Temperature-Ion-pH Triple Responsive Gellan Gum as In Situ Hydrogel for Long-Acting Cancer Treatment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of GSH-GG
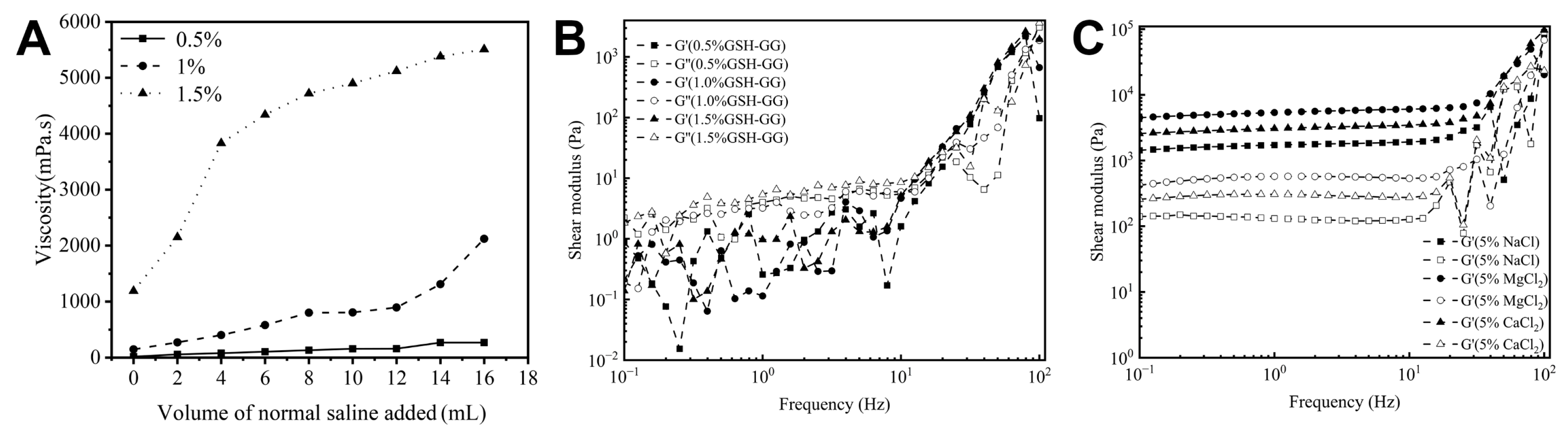
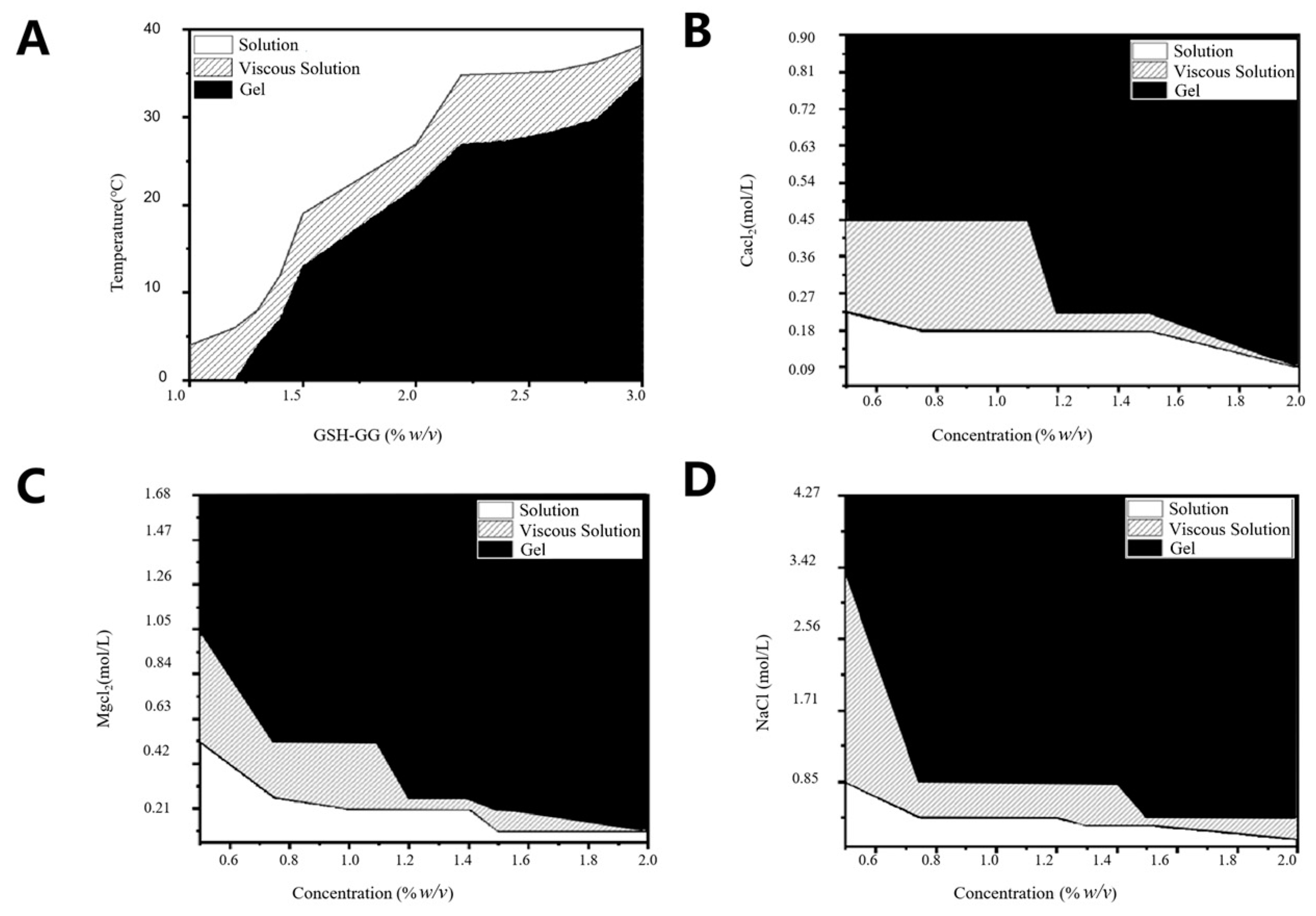
2.2. Effect of Temperature and Cations on Gelling Behavior of GSH-GG
2.3. Injectability Study
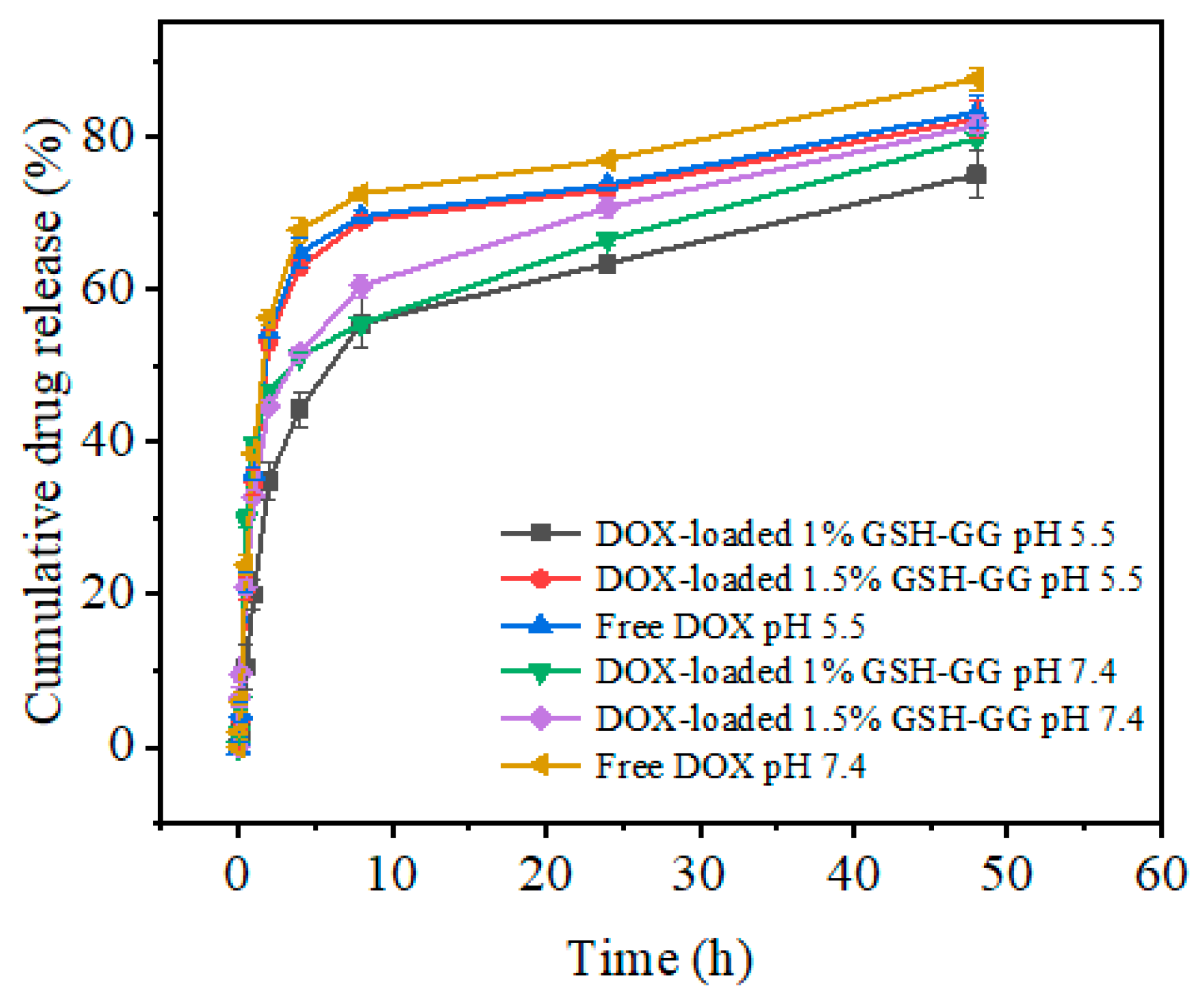
2.4. In Vitro Release
2.5. Cytotoxicity Assay
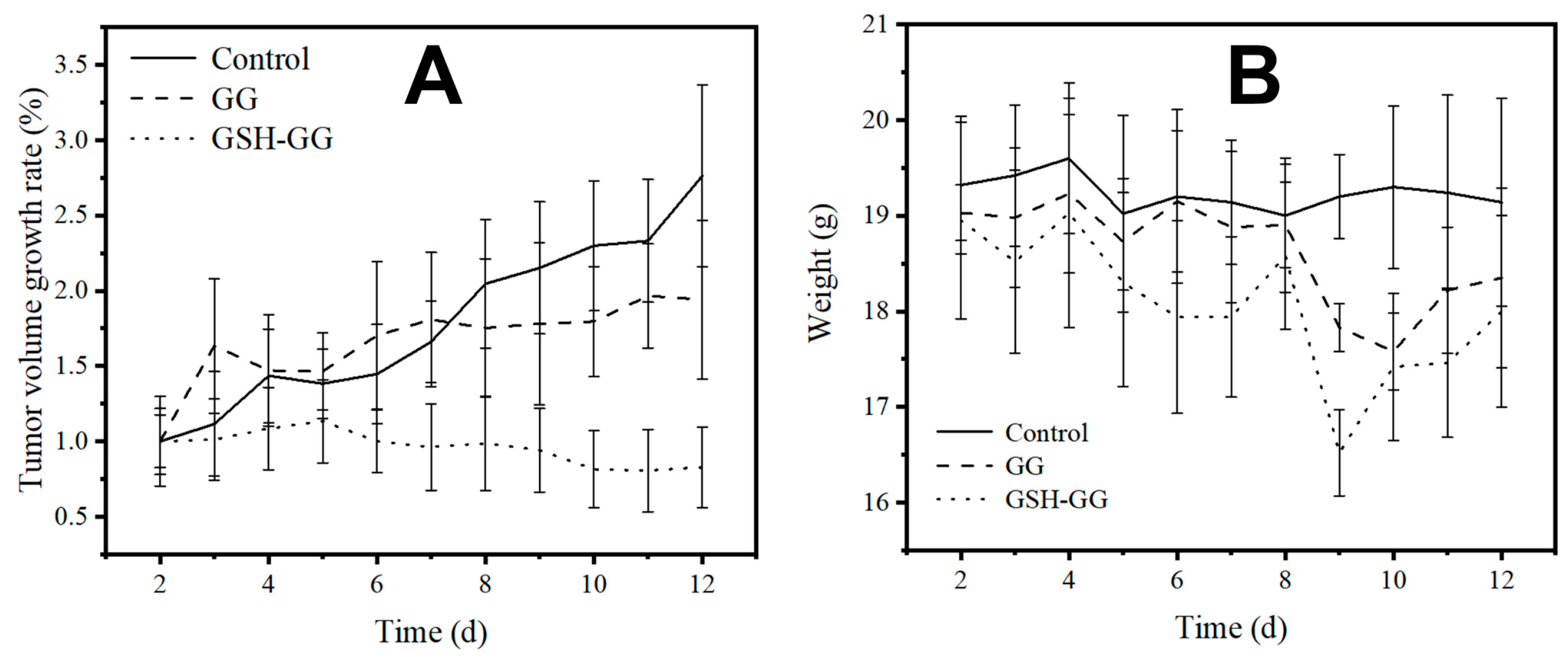
2.6. In Vivo Anticancer Assay
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of the GSH-GG
4.3. Gel Formation Assay
4.4. The Preparation of DOX-Loaded GSH-GG
4.5. Viscosity
4.6. Rheology of GSH-GG Hydrogel Measurements
4.7. Injectability Studies
4.8. In Vitro Adhesion
4.9. Effect of Temperature on Gelling Behavior of GSH-GG
4.10. Effect of Cations on Gelling Behavior of GSH-GG
4.11. In Vitro Drug Release of DOX-Loaded GSH-GG Hydrogels
4.12. Cytotoxicity
4.13. In Vivo Anti-Tumor Effect
4.14. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Brenner, D.R.; Weir, H.K.; Demers, A.A.; Ellison, L.F.; Louzado, C.; Shaw, A.; Turner, D.; Woods, R.R.; Smith, L.M. Projected estimates of cancer in Canada in 2020. Cmaj 2020, 192, E199–E205. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Zhang, S.; Chen, Z.; Chen, A.T.; Ma, J.; Deng, G.; Xu, W.; Zhou, J.; Yu, Z.-Q.; Yao, G.; et al. Synergistic Chemotherapy for Breast Cancer and Breast Cancer Brain Metastases via Paclitaxel-Loaded Oleanolic Acid Nanoparticles. Mol. Pharm. 2020, 17, 1343–1351. [Google Scholar] [CrossRef]
- Bathara, M.; Date, T.; Chaudhari, D.; Ghadi, R.; Kuche, K.; Jain, S. Exploring the promising potential of high permeation vesicle-mediated localized transdermal delivery of docetaxel in breast cancer to overcome the limitations of systemic chemotherapy. Mol. Pharm. 2020, 17, 2473–2486. [Google Scholar] [CrossRef]
- Zancanella, P.; Oliveira, D.M.L.; De Oliveira, B.H.; Woiski, T.D.; Pinto, C.C.; Santana, M.H.A.; Souto, E.B.; Severino, P. Mitotane liposomes for potential treatment of adrenal cortical carcinoma: Ex vivo intestinal permeation and in vivo bioavailability. Pharm. Dev. Technol. 2020, 25, 949–961. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, D.; Zeng, K.; Li, D.; Qin, L.; Cai, Y.; Jin, J. Simultaneous Delivery of antimiR-21 and Doxorubicin by Graphene Oxide for Reducing Toxicity in Cancer Therapy. ACS Omega 2020, 5, 14437–14443. [Google Scholar] [CrossRef] [PubMed]
- Wathoni, N.; Ny Nguyen, A.; Rusdin, A.; Umar, A.K.; Mohammed, A.F.A.; Motoyama, K.; Joni, I.M.; Muchtaridi, M. Enteric-Coated Strategies in Colorectal Cancer Nanoparticle Drug Delivery System. Drug Des. Dev. Ther. 2020, 14, 4387–4405. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, S.; Yu, F.; Lv, J.; Zhao, R.; Hu, F.; Yuan, H. Dual-channel theranostic system for quantitative self-indication and low-temperature synergistic therapy of cancer. Small 2021, 1, 2007953. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Z.; Chen, S.; Cui, P.; Qiu, L.; Zhou, S.; Jiang, H.; Jiang, P.; Wang, J. Modulation of Aggregation-Caused Quenching to Aggregation-Induced Emission: Finding a Biocompatible Polymeric Theranostics Platform for Cancer Therapy. Macromol. Rapid Commun. 2021, 42, 2100264. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Xinyu, H.; Jabeen, A.; Ahsan, A.; Seidu, T.A.; Kutoka, P.T.; Wang, B. Enhanced cellular uptake and cytotoxicity of vorinostat through encapsulation in TPGS-modified liposomes. Colloids Surf. B Biointerfaces 2021, 199, 111523. [Google Scholar] [CrossRef] [PubMed]
- Schneible, J.D.; Young, A.T.; Daniele, M.A.; Menegatti, S. Chitosan Hydrogels for Synergistic Delivery of Chemotherapeutics to Triple Negative Breast Cancer Cells and Spheroids. Pharm. Res. 2020, 37, 142. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, R.; Soleimani, K.; Derakhshankhah, H.; Haghshenas, B.; Rezaei, A.; Massoumi, B.; Farnudiyan-Habibi, A.; Samadian, H.; Jaymand, M. Multi-stimuli-responsive magnetic hydrogel based on Tragacanth gum as a de novo nanosystem for targeted chemo/hyperthermia treatment of cancer. J. Mater. Res. 2021, 36, 858–869. [Google Scholar] [CrossRef]
- Amano, Y.; Sakura, K.L.; Ohta, S.; Ito, T. Cisplatin–Chelated Iminodiacetic Acid–Conjugated Hyaluronic Acid Nanogels for the Treatment of Malignant Pleural Mesothelioma in Mice. Mol. Pharm. 2022, 19, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Gao, Q.; Tang, J.; Jiang, Y.; Yang, L.; Shi, X.; Chen, Y.; Zhang, Y.; Fu, S.; Lin, S. Anti-tumor effect of local injectable hydrogel-loaded endostatin alone and in combination with radiotherapy for lung cancer. Drug Deliv. 2021, 28, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Nah, H.; Moon, H.J.; Lee, S.J.; Heo, D.N.; Kwon, I.K. Controllable delivery system: A temperature and pH-responsive injectable hydrogel from succinylated chitosan. Appl. Surf. Sci. 2020, 528, 146812. [Google Scholar] [CrossRef]
- Pushpamalar, J.; Meganathan, P.; Tan, H.L.; Dahlan, N.A.; Ooi, L.-T.; Neerooa, B.N.H.M.; Essa, R.Z.; Shameli, K.; Teow, S.-Y. Development of a Polysaccharide-Based Hydrogel Drug Delivery System (DDS): An Update. Gels 2021, 7, 153. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Shah, L.A.; Shah, M.; Jamil, I. Engineering of 3D polymer network hydrogels for biomedical applications: A review. Polym. Bull. 2021, 79, 2685–2705. [Google Scholar] [CrossRef]
- Iglesias, N.; Galbis, E.; Valencia, C.; Díaz-Blanco, M.J.; Lacroix, B.; de-Paz, M.V. Biodegradable double cross-linked chitosan hydrogels for drug delivery: Impact of chemistry on rheological and pharmacological performance. Int. J. Biol. Macromol. 2020, 165, 2205–2218. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Sharma, U. Formulation and Characterization of Atenolol-loaded Gellan Gum Nanoparticles. Indian J. Pharm. Sci. 2021, 83, 60–65. [Google Scholar] [CrossRef]
- Okur, N.U.; Yağcılar, A.P.; Siafaka, P.I. Promising polymeric drug carriers for local delivery: The case of in situ gels. Curr. Drug Deliv. 2020, 17, 675–693. [Google Scholar] [CrossRef]
- Rukmanikrishnan, B.; Jo, C.; Choi, S.; Ramalingam, S.; Lee, J. Flexible Ternary Combination of Gellan Gum, Sodium Carboxymethyl Cellulose, and Silicon Dioxide Nanocomposites Fabricated by Quaternary Ammonium Silane: Rheological, Thermal, and Antimicrobial Properties. ACS Omega 2020, 5, 28767–28775. [Google Scholar] [CrossRef] [PubMed]
- Rim, M.A.; Choi, J.H.; Park, A.; Youn, J.; Lee, S.; Kim, N.E.; Song, J.E.; Khang, G. Characterization of Gelatin/Gellan Gum/Glycol Chitosan Ternary Hydrogel for Retinal Pigment Epithelial Tissue Reconstruction Materials. ACS Appl. Bio Mater. 2020, 3, 6079–6087. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zheng, X.; Chen, J.; Xu, Y.; Du, X.; Wang, C.; Cui, P.; Qiu, L.; Jiang, P.; Ni, X.; et al. Liposome Loaded Ion/Temperature Dual Responsive Gellan Gum Hydrogel as Potential Nasal-To-Brain Delivery System. J. Biomed. Nanotechnol. 2022, 18, 571–580. [Google Scholar] [CrossRef]
- Yu, L.; Ding, J. Injectable hydrogels as unique biomedical materials. Chem. Soc. Rev. 2008, 37, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yao, P. Injectable thermo-responsive hydrogel composed of xanthan gum and methylcellulose double networks with shear-thinning property. Carbohydr. Polym. 2015, 132, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.; Thao, N.; Thuy, B.; Tran, V.; Nguyen, T.; Nguyen, N. Silk fibroin hydrogel containing Sesbania sesban L. extract for rheumatoid arthritis treatment. Drug Deliv. 2022, 29, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Paulsson, M.; Hägerström, H.; Edsman, K. Rheological studies of the gelation of deacetylated gellan gum (Gelrite®) in physiological conditions. Eur. J. Pharm. Sci. 1999, 9, 99–105. [Google Scholar] [CrossRef]
- Fernández-Ferreiro, A.; Barcia, M.G.; Gil-Martínez, M.; Vieites-Prado, A.; Lema, I.; Argibay, B.; Méndez, J.B.; Lamas, M.J.; Otero-Espinar, F.J. In vitro and in vivo ocular safety and eye surface permanence determination by direct and Magnetic Resonance Imaging of ion-sensitive hydrogels based on gellan gum and kappa-carrageenan. Eur. J. Pharm. Biopharm. 2015, 94, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Clegg, J.R.; Anselmo, A.C.; Mitragotri, S. Hydrogels in the clinic. Bioeng. Transl. Med. 2020, 5, e10158. [Google Scholar] [CrossRef] [PubMed]
- Kashani, H.; Madrakian, T.; Afkhami, A. Development of modified polymer dot as stimuli-sensitive and 67Garadio-carrier, for investigation of in vitro drug delivery, in vivoimaging and drug release kinetic. J. Pharm. Biomed. Anal. 2021, 203, e114217. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Li, W.; Liu, D.; Wu, S.; Song, B.; Ma, J.; Chen, D.; Hu, H. Tumor Microenvironment Stimuli-Responsive Nanoparticles for Programmed Anticancer Drug Delivery. Mol. Pharm. 2020, 17, 1516–1526. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Wang, Z.; Wang, C. Recent Progress in Polymeric AIE-Active Drug Delivery Systems: Design and Application. Mol. Pharm. 2021, 18, 3951–3965. [Google Scholar] [CrossRef]
- Wang, C.; Chen, S.; Wang, Y.; Liu, K.; Hu, F.; Sun, J.; Yuan, H. Lipase-triggered water-responsive “Pandora’s Box” for cancer therapy: Toward induced neighboring effect and enhanced drug penetration. Adv. Mater. 2018, 30, 1706407. [Google Scholar] [CrossRef] [PubMed]
- Najafipour, A.; Gharieh, A.; Fassihi, A.; Sadeghi-Aliabadi, S.; Mahdavian, A. MTX-loaded dual thermoresponsive and pH-responsive magnetic hydrogel nanocomposite particles for combined controlled drug delivery and hyperthermia therapy of cancer. Mol. Pharm. 2020, 18, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Cunha, P.L.; Castro, R.R.; Rocha, F.A.; de Paula, R.C.; Feitosa, J.P. Low viscosity hydrogel of guar gum: Preparation and physicochemical characterization. Int. J. Biol. Macromol. 2005, 37, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Seif-Naraghi, S.B.; Singelyn, J.M.; Salvatore, M.A.; Osborn, K.G.; Wang, J.J.; Sampat, U.; Kwan, O.L.; Strachan, G.M.; Wong, J.; Schup-Magoffin, P.J.; et al. Safety and Efficacy of an Injectable Extracellular Matrix Hydrogel for Treating Myocardial Infarction. Sci. Transl. Med. 2013, 5, 173ra25. [Google Scholar] [CrossRef]
- Schiavi, A.; Cuccaro, R.; Troia, A. Strain-rate and temperature dependent material properties of Agar and Gellan Gum used in biomedical applications. J. Mech. Behav. Biomed. Mater. 2016, 53, 119–130. [Google Scholar] [CrossRef]
- Kirchmajer, D.M.; Steinhoff, B.; Warren, H.; Clark, R.; Panhuis, M.I.H. Enhanced gelation properties of purified gellan gum. Carbohydr. Res. 2014, 388, 125–129. [Google Scholar] [CrossRef]
- Tao, S.; Yu, F.; Song, Y.; Zhou, W.; Lv, J.; Zhao, R.; Wang, C.; Hu, F.; Yuan, H. Water/pH dual responsive in situ calcium supplement collaborates simvastatin for osteoblast promotion mediated osteoporosis therapy via oral medication. J. Control. Release 2021, 329, 121–135. [Google Scholar] [CrossRef]
- Wang, J.; Tao, S.; Jin, X.; Song, Y.; Zhou, W.; Lou, H.; Zhao, R.; Wang, C.; Hu, F.; Yuan, H. Calcium Supplement by Tetracycline guided amorphous Calcium Carbonate potentiates Osteoblast promotion for Synergetic Osteoporosis Therapy. Theranostics 2020, 10, 8591–8605. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Li, H.; Yin, C.; Tang, F. Research progress in the application of in situ hydrogel system in tumor treatment. Drug Deliv. 2020, 27, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Thakur, S.; Singh, N.; Kaur, S.; Jain, S.K. Novel Gellan Gum-Based In Situ Nanovesicle Formulation of Docetaxel for Its Localized Delivery Using Depot Formation. AAPS PharmSciTech 2021, 22, 165. [Google Scholar] [CrossRef] [PubMed]


| Conditions | Mathematical Models | R2 | K | n | Release Mechanism |
|---|---|---|---|---|---|
| pH = 7.4, 1.5% GSH-GG | Zero-order | 0.57611 | 1.29968 | - | |
| First-order | 0.9254 | 0.50898 | - | ||
| Higuchi | 0.80759 | 10.6925 | - | ||
| Korsmeyer–Peppas | 0.98972 | 970.346 | 0.01249 | Fickian diffusion | |
| pH = 5.5, 1.5% GSH-GG | Zero-order | 0.40511 | 1.30751 | - | |
| First-order | 0.97956 | 0.59000 | - | ||
| Higuchi | 0.65597 | 11.2961 | - | ||
| Korsmeyer–Peppas | 0.94492 | 128,942 | 0.00011 | Fickian diffusion |
| Group | Tumor | Type of Administration |
|---|---|---|
| 1 | + | Saline |
| 2 | + | DOX-loaded GG |
| 3 | + | DOX-loaded GSH-GG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Zheng, X.; Yi, K.; Du, X.; Wang, C.; Cui, P.; Jiang, P.; Ni, X.; Qiu, L.; Wang, J. Temperature-Ion-pH Triple Responsive Gellan Gum as In Situ Hydrogel for Long-Acting Cancer Treatment. Gels 2022, 8, 508. https://doi.org/10.3390/gels8080508
Zhou S, Zheng X, Yi K, Du X, Wang C, Cui P, Jiang P, Ni X, Qiu L, Wang J. Temperature-Ion-pH Triple Responsive Gellan Gum as In Situ Hydrogel for Long-Acting Cancer Treatment. Gels. 2022; 8(8):508. https://doi.org/10.3390/gels8080508
Chicago/Turabian StyleZhou, Shuwen, Xinmeng Zheng, Ke Yi, Xuancheng Du, Cheng Wang, Pengfei Cui, Pengju Jiang, Xinye Ni, Lin Qiu, and Jianhao Wang. 2022. "Temperature-Ion-pH Triple Responsive Gellan Gum as In Situ Hydrogel for Long-Acting Cancer Treatment" Gels 8, no. 8: 508. https://doi.org/10.3390/gels8080508






