Gene Regulations upon Hydrogel-Mediated Drug Delivery Systems in Skin Cancers—An Overview
Abstract
1. Introduction
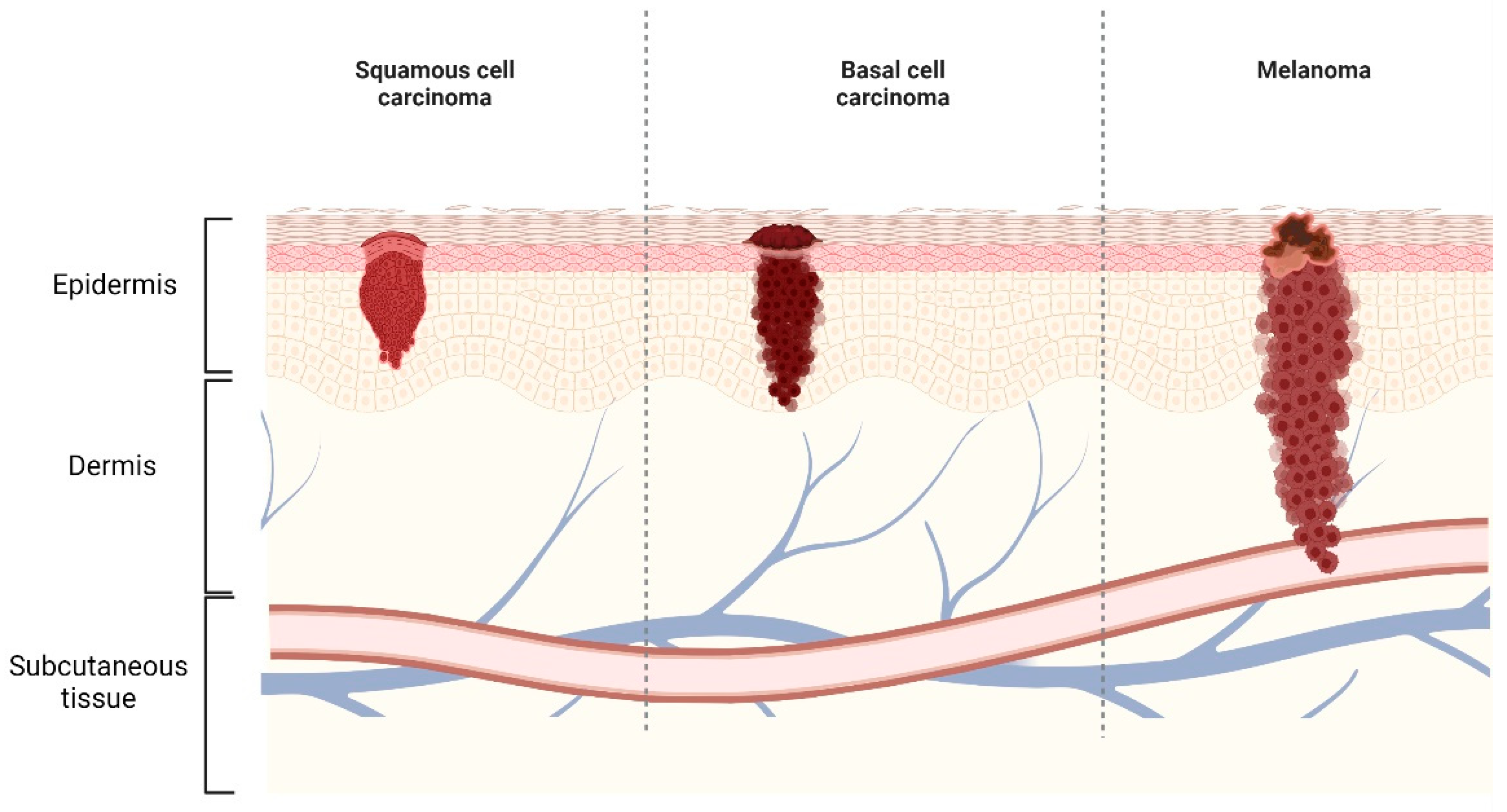
2. Skin Cancer
2.1. NMSC
2.2. Melanoma
2.3. Current Treatments and Drugs for Skin Cancers
3. Hydrogel: Promising Drug Delivery Systems to Treat Skin Cancers
4. A Brief Background on the Use of Hydrogel-Based Drug Delivery System to Regulate Skin Cancer Related Genes
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- You, L.; Cho, J.Y. The regulatory role of Korean ginseng in skin cells. J. Ginseng Res. 2021, 45, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.; Garg, A. An insight of techniques for the assessment of permeation flux across the skin for optimization of topical and transdermal drug delivery systems. J. Drug Deliv. Sci. Technol. 2021, 62, 102355. [Google Scholar] [CrossRef]
- Bouwstra, J.A.; Ponec, M. The skin barrier in healthy and diseased state. Biochim. Biophys. Acta (BBA) Biomembr. 2006, 1758, 2080–2095. [Google Scholar] [CrossRef]
- Katz, M.; Poulsen, B.J. Absorption of drugs through the skin. In Concepts in Biochemical Pharmacology; Springer: Berlin/Heidelberg, Germany, 1971; pp. 103–174. [Google Scholar]
- Haque, T.; Rahman, K.M.; Thurston, D.E.; Hadgraft, J.; Lane, M.E. Topical therapies for skin cancer and actinic keratosis. Eur. J. Pharm. Sci. 2015, 77, 279–289. [Google Scholar] [CrossRef]
- Marzi, M.; Rostami Chijan, M.; Zarenezhad, E. Hydrogels as promising therapeutic strategy for the treatment of skin cancer. J. Mol. Struct. 2022, 1262, 133014. [Google Scholar] [CrossRef]
- Vishnubhakthula, S.; Elupula, R.; Durán-Lara, E.F. Recent advances in hydrogel-based drug delivery for melanoma cancer therapy: A mini review. J. Drug Deliv. 2017, 2017, 7275985. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.M. From precursor to cancer: Field cancerization and the opportunities for therapy. In Non-Surgical Treatment of Keratinocyte Skin Cancer; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–7. [Google Scholar]
- Gordon, R. Skin cancer: An overview of epidemiology and risk factors. Semin. Oncol. Nurs. 2013, 29, 160–169. [Google Scholar] [CrossRef]
- Maddodi, N.; Setaluri, V. Role of UV in cutaneous melanoma. Photochem. Photobiol. 2008, 84, 528–536. [Google Scholar] [CrossRef]
- Jerant, A.F.; Johnson, J.T.; Sheridan, C.D.; Caffrey, T.J. Early detection and treatment of skin cancer. Am. Fam. Physician 2000, 62, 357–368. [Google Scholar]
- Motley, R.; Kersey, P.; Lawrence, C. Multiprofessional guidelines for the management of the patient with primary cutaneous squamous cell carcinoma. Br. J. Dermatol. 2002, 146, 18–25. [Google Scholar] [CrossRef]
- Simões, M.F.; Sousa, J.S.; Pais, A.C. Skin cancer and new treatment perspectives: A review. Cancer Lett. 2015, 357, 8–42. [Google Scholar] [CrossRef] [PubMed]
- Nickoloff, B.J.; Qin, J.-Z.; Chaturvedi, V.; Bacon, P.; Panella, J.; Denning, M.F. Life and death signaling pathways contributing to skin cancer. J. Investig. Dermatol. Symp. Proc. 2002, 7, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Cobanoglu, H.B.; Constantinides, M.; Ural, A. Nonmelanoma skin cancer of the head and neck: Molecular mechanisms. Facial Plast. Surg. Clin. 2012, 20, 437–443. [Google Scholar] [CrossRef]
- Uribe, P.; Gonzalez, S. Epidermal growth factor receptor (EGFR) and squamous cell carcinoma of the skin: Molecular bases for EGFR-targeted therapy. Pathol. Res. Pract. 2011, 207, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Toll, A.; Salgado, R.; Yébenes, M.; Martín-Ezquerra, G.; Gilaberte, M.; Baró, T.; Solé, F.; Alameda, F.; Espinet, B.; Pujol, R.M. Epidermal growth factor receptor gene numerical aberrations are frequent events in actinic keratoses and invasive cutaneous squamous cell carcinomas. Exp. Dermatol. 2010, 19, 151–153. [Google Scholar] [CrossRef]
- Coleman, W.P.; Loria, P.R.; Reed, R.J.; Krementz, E.T. Acral lentiginous melanoma. Arch. Dermatol. 1980, 116, 773–776. [Google Scholar] [CrossRef]
- Abildgaard, C.; Guldberg, P. Molecular drivers of cellular metabolic reprogramming in melanoma. Trends Mol. Med. 2015, 21, 164–171. [Google Scholar] [CrossRef]
- Paluncic, J.; Kovacevic, Z.; Jansson, P.J.; Kalinowski, D.; Merlot, A.M.; Huang, M.L.-H.; Lok, H.C.; Sahni, S.; Lane, D.J.; Richardson, D.R. Roads to melanoma: Key pathways and emerging players in melanoma progression and oncogenic signaling. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2016, 1863, 770–784. [Google Scholar] [CrossRef]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef]
- Veer, L.J.V.T.; Burgering, B.M.; Versteeg, R.; Boot, A.J.; Ruiter, D.J.; Osanto, S.; Schrier, P.I.; Bos, J.L. N-ras mutations in human cutaneous melanoma from sun-exposed body sites. Mol. Cell. Biol. 1989, 9, 3114–3116. [Google Scholar] [CrossRef]
- Curtin, J.A.; Busam, K.; Pinkel, D.; Bastian, B.C. Somatic Activation of KIT in Distinct Subtypes of Melanoma. J. Clin. Oncol. 2006, 24, 4340–4346. [Google Scholar] [CrossRef] [PubMed]
- Dahl, C.; Abildgaard, C.; Riber-Hansen, R.; Steiniche, T.; Lade-Keller, J.; Guldberg, P. KIT Is a Frequent Target for Epigenetic Silencing in Cutaneous Melanoma. J. Investig. Dermatol. 2015, 135, 516–524. [Google Scholar] [CrossRef]
- Van Raamsdonk, C.D.; Bezrookove, V.; Green, G.; Bauer, J.; Gaugler, L.; O’Brien, J.M.; Simpson, E.M.; Barsh, G.S.; Bastian, B.C. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature 2009, 457, 599–602. [Google Scholar] [CrossRef] [PubMed]
- Van Raamsdonk, C.D.; Griewank, K.G.; Crosby, M.B.; Garrido, M.C.; Vemula, S.; Wiesner, T.; Obenauf, A.C.; Wackernagel, W.; Green, G.; Bouvier, N.; et al. Mutations in GNA11 in Uveal Melanoma. N. Engl. J. Med. 2010, 363, 2191–2199. [Google Scholar] [CrossRef] [PubMed]
- Freedberg, D.E.; Rigas, S.H.; Russak, J.; Gai, W.; Kaplow, M.; Osman, I.; Turner, F.; Randerson-Moor, J.A.; Houghton, A.; Busam, K.; et al. Frequent p16-Independent Inactivation of p14ARF in Human Melanoma. JNCI J. Natl. Cancer Inst. 2008, 100, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, G.; Dahl, C.; Staaf, J.; Sandberg, T.; Bendahl, P.O.; Ringnér, M.; Guldberg, P.; Borg, Å. Genomic profiling of malignant melanoma using tiling-resolution arrayCGH. Oncogene 2007, 26, 4738–4748. [Google Scholar] [CrossRef] [PubMed]
- Bartkova, J.; Lukas, J.; Guldberg, P.; Alsner, J.; Kirkin, A.F.; Zeuthen, J.; Bartek, J. The p16-cyclin D/Cdk4-pRb Pathway as a Functional Unit Frequently Altered in Melanoma Pathogenesis1. Cancer Res. 1996, 56, 5475–5483. [Google Scholar]
- Cully, M.; You, H.; Levine, A.J.; Mak, T.W. Beyond PTEN mutations: The PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat. Rev. Cancer 2006, 6, 184–192. [Google Scholar] [CrossRef]
- Guldberg, P.; Straten, P.T.; Ahrenkiel, V.; Seremet, T.; Kirkin, A.F.; Zeuthen, J. Somatic mutation of the Peutz-Jeghers syndrome gene, LKB1/STK11, in malignant melanoma. Oncogene 1999, 18, 1777–1780. [Google Scholar] [CrossRef]
- Rowan, A.; Tomlinson, I.; Bataille, V.; MacKie, R.; Healy, E.; Bicknell, D.; Bodmer, W. Somatic Mutations in the Peutz-Jegners (LKB1/STKII) Gene in Sporadic Malignant Melanomas. J. Investig. Dermatol. 1999, 112, 509–511. [Google Scholar] [CrossRef]
- Bertolotto, C.; Lesueur, F.; Giuliano, S.; Strub, T.; de Lichy, M.; Bille, K.; Dessen, P.; d’Hayer, B.; Mohamdi, H.; Remenieras, A.; et al. A SUMOylation-defective MITF germline mutation predisposes to melanoma and renal carcinoma. Nature 2011, 480, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Shtivelman, E.; Davies, M.Q.A.; Hwu, P.; Yang, J.; Lotem, M.; Oren, M.; Flaherty, K.T.; Fisher, D.E. Pathways and therapeutic targets in melanoma. Oncotarget 2014, 5, 1701–1752. [Google Scholar] [CrossRef] [PubMed]
- Maertens, O.; Johnson, B.; Hollstein, P.; Frederick, D.T.; Cooper, Z.A.; Messiaen, L.; Bronson, R.T.; McMahon, M.; Granter, S.; Flaherty, K.; et al. Elucidating Distinct Roles for NF1 in Melanomagenesis. Cancer Discov. 2013, 3, 338–349. [Google Scholar] [CrossRef]
- Kraehn, G.M.; Utikal, J.; Udart, M.; Greulich, K.M.; Bezold, G.; Kaskel, P.; Leiter, U.; Peter, R.U. Extra c-myc oncogene copies in high risk cutaneous malignant melanoma and melanoma metastases. Br. J. Cancer 2001, 84, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Polsky, D.; Cordon-Cardo, C. Oncogenes in melanoma. Oncogene 2003, 22, 3087–3091. [Google Scholar] [CrossRef]
- Sauter, E.R.; Yeo, U.-C.; von Stemm, A.; Zhu, W.; Litwin, S.; Tichansky, D.S.; Pistritto, G.; Nesbit, M.; Pinkel, D.; Herlyn, M. Cyclin D1 is a candidate oncogene in cutaneous melanoma. Cancer Res. 2002, 62, 3200–3206. [Google Scholar]
- Muthusamy, V.; Hobbs, C.; Nogueira, C.; Cordon-Cardo, C.; McKee, P.H.; Chin, L.; Bosenberg, M.W. Amplification of CDK4 and MDM2 in malignant melanoma. Genes Chromosomes Cancer 2006, 45, 447–454. [Google Scholar] [CrossRef]
- Polsky, D.; Melzer, K.; Hazan, C.; Panageas, K.S.; Busam, K.; Drobnjak, M.; Kamino, H.; Spira, J.G.; Kopf, A.W.; Houghton, A.; et al. HDM2 Protein Overexpression and Prognosis in Primary Malignant Melanoma. JNCI J. Natl. Cancer Inst. 2002, 94, 1803–1806. [Google Scholar] [CrossRef]
- Omholt, K.; Kröckel, D.; Ringborg, U.; Hansson, J. Mutations of PIK3CA are rare in cutaneous melanoma. Melanoma Res. 2006, 16, 197–200. [Google Scholar] [CrossRef]
- Davies, M.A.; Stemke-Hale, K.; Tellez, C.; Calderone, T.L.; Deng, W.; Prieto, V.G.; Lazar, A.J.F.; Gershenwald, J.E.; Mills, G.B. A novel AKT3 mutation in melanoma tumours and cell lines. Br. J. Cancer 2008, 99, 1265–1268. [Google Scholar] [CrossRef]
- Boone, B.; Jacobs, K.; Ferdinande, L.; Taildeman, J.; Lambert, J.; Peeters, M.; Bracke, M.; Pauwels, P.; Brochez, L. EGFR in melanoma: Clinical significance and potential therapeutic target. J. Cutan Pathol. 2011, 38, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Zhang, X.; Liu, J.; Estrem, S.; Li, S.; Gong, X.-Q.; Buchanan, S.; Henry, J.R.; Starling, J.J.; Peng, S.-B. Reactivation of mitogen-activated protein kinase (MAPK) pathway by FGF receptor 3 (FGFR3)/Ras mediates resistance to vemurafenib in human B-RAF V600E mutant melanoma. J. Biol. Chem. 2012, 287, 28087–28098. [Google Scholar] [CrossRef]
- Easty, D.J.; Gray, S.G.; O’Byrne, K.J.; O’Donnell, D.; Bennett, D.C. Receptor tyrosine kinases and their activation in melanoma. Pigment Cell Melanoma Res. 2011, 24, 446–461. [Google Scholar] [CrossRef] [PubMed]
- Hayward, N.K.; Wilmott, J.S.; Waddell, N.; Johansson, P.A.; Field, M.A.; Nones, K.; Patch, A.-M.; Kakavand, H.; Alexandrov, L.B.; Burke, H.; et al. Whole-genome landscapes of major melanoma subtypes. Nature 2017, 545, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wang, H.; Li, C. Signal pathways of melanoma and targeted therapy. Signal Transduct. Target. Ther. 2021, 6, 424. [Google Scholar] [CrossRef] [PubMed]
- Garraway, L.A.; Widlund, H.R.; Rubin, M.A.; Getz, G.; Berger, A.J.; Ramaswamy, S.; Beroukhim, R.; Milner, D.A.; Granter, S.R.; Du, J.; et al. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature 2005, 436, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Soufir, N.; Avril, M.-F.; Chompret, A.; Demenais, F.; Bombled, J.; Spatz, A.; Stoppa-Lyonnet, D.; Group, F.F.M.S.; Bénard, J.; de Paillerets, B.B. Prevalence of p16 and CDK4 germline mutations in 48 melanoma-prone families in France. Hum. Mol. Genet. 1998, 7, 209–216. [Google Scholar] [CrossRef]
- Zuo, L.; Weger, J.; Yang, Q.; Goldstein, A.M.; Tucker, M.A.; Walker, G.J.; Hayward, N.; Dracopoli, N.C. Germline mutations in the p16INK4a binding domain of CDK4 in familial melanoma. Nat. Genet. 1996, 12, 97–99. [Google Scholar] [CrossRef]
- Krishnan, V.; Mitragotri, S. Nanoparticles for topical drug delivery: Potential for skin cancer treatment. Adv. Drug Deliv. Rev. 2020, 153, 87–108. [Google Scholar] [CrossRef]
- Ishizuki, S.; Nakamura, Y. Evidence from Clinical Studies Related to Dermatologic Surgeries for Skin Cancer. Cancers 2022, 14, 3835. [Google Scholar] [CrossRef]
- Nanamori, H.; Sawada, Y. Epigenetic Modification of PD-1/PD-L1-Mediated Cancer Immunotherapy against Melanoma. Int. J. Mol. Sci. 2022, 23, 1119. [Google Scholar]
- Chinembiri, T.N.; Du Plessis, L.H.; Gerber, M.; Hamman, J.H.; Du Plessis, J. Review of natural compounds for potential skin cancer treatment. Molecules 2014, 19, 11679–11721. [Google Scholar] [PubMed]
- Fahradyan, A.; Howell, A.C.; Wolfswinkel, E.M.; Tsuha, M.; Sheth, P.; Wong, A.K. Updates on the management of non-melanoma skin cancer (NMSC). Healthcare 2017, 5, 82. [Google Scholar]
- Skok, K.; Zidarič, T.; Orthaber, K.; Pristovnik, M.; Kostevšek, N.; Rožman, K.Ž.; Šturm, S.; Gradišnik, L.; Maver, U.; Maver, T. Novel Methacrylate-Based Multilayer Nanofilms with Incorporated FePt-Based Nanoparticles and the Anticancer Drug 5-Fluorouracil for Skin Cancer Treatment. Pharmaceutics 2022, 14, 689. [Google Scholar] [PubMed]
- Yu, N.; Luo, X.; Wei, T.; Zhang, Q.; Yu, J.; Wu, L.; Su, J.; Chen, M.; Huang, K.; Li, F. Dermabrasion combined with photodynamic therapy: A new option for the treatment of non-melanoma skin cancer. Lasers Med. Sci. 2022, 37, 1255–1263. [Google Scholar] [PubMed]
- Tang, X.; Chen, X.; Zhang, S.; Gu, X.; Wu, R.; Huang, T.; Zhou, Z.; Sun, C.; Ling, J.; Liu, M. Silk-Inspired In Situ Hydrogel with Anti-Tumor Immunity Enhanced Photodynamic Therapy for Melanoma and Infected Wound Healing. Adv. Funct. Mater. 2021, 31, 2101320. [Google Scholar]
- Lucena, S.R.; Salazar, N.; Gracia-Cazaña, T.; Zamarrón, A.; González, S.; Juarranz, Á.; Gilaberte, Y. Combined treatments with photodynamic therapy for non-melanoma skin cancer. Int. J. Mol. Sci. 2015, 16, 25912–25933. [Google Scholar]
- Kanekura, T.; Higashi, Y.; Kanzaki, T. Inhibitory effects of 9-cis-retinoic acid and pyrrolidinedithiocarbamate on cyclooxygenase (COX)-2 expression and cell growth in human skin squamous carcinoma cells. Cancer Lett. 2000, 161, 177–183. [Google Scholar] [CrossRef]
- Buckman, S.; Gresham, A.; Hale, P.; Hruza, G.; Anast, J.; Masferrer, J.; Pentland, A.P. COX-2 expression is induced by UVB exposure in human skin: Implications for the development of skin cancer. Carcinogenesis 1998, 19, 723–729. [Google Scholar]
- Fecker, L.F.; Stockfleth, E.; Braun, F.K.; Rodust, P.M.; Schwarz, C.; Köhler, A.; Leverkus, M.; Eberle, J. Enhanced death ligand-induced apoptosis in cutaneous SCC cells by treatment with diclofenac/hyaluronic acid correlates with downregulation of c-FLIP. J. Investig. Dermatol. 2010, 130, 2098–2109. [Google Scholar]
- Sligh, J.E., Jr. New therapeutic options for actinic keratosis and basal cell carcinoma. Semin. Cutan Med. Surg. 2014, 33, S76–S80. [Google Scholar] [CrossRef][Green Version]
- Rosen, R.H.; Gupta, A.K.; Tyring, S.K. Dual mechanism of action of ingenol mebutate gel for topical treatment of actinic keratoses: Rapid lesion necrosis followed by lesion-specific immune response. J. Am. Acad. Dermatol. 2012, 66, 486–493. [Google Scholar] [PubMed]
- Kedei, N.; Lundberg, D.J.; Toth, A.; Welburn, P.; Garfield, S.H.; Blumberg, P.M. Characterization of the interaction of ingenol 3-angelate with protein kinase C. Cancer Res. 2004, 64, 3243–3255. [Google Scholar] [PubMed]
- Skeel, R.T.; Khleif, S.N. Handbook of Cancer Chemotherapy; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011. [Google Scholar]
- Luke, J.J.; Schwartz, G.K. Chemotherapy in the management of advanced cutaneous malignant melanoma. Clin. Dermatol. 2013, 31, 290–297. [Google Scholar] [PubMed]
- Landi, M.M.; Ahmadi, S.A.; Akhgar, M.R.; Ghazanfari, D. Theoretical study of the tautomerization of Carmustine in a biological media as an anti-cancer drug. J. Part. Sci. Technol. 2021, 7, 51–57. [Google Scholar]
- Larkin, J.M.G.; Hughes, S.A.; Beirne, D.A.; Patel, P.M.; Gibbens, I.M.; Bate, S.C.; Thomas, K.; Eisen, T.G.; Gore, M.E. A Phase I/II study of lomustine and temozolomide in patients with cerebral metastases from malignant melanoma. Br. J. Cancer 2007, 96, 44–48. [Google Scholar] [CrossRef]
- Livshits, Z.; Rao, R.B.; Smith, S.W. An approach to chemotherapy-associated toxicity. Emerg. Med. Clin. 2014, 32, 167–203. [Google Scholar]
- Rowinsky, E.K.; Donehower, R.C.; Jones, R.J.; Tucker, R.W. Microtubule changes and cytotoxicity in leukemic cell lines treated with taxol. Cancer Res. 1988, 48, 4093–4100. [Google Scholar]
- Manfredi, J.J.; Horwitz, S.B. Taxol: An antimitotic agent with a new mechanism of action. Pharmacol. Ther. 1984, 25, 83–125. [Google Scholar]
- Mhaidat, N.M.; Wang, Y.; Kiejda, K.A.; Zhang, X.D.; Hersey, P. Docetaxel-induced apoptosis in melanoma cells is dependent on activation of caspase-2. Mol. Cancer Ther. 2007, 6, 752–761. [Google Scholar]
- Meyer, T.; Surber, C.; French, L.E.; Stockfleth, E. Resiquimod, a topical drug for viral skin lesions and skin cancer. Expert Opin. Investig. Drugs 2013, 22, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Liner, K.; Brown, C.; McGirt, L.Y. Clinical potential of mechlorethamine gel for the topical treatment of mycosis fungoides-type cutaneous T-cell lymphoma: A review on current efficacy and safety data. Drug Des. Dev. Ther. 2018, 12, 241–254. [Google Scholar] [CrossRef]
- Minchinton, A.I.; Tannock, I.F. Drug penetration in solid tumours. Nat. Rev. Cancer 2006, 6, 583–592. [Google Scholar]
- Fujimura, T.; Kambayashi, Y.; Fujisawa, Y.; Hidaka, T.; Aiba, S. Tumor-associated macrophages: Therapeutic targets for skin cancer. Front. Oncol. 2018, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, S.R.; Tampa, M.; Mitran, C.I.; Mitran, M.I.; Caruntu, C.; Caruntu, A.; Lupu, M.; Matei, C.; Constantin, C.; Neagu, M. Tumour microenvironment in skin carcinogenesis. Tumor Microenviron. Organs 2020, 1226, 123–142. [Google Scholar]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [PubMed]
- Ghafouri-Fard, S.; Ghafouri-Fard, S. Immunotherapy in nonmelanoma skin cancer. Immunotherapy 2012, 4, 499–510. [Google Scholar] [CrossRef]
- Thambi, T.; Li, Y.; Lee, D.S. Injectable hydrogels for sustained release of therapeutic agents. J. Control. Release 2017, 267, 57–66. [Google Scholar] [CrossRef]
- Phan, V.H.G.; Le, T.M.D.; Janarthanan, G.; Ngo, P.-K.T.; Lee, D.S.; Thambi, T. Development of bioresorbable smart injectable hydrogels based on thermo-responsive copolymer integrated bovine serum albumin bioconjugates for accelerated healing of excisional wounds. J. Ind. Eng. Chem. 2021, 96, 345–355. [Google Scholar] [CrossRef]
- Duong, H.T.T.; Thambi, T.; Yin, Y.; Kim, S.H.; Nguyen, T.L.; Phan, V.H.G.; Kim, J.; Jeong, J.H.; Lee, D.S. Degradation-regulated architecture of injectable smart hydrogels enhances humoral immune response and potentiates antitumor activity in human lung carcinoma. Biomaterials 2020, 230, 119599. [Google Scholar] [CrossRef]
- Giang Phan, V.H.; Duong, H.T.T.; Thambi, T.; Nguyen, T.L.; Turabee, M.H.; Yin, Y.; Kim, S.H.; Kim, J.; Jeong, J.H.; Lee, D.S. Modularly engineered injectable hybrid hydrogels based on protein-polymer network as potent immunologic adjuvant in vivo. Biomaterials 2019, 195, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Luu, C.H.; Nguyen, G.; Le, T.-T.; Nguyen, T.-M.N.; Giang Phan, V.H.; Murugesan, M.; Mathiyalagan, R.; Jing, L.; Janarthanan, G.; Yang, D.C.; et al. Graphene Oxide-Reinforced Alginate Hydrogel for Controlled Release of Local Anesthetics: Synthesis, Characterization, and Release Studies. Gels 2022, 8, 246. [Google Scholar] [CrossRef] [PubMed]
- Buwalda, S.J.; Boere, K.W.M.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Khare, A.R. Preparation, structure and diffusional behavior of hydrogels in controlled release. Adv. Drug Deliv. Rev. 1993, 11, 1–35. [Google Scholar] [CrossRef]
- Thambi, T.; Giang Phan, V.H.; Kim, S.H.; Duy Le, T.M.; Duong, H.T.T.; Lee, D.S. Smart injectable biogels based on hyaluronic acid bioconjugates finely substituted with poly(β-amino ester urethane) for cancer therapy. Biomater. Sci. 2019, 7, 5424–5437. [Google Scholar] [CrossRef]
- Kim, S.H.; Thambi, T.; Giang Phan, V.H.; Lee, D.S. Modularly engineered alginate bioconjugate hydrogel as biocompatible injectable scaffold for in situ biomineralization. Carbohydr. Polym. 2020, 233, 115832. [Google Scholar] [CrossRef]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef]
- Sun, T.L.; Kurokawa, T.; Kuroda, S.; Ihsan, A.B.; Akasaki, T.; Sato, K.; Haque, M.; Nakajima, T.; Gong, J.P. Physical hydrogels composed of polyampholytes demonstrate high toughness and viscoelasticity. Nat. Mater. 2013, 12, 932–937. [Google Scholar] [CrossRef]
- Pang, X.; Wu, J.; Chu, C.-C.; Chen, X. Development of an arginine-based cationic hydrogel platform: Synthesis, characterization and biomedical applications. Acta Biomater. 2014, 10, 3098–3107. [Google Scholar] [CrossRef]
- Gil, M.S.; Cho, J.; Thambi, T.; Giang Phan, V.H.; Kwon, I.; Lee, D.S. Bioengineered robust hybrid hydrogels enrich the stability and efficacy of biological drugs. J. Control. Release 2017, 267, 119–132. [Google Scholar] [CrossRef]
- Anwary, M.; Kumar, P.; du Toit, L.C.; Choonara, Y.E.; Pillay, V. Polymeric, injectable, intravitreal hydrogel devices for posterior segment applications and interventions. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Phan, V.H.G.; Thambi, T.; Kim, B.S.; Huynh, D.P.; Lee, D.S. Engineering highly swellable dual-responsive protein-based injectable hydrogels: The effects of molecular structure and composition in vivo. Biomater. Sci. 2017, 5, 2285–2294. [Google Scholar] [CrossRef] [PubMed]
- Cipriano, B.H.; Banik, S.J.; Sharma, R.; Rumore, D.; Hwang, W.; Briber, R.M.; Raghavan, S.R. Superabsorbent hydrogels that are robust and highly stretchable. Macromolecules 2014, 47, 4445–4452. [Google Scholar] [CrossRef]
- Omidian, H.; Rocca, J.G.; Park, K. Advances in superporous hydrogels. J. Control. Release 2005, 102, 3–12. [Google Scholar] [CrossRef]
- Lee, J.H. Injectable hydrogels delivering therapeutic agents for disease treatment and tissue engineering. Biomater. Res. 2018, 22, 27. [Google Scholar] [CrossRef]
- Turabee, M.H.; Thambi, T.; Duong, H.T.T.; Jeong, J.H.; Lee, D.S. A pH- and temperature-responsive bioresorbable injectable hydrogel based on polypeptide block copolymers for the sustained delivery of proteins in vivo. Biomater. Sci. 2018, 6, 661–671. [Google Scholar] [CrossRef]
- Turabee, M.H.; Thambi, T.; Lym, J.S.; Lee, D.S. Bioresorbable polypeptide-based comb-polymers efficiently improves the stability and pharmacokinetics of proteins in vivo. Biomater. Sci. 2017, 5, 837–848. [Google Scholar] [CrossRef]
- Harrison, I.P.; Spada, F. Hydrogels for Atopic Dermatitis and Wound Management: A Superior Drug Delivery Vehicle. Pharmaceutics 2018, 10, 71. [Google Scholar] [CrossRef]
- Kim, S.H.; Thambi, T.; Lym, J.S.; Giang Phan, V.H.; Lee, D.S. Tunable Engineering of Heparinized Injectable Hydrogels for Affinity-Based Sustained Delivery of Bioactive Factors. Macromol. Mater. Eng. 2019, 304, 1900279. [Google Scholar] [CrossRef]
- Thambi, T.; Phan, V.H.G.; Lee, D.S. Stimuli-Sensitive Injectable Hydrogels Based on Polysaccharides and Their Biomedical Applications. Macromol. Rapid Commun. 2016, 37, 1881–1896. [Google Scholar] [CrossRef]
- Zagórska-Dziok, M.; Sobczak, M. Hydrogel-based active substance release systems for cosmetology and dermatology application: A review. Pharmaceutics 2020, 12, 396. [Google Scholar] [CrossRef] [PubMed]
- Thambi, T.; Giang Phan, V.H.; Kim, S.H.; Duy Le, T.M.; Lee, D.S. Hyaluronic acid decorated pH- and temperature-induced injectable bioconjugates for sustained delivery of bioactive factors and highly efficient wound regeneration. New J. Chem. 2019, 43, 18979–18982. [Google Scholar] [CrossRef]
- Phan, V.H.G.; Murugesan, M.; Manivasagan, P.; Nguyen, T.L.; Phan, T.-H.; Luu, C.H.; Ho, D.-K.; Li, Y.; Kim, J.; Lee, D.S.; et al. Injectable Hydrogel Based on Protein-Polyester Microporous Network as an Implantable Niche for Active Cell Recruitment. Pharmaceutics 2022, 14, 709. [Google Scholar] [CrossRef] [PubMed]
- Phan, V.H.G.; Lee, E.; Maeng, J.H.; Thambi, T.; Kim, B.S.; Lee, D.; Lee, D.S. Pancreatic cancer therapy using an injectable nanobiohybrid hydrogel. RSC Adv. 2016, 6, 41644–41655. [Google Scholar] [CrossRef]
- Peppas, N.A.; Van Blarcom, D.S. Hydrogel-based biosensors and sensing devices for drug delivery. J. Control. Release 2016, 240, 142–150. [Google Scholar] [CrossRef]
- Calvert, P. Hydrogels for soft machines. Adv. Mater. 2009, 21, 743–756. [Google Scholar] [CrossRef]
- Kirschner, C.M.; Anseth, K.S. Hydrogels in healthcare: From static to dynamic material microenvironments. Acta Mater. 2013, 61, 931–944. [Google Scholar] [CrossRef]
- Chittasupho, C.; Angklomklew, J.; Thongnopkoon, T.; Senavongse, W.; Jantrawut, P.; Ruksiriwanich, W. Biopolymer hydrogel scaffolds containing doxorubicin as a localized drug delivery system for inhibiting lung cancer cell proliferation. Polymers 2021, 13, 3580. [Google Scholar] [CrossRef]
- Javanbakht, S.; Namazi, H. Doxorubicin loaded carboxymethyl cellulose/graphene quantum dot nanocomposite hydrogel films as a potential anticancer drug delivery system. Mater. Sci. Eng. C 2018, 87, 50–59. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, W.; Zhao, J.; Wu, C.; Ye, C.; Huang, M.; Wang, S. Preparation of injectable temperature-sensitive chitosan-based hydrogel for combined hyperthermia and chemotherapy of colon cancer. Carbohydr. Polym. 2019, 222, 115039. [Google Scholar] [CrossRef]
- Xin, H.; Naficy, S. Drug delivery based on stimuli-responsive injectable hydrogels for breast cancer therapy: A review. Gels 2022, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Jin, J.-O. Attachable hydrogel containing indocyanine green for selective photothermal therapy against melanoma. Biomolecules 2020, 10, 1124. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xu, W.; Zhao, Z.; Ling, G.; Zhang, P. Intelligent nanocomposite hydrogels with simultaneous photothermal antitumor and antibacterial efficacy for cutaneous melanoma treatment. Compos. Part B Eng. 2022, 243, 110130. [Google Scholar] [CrossRef]
- Jung, J.M.; Kim, S.H.; Giang Phan, V.H.; Thambi, T.; Lee, D.S. Therapeutic effects of boronate ester cross-linked injectable hydrogels for the treatment of hepatocellular carcinoma. Biomater. Sci. 2021, 9, 7275–7286. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef] [PubMed]
- Prasannan, A.; Tsai, H.-C.; Hsiue, G.-H. Formulation and evaluation of epinephrine-loaded poly (acrylic acid-co-N-isopropylacrylamide) gel for sustained ophthalmic drug delivery. React. Funct. Polym. 2018, 124, 40–47. [Google Scholar] [CrossRef]
- Carthew, J.; Donderwinkel, I.; Shrestha, S.; Truong, V.X.; Forsythe, J.S.; Frith, J.E. In situ miRNA delivery from a hydrogel promotes osteogenesis of encapsulated mesenchymal stromal cells. Acta Biomater. 2020, 101, 249–261. [Google Scholar] [CrossRef]
- Xu, L.; Bai, X.; Yang, J.; Li, J.; Xing, J.; Yuan, H.; Xie, J.; Li, J. Preparation and characterisation of a gellan gum-based hydrogel enabling osteogenesis and inhibiting Enterococcus faecalis. Int. J. Biol. Macromol. 2020, 165, 2964–2973. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, X.; Gao, J.; Zhao, Y.; Zhao, Y.; Guo, L.; Chen, C.; Duan, Z.; Li, P.; Wei, L. Intra-articular injection of cross-linked hyaluronic acid-dexamethasone hydrogel attenuates osteoarthritis: An experimental study in a rat model of osteoarthritis. Int. J. Mol. Sci. 2016, 17, 411. [Google Scholar] [CrossRef]
- Diniz, F.R.; Maia, R.C.A.; Rannier Andrade, L.; Andrade, L.N.; Vinicius Chaud, M.; da Silva, C.F.; Corrêa, C.B.; de Albuquerque Junior, R.L.C.; Pereira da Costa, L.; Shin, S.R. Silver nanoparticles-composing alginate/gelatine hydrogel improves wound healing in vivo. Nanomaterials 2020, 10, 390. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Singh, T.R.R.; Morrow, D.I.; Woolfson, A.D. Microneedle-Mediated Transdermal and Intradermal Drug Delivery; John and Wiley and Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Brown, M.; Jones, S. Hyaluronic acid: A unique topical vehicle for the localized delivery of drugs to the skin. J. Eur. Acad. Dermatol. Venereol. 2005, 19, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.; Figueiras, A.; Veiga, F. Modular Hydrogels for Drug Delivery. J. Biomater. Nanobiotechnol. 2012, 3, 18938. [Google Scholar]
- Langer, R. New methods of drug delivery. Science 1990, 249, 1527–1533. [Google Scholar] [CrossRef]
- Overstreet, D.J.; Dutta, D.; Stabenfeldt, S.E.; Vernon, B.L. Injectable hydrogels. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 881–903. [Google Scholar] [CrossRef]
- Harush-Frenkel, O.; Rozentur, E.; Benita, S.; Altschuler, Y. Surface Charge of Nanoparticles Determines Their Endocytic and Transcytotic Pathway in Polarized MDCK Cells. Biomacromolecules 2008, 9, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhou, X.; Mao, Z.; Yu, D.; Wang, B.; Gao, C. Uptake of hydrogel particles with different stiffness and its influence on HepG2 cell functions. Soft Matter 2012, 8, 9235–9245. [Google Scholar] [CrossRef]
- Thambi, T.; Deepagan, V.G.; Yoon, H.Y.; Han, H.S.; Kim, S.-H.; Son, S.; Jo, D.-G.; Ahn, C.-H.; Suh, Y.D.; Kim, K.; et al. Hypoxia-responsive polymeric nanoparticles for tumor-targeted drug delivery. Biomaterials 2014, 35, 1735–1743. [Google Scholar] [CrossRef]
- Thambi, T.; Yoon, H.Y.; Kim, K.; Kwon, I.C.; Yoo, C.K.; Park, J.H. Bioreducible Block Copolymers Based on Poly(Ethylene Glycol) and Poly(γ-Benzyl l-Glutamate) for Intracellular Delivery of Camptothecin. Bioconjugate Chem. 2011, 22, 1924–1931. [Google Scholar] [CrossRef]
- Lei, T.; Srinivasan, S.; Tang, Y.; Manchanda, R.; Nagesetti, A.; Fernandez-Fernandez, A.; McGoron, A.J. Comparing cellular uptake and cytotoxicity of targeted drug carriers in cancer cell lines with different drug resistance mechanisms. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 324–332. [Google Scholar] [CrossRef]
- Jang, J.Y.; Le, T.M.D.; Ko, J.H.; Ko, Y.-J.; Lee, S.M.; Kim, H.J.; Jeong, J.H.; Thambi, T.; Lee, D.S.; Son, S.U. Triple-, Double-, and Single-Shelled Hollow Spheres of Sulfonated Microporous Organic Network as Drug Delivery Materials. Chem. Mater. 2019, 31, 300–304. [Google Scholar] [CrossRef]
- Banquy, X.; Suarez, F.; Argaw, A.; Rabanel, J.-M.; Grutter, P.; Bouchard, J.-F.; Hildgen, P.; Giasson, S. Effect of mechanical properties of hydrogel nanoparticles on macrophage cell uptake. Soft Matter 2009, 5, 3984–3991. [Google Scholar] [CrossRef]
- Kalal, B.S.; Upadhya, D.; Pai, V.R. Chemotherapy resistance mechanisms in advanced skin cancer. Oncol. Rev. 2017, 11, 326. [Google Scholar] [CrossRef]
- Sepantafar, M.; Maheronnaghsh, R.; Mohammadi, H.; Radmanesh, F.; Hasani-Sadrabadi, M.M.; Ebrahimi, M.; Baharvand, H. Engineered hydrogels in cancer therapy and diagnosis. Trends Biotechnol. 2017, 35, 1074–1087. [Google Scholar] [CrossRef] [PubMed]
- Manivasagan, P.; Joe, A.; Han, H.-W.; Thambi, T.; Selvaraj, M.; Chidambaram, K.; Kim, J.; Jang, E.-S. Recent advances in multifunctional nanomaterials for photothermal-enhanced Fenton-based chemodynamic tumor therapy. Mater. Today Bio 2022, 13, 100197. [Google Scholar] [CrossRef]
- Gil, M.S.; Thambi, T.; Phan, V.H.G.; Kim, S.H.; Lee, D.S. Injectable hydrogel-incorporated cancer cell-specific cisplatin releasing nanogels for targeted drug delivery. J. Mater. Chem. B 2017, 5, 7140–7152. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Phan, V.H.G.; Lee, D.S.; Thambi, T.; Huynh, D.P. Bioresorbable pH- and temperature-responsive injectable hydrogels-incorporating electrosprayed particles for the sustained release of insulin. Polym. Degrad. Stab. 2019, 162, 36–46. [Google Scholar] [CrossRef]
- Phan, V.H.G.; Thambi, T.; Duong, H.T.T.; Lee, D.S. Poly(amino carbonate urethane)-based biodegradable, temperature and pH-sensitive injectable hydrogels for sustained human growth hormone delivery. Sci. Rep. 2016, 6, 29978. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, H.; Qiao, S.; Zhang, L.; Wang, T.; Meng, Q.; Chen, X.; Lin, F.-H.; Guo, K.; Li, C. Hydrogels bearing bioengineered mimetic embryonic microenvironments for tumor reversion. J. Mater. Chem. B 2016, 4, 6183–6191. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Deng, J.; Cao, J.; Wu, H.; Feng, G.; Zhang, R.; Ran, B.; Hu, K.; Cao, H.; Zhu, X. Nano-hydroxyapatite-evoked immune response synchronized with controllable immune adjuvant release for strengthening melanoma-specific growth inhibition. Acta Biomater. 2022, 145, 159–171. [Google Scholar] [CrossRef]
- Karavasili, C.; Andreadis, D.A.; Katsamenis, O.L.; Panteris, E.; Anastasiadou, P.; Kakazanis, Z.; Zoumpourlis, V.; Markopoulou, C.K.; Koutsopoulos, S.; Vizirianakis, I.S. Synergistic antitumor potency of a self-assembling peptide hydrogel for the local co-delivery of doxorubicin and curcumin in the treatment of head and neck cancer. Mol. Pharm. 2019, 16, 2326–2341. [Google Scholar] [CrossRef]
- Wu, X.; Wu, Y.; Ye, H.; Yu, S.; He, C.; Chen, X. Interleukin-15 and cisplatin co-encapsulated thermosensitive polypeptide hydrogels for combined immuno-chemotherapy. J. Control. Release 2017, 255, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; He, C.; Quan, F.; Yu, S.; Chen, X. DOX/IL-2/IFN-γ co-loaded thermo-sensitive polypeptide hydrogel for efficient melanoma treatment. Bioact. Mater. 2018, 3, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Shin-Ya, M.; Takeda, S.; Hashimoto, Y.; Mukai, S.A.; Sawada, S.I.; Adachi, T.; Akiyoshi, K.; Miki, T.; Mazda, O. Cycloamylose-nanogel drug delivery system-mediated intratumor silencing of the vascular endothelial growth factor regulates neovascularization in tumor microenvironment. Cancer Sci. 2014, 105, 1616–1625. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fang, M.; Zhang, J.; Wang, J.; Song, Y.; Shi, J.; Li, W.; Wu, G.; Ren, J.; Wang, Z. Hydrogel dual delivered celecoxib and anti-PD-1 synergistically improve antitumor immunity. Oncoimmunology 2016, 5, e1074374. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ye, T.; Ye, C.; Wan, C.; Yuan, S.; Liu, Y.; Li, T.; Jiang, F.; Lovell, J.F.; Jin, H. Secretions from hypochlorous acid-treated tumor cells delivered in a melittin hydrogel potentiate cancer immunotherapy. Bioact. Mater. 2022, 9, 541–553. [Google Scholar] [CrossRef]
- Sun, R.; Chen, Y.; Yang, Q.; Zhang, W.; Guo, L.; Feng, M. Polysaccharide hydrogels regulate macrophage polarization and enhance the anti-tumor efficacy of melanoma. Int. J. Pharm. 2022, 613, 121390. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, D.; Sun, X.; Song, X.; Yan, K.C.; Liang, H. Polyvinyl alcohol/gelatin hydrogels regulate cell adhesion and chromatin accessibility. Int. J. Biol. Macromol. 2022, 219, 672–684. [Google Scholar] [CrossRef]
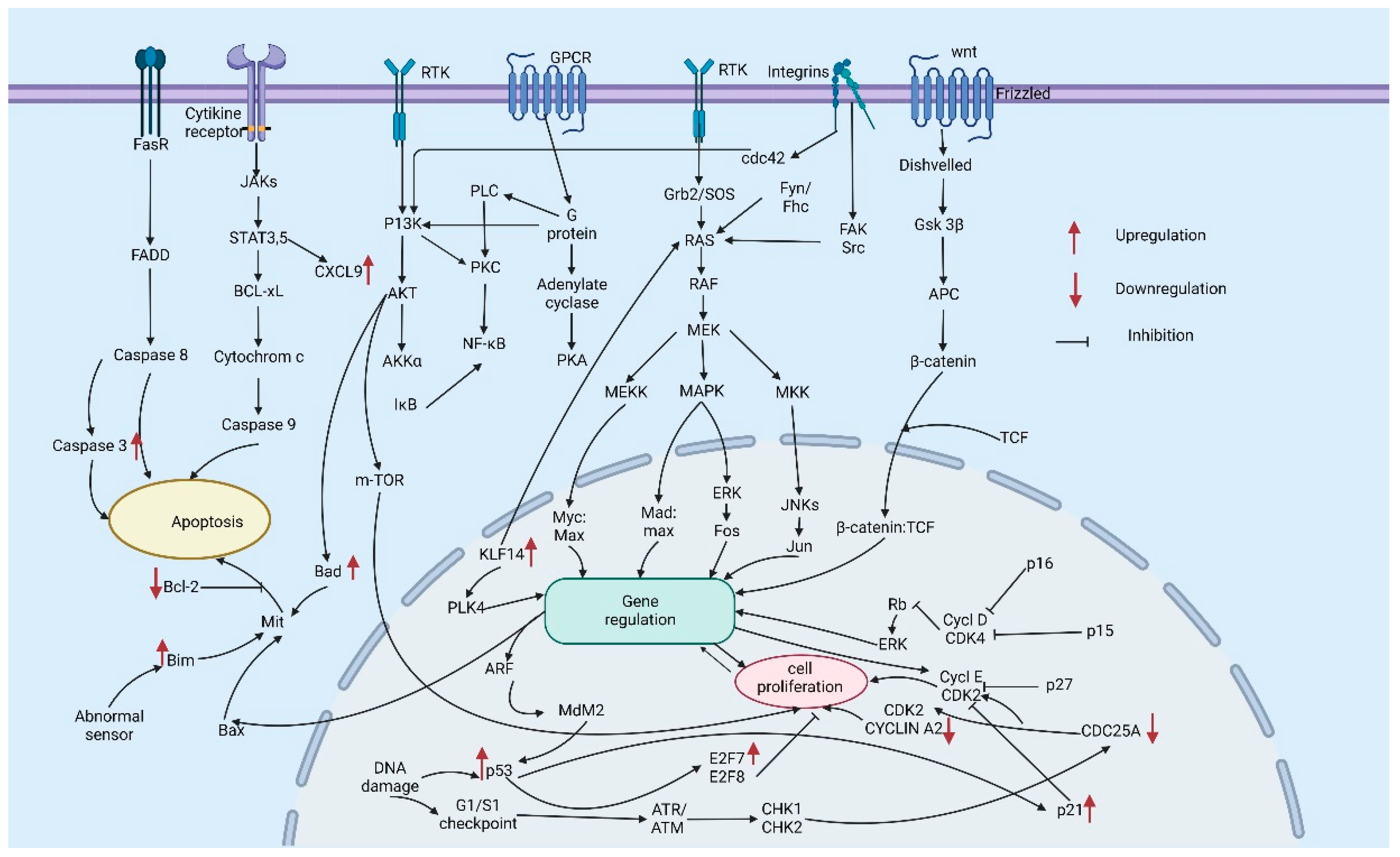
| Gene | Pathway | Regulation | References |
|---|---|---|---|
| BRAF | RAS–RAF–MEK–ERK mitogen-activated protein kinase (MAPK) pathway | Somatic missense mutation-valine-to-glutamic acid substitution at position 599 (V599E) | [21] |
| NRAS | RAS–RAF–MEK–ERK MAPK pathway | Substitution mutations | [22] |
| KIT | mitogen-activated protein (MAP) kinase and phosphatidylinositol 3 (PI3) kinase pathways, PI3K–AKT–mTOR, JAK–STAT | Somatic mutations- on exon 11 (L576P or exon 13 (K642E) | [23,24] |
| GNAQ | MAPK pathways | Somatic mutations—glutamine at position 209 (Q209) is mutated to either leucine or proline | [25] |
| GNA11 | MAPK pathway | Somatic mutations—glutamine at position 209 (Q209) is mutated to either leucine or proline | [26] |
| CDKN2A (p14ARF) | MDM2–p53 | Deletion | [27,28] |
| CDKN2A (p16INK4A) | p16INK4A–cyclin D/CDK4–RB checkpoint | ‘G’ TO ‘A’ transition at the first nucleotide of the splice donor site of intron 2 | [28,29] |
| PTEN | oncogenic phosphatidylinositol-3-kinase (PI3K) signaling pathway | Deletion or mutation leads to constitutive activation of this pathway | [30] |
| LKB1 | LKB1–AMPK | ‘C’ to ‘T’ transition, resulting in the substitution of the normal glutamine codon (CAG) with a premature termination codon (TAG) | [31,32] |
| MITF | MITF–PGC1a Transcription, lineage, cell cycle | Amplification/germline missense substitution | [33,34] |
| NF1/neurofibromin | PI3K and MAPK pathways | Mutation | [35] |
| MYC | Amplification | [36] | |
| Cyclin D1 | RAS/MAPK pathways | Amplification | [37,38] |
| CDK4 | Cell cycle, G1/S cyclin-dependent kinase | Amplification or Point mutation | [39] |
| HDM2 | P53 | Amplification | [40] |
| PIK3CA | PI3K–AKT–mTOR | Missense mutations | [41] |
| AKT1,AKT2, AKT3 | PI3K–AKT–mTOR | Oncogenic mutation | [42] |
| ERBB4 | Receptor tyrosine kinases | Amplification | [43] |
| fibroblast growth factor receptor 3 (FGFR3) | Ras/MAPK | Amplification, gain-of-function mutations | [44,45] |
| MET | PI3K, MAPK | Amplification/single-nucleotide variations | [46] |
| Drug | Origin | Molecular Weight (g/mol) | Structure | Route of Administration | Effect | References |
|---|---|---|---|---|---|---|
| Alitretinoin | Synthetic/Natural | 300.4 | 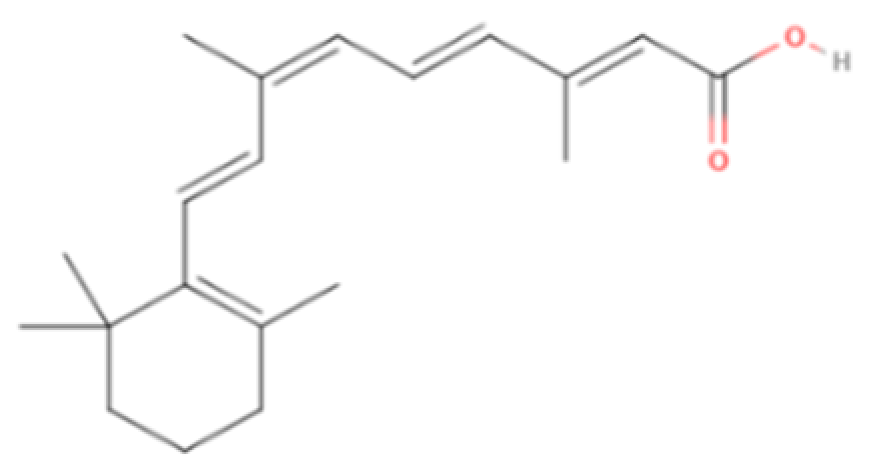 | Topical | Inhibit cyclooxygenase (COX)-2 expression, suppress cell growth | [5,60] |
| Diclofenac sodium | Synthetic | 318.1 | 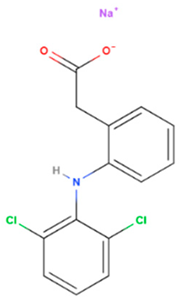 | Topical | Cycloxygenase-2 enzyme overexpression & increase apoptosis | [61,62] |
| Fluorouracil | Synthetic | 130.1 | 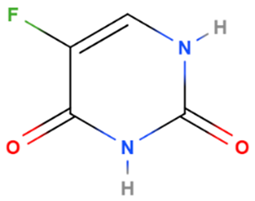 | Topical | Enzyme responsible for synthesis of thymidine, one of the pyrimidine nucleosides of DNA has been inhibited | [5] |
| Imiquimod | Synthetic | 240.3 |  | Topical | Through toll-like receptor 7 (TLR7) an immune response which upregulates cytokines and consequently leads to apoptosis has been triggered | [5,63] |
| Ingenol mebutate | Synthetic/Natural | 430.5 | 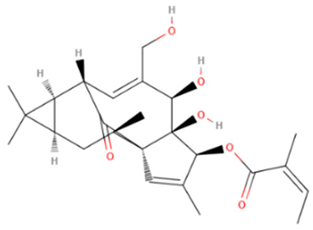 | Topical | Upregulate rapid lesion necro sis and neutrophil-mediated cellular cytotoxicity, Modulation of protein kinase C isoforms | [64,65] |
| Cisplatin | Synthetic | 301.1 | 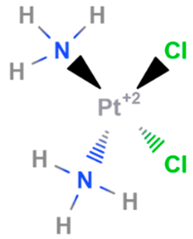 | Intravenous | DNA cross linking agents | [66] |
| Dacarbazine | Synthetic | 182.18 | 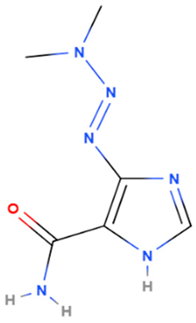 | Intravenous | Alkylating agents that damage DNA by introducing alkyl groups to guanine bases, eventually causing cell death via apoptosis and other cell death mechanisms | [7,67] |
| Temozolomide | Synthetic | 194.151 |  | intravenous infusion | Alkylating agents that damage DNA by introducing alkyl groups to guanine bases, eventually causing cell death via apoptosis and other cell death mechanisms | [67] |
| Carmustine | Synthetic | 214.05 |  | intravenous infusion | DNA binding and DNA alkylation by a nitrogenous base within a duplex | [68] |
| Lomustine | Synthetic | 233.69 | 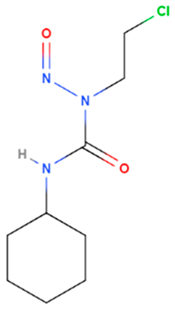 | oral | Alkylation of the O6 position of guanine-containing bases in DNA and the enzyme O6 -alkylguanine transferase mediates | [69] |
| Vincristine | Natural | 824.958 |  | intravenous infusion | Inhibition of microtubule polymerization; microtubule destabilization | [70] |
| Vinblastine | Natural | 810.974 | 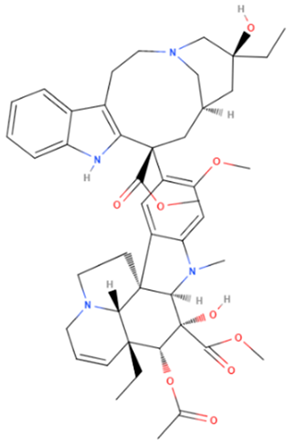 | intravenous infusion | Inhibition of microtubule polymerization; microtubule destabilization | [70] |
| Carboplatin | Synthetic | 371.249 | 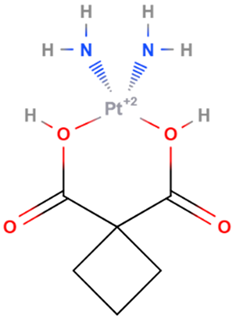 | intravenous infusion | DNA alkylation: formation of DNA crosslinks and adducts; inhibition of DNA synthesis | [7,70] |
| Taxol/Paclitaxel | Natural | 853.906 |  | intravenous infusion | Interacts with microtubules causing polymerization and stabilization of the tubulin polymer which prevents successful completion of cell division | [71,72] |
| Docetaxel | Natural | 807.879 |  | intravenous infusion | Binds to β-tubulins in the cell and causes their polymerization, induces caspase-2 dependent apoptosis | [73] |
| Resiquimod | Synthetic | 314.4 |  | Topical | Stimulates immune responses through toll-like receptors (TLR) 7 and 8 dependent pathway activation | [74] |
| Mechlorethamine | Synthetic | 156.05 | 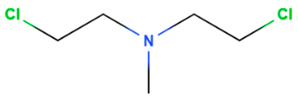 | Topical | Cancer cell growth postulated by DNA cross-linking and depurination, or abnormal base paring. | [75] |
| Hydrogel | Gene | Pathway | Effect | In Vivo/In Vitro | Cancer | Reference |
|---|---|---|---|---|---|---|
| Hyaluronic acid scaffold loaded with Nodal Signaling Crypto 1 receptor antibodies (2B11) | Kit, Itga4, Hapln1, Irf8, Trail, Casp1, Aim2, and Irf1 | Apoptosis pathways, MAPK pathways and PI3K–AKT–mTOR, JAK–STAT | Upregulation of tumor suppressor genes and the downregulation of oncogenes | In vivo and in vitro | Melanoma | [142] |
| mPEG-b-PELG hydrogel encapsulating f interleukin-15 (IL-15) and cisplatin | Cyclin A2, CDK2, and Cdc25A | Cell cycle | Significant decrease in the expressions of genes and cell cycle arrest | Ex vivo | Melanoma | [145] |
| Poly Lactic-co-Glycolic Acid (PLGA)-polyethylene glycol-PLGA hydrogel encapsulating, nano-hydroxyapatite and granulocyte-macrophage colony-stimulating factor | E2Fs family genes and PLK1, KLF14, KLF11 | Cell cycle | Cell cycle arrested in the G2/M phase, apoptosis | In vitro | Melanoma | [143] |
| PELG-PEG-PELG loaded with DOX, IL-2, and IFN-g | Bcl-2 Caspase 3 | Janus kinase, JAK/STAT and mitochondrial signal pathways | Induces apoptosis | In vitro | Melanoma | [146] |
| Olesterol-bearing cycloamylose with spermine group nanogel carrying VEGF-specific short interfering RNA | Vascular endothelial growth factor (VEGF) | Angiogenesis | In vitro | [147] | ||
| peptide hydrogel (ac-(RADA)4-CONH2) loaded with curcumin and doxorubicin | p53, p21, BAX, BAD, Cdk2, Bcl-2, c-myc and CyclD1 | Apoptosis pathways, | High rate of apoptosis | In vitro | Head and neck squamous cell carcinoma | [144] |
| Alginate hydrogel bearing loaded with anti-PD-1 monoclonal antibody and celecoxib | IL-1b, IL-6, CXCL9 and CXCL10 | Programmed death 1 (PD-1) signaling pathway | Increases the expression of two anti-angiogenic chemokines and suppresses the intra tumoral production of interleukin (IL)-1, IL-6, and cycloxygenase-2 (COX2) | In vitro | Melanoma | [148] |
| RADA24-melittin fusion peptide hydrogel loaded with cell-derived secretions from cells exposed to HOCl | IFN-α, IFN-β, IL-6 | cGAS-STING pathway and PD-1 signaling pathway | Increased tumor cell death, cytotoxic T lymphocyte infiltration, and tumor-associated macrophage reprogramming towards an M1 phenotype | In vitro | Melanoma | [149] |
| N-succinyl chitosan and oxidized dextran hydrogel loaded with doxorubicin | CD206, Arginase-1, TNF-α and Inos | p65 NF-kappaB and P53 pathway | Induced macrophages to produce anti-tumorigenic cytokines such as TNF-α, iNOS, IL-6 and IL-1β | In vivo and in vitro | Melanoma | [150] |
| Polyvinyl alcohol/gelatin | mechanotransduction related genes and transposase-accessible chromatin | MAPK pathway and MKL1/SRF pathway | Poor cell adhesion and increased chromatin accessibility | in vitro | melanoma | [151] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathiyalagan, R.; Kariyarath Valappil, A.; Yang, D.C.; Kang, S.C.; Thambi, T. Gene Regulations upon Hydrogel-Mediated Drug Delivery Systems in Skin Cancers—An Overview. Gels 2022, 8, 560. https://doi.org/10.3390/gels8090560
Mathiyalagan R, Kariyarath Valappil A, Yang DC, Kang SC, Thambi T. Gene Regulations upon Hydrogel-Mediated Drug Delivery Systems in Skin Cancers—An Overview. Gels. 2022; 8(9):560. https://doi.org/10.3390/gels8090560
Chicago/Turabian StyleMathiyalagan, Ramya, Anjali Kariyarath Valappil, Deok Chun Yang, Se Chan Kang, and Thavasyappan Thambi. 2022. "Gene Regulations upon Hydrogel-Mediated Drug Delivery Systems in Skin Cancers—An Overview" Gels 8, no. 9: 560. https://doi.org/10.3390/gels8090560
APA StyleMathiyalagan, R., Kariyarath Valappil, A., Yang, D. C., Kang, S. C., & Thambi, T. (2022). Gene Regulations upon Hydrogel-Mediated Drug Delivery Systems in Skin Cancers—An Overview. Gels, 8(9), 560. https://doi.org/10.3390/gels8090560









