Preparation of Various Nanomaterials via Controlled Gelation of a Hydrophilic Polymer Bearing Metal-Coordination Units with Metal Ions
Abstract
:1. Introduction
2. Results and Discussion
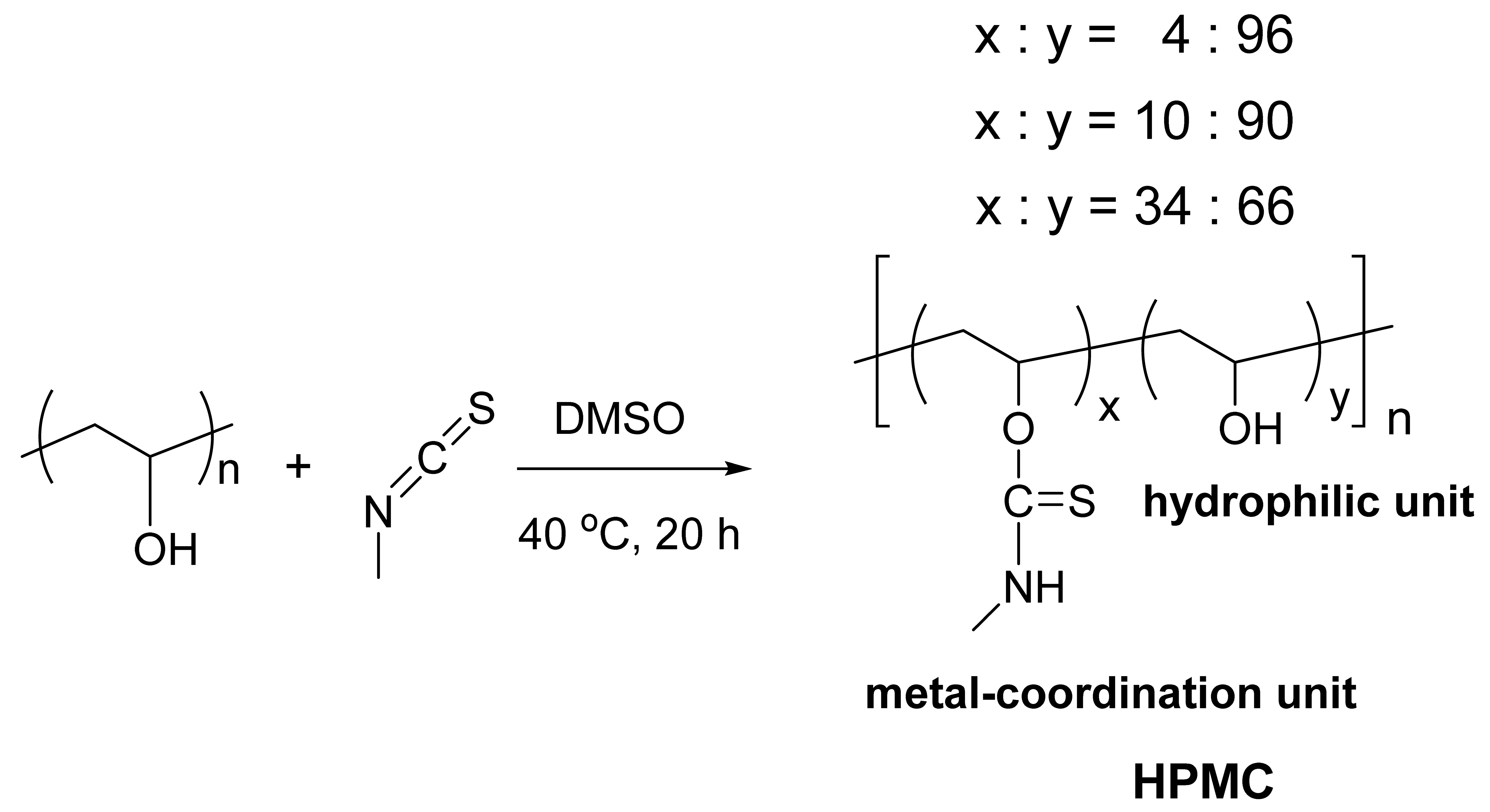
2.1. Synthesis of HPMC and Its Gelation Behavior with Metal Ions
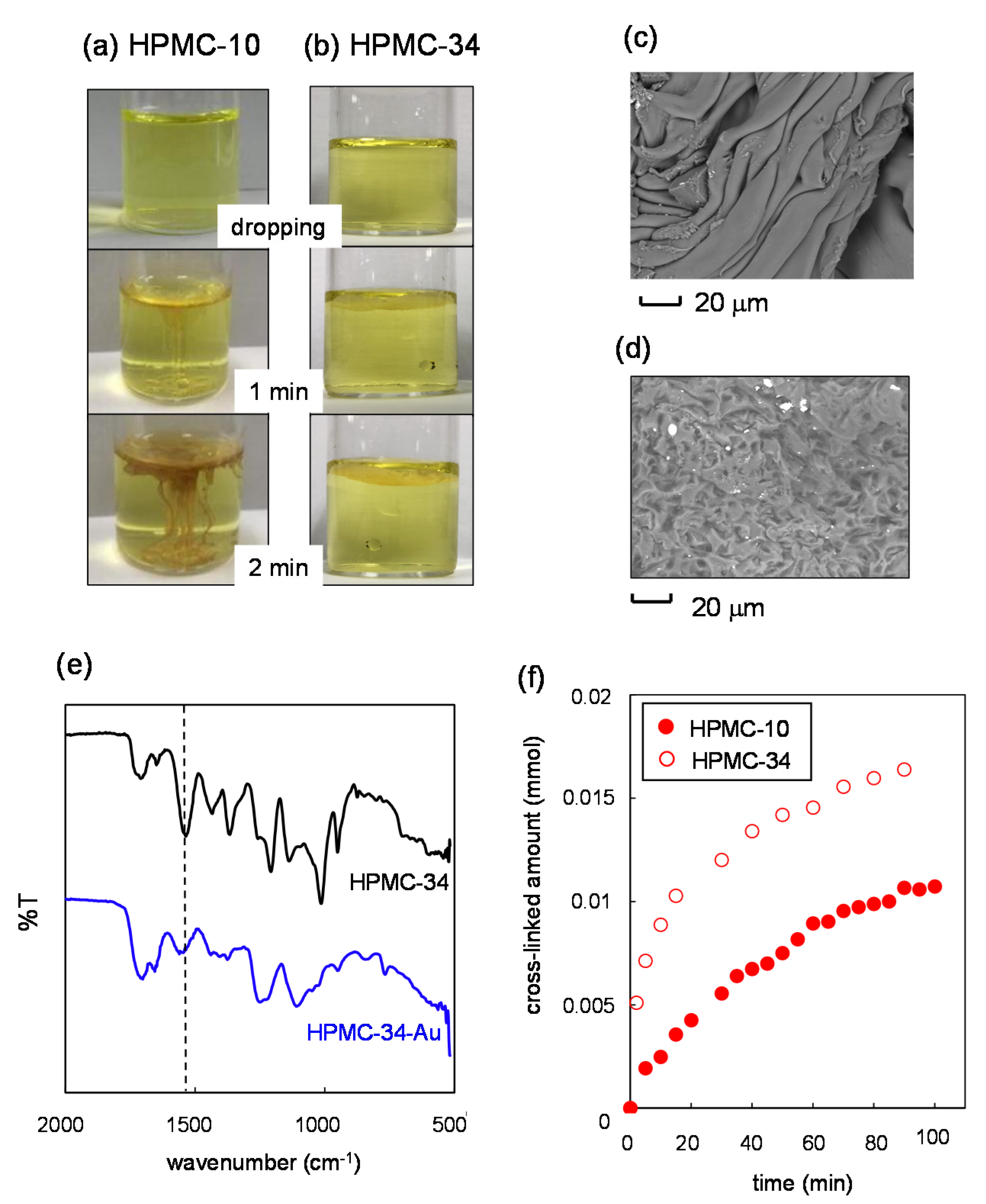
2.2. Synthesis of Nanosheets
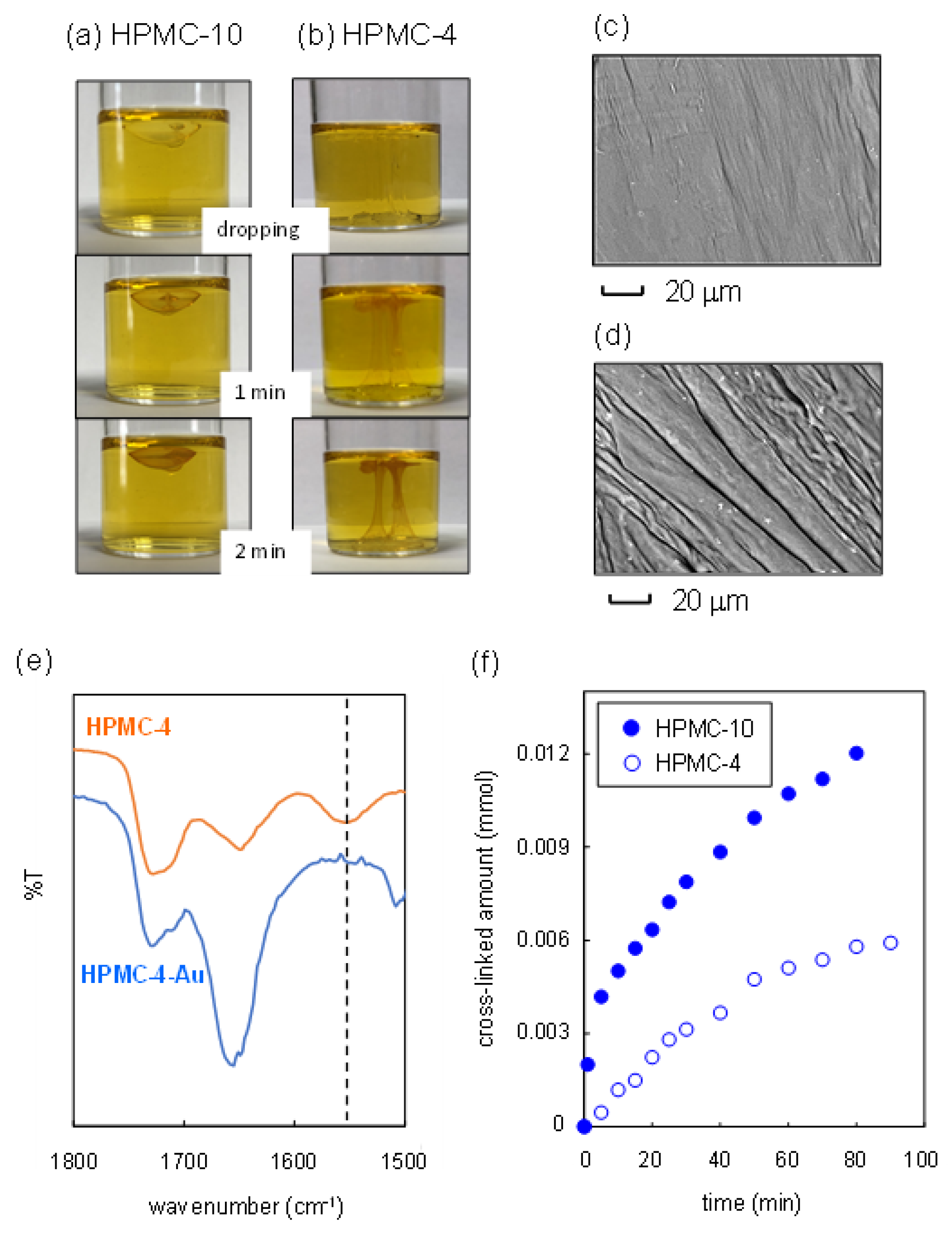
2.3. Synthesis of Nanofiber
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Instruments
4.3. Gelation of HPMC and Metal Ions
4.4. Synthesis of Au Nanosheets
4.5. Synthesis of Pd Nanofibers
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Masalamani, N.; Bakhsh, E.M.; Khan, S.B.; Danish, E.Y.; Akhtarm, K.; Fagieh, T.M.; Su, X.T.; Asiri, A.M. Chitosan@carboxymethylcellulose/CuO-Co2O3 nanoadsorbent as a super catalyst for the removal of water pollutants. Gels 2022, 8, 91. [Google Scholar] [CrossRef] [PubMed]
- Valot, L.; Maumus, M.; Brunel, L.; Martinez, J.; Amblard, M.; Noel, D.; Mehdi, A.; Subra, G. A Collagen-mimetic organic-inorganic hydrogel for cartilage engineering. Gels 2021, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Paraskevopoulou, P.; Raptopoulos, G.; Leontaridou, F.; Papastergiou, M.; Sakellari, A.; Karavoltsos, S. Evaluation of polyurea-crosslinked alginate aerogels for seawater decontamination. Gels 2021, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Bellotto, O.; Cringoli, M.C.; Perathoner, S.; Fornasiero, P.; Marchesan, S. Peptide gelators to template inorganic nanoparticle formation. Gels 2021, 7, 14. [Google Scholar] [CrossRef]
- Weinberger, C.; Kuchling, D.; Tiemann, M. Hydrogels as porogens for nanoporous inorganic materials. Gels 2018, 4, 83. [Google Scholar] [CrossRef] [Green Version]
- Riva, L.; Lotito, A.D.; Punta, C.; Sacchetti, A. Zinc- and copper-loaded nanosponges from cellulose nanofibers hydrogels: New heterogeneous catalysts for the synthesis of aromatic acetals. Gels 2022, 8, 54. [Google Scholar] [CrossRef]
- Takeno, H.; Suto, N. Robust and highly stretchable chitosan nanofiber/alumina-coated silica/carboxylated poly(vinyl alcohol)/borax composite hydrogels constructed by multiple crosslinking. Gels 2022, 8, 6. [Google Scholar] [CrossRef]
- Zhao, Q.; Mu, S.; Liu, X.; Qiu, G.; Astruc, D.; Gu, H. Gallol-tethered injectable AuNP hydrogel with desirable self-healing and catalytic properties. Macromol. Chem. Phys. 2019, 220, 1800427. [Google Scholar] [CrossRef]
- Pena, N.; Maldonao, M.; Bonham, A.J.; Aguado, B.A.; Dominguez-Alfaro, A.; Laughter, M.; Rowland, T.J.; Bardill, J.; Farnsworth, N.L.; Ramon, N.A.; et al. Gold nanoparticle-functionalized reverse thermal gel for tissue engineering applications. ACS Appl. Mater. Interfaces 2019, 11, 18671–18680. [Google Scholar] [CrossRef]
- Li, W.; Chu, K.; Liu, L. Zwitterionic gel coating endows gold nanoparticles with ultrastability. Langmuir 2019, 35, 1369–1378. [Google Scholar] [CrossRef]
- Kim, J.; Chan Hong, S.; Bae, G.N.; Jung, J.H. Electrospun magnetic nanoparticle-decorated nanofiber filter and its application to high-efficiency air filtration. Environ. Sci. Technol. 2017, 51, 11967–11975. [Google Scholar] [CrossRef] [PubMed]
- Chee, W.K.; Lim, H.N.; Zainal, Z.; Harrison, I.; Huang, N.M.; Andou, Y.; Chong, K.F.; Pendikumar, A. Electrospun nanofiber membranes as ultratin flexible supercapacitors. RSC Adv. 2017, 7, 12033–12040. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, Y.; Sun, G.; Zhang, G.; Liu, H.; Du, J.; Yang, S.; Bai, J.; Yang, Q. Fabrication of Au/PVP nanofiber composites by electrospinning. J. Appl. Polym. Sci. 2007, 105, 3618–3622. [Google Scholar] [CrossRef]
- Ding, H.; Khan, S.T.; Liu, J.J.; Sun, L.Y. Gelation based on host-guest interactions induced by multi-functionalize nanosheets. Gels 2021, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, Y.; Wu, Z.; Zhang, Z.; Hu, N.; Peng, C. Three-dimentional MoS2/reduced graphene oxide nanosheets/graphene quantum dots hybrids for high-performance room-temperature NO2 gas sensors. Nanomaterials 2022, 12, 901. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Zhao, X. Nanosheet-assembled MnO-integrated electrode based on the low-temperature and green chemical route. Crystals 2022, 12, 115. [Google Scholar] [CrossRef]
- Zhao, M.; Huang, Y.; Peng, Y.; Huang, Z.; Ma, Q.; Zhang, H. Two-dimentional metal-organic framework nanosheets: Synthesis and applications. Chem. Soc. Rev. 2018, 47, 6267–6295. [Google Scholar] [CrossRef]
- Tan, C.; Cao, X.; Xu, X.-J.; He, Q.; Yang, J.; Zhang, H. Recent advances in ultrathin two-dimentional nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, Z.; Wong, W.-Y. Synthesis and characterization of a large-sized π-conjugated copper(II) complex nanosheet. J. Inorg. Organomet. Polym. Mater. 2020, 30, 254–258. [Google Scholar] [CrossRef]
- Tsukamoto, T.; Takada, K.; Sakamoto, R.; Matsuoka, R.; Toyoda, R.; Maeda, H.; Yagi, T.; Nishikawa, M.; Shinjo, N.; Amano, S.; et al. Coordination nanosheets based on terpyridine-zinc(II) complexes: As photoactive host materials. J. Am. Chem. Soc. 2017, 139, 5359–5366. [Google Scholar] [CrossRef]
- Sakamoto, R.; Hoshiko, K.; Liu, Q.; Yagi, T.; Nagayama, T.; Kusaka, S.; Tsuchiya, M.; Kitagawa, Y.; Wong, W.-Y.; Nishihara, H. A photofunctional bottom-up bis(dipyrrinato)zinc(II) complex nanosheet. Nat. Commun. 2015, 6, 6713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takada, K.; Sakamoto, R.; Yi, S.-T.; Katagiri, S.; Kambe, T.; Nishihara, H. Electrochromic bis(terpyridine)metal complex nanosheets. J. Am. Chem. Soc. 2015, 137, 4681–4689. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T.; Sakamoto, R.; Hoshiko, K.; Takada, K.; Miyachi, M.; Ryu, J.-H.; Sasaki, S.; Kim, J.; Nakazato, K.; Takata, M.; et al. π-Conjugated nickel bis(dithiolene) complex nanosheet. J. Am. Chem. Soc. 2013, 135, 2462–2465. [Google Scholar] [CrossRef] [PubMed]
- Nagai, D.; Kubo, A.; Morita, M.; Shimazaki, N.; Maki, Y.; Takeno, H.; Mori, M.; Uehara, H.; Yamanobe, T. Pd-and Au-Induced circular fibrous and polymer gelation via thiocarbonyl groups and high Pd catalyst activity. ACS Appl. Polym. Mater. 2020, 2, 2211–2219. [Google Scholar] [CrossRef]
- Nagai, D.; Morita, M.; Yamanobe, T. Synthesis of nanosheets containing uniformly dispersed PdII ions at an aqueous/aqueous interface: Development of a highly active nanosheet catalyst for Mizoroki-Heck reaction. ACS Omega 2020, 5, 18484–18489. [Google Scholar] [CrossRef]
- Park, J.; Won, S.W.; Mao, I.; Kwak, I.S.; Yun, Y.S. Recovery of Pd(II) from hydrochloric solution using polyallylamine hydrochloride-modified Escherichia coli biomass. J. Hazard. Mater. 2010, 181, 2211–2219. [Google Scholar] [CrossRef]
- Huang, M.R.; Peng, Q.Y.; Li, X.G. Rapid and effective adsorption of lead ions on fine poly(phenylenediamine) microparticles. Chem.-Eur. J. 2006, 12, 4341–4350. [Google Scholar] [CrossRef]
- NIST X-Ray Photoelectron Spectroscopy Database (NIST XPS Database, Selected Element Search Menu). Available online: https://srdata.nist.gov/xps/ (accessed on 20 May 2022).
- Liu, Z.; Yuan, X.; Yu, Y.; Zhang, Q.; Leong, D.T.; Lee, J.Y.; Xie, J. From aggregation-induced emission of Au(I)-thiolate complexes to ultrabright Au(0)@Au(I)-thiolate core-shell nanoclusters. J. Am. Chem. Soc. 2012, 134, 16662–16670. [Google Scholar]
- Shin, H.-S.; Huh, S. Au/Au@polythiophene core/shell nanoparticles for heterogeneous catalysis of nitroarenes. ACS Appl. Mater. Interfaces 2012, 4, 6324–6331. [Google Scholar] [CrossRef]
- Gniewek, A.; Trzecial, A.; Ziolkowski, J.; Kepinski, L.; Wrzyszcz, J.; Tylus, W. Pd-PVP colloid as catalyst for Heck and carbonylation reactions: TEM and XPS studies. J. Catal. 2005, 229, 332–343. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagai, D.; Isobe, N.; Inoue, T.; Okamoto, S.; Maki, Y.; Yamanobe, T. Preparation of Various Nanomaterials via Controlled Gelation of a Hydrophilic Polymer Bearing Metal-Coordination Units with Metal Ions. Gels 2022, 8, 435. https://doi.org/10.3390/gels8070435
Nagai D, Isobe N, Inoue T, Okamoto S, Maki Y, Yamanobe T. Preparation of Various Nanomaterials via Controlled Gelation of a Hydrophilic Polymer Bearing Metal-Coordination Units with Metal Ions. Gels. 2022; 8(7):435. https://doi.org/10.3390/gels8070435
Chicago/Turabian StyleNagai, Daisuke, Naoki Isobe, Tatsushi Inoue, Shusuke Okamoto, Yasuyuki Maki, and Takeshi Yamanobe. 2022. "Preparation of Various Nanomaterials via Controlled Gelation of a Hydrophilic Polymer Bearing Metal-Coordination Units with Metal Ions" Gels 8, no. 7: 435. https://doi.org/10.3390/gels8070435
APA StyleNagai, D., Isobe, N., Inoue, T., Okamoto, S., Maki, Y., & Yamanobe, T. (2022). Preparation of Various Nanomaterials via Controlled Gelation of a Hydrophilic Polymer Bearing Metal-Coordination Units with Metal Ions. Gels, 8(7), 435. https://doi.org/10.3390/gels8070435





