrogel: A review. Carbohydr. Polym. 2019, 205, 225–235. [Google Scholar] [CrossRef]
- Gibbs, D.M.R.; Black, C.R.M.; Dawson, J.I.; Oreffo, R.O.C. A review of hydrogel use in fracture healing and bone regeneration. J. Tissue Eng. Regen. Med. 2014, 10, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Kolambkar, Y.M.; Dupont, K.M.; Boerckel, J.D.; Huebsch, N.; Mooney, D.J.; Hutmacher, D.W.; Guldberg, R.E. An alginate-based hybrid system for growth factor delivery in the functional repair of large bone defects. Biomaterials 2011, 32, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Gao, M.; Syed, S.; Zhuang, J.; Xu, X.; Zhang, X.-Q. Bioactive hydrogels for bone regeneration. Bioact. Mater. 2018, 3, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yu, C.; Xiong, Y.; Chen, K.; Liu, P.; Panayi, A.C.; Xiao, X.; Feng, Q.; Mi, B.; Liu, G. Multifunctional hydrogel enhances bone regeneration through sustained release of Stromal Cell-Derived Factor-1α and exosomes. Bioact. Mater. 2023, 25, 460–471. [Google Scholar] [CrossRef]
- Li, D.; Zhou, J.; Zhang, M.; Ma, Y.; Yang, Y.; Han, X.; Wang, X. Long-term delivery of alendronate through an injectable tetra-PEG hydrogel to promote osteoporosis therapy. Biomater. Sci. 2020, 8, 3138–3146. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, G.; Ruan, H.; Chen, K.; Cai, Z.; Lu, G.; Li, R.; Deng, L.; Cai, M.; Cui, W. Capturing Magnesium Ions via Microfluidic Hydrogel Microspheres for Promoting Cancellous Bone Regeneration. ACS Nano 2021, 15, 13041–13054. [Google Scholar] [CrossRef]
- Gong, Y.; Bu, Y.; Li, Y.; Hao, D.; He, B.; Kong, L.; Huang, W.; Gao, X.; Zhang, B.; Qu, Z.; et al. Hydrogel-based delivery system applied in the local anti-osteoporotic bone defects. Front. Bioeng. Biotechnol. 2022, 10, 1058300. [Google Scholar] [CrossRef]
- Bai, H.; Zhao, Y.; Wang, C.; Wang, Z.; Wang, J.; Liu, H.; Feng, Y.; Lin, Q.; Li, Z.; Liu, H. Enhanced osseointegration of three-dimensional supramolecular bioactive interface through osteoporotic microenvironment regulation. Theranostics 2020, 10, 4779–4794. [Google Scholar] [CrossRef]
- Ding, W.; Zhou, Q.; Lu, Y.; Wei, Q.; Tang, H.; Zhang, D.; Liu, Z.; Wang, G.; Wu, D. ROS-scavenging hydrogel as protective carrier to regulate stem cells activity and promote osteointegration of 3D printed porous titanium prosthesis in osteoporosis. Front. Bioeng. Biotechnol. 2023, 11, 1103611. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.-B.; Kwon, Y.; Kim, J.; Hong, K.H.; Kim, S.-E.; Song, H.-R.; Kim, Y.-M.; Song, S.-C. Injectable polymeric nanoparticle hydrogel system for long-term anti-inflammatory effect to treat osteoarthritis. Bioact. Mater. 2022, 7, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Vinikoor, T.; Dzidotor, G.K.; Le, T.T.; Liu, Y.; Kan, H.-M.; Barui, S.; Chorsi, M.T.; Curry, E.J.; Reinhardt, E.; Wang, H.; et al. Injectable and biodegradable piezoelectric hydrogel for osteoarthritis treatment. Nat. Commun. 2023, 14, 6257. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.-L.; Zhang, L.-N.; Bohara, R.; Martin-Saldaña, S.; Yang, F.; Zhao, Y.-Y.; Xie, Y.; Bu, Y.-Z.; Pandit, A. Adhesive hydrogels in osteoarthritis: From design to application. Mil. Med. Res. 2023, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Qiu, Y.; Qu, L.; Wang, Q.; Zhou, Q. Hydrogels for Treatment of Different Degrees of Osteoarthritis. Front. Bioeng. Biotechnol. 2022, 10, 858656. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, G.; Cao, Q.; Zhao, T.; Bian, Y.; Zhu, W.; Weng, X. Recent Developments and Current Applications of Organic Nanomaterials in Cartilage Repair. Bioengineering 2022, 9, 390. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef]
- Ghandforoushan, P.; Alehosseini, M.; Golafshan, N.; Castilho, M.; Dolatshahi-Pirouz, A.; Hanaee, J.; Davaran, S.; Orive, G. Injectable hydrogels for cartilage and bone tissue regeneration: A review. Int. J. Biol. Macromol. 2023, 246, 125674. [Google Scholar] [CrossRef]
- Jung, S.W.; Oh, S.H.; Lee, I.S.; Byun, J.-H.; Lee, J.H. In Situ Gelling Hydrogel with Anti-Bacterial Activity and Bone Healing Property for Treatment of Osteomyelitis. Tissue Eng. Regen. Med. 2019, 16, 479–490. [Google Scholar] [CrossRef]
- Xie, X.; Wei, J.; Zhang, B.; Xiong, W.; He, Z.; Zhang, Y.; Gao, C.; Zhao, Y.; Liu, B. A self-assembled bilayer polypeptide-engineered hydrogel for spatiotemporal modulation of bactericidal and anti-inflammation process in osteomyelitis treatment. J. Nanobiotechnol. 2022, 20, 416. [Google Scholar] [CrossRef]
- Xin, W.; Gao, Y.; Yue, B. Recent Advances in Multifunctional Hydrogels for the Treatment of Osteomyelitis. Front. Bioeng. Biotechnol. 2022, 10, 865250. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-Y.; Lin, T.-Y.; Yeh, Y.-C. Hydrogel-Based Strategies for the Management of Osteomyelitis. ACS Biomater. Sci. Eng. 2023, 9, 1843–1861. [Google Scholar] [CrossRef] [PubMed]
- Vega, S.L.; Kwon, M.Y.; Burdick, J.A. Recent advances in hydrogels for cartilage tissue engineering. Eur. Cells Mater. 2017, 33, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Escalante, S.; Rico, G.; Becerra, J.; Román, J.S.; Vázquez-Lasa, B.; Aguilar, M.R.; Durán, I.; García-Fernández, L. Chemically crosslinked hyaluronic acid-chitosan hydrogel for application on cartilage regeneration. Front. Bioeng. Biotechnol. 2022, 10, 1058355. [Google Scholar] [CrossRef]
- Spiller, K.L.; Maher, S.A.; Lowman, A.M. Hydrogels for the Repair of Articular Cartilage Defects. Tissue Eng. Part B Rev. 2011, 17, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Zhang, S.; Liu, X.; Zhao, Y.; Yang, J.; Chai, G.; Wang, N.; Ma, S.; Liu, W.; Ding, C. Hydrogel Tissue Bioengineered Scaffolds in Bone Repair: A Review. Molecules 2023, 28, 7039. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Hu, Y.; Wang, S.; Chen, X.; Jiang, Y.; Su, J. Fabrication of physical and chemical crosslinked hydrogels for bone tissue engineering. Bioact. Mater. 2022, 12, 327–339. [Google Scholar] [CrossRef]
- Sun, Y.; Nan, D.; Jin, H.; Qu, X. Recent advances of injectable hydrogels for drug delivery and tissue engineering applications. Polym. Test. 2020, 81, 106283. [Google Scholar] [CrossRef]
- Almawash, S.; Osman, S.K.; Mustafa, G.; El Hamd, M.A. Current and Future Prospective of Injectable Hydrogels—Design Challenges and Limitations. Pharmaceuticals 2022, 15, 371. [Google Scholar] [CrossRef]
- Olov, N.; Bagheri-Khoulenjani, S.; Mirzadeh, H. Injectable hydrogels for bone and cartilage tissue engineering: A review. Prog. Biomater. 2022, 11, 113–135. [Google Scholar] [CrossRef] [PubMed]
- Martorell, L.; López-Fernández, A.; García-Lizarribar, A.; Sabata, R.; Gálvez-Martín, P.; Samitier, J.; Vives, J. Preservation of critical quality attributes of mesenchymal stromal cells in 3D bioprinted structures by using natural hydrogel scaffolds. Biotechnol. Bioeng. 2023, 120, 2717–2724. [Google Scholar] [CrossRef]
- Fatimi, A.; Okoro, O.V.; Podstawczyk, D.; Siminska-Stanny, J.; Shavandi, A. Natural Hydrogel-Based Bio-Inks for 3D Bioprinting in Tissue Engineering: A Review. Gels 2022, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Chyzy, A.; Plonska-Brzezinska, M.E. Hydrogel Properties and Their Impact on Regenerative Medicine and Tissue Engineering. Molecules 2020, 25, 5795. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xiong, J.; Wang, D.; Zhang, J.; Yang, L.; Sun, S.; Liang, Y. 3D Bioprinting of Hydrogels for Cartilage Tissue Engineering. Gels 2021, 7, 144. [Google Scholar] [CrossRef]
- Yang, X.; Li, S.; Ren, Y.; Qiang, L.; Liu, Y.; Wang, J.; Dai, K. 3D printed hydrogel for articular cartilage regeneration. Compos. Part B Eng. 2022, 237, 109863. [Google Scholar] [CrossRef]
- Yang, Z.; Yi, P.; Liu, Z.; Zhang, W.; Mei, L.; Feng, C.; Tu, C.; Li, Z. Stem Cell-Laden Hydrogel-Based 3D Bioprinting for Bone and Cartilage Tissue Engineering. Front. Bioeng. Biotechnol. 2022, 10, 865770. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Ma, Y.; Hou, C.; Gao, F.; Zhang, Y.; Ruan, C.; Pan, H.; Lu, W.W.; Liu, W. 3D-Printed High Strength Bioactive Supramolecular Polymer/Clay Nanocomposite Hydrogel Scaffold for Bone Regeneration. ACS Biomater. Sci. Eng. 2017, 3, 1109–1118. [Google Scholar] [CrossRef]
- Kang, X.; Zhang, X.-B.; Gao, X.-D.; Hao, D.-J.; Li, T.; Xu, Z.-W. Bioprinting for bone tissue engineering. Front. Bioeng. Biotechnol. 2022, 10, 1036375. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Yang, Z.; Ma, R.; Aimaijiang, M.; Xu, J.; Zhang, Y.; Zhou, Y. Four-Dimensional Printing and Shape Memory Materials in Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 814. [Google Scholar] [CrossRef]
- Wan, Z.; Zhang, P.; Liu, Y.; Lv, L.; Zhou, Y. Four-dimensional bioprinting: Current developments and applications in bone tissue engineering. Acta Biomater. 2020, 101, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, L.; Liu, K.; Gao, F. Hydrogel scaffolds in bone regeneration: Their promising roles in angiogenesis. Front. Pharmacol. 2023, 14, 1050954. [Google Scholar] [CrossRef] [PubMed]
- Short, A.R.; Koralla, D.; Deshmukh, A.; Wissel, B.; Stocker, B.; Calhoun, M.; Dean, D.; Winter, J.O. Hydrogels that allow and facilitate bone repair, remodeling, and regeneration. J. Mater. Chem. B 2015, 3, 7818–7830. [Google Scholar] [CrossRef] [PubMed]
- Shekaran, A.; García, J.R.; Clark, A.Y.; Kavanaugh, T.E.; Lin, A.S.; Guldberg, R.E.; García, A.J. Bone regeneration using an alpha 2 beta 1 integrin-specific hydrogel as a BMP-2 delivery vehicle. Biomaterials 2014, 35, 5453–5461. [Google Scholar] [CrossRef] [PubMed]
- Mi, L.; Liu, H.; Gao, Y.; Miao, H.; Ruan, J. Injectable nanoparticles/hydrogels composite as sustained release system with stromal cell-derived factor-1α for calvarial bone regeneration. Int. J. Biol. Macromol. 2017, 101, 341–347. [Google Scholar] [CrossRef]
- Zhang, F.; Lv, M.; Wang, S.; Li, M.; Wang, Y.; Hu, C.; Hu, W.; Wang, X.; Wang, X.; Liu, Z.; et al. Ultrasound-triggered biomimetic ultrashort peptide nanofiber hydrogels promote bone regeneration by modulating macrophage and the osteogenic immune microenvironment. Bioact. Mater. 2024, 31, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.-L.; Fu, W.; Ding, Y.-J.; Hettinghouse, A.; Lendhey, M.; Schwarzkopf, R.; Kennedy, O.D.; Liu, C.-J. Progranulin derivative Atsttrin protects against early osteoarthritis in mouse and rat models. Arthritis Res. Ther. 2017, 19, 280. [Google Scholar] [CrossRef]
- Moradi, L.; Witek, L.; Nayak, V.V.; Pereira, A.C.; Kim, E.; Good, J.; Liu, C.-J. Injectable hydrogel for sustained delivery of progranulin derivative Atsttrin in treating diabetic fracture healing. Biomaterials 2023, 301, 122289. [Google Scholar] [CrossRef]
- Wang, S.; He, W.; Wang, H.; Liu, D.; Wang, M.; Yang, H.; Pan, G.; Li, B. Hematoma-like dynamic hydrogelation through natural glycopeptide molecular recognition for infected bone fracture repair. Bioact. Mater. 2023, 30, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhou, Z.; Li, Q.; Li, W.; Li, X.; Li, G.; Fan, J.; Yu, L.; Yin, G. Dendronized chitosan hydrogel with GIT1 to accelerate bone defect repair through increasing local neovascular amount. Bone Rep. 2023, 19, 101712. [Google Scholar] [CrossRef]
- Moussi, H.; Weiss, P.; Le Bideau, J.; Gautier, H.; Charbonnier, B. Injectable macromolecule-based calcium phosphate bone substitutes. Mater. Adv. 2022, 3, 6125–6141. [Google Scholar] [CrossRef]
- Fatimi, A.; Tassin, J.-F.; Axelos, M.A.V.; Weiss, P. The stability mechanisms of an injectable calcium phosphate ceramic suspension. J. Mater. Sci. Mater. Med. 2010, 21, 1799–1809. [Google Scholar] [CrossRef] [PubMed]
- Fatimi, A.; Tassin, J.-F.; Turczyn, R.; Axelos, M.A.; Weiss, P. Gelation studies of a cellulose-based biohydrogel: The influence of pH, temperature and sterilization. Acta Biomater. 2009, 5, 3423–3432. [Google Scholar] [CrossRef] [PubMed]
- Fatimi, A.; Tassin, J.-F.; Bosco, J.; Deterre, R.; Axelos, M.A.V.; Weiss, P. Injection of calcium phosphate pastes: Prediction of injection force and comparison with experiments. J. Mater. Sci. Mater. Med. 2012, 23, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Sen, K.S.; Campos, D.F.D.; Köpf, M.; Blaeser, A.; Fischer, H. The Effect of Addition of Calcium Phosphate Particles to Hydrogel-Based Composite Materials on Stiffness and Differentiation of Mesenchymal Stromal Cells toward Osteogenesis. Adv. Health Mater. 2018, 7, e1800343. [Google Scholar] [CrossRef]
- Svarca, A.; Grava, A.; Dubnika, A.; Ramata-Stunda, A.; Narnickis, R.; Aunina, K.; Rieksta, E.; Boroduskis, M.; Jurgelane, I.; Locs, J.; et al. Calcium Phosphate/Hyaluronic Acid Composite Hydrogels for Local Antiosteoporotic Drug Delivery. Front. Bioeng. Biotechnol. 2022, 10, 917765. [Google Scholar] [CrossRef] [PubMed]
- Soliman, T.; Ali, Z.; Zayed, M.; Sabry, D.; AbuBakr, N. Assessing the bone-healing potential of bone marrow mesenchymal stem cells in jawbone osteoporosis in albino rats. Dent. Med. Probl. 2022, 59, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Zhong, H.; Huang, S.; Lai, W.; Huang, Y.; Sun, C.; Zhang, Y.; Zheng, S. Reactive Oxygen Species Scavenging Hydrogel Regulates Stem Cell Behavior and Promotes Bone Healing in Osteoporosis. Tissue Eng. Regen. Med. 2023, 20, 981–992. [Google Scholar] [CrossRef]
- Kim, G.; Park, Y.S.; Lee, Y.; Jin, Y.M.; Choi, D.H.; Ryu, K.-H.; Park, Y.J.; Park, K.D.; Jo, I. Tonsil-derived mesenchymal stem cell-embedded in situ crosslinkable gelatin hydrogel therapy recovers postmenopausal osteoporosis through bone regeneration. PLoS ONE 2018, 13, e0200111. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.-S.; Cui, X.; Hou, R.-X.; Li, Q.; Deng, H.-X.; Fu, J. Tough biodegradable chitosan–gelatin hydrogels via in situ precipitation for potential cartilage tissue engineering. RSC Adv. 2015, 5, 55640–55647. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, Y.; Shen, J.; Cai, Z.; Zhao, C.; Chen, H.; Luo, X.; Hu, N.; Cui, W.; Huang, W. Injectable hydrogel microspheres with self-renewable hydration layers alleviate osteoarthritis. Sci. Adv. 2022, 8, eabl6449. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yang, J.; Zhao, W.; Wang, H.; Sun, Y.; Chen, Y.; Luo, J.; Deng, L.; Xu, X.; Cui, W.; et al. Biomimetic injectable hydrogel microspheres with enhanced lubrication and controllable drug release for the treatment of osteoarthritis. Bioact. Mater. 2021, 6, 3596–3607. [Google Scholar] [CrossRef] [PubMed]
- Rigogliuso, S.; Salamone, M.; Barbarino, E.; Barbarino, M.; Nicosia, A.; Ghersi, G. Production of Injectable Marine Collagen-Based Hydrogel for the Maintenance of Differentiated Chondrocytes in Tissue Engineering Applications. Int. J. Mol. Sci. 2020, 21, 5798. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Yuan, L.; Li, J.; Wang, Z.; Chen, J.; Guo, C.; Mo, X.; Yan, Z. Injectable double-crosslinked hydrogels with kartogenin-conjugated polyurethane nano-particles and transforming growth factor β3 for in-situ cartilage regeneration. Mater. Sci. Eng. C 2020, 110, 110705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Hu, P.; Liu, T.; Li, Z.; Huang, Y.; Liao, J.; Hamid, M.R.; Wen, L.; Wang, T.; Mo, C.; et al. Kartogenin hydrolysis product 4-aminobiphenyl distributes to cartilage and mediates cartilage regeneration. Theranostics 2019, 9, 7108–7121. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Chen, P.; Chen, Y.; Li, M.; Chen, C.; Lu, H. 3D-Printed Extracellular Matrix/Polyethylene Glycol Diacrylate Hydrogel Incorporating the Anti-inflammatory Phytomolecule Honokiol for Regeneration of Osteochondral Defects. Am. J. Sports Med. 2020, 48, 2808–2818. [Google Scholar] [CrossRef]
- Rauf, A.; Olatunde, A.; Imran, M.; Alhumaydhi, F.A.; Aljohani, A.S.M.; Khan, S.A.; Uddin, M.S.; Mitra, S.; Bin Emran, T.; Khayrullin, M.; et al. Honokiol: A review of its pharmacological potential and therapeutic insights. Phytomedicine 2021, 90, 153647. [Google Scholar] [CrossRef]
- Nemeth, C.L.; Janebodin, K.; Yuan, A.E.; Dennis, J.E.; Reyes, M.; Kim, D.-H. Enhanced Chondrogenic Differentiation of Dental Pulp Stem Cells Using Nanopatterned PEG-GelMA-HA Hydrogels. Tissue Eng. Part A 2014, 20, 2817–2829. [Google Scholar] [CrossRef]
- Thakur, A.; Jaiswal, M.K.; Peak, C.W.; Carrow, J.K.; Gentry, J.; Dolatshahi-Pirouz, A.; Gaharwar, A.K. Injectable shear-thinning nanoengineered hydrogels for stem cell delivery. Nanoscale 2016, 8, 12362–12372. [Google Scholar] [CrossRef] [PubMed]
- Sá-Lima, H.; Caridade, S.G.; Mano, J.F.; Reis, R.L. Stimuli-responsive chitosan-starch injectable hydrogels combined with encapsulated adipose-derived stromal cells for articular cartilage regeneration. Soft Matter 2010, 6, 5184–5195. [Google Scholar] [CrossRef]
- Hafezi, M.; Khorasani, S.N.; Zare, M.; Neisiany, R.E.; Davoodi, P. Advanced Hydrogels for Cartilage Tissue Engineering: Recent Progress and Future Directions. Polymers 2021, 13, 4199. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, P.; Li, X.; Xu, Y.; Lu, G.; Jiang, Q.; Sun, Y.; Fan, Y.; Zhang, X. A di-self-crosslinking hyaluronan-based hydrogel combined with type I collagen to construct a biomimetic injectable cartilage-filling scaffold. Acta Biomater. 2020, 111, 197–207. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Leon-Oliva, D.; Boaru, D.L.; Perez-Exposito, R.E.; Fraile-Martinez, O.; García-Montero, C.; Diaz, R.; Bujan, J.; García-Honduvilla, N.; Lopez-Gonzalez, L.; Álvarez-Mon, M.; et al. Advanced Hydrogel-Based Strategies for Enhanced Bone and Cartilage Regeneration: A Comprehensive Review. Gels 2023, 9, 885. https://doi.org/10.3390/gels9110885
De Leon-Oliva D, Boaru DL, Perez-Exposito RE, Fraile-Martinez O, García-Montero C, Diaz R, Bujan J, García-Honduvilla N, Lopez-Gonzalez L, Álvarez-Mon M, et al. Advanced Hydrogel-Based Strategies for Enhanced Bone and Cartilage Regeneration: A Comprehensive Review. Gels. 2023; 9(11):885. https://doi.org/10.3390/gels9110885
Chicago/Turabian StyleDe Leon-Oliva, Diego, Diego Liviu Boaru, Roque Emilio Perez-Exposito, Oscar Fraile-Martinez, Cielo García-Montero, Raul Diaz, Julia Bujan, Natalio García-Honduvilla, Laura Lopez-Gonzalez, Melchor Álvarez-Mon, and et al. 2023. "Advanced Hydrogel-Based Strategies for Enhanced Bone and Cartilage Regeneration: A Comprehensive Review" Gels 9, no. 11: 885. https://doi.org/10.3390/gels9110885
APA StyleDe Leon-Oliva, D., Boaru, D. L., Perez-Exposito, R. E., Fraile-Martinez, O., García-Montero, C., Diaz, R., Bujan, J., García-Honduvilla, N., Lopez-Gonzalez, L., Álvarez-Mon, M., Saz, J. V., de la Torre, B., & Ortega, M. A. (2023). Advanced Hydrogel-Based Strategies for Enhanced Bone and Cartilage Regeneration: A Comprehensive Review. Gels, 9(11), 885. https://doi.org/10.3390/gels9110885











