Thermal Insulation Performance of SiC-Doped Silica Aerogels under Large Temperature and Air Pressure Differences
Abstract
1. Introduction
2. Experiment
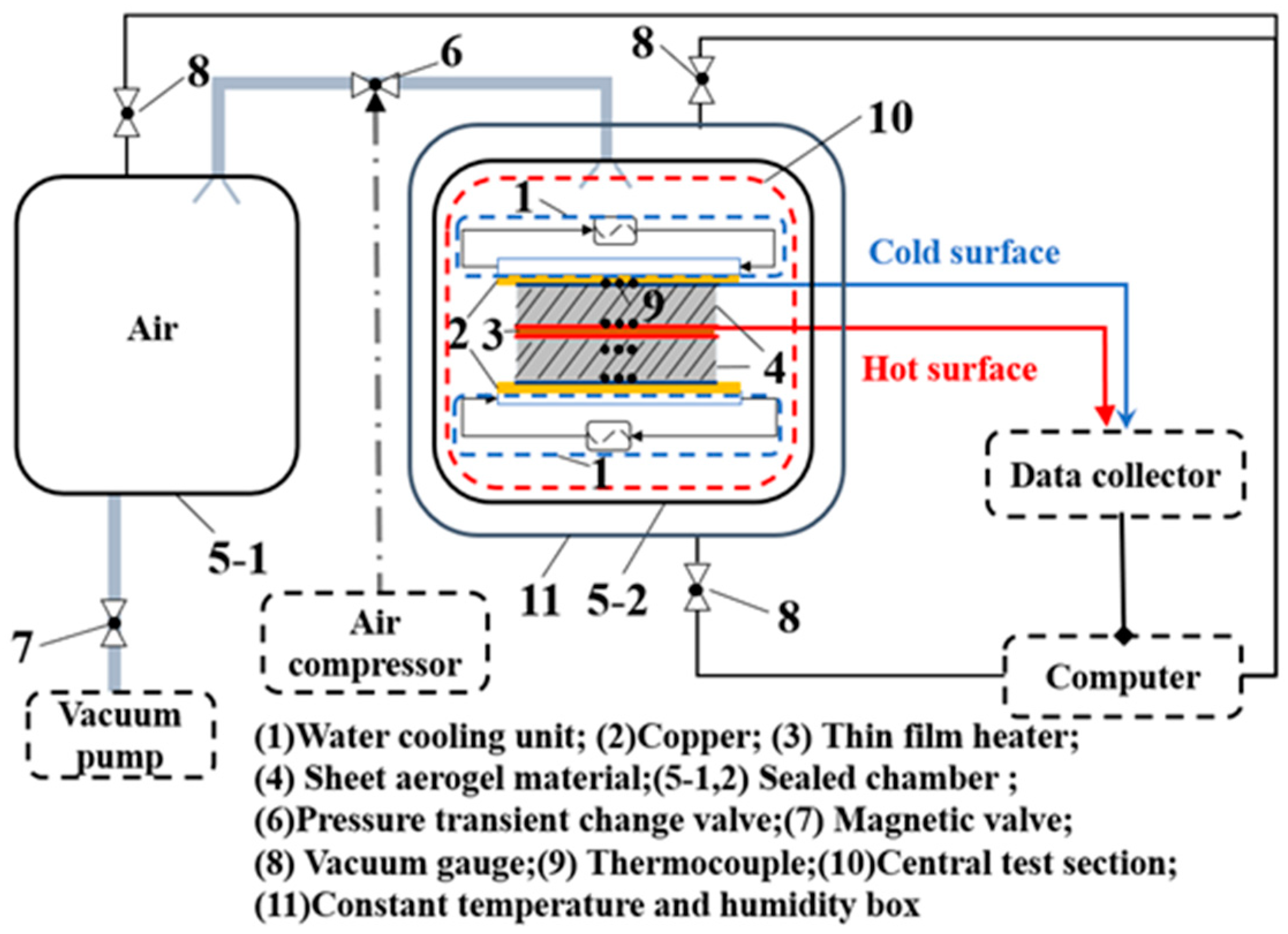
2.1. Experimental System
2.2. Material and Testing Procedure
- (1)
- Dehydrate two identical samples in a drying oven to remove water vapor.
- (2)
- Put the specimens sandwiched with the heater, thermocouples, and cooling units and place the main test section into the sealed chamber, as shown in Figure 1.
- (3)
- Set the initial and ambient temperature identical and run the system.
- (4)
- Turn on the D.C. power supply when the specimen’s heat transfer reaches the initial steady state; change the atmospheric pressure through the pressure transient valve at the first steady state.
- (5)
- Record the experimental data of the whole process by the thermocouples. Shut down the test system after the second steady state.
3. Theoretical Analysis Model
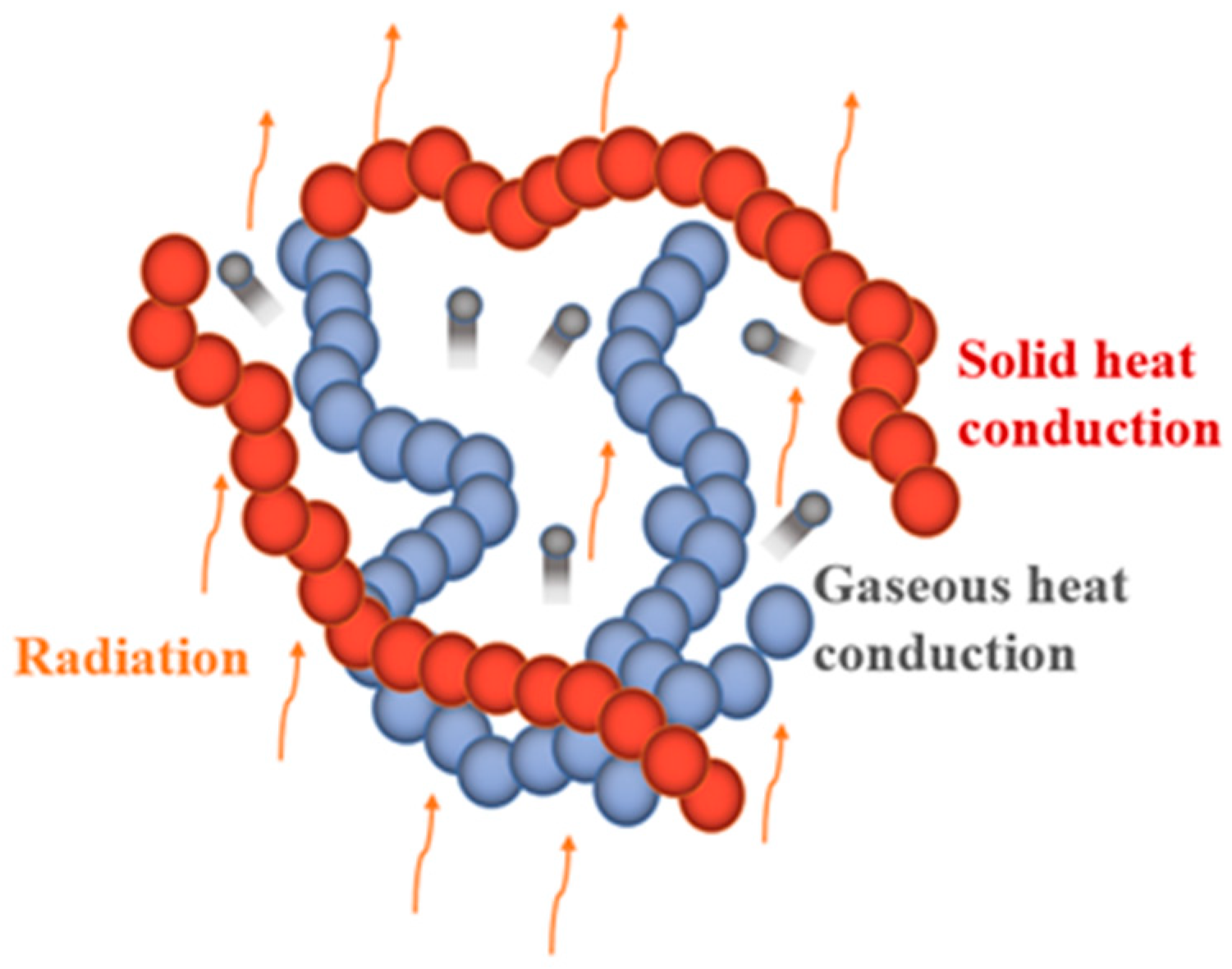
3.1. Thermal Conductivity
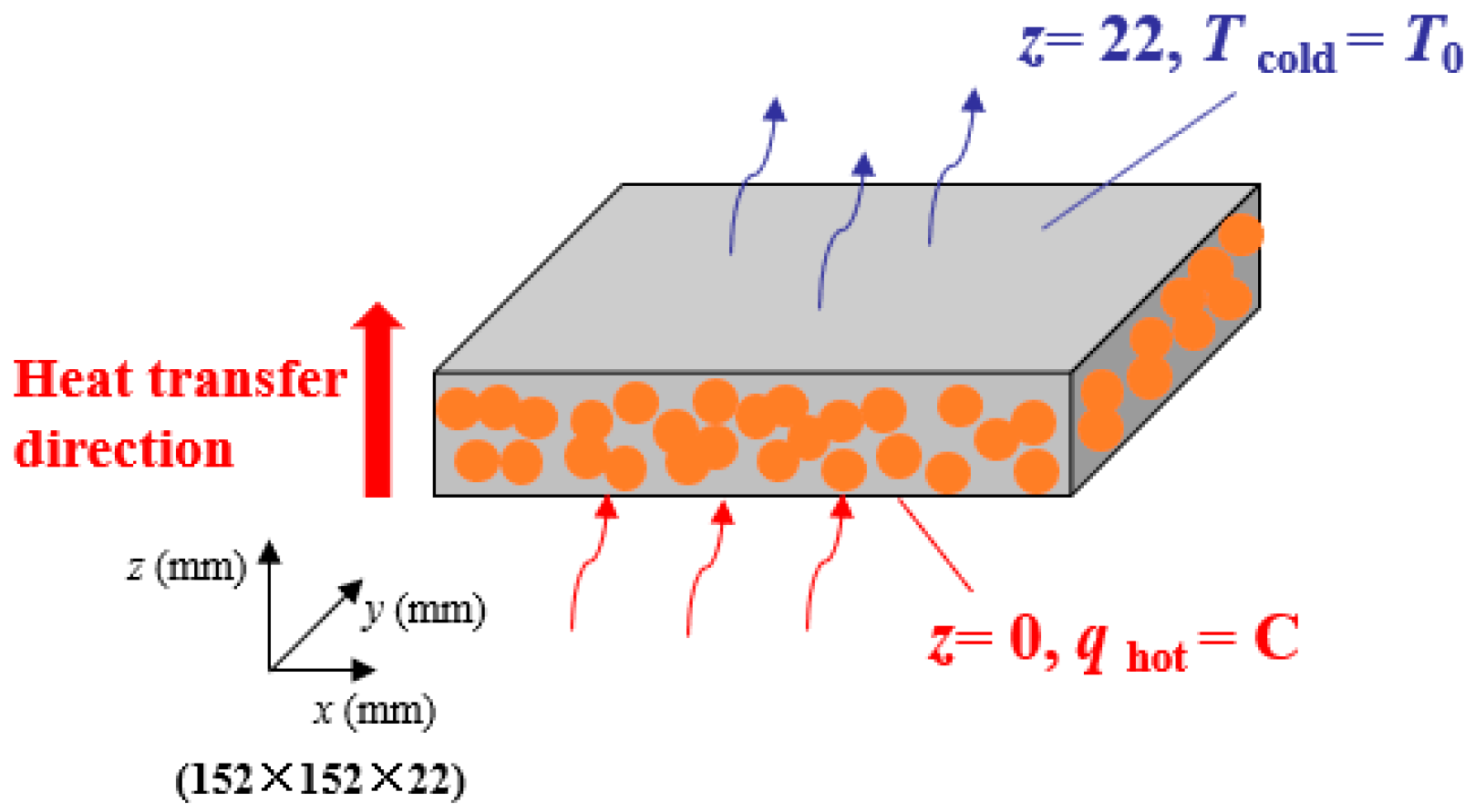
3.2. 3-D Unsteady-State Heat Transfer Model
4. Results and Discussion
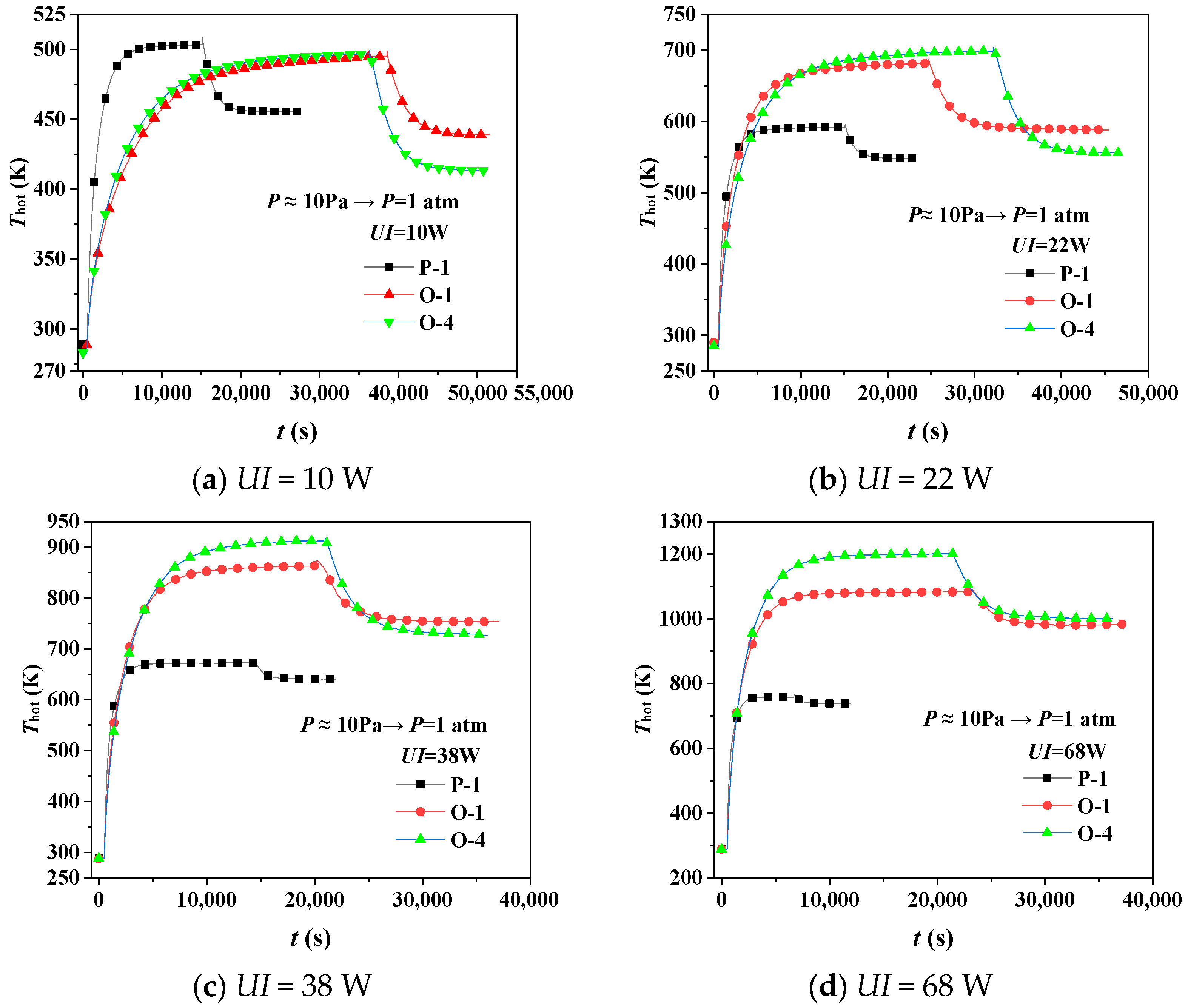
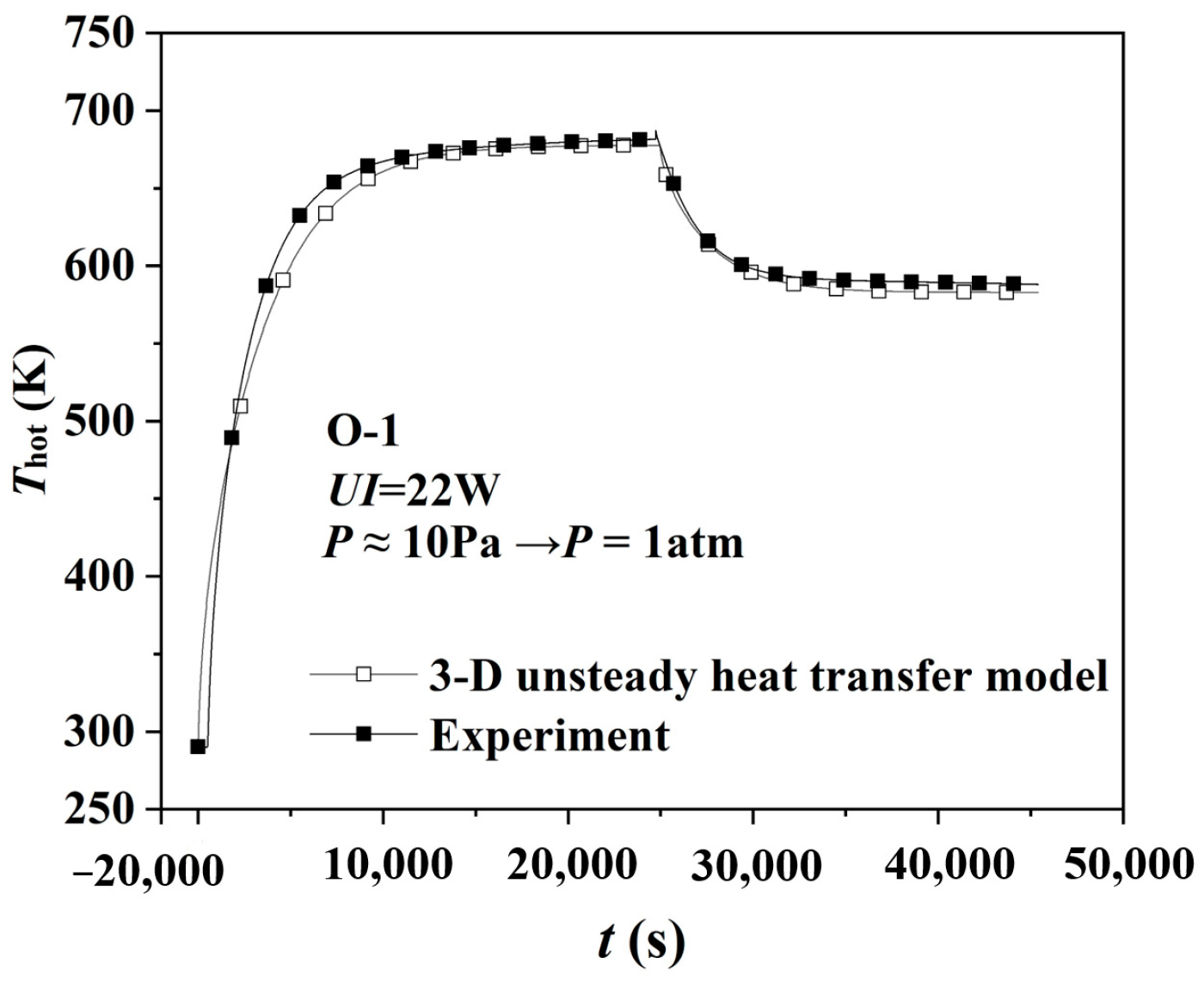
4.1. Thermal Insulation Performance by Experiment
4.2. Correlative Thermal Conductivity of SiC-Doped Silica Aerogel
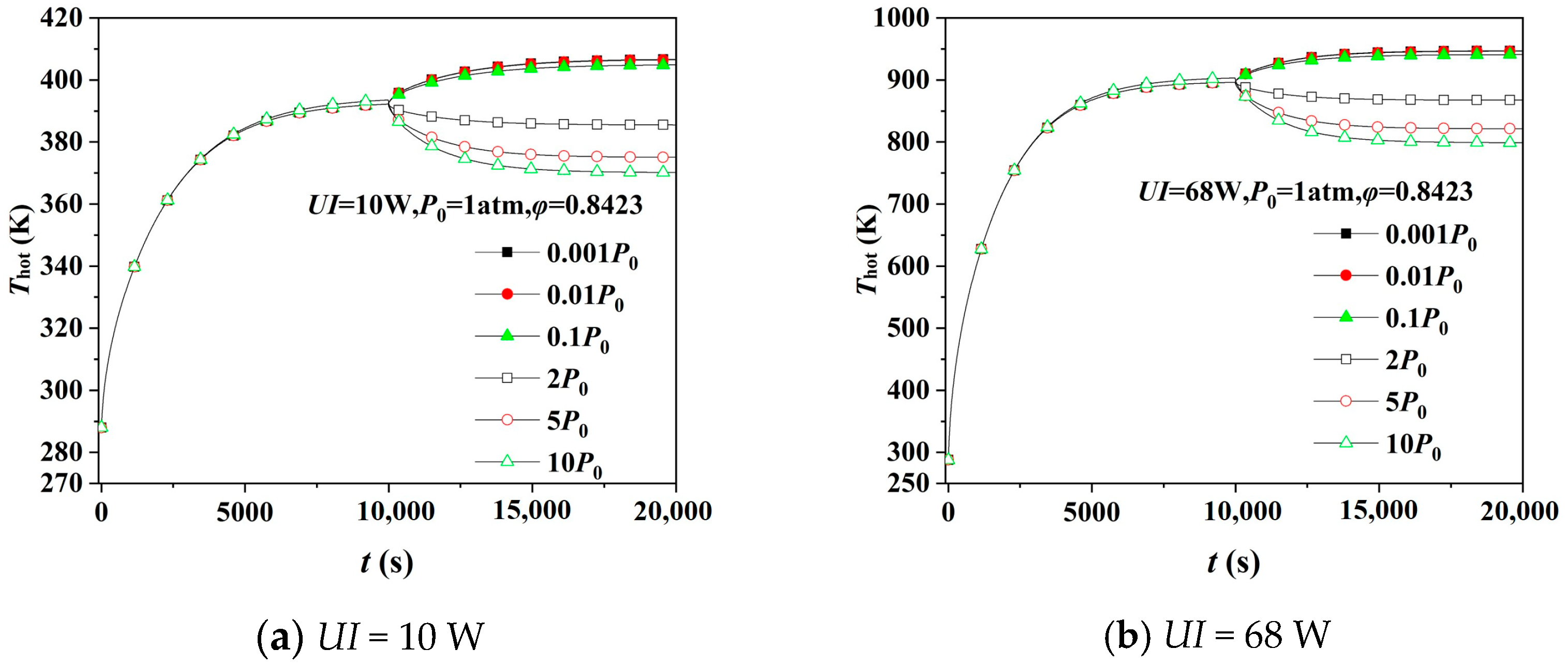
4.3. Hot Surface’s Temperature Variation by Simulation at Transient Pressure Change
4.4. Hot Surface’s Temperature at Different Porosity
5. Conclusions
- (1)
- Transient pressure changes the heat transfer of the gas inside the aerogel, affecting the gaseous thermal conductivity and the thermal insulation performance. When the pressure decreases instantaneously, the hot surface’s temperature increases, and its thermal insulation performance is improved, and vice versa. The more extensive the transient pressure change is, the more significant the thermal insulation performance’s variation will be.
- (2)
- When Tcold = 15 °C and ΔT = 171~912 K, the effective thermal conductivities of SiC-doped silica aerogel with 0, 1, and 5.84% SiC are 0.02220~0.06901, 0.02304~0.04077, and 0.02228~0.03550 W·m−1·K−1 at P ≈ 10 Pa, and 0.02856~0.07207, 0.03165~0.04665, and 0.03653~0.04541 W·m−1·K−1 at P = 1 atm, respectively.
- (3)
- The higher SiC content increases the solid thermal conductivity of the aerogel, but the ability to restrain thermal radiation is more significant at large temperature differences.
- (4)
- Aerogels with greater porosity are strongly influenced by the movement of the internal gas molecules at transient pressure changes. The larger the porosity of the aerogel, the greater the influence of the internal molecular motion, the more noticeable change of the gaseous thermal conductivity, and the more significant change in thermal insulation performance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, F.; Jiang, Y.; Feng, J.; Cai, H.; Feng, J.; Li, L. Thermally insulating, fiber-reinforced alumina–silica aerogel composites with ultra-low shrinkage up to 1500 °C. Chem. Eng. J. 2021, 411, 128402. [Google Scholar] [CrossRef]
- Lee, K.-J.; Lee, J.M.; Nam, K.S.; Hwang, H. Thermal Gelation for Synthesis of Surface-Modified Silica Aerogel Powders. Gels 2021, 7, 242. [Google Scholar] [CrossRef]
- Song, Q.; Miao, C.; Sai, H.; Gu, J.; Wang, M.; Jiang, P.; Wang, Y.; Fu, R.; Wang, Y. Silica-Bacterial Cellulose Composite Aerogel Fibers with Excellent Mechanical Properties from Sodium Silicate Precursor. Gels 2022, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Lun, Z.; Gong, L.; Zhang, Z.; Deng, Y.; Zhou, Y.; Pan, Y.; Cheng, X. Improvement of the Thermal Insulation Performance of Silica Aerogel by Proper Heat Treatment: Microporous Structures Changes and Pyrolysis Mechanism. Gels 2022, 8, 141. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, Y.; Hara, Y.; Sakuma, W.; Saito, T.; Nakanishi, K.; Kanamori, K. Colorless transparent melamine–formaldehyde aerogels for thermal insulation. ACS Appl. Nano Mater. 2020, 3, 49–54. [Google Scholar] [CrossRef]
- Noman, M.T.; Amor, N.; Ali, A.; Petrik, S.; Coufal, R.; Adach, K.; Fijalkowski, M. Aerogels for Biomedical, Energy and Sensing Applications. Gels 2021, 7, 264. [Google Scholar] [CrossRef] [PubMed]
- Çok, S.S.; Gizli, N. Microstructural properties and heat transfer characteristics of in-situ modified silica aerogels prepared with different organosilanes. Int. J. Heat Mass Transf. 2022, 188, 122618. [Google Scholar]
- Han, Y.F.; Li, S.; Liu, H.-D.; Cui, W. Lattice Boltzmann method simulation of the gas heat conduction of nanoporous material. Therm. Sci. 2020, 24, 3749–3756. [Google Scholar] [CrossRef]
- Kistler, S. The relation between heat conductivity and structure in silica aerogel. J. Phys. Chem. 2002, 39, 79–86. [Google Scholar] [CrossRef]
- Zeng, S.; Hunt, A.; Greif, R. Mean free path and apparent thermal conductivity of a gas in a porous medium. J. Heat Transf. 1995, 117, 758–761. [Google Scholar] [CrossRef]
- Zhang, H.; Hong, C.; Qiao, Y. Synthesis, structural and thermal properties of nano-porous SiO2-based aerogels. Adv. Nanocomposites-Synth. Charact. Ind. Appl. 2011, 40–60. [Google Scholar] [CrossRef][Green Version]
- Pang, H.-Q.; Zhang, R.; Yang, H.-L.; Li, Z.-Y.; Xu, H.-B. Preparation and thermal insulation performance characterization of endothermic opacifier doped silica aerogel. Int. J. Therm. Sci. 2022, 174, 107431. [Google Scholar] [CrossRef]
- Balamurugan, S.; Naresh, N.; Prakash, I.; Satyanarayana, N. Capacity fading mechanism of Li2O loaded NiFe2O4/SiO2 aerogel anode for lithium-ion battery: Ex-situ XPS analysis. Appl. Surf. Sci. 2021, 535, 147677. [Google Scholar] [CrossRef]
- He, Y.-L.; Xie, T. Advances of thermal conductivity models of nanoscale silica aerogel insulation material. Appl. Therm. Eng. 2015, 81, 28–50. [Google Scholar] [CrossRef]
- Liu, L.; Shan, X.; Hu, X.; Lv, W.; Wang, J. Superhydrophobic Silica Aerogels and Their Layer-by-Layer Structure for Thermal Management in Harsh Cold and Hot Environments. ACS Nano 2021, 15, 19771–19782. [Google Scholar] [CrossRef] [PubMed]
- Hebalkar, N.; Kollipara, K.S.; Ananthan, Y.; Sudha, M.K. Nanoporous aerogels for defense and aerospace applications. In Handbook of Advanced Ceramics and Composites: Defense, Security, Aerospace and Energy Applications; Springer: Cham, Switzerland, 2020; pp. 121–163. [Google Scholar]
- Zeng, S.; Hunt, A.; Greif, R. Transport properties of gas in silica aerogel. J. Non-Cryst. Solids 1995, 186, 264–270. [Google Scholar] [CrossRef]
- Spagnol, S.; Lartigue, B.; Trombe, A.; Despetis, F. Experimental investigations on the thermal conductivity of silica aerogels by a guarded thin-film-heater method. J. Heat Transf. 2009, 131, 074501. [Google Scholar] [CrossRef]
- Zhang, H.; Fang, W.; Li, Z.; Tao, W. The influence of gaseous heat conduction to the effective thermal conductivity of nano-porous materials. Int. Commun. Heat Mass Transf. 2015, 68, 158–161. [Google Scholar] [CrossRef]
- ASTM Committee C-16 on Thermal Insulation. Standard Test Method for Steady-State Heat Flux Measurements and Thermal Transmission Properties by Means of the Guarded-Hot-Plate Apparatus; Designation: C177–13; ASTM International: West Conshohocken, PA, USA, 2004. [Google Scholar]
- G. 329-87, Test Method for Steady-State Thermal Conductivity of Heat Insulating Materials over Moderate Temperature; China Military Standards Press: Beijing, China, 1987.
- Yildirim, G.; Genç, S. Experimental study on heat transfer of the magnetorheological fluids. Smart Mater. Struct. 2013, 22, 085001. [Google Scholar] [CrossRef]
- Zhu, D.; Miller, R.A.; Nagaraj, B.A.; Bruce, R.W. Thermal conductivity of EB-PVD thermal barrier coatings evaluated by a steady-state laser heat flux technique. Surf. Coat. Technol. 2001, 138, 1–8. [Google Scholar] [CrossRef]
- Liu, H.; Xia, X.; Ai, Q.; Xie, X.; Sun, C. Experimental investigations on temperature-dependent effective thermal conductivity of nanoporous silica aerogel composite. Exp. Therm. Fluid Sci. 2017, 84, 67–77. [Google Scholar] [CrossRef]
- Hao-Qiang, P.; Zeng-Yao, L. Experimental investigations on the thermal insulation performance of SiC opacifier doped silica aerogel at large temperature difference. Int. J. Therm. Sci. 2021, 160, 106681. [Google Scholar] [CrossRef]
- Noroozi, M.; Panahi-Sarmad, M.; Bahramian, A.R.; Sharif, A. Theoretical investigation of heat transfer in structurally graded silica aerogels with pores diameter changing. J. Therm. Anal. Calorim. 2019, 135, 1713–1721. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, H.; Zhao, J.; Liu, J.; Xia, X.; Wu, X. Thermal conductivity of micro/nano-porous polymers: Prediction models and applications. Front. Phys. 2022, 17, 23202. [Google Scholar] [CrossRef]
- Xie, T.; He, Y.-L.; Hu, Z.-J. Theoretical study on thermal conductivities of silica aerogel composite insulating material. Int. J. Heat Mass Transf. 2013, 58, 540–552. [Google Scholar] [CrossRef]
- Wei, G.; Liu, Y.; Zhang, X.; Yu, F.; Du, X. Thermal conductivities study on silica aerogel and its composite insulation materials. Int. J. Heat Mass Transf. 2011, 54, 2355–2366. [Google Scholar] [CrossRef]
- Zhu, C.-Y.; Li, Z.-Y. Modeling of the apparent solid thermal conductivity of aerogel. Int. J. Heat Mass Transf. 2018, 120, 724–730. [Google Scholar] [CrossRef]
- Warrier, P.; Yuan, Y.; Beck, M.P.; Teja, A.S. Heat transfer in nanoparticle suspensions: Modeling the thermal conductivity of nanofluids. AlChE J. 2010, 56, 3243–3256. [Google Scholar] [CrossRef]
- Dai, Y.-J.; Tang, Y.-Q.; Fang, W.-Z.; Zhang, H.; Tao, W.-Q. A theoretical model for the effective thermal conductivity of silica aerogel composites. Appl. Therm. Eng. 2018, 128, 1634–1645. [Google Scholar] [CrossRef]
- Wan, P.; Wang, J. Highly porous nano-SiC with very low thermal conductivity and excellent high temperature behavior. J. Eur. Ceram. Soc. 2018, 38, 463–467. [Google Scholar] [CrossRef]
- Qu, Z.; Fu, Y.; Liu, Y.; Zhou, L. Approach for predicting effective thermal conductivity of aerogel materials through a modified lattice Boltzmann method. Appl. Therm. Eng. 2018, 132, 730–739. [Google Scholar] [CrossRef]
- Zhao, J.-J.; Duan, Y.-Y.; Wang, X.-D.; Zhang, X.-R.; Han, Y.-H.; Gao, Y.-B.; Lv, Z.-H.; Yu, H.-T.; Wang, B.-X. Optical and radiative properties of infrared opacifier particles loaded in silica aerogels for high temperature thermal insulation. Int. J. Therm. Sci. 2013, 70, 54–64. [Google Scholar] [CrossRef]
- Zhao, J.-J.; Duan, Y.-Y.; Wang, X.-D.; Wang, B.-X. Radiative properties and heat transfer characteristics of fiber-loaded silica aerogel composites for thermal insulation. Int. J. Heat Mass Transf. 2012, 55, 5196–5204. [Google Scholar] [CrossRef]
- Yang, J.; He, F.; Fan, Y.; Hu, Z.; Li, J. Measurement and estimate of thermophysical parameters of SiO2 aerogel. Aerosp Mater Technol 2013, 2, 92. [Google Scholar]
- Howell, J.R.; Mengüç, M.P.; Daun, K.; Siegel, R. Thermal Radiation Heat Transfer; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Tao, W.Q. Numerical Heat Transfer; Xi’an Jiaotong University Press: Xi’an, China, 2001. [Google Scholar]

| No. | Matrix Silica Aerogel (fv, %) | SiC (fv, %) | Porosity (φ, %) | Density (ρ, kg·m−3) |
|---|---|---|---|---|
| P−1 | 99.49 | 0 | 89.87 | 346.39 |
| O−1 | 98.49 | 1 | 84.23 | 354.06 |
| O−4 | 93.65 | 5.84 | 88.57 | 387.14 |
| P/W | Correlative Thermal Conductivity/(W·m−1·K−1) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P−1 | O−1 | O−4 | ||||||||||
| ΔT /K | Steady State 1st | ΔT /K | Steady State 2nd | ΔT /K | Steady State 1st | ΔT /K | Steady State 2nd | Δ /K | Steady State 1st | ΔT /K | Steady State 2nd | |
| 10 | 214.33 | 0.02220 | 455.57 | 0.02856 | 171.09 | 0.02304 | 150.92 | 0.03165 | 208.54 | 0.02223 | 125.46 | 0.03653 |
| 22 | 303.92 | 0.03459 | 548.40 | 0.04040 | 398.53 | 0.02643 | 300.13 | 0.03515 | 410.81 | 0.02532 | 267.97 | 0.03867 |
| 38 | 384.53 | 0.04726 | 640.51 | 0.05158 | 585.01 | 0.03091 | 465.75 | 0.03882 | 624.01 | 0.02901 | 438.56 | 0.04129 |
| 68 | 470.12 | 0.06901 | 738.23 | 0.07207 | 759.16 | 0.04077 | 695.02 | 0.04665 | 912.37 | 0.03550 | 713.30 | 0.04541 |
| Pressure/(atm) | P0 | 0.1P0 | 0.01P0 | 0.001P0 | 2P0 | 5P0 | 10P0 |
| /(W·m−1·K−1) | 0.05361 | 0.04958 | 0.04916 | 0.04911 | 0.05587 | 0.06069 | 0.06336 |
| Change rate/(%) | / | −7.52 | −8.30 | −8.39 | +4.22 | +13.21 | +18.19 |
| Porosity | P0 | 10P0 | 0.01P0 | ||
|---|---|---|---|---|---|
| /(W·m−1·K−1) | /(W·m−1·K−1) | Change Rate/(%) | /(W·m−1·K−1) | Change Rate/(%) | |
| 0.7 | 0.05691 | 0.07575 | +33.10 | 0.04863 | −14.55 |
| 0.8 | 0.05402 | 0.08072 | +49.43 | 0.04687 | −13.24 |
| 0.9 | 0.04951 | 0.08670 | +75.12 | 0.04518 | −8.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.-N.; Pang, H.-Q.; Fan, T.-H.; Ye, Q.; Cai, Q.-L.; Wu, X. Thermal Insulation Performance of SiC-Doped Silica Aerogels under Large Temperature and Air Pressure Differences. Gels 2022, 8, 320. https://doi.org/10.3390/gels8050320
Zhang S-N, Pang H-Q, Fan T-H, Ye Q, Cai Q-L, Wu X. Thermal Insulation Performance of SiC-Doped Silica Aerogels under Large Temperature and Air Pressure Differences. Gels. 2022; 8(5):320. https://doi.org/10.3390/gels8050320
Chicago/Turabian StyleZhang, Sheng-Nan, Hao-Qiang Pang, Ting-Hui Fan, Qing Ye, Qi-Lin Cai, and Xi Wu. 2022. "Thermal Insulation Performance of SiC-Doped Silica Aerogels under Large Temperature and Air Pressure Differences" Gels 8, no. 5: 320. https://doi.org/10.3390/gels8050320
APA StyleZhang, S.-N., Pang, H.-Q., Fan, T.-H., Ye, Q., Cai, Q.-L., & Wu, X. (2022). Thermal Insulation Performance of SiC-Doped Silica Aerogels under Large Temperature and Air Pressure Differences. Gels, 8(5), 320. https://doi.org/10.3390/gels8050320






