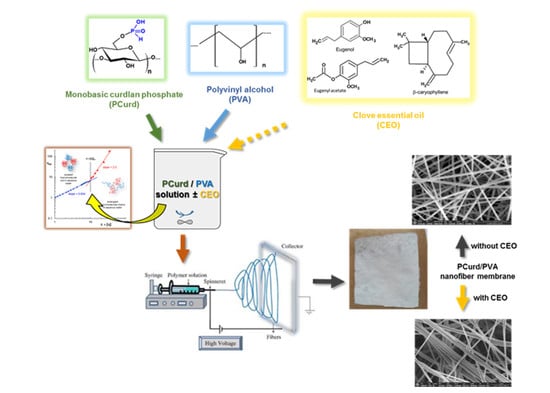
Phosphorylated Curdlan Gel/Polyvinyl Alcohol Electrospun Nanofibres Loaded with Clove Oil with Antibacterial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Polymer Solution Preparation and Characterisation
2.2.2. Behaviour of PVA, PCurd and Their Mixture in Aqueous Solution
2.2.3. Electrospinning Process of PCurd/PVA Solutions
2.2.4. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR–FTIR)
2.2.5. Scanning Electron Microscopy (SEM)
2.2.6. Mechanical Properties of the Electrospun Membranes
2.2.7. Determination of Total CEO Content from the CEO-Loaded Electrospun Membrane
2.2.8. In Vitro CEO Release from PCurd/PVA Electrospun Membrane
2.2.9. Phytochemical Proprieties of the CEO and CEO-Loaded Electrospun Membranes
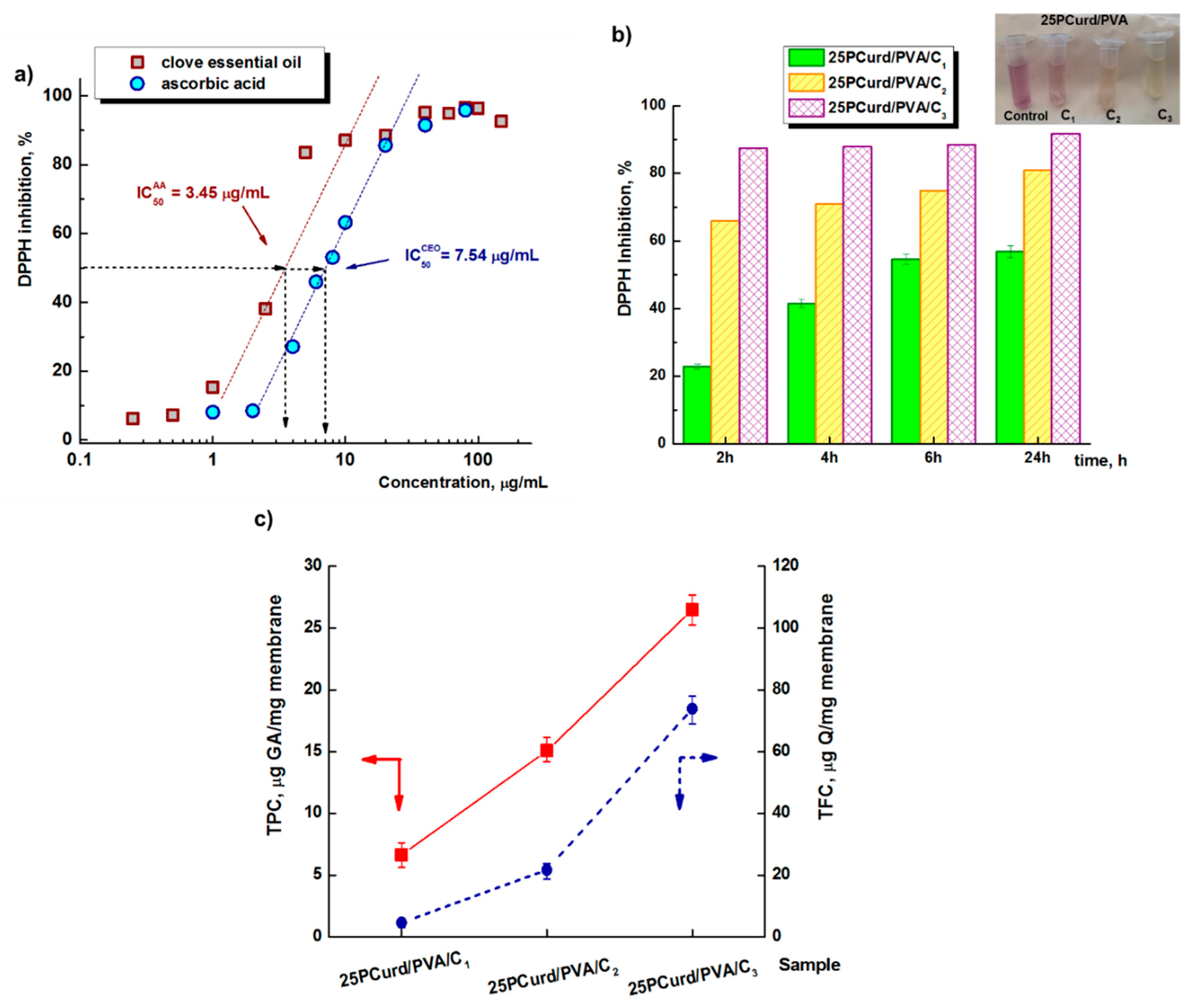
DPPH Free-Radical Scavenging Activity
Determination of Total Phenolic Content (TPC)
Total Flavonoid Content (TFC)
2.2.10. Antibacterial Assays
2.2.11. Cell Viability Assay of Electrospun Nanofibres Membrane
2.2.12. Statistical Analysis
3. Results and Discussions
3.1. Behaviour of PVA, PCurd and Their Mixture in Aqueous Solution

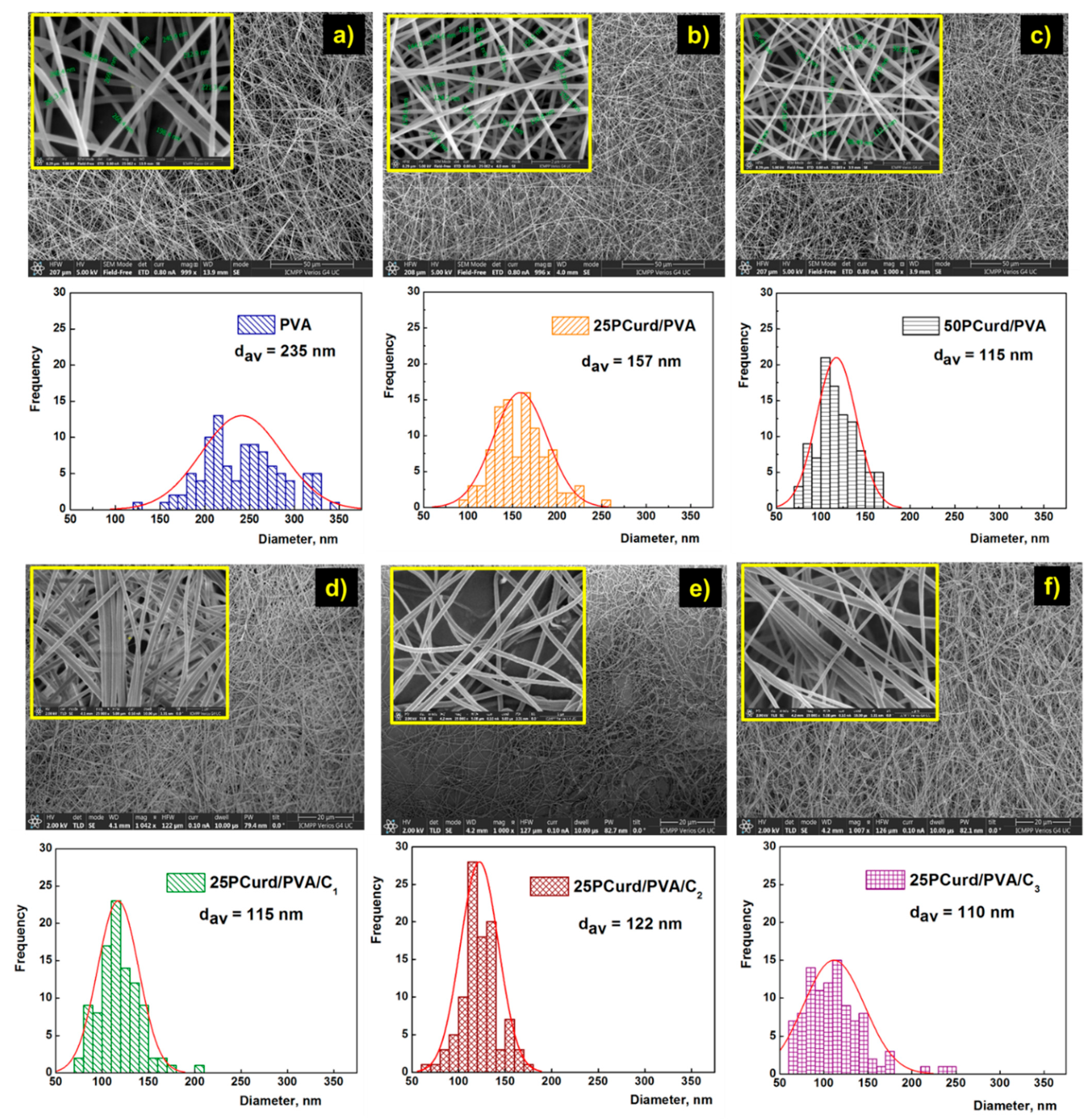
3.2. Membrane Characterisation
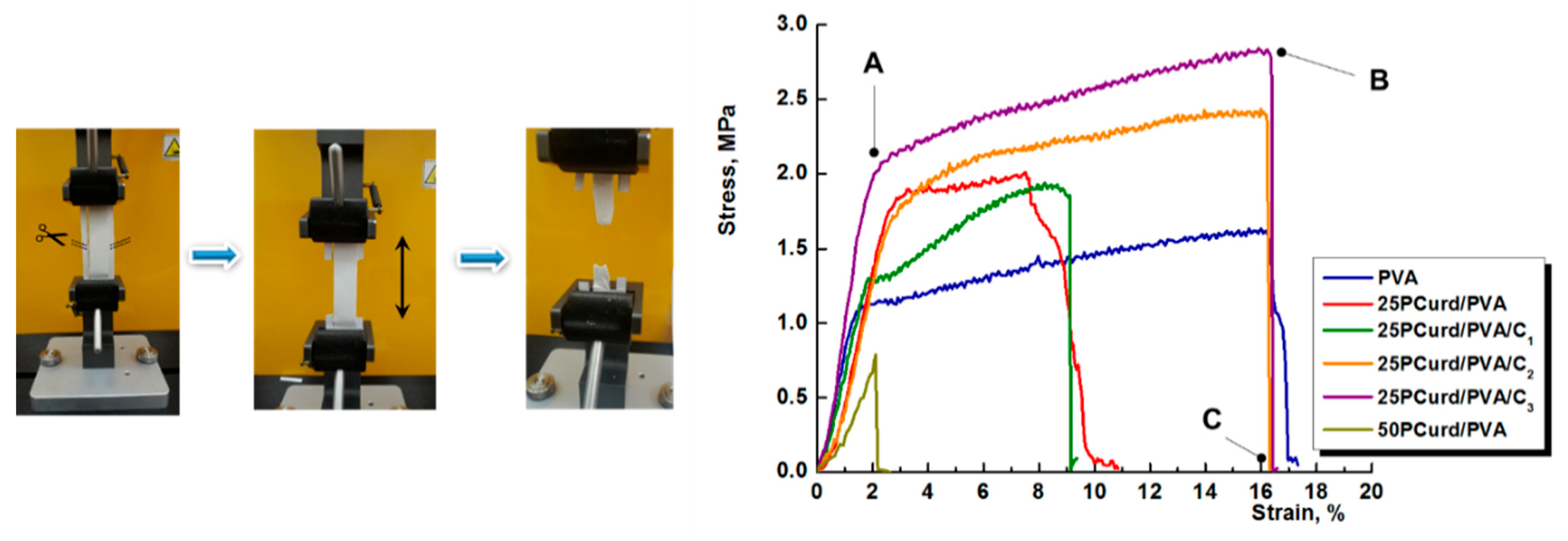
3.3. Mechanical Properties
3.4. In Vitro CEO Release
3.5. Phytochemical Proprieties of the CEO-Loaded Electrospun Membranes
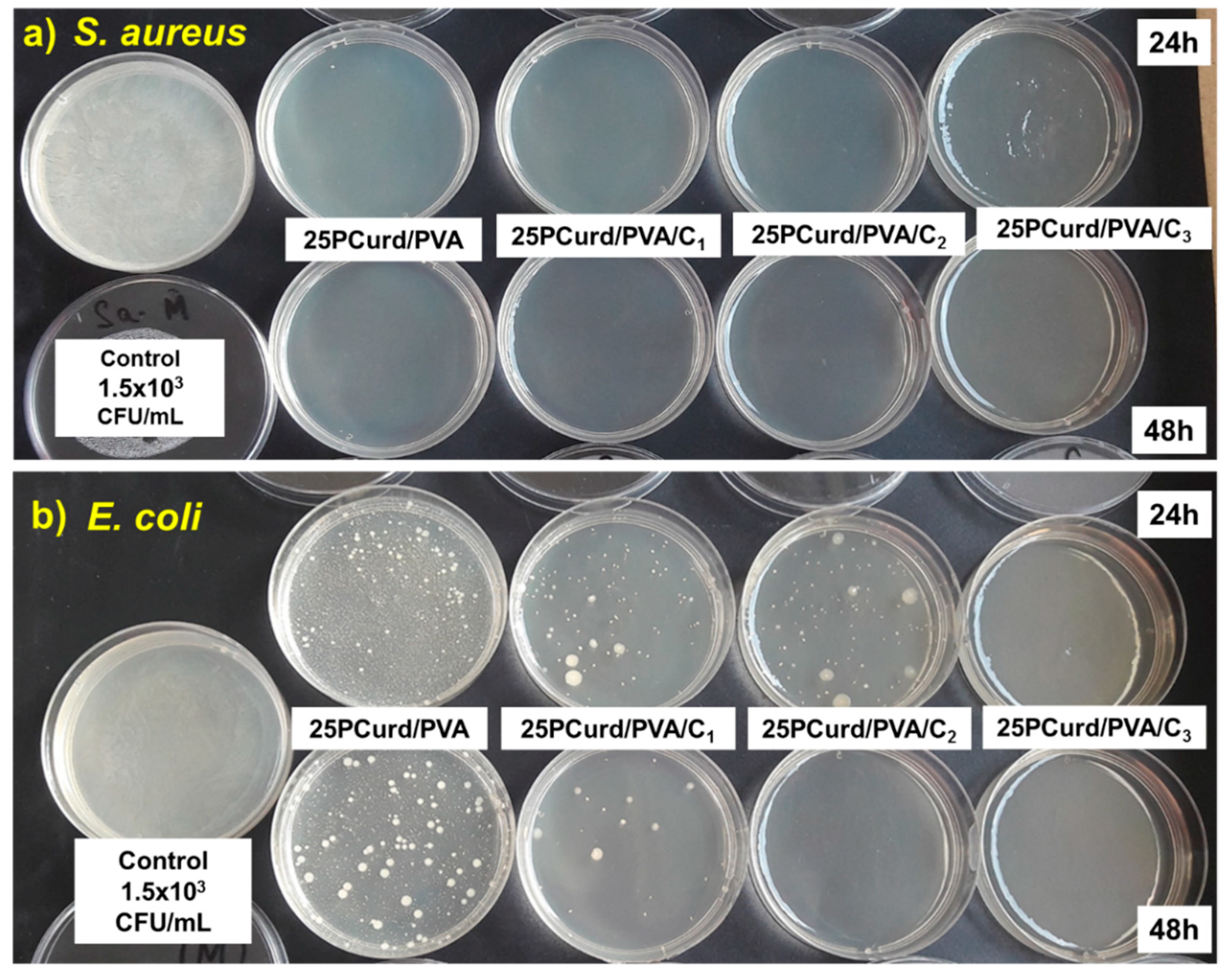
3.6. Antimicrobial Test
3.7. Time Kill Assay Test
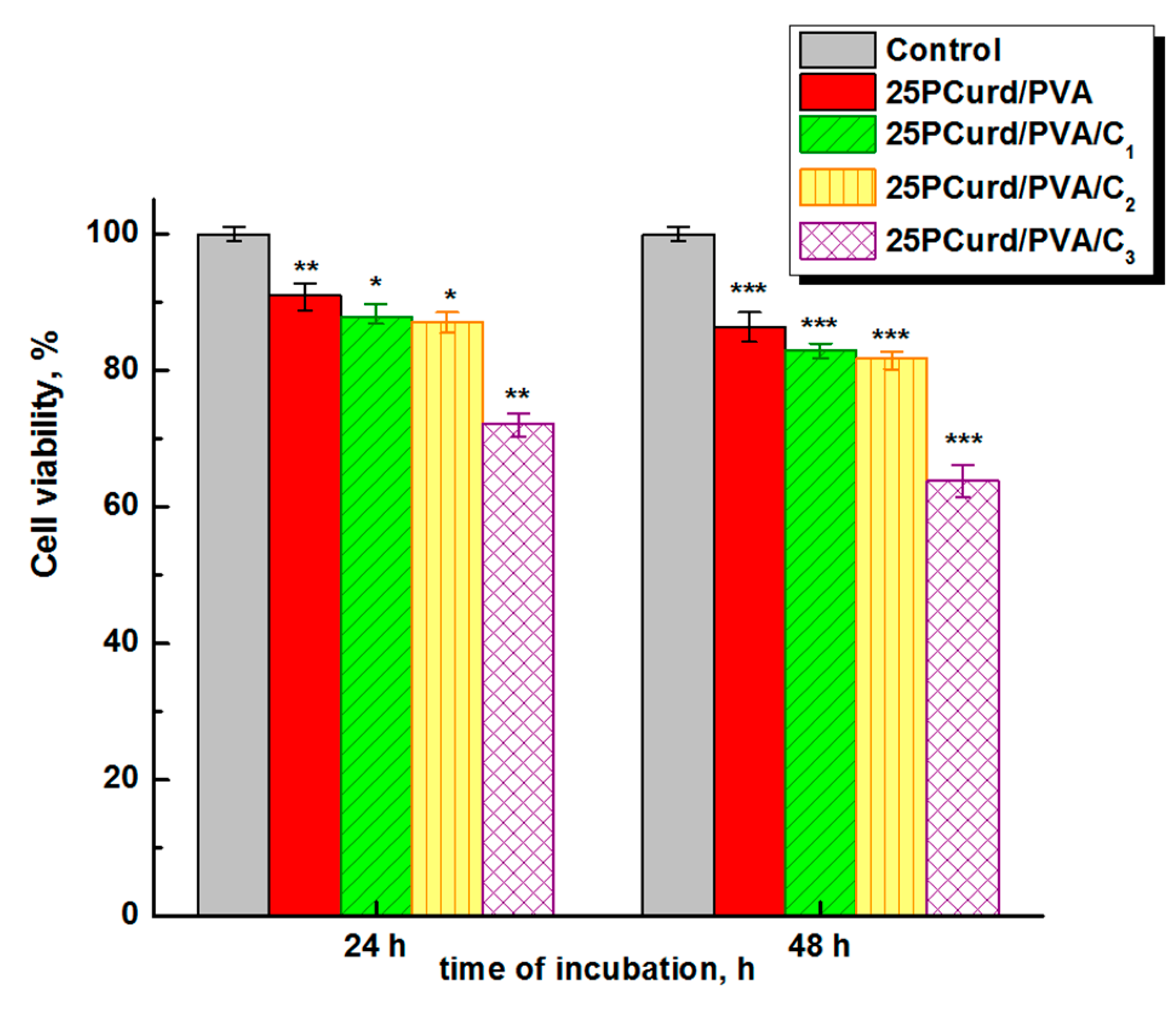
3.8. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zahedi, P.; Rezaeian, I.; Ranaei-Siadat, S.O.; Jafari, S.H.; Supaphol, P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Adv. Technol. 2010, 2, 77–95. [Google Scholar] [CrossRef]
- Edwards, R.; Harding, K.G. Bacteria and wound healing. Curr. Opin. Infect. Dis. 2004, 17, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Yougbare, S.; Chang, T.-K.; Tan, S.-H.; Kuo, J.-C.; Hsu, P.-H.; Su, C.-Y.; Kuo, T.-R. Antimicrobial gold nanoclusters: Recent developments and future perspectives. Int. J. Mol. Sci. 2019, 20, 2924. [Google Scholar] [CrossRef] [Green Version]
- Cacciotti, I.; Ciocci, M.; Di Giovanni, E.; Nanni, F.; Melino, S. Hydrogen sulfide-releasing fibrous membranes: Potential patches for stimulating human stem cells proliferation and viability under oxidative stress. Int. J. Mol. Sci. 2018, 19, 2368. [Google Scholar] [CrossRef] [Green Version]
- Abrigo, M.; McArthur, S.L.; Kingshott, P. Electrospun nanofibers as dressings for chronic wound care: Advances, challenges, and future prospects. Macromol. Biosci. 2014, 14, 772–792. [Google Scholar] [CrossRef]
- Fatahian, R.; Mirjalili, M.; Khajavi, R.; Rahimi, M.K.; Nasirizadeh, N. Fabrication of antibacterial and hemostatic electrospun PVA nanofbers for wound healing. SN Appl. Sci. 2020, 2, 1288. [Google Scholar] [CrossRef]
- Hashmi, M.; Ullah, S.; Kim, I.S. Electrospun Momordica charantia incorporated polyvinyl alcohol (PVA) nanofibers for antibacterial applications. Mater. Today Commun. 2020, 24, 101161. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Loutfy, S.A.; Hussein, Y.; Kenawy, E.R.S. Recent advances in PVA-polysaccharide based hydrogels and electrospun nanofibers in biomedical applications: A review. Int. J. Biol. Macromol. 2021, 187, 755–768. [Google Scholar] [CrossRef]
- Ji, X.; Guo, J.; Guan, F.; Liu, Y.; Yang, Q.; Zhang, X.; Xu, Y. Preparation of electrospun polyvinyl alcohol/nanocellulose composite film and evaluation of its biomedical performance. Gels 2021, 7, 223. [Google Scholar] [CrossRef]
- Al-Moalemi, H.A.; Razak, S.I.A.; Bohari, S.P.M. Electrospun sodium alginate/poly(ethylene oxide) nanofibers for wound healing applications: Challenges and future directions. Cellul. Chem. Technol. 2022, 56, 251–270. [Google Scholar] [CrossRef]
- Chen, J.; Seviour, R. Medicinal importance of fungal β-(1→3), (1→6)-glucans. Mycol. Res. 2007, 111, 635–6520. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, M.S.; Bellini, M.F.; Angeli, J.P.F.; Oliveira, R.J.; Silva, A.F.; Ribeiro, L.R. beta-Glucans in promoting health: Prevention against mutation and cancer. Mutat. Res. 2008, 658, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vetvickova, J. β-1,3-Glucan in cancer treatment. Am. J. Immunol. 2012, 8, 38–43. [Google Scholar]
- Bao, M.; Ehexige, E.; Xu, J.; Ganbold, T.; Han, S.; Baigude, H. Oxidized curdlan activates dendritic cells and enhances antitumor immunity. Carbohydr. Polym. 2021, 264, 117988. [Google Scholar] [CrossRef]
- van den Berg, L.M.; Zijlstra-Willems, E.M.; Richters, C.D.; Ulrich, M.M.W.; Geijtenbeek, T.B.H. Dectin-1 activation induces proliferation and migration of human keratinocytes enhancing wound re-epithelialization. Cell. Immunol. 2014, 289, 49–54. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, H. The effect of carboxymethylation on the macromolecular conformation of the (1→3)-β-D-glucan of curdlan in water. Carbohydr. Polym. 2021, 272, 118456. [Google Scholar] [CrossRef]
- Suflet, D.M.; Nicolescu, A.; Popescu, I.; Chitanu, G.C. Phosphorylated polysaccharides. 3. Synthesis of phosphorylated curdlan and its polyelectrolyte behavior compared with other phosphorylated polysaccharides. Carbohydr. Polym. 2011, 84, 1176–1181. [Google Scholar] [CrossRef]
- Osawa, Z.; Morota, T.; Hatanaka, K.; Akaike, T.; Matsuzaki, K.; Nakashima, H.; Yamamoto, N.; Suzuki, E.; Miyano, H.; Mimura, T.; et al. Synthesis of sulfated derivatives of curdlan and their anti-HIV activity. Carbohydr. Polym. 1993, 21, 283–288. [Google Scholar] [CrossRef]
- Popescu, I.; Pelin, I.M.; Ailiesei, G.L.; Ichim, D.L.; Suflet, D.M. Amphiphilic polysaccharide based on curdlan: Synthesis and behavior in aqueous solution. Carbohydr. Polym. 2019, 224, 115157. [Google Scholar] [CrossRef]
- Basha, R.Y.; Kumar, T.S.S.; Doble, M. Electrospun nanofibers of curdlan (β-1,3 Glucan) blend as a potential skin scaffold material. Macromol. Mater. Eng. 2017, 302, 1600417. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Wu, J.; Shi, Z.; Zhao, P.; Su, H.; Wang, Q.; Jin, L. Nanofiber orodispersible films based on carboxymethyl curdlan and PEO: New delivery system for amlodipine besylate. Colloids Surf. A Physicochem. Eng. 2022, 635, 128096. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Abdelgawad, A.M.; Salas, C.; Rojas, O.J. Curdlan in fibers as carriers of tetracycline hydrochloride: Controlled release and antibacterial activity. Carbohydr. Polym. 2016, 154, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Toullec, C.; Le Bideau, J.; Geoffroy, V.; Halgand, B.; Buchtova, N.; Molina-Peña, R.; Garcion, E.; Avril, S.; Sindji, L.; Dube, A.; et al. Curdlan–chitosan electrospun fibers as potential scaffolds for bone regeneration. Polymers 2021, 13, 526. [Google Scholar] [CrossRef] [PubMed]
- Popescu, I.; Pelin, I.M.; Butnaru, M.; Fundueanu, G.; Suflet, D.M. Phosphorylated curdlan microgels. Preparation, characterization, and in vitro drug release studies. Carbohydr. Polym. 2013, 94, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Suflet, D.M.; Popescu, I.; Prisacaru, A.I.; Pelin, I.M. Synthesis and characterization of curdlan—phosphorylated curdlan based hydrogels for drug release. Int. J. Polym. Mater. Polym. Biomater. 2020, 70, 870–879. [Google Scholar] [CrossRef]
- Adamu, B.F.; Gao, J.; Jhatial, A.K.; Kumelachew, D.M. A review of medicinal plant-based bioactive electrospun nano fibrous wound dressings. Mater. Des. 2021, 209, 109942. [Google Scholar] [CrossRef]
- Han, X.; Parker, T.L. Anti-inflammatory activity of clove (Eugenia caryophyllata) essential oil in human dermal fibroblasts. Pharm. Biol. 2017, 55, 1619–1622. [Google Scholar] [CrossRef] [Green Version]
- Chaieb, K.; Hajlaoui, H.; Zmantar, T.; Kahla-Nakbi, A.B.; Rouabhia, M.; Mahdouani, K.; Bakhrouf, A. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): A short review. Phytother. Res. 2007, 21, 501–506. [Google Scholar] [CrossRef]
- Solaberrieta, I.; Jiménez, A.; Cacciotti, I.; Garrigós, M.C. Encapsulation of bioactive compounds from Aloe Vera Agrowastes in electrospun poly (ethylene oxide) nanofibers. Polymers 2020, 12, 1323. [Google Scholar] [CrossRef]
- Qin, M.; Mou, X.-J.; Dong, W.-H.; Liu, J.-X.; Liu, H.; Dai, Z.; Huang, X.-W.; Wang, N.; Yan, X. In Situ electrospinning wound healing films composed of zein and clove essential oil. Macromol. Mater. Eng. 2020, 305, 1900790. [Google Scholar] [CrossRef]
- Berechet, M.D.; Gaidau, C.; Miletic, A.; Pilic, B.; Simion, D.; Rapa, M.; Stanca, M.; Constantinescu, R. Nanofibers based on concentrated collagen hydrolysate loaded with essential oils. J. Nanotechnol. Nanomater. 2021, 2, 25–40. [Google Scholar]
- Tarus, B.K.; Fadel, N.; Al-Oufy, A.; El-Messiry, M. Investigation of mechanical properties of electrospun poly (vinyl chloride) polymer nanoengineered composite. J. Eng. Fibers Fabr. 2020, 15, 1–10. [Google Scholar] [CrossRef]
- Simon, A.; Amaro, M.I.; Healy, A.M.; Cabral, L.M.; de Sousa, V.P. Comparative evaluation of rivastigmine permeation from a transdermal system in the Franz cell using synthetic membranes and pig ear skin with in vivo-in vitro correlation. Int. J. Pharm. 2016, 512, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I.; Elmastas, M.; Aboul-Enein, H.Y. Antioxidant activity of clove oil—A powerful antioxidant source. Arab. J. Chem. 2012, 5, 489–499. [Google Scholar] [CrossRef] [Green Version]
- Adaramola, B.; Onigbinde, A. Effect of extraction solvent on the phenolic content, flavonoid content and antioxidant capacity of clove bud. IOSR J. Pharm. Biol. Sci. 2016, 11, 33–38. [Google Scholar]
- Serbezeanu, D.; Bargan, A.; Homocianu, M.; Aflori, M.; Rîmbu, C.M.; Enache, A.A.; Vlad-Bubulac, T. Electrospun polyvinyl alcohol loaded with phytotherapeutic agents for wound healing applications. Nanomaterials 2021, 11, 3336. [Google Scholar] [CrossRef]
- ASTM E2315-16; Standard Guide for Assessment of Antimicrobial Activity Using a Time-Kill Procedure. ASTM International: West Conshohocken, PA, USA, 2016.
- Vetcher, A.; Gearheart, R.; Morozov, V. Correlation of morphology of electrospun fibers with rheology of linear polyacrylamide solution. Polym. J. 2007, 39, 878–881. [Google Scholar] [CrossRef] [Green Version]
- Wolf, B.A. Polyelectrolytes revisited: Reliable determination of intrinsic viscosities. Macromol. Rapid Commun. 2007, 28, 164–170. [Google Scholar] [CrossRef]
- Wolf, B.A. Viscosity of polymer solutions over the full range of composition: A thermodynamically inspired two-parameter approach. Ind. Eng. Chem. Res. 2015, 54, 4672–4680. [Google Scholar] [CrossRef]
- Wolf, B.A. Coil overlap in moderately concentrated polyelectrolyte solutions: Effects of self-shielding as compared with salt-shielding as a function of chain length. RSC Adv. 2016, 6, 38004–38011. [Google Scholar] [CrossRef]
- Ghimici, L.; Suflet, M.D. Phosphorylated polysaccharide derivatives as efficient separation agents for zinc and ferric oxides particles from water. Sep. Purif. Technol. 2015, 144, 31–36. [Google Scholar] [CrossRef]
- Bercea, M.; Wolf, B.A. Intrinsic viscosities of polymer blends: Sensitive probes of specific interactions between the counterions of polyelectrolytes and uncharged macromolecules. Macromolecules 2018, 51, 7483–7490. [Google Scholar] [CrossRef]
- Bercea, M.; Wolf, B.A. Detection of polymer compatibility by means of self-organization: Poly(ethylene oxide) and poly(sodium 4-styrenesulfonate). Soft Matter 2021, 17, 5214–5220. [Google Scholar] [CrossRef]
- Xiong, X.; Huang, X.; Wolf, B.A. A versatile viscometric method for the study of dissolved proteins, exemplified for casein micelles in ammoniacal solutions. Food Hydrocoll. 2017, 72, 195–201. [Google Scholar] [CrossRef]
- Bercea, M.; Morariu, S.; Rusu, D. In-situ gelation of aqueous solutions of entangled poly(vinyl alcohol). Soft Matter 2013, 9, 1244–1253. [Google Scholar] [CrossRef]
- McKee, M.G.; Wilkes, G.L.; Colby, R.H.; Long, T.E. Correlations of solution rheology with electrospun fiber formation of linear and branched polyesters. Macromolecules 2004, 37, 1760–1767. [Google Scholar] [CrossRef]
- Tang, C.; Saquing, C.D.; Harding, J.R.; Khan, S.A. In situ cross-linking of electrospun poly(vinyl alcohol) nanofibers. Macromolecules 2010, 45, 630–637. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Hashimoto, T. Impact of entanglement density on solution electrospinning: A phenomenological model for fiber diameter. Macromolecules 2016, 49, 7985–7996. [Google Scholar] [CrossRef]
- Suflet, D.M.; Pelin, I.M.; Dinu, V.M.; Lupu, M.; Popescu, I. Hydrogels based on monobasic curdlan phosphate for biomedical applications. Cellul. Chem. Technol. 2019, 53, 897–906. [Google Scholar] [CrossRef]
- Tarhan, I. A robust method for simultaneous quantification of eugenol, eugenyl acetate, and β-caryophyllene in clove essential oil by vibrational spectroscopy. Phytochemistry 2021, 191, 112928. [Google Scholar] [CrossRef]
- Behbahani, B.A.; Noshad, M.; Falah, F. Study of chemical structure, antimicrobial, cytotoxic and mechanism of action of Syzygium aromaticum essential oil on foodborne pathogens. Potravin. Slovak J. Food Sci. 2019, 13, 875–883. [Google Scholar] [CrossRef] [Green Version]
- Stoleru, E.; Dumitriu, R.P.; Ailiesei, G.-L.; Yilmaz, C.; Brebu, M. Synthesis of bioactive materials by in situ one-step direct loading of Syzygium aromaticum essential oil into chitosan-based hydrogels. Gels 2022, 8, 225. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.-H.; Inai, R.; Kotaki, M.; Ramakrishna, S. Systematic parameter study for ultra-fine fiber fabrication via electrospinning process. Polymer 2005, 46, 6128–6134. [Google Scholar] [CrossRef]
- McKee, M.G.; Hunley, M.T.; Layman, J.M.; Long, T.E. Solution rheological behavior and electrospinning of cationic polyelectrolytes. Macromolecules 2006, 39, 575–583. [Google Scholar] [CrossRef]
- Rad, Z.P.; Mokhtari, J.; Abbasi, M. Preparation and characterization of Calendula officinalis-loaded PCL/gum arabic nanocomposite scaffolds for wound healing applications. Iran. Polym. J. 2019, 28, 51–63. [Google Scholar]
- Hameed, M.; Rasul, A.; Waqas, M.K.; Saadullah, M.; Aslam, N.; Abbas, G.; Latif, S.; Afzal, H.; Inam, S.; Shah, P.A. Formulation and evaluation of a clove oil-encapsulated nanofiber formulation for effective wound-healing. Molecules 2021, 26, 2491. [Google Scholar] [CrossRef]
- ElMessiry, M.; Fadel, N. The tensile properties of electrospun Polyvinyl chloride and cellulose acetate (PVC/CA) bi-component polymers nanofibers. Alex. Eng. J. 2019, 58, 885–890. [Google Scholar] [CrossRef]
- Cacciotti, I.; Calderone, M.; Bianco, A. Tailoring the properties of electrospun PHBV mats: Co-solution blending and selective removal of PEO. Eur. Polym. J. 2013, 49, 3210–3222. [Google Scholar] [CrossRef]
- Soud, S.A.; Hasoon, B.A.; Abdulwahab, A.I.; Hussein, N.N.; Maeh, R.K. Synthesis and characterization of plant extracts loaded PVA/PVP blend films and evaluate their biological activities. EurAsian J. Biosci. 2020, 14, 2921–2931. [Google Scholar]
- Rather, A.H.; Wani, T.U.; Khan, R.S.; Pant, B.; Park, M.; Sheikh, F.A. Prospects of polymeric nanofibers loaded with essential oils for biomedical and food-packaging applications. Int. J. Mol. Sci. 2021, 22, 4017. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Reis, R.L.; Ramakrishna, S.; Kundu, S.C. Functionalized silk fibroin nanofibers as drug carriers: Advantages and challenges. J. Control. Release 2020, 321, 324–347. [Google Scholar] [CrossRef] [PubMed]
- Jirovetz, L.; Buchbauer, G.; Stoilova, I.; Stoyanova, A.; Krastanov, A.; Schmidt, E. Chemical composition and antioxidant properties of clove leaf essential oil. J. Agric. Food Chem. 2006, 54, 6303–6307. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-W.; Yu, C.-W.; Wu, S.-C.; Yih, K.-H. DPPH free-radical scavenging activity, total phenolic contents and chemical composition analysis of forty-two kinds of essential oils. J. Food Drug Anal. 2009, 17, 386–395. [Google Scholar] [CrossRef]
- Sohilait, H.J.; Kainama, H. Free radical scavenging activity of essential oil of Eugenia caryophylata from Amboina Island and derivatives of Eugenol. Open Chem. 2019, 17, 422–428. [Google Scholar] [CrossRef]
- Hadidi, M.; Pouramin, S.; Adinepour, F.; Haghani, S.; Jafari, S.M. Chitosan nanoparticles loaded with clove essential oil: Characterization, antioxidant and antibacterial activities. Carbohydr. Polym. 2020, 236, 116075. [Google Scholar] [CrossRef] [PubMed]
- Tavares, T.D.; Antunes, J.C.; Padrão, J.; Ribeiro, A.I.; Zille, A.; Amorim, M.T.P.; Ferreira, F.; Felgueiras, H.P. Activity of Specialized Biomolecules against Gram-Positive and Gram-Negative Bacteria. Antibiotics 2020, 9, 314. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Demartino, L.; Coppola, L.; Defeo, V. Effect of essentials oils on pathogenic bacteria. Pharmaceutics 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Gopi, M.; Karthik, K.; Manjunathchar, H.V.; Tamilmahan, P.; Kesavan, M.; Dashprakash, M.; Balaraju, B.L.; Purushothaman, M.R. Essential oils as a feed additive in poultry nutrition. Adv. Anim. Vet. Sci. 2014, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.-G.; Liu, T.; Hu, Q.-P.; Cao, X.-M. Chemical composition, antibacterial properties and mechanism of action of essential oil from clove buds against Staphylococcus aureus. Molecules 2016, 21, 1194. [Google Scholar] [CrossRef]
- CLSI Document M100-S19; Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2010.
- Minarini, L.A.D.R.; Andrade, L.N.D.; De Gregorio, E.; Grosso, F.; Naas, T.; Zarrilli, R.; Camargo, I.L.B.C. Editorial: Antimicrobial resistance as a Global Public Health problem: How can we address it? Front. Public Health 2020, 8, 612844. [Google Scholar] [CrossRef]
- WHO—World Health Organization, Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 17 January 2022).
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef] [PubMed]
- Suflet, D.M.; Popescu, I.; Pelin, I.M.; Ichim, D.L.; Daraba, O.M.; Constantin, M.; Fundueanu, G. Dual cross-linked chitosan/PVA hydrogels containing silver nanoparticles with antimicrobial properties. Pharmaceutics 2021, 13, 1461. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Xu, Y.; Shi, X.; Zhang, M.; Huang, Y.; Liang, Y.; Chen, Y.; Ji, W.; Kim, J.R.; et al. Engineering of hollow polymeric nanosphere-supported imidazolium-based ionic liquids with enhanced antimicrobial activities. Nano Res. 2022, 15, 5556–5568. [Google Scholar] [CrossRef]
- Prashar, A.; Locke, I.C.; Evans, C.S. Cytotoxicity of clove (Syzygium aromaticum) oil and its major components to human skin cells. Cell Prolif. 2006, 39, 241–248. [Google Scholar] [CrossRef] [PubMed]


| Sample Code | PCurd/PVA Ratio, (v/v) | [η] (dL/g) | β | γ | centangled (g/dL) |
|---|---|---|---|---|---|
| PCurd | 100/0 | 26.032 ± 0.8707 | 0.6338 ± 0.0117 | −0.0123 ± 0.0004 | <1.9975 |
| 50PCurd/PVA | 50/50 | 9.808 ± 0.6121 | 0.5917 ± 0.0241 | −0.0135 ± 0.0010 | 2.0392 |
| 25PCurd/PVA | 25/75 | 5.227 ± 0.2897 | 0.6297 ± 0.0306 | −0.0279 ± 0.0019 | 1.9133 |
| PVA | 0/100 | 0.792 ± 0.0132 | 0.0750 ± 0.0036 | 0.0109 ± 0.0018 | 2.6509 |
| Sample Code | Electrospinning Composition | dav (nm) | μg CEO/ mg Membrane | Mechanical Properties | ||||
|---|---|---|---|---|---|---|---|---|
| PCurd/PVA Ratio, (v/v) | PCurd Content, (%, w/w) | CEO (%, w/w) | σ (MPa) | εb (%) | Young’s Modulus (MPa) | |||
| PVA | 100/0 | 0 | - | 235 ± 7.37 | - | 1.63 ± 0.17 | 16.08 ± 1.85 | 0.924 ± 0.19 |
| 25PCurd/PVA | 25/75 | 17 | - | 157 ± 3.09 | - | 1.97 ± 0.16 | 7.58 ± 0.90 | 0.861 ± 0.13 |
| 50PCurd/PVA | 50/50 | 37 | - | 115 ± 3.10 | - | 0.788 ± 0.08 | 2.01 ± 0.45 | 0.392 ± 0.05 |
| 25PCurd/PVA/C1 | 25/75 | 17 | 2.70 | 115 ± 1.36 | 2.88 | 1.93 ± 0.14 | 8.73 ± 0.55 | 0.831 ± 0.12 |
| 25PCurd/PVA/C2 | 27/75 | 17 | 5.26 | 122 ± 2.07 | 5.63 | 2.40 ± 0.21 | 16.13 ± 1.8 | 0.861 ± 0.10 |
| 25PCurd/PVA/C3 | 27/75 | 17 | 35.71 | 110 ± 1.47 | 27.12 | 2.77 ± 0.25 | 16.38 ± 2.5 | 1.309 ± 0.50 |
| Sample Code | μg CEO/Disc | S. Aureus (mm) | E. coli (mm) |
|---|---|---|---|
| 25PCurd/PVA | - | 7.00 | 7.00 |
| 25PCurd/PVA/C1 | 10.35 | 7.93 | 7.10 |
| 25 PCurd/PVA/C2 | 57.49 | 8.12 | 7.30 |
| 25PCurd/PVA/C3 | 162.71 | 8.97 | 8.10 |
| Gentamicin (10 μg) | - | 24 | 22 |
| Bacteria Strains | M(+) | Samples | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 25PCurd/PVA | 25PCurd/PVA/C1 | 25PCurd/PVA/C2 | 25PCurd/PVA/C3 | |||||||
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |||
| S. aureus ATCC 25923 | CFU/mL | 1.5 × 108 | 0 × 100 | 0 × 100 | 0 × 100 | 0 × 100 | 0 × 100 | 0 × 100 | 0 × 100 | 0 × 100 |
| Log10 (CFU/mL) | 8.176 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Log Reduction | - | 8.176 | 8.176 | 8.176 | 8.176 | 8.176 | 8.176 | 8.176 | 8.176 | |
| LR (%) | - | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| E. coli ATCC 25922 | CFU/mL | 1.5 × 108 | 23.3 × 102 | 11.68 × 102 | 9.2 × 101 | 2.2 × 101 | 8.3 × 101 | 1 × 100 | 0 × 100 | 0 × 100 |
| Log10 (CFU/mL) | 8.176 | 3.367 | 3.067 | 1.963 | 1.342 | 1.919 | 0 | 0 | 0 | |
| Log Reduction | - | 4.809 | 5.109 | 6.213 | 6.834 | 6.257 | 6.257 | 8.176 | 8.176 | |
| LR (%) | - | 99.920 | 99.999 | 99.999 | 99.999 | 99.999 | 99.999 | 100 | 100 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suflet, D.M.; Popescu, I.; Pelin, I.M.; David, G.; Serbezeanu, D.; Rîmbu, C.M.; Daraba, O.M.; Enache, A.A.; Bercea, M. Phosphorylated Curdlan Gel/Polyvinyl Alcohol Electrospun Nanofibres Loaded with Clove Oil with Antibacterial Activity. Gels 2022, 8, 439. https://doi.org/10.3390/gels8070439
Suflet DM, Popescu I, Pelin IM, David G, Serbezeanu D, Rîmbu CM, Daraba OM, Enache AA, Bercea M. Phosphorylated Curdlan Gel/Polyvinyl Alcohol Electrospun Nanofibres Loaded with Clove Oil with Antibacterial Activity. Gels. 2022; 8(7):439. https://doi.org/10.3390/gels8070439
Chicago/Turabian StyleSuflet, Dana M., Irina Popescu, Irina M. Pelin, Geta David, Diana Serbezeanu, Cristina M. Rîmbu, Oana M. Daraba, Alin A. Enache, and Maria Bercea. 2022. "Phosphorylated Curdlan Gel/Polyvinyl Alcohol Electrospun Nanofibres Loaded with Clove Oil with Antibacterial Activity" Gels 8, no. 7: 439. https://doi.org/10.3390/gels8070439
APA StyleSuflet, D. M., Popescu, I., Pelin, I. M., David, G., Serbezeanu, D., Rîmbu, C. M., Daraba, O. M., Enache, A. A., & Bercea, M. (2022). Phosphorylated Curdlan Gel/Polyvinyl Alcohol Electrospun Nanofibres Loaded with Clove Oil with Antibacterial Activity. Gels, 8(7), 439. https://doi.org/10.3390/gels8070439