Active Neutralizing Mats for Corrosive Chemical Storage
Abstract
:1. Introduction
2. Results and Discussion
2.1. Evaluation of Neutralizing Efficiency
2.1.1. Direct Titrations
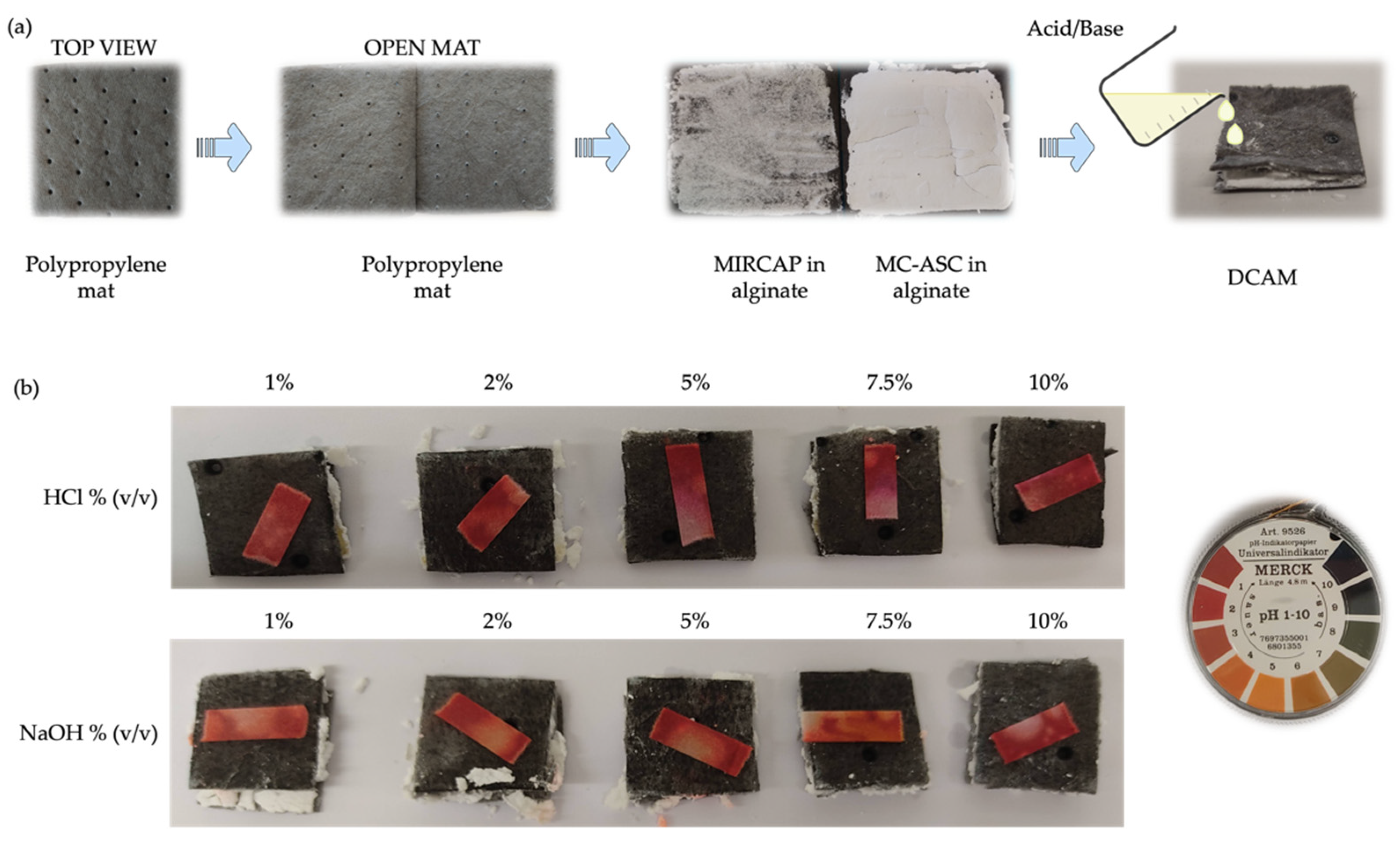
2.1.2. PH Strip Measurement Tests of Simulated Leakage
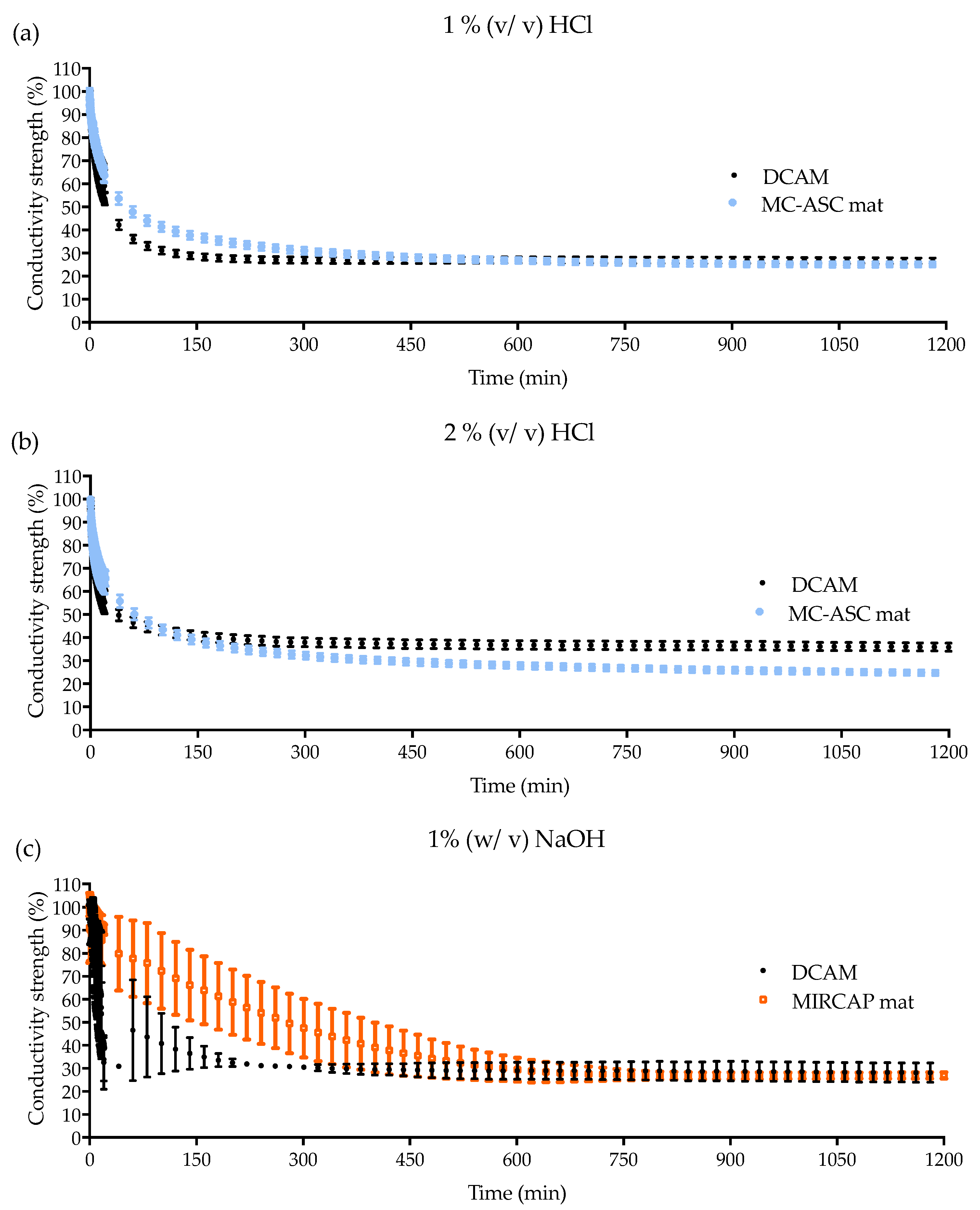
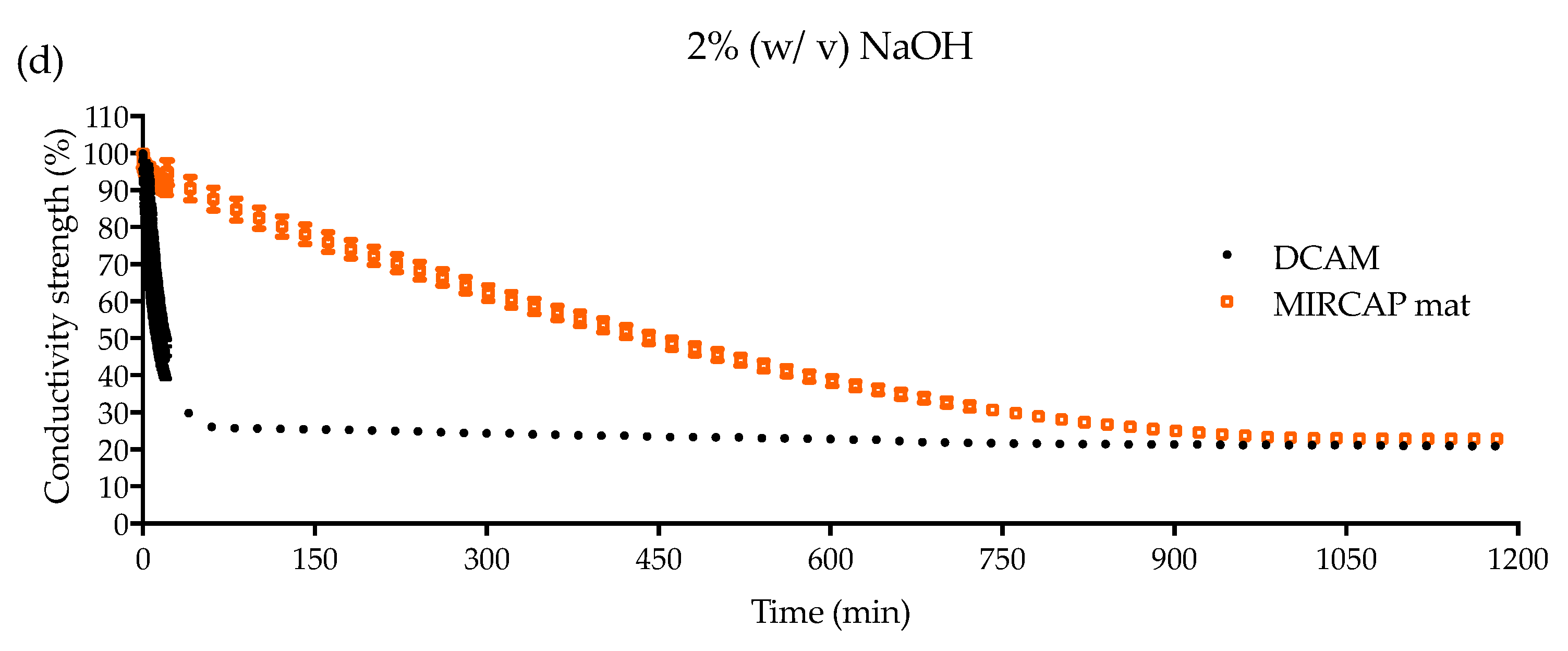
2.1.3. Conductivity Analysis
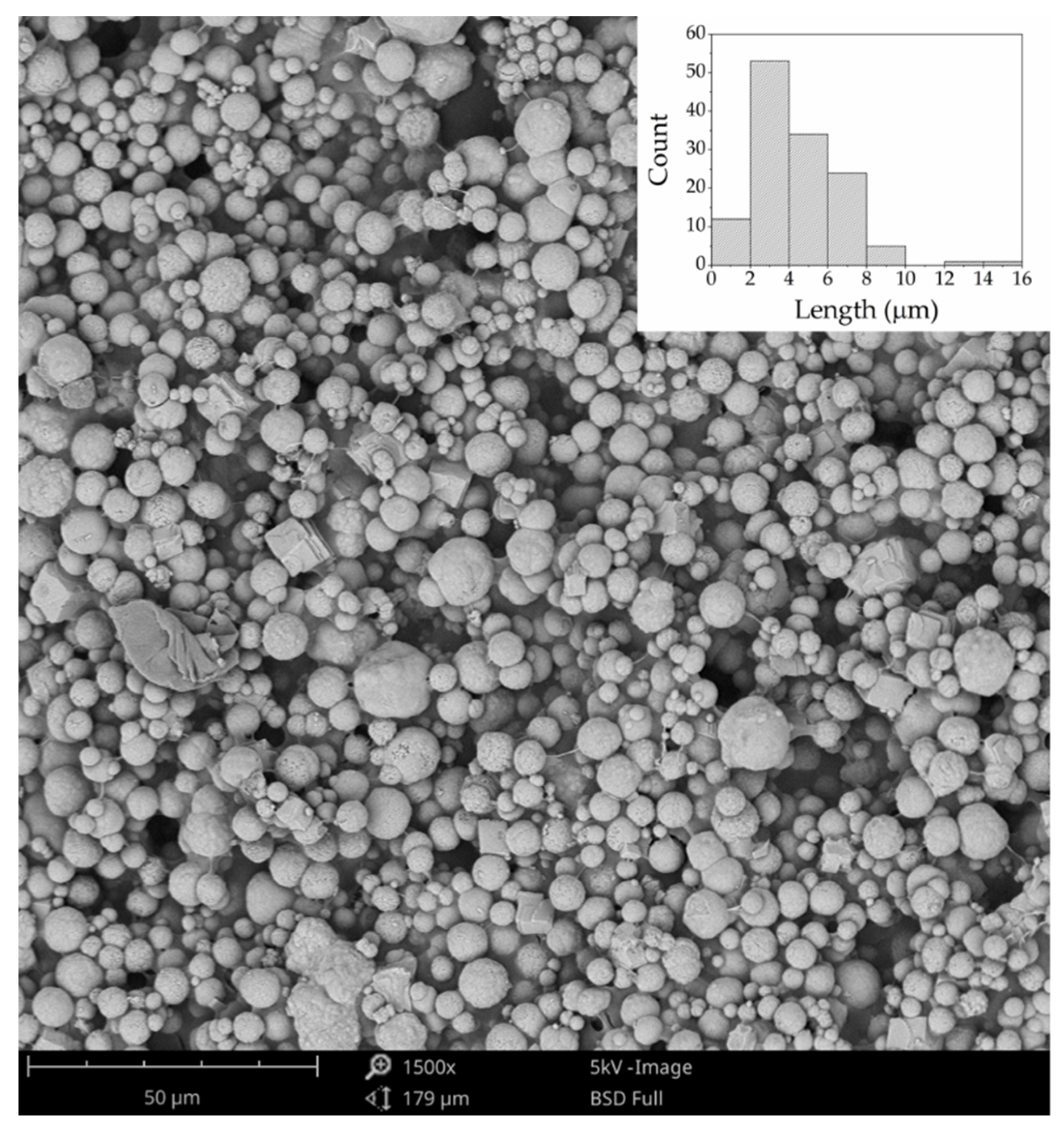
2.2. Scanning Electron Microscopy (SEM) Analysis
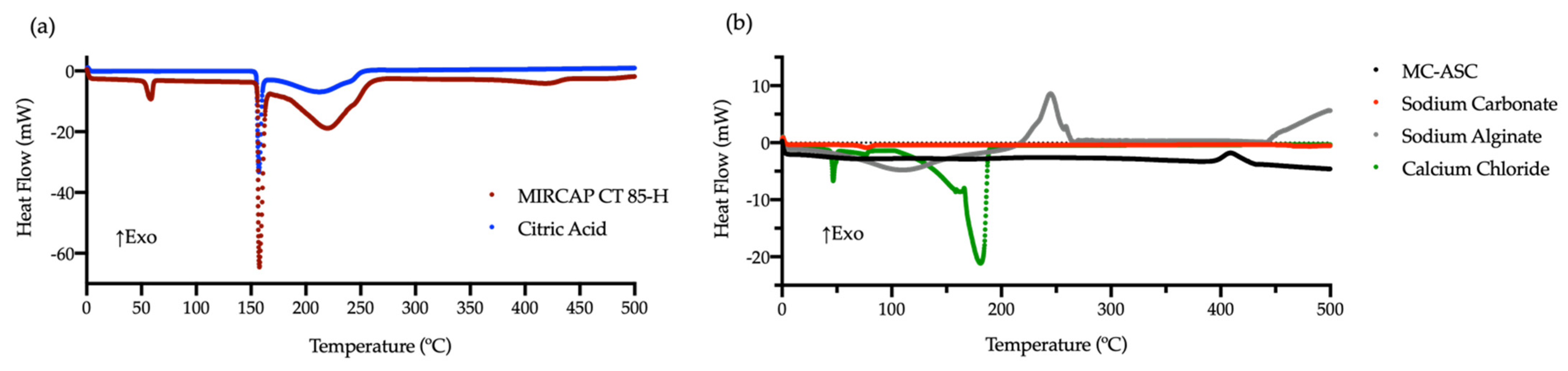
2.3. Thermal Analysis
3. Conclusions
4. Materials and Methods
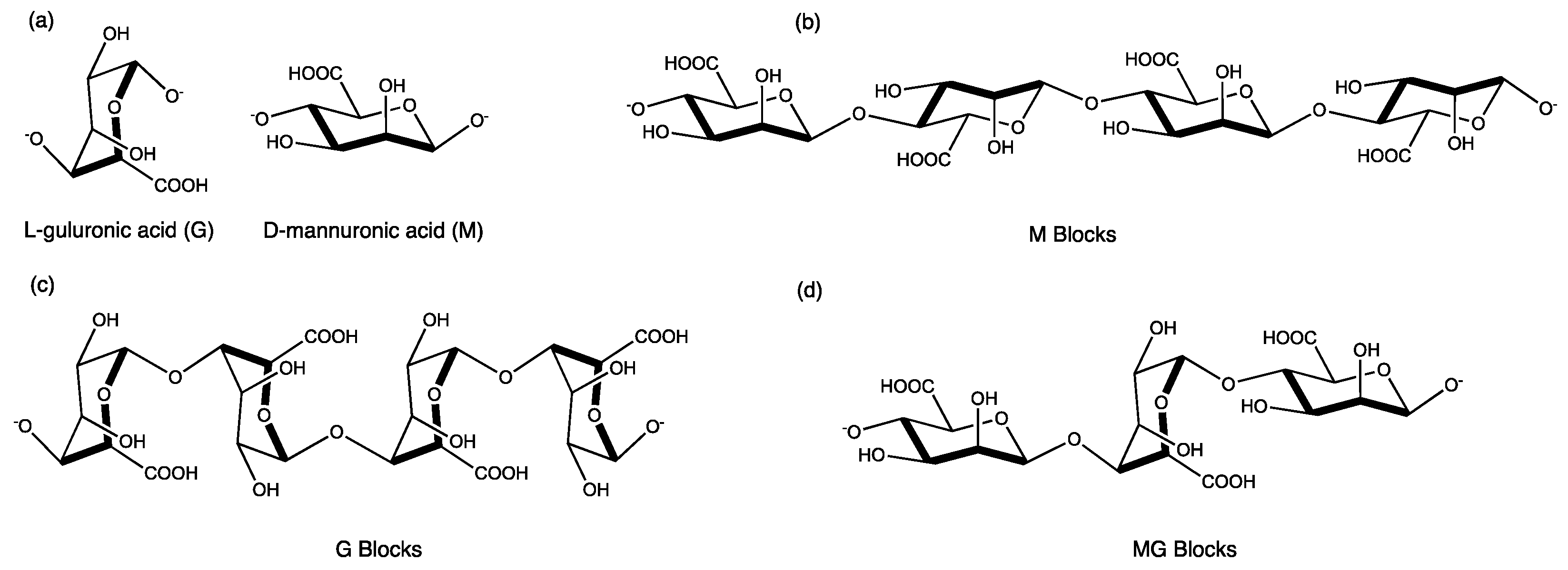
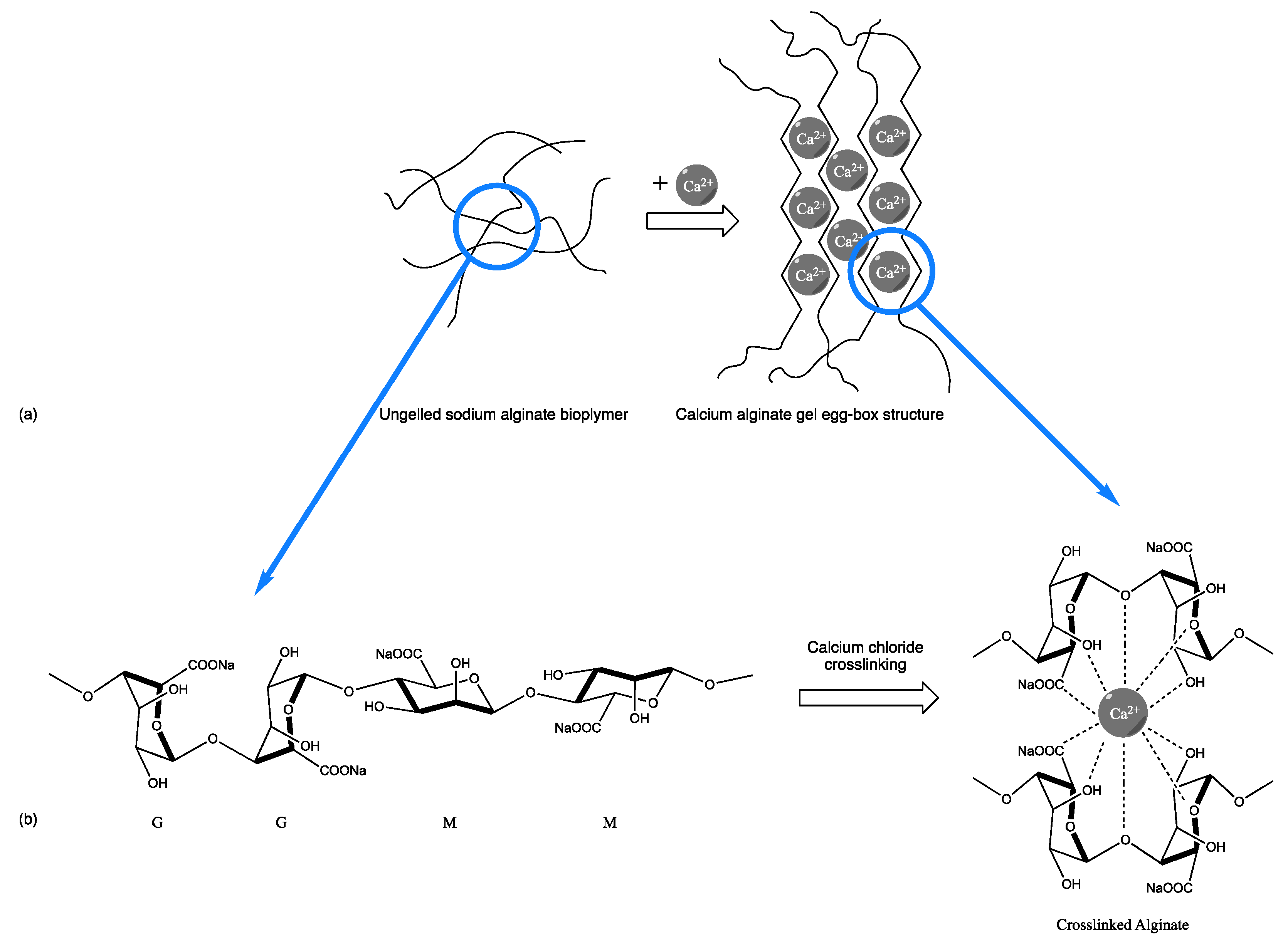
4.1. Materials
4.2. Methods
4.2.1. Direct Titrations
4.2.2. Microcapsules Production
4.2.3. Scanning Electron Microscope (SEM)
4.2.4. Neutralizing Mats Preparation
4.2.5. Conductivity and PH
4.2.6. Thermal Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Asiry, S.; Ang, L.-C. Laboratory Safety: Chemical and Physical Hazards. In Biobanking; Springer: Berlin/Heidelberg, Germany, 2019; pp. 243–252. [Google Scholar] [CrossRef]
- Zhang, C. Analysis of Fire Safety System for Storage Enterprises of Dangerous Chemicals. Procedia Eng. 2018, 211, 986–995. [Google Scholar] [CrossRef]
- Vergara-Rubio, A.; Ribba, L.; Picón, D.; Candal, R.; Goyanes, S. A Highly Efficient Nanostructured Sorbent of Sulfuric Acid from Ecofriendly Electrospun Poly(vinyl alcohol) Mats. Ind. Eng. Chem. Res. 2022, 61, 2091–2099. [Google Scholar] [CrossRef]
- Zaffora, A.; Culcasi, A.; Gurreri, L.; Cosenza, A.; Tamburini, A.; Santamaria, M.; Micale, G. Energy Harvesting by Waste Acid/Base Neutralization via Bipolar Membrane Reverse Electrodialysis. Energies 2020, 13, 5510. [Google Scholar] [CrossRef]
- First Mats Ltd. Available online: https://www.firstmats.co.uk/ (accessed on 1 February 2021).
- Global Spill Australia. Available online: https://www.globalspill.com.au (accessed on 1 February 2021).
- Fisher Scientific UK Ltd. Available online: https://www.fishersci.co.uk (accessed on 1 February 2021).
- New Pig, UK. Available online: https://www.newpig.eu/ (accessed on 1 February 2021).
- Choi, H.; Song, Y.K.; Kim, K.Y.; Park, J.M. Encapsulation of triethanolamine as organic corrosion inhibitor into nanoparticles and its active corrosion protection for steel sheets. Surf. Coat. Technol. 2012, 206, 2354–2362. [Google Scholar] [CrossRef]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate gel particles—A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2015, 57, 1133–1152. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.; Padrão, J.; Ribeiro, A.I.; Fernandes, R.D.V.; Melro, L.; Nicolau, T.; Mehravani, B.; Alves, C.; Rodrigues, R.; Zille, A. Polysaccharides and Metal Nanoparticles for Functional Textiles: A Review. Nanomaterials 2022, 12, 1006. [Google Scholar] [CrossRef]
- Smith, A.M.; Miri, T. Alginates in Foods. In Practical Food Rheology; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2010; Volume Chapter 6, pp. 113–132. [Google Scholar]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.-H.; Kim, S.-K. Alginate composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef]
- Ahmad, A.; Mubarak, N.M.; Jannat, F.T.; Ashfaq, T.; Santulli, C.; Rizwan, M.; Najda, A.; Bin-Jumah, M.; Abdel-Daim, M.M.; Hussain, S.; et al. A Critical Review on the Synthesis of Natural Sodium Alginate Based Composite Materials: An Innovative Biological Polymer for Biomedical Delivery Applications. Processes 2021, 9, 137. [Google Scholar] [CrossRef]
- Chen, K.; Wang, F.; Liu, S.; Wu, X.; Xu, L.; Zhang, D. In situ reduction of silver nanoparticles by sodium alginate to obtain silver-loaded composite wound dressing with enhanced mechanical and antimicrobial property. Int. J. Biol. Macromol. 2020, 148, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Wang, Y.; Qi, H.; Shi, C.; Wei, G.; Xiao, L.; Huang, Z.; Liu, S.; Yu, H.; Teng, C.; et al. Nanocomposite sponges of sodium alginate/graphene oxide/polyvinyl alcohol as potential wound dressing: In vitro and in vivo evaluation. Compos. Part B Eng. 2019, 167, 396–405. [Google Scholar] [CrossRef]
- Wilkinson, J.; Abd-Elaziz, K.; den Daas, I.; Wemer, J.; van Haastert, M.; Hodgkinson, V.; Foster, M.; Coyle, C. Two placebo-controlled crossover studies in healthy subjects to evaluate gastric acid neutralization by an alginate–antacid formulation (Gaviscon Double Action). Drug Dev. Ind. Pharm. 2018, 45, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Afshar, M.; Dini, G.; Vaezifar, S.; Mehdikhani, M.; Movahedi, B. Preparation and characterization of sodium alginate/polyvinyl alcohol hydrogel containing drug-loaded chitosan nanoparticles as a drug delivery system. J. Drug Deliv. Sci. Technol. 2020, 56, 101530. [Google Scholar] [CrossRef]
- Yang, J.-S.; Xie, Y.-J.; He, W. Research progress on chemical modification of alginate: A review. Carbohydr. Polym. 2011, 84, 33–39. [Google Scholar] [CrossRef]
- Gao, X.; Guo, C.; Hao, J.; Zhao, Z.; Long, H.; Li, M. Adsorption of heavy metal ions by sodium alginate based adsorbent-a review and new perspectives. Int. J. Biol. Macromol. 2020, 164, 4423–4434. [Google Scholar] [CrossRef] [PubMed]
- Sachan, N.; Pushkar, S.; Jha, A.; Bhattcharya, A. Sodium alginate: The wonder polymer for controlled drug delivery. J. Pharm. Res. 2009, 2, 1191–1199. [Google Scholar]
- Apelblat, A. Citric Acid; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- VEDEQSA. Available online: https://www.vedeqsa.com/ (accessed on 2 May 2021).
- Watson Inc. Available online: https://www.watson-inc.com/product/citric-acid-85-palm/ (accessed on 2 May 2021).
- HSE—Health and Safety Executive. Chemical Warehousing: The Storage of Packaged Dangerous Substances, 4th ed.; HSE Books: London, UK, 2009; p. 56. [Google Scholar]
- Artemov, V.G.; Volkov, A.A.; Sysoev, N.N.; Volkov, A.A. Conductivity of aqueous HCl, NaOH and NaCl solutions: Is water just a substrate? EPL Europhys. Lett. 2015, 109, 26002. [Google Scholar] [CrossRef]
- Munjal, S.; Singh, A. The Arrhenius Acid and Base Theory; IntechOpen Limited: London, UK, 2019. [Google Scholar] [CrossRef]
- Gaeini, M.; Rouws, A.L.; Salari, J.W.O.; Zondag, H.A.; Rindt, C.C.M. Characterization of microencapsulated and impregnated porous host materials based on calcium chloride for thermochemical energy storage. Appl. Energy 2018, 212, 1165–1177. [Google Scholar] [CrossRef]
- Karunadasa, K.S.P.; Manoratne, C.H.; Pitawala, H.M.T.G.A.; Rajapakse, R.M.G. Relative stability of hydrated/anhydrous products of calcium chloride during complete dehydration as examined by high-temperature X-ray powder diffraction. J. Phys. Chem. Solids 2018, 120, 167–172. [Google Scholar] [CrossRef]
- Fraissler, G.; Jöller, M.; Brunner, T.; Obernberger, I. Influence of dry and humid gaseous atmosphere on the thermal decomposition of calcium chloride and its impact on the remove of heavy metals by chlorination. Chem. Eng. Process. Process Intensif. 2009, 48, 380–388. [Google Scholar] [CrossRef]
- Newkirk, A.E.; Aliferis, I. Drying and Decomposition of Sodium Carbonate. Anal. Chem. 2002, 30, 982–984. [Google Scholar] [CrossRef]
- Gumieniczek, A.; Trębacz, H.; Komsta, Ł.; Atras, A.; Jopa, B.; Szumiło, M.; Popiołek, Ł. DSC, FT-IR, NIR, NIR-PCA and NIR-ANOVA for determination of chemical stability of diuretic drugs: Impact of excipients. Open Chem. 2018, 16, 116–132. [Google Scholar] [CrossRef] [Green Version]
- Harris, J.D.; Rusch, A.W. Identifying Hydrated Salts Using Simultaneous Thermogravimetric Analysis and Differential Scanning Calorimetry. J. Chem. Educ. 2012, 90, 235–238. [Google Scholar] [CrossRef]
- Wyrzykowski, D.; Hebanowska, E.; Nowak-Wiczk, G.; Makowski, M.; Chmurzyński, L. Thermal behaviour of citric acid and isomeric aconitic acids. J. Therm. Anal. Calorim. 2010, 104, 731–735. [Google Scholar] [CrossRef] [Green Version]


| Citric Acid (g) | MIRCAP CT 85-H (g) | Na2CO3 (g) | MC-ASC (g) | |
|---|---|---|---|---|
| HCl (% (v/v)) | ||||
| 1 | - | - | 0.459 ± 0.004 | 0.475 ± 0.043 |
| 2 | - | - | 0.891 ± 0.010 | 0.946 ± 0.073 |
| 10 | - | - | 4.321 ± 0.025 | 4.413 ± 0.204 |
| NaOH (% (v/v)) | ||||
| 1 | 0.419 ± 0.004 | 0.505 ± 0.004 | - | - |
| 2 | 0.788 ± 0.024 | 0.954 ± 0.054 | - | - |
| 10 | 3.922 ± 0.055 | 4.639 ± 0.136 | - | - |
| DSC | TGA | ||||||
|---|---|---|---|---|---|---|---|
| Temperature (°C) | Enthalpy (J.g−1) | DTG Peak (°C) | Tonset (°C) | Toffset (°C) | Weight Loss (%) | Residual Weight at 920 °C (%) | |
| CaCl2.2H2O | 46.7 | −26.8 | 49.1 | 49.0 | 102.5 | 10 | 19.5 |
| 180.9 | −855.6 | 140.1; 154.3 | 102.9 | 168.2 | 30 | ||
| 768.9 | 692.8 | 914.8 | 33 | ||||
| Sodium Alginate | 107.8 | −233.6 | 77.5 | 28.2 | 160.8 | 14 | 0 |
| 244.8 | 183.1 | 246.2 | 233.9 | 262.0 | 32 | ||
| 842.8 | 605.5 | 920.0 | 45 | ||||
| Na2CO3 | 77.1 | −12.4 | 76.4 | 23.9 | 860.0 | 10 | 85.9 |
| 356.0 | Tg | 888.2 | 861.1 | 920.0 | 4 | ||
| MC-ASC | 61.6 | Tg | 764.9 | 25.5 | 644.9 | 4 | 53.9 |
| 409.4 | 26.5 | 646.2 | 780.1 | 42 | |||
| Citric Acid | 157.2 | −200.6 | 201.3 | 163.8 | 292.2 | 88 | 0.3 |
| 214.5 | −449.5 | ||||||
| MIRCAP CT 85-H | 58.6 | −24.3 | 211.9 | 176.6 | 312.5 | 76 | 0 |
| 157.6 | −177.0 | ||||||
| 221.0 | −387.9 | 375.1 | 312.5 | 456.6 | 20 | ||
| 420.9 | −21.0 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, R.D.V.; Melro, L.; Padrão, J.; Ribeiro, A.I.; Mehravani, B.; Monteiro, F.; Pereira, E.; Martins, M.S.; Dourado, N.; Zille, A. Active Neutralizing Mats for Corrosive Chemical Storage. Gels 2022, 8, 489. https://doi.org/10.3390/gels8080489
Fernandes RDV, Melro L, Padrão J, Ribeiro AI, Mehravani B, Monteiro F, Pereira E, Martins MS, Dourado N, Zille A. Active Neutralizing Mats for Corrosive Chemical Storage. Gels. 2022; 8(8):489. https://doi.org/10.3390/gels8080489
Chicago/Turabian StyleFernandes, Rui D. V., Liliana Melro, Jorge Padrão, Ana Isabel Ribeiro, Behnaz Mehravani, Filipa Monteiro, Eduardo Pereira, Marcos S. Martins, Nuno Dourado, and Andrea Zille. 2022. "Active Neutralizing Mats for Corrosive Chemical Storage" Gels 8, no. 8: 489. https://doi.org/10.3390/gels8080489
APA StyleFernandes, R. D. V., Melro, L., Padrão, J., Ribeiro, A. I., Mehravani, B., Monteiro, F., Pereira, E., Martins, M. S., Dourado, N., & Zille, A. (2022). Active Neutralizing Mats for Corrosive Chemical Storage. Gels, 8(8), 489. https://doi.org/10.3390/gels8080489










