Biological Thermal Performance of Organic and Inorganic Aerogels as Patches for Photothermal Therapy
Abstract
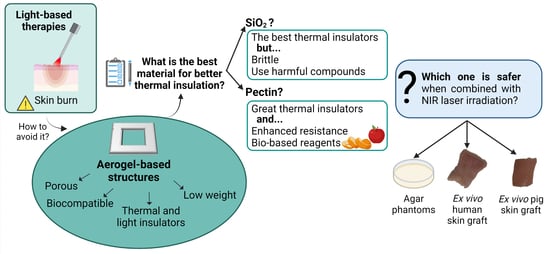
:1. Introduction
2. Results and Discussion
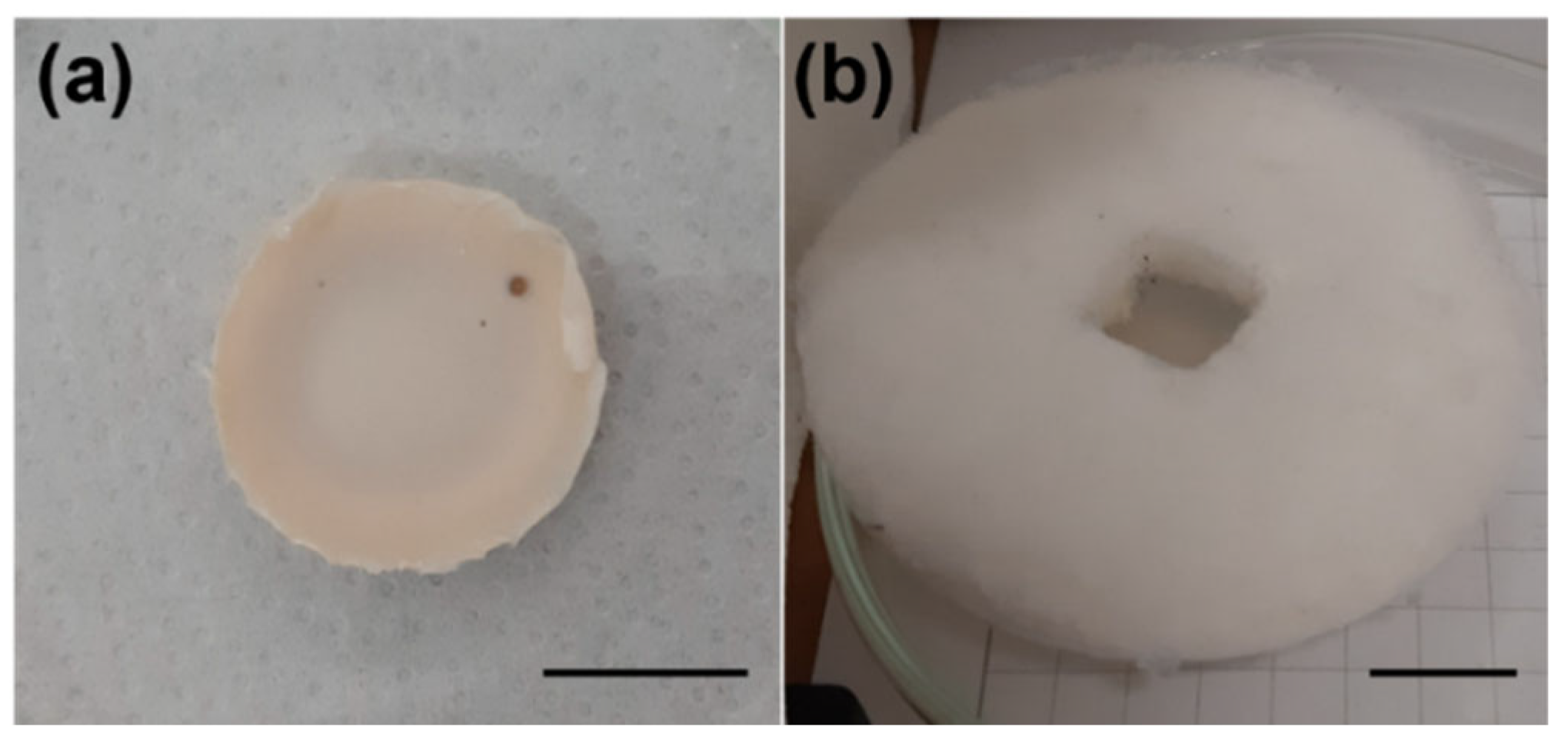
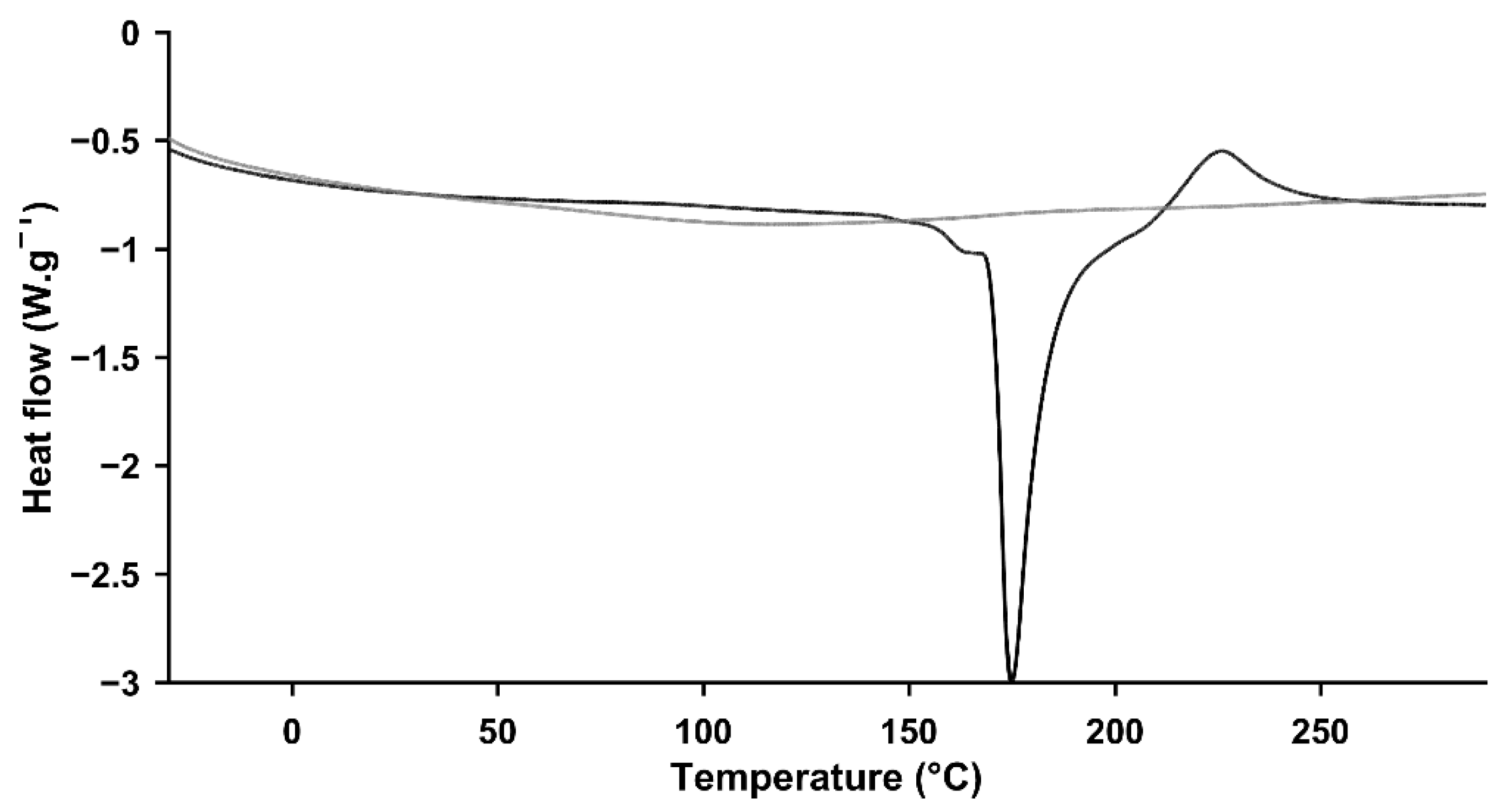
2.1. Physicochemical Characterization
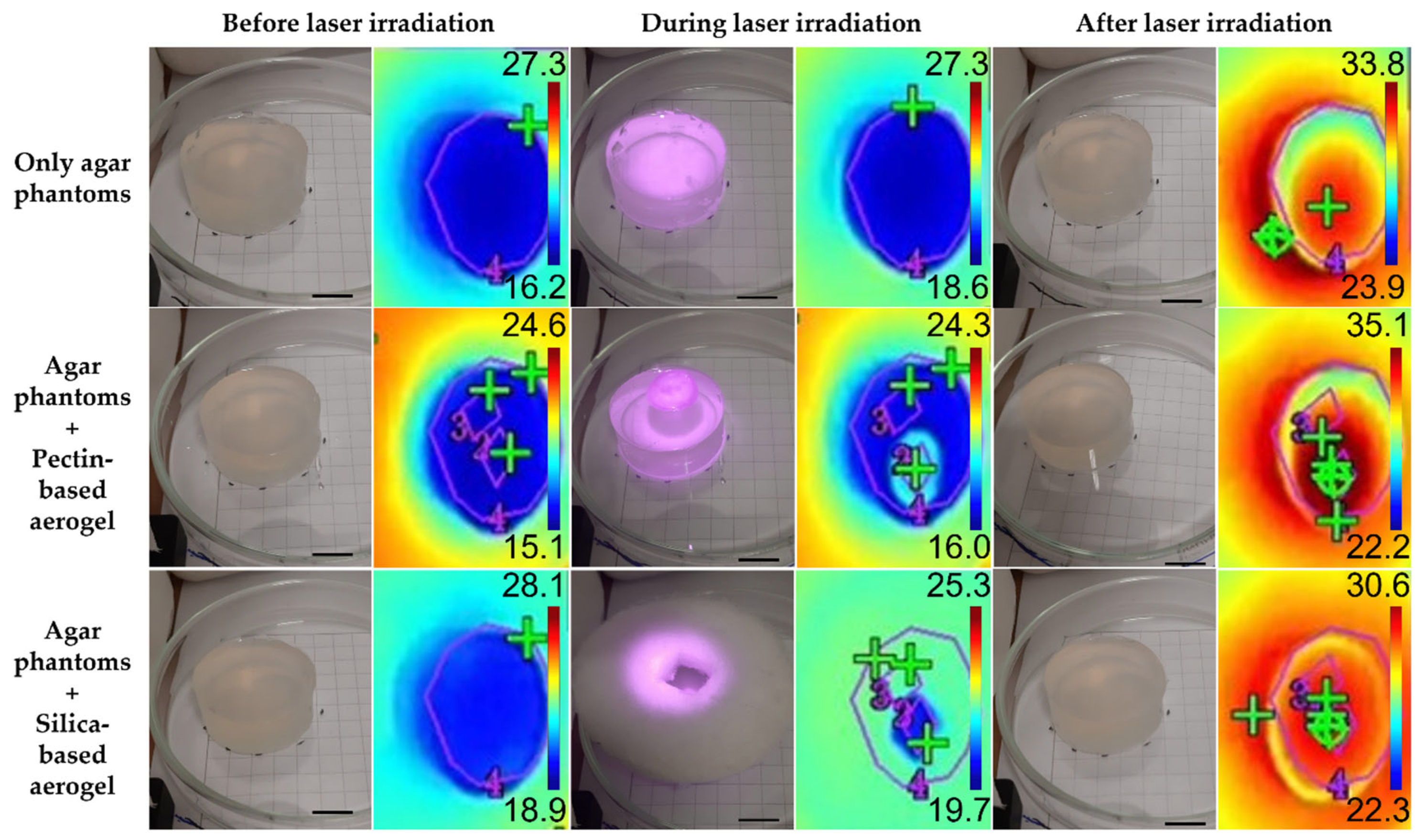
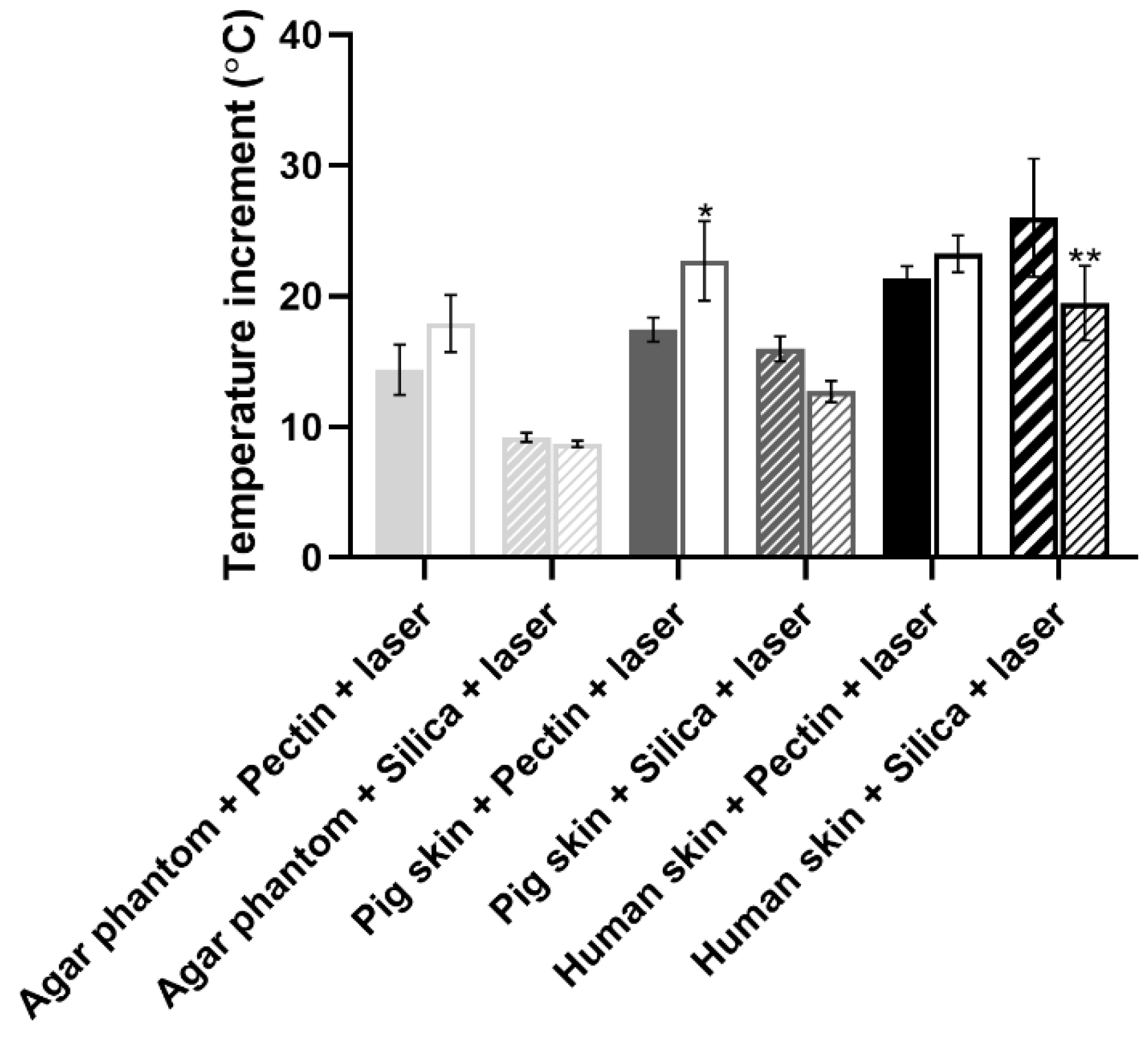
2.2. Biological Performance of Aerogels When Combined with NIR Irradiation
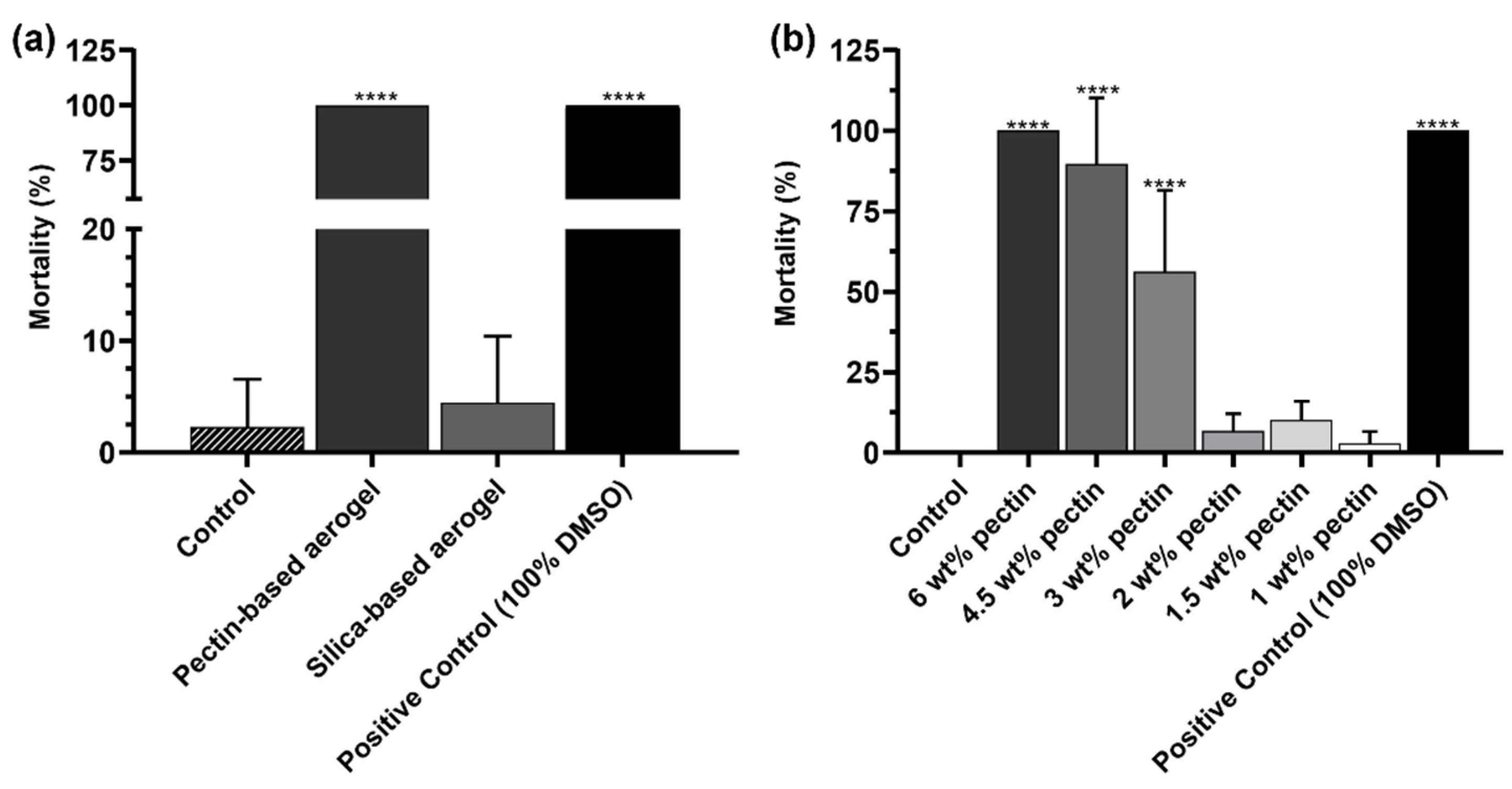
2.3. Preliminary In Vivo Safety Assays Using Artemia salina Model
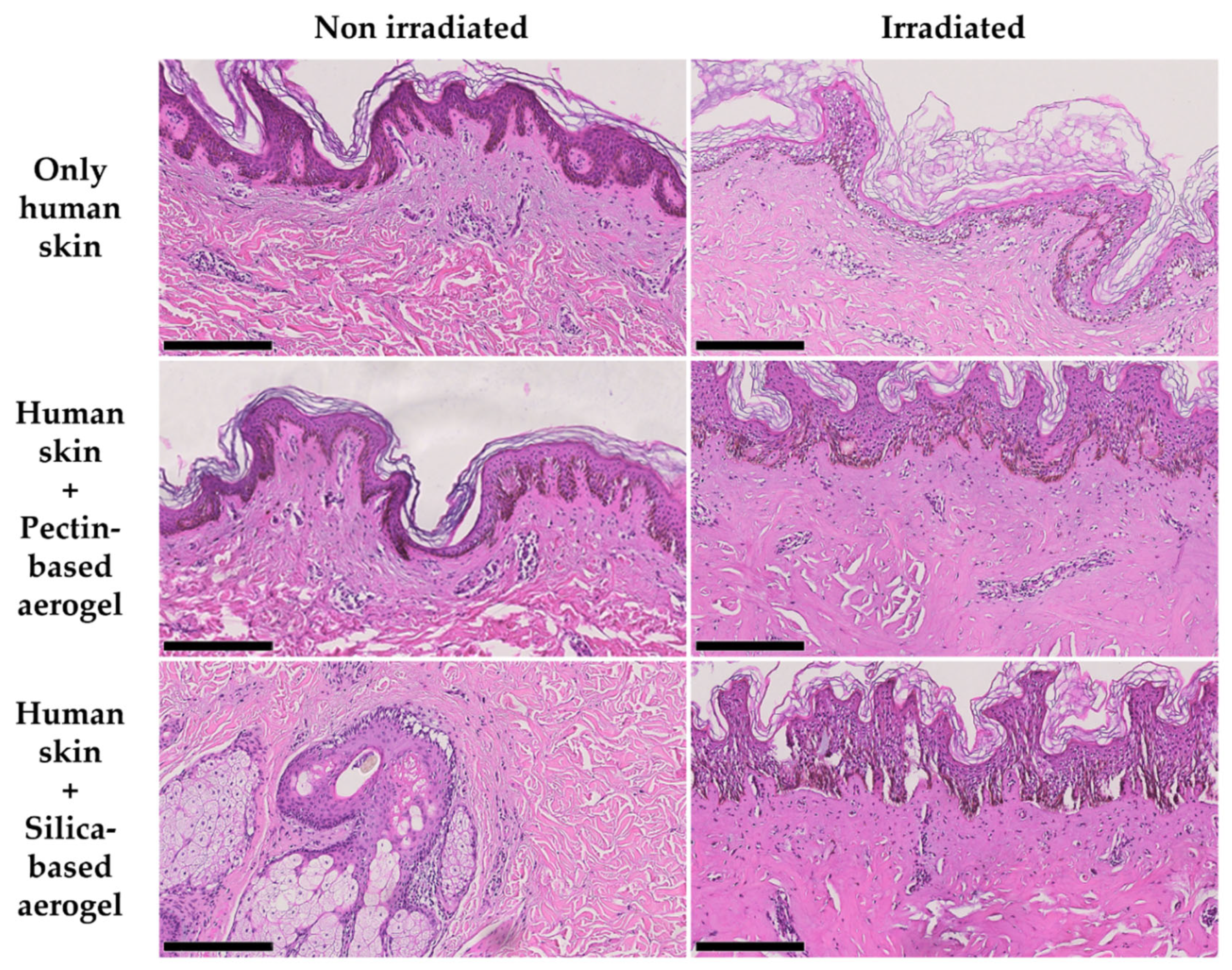
2.4. Human Skin Compatibility Tests
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Pectin-Based Structures
4.2.1. Pectin Hydrogel Preparation
4.2.2. Supercritical Drying
4.3. Preparation of Silica-Based Structures
4.4. Physicochemical Characterization of the Aerogels
4.5. Biological Performance of Aerogels When Combined with NIR Irradiation
4.6. Preliminary In Vivo Safety Assays Using Artemia salina Model
4.7. Human Skin Compatibility Tests
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, L.; Zhang, S.; Ying, Z.; Liu, J.; Zhou, Y.; Chen, F. Engineering of aerogel-based biomaterials for biomedical applications. Int. J. Nanomed. 2020, 15, 2363–2378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maleki, H.; Durães, L.; García-González, C.A.; del Gaudio, P.; Portugal, A.; Mahmoudi, M. Synthesis and biomedical applications of aerogels: Possibilities and challenges. Adv. Colloid Interface Sci. 2016, 236, 1–27. [Google Scholar] [CrossRef]
- García-González, C.A.; Budtova, T.; Durães, L.; Erkey, C.; Del Gaudio, P.; Gurikov, P.; Koebel, M.; Liebner, F.; Neagu, M.; Smirnova, I. An Opinion Paper on Aerogels for Biomedical and Environmental Applications. Molecules 2019, 24, 1815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smirnova, I.; Gurikov, P. Aerogels in chemical engineering: Strategies toward tailor-made aerogels. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 307–334. [Google Scholar] [CrossRef] [PubMed]
- Kistler, S.S. Coherent Expanded Aerogels and Jellies. Nature 1931, 127, 741. [Google Scholar] [CrossRef]
- Vareda, J.P.; Lamy-Mendes, A.; Durães, L. A reconsideration on the definition of the term aerogel based on current drying trends. Microporous Mesoporous Mater. 2018, 258, 211–216. [Google Scholar] [CrossRef]
- Alemán, J.V.; Chadwick, A.V.; He, J.; Hess, M.; Horie, K.; Jones, R.G.; Kratochvíl, P.; Meisel, I.; Mita, I.; Moad, G.; et al. Definitions of terms relating to the structure and processing of sols, gels, networks, and inorganic-organic hybrid materials (IUPAC Recommendations 2007). Pure Appl. Chem. 2007, 79, 1801–1829. [Google Scholar] [CrossRef]
- Nita, L.E.; Ghilan, A.; Rusu, A.G.; Neamtu, I.; Chiriac, A.P. New Trends in Bio-Based Aerogels. Pharmaceutics 2020, 12, 449. [Google Scholar] [CrossRef]
- Ferreira-Gonçalves, T.; Constantin, C.; Neagu, M.; Reis, C.P.; Sabri, F.; Simón-Vázquez, R. Safety and efficacy assessment of aerogels for biomedical applications. Biomed. Pharmacother. 2021, 144, 112356. [Google Scholar] [CrossRef]
- Horvat, G.; Fajfar, T.; Perva Uzunalić, A.; Knez, Ž.; Novak, Z. Thermal properties of polysaccharide aerogels. J. Therm. Anal. Calorim. 2017, 127, 363–370. [Google Scholar] [CrossRef]
- Tevlek, A.; Atya, A.M.N.; Almemar, M.; Duman, M.; Gokcen, D.; Ganin, A.Y.; Yiu, H.H.P.; Aydin, H.M. Synthesis of Conductive Carbon Aerogels Decorated with β-Tricalcium Phosphate Nanocrystallites. Sci. Rep. 2020, 10, 5758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linhares, T.; Carneiro, V.H.; Merillas, B.; Pessoa de Amorim, M.T.; Durães, L. Textile waste-reinforced cotton-silica aerogel composites for moisture regulation and thermal/acoustic barrier. J. Sol-Gel Sci. Technol. 2022, 102, 574–588. [Google Scholar] [CrossRef]
- Santos-Rosales, V.; Ardao, I.; Alvarez-Lorenzo, C.; Ribeiro, N.; Oliveira, A.L.; García-González, C.A. Sterile and Dual-Porous Aerogels Scaffolds Obtained through a Multistep Supercritical CO2-Based Approach. Molecules 2019, 24, 871. [Google Scholar] [CrossRef] [Green Version]
- Tkalec, G.; Knez, Ž.; Novak, Z. Formation of polysaccharide aerogels in ethanol. RSC Adv. 2015, 5, 77362–77371. [Google Scholar] [CrossRef]
- Zhao, S.; Siqueira, G.; Drdova, S.; Norris, D.; Ubert, C.; Bonnin, A.; Galmarini, S.; Ganobjak, M.; Pan, Z.; Brunner, S.; et al. Additive manufacturing of silica aerogels. Nature 2020, 584, 387–392. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; Portugal, A. An overview on silica aerogels synthesis and different mechanical reinforcing strategies. J. Non. Cryst. Solids 2014, 385, 55–74. [Google Scholar] [CrossRef] [Green Version]
- Cao, O.T.H.; Thibthong, P.P.; Thai, Q.B.; Tran, T.D.; Huynh, H.K.P.; Nguyen, S.T. Composite aerogel for heat insulation. Chem. Eng. Trans. 2020, 78, 361–366. [Google Scholar] [CrossRef]
- Ghica, M.E.; Almeida, C.M.R.; Fonseca, M.; Portugal, A.; Durães, L. Optimization of polyamide pulp-reinforced silica aerogel composites for thermal protection systems. Polymers 2020, 12, 1278. [Google Scholar] [CrossRef] [PubMed]
- Linhares, T.; Pessoa De Amorim, M.T.; Durães, L. Silica aerogel composites with embedded fibres: A review on their preparation, properties and applications. J. Mater. Chem. A 2019, 7, 22768–22802. [Google Scholar] [CrossRef]
- Groult, S.; Budtova, T. Thermal conductivity/structure correlations in thermal super-insulating pectin aerogels. Carbohydr. Polym. 2018, 196, 73–81. [Google Scholar] [CrossRef]
- García-González, C.A.; Carenza, E.; Zeng, M.; Smirnova, I.; Roig, A. Design of biocompatible magnetic pectin aerogel monoliths and microspheres. RSC Adv. 2012, 2, 9816–9823. [Google Scholar] [CrossRef]
- Iglesias-Mejuto, A.; García-González, C.A. 3D-printed alginate-hydroxyapatite aerogel scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2021, 131, 112525. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Mejuto, A.; García-González, C.A. 3D-Printed, Dual Crosslinked and Sterile Aerogel Scaffolds for Bone Tissue Engineering. Polymers 2022, 14, 1211. [Google Scholar] [CrossRef] [PubMed]
- Santos-Rosales, V.; Iglesias-Mejuto, A.; García-González, C.A. Solvent-free approaches for the processing of scaffolds in regenerative medicine. Polymers 2020, 12, 533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, S.; Zhang, Y.N.; Wan, J.; Cui, R.; Yu, X.; Zhao, G.; Lin, K. A novel multifunctional carbon aerogel-coated platform for osteosarcoma therapy and enhanced bone regeneration. J. Mater. Chem. B 2020, 8, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.; Jing, G.; Liang, X.; Qiu, M.; Li, Z.; Cao, R.; Li, X.; Fan, D.; Zhang, H. Graphene oxide/black phosphorus nanoflake aerogels with robust thermo-stability and significantly enhanced photothermal properties in air. Nanoscale 2017, 9, 8096–8101. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Huang, L.Z.; Wang, P.L.; Mai, T.; Ma, M.G. Cellulose nanofiber/molybdenum disulfide aerogels for ultrahigh photothermal effect. J. Colloid Interface Sci. 2022, 624, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Wang, H.; He, B.; Zeng, L.; Tan, T.; Cao, H.; He, X.; Zhang, Z.; Guo, S.; Li, Y. Current Approaches of Photothermal Therapy in Treating Cancer Metastasis with Nanotherapeutics. Theranostics 2016, 6, 762–772. [Google Scholar] [CrossRef]
- Liu, T.-M.; Conde, J.; Lipiński, T.; Bednarkiewicz, A.; Huang, C.-C. Smart NIR linear and nonlinear optical nanomaterials for cancer theranostics: Prospects in photomedicine. Prog. Mater. Sci. 2017, 88, 89–135. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008, 23, 217–228. [Google Scholar] [CrossRef]
- Bao, Z.; Liu, X.; Liu, Y.; Liu, H.; Zhao, K. Near-infrared light-responsive inorganic nanomaterials for photothermal therapy. Asian J. Pharm. Sci. 2016, 11, 349–364. [Google Scholar] [CrossRef] [Green Version]
- Silva, C.O.; Petersen, S.B.; Reis, C.P.; Rijo, P.; Molpeceres, J.; Fernandes, A.S.; Gonçalves, O.; Gomes, A.C.; Correia, I.; Vorum, H.; et al. EGF functionalized polymer-coated gold nanoparticles promote EGF photostability and EGFR internalization for photothermal therapy. PLoS ONE 2016, 11, e0165419. [Google Scholar] [CrossRef] [Green Version]
- Ferreira-Gonçalves, T.; Ferreira, D.; Ferreira, H.A.; Reis, C.P. Nanogold-based materials in medicine: From their origins to their future. Nanomedicine 2021, 16, 2695–2723. [Google Scholar] [CrossRef] [PubMed]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37–R61. [Google Scholar] [CrossRef]
- Deng, X.; Shao, Z.; Zhao, Y. Solutions to the Drawbacks of Photothermal and Photodynamic Cancer Therapy. Adv. Sci. 2021, 8, 2002504. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; Coelho, J.M.P.; Vieira, P. Optical design of a variable angle irradiation system for skin cancer laser phototherapy. EPJ Web Conf. 2020, 238, 12010. [Google Scholar] [CrossRef]
- Guerreiro, J.; Vieira, P.; Coelho, J.M.P. Evaluation of Three Iterative Algorithms for Phase Modulation Regarding Their Application in Concentrating Light Inside Biological Tissues for Laser Induced Photothermal Therapy. Photonics 2021, 8, 355. [Google Scholar] [CrossRef]
- Costa, E.; Ferreira-Gonçalves, T.; Cardoso, M.; Coelho, J.M.P.; Gaspar, M.M.; Faísca, P.; Ascensão, L.; Cabrita, A.S.; Reis, C.P.; Figueiredo, I.V. A step forward in breast cancer research: From a natural-like experimental model to a preliminary photothermal approach. Int. J. Mol. Sci. 2020, 21, 9681. [Google Scholar] [CrossRef]
- Amaral, M.; Charmier, A.J.; Afonso, R.A.; Catarino, J.; Faísca, P.; Carvalho, L.; Ascensão, L.; Coelho, J.M.P.; Manuela Gaspar, M.; Reis, C.P. Gold-based nanoplataform for the treatment of anaplastic thyroid carcinoma: A step forward. Cancers 2021, 13, 1242. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.; Ferreira-Gonçalves, T.; Figueiredo, I.V.; Rodrigues, C.M.P.; Ferreira, H.; Ferreira, D.; Viana, A.S.; Faísca, P.; Gaspar, M.M.; Coelho, J.M.P.; et al. Proof-of-concept study of multifunctional hybrid nanoparticle system combined with nir laser irradiation for the treatment of melanoma. Biomolecules 2021, 11, 511. [Google Scholar] [CrossRef] [PubMed]
- Horvat, G.; Pantic, M.; Knez, Ž.; Novak, Z. A Brief Evaluation of Pore Structure Determination for Bioaerogels. Gels 2022, 8, 438. [Google Scholar] [CrossRef] [PubMed]
- White, R.J.; Budarin, V.L.; Clark, J.H. Pectin-derived porous materials. Chem. Eur. J. 2010, 16, 1326–1335. [Google Scholar] [CrossRef]
- Veronovski, A.; Tkalec, G.; Knez, Z.; Novak, Z. Characterisation of biodegradable pectin aerogels and their potential use as drug carriers. Carbohydr. Polym. 2014, 113, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquérol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Karaki, N.; Aljawish, A.; Muniglia, L.; Humeau, C.; Jasniewski, J. Physicochemical characterization of pectin grafted with exogenous phenols. Food Hydrocoll. 2016, 60, 486–493. [Google Scholar] [CrossRef]
- Mustari, A.; Nishidate, I.; Wares, M.A.; Maeda, T.; Kawauchi, S.; Sato, S.; Sato, M.; Aizu, Y. Agarose-based tissue mimicking optical phantoms for diffuse reflectance spectroscopy. J. Vis. Exp. 2018, 2018, 57578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.A.; Zachary, J.F. Chapter 1—Mechanisms and Morphology of Cellular Injury, Adaptation, and Death11For a glossary of abbreviations and terms used in this chapter see E-Glossary 1-1. In Pathologic Basis of Veterinary Disease, 6th ed.; Miller, M.A., Zachary, J.F., Eds.; Mosby: Maryland Heights, MO, USA, 2017; pp. 2–43.e19. ISBN 978-0-323-35775-3. [Google Scholar]
- Amaral, S.D.C.; Barbieri, S.F.; Ruthes, A.C.; Bark, J.M.; Winnischofer, S.M.B.; Silveira, J.L.M. Cytotoxic effect of crude and purified pectins from Campomanesia xanthocarpa Berg on human glioblastoma cells. Carbohydr. Polym. 2019, 224, 115140. [Google Scholar] [CrossRef]
- Hawach, V.; Boujaoude, M.A.; Abdel-Massih, R.M. The Cytotoxic and Anti-proliferative Activity of High Molecular Weight Pectin and Modified Citrus Pectin. Funct. Foods Health Dis. 2016, 6, 587–601. [Google Scholar] [CrossRef]
- Remuinan-Pose, P.; López-Iglesias, C.; Iglesias-Mejuto, A.; Mano, J.F.; García-González, C.A.; Rial-Hermida, M.I. Preparation of Vancomycin-Loaded Aerogels Implementing Inkjet Printing and Superhydrophobic Surfaces. Gels 2022, 8, 417. [Google Scholar] [CrossRef] [PubMed]
- Merillas, B.; Martín-De León, J.; Villafañe, F.; Rodríguez-Pérez, M.Á. Optical Properties of Polyisocyanurate–Polyurethane Aerogels: Study of the Scattering Mechanisms. Nanomaterials 2022, 12, 1522. [Google Scholar] [CrossRef]
- Bostain, D.A.; Brenizer, J.S., Jr.; Norris, P.M. Neutron Radioscopic Measurement of Water Adsorption Coefficients in Aerogels. Res. Nondestruct. Eval. 2002, 14, 47–57. [Google Scholar] [CrossRef]
- Hamidi, M.R.; Jovanova, B.; Panovska, T.K. Toxicological evaluation of the plant products using Brine Shrimp (Artemia salina L.) model. Maced. Pharm. Bull. 2014, 60, 9–18. [Google Scholar] [CrossRef]
- Ferreira-Gonçalves, T.; Gaspar, M.M.; Coelho, J.M.P.; Marques, V.; Viana, A.S.; Ascensão, L.; Carvalho, L.; Rodrigues, C.M.P.; Ferreira, H.A.; Ferreira, D.; et al. The Role of Rosmarinic Acid on the Bioproduction of Gold Nanoparticles as Part of a Photothermal Approach for Breast Cancer Treatment. Biomolecules 2022, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Marzulli, F.N.; Maibach, H.I. Contact allergy: Predictive testing in man. Contact Dermat. 1976, 2, 1–17. [Google Scholar] [CrossRef] [PubMed]




| Aerogel | Volume Shrinkage % | ρbulk (g cm−3) | ABET (m2 g−1) | Vp,BJH (cm3 g−1) | dp,BJH (nm) |
|---|---|---|---|---|---|
| Pectin-based | 83.5 ± 5.5 | 0.334 ± 0.092 | 330 ± 17 | 1.3 ± 0.1 | 10.3 ± 0.5 |
| Silica-based | 21.8 ± 2.8 **** | 0.159 ± 0.009 | 726 ± 36 **** | 2.4 ± 0.1 | 9.1 ± 0.5 |
| Test Group | Non-Irradiated | Irradiated |
|---|---|---|
| Only human skin | NO | ++ |
| Human skin + Pectin-based aerogel | + | +++ |
| Human skin + Silica-based aerogel | + | +++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira-Gonçalves, T.; Iglesias-Mejuto, A.; Linhares, T.; Coelho, J.M.P.; Vieira, P.; Faísca, P.; Catarino, J.; Pinto, P.; Ferreira, D.; Ferreira, H.A.; et al. Biological Thermal Performance of Organic and Inorganic Aerogels as Patches for Photothermal Therapy. Gels 2022, 8, 485. https://doi.org/10.3390/gels8080485
Ferreira-Gonçalves T, Iglesias-Mejuto A, Linhares T, Coelho JMP, Vieira P, Faísca P, Catarino J, Pinto P, Ferreira D, Ferreira HA, et al. Biological Thermal Performance of Organic and Inorganic Aerogels as Patches for Photothermal Therapy. Gels. 2022; 8(8):485. https://doi.org/10.3390/gels8080485
Chicago/Turabian StyleFerreira-Gonçalves, Tânia, Ana Iglesias-Mejuto, Teresa Linhares, João M. P. Coelho, Pedro Vieira, Pedro Faísca, José Catarino, Pedro Pinto, David Ferreira, Hugo A. Ferreira, and et al. 2022. "Biological Thermal Performance of Organic and Inorganic Aerogels as Patches for Photothermal Therapy" Gels 8, no. 8: 485. https://doi.org/10.3390/gels8080485
APA StyleFerreira-Gonçalves, T., Iglesias-Mejuto, A., Linhares, T., Coelho, J. M. P., Vieira, P., Faísca, P., Catarino, J., Pinto, P., Ferreira, D., Ferreira, H. A., Gaspar, M. M., Durães, L., García-González, C. A., & Reis, C. P. (2022). Biological Thermal Performance of Organic and Inorganic Aerogels as Patches for Photothermal Therapy. Gels, 8(8), 485. https://doi.org/10.3390/gels8080485