Mechano-Chemical Effect of Gelatin- and HA-Based Hydrogels on Human Retinal Progenitor Cells
Abstract
1. Introduction
2. Results
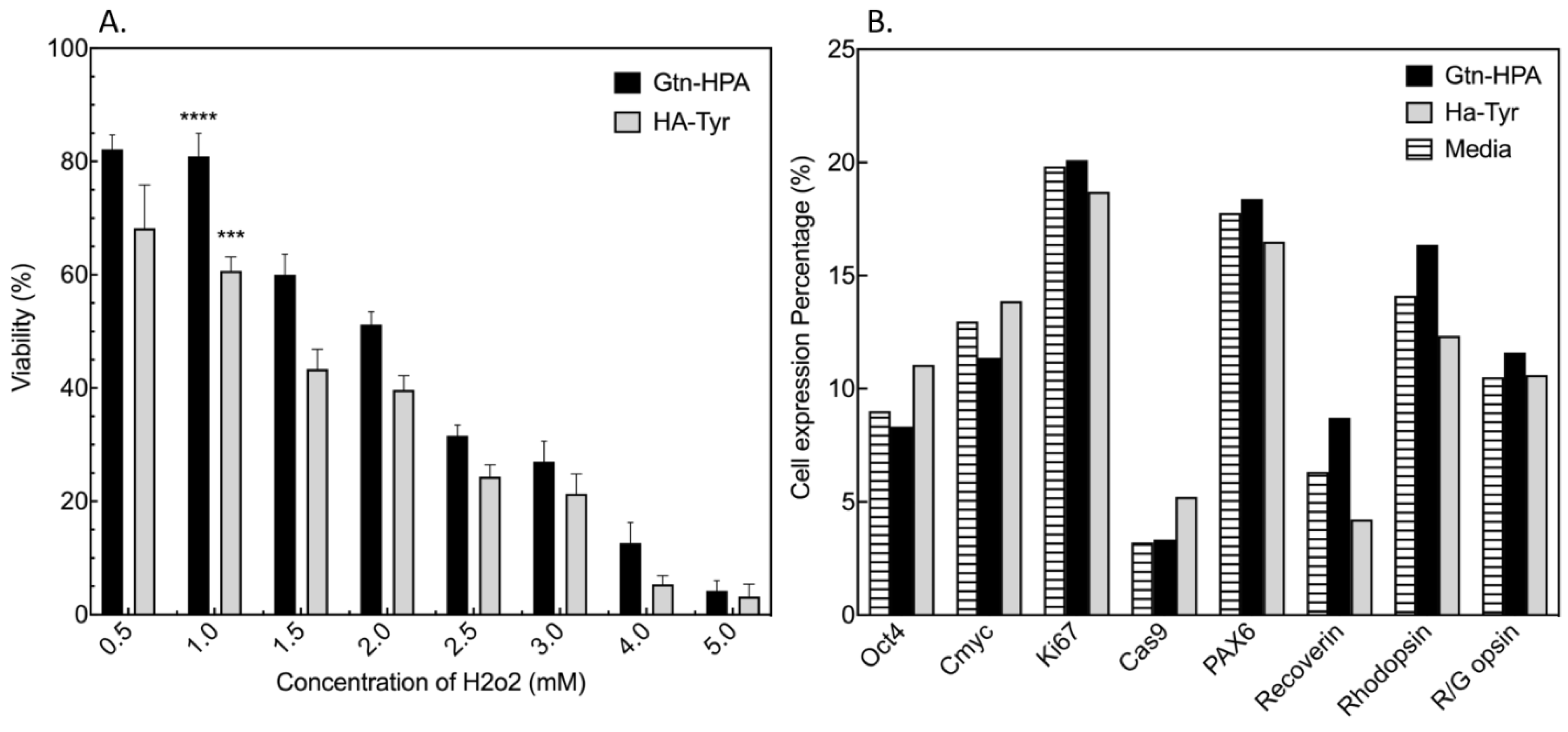
2.1. Testing of Short-Term Cell Viability and Phenotype in Gtn-HPA and HA-Tyr Hydrogels
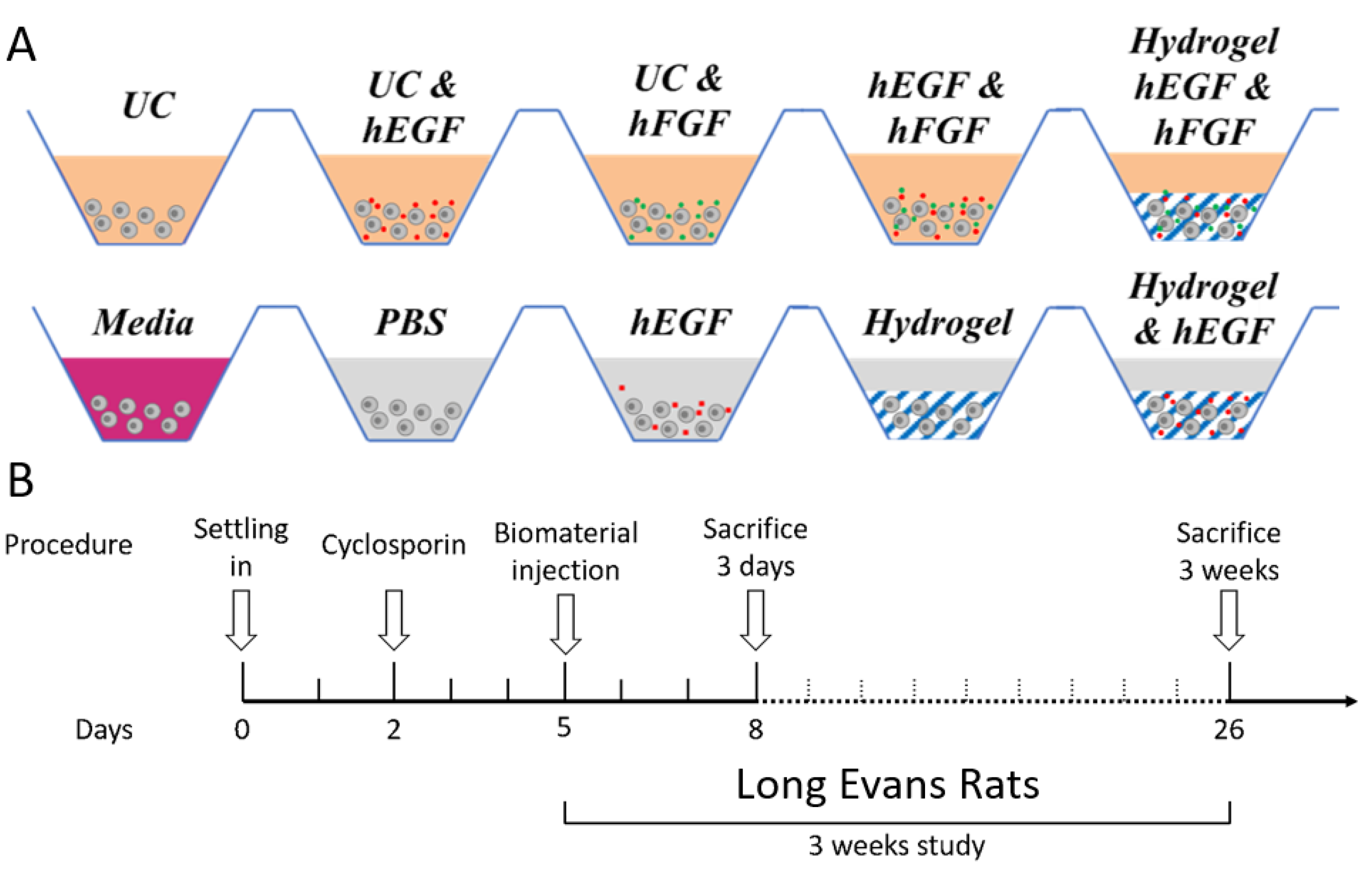
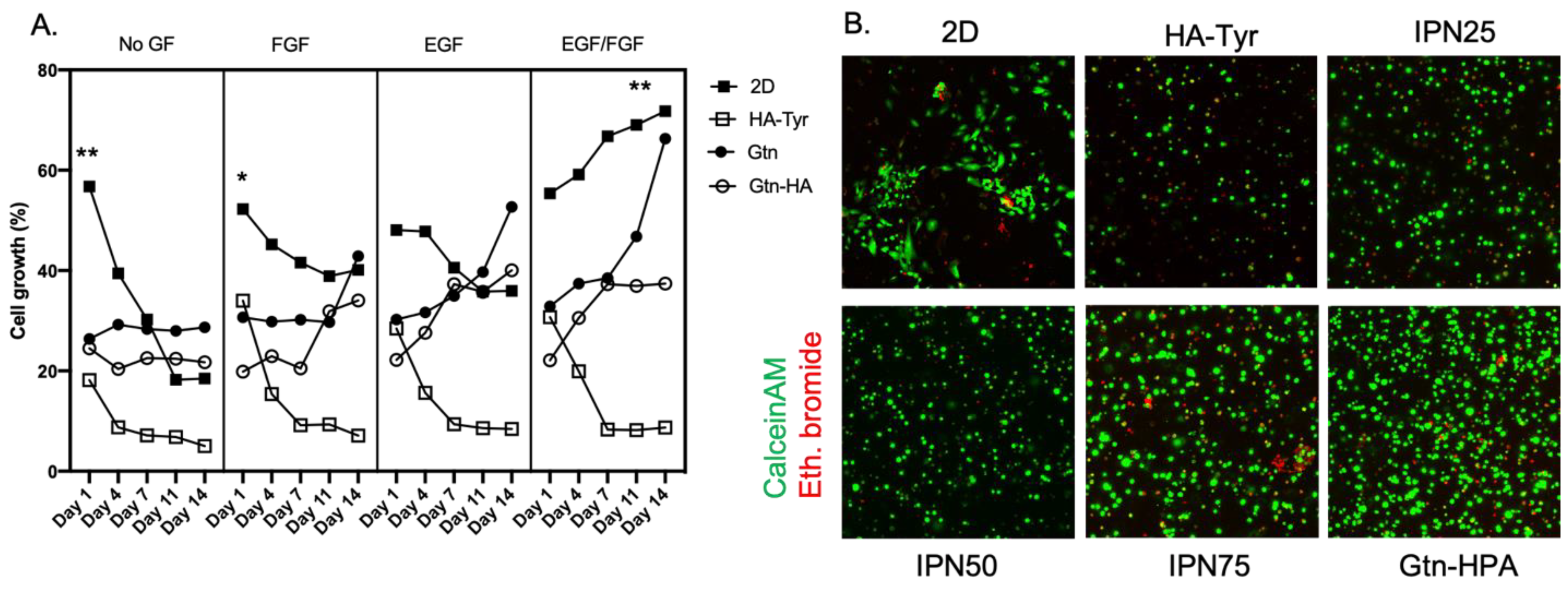
2.2. Improvement of Viability and Proliferation of hRPCs Encapsulated in Biocompatible Hydrogels
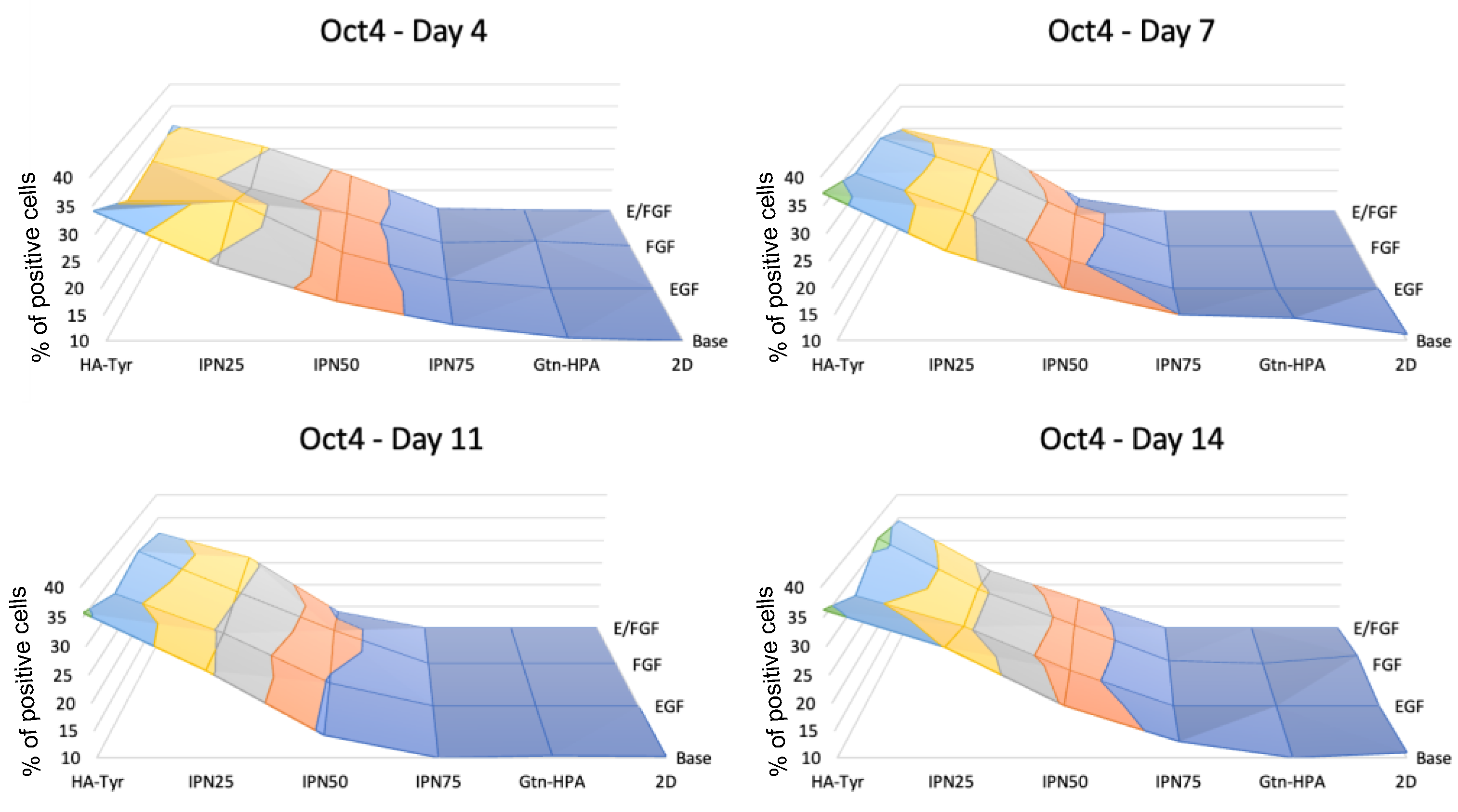
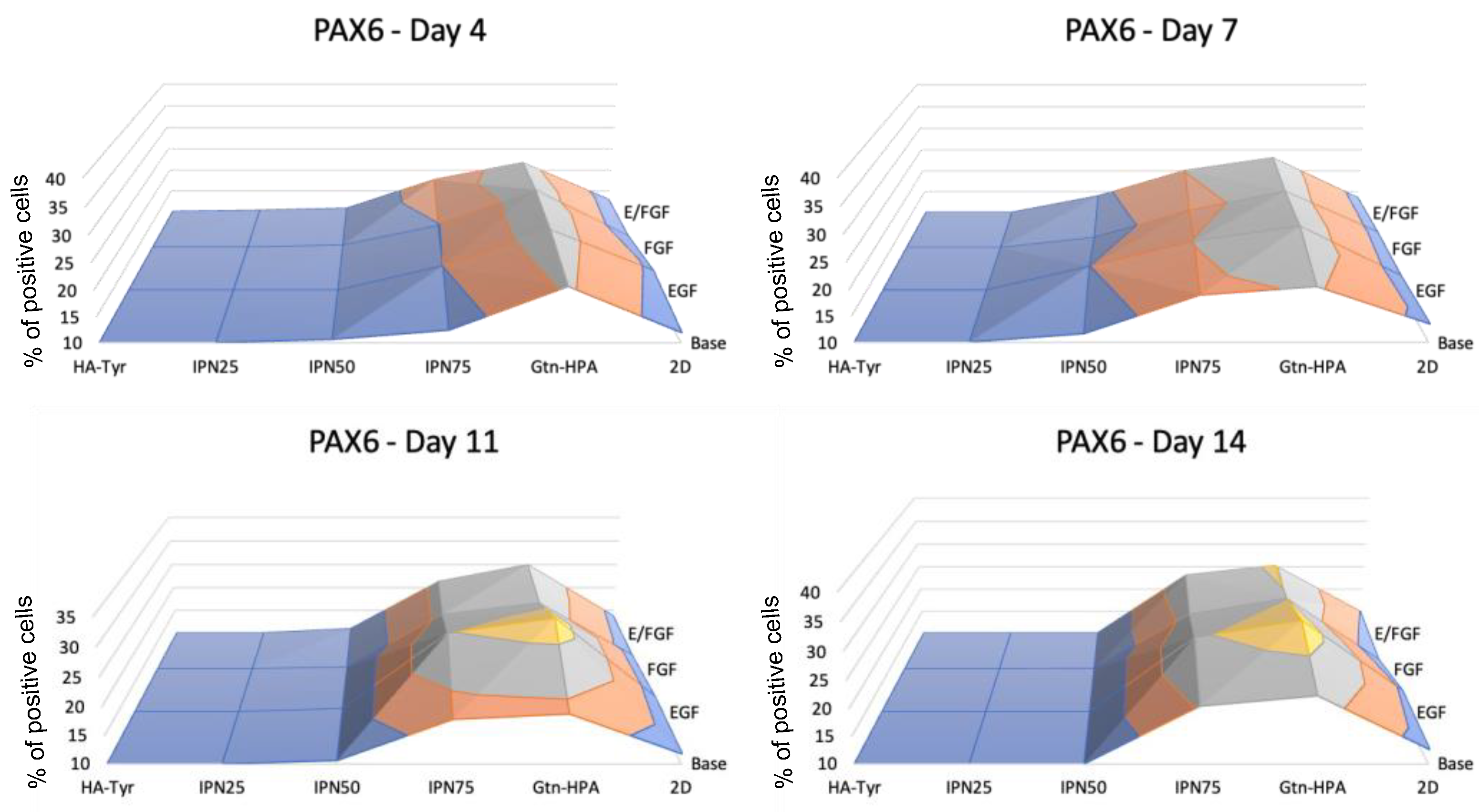
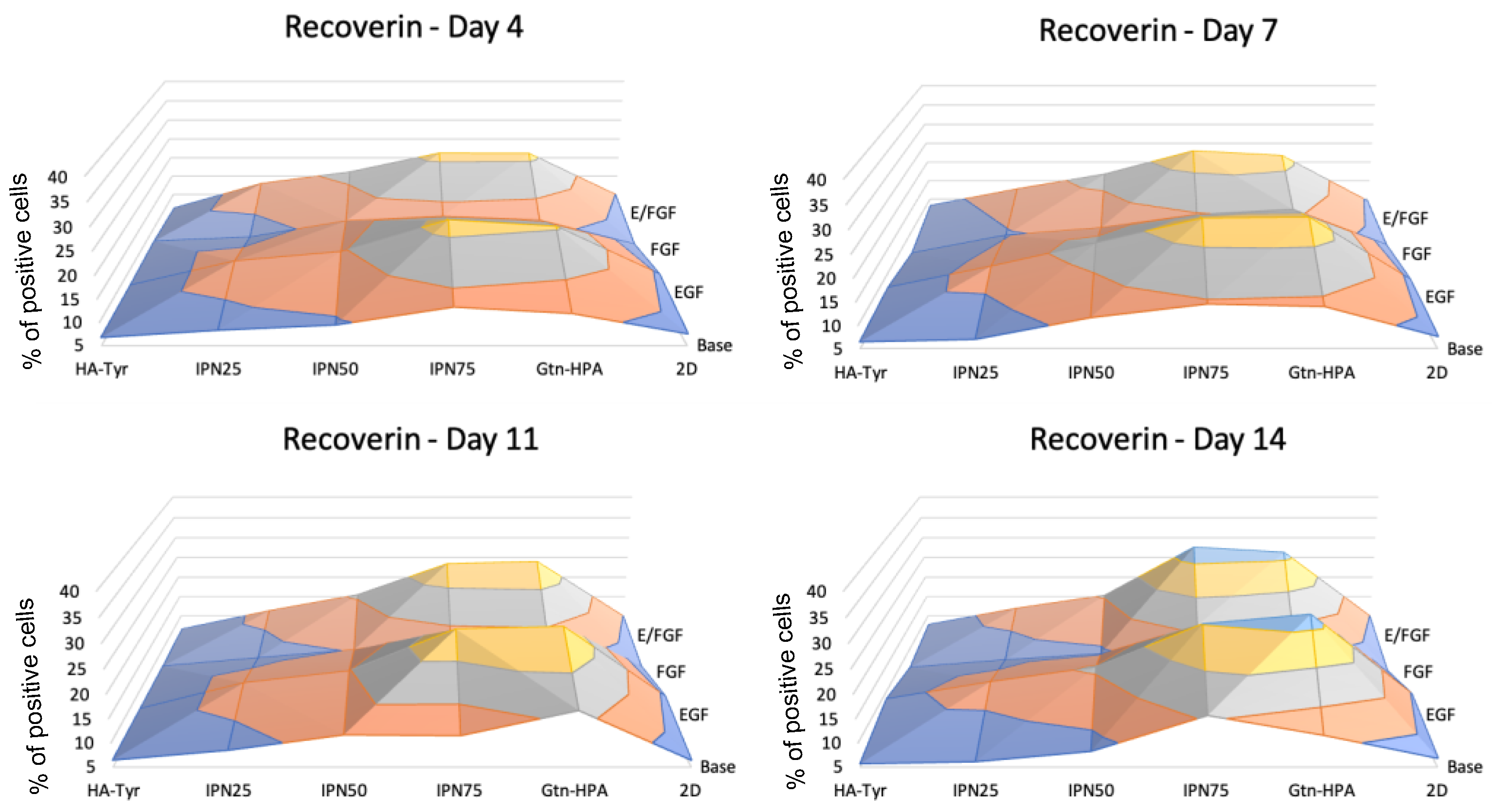
2.3. Encapsulated hRPC Differentiation Is Driven by the Interpenetrating Network Content
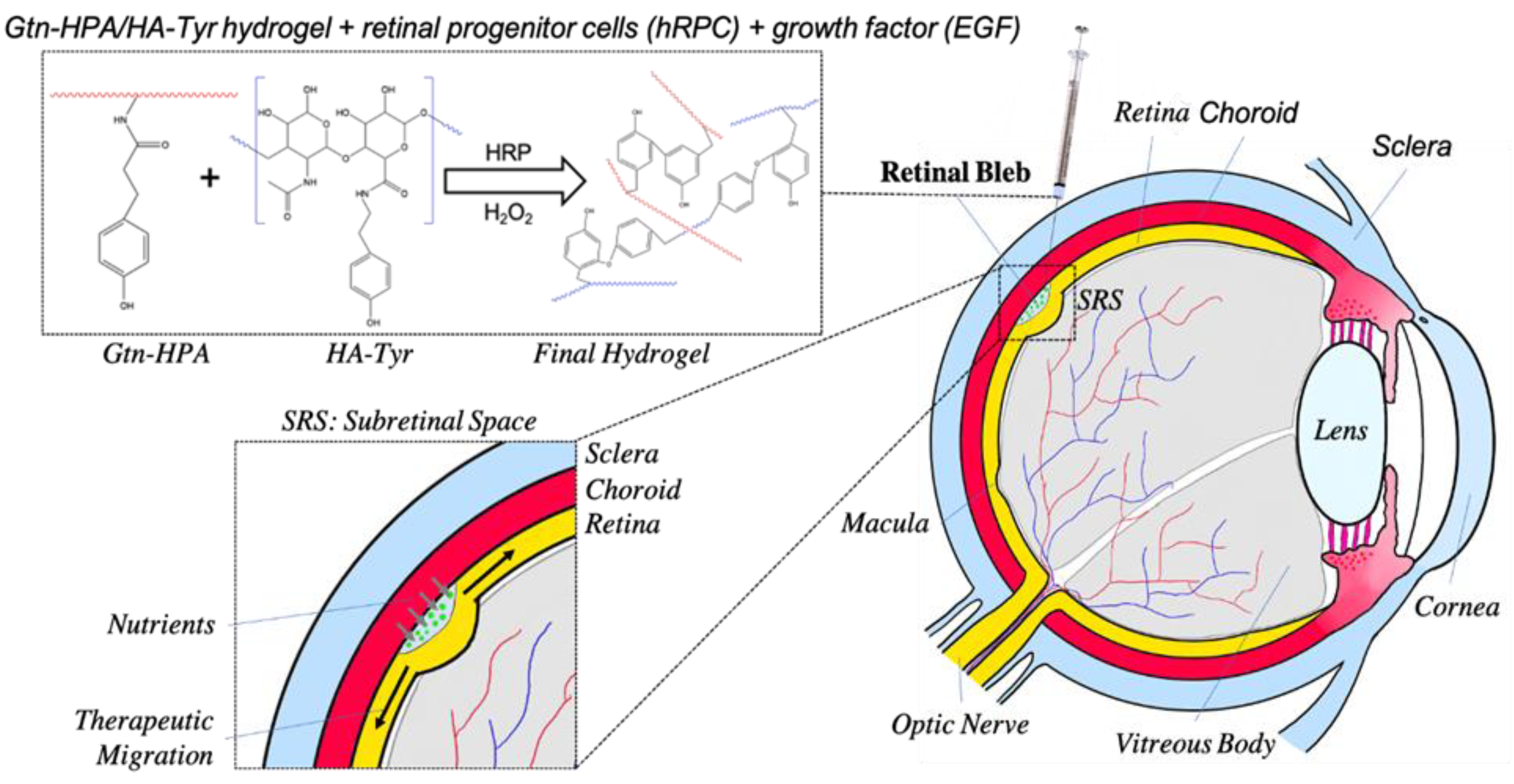
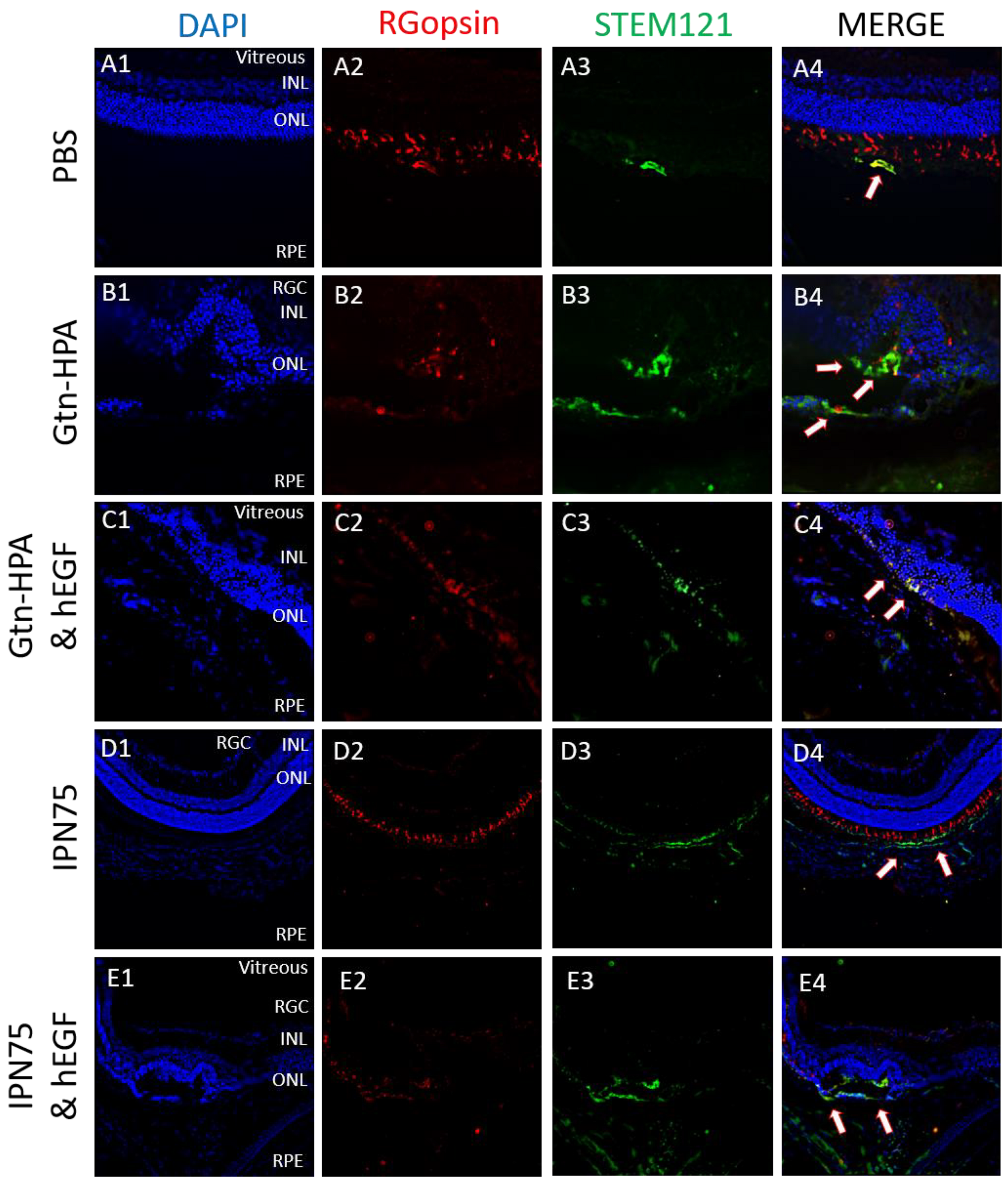
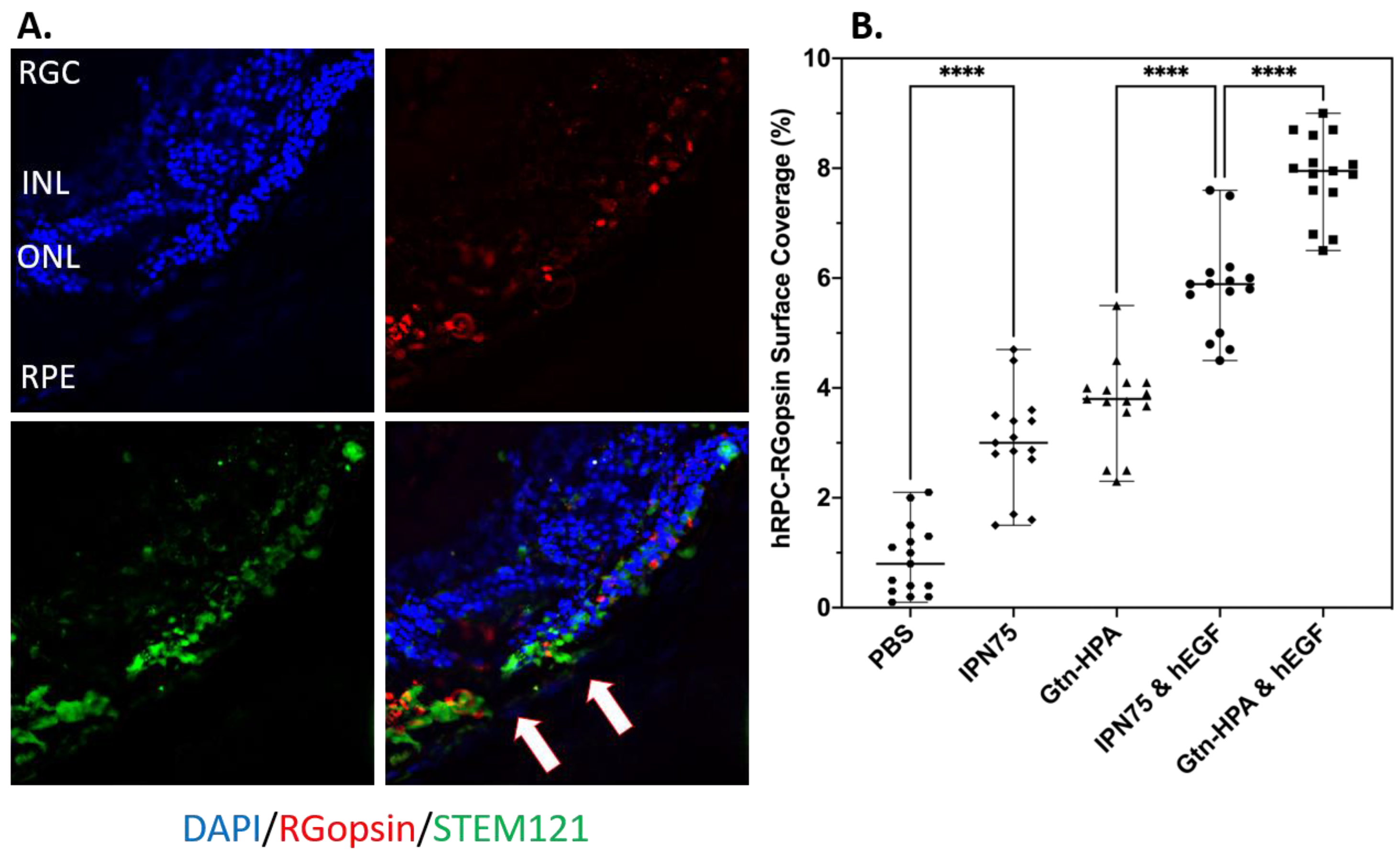
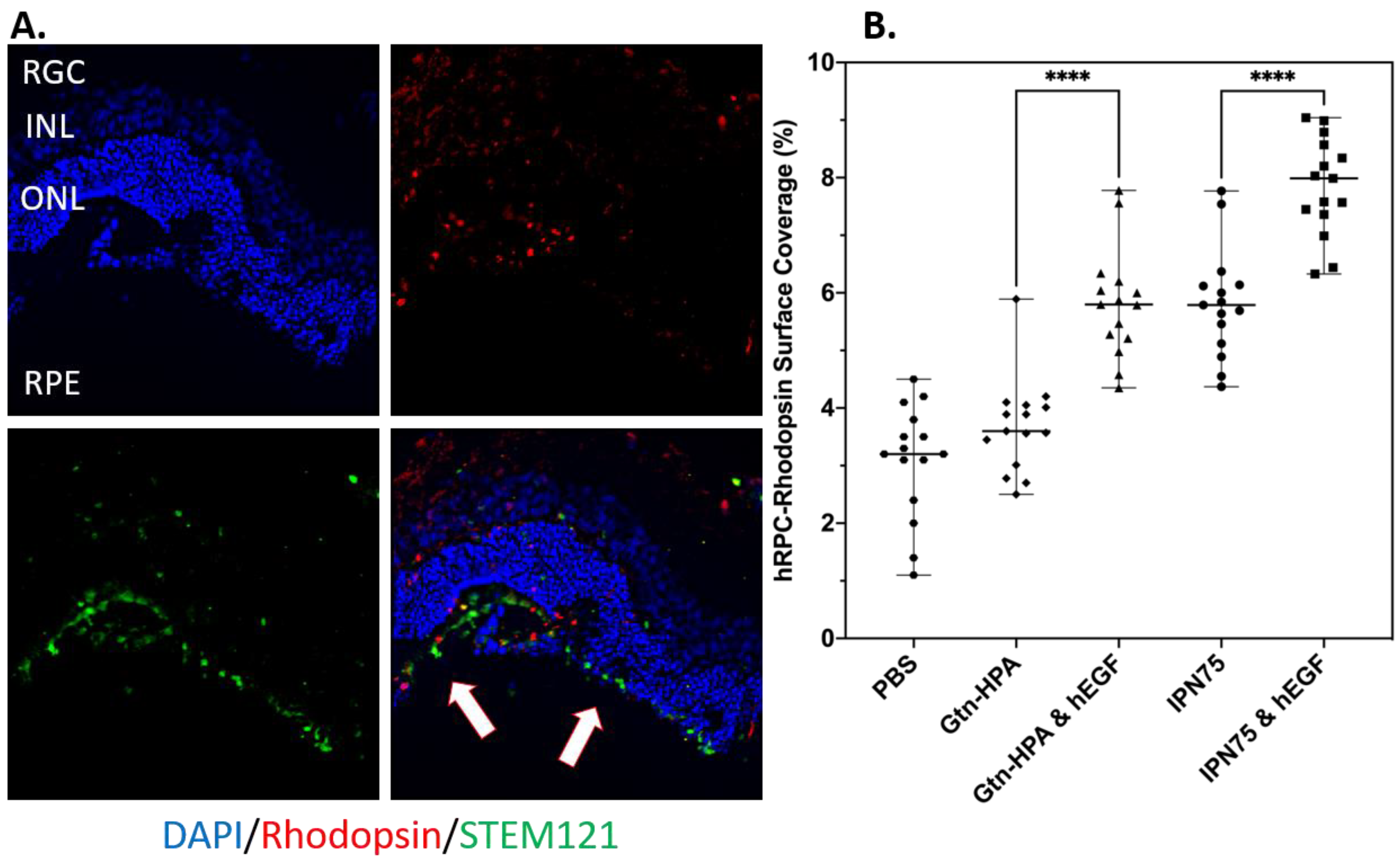
2.4. Subretinal Transplantation of Encapsulated hRPCs in Different Hydrogels
2.5. Immunoresponse from Host for Encapsulated hRPCs in Different Hydrogels
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Human Retinal Progenitor Cell Culture
5.2. Hydrogel Preparation and Crosslinking
5.3. Viability and Proliferation Assays
5.4. Phenotype Assay with Flow Cytometry
5.5. Oscillatory Rheology Measurement of Gel Stiffness
5.6. In Vivo Xenograft Study—Animals and Surgery
5.7. Control and Measured Outcomes
- % of cells engrafted expressing STEM121 (Human),
- % of cells engrafted expressing rhodopsin (Rods),
- % of cells engrafted expressing R/G opsin (Cones),
- % of cells engrafted expressing DAPI (Nuclei), and
- Position of engrafted in cells in retinal layer.
5.8. Tissue Processing
5.9. Immunofluorescence Staining
5.10. Image Processing and Analysis
5.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, D.; Wang, S.B.; Xia, T.; Tainsh, L.; Ghiassi-Nejad, M.; Xu, T.; Peng, S.; Adelman, R.A.; Rizzolo, L.J. A biodegradable scaffold enhances differentiation of embryonic stem cells into a thick sheet of retinal cells. Biomaterials 2018, 154, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Baranov, P.; Patel, S.; Ouyang, H.; Quach, J.; Wu, F.; Qiu, A.; Luo, H.; Hicks, C.; Zeng, J.; et al. Human retinal progenitor cell transplantation preserves vision. J. Biol. Chem. 2014, 289, 6362–6371. [Google Scholar] [CrossRef] [PubMed]
- Safety of Repeat Intravitreal Injection of Human Retinal Progenitor Cells (jCell) in Adult Subjects With Retinitis Pigmentosa. Available online: https://clinicaltrials.gov/ct2/show/NCT04604899 (accessed on 25 November 2022).
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Waldron, P.V.; Di Marco, F.; Kruczek, K.; Ribeiro, J.; Graca, A.B.; Hippert, C.; Aghaizu, N.D.; Kalargyrou, A.A.; Barber, A.C.; Grimaldi, G.; et al. Transplanted Donor- or Stem Cell-Derived Cone Photoreceptors Can Both Integrate and Undergo Material Transfer in an Environment-Dependent Manner. Stem Cell Rep. 2018, 10, 406–421. [Google Scholar] [CrossRef] [PubMed]
- Kador, K.E.; Goldberg, J.L. Scaffolds and stem cells: Delivery of cell transplants for retinal degenerations. Expert Rev. Ophthalmol. 2012, 7, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.V.; Bull, N.D.; Martin, K.R. Identification of barriers to retinal engraftment of transplanted stem cells. Investig. Ophthalmol. Vis. Sci. 2010, 51, 960–970. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Cao, H.Q.; Shi, L.Y.; Ng, S.Y.; Stanton, L.W.; Chew, S.Y. Nanofiber topography and sustained biochemical signaling enhance human mesenchymal stem cell neural commitment. Acta Biomater. 2012, 8, 1290–1302. [Google Scholar] [CrossRef] [PubMed]
- Baranov, P.; Regatieri, C.; Melo, G.; Clissold, H.; Young, M. Synthetic peptide-acrylate surface for self-renewal of human retinal progenitor cells. Tissue Eng. Part C Methods 2013, 19, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Marsano, A.; Maidhof, R.; Wan, L.Q.; Wang, Y.; Gao, J.; Tandon, N.; Vunjak-Novakovic, G. Scaffold stiffness affects the contractile function of three-dimensional engineered cardiac constructs. Biotechnol. Prog. 2010, 26, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Klassen, H.; Ziaeian, B.; Kirov, I.I.; Young, M.J.; Schwartz, P.H. Isolation of retinal progenitor cells from post-mortem human tissue and comparison with autologous brain progenitors. J. Neurosci. Res. 2004, 77, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Zeineddine, D.; Hammoud, A.A.; Mortada, M.; Boeuf, H. The Oct4 protein: More than a magic stemness marker. Am. J. Stem Cells 2014, 3, 74–82. [Google Scholar] [PubMed]
- Evans, N.D.; Minelli, C.; Gentleman, E.; LaPointe, V.; Patankar, S.N.; Kallivretaki, M.; Chen, X.; Roberts, C.J.; Stevens, M.M. Substrate stiffness affects early differentiation events in embryonic stem cells. Eur. Cell Mater. 2009, 18, 1–13; discussion 13–14. [Google Scholar] [CrossRef] [PubMed]
- Remez, L.A.; Onishi, A.; Menuchin-Lasowski, Y.; Biran, A.; Blackshaw, S.; Wahlin, K.J.; Zack, D.J.; Ashery-Padan, R. Pax6 is essential for the generation of late-born retinal neurons and for inhibition of photoreceptor-fate during late stages of retinogenesis. Dev. Biol. 2017, 432, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Dromel, P.C.; Singh, D.; Andres, E.; Likes, M.; Kurisawa, M.; Alexander-Katz, A.; Spector, M.; Young, M. A bioinspired gelatin-hyaluronic acid-based hybrid interpenetrating network for the enhancement of retinal ganglion cells replacement therapy. NPJ Regen. Med. 2021, 6, 85. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.T.; Wang, A.; Nguyen, A.B.; Iyer, J.; Tran, S.D. Recent Advances in Hydrogels: Ophthalmic Applications in Cell Delivery, Vitreous Substitutes, and Ocular Adhesives. Biomedicines 2021, 9, 1203. [Google Scholar] [CrossRef] [PubMed]
- Vilema-Enriquez, G.; Arroyo, A.; Grijalva, M.; Amador-Zafra, R.I.; Camacho, J. Molecular and Cellular Effects of Hydrogen Peroxide on Human Lung Cancer Cells: Potential Therapeutic Implications. Oxid. Med. Cell Longev. 2016, 2016, 1908164. [Google Scholar] [CrossRef] [PubMed]
- Klassen, H.J.; Ng, T.F.; Kurimoto, Y.; Kirov, I.; Shatos, M.; Coffey, P.; Young, M.J. Multipotent retinal progenitors express developmental markers, differentiate into retinal neurons, and preserve light-mediated behavior. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4167–4173. [Google Scholar] [CrossRef]
- Vazin, T.; Freed, W.J. Human embryonic stem cells: Derivation, culture, and differentiation: A review. Restor. Neurol. Neurosci. 2010, 28, 589–603. [Google Scholar] [CrossRef]
- Colombe Dromel, P.; Singh, D.; Alexander-Katz, A.; Kurisawa, M.; Spector, M.; Young, M. Injectable gelatin hydroxyphenyl propionic acid hydrogel protects human retinal progenitor cells (hRPCs) from shear stress applied during small-bore needle injection. Appl. Mater. Today 2020, 19, 100602. [Google Scholar] [CrossRef]
- Park, J.; Baranov, P.; Aydin, A.; Abdelgawad, H.; Singh, D.; Niu, W.; Kurisawa, M.; Spector, M.; Young, M.J. In Situ Cross-linking Hydrogel as a Vehicle for Retinal Progenitor Cell Transplantation. Cell Transpl. 2019, 28, 596–606. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dromel, P.C.; Singh, D.; Alexander-Katz, A.; Kurisawa, M.; Spector, M.; Young, M. Mechano-Chemical Effect of Gelatin- and HA-Based Hydrogels on Human Retinal Progenitor Cells. Gels 2023, 9, 58. https://doi.org/10.3390/gels9010058
Dromel PC, Singh D, Alexander-Katz A, Kurisawa M, Spector M, Young M. Mechano-Chemical Effect of Gelatin- and HA-Based Hydrogels on Human Retinal Progenitor Cells. Gels. 2023; 9(1):58. https://doi.org/10.3390/gels9010058
Chicago/Turabian StyleDromel, Pierre C., Deepti Singh, Alfredo Alexander-Katz, Motoichi Kurisawa, Myron Spector, and Michael Young. 2023. "Mechano-Chemical Effect of Gelatin- and HA-Based Hydrogels on Human Retinal Progenitor Cells" Gels 9, no. 1: 58. https://doi.org/10.3390/gels9010058
APA StyleDromel, P. C., Singh, D., Alexander-Katz, A., Kurisawa, M., Spector, M., & Young, M. (2023). Mechano-Chemical Effect of Gelatin- and HA-Based Hydrogels on Human Retinal Progenitor Cells. Gels, 9(1), 58. https://doi.org/10.3390/gels9010058






