Temperature- and Salt-Resistant Micro-Crosslinked Polyampholyte Gel as Fluid-Loss Additive for Water-Based Drilling Fluids
Abstract
:1. Introduction
2. Results and Discussion
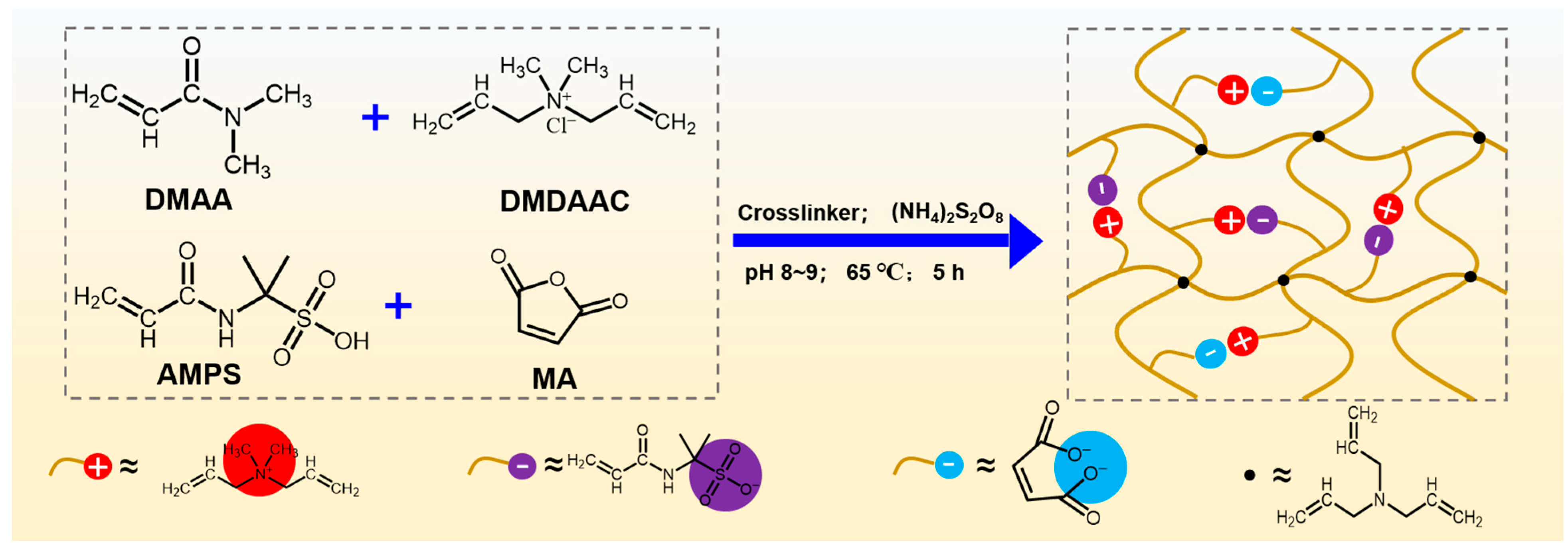
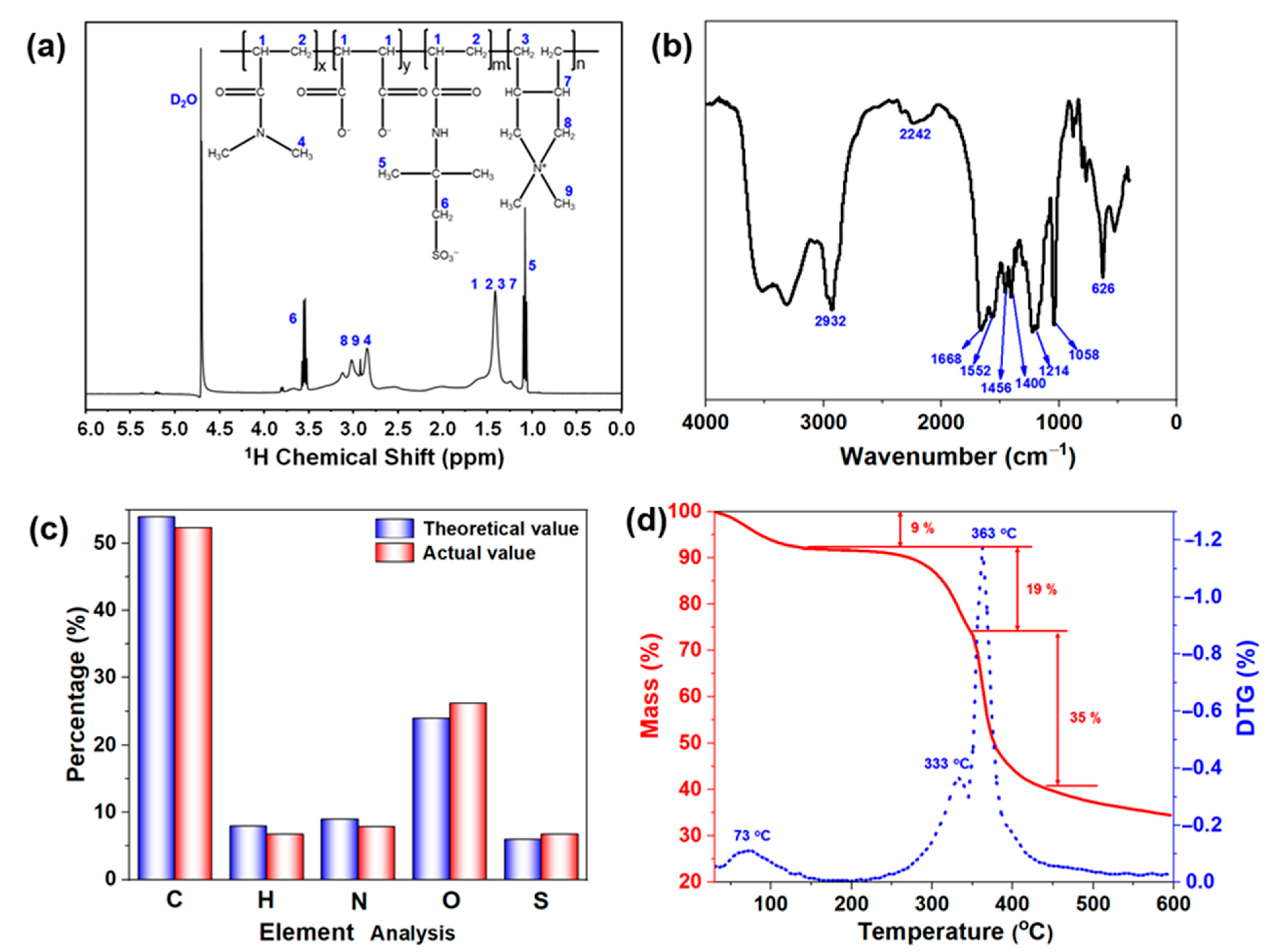
2.1. Characterization of DDAM
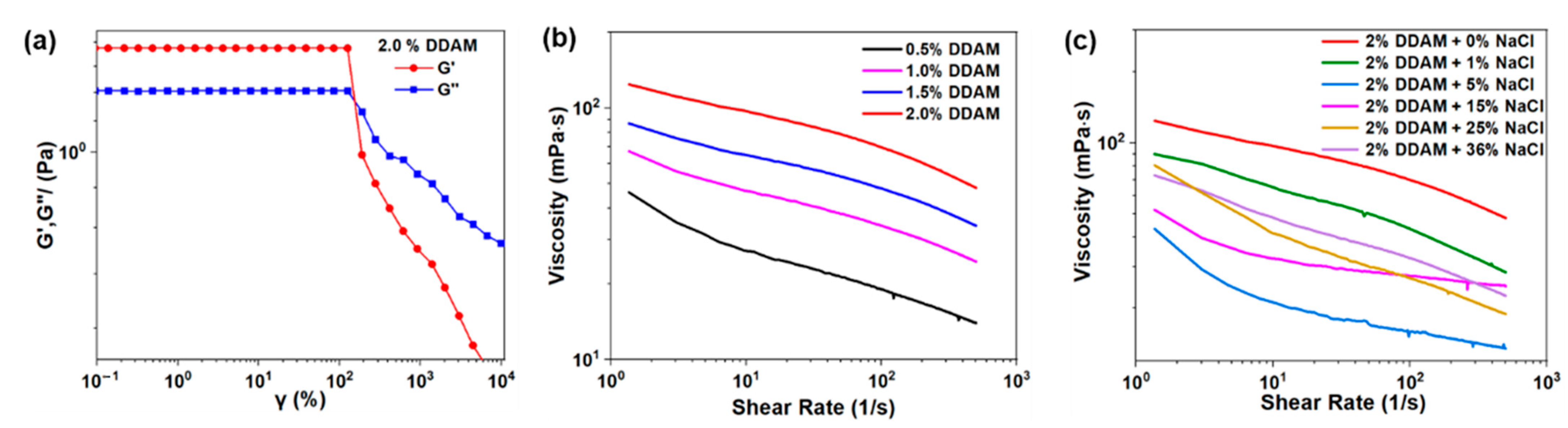
2.2. Rheological Properties of DDAM Solutions and DDAM-Based Drilling Fluid
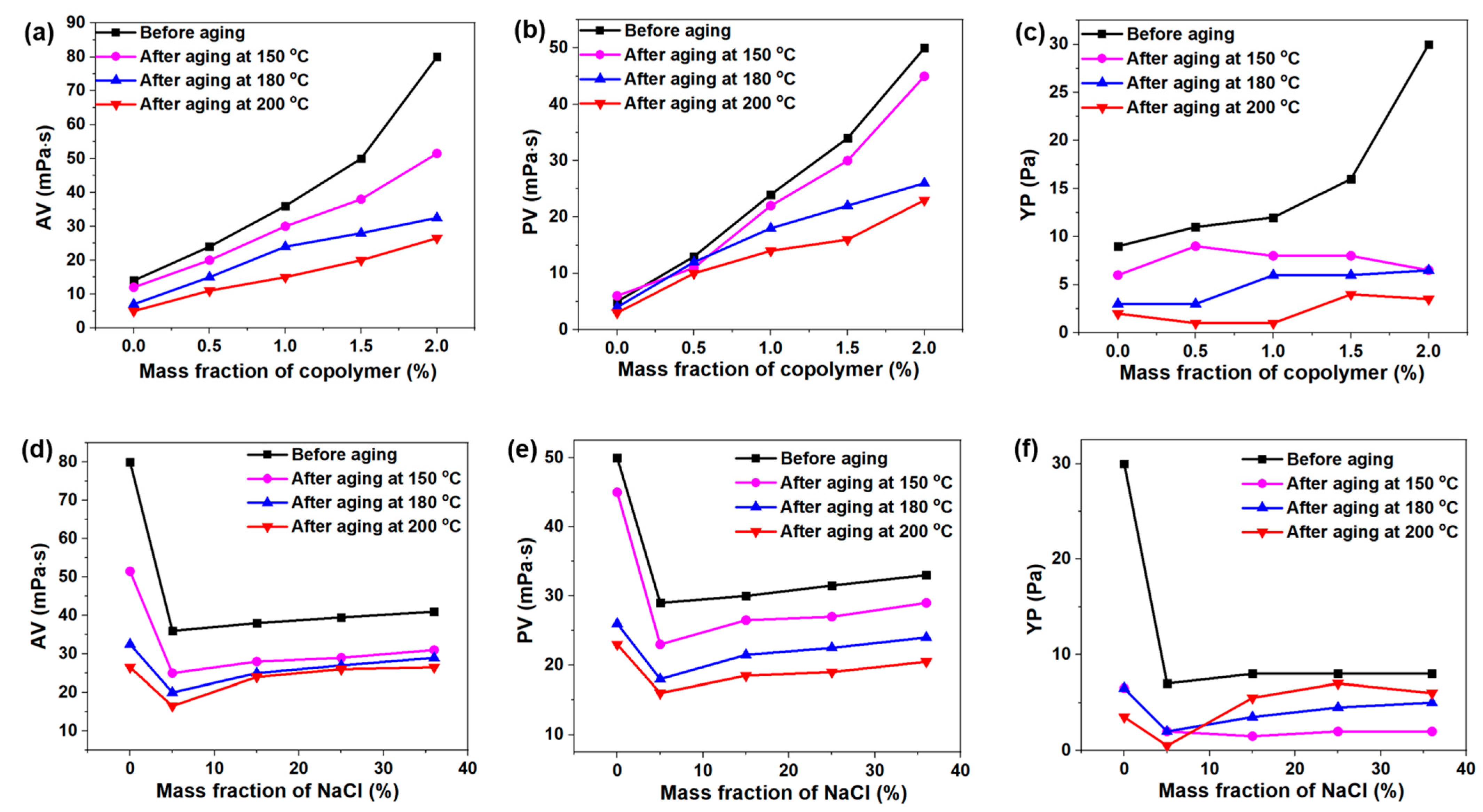
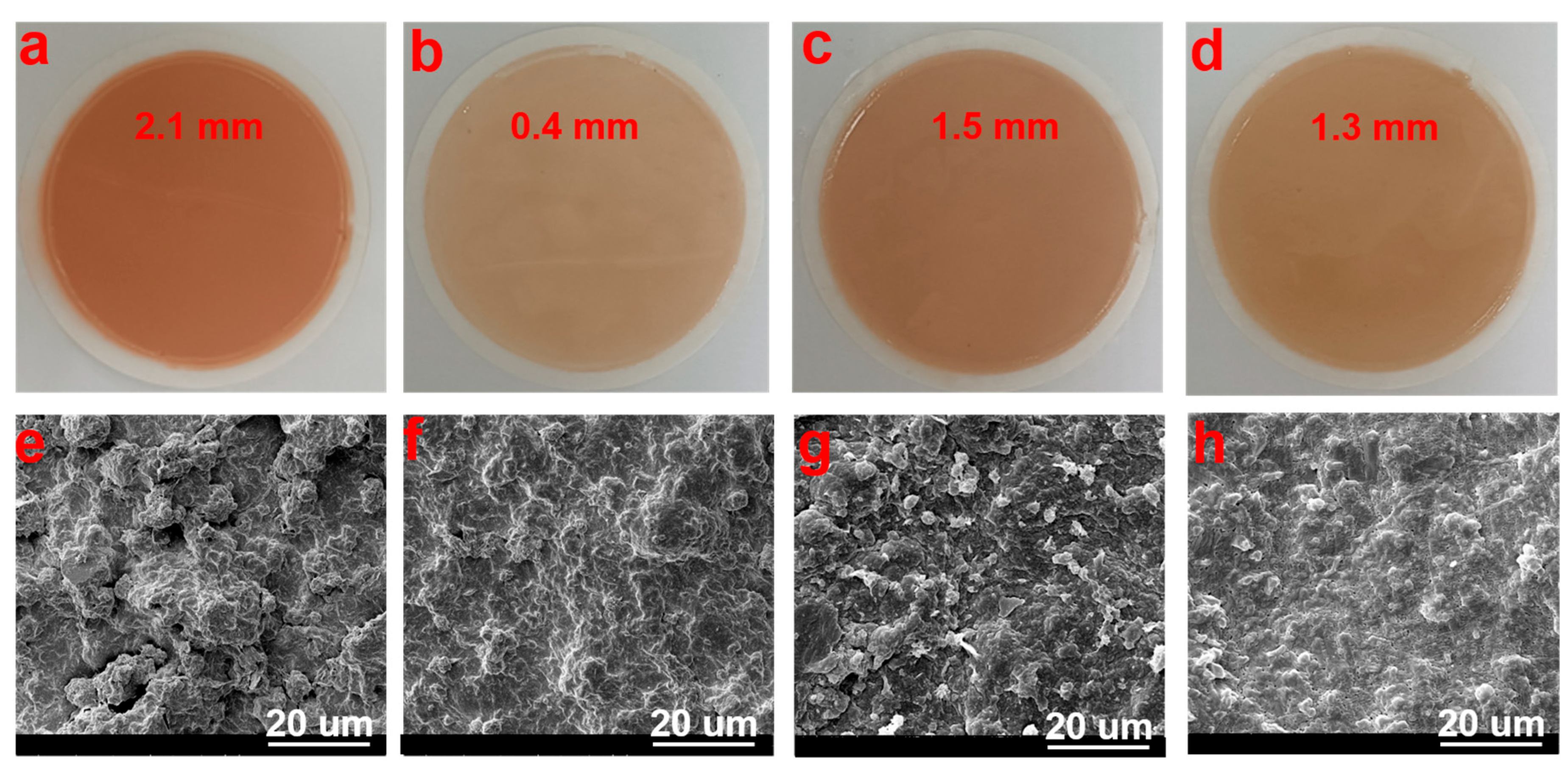
2.3. Fluid Loss Analysis of DDAM-Based Drilling Fluid
2.4. Mechanistic Analysis
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of DDAM
4.3. Methods
4.3.1. DDAM Characterization
4.3.2. Preparation of Water-Based Drilling Fluids (WDFs)
4.3.3. American Petroleum Institute (API) Fluid-Loss Test
4.3.4. Rheology Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, J.; Zhang, X.; Lv, K.; Liu, J.; Xiu, Z.; Wang, Z.; Huang, X.; Bai, Y.; Wang, J.; Jin, J. Synthesis of hydrophobic associative polymers to improve the rheological and filtration performance of drilling fluids under high temperature and high salinity conditions. J. Pet. Sci. Eng. 2022, 209, 109808. [Google Scholar] [CrossRef]
- Jiang, G.; Sun, J.; He, Y.; Cui, K.; Dong, T.; Yang, L.; Yang, X.; Wang, X. Novel water-based drilling and completion fluid technology to improve wellbore quality during drilling and protect unconventional reservoirs. Engineering 2021, accepted in press. [Google Scholar] [CrossRef]
- Huang, X.; Shen, H.; Sun, J.; Lv, K.; Liu, J.; Dong, X.; Luo, S. Nano laponite as a potential shale inhibitor in water based drilling fluid for stabilizing wellbore stability and mechanism study. ACS Appl. Mater. Interfaces 2018, 10, 33252–33259. [Google Scholar] [CrossRef]
- Pakdamana, E.; Osfouria, S.; Azinb, R.; Niknamc, H.; Roohid, A. Improving the rheology, lubricity, and differential sticking properties of water-based drilling muds at high temperatures using hydrophilic gilsonite nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2019, 582, 123930. [Google Scholar] [CrossRef]
- Kamali, F.; Saboori, R.; Sabbaghi, S. Fe3O4-CMC nanocomposite performance evaluation as rheology modifier and fluid loss control characteristic additives in water-based drilling fluid. J. Pet. Sci. Eng. 2021, 205, 108912. [Google Scholar] [CrossRef]
- Li, M.; Ren, S.; Zhang, X.; Liu, J.; Dong, L.; Lei, T.; Lee, S.; Wu, Q. Surface-chemistry-tuned cellulose nanocrystals in a bentonite suspension for water-based drilling fluids. ACS Appl. Nano Mater. 2018, 1, 7039–7051. [Google Scholar] [CrossRef]
- Balding, P.; Li, M.; Wu, Q.; Volkovinsky, R.; Russo, P. Cellulose nanocrystal-polyelectrolyte hybrids for bentonite water based drilling fluids. ACS Appl. Bio Mater. 2020, 3, 3015–3027. [Google Scholar] [CrossRef]
- Ahmad, H.; Kamal, M.; Al-Harthi, M. High molecular weight copolymers as rheology modifier and fluid loss additive for water-based drilling fluids. J. Mol. Liq. 2018, 252, 133–143. [Google Scholar] [CrossRef]
- Cao, J.; Meng, L.; Yang, Y.; Zhu, Y.; Wang, X.; Yao, C.; Sun, M.; Zhong, H. Novel acrylamide/2-acrylamide-2-methylpropanesulfonic acid/4vinylpyridine terpolymer as an anti-calcium contamination fluid loss additive for water-based drilling fluids. Energy Fuels 2017, 31, 11963–11970. [Google Scholar] [CrossRef]
- Jia, X.; Zhao, X.; Chen, B.; Egwu, S.; Huang, Z. Polyanionic cellulose/hydrophilic monomer copolymer grafted silica nanocomposites as HTHP drilling fluid-loss control agent for water-based drilling fluids. Appl. Surf. Sci. 2022, 578, 152089. [Google Scholar] [CrossRef]
- Sun, J.; Bai, Y.; Cheng, R.; Lyu, K.; Liu, F.; Feng, J.; Lei, S.; Zhang, J.; Hao, H. Research progress and prospect of plugging technologies for fractured formation with severe lost circulation. Pet. Explor. Dev. 2021, 48, 732–743. [Google Scholar] [CrossRef]
- Liu, F.; Jiang, G.; Peng, S.; He, Y.; Wang, J. Amphoteric polymer as an anti-calcium contamination fluid-loss additive in water-based drilling fluids. Energy Fuels 2016, 30, 7221–7228. [Google Scholar] [CrossRef]
- Tan, X.; Duan, L.; Han, W.; Li, Y.; Guo, M. A zwitterionic copolymer as rheology modifier and fluid loss agents for water-based drilling fluids. Polymers 2021, 13, 3120. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Jiang, G.; Yang, J.; Yang, L.; Li, X.; He, Y.; Chang, X. Novel N, N-dimethylacrylamide copolymer containing multiple rigid comonomers as a filtrate reducer in water-based drilling fluids and mechanism study. J. Appl. Polym. Sci. 2021, 138, e51001. [Google Scholar] [CrossRef]
- Zhu, W.; Zheng, X.; Shi, J.; Wang, Y. A high-temperature resistant colloid gas aphron drilling fluid system prepared by using a novel graft copolymer xanthan gum-AA/AM/AMPS. J. Pet. Sci. Eng. 2021, 205, 108821. [Google Scholar] [CrossRef]
- Zhu, w.; Zheng, X. Effective modified xanthan gum fluid loss agent for high temperature water-based drilling fluid and the filtration control mechanism. ACS Omega 2021, 6, 23788–23801. [Google Scholar] [CrossRef]
- Huo, J.; Peng, Z.; Ye, Z.; Feng, Q.; Zheng, Y.; Zhang, J.; Liu, X. Investigation of synthesized polymer on the rheological and filtration performance of water-based drilling fluid system. J. Petrol. Sci. Eng. 2018, 165, 655–663. [Google Scholar] [CrossRef]
- Aghdam, S.B.; Moslemizadeh, A.; Kowsari, E.; Asghari, N. Synthesis and performance evaluation of a novel polymeric fluid loss controller in water-based drilling fluids: High-temperature and high-salinity conditions. J. Nat. Gas Sci. Eng. 2020, 83, 103576. [Google Scholar] [CrossRef]
- Huang, W.; Leong, Y.K.; Chen, T.; Au, P.I.; Liu, X.; Qiu, Z. Surface chemistry and rheological properties of API bentonite drilling fluid: pH effect, yield stress, zeta potential and ageing behaviour. J. Petrol. Sci. Eng. 2016, 146, 561–569. [Google Scholar] [CrossRef] [Green Version]
- Mao, H.; Qiu, Z.; Shen, Z.; Huang, W. Hydrophobic associated polymer based silica nanoparticles composite with core-shell structure as a filtrate reducer for drilling fluid at utra-high temperature. J. Petrol. Sci. Eng. 2015, 129, 1–14. [Google Scholar] [CrossRef]
- Li, M.; Tang, Z.; Liu, C.; Huang, R.; Koo, M.; Zhou, G.; Wu, Q. Water-redispersible cellulose nanofiber and polyanionic cellulose hybrids for high-performance water-based drilling fluids. Ind. Eng. Chem. Res. 2020, 59, 14352–14363. [Google Scholar] [CrossRef]
- Zhong, H.; Kong, X.; Chen, S.; Grady, B.P.; Qiu, Z. Preparation, characterization and filtration control properties of crosslinked starch nano spheres in water-based drilling fluids. J. Mol. Liq. 2021, 325, 115221. [Google Scholar] [CrossRef]
- Guo, W.; Peng, B. Synthesis and characterization of starch-graft-polyacrylamide copolymers and their application as filtration control agents in drilling fluid. J. Vinyl Addit. Technol. 2012, 18, 261–266. [Google Scholar] [CrossRef]
- Sun, J.; Chang, X.; Lv, K.; Wang, J.; Zhang, F.; Jin, J.; Zhou, X.; Dai, Z. Environmentally friendly and salt-responsive polymer brush based on lignin nanoparticle as fluid-loss additive in water-based drilling fluids. Colloids Surf. A Physicochem. Eng. Asp. 2021, 621, 126482. [Google Scholar] [CrossRef]
- Li, M.; Wu, Q.; Han, J.; Mei, C.; Lei, T.; Lee, S.; Gwon, J. Overcoming salt contamination of bentonite water-based drilling fluids with blended dual functionalized cellulose nanocrystals. ACS Sustain. Chem. Eng. 2020, 8, 11569–11578. [Google Scholar] [CrossRef]
- Jiang, G.; He, Y.; Cui, W.; Yang, L.; Ye, C. A saturated saltwater drilling fluid based on salt-responsive polyampholytes. Petrol. Explor. Dev. 2019, 46, 401–406. [Google Scholar] [CrossRef]
- Liu, J.; Dai, Z.; Xu, K.; Yang, Y.; Lv, K.; Huang, X.; Sun, J. Water-based drilling fluid containing bentonite/poly(sodium 4-styrenesulfonate) composite for ultra high temperature ultra deep drilling and its field performance. SPE J. 2020, 25, 1193–1203. [Google Scholar] [CrossRef]
- Bai, X.; Yang, Y.; Xiao, D.; Pu, X.; Wang, X. Synthesis, characterization, and performance evaluation of the AM/AMPS/DMDAAC/SSS quadripolymer as a fluid loss additive for water-based drilling fluid. J. Appl. Polym. Sci. 2015, 132, 41762. [Google Scholar] [CrossRef]
- Ma, J.; Cui, P.; Zhao, L.; Huang, R. Synthesis and solution behavior of hydrophobic association water-soluble polymers containing arylalkyl group. Eur. Polym. J. 2002, 38, 1627–1633. [Google Scholar] [CrossRef]
- Xie, B.; Liu, X. Thermo-thickening behavior of LCST-based copolymer viscosifier for water-based drilling fluids. J. Petrol. Sci. Eng. 2017, 154, 244–251. [Google Scholar] [CrossRef]
- Sun, J.; Chang, X.; Zhang, F.; Bai, Y.; Lv, K.; Wang, J.; Zhou, X.; Wang, B. Salt-responsive zwitterionic polymer brush based on modified silica nanoparticles as a fluid-loss additive in water-based drilling fluids. Energy Fuels 2020, 34, 1669–1679. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Sun, J.; Lv, K.; Ji, Y.; Liu, J.; Huang, X.; Bai, Y.; Wang, J.; Jin, J.; Shi, S. Temperature- and Salt-Resistant Micro-Crosslinked Polyampholyte Gel as Fluid-Loss Additive for Water-Based Drilling Fluids. Gels 2022, 8, 289. https://doi.org/10.3390/gels8050289
Li J, Sun J, Lv K, Ji Y, Liu J, Huang X, Bai Y, Wang J, Jin J, Shi S. Temperature- and Salt-Resistant Micro-Crosslinked Polyampholyte Gel as Fluid-Loss Additive for Water-Based Drilling Fluids. Gels. 2022; 8(5):289. https://doi.org/10.3390/gels8050289
Chicago/Turabian StyleLi, Jian, Jinsheng Sun, Kaihe Lv, Yuxi Ji, Jingping Liu, Xianbin Huang, Yingrui Bai, Jintang Wang, Jiafeng Jin, and Shenglong Shi. 2022. "Temperature- and Salt-Resistant Micro-Crosslinked Polyampholyte Gel as Fluid-Loss Additive for Water-Based Drilling Fluids" Gels 8, no. 5: 289. https://doi.org/10.3390/gels8050289
APA StyleLi, J., Sun, J., Lv, K., Ji, Y., Liu, J., Huang, X., Bai, Y., Wang, J., Jin, J., & Shi, S. (2022). Temperature- and Salt-Resistant Micro-Crosslinked Polyampholyte Gel as Fluid-Loss Additive for Water-Based Drilling Fluids. Gels, 8(5), 289. https://doi.org/10.3390/gels8050289








