Construction and Tribological Properties of Biomimetic Cartilage-Lubricating Hydrogels
Abstract
:1. Introduction
2. Results and Discussion
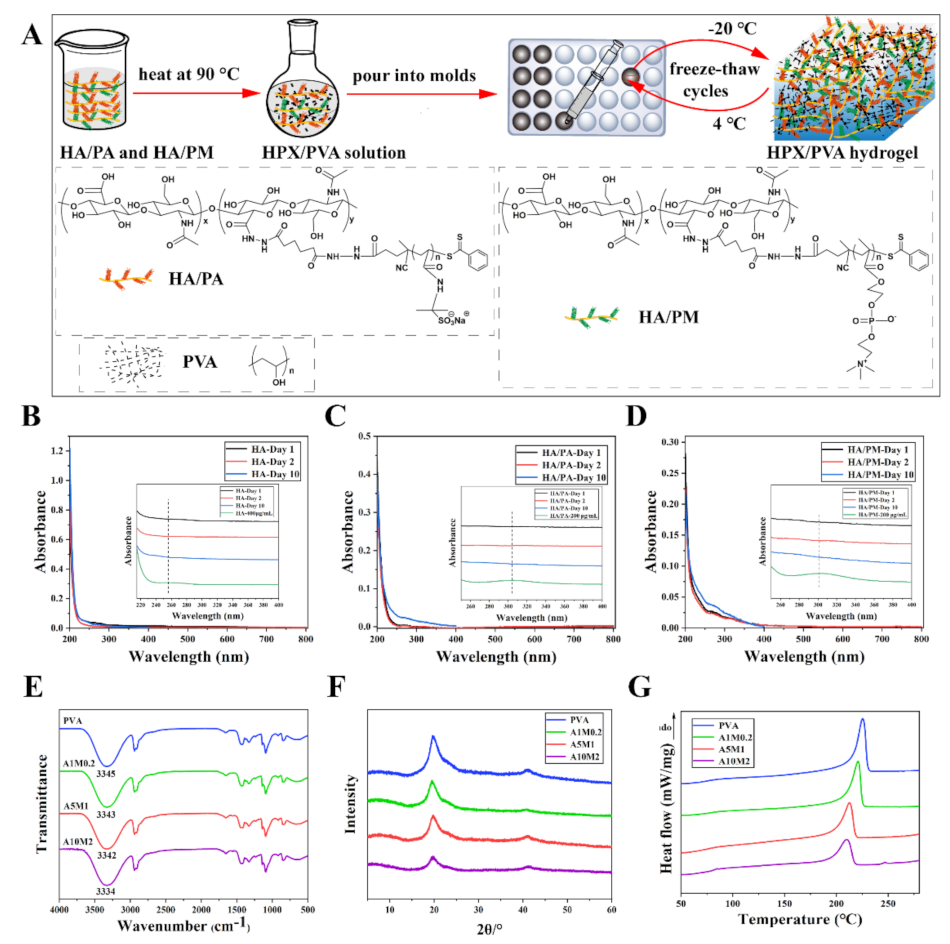
2.1. Construction of the HPX/PVA Hydrogels
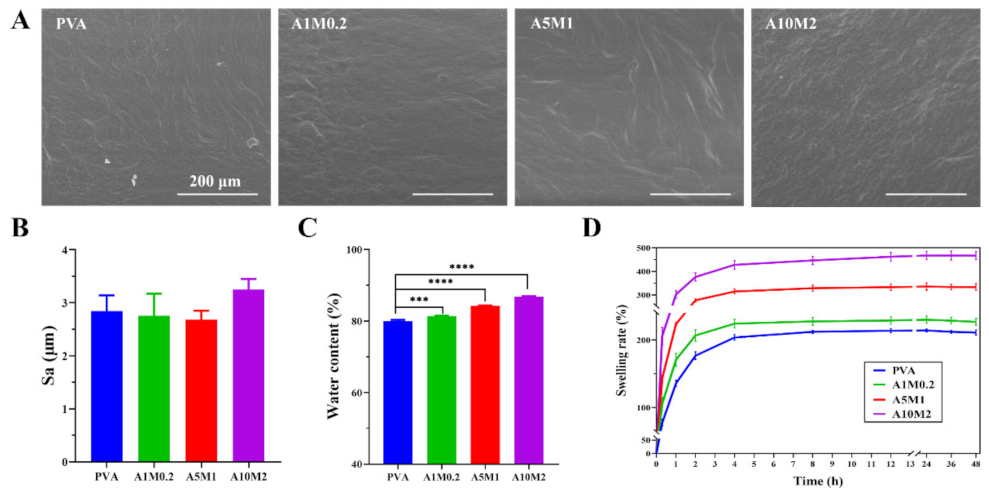
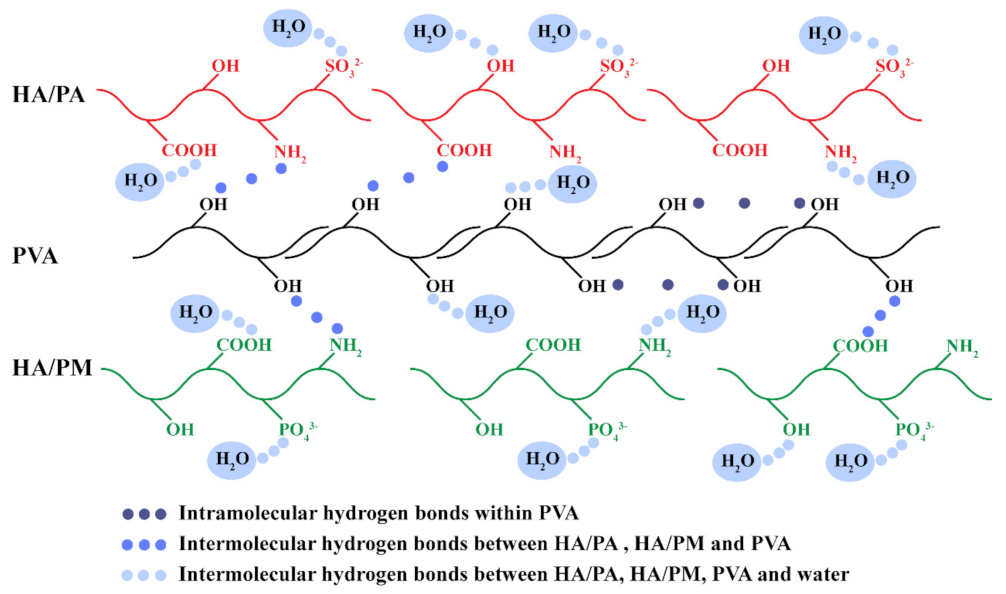
2.2. Physicochemical Properties of the HPX/PVA Hydrogels
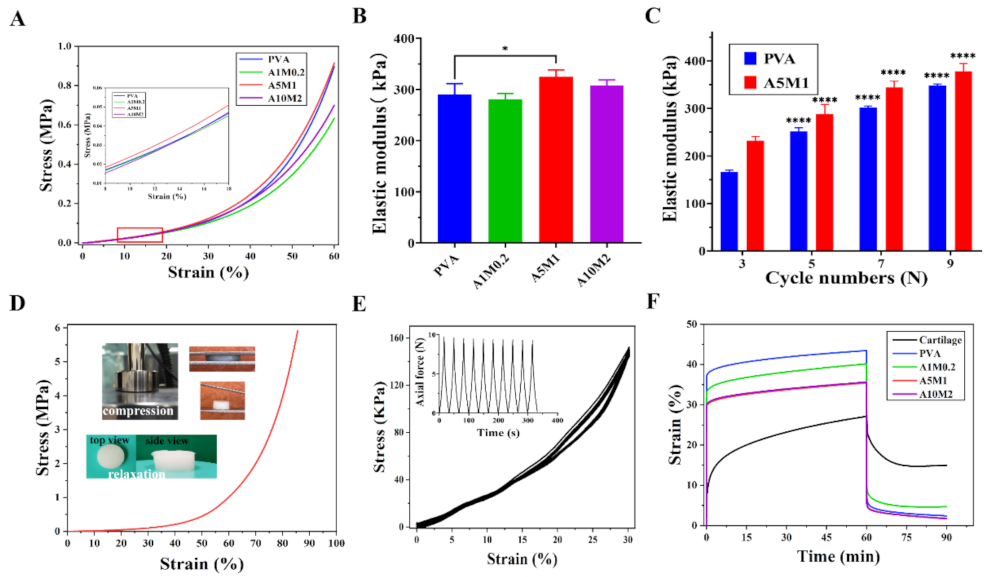
2.3. Mechanical Properties of the HPX/PVA Hydrogels
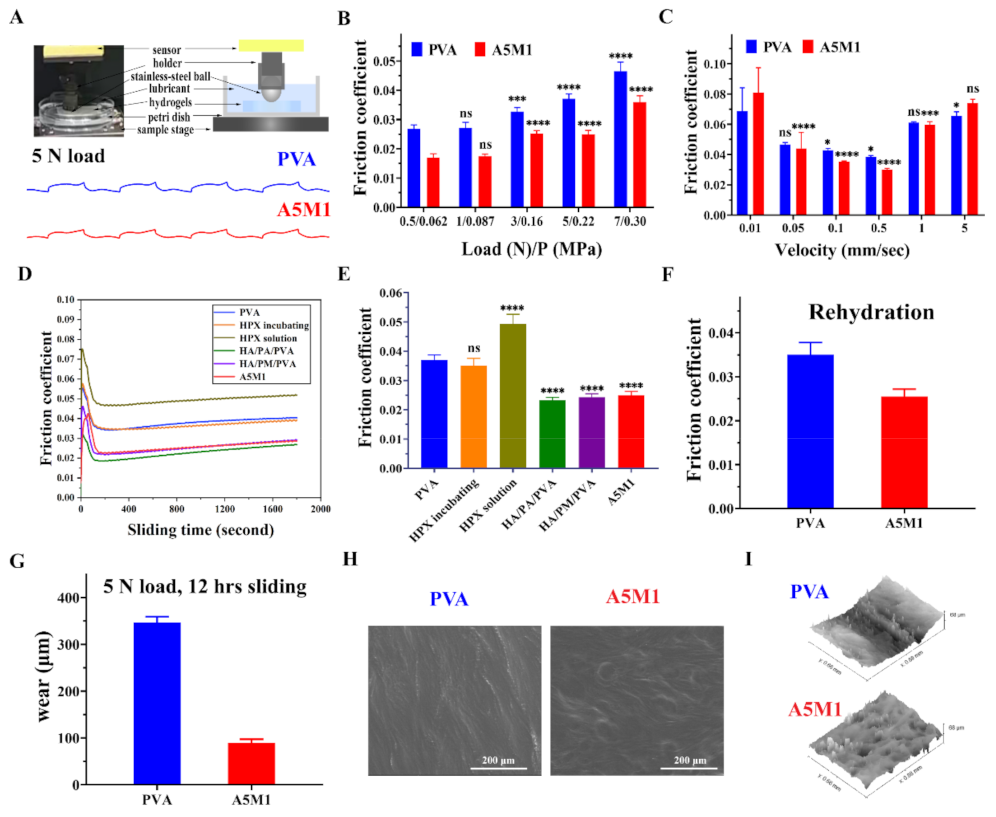
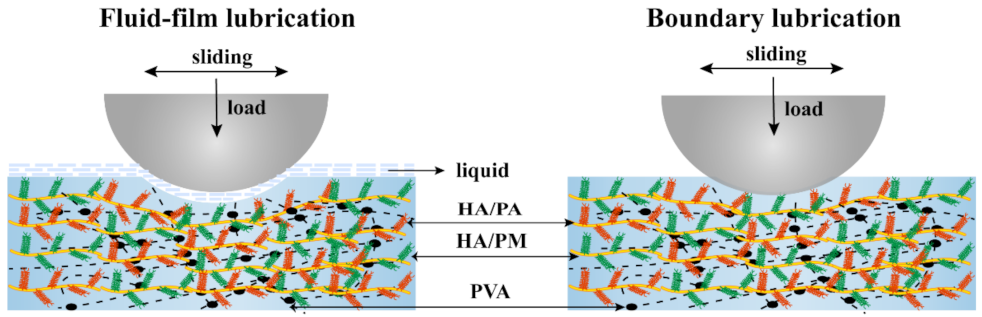
2.4. Friction and Wear Properties of the HPX/PVA Hydrogels
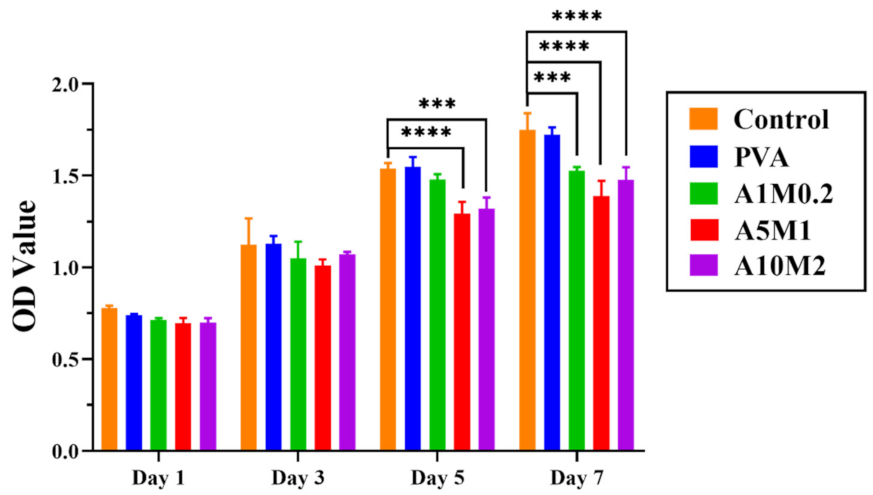
2.5. Cytotoxicity Properties of the HPX/PVA Hydrogels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of the HPX/PVA Hydrogels
4.3. Characterization of Hydrogels
4.3.1. Elemental Analyses of Hydrogels
4.3.2. UV-Visible Spectroscopy of Hydrogel Extracts
4.3.3. Reflection Fourier Transform Infrared of Hydrogels
4.3.4. X-ray Diffraction of Hydrogels
4.3.5. Differential Scanning Calorimetry of Hydrogels
4.3.6. Surface Morphology and Roughness
4.3.7. Water Content, Swelling Ratio, and Contact Angle
4.4. Compression Mechanical Testing
4.5. Creep Testing
4.6. Friction and Wear Measurements
4.7. Cytocompatibility
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forster, H.; Fisher, J. The influence of loading time and lubricant on the friction of articular cartilage. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1996, 210, 109–119. [Google Scholar] [CrossRef]
- Jahn, S.; Klein, J. Lubrication of articular cartilage. Phys. Today 2018, 71, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.F.; Kluzek, M.; Iuster, N.; Shimoni, E.; Kampf, N.; Goldberg, R.; Klein, J. Cartilage-inspired, lipid-based boundary-lubricated hydrogels. Science 2020, 370, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Jahn, S.; Seror, J.; Klein, J. Lubrication of Articular Cartilage. Annu. Rev. Biomed. Eng. 2018, 18, 235–258. [Google Scholar] [CrossRef] [PubMed]
- McCutchen, C. Mechanism of animal joints: Sponge-hydrostatic and weeping bearings. Nature 1959, 184, 1284–1285. [Google Scholar] [CrossRef]
- Xie, R.J.; Yao, H.; Mao, A.S.; Zhu, Y.; Qi, D.W.; Jia, Y.G.; Gao, M.; Chen, Y.H.; Wang, L.; Wang, D.-A.; et al. Biomimetic cartilage-lubricating polymers regenerate cartilage in rats with early osteoarthritis. Nat. Biomed. Eng. 2021, 5, 1189–1201. [Google Scholar] [CrossRef]
- Seror, J.; Merkher, Y.; Kampf, N.; Collinson, L.; Day, A.J.; Maroudas, A.; Klein, J. Normal and Shear Interactions between Hyaluronan–Aggrecan Complexes Mimicking Possible Boundary Lubricants in Articular Cartilage in Synovial Joints. Biomacromolecules 2012, 13, 3823–3832. [Google Scholar] [CrossRef]
- Seror, J.; Merkher, Y.; Kampf, N.; Collinson, L.; Day, A.J.; Maroudas, A.; Klein, J. Articular Cartilage Proteoglycans as Boundary Lubricants: Structure and Frictional Interaction of Surface-Attached Hyaluronan and Hyaluronan-Aggrecan Complexes. Biomacromolecules 2011, 12, 3432–3443. [Google Scholar] [CrossRef]
- Banquy, X.; Lee, D.W.; Das, S.; Hogan, J.; Israelachvili, J.N. Shear-Induced Aggregation of Mammalian Synovial Fluid Components under Boundary Lubrication Conditions. Adv. Funct. Mater. 2014, 24, 3152–3161. [Google Scholar] [CrossRef] [Green Version]
- Goldring, S.R.; Goldring, M.B. Changes in the osteochondral unit during osteoarthritis: Structure, function and cartilage-bone crosstalk. Nat. Rev. Rheumatol. 2016, 12, 632–644. [Google Scholar] [CrossRef]
- Sellam, J.; Berenbaum, F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 2010, 6, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Morgese, G.; Benetti, E.M.; Zenobi-Wong, M. Molecularly engineered biolubricants for articular cartilage. Adv. Healthc. Mater. 2018, 7, 1701463. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar]
- Revell, C.M.; Athanasiou, K.A. Success rates and immunologic responses of autogenic, allogenic, and xenogenic treatments to repair articular cartilage defects. Tissue Eng. Part B Rev. 2009, 15, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, F.; Kim, M.; Nahri, S.Y.; Mauck, R.L.; Pleshko, N. Near-Infrared Spectroscopy Predicts Compositional and Mechanical Properties of Hyaluronic Acid-Based Engineered Cartilage Constructs. Tissue. Eng. Part A 2018, 24, 106–116. [Google Scholar] [CrossRef]
- Gomoll, A.H.; Farr, J.; Gillogly, S.D.; Kercher, J.S.; Minas, T. Surgical management of articular cartilage defects of the knee. Instr. Course Lect. 2011, 60, 461–483. [Google Scholar]
- Accardi, M.A.; Dini, D.; Cann, P.M. Experimental and numerical investigation of the behaviour of articular cartilage under shear loading—interstitial fluid pressurisation and lubrication mechanisms. Tribol. Int. 2011, 44, 565–578. [Google Scholar] [CrossRef]
- Peppas, N.A.; Korsmeyer, R. Dynamically swelling hydrogels in controlled release applications. In Hydrogels in Medicine and Pharmacy; Peppas, N.A., Ed.; CRC Press: Boca Raton, FL, USA, 1987; Volume 3, pp. 109–136. [Google Scholar]
- Stammen, J.A.; Williams, S.; Ku, D.N.; Guldberg, R.E. Mechanical properties of a novel PVA hydrogel in shear and unconfined compression. Biomaterials 2001, 22, 799–806. [Google Scholar] [CrossRef]
- Farsi, M.; Asefnejad, A.; Baharifar, H. A hyaluronic acid/PVA electrospun coating on 3D printed PLA scaffold for orthopedic application. Prog. Biomater. 2022, 11, 67–77. [Google Scholar] [CrossRef]
- Sepahdar, A.; Nazbar, A.; Bahadorikhalili, S.; Rezaei, G.; Shokrgozar, M.A.; Dehghan, M.M.; Javar, H.A.; Bonakdar, S. Cartilage tissue regeneration using kartogenin loaded hybrid scaffold for the chondrogenic of adipose mesenchymal stem cells. J. Drug Deliv. Sci. Technol. 2022, 73, 103384. [Google Scholar] [CrossRef]
- Blum, M.M.; Ovaert, T.C. Investigation of friction and surface degradation of innovative boundary lubricant functionalized hydrogel material for use as artificial articular cartilage. Wear 2013, 301, 201–209. [Google Scholar] [CrossRef]
- Yarimitsu, S.; Sasaki, S.; Murakami, T.; Suzuki, A. Evaluation of lubrication properties of hydrogel artificial cartilage materials for joint prosthesis. Biosurface Biotribol. 2016, 2, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Osaheni, A.O.; Finkelstein, E.B.; Mather, P.T.; Blum, M.M. Synthesis and characterization of a zwitterionic hydrogel blend with low coefficient of friction. Acta. Biomater. 2016, 46, 245–255. [Google Scholar] [CrossRef]
- Osaheni, A.O.; Ash-Shakoor, A.; Gitsov, I.; Mather, P.T.; Blum, M.M. Synthesis and characterization of zwitterionic polymer brush functionalized hydrogels with ionic responsive coefficient of friction. Langmuir 2020, 36, 3932–3940. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Xiong, D.S.; Zhao, X.D. A bionic PEEK composite structure with negatively charged surface adsorbing molecular brushes possessing improved biotribological properties for artificial joints. Tribol. Int. 2021, 155, 106808. [Google Scholar] [CrossRef]
- Xu, P.P.; Du, M.; Zheng, Q. Influence of Preparation Parameters on the Frictional Behavior of PVA Hydrogel Against Glass Substrate. Acta. Polym. Sin. 2014, 5, 708–714. [Google Scholar]
- Branco, A.C.; Oliveira, A.S.; Monteiro, I.; Nolasco, P.; Silva, D.C.; Figueiredo-Pina, C.G.; Colaço, R.; Serro, A.P. PVA-based hydrogels loaded with diclofenac for cartilage replacement. Gels 2022, 8, 143. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Zhang, J.Y.; Liu, Z.Z.; Lin, Q.N.; Liu, X.L.; Bao, C.Y.; Wang, Y.; Zhu, L.Y. Tissue-Integratable and Biocompatible Photogelation by the Imine Crosslinking Reaction. Adv. Mater. 2016, 28, 2724–2730. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Chen, H.; Gao, R.C.; Li, L.H.; Yang, X.Q.; Wu, Y.Y.; Hu, X. Degradation of hyaluronic acid derived from tilapia eyeballs by a combinatorial method of microwave, hydrogen peroxide, and ascorbic acid. Polym. Degrad. Stabil. 2015, 112, 117–121. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, J.; Kang, D.; Zhang, H.B. Development of a complex hydrogel of hyaluronan and PVA embedded with silver nanoparticles and its facile studies on Escherichia coli. J. Biomater. Sci. Polym. Ed. 2013, 24, 1410–1425. [Google Scholar] [CrossRef]
- Fan, L.H.; Yang, H.; Yang, J.; Peng, M.; Hu, J. Preparation and characterization of chitosan/gelatin/PVA hydrogel for wound dressings. Carbohyd. Polym. 2016, 146, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Banno, M.; Ohta, K.; Yamaguchi, S.; Hirai, S.; Tominaga, K. Vibrational Dynamics of Hydrogen-Bonded Complexes in Solutions Studied with Ultrafast Infrared Pump-Probe Spectroscopy. Acc. Chem. Res. 2009, 42, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
- Colvin, B.G. Crystal structure of polyvinyl alcohol. Nature 1974, 248, 756–759. [Google Scholar] [CrossRef]
- Khodiri, A.A.; Al-Ashry, M.Y.; El-Shamy, A.G. Novel hybrid nanocomposites based on polyvinyl alcohol/graphene/magnetite nanoparticles for high electromagnetic shielding performance. J. Alloy Compd. 2020, 847, 156430. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and morphology of freeze/thawed PVA hydrogels. Macromolecules 2000, 33, 2472–2479. [Google Scholar] [CrossRef]
- Higuchi, A.; Iijima, T. DSC investigation of the states of water in poly(vinyl alcohol) membranes. Polymer 1985, 26, 1207–1211. [Google Scholar] [CrossRef]
- Lin, W.F.; Klein, J. Recent Progress in Cartilage Lubrication. Adv. Mater. 2021, 33, 2005513. [Google Scholar] [CrossRef]
- Pinheiro, A.; Cooley, A.; Liao, J.; Prabhu, R.; Elder, S. Comparison of natural crosslinking agents for the stabilization of xenogenic articular cartilage. J. Orthop. Res. 2016, 34, 1037–1046. [Google Scholar] [CrossRef]
- Wang, Z.N.; Li, J.J.; Liu, Y.H.; Luo, J.B. Synthesis and characterizations of zwitterionic copolymer hydrogels with excellent lubrication behavior. Tribol. Int. 2020, 143, 106026. [Google Scholar] [CrossRef]
- Gao, F.; Xu, Z.Y.; Liang, Q.F.; Li, H.F.; Peng, L.Q.; Wu, M.M.; Zhao, X.L.; Cui, X.; Ruan, C.S.; Liu, W.G. Osteochondral Regeneration with 3D-Printed Biodegradable High-Strength Supramolecular Polymer Reinforced-Gelatin Hydrogel Scaffolds. Adv. Sci. 2019, 6, 1900867. [Google Scholar] [CrossRef] [Green Version]
- Holloway, J.L.; Lowman, A.M.; Palmese, G.R. The role of crystallization and phase separation in the formation of physically cross-linked PVA hydrogels. Soft Matter 2013, 9, 826–833. [Google Scholar] [CrossRef]
- Means, A.K.; Shrode, C.S.; Whitney, L.V.; Ehrhardt, D.A.; Grunlan, M.A. Double Network Hydrogels that Mimic the Modulus, Strength, and Lubricity of Cartilage. Biomacromolecules 2019, 20, 2034–2042. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Liu, J.L.; Yang, X.H.; Zhang, D.K. Preparation, optimization and property of PVA-HA/PAA composite hydrogel. Mat. Sci. Eng. C-Mater. 2017, 78, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef]
- Mow, V.C.; Holmes, M.H.; Lai, W.M. Fluid transport and mechanical properties of articular cartilage: A review. J. Biomech. 1984, 17, 377–394. [Google Scholar] [CrossRef]
- Silbert, G.; Kampf, N.; Klein, J. Normal and Shear Forces between Charged Solid Surfaces Immersed in Cationic Surfactant Solution: The Role of the Alkyl Chain Length. Langmuir 2014, 30, 5097–5104. [Google Scholar] [CrossRef]
- Caligaris, M.; Ateshian, G.A. Effects of sustained interstitial fluid pressurization under migrating contact area, and boundary lubrication by synovial fluid, on cartilage friction. Osteoarthr. Cartil. 2008, 16, 1220–1227. [Google Scholar] [CrossRef] [Green Version]
- Bonnevie, E.D.; Baro, V.J.; Wang, L.; Burris, D.L. In Situ Studies of Cartilage Microtribology: Roles of Speed and Contact Area. Tribol. Lett. 2011, 41, 83–95. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, R.; Kopacz, M.; Ateshian, G.A. Experimental verification of the role of interstitial fluid pressurization in cartilage lubrication. J. Orthop. Res. 2004, 22, 565–570. [Google Scholar] [CrossRef]
- Rong, M.M.; Liu, H.; Scaraggi, M.; Bai, Y.Y.; Bao, L.Y.; Ma, S.H.; Ma, Z.F.; Cai, M.R.; Dini, D.; Zhou, F. High Lubricity Meets Load Capacity: Cartilage Mimicking Bilayer Structure by Brushing Up Stiff Hydrogels from Subsurface. Adv. Funct. Mater. 2020, 30, 2004062. [Google Scholar] [CrossRef]
| Elements | PVA | A1M0.2 | A5M1 | A10M2 |
|---|---|---|---|---|
| C% | 47.55 | 48.55 | 49.08 | 47.42 |
| H% | 6.84 | 8.14 | 8.27 | 8.15 |
| N% | 0 | 0.02 | 0.21 | 0.33 |
| S% | 0 | 0.01 | 0.12 | 0.22 |
| P% | 0 | 0.01 | 0.03 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Liu, S.; Yuan, Z.; Yang, H.; Xie, R.; Ren, L. Construction and Tribological Properties of Biomimetic Cartilage-Lubricating Hydrogels. Gels 2022, 8, 415. https://doi.org/10.3390/gels8070415
Chen Q, Liu S, Yuan Z, Yang H, Xie R, Ren L. Construction and Tribological Properties of Biomimetic Cartilage-Lubricating Hydrogels. Gels. 2022; 8(7):415. https://doi.org/10.3390/gels8070415
Chicago/Turabian StyleChen, Qiuyi, Sa Liu, Zhongrun Yuan, Hai Yang, Renjian Xie, and Li Ren. 2022. "Construction and Tribological Properties of Biomimetic Cartilage-Lubricating Hydrogels" Gels 8, no. 7: 415. https://doi.org/10.3390/gels8070415
APA StyleChen, Q., Liu, S., Yuan, Z., Yang, H., Xie, R., & Ren, L. (2022). Construction and Tribological Properties of Biomimetic Cartilage-Lubricating Hydrogels. Gels, 8(7), 415. https://doi.org/10.3390/gels8070415








