1. Introduction
The use of foam gel fracturing fluid is a great achievement of liquid technology. Foamed fracturing fluid is formed by dispersing N2 or CO2 in water, acid, methanol/water mixture, or hydrocarbon liquid as bubbles, and is usually a two-phase mixture of 70%–80% dryness gas (N2 or CO2) and fracturing of fluid (water-based polymer solution). Foam gel fracturing fluid is essentially a kind of gas-in-liquid emulsion, and bubbles provide high viscosity and excellent proppant-carrying capacity. Because it has the characteristics of low reservoir damage, strong flowback ability, low fluid loss, high fluid efficiency, proper viscosity, and strong sand-carrying capacity, it occupies a very important position in fracturing fluid systems.
To solve the shortcomings and defects of conventional fracturing technology, researchers began to study foam fracturing technology in the 1970s [
1]. Since foam fracturing was first completed in Lincoln County, West Virginia, USA, foam fracturing technology has developed from the initial N
2 foam fracturing to the present CO
2 foam fracturing. In 1986, in the Federal Republic of Germany, 60% CO
2 foam gel fracturing fluid was used in the carboniferous gas reservoir in Fez Dolf, which was buried 3400−3650 m underground. The fracturing was successful, and the natural gas production increased by nearly 12 times after fracturing [
2]. By the 1990s, about 90% of gas wells and 30% of oil wells in the United States and Canada had adopted CO
2 foam fracturing technology [
3]. Nowadays, it is very common to use foam fracturing technology for fracturing worldwide. In the United States, about 3600 foam fracturing operations are carried out every year, which not only has a high success rate but also has an obvious effect on increasing production. Foams have been considered the most attractive and preferred fluid for fracturing unconventional reservoirs due to their ability to reduce formation damage and improve the recovery of injected fluid [
4,
5]. Gel and foam systems, as the two most widely used plugging agents for lost circulation control, have achieved positive progress in both laboratory experiments and field applications in recent decades [
6,
7]. Wang et al. (2022) developed a composite gel foam plugging system, which is used to plug and control flooding for heterogeneous reservoirs, and it showed better plugging and recovering performance for field applications [
8].
The characteristics of CO
2 foam fracturing fluid can also influence the propagation of hydraulic fractures. Several authors reported that the high performance of CO
2 foam is attributed to its unique and favorable rheological characteristics [
9,
10,
11,
12,
13]. However, due to the complex nature of foam, it is difficult to understand and model its flow behavior, especially under operating conditions. The versatility and uniqueness of foam are attributed to its enormously high viscosity profile compared to its base fluids and the efficiency of foam fracturing is dictated by the complex non-Newtonian behavior of foam [
14,
15,
16,
17,
18]. Numerous authors agree that the design of fracturing treatments highly depends on foam rheology and it governs the overall process performance [
19,
20,
21,
22,
23,
24,
25,
26]. Foam rheology also determines the properties of the fracture network that may help in obtaining the required fracture geometry. At present, due to the limitation of equipment conditions and research methods, the research on the rheological properties of fracturing fluid under simulated field construction conditions has not been reported. The prediction of foam rheological behavior is a complex task and the direct determination of foam rheology under operating conditions is still considered a challenge [
27]. Fu et al. (2021) investigated the rheology and stability of nanoparticle-stabilized CO
2 foams under reservoir conditions (high temperature and high pressure) for fracturing applications [
28]. Li et al. (2022) investigated the rheology properties of thickened liquid CO
2 by measuring the viscosity of thickened liquid CO
2 in different physical parameters of this prepared thickener and explained the causes of the rheological changes [
29]. Kadafur et al. (2022) investigated the rheology of a CO
2 foamed chelating agent, L-glutamic acid-N, N-diacetic acid (GLDA), which was conducted at 100 C, 1000 psi, 3.5 pH level, and various water salinities, resembling harsh reservoir conditions [
30]. Tariq et al. (2022) established a data pool, which was analyzed using four machine learning techniques: Artificial Neural Network (ANN), Decision Trees (DT), Random Forest Regressor (RFR), and K-Nearest Neighbor (KNN), and it provides a simplified ANN-based model which can be used on the fly to predict the effective bulk foam viscosity in both laboratory and field conditions [
31].
Presently, the limit pressure of the experimental system for studying the rheological properties of CO2 foam gel fracturing fluid is only 2000 psi (13.8 MPa). Under the condition of simulating tubing or formation temperature (30−50 °C), the gas phase of the foam gel fracturing fluid is in a gas state, so the foam gel fracturing fluid in the experiment is in a gas-liquid two-phase flow, while for the actual fracturing technology, the pumping pressure is extremely high, reaching tens of MPa. At the same time, the fluid temperature in the wellbore or formation fracture is also high. For CO2 foam gel fracturing fluid, CO2 is in a supercritical fluid state. The physical property of the supercritical fluid is closer to that of liquid, and the rheological property at this time is closer to that of a liquid–liquid emulsion. Most of the literature has investigated the rheological performance of the CO2 foam gel fracturing fluid unfoamed and ignored the different foam qualities’ effects in the foaming process, and the experimental temperature and pressure are so low that they are unable to simulate actual field fracturing conditions. Therefore, it is very important to study the rheological properties of foam gel fracturing fluid in the two states of supercooled liquid (unfoamed) and supercritical state (foamed) under simulated actual construction conditions—high pressure (tens of MPa) and high shear rate—for the effective implementation of fracturing technology, the selection of reasonable fracturing parameters, more accurate fracturing prediction, and the evaluation of fracturing effects.
To simulate the rheological performance of CO2 foam fracturing fluid in the two states of foamed and unfoamed under field operation conditions, the formula of the gel fracturing fluid is obtained through experimental optimization firstly, and the viscosity, static sand setting performance, and rheological performance of the foam gel fracturing fluid are experimentally evaluated. The goal is to obtain a foaming gel fracturing fluid with good performance parameters and which is mixed with liquid CO2. This paper selects the foam fracturing fluid formed by CO2 and gel fracturing fluid and studies the rheological properties of CO2 foam fracturing fluid under high temperature (65 °C) and high pressure (30 MPa), considering the two states of supercooled liquid and supercritical through indoor pipe flow experiments. The effects of temperature (15–90 °C), pressure (10, 20, 30 MPa), shear rate (100–3000 s−1), foam quality (0, 45, 55, 65, 75%), and other factors on the rheological properties of fracturing fluid are investigated, which is of great significance for better understanding and evaluating the flow characteristics of CO2 foam gel fracturing fluid and on-site fracturing construction design.
2. Results and Discussion
2.1. Experimental Study on the Rheological Characteristics of CO2 Foam Gel Fracturing Fluid
The effects of temperature, pressure, shear rate, and foam quality on the rheological properties of fracturing fluid are considered. In this experiment, the inner diameter of the test instrument pipeline was 12 mm. The effective viscosity of the CO2 foam gel fracturing fluid changed with the shear rate at 20 MPa, 30 MPa, and 40 MPa, and the temperature changed from 0 to 80 °C.
2.1.1. Effect of Shear Rate on the Effective Viscosity of CO2 Foam Gel Fracturing Fluid
In actual fracturing construction, high-pressure supercooled liquid CO2 is often mixed with guanidine gum and then injected into the formation by tubing for fracturing. As the fracturing fluid enters the formation, the temperature gradually rises, and the high-pressure CO2 completely changes from supercooled liquid to a supercritical state, and the effects of CO2 in the two states on the effective viscosity of CO2 foam gel fracturing fluid are completely different. Therefore, the research on the influence of various factors on the effective viscosity of CO2 foam gel fracturing fluid is divided into two processes for analysis. The CO2 foam fracturing fluid with CO2 in the liquid state is defined as the fracturing fluid system under unfoamed conditions, and the CO2 foam fracturing fluid with CO2 in the supercritical state is defined as the fracturing fluid system under foamed conditions.
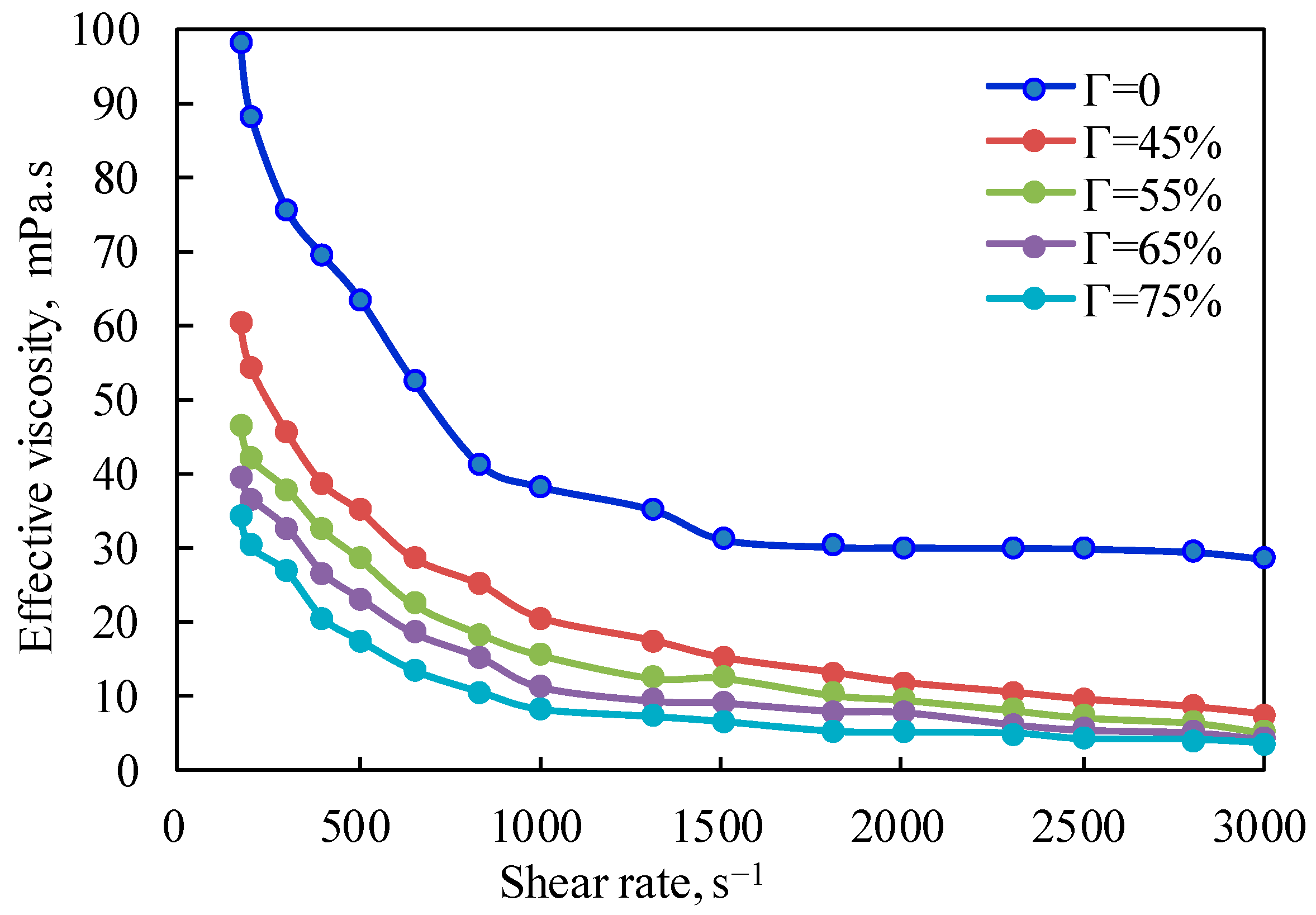
Figure 1 is the curve of the effective viscosity of the fracturing fluid under the unfoamed condition, with a pressure of 10 MPa and a temperature of 20 °C changing with the shear rate. It can be seen from the figure that the effective viscosity of the fluid decreases exponentially with the increase in the shear rate at the same temperature, which fully shows that the unfoamed fracturing fluid is a typical shear-thinning non-Newtonian fluid, and the changing trend when the shear rate is lower than 500 s
−1 is other than that when the shear rate is higher than 500 s
−1. The shear-thinning characteristics of the foam system in the unfoamed state are mainly due to the influence of shear on the base liquid of the gel fracturing fluid. Linear guanidine gum is a long-chain polymer without a cross-linking structure. Increasing the shear rate will reduce the intermolecular interaction force caused by polymer molecular entanglement and hydrogen bonding, which will lead to a decrease in effective viscosity.
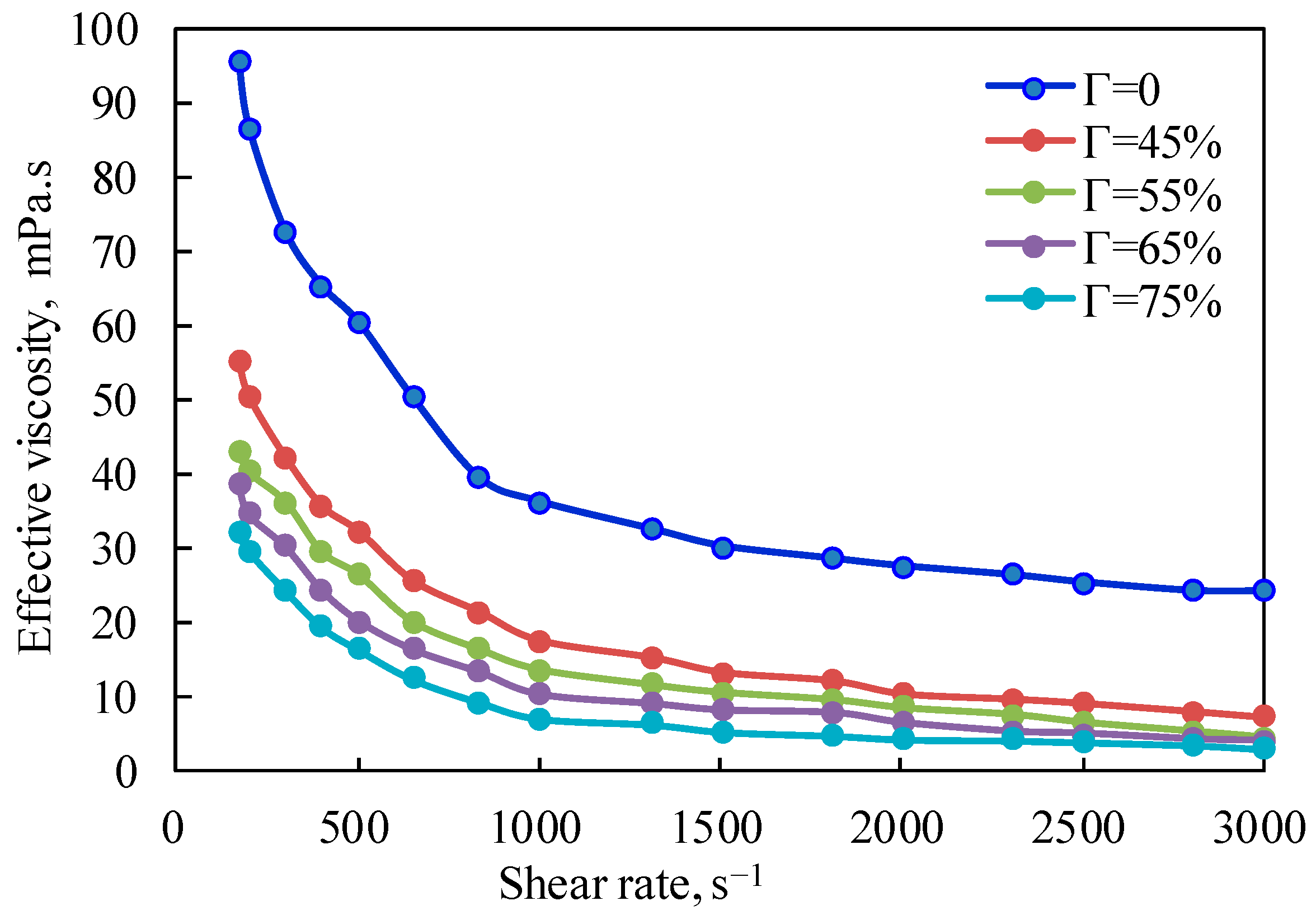
Figure 2 is the curve of the effective viscosity of CO
2 foam gel fracturing fluid with a shear rate at a pressure of 10 MPa and a temperature of 65 °C. It can be seen from the figure that the effective viscosity of CO
2 foam gel fracturing fluid decreases exponentially with the increase in the shear rate at the same pressure, indicating that the CO
2 foam gel fracturing fluid system is a typical shear-thinning non-Newtonian fluid, with a changing trend when the shear rate is lower than 1000 s
−1. It can be seen from the figure that for CO
2 foam gel fracturing fluid during foaming, CO
2 is in a supercritical state, and its physical properties are increasingly close to those of gas. At this time, the emulsion formed by guanidine gum solution and supercritical CO
2, which are two limited miscible fluids, is closer to the traditional foam system. The weakening effect of shearing on the viscosity of CO
2 foam fluid is mainly reflected in two aspects: on the one hand, the shearing mentioned above will reduce the intermolecular interaction force caused by polymer molecular entanglement and hydrogen bonding; on the other hand, it is due to the destruction of the internal-phase CO
2 foam structure by shearing.
2.1.2. Effect of Foam Quality on the Effective Viscosity of CO2 Foam Gel Fracturing Fluid
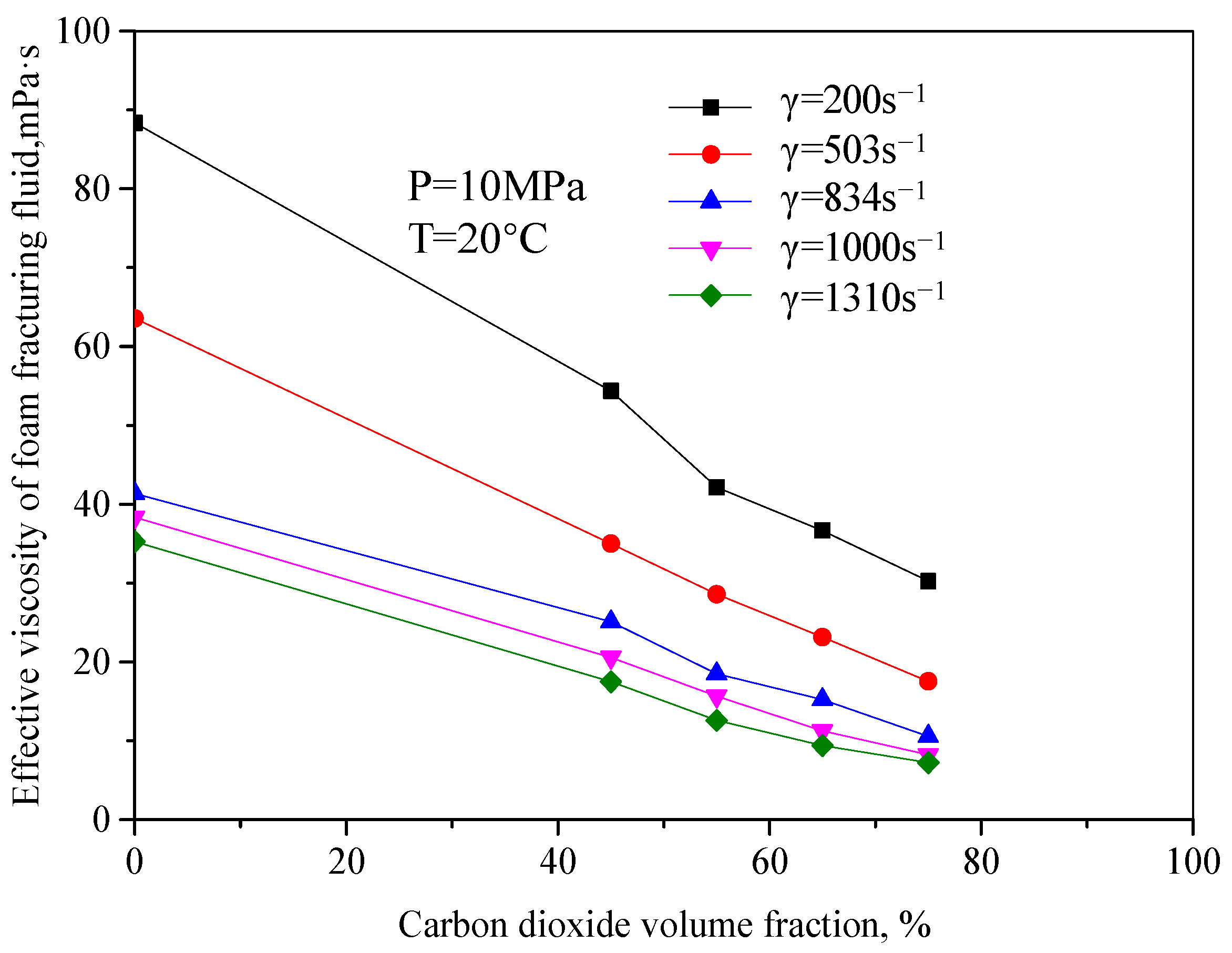
Figure 3 is the curve of the variation of effective viscosity of fracturing fluid with a CO
2 volume fraction when the pressure is 10 MPa and the temperature is 20 °C. It can be seen from the figure that the effective viscosity of unfoamed fracturing fluid decreases with the increase in the CO
2 volume fraction, and the change range is large. The main reason is that the unfoamed CO
2 is in the supercooled liquid form, similar to Newtonian fluid in this mixed system. At this time, CO
2 has little significance for the viscosity increase in the whole system. On the contrary, the increase in the CO
2 volume fraction will dilute the guanidine gum base liquid. When the volume shares of CO
2 increase to a certain extent, the fluid-structure will suddenly change, from the previous guanidine gum base liquid as the continuous phase to the liquid one. When the guanidine gum base liquid changes from the external phase to the internal phase, the continuous phase of the fluid becomes the liquid CO
2, which greatly reduces the viscosity of the whole system.
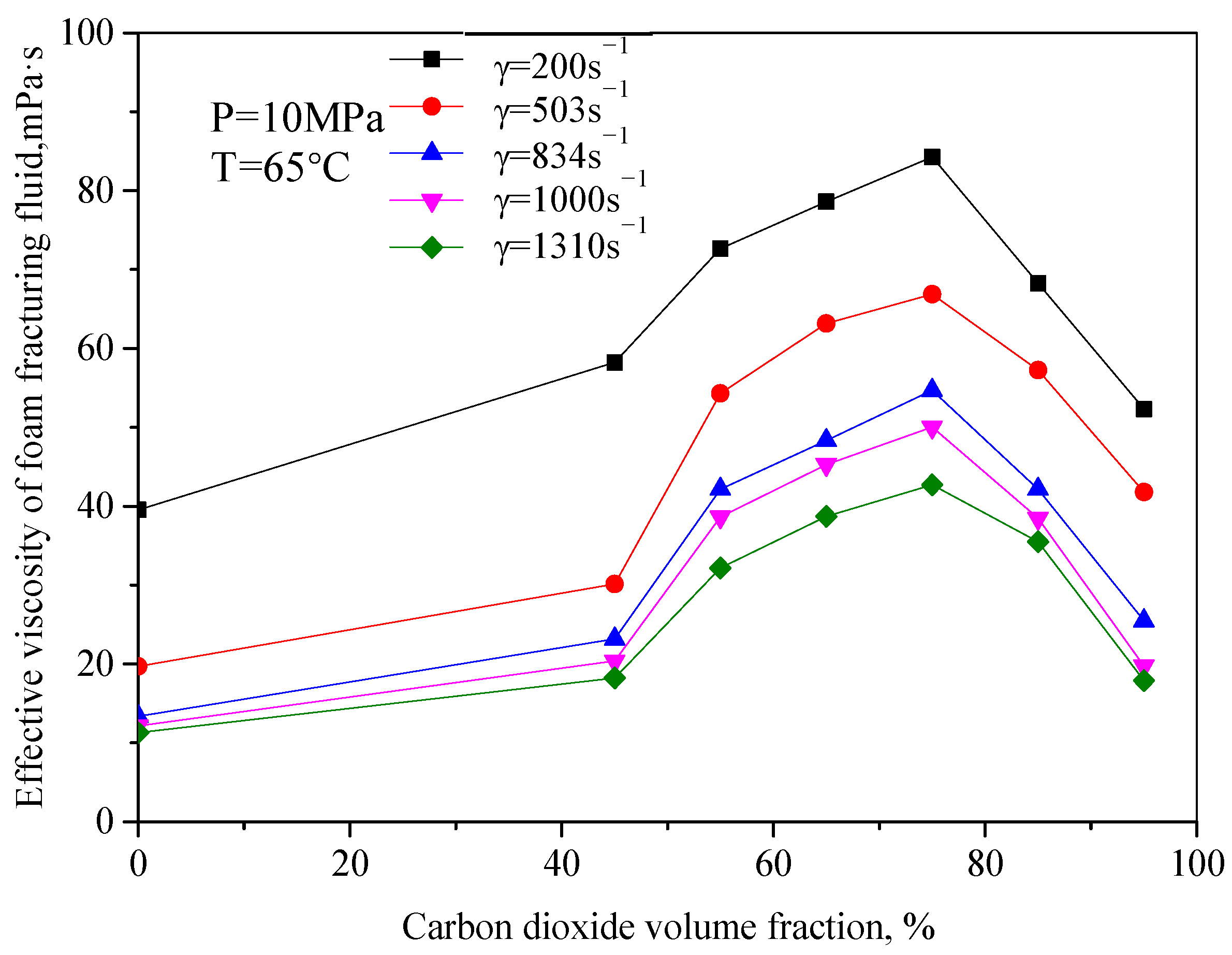
Figure 4 is the variation law curve of the effective viscosity of CO
2 foam gel fracturing fluid with foam quality under foaming conditions, with a pressure of 10 MPa and temperature of 65 °C. It can be seen from the figure that the change rule of effective viscosity with foam quality is opposite to that without foam. The increase in foam quality makes the effective viscosity of the whole system increase, and the increased range is large. For example, when the shear rate is 834 s
−1, the viscosity of foam gel fracturing fluid increases from 26.45 mPa·s to 56.32 mPa·s, with an increasing range of 113%. When the foam mass is 75%, the effective viscosity reaches the maximum value, and when the foam mass is more than 75%, the viscosity of foam gel fracturing fluid decreases obviously. In the research of this system, it can be seen that when the foam mass is more than 55%, with the increase in foam mass, the number of bubbles in the foam system increases, the mutual interference, and deformation among bubbles increase, the bubble structure becomes denser, and the viscosity of the foam system continues to increase.
2.1.3. Effect of Temperature on Effective Viscosity of CO2 Foam Gel Fracturing Fluid
The effective viscosity of CO2 foam gel fracturing fluid with a foam mass of 45−75% was tested at elevated temperatures, and the influence of temperature on the effective viscosity of CO2 foam gel fracturing fluid was analyzed. In the experiment, a pipe diameter of 12 mm, a shear rate of 170 s−1, a heating rate of 1 °C/min, and a temperature of 80 °C were selected, and the viscosity−temperature characteristics of CO2 foam gel fracturing fluid under conditions of 10−40 MPa were tested.
Figure 5 and
Figure 6 are the curves of the effective viscosity of the fracturing fluid with temperature in an unfoamed state, pressure 20 MPa, and different shear rates and CO
2 volume fractions. The temperature ranges from 5 to 25 °C, the shear rate ranges from 503 s
−1 to 1500 s
−1, and the volume fraction of CO
2 from 45 to 75%. It can be seen from the figure that the effective viscosity of unfoamed fluid decreases with the increase in temperature, showing an exponentially decreasing trend. The main reason is the influence of temperature on the rheological properties of the guanidine gum base liquid: with the increase in temperature, the movement activity of guanidine gum molecules increases, and the thermal fracture of the hydrogen bonds of linear guanidine gum in the n liquid system is accelerated, so that the activation energy of guanidine gum base liquid decreases, which comprehensively shows that the effective viscosity of the solution decreases.
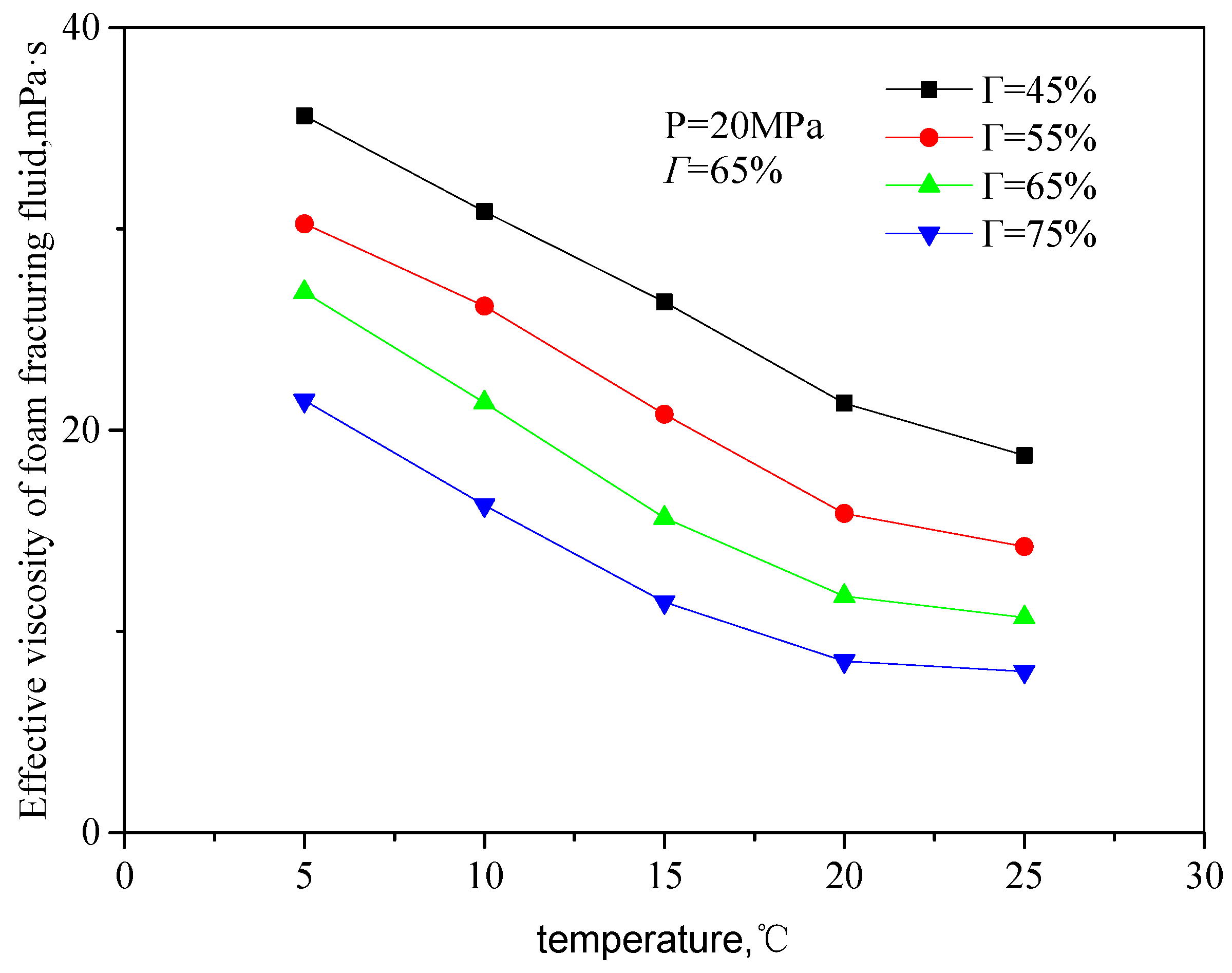
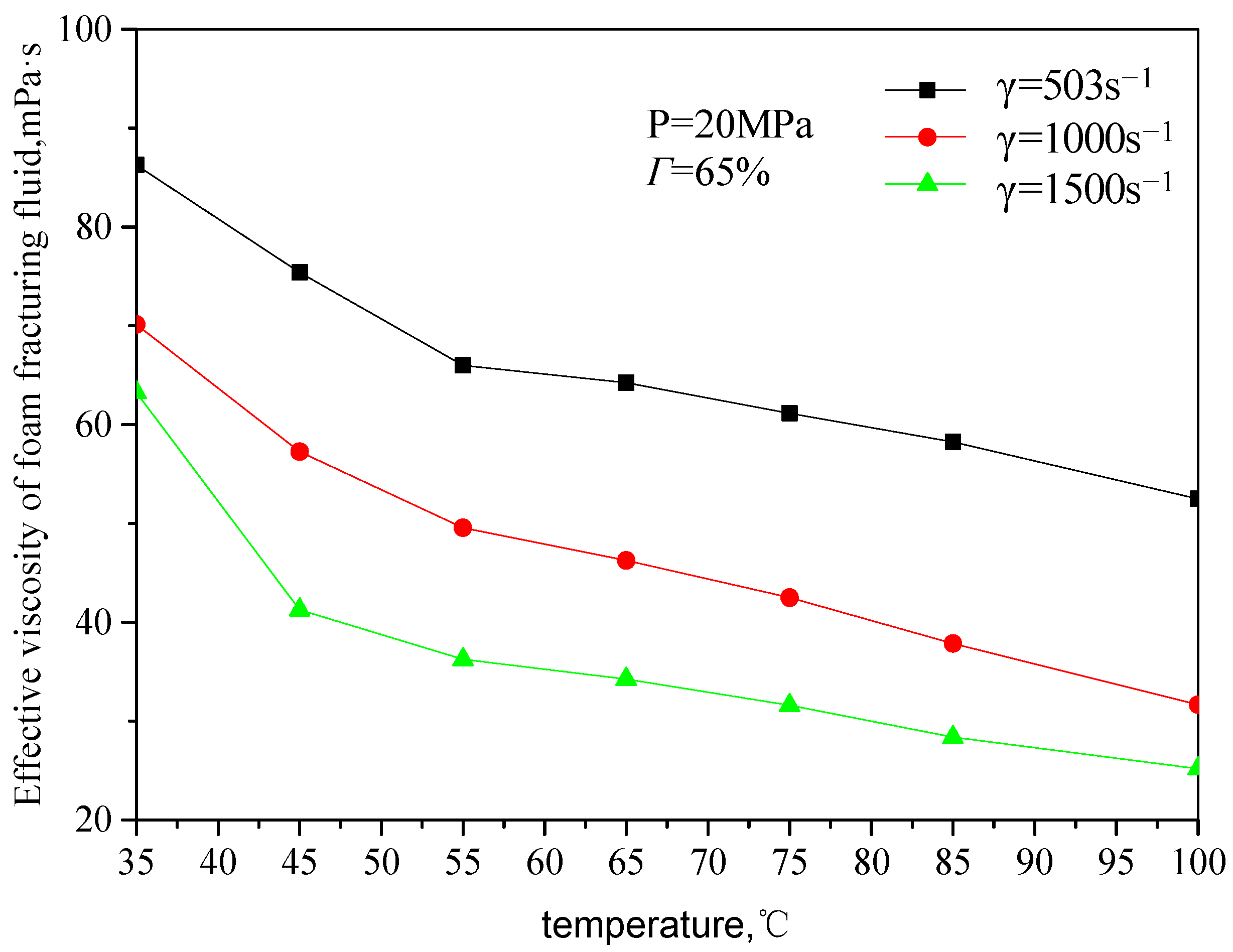
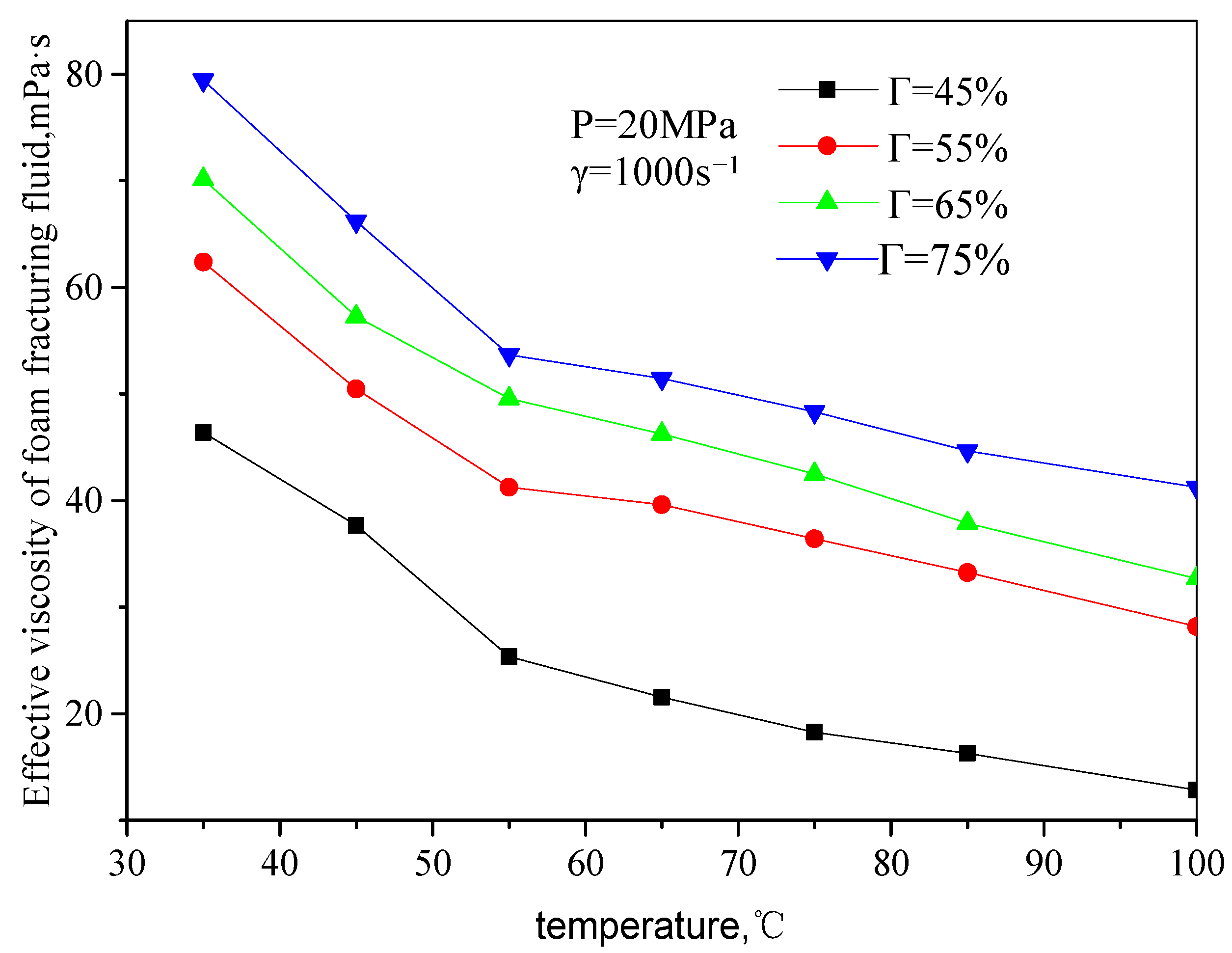
Figure 7 and
Figure 8 are the curves of the effective viscosity of CO
2 foam gel fracturing fluid with temperature under the foaming condition, with a pressure of 20 MPa, different shear rates, and foam quality. The temperature ranges from 35 to 75 °C, the shear rate ranges from 503 s
−1 to 1500 s
−1, and the foam mass ranges from 45 to 75%. It can be seen from the figure that the effective viscosity−temperature characteristics of CO
2 foam gel fracturing fluid during foaming are consistent with those of unfoamed fracturing fluid, showing an exponentially decreasing trend. At the same time, when the temperature is greater than 55 °C, the variation ranges of the effective viscosity of foam gel fracturing fluid with temperature becomes smaller.
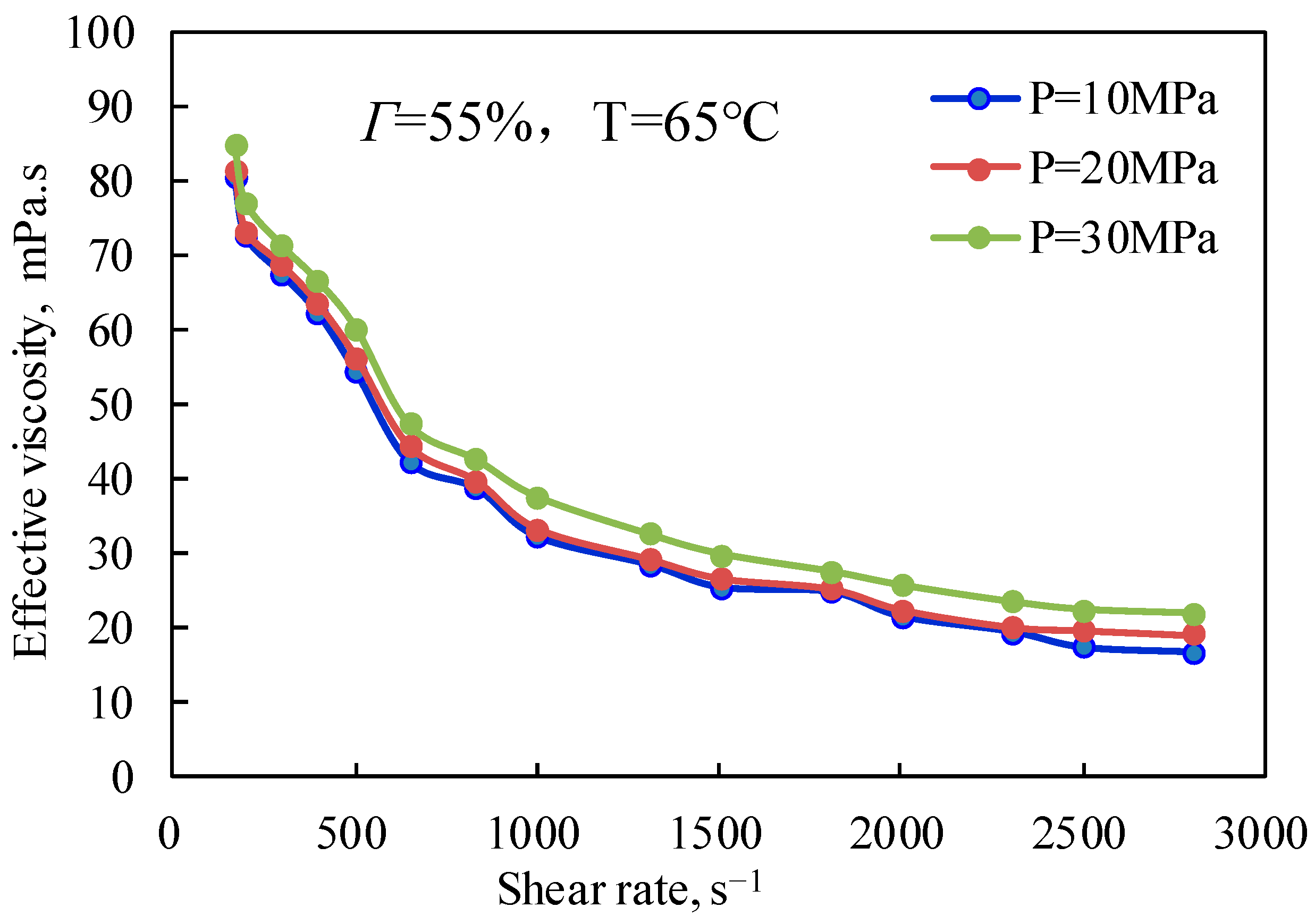
2.1.4. Effect of Pressure on the Effective Viscosity of CO2 Foam Gel Fracturing Fluid (Shear Rate)
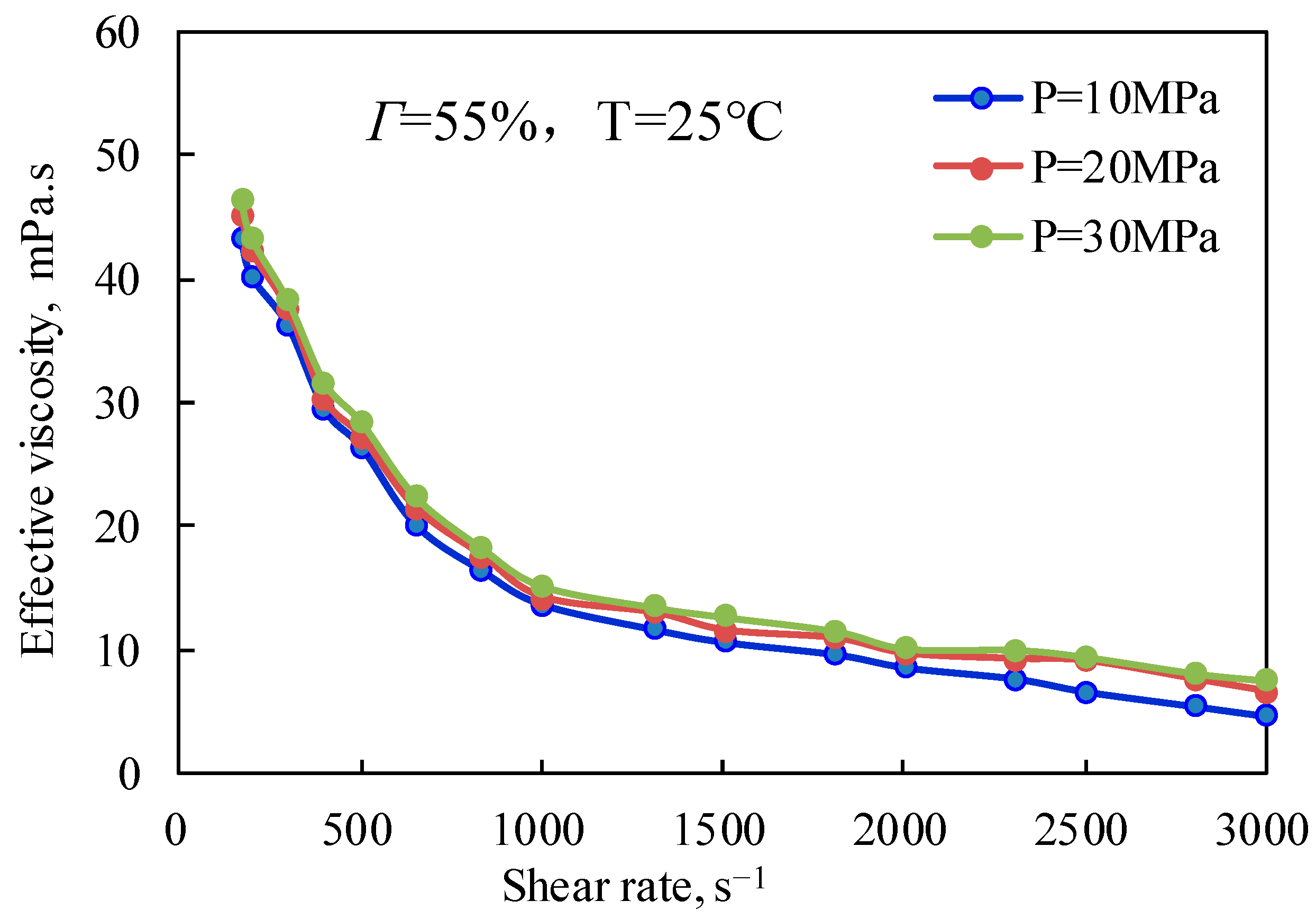
Figure 9 and
Figure 10 are the curves of the effective viscosity of CO
2 foam gel fracturing fluid with a shear rate under different pressure conditions and under unfoamed and foamed conditions at 25 °C and 65 °C, respectively. It can be seen from
Figure 9 that the effective viscosity of the unfoamed fracturing fluid system is affected very little by pressure, and the effective viscosity increases slightly with the increase in experimental pressure. The influence of pressure on the effective viscosity of fracturing fluid without foaming is mainly that pressure can effectively change the interaction between guar gum molecules, making the linear structure change and improving the stability of the linear structure, which shows that the increase in pressure strengthens the long-chain linear structure of guar gum, slows down the damage of shear to the linear structure, and makes the viscosity of the solution increase to a certain extent.
Figure 10 shows the variation law of the effective viscosity of CO
2 foam gel fracturing fluid with pressure under the conditions of foaming at 65 °C, 55% foam mass, and 10 MPa, 20 MPa, and 30 MPa respectively. Similar to the unfoamed condition, the effective viscosity is less affected by pressure due to the pressure’s influence on bubble size and distribution in the foam gel fracturing fluid. The results show that the diameter of bubbles in the foam system gradually decreases with the increase in pressure, and the higher the pressure, the more uniform the bubble size distribution. On the one hand, the stability of bubbles is increased, while on the other hand, the nonlinear interaction between bubbles is enhanced, and the viscosity of the foam gel fracturing fluid is improved as a whole.
2.2. Changes in the Rheological Parameters of CO2 Foam Gel Fracturing Fluid with Various Factors
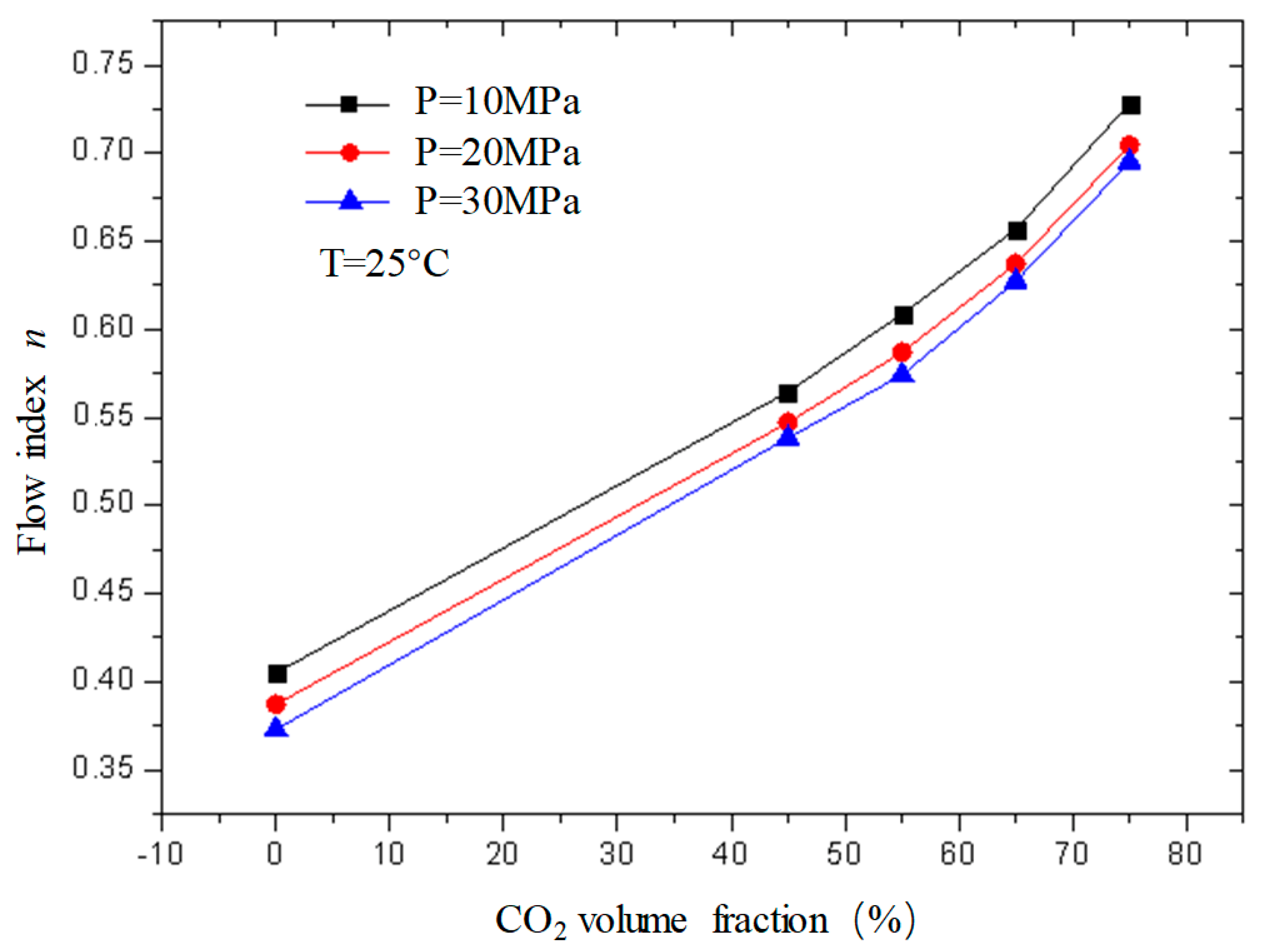
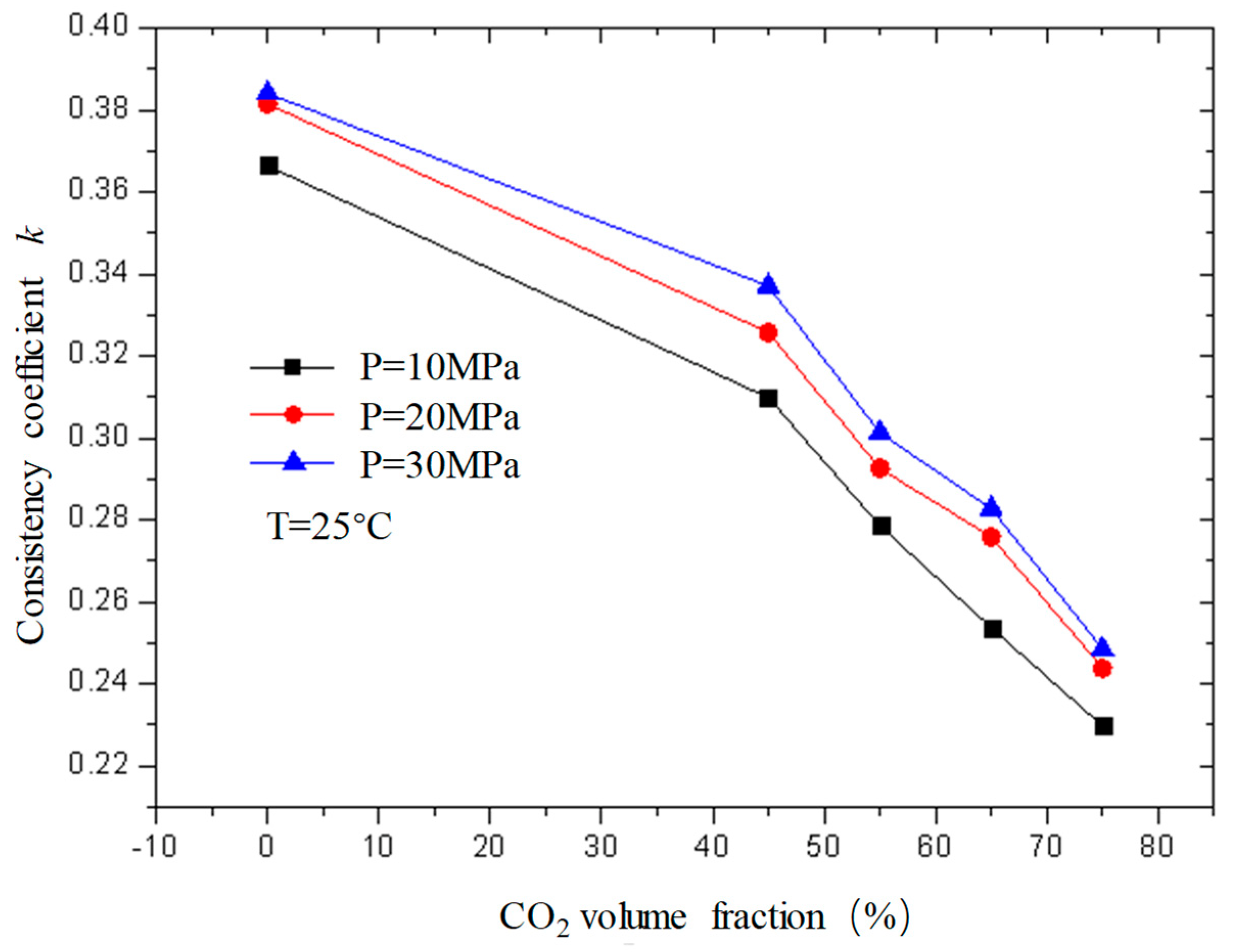
Figure 11 and
Figure 12 are curves of the flow index and consistency index of fracturing fluid with CO
2 volume fraction when the temperature is 25 °C and the pressure is 10 MPa, 20 MPa, and 30 MPa, respectively. It can be seen from the figure that, when unfoamed, the flow index gradually increases with the increase in CO
2 volume fraction, while the consistency index decreases. Under experimental conditions, the change range of the flow index is 0.37−0.72; the variation range of the consistency index is 0.23−0.39.
The flow index is a parameter used to describe the non-Newtonian property of the fluid. It can be seen from the figure that the flow index n of the unfoamed fracturing fluid is less than 1, indicating that the fluid is a shear-thinning non-Newtonian fluid, and the value of n is closer to 1 with the increase in CO2 volume fraction, indicating that the fluid property of the fracturing fluid gradually changes to a Newtonian fluid with the increase in the CO2 volume fraction, which is precise because the unfoamed CO2 exists as a supercooled liquid similar to a Newtonian fluid, and its non-Newtonian property weakens with the increase in the CO2 volume fraction.
2.2.1. Effect of Pressure on the Effective Viscosity of CO2 Foam Gel Fracturing Fluid (Foam Quality)
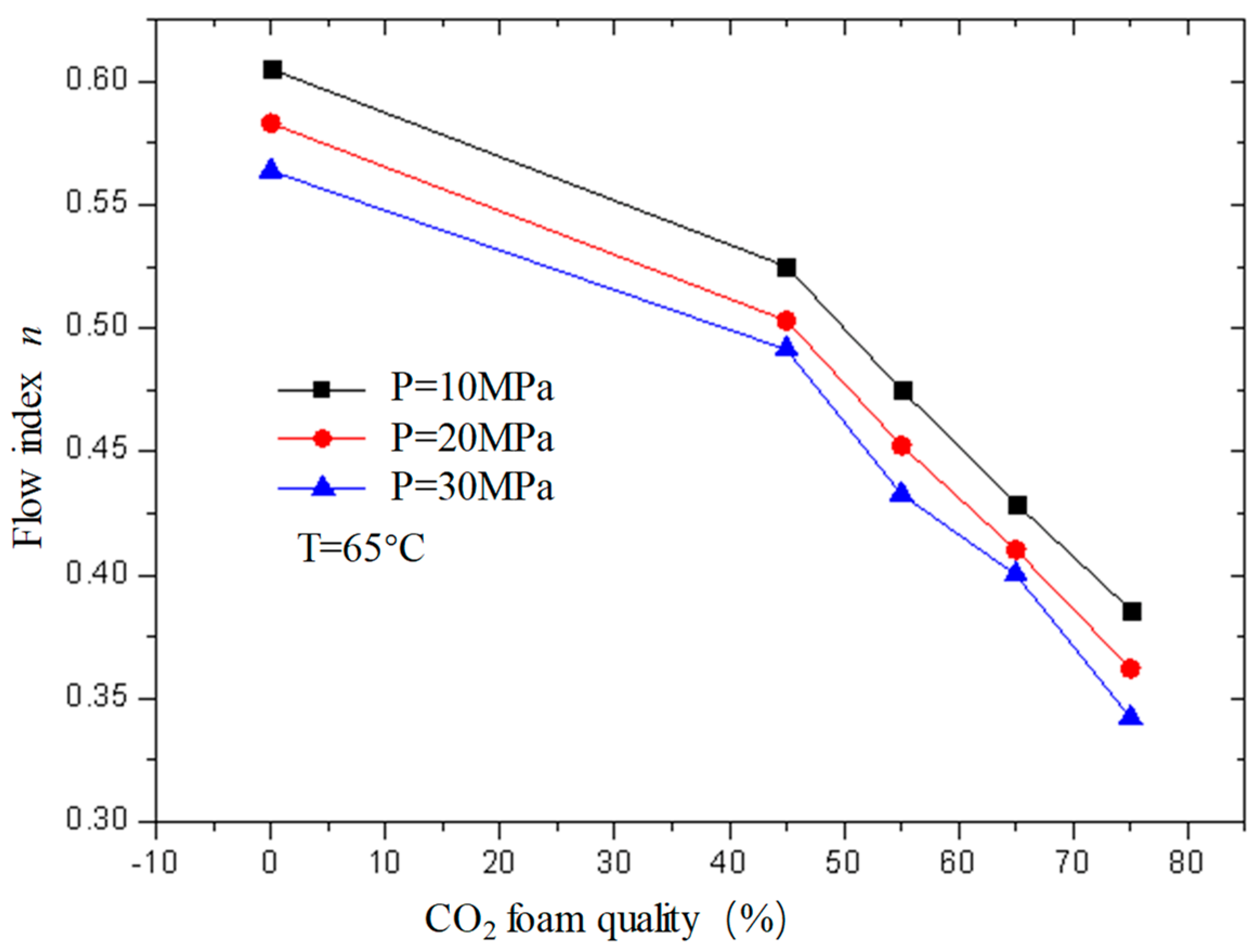
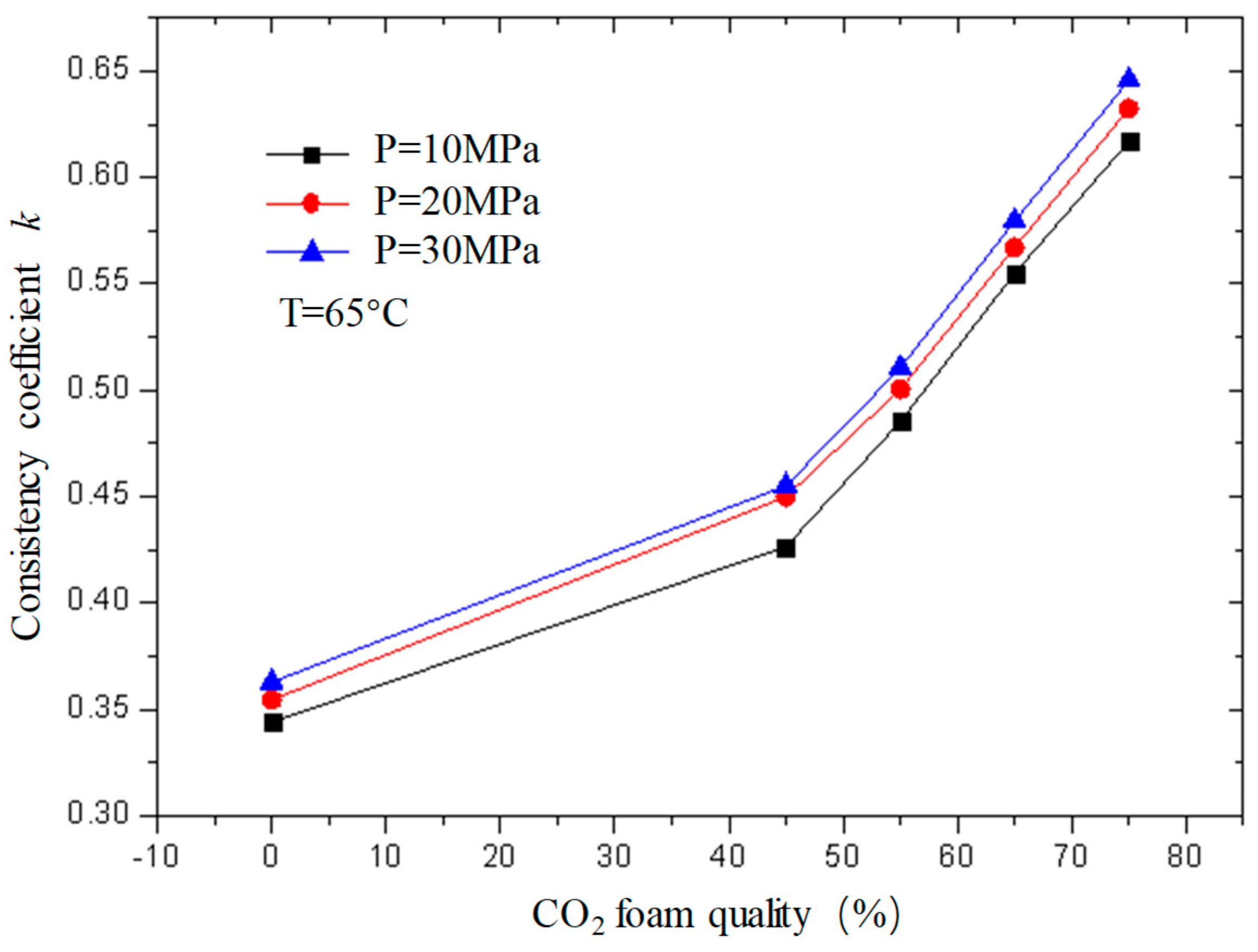
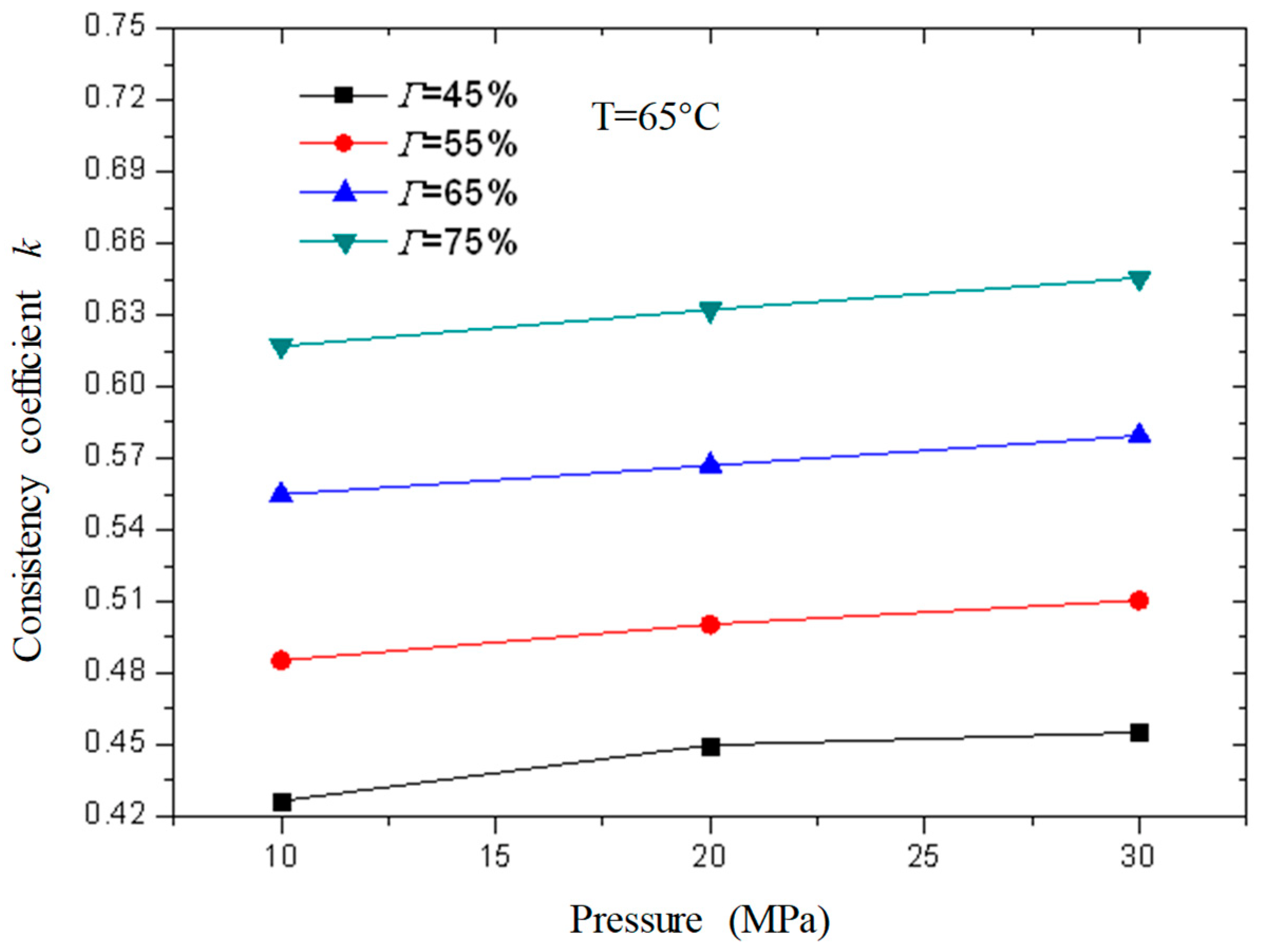
Figure 13 and
Figure 14 are the curves of the flow index and consistency index of CO
2 foam gel fracturing fluid with foam quality when the temperature is 65 °C and the pressure is 10 MPa, 20 MPa, and 30 MPa, respectively. As can be seen from the figure, with the increase in foam quality, the flow index gradually decreases, and the consistency index increases, and from the perspective of the change range, it starts to increase substantially when the foam quality is 55%. This shows that the CO
2 foam gel fracturing fluid in the foaming stage still belongs to the shear-thinning non-Newtonian fluid, and with the increase in foam quality, the non-Newtonian fluid properties of the foam gel fracturing fluid gradually increase.
2.2.2. Effect of Temperature on the Rheological Parameters of CO2 Foam Gel Fracturing Fluid
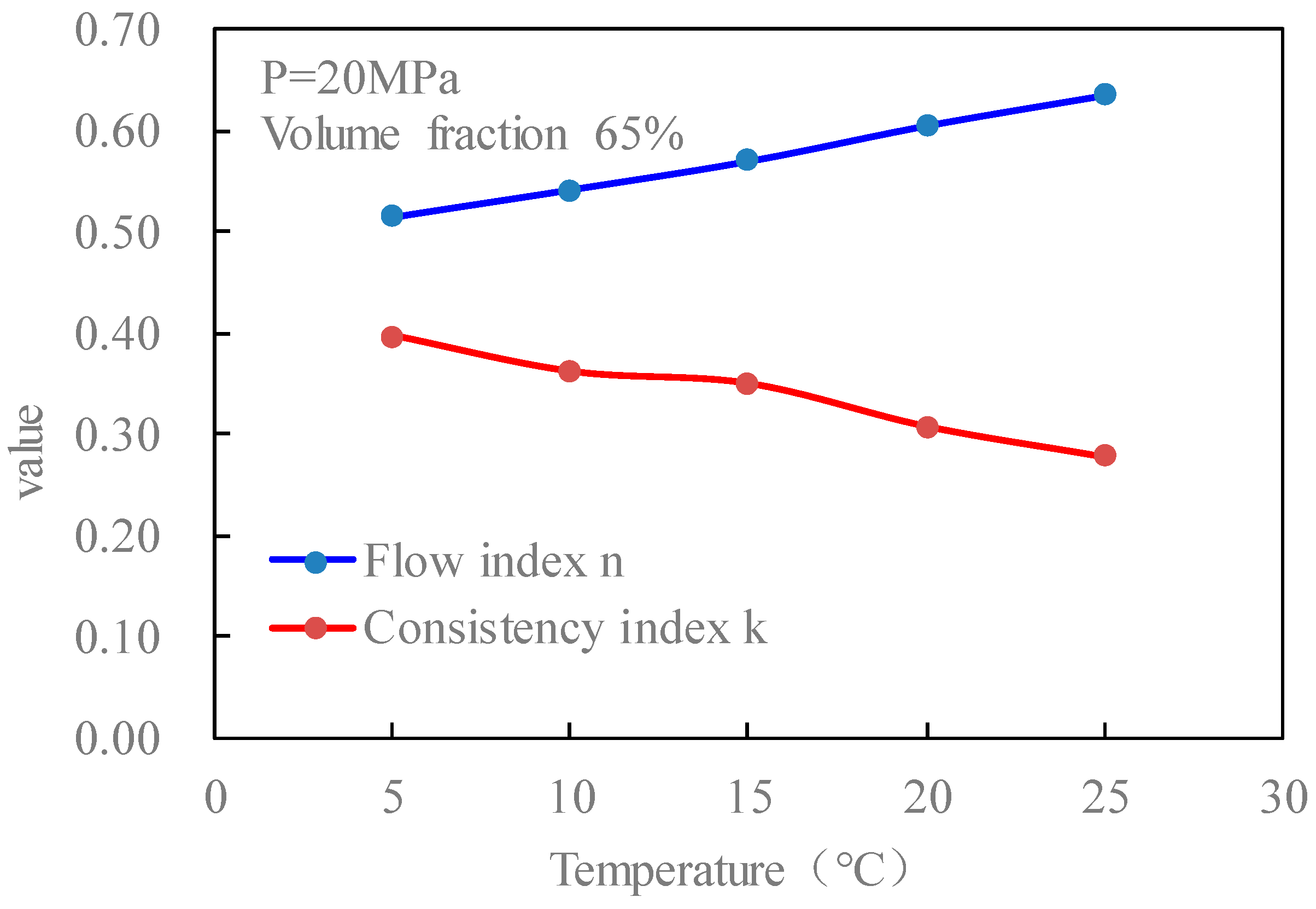
Figure 15 is the curves of the flow index and consistency index of fracturing fluid with temperature when the pressure is 20 MPa and the volume fraction of CO
2 is 65% without foaming. It can be seen from the figure that with the increase in temperature, the flow index of the fluid increases, and the consistency index decreases.
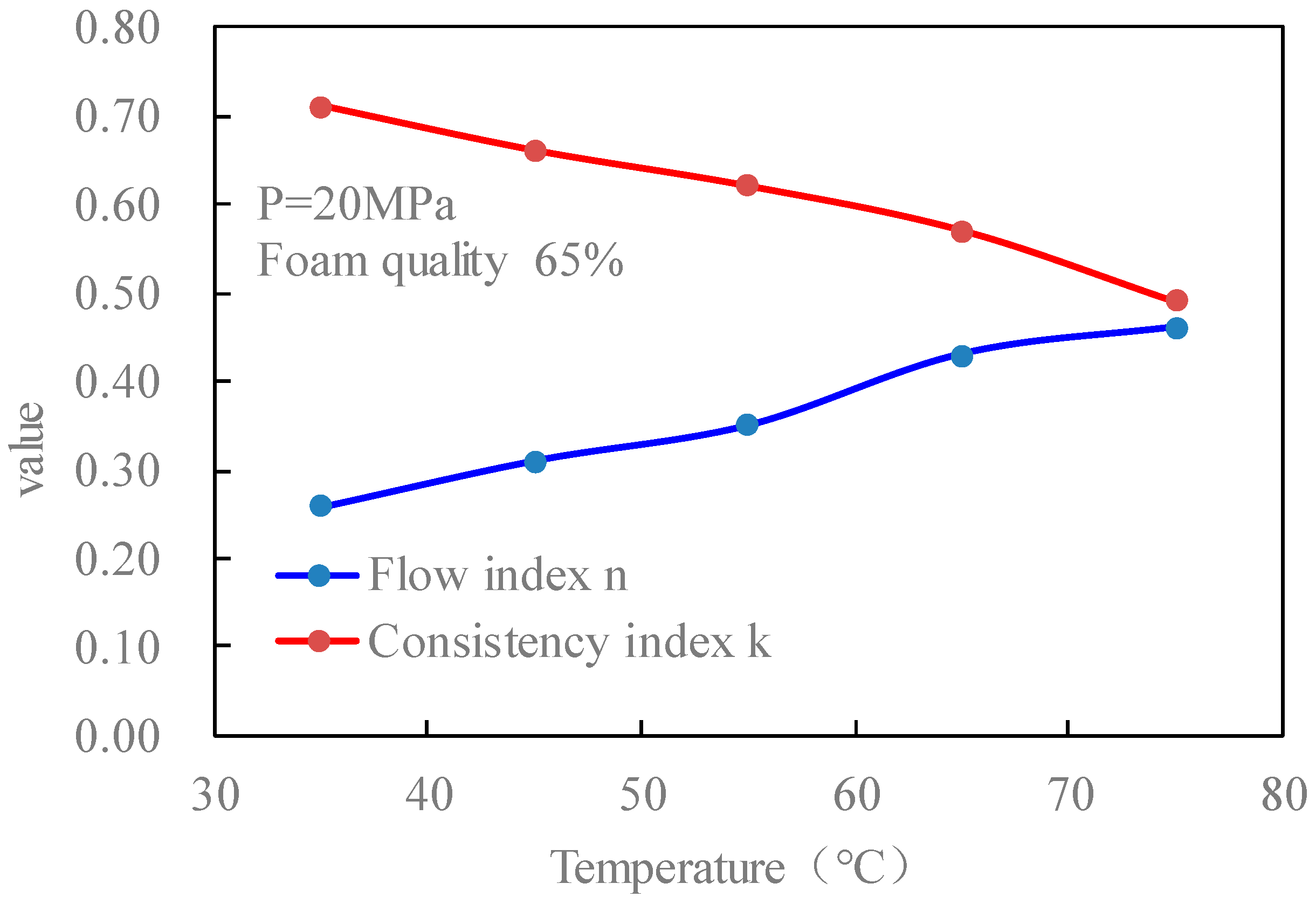
Figure 16 is the curves of the flow index and consistency index of the CO
2 foam gel fracturing fluid with temperature when the pressure is 20 MPa and the foam mass is 65%. It can be seen that the change rule of rheological parameters of foamed fracturing fluid with temperature is consistent with that of unfoamed fracturing fluid, which shows that with the increase in temperature, the flow index of fluid increases and the consistency index decreases. From the action mechanism, it is the same as that of the previous temperature on the viscosity characteristics of CO
2 foamed fracturing fluid.
2.2.3. Effect of Pressure on the Rheological Parameters of CO2 Foam Gel Fracturing Fluid
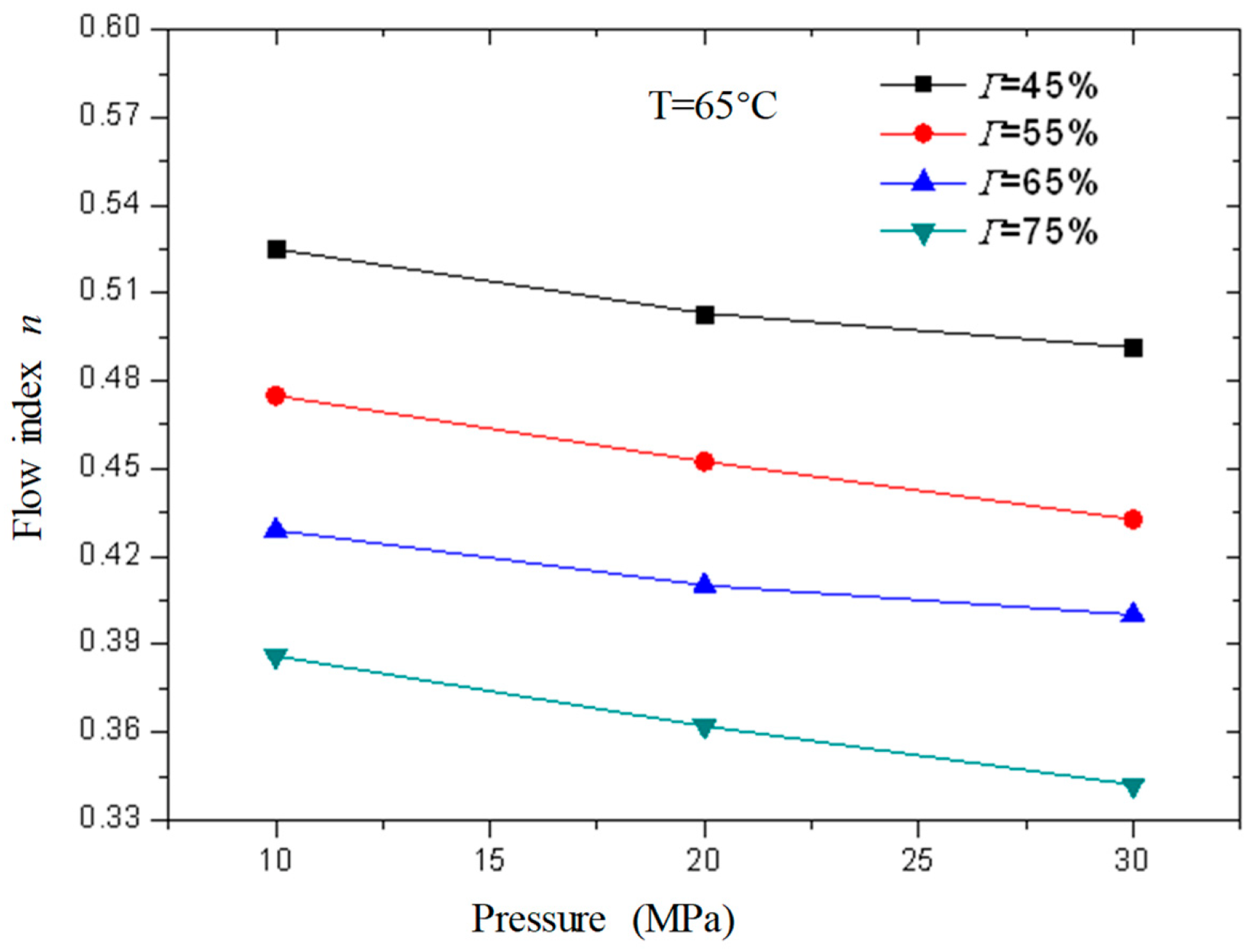
Figure 17 and
Figure 18 are curves of the rheological parameters of CO
2 foam gel fracturing fluid changing with pressure when the temperature is 65 °C and the foam quality is 45%, 55%, 65%, and 75% respectively. It can be seen from the figures that with the increase in pressure, the change of fluid flow index and consistency index is very small, which shows that the influence of pressure on the rheological properties of the foam gel fracturing fluid is almost negligible compared with temperature and foam quality. Meanwhile, the influence of pressure on the rheological properties of the foam gel fracturing fluid is negligible.
2.3. Sensitivity Analysis
Sensitivity analysis is a method to analyze system stability in system analysis. There is a system, and its system characteristics, P, are mainly determined by n factors a = {a1, a2, …, an} and P = f (a1, a2, …, an). In a certain reference state, a* = {a1*, a2*, …, an*}, the system characteristic is P*. Let each factor change within its possible range, and analyze the trend and degree of the deviation of the system characteristic P from the benchmark state P* due to these factors. This analysis method is called sensitivity analysis.
We define the dimensionless sensitivity function and sensitivity factor. That is, the ratio of the relative error of the system characteristic
P to the relative error of the parameter
ak is defined as the sensitivity function
Sk(
ak) of the parameter
ak.
When
is small,
Sk(
ak) can be approximately expressed as:
Sk*, k = 1, 2, …, n, is a set of dimensionless non-negative real numbers. The larger the Sk* value, the more sensitive P is to ak in the reference state. Through the comparison of Sk*, the sensitivity of system characteristics to various factors can be compared and evaluated.
To understand the sensitivity of the rheological properties of foam gel fracturing fluid to various factors, the above sensitivity analysis method was used to analyze the main factors affecting the rheological properties of foam gel fracturing fluid, and the sensitivity of each factor was compared. The characteristics of the system, that is, the rheological characteristics of foam gel fracturing fluid, are characterized by the effective viscosity of the system. The parameters for sensitivity analysis are shear rate, pressure, foam quality, and temperature. Observe the shape of the rheological curve and establish the functional relationship between effective viscosity and shear rate, temperature, and other parameters to obtain the sensitivity function, and then calculate the sensitivity factor. After analysis, the sensitivity values of each parameter are obtained (
Table 1), which are ranked by size, followed by foam quality, temperature, shear rate, and pressure. It can be seen that among all the factors, foam quality and temperature are the main influencing factors. Therefore, the performance of CO
2 foam gel fracturing can be mainly regulated by the two parameters, which is helpful for application in shale gas reservoir fracturing.
3. Conclusions
The formula of the gel fracturing fluid was obtained through experimental optimization, which evaluated experimentally the viscosity, residue, surface tension, sand-carrying capacity, and interfacial tension of the gel fracturing fluid. The core damage rate of the gel fracturing fluid is less than 19%, the shear time is 90 min at 170 s−1 and 90 °C, and the viscosity of the fracturing fluid is >50 mPa·s.
The rheological properties of CO2 foam gel fracturing fluid and its influencing factors were studied experimentally. For the foam gel fracturing fluid with CO2 in supercooled liquid and at a supercritical state, the effects of temperature, pressure, shear rate, and foam quality on the rheological properties of the gel fracturing fluid were considered. The main conclusions are as follows:
In the unfoamed state, the effective viscosity of foam gel fluid decreases exponentially with the increase in shear rate, gradually decreases with the increase in CO2 volume fraction, and the effective viscosity of fluid decreases with the increase in temperature. The effective viscosity is little affected by pressure. In the foaming state, the change rule of effective viscosity with shear rate is the same as that without foaming, the change rule with foam quality is opposite to that without foaming, and the effective viscosity−temperature characteristics of fluid are the same as that without foaming.
Without foaming, the foam quality increases, the flow index gradually increases, and the consistency index decreases. With the increase in temperature, the flow index of fluid increases, and the consistency index decreases. When foaming, the foam quality increases, the flow index gradually decreases, and the consistency index increases. When the foam quality is about 50%, a sudden change begins. With the increase in temperature, the flow index of fluid increases, and the consistency index decreases. With the increase in pressure, the flow index and consistency index of fluid change very little.
Based on the sensitivity analysis method, the influencing factors of the rheological behavior of CO2 foam gel fracturing fluid are foam quality, temperature, shear rate, and pressure, in turn, which provides a theoretical basis for CO2 foam fracturing technology.