Application of Sol–Gels for Treatment of Gynaecological Conditions—Physiological Perspectives and Emerging Concepts in Intravaginal Drug Delivery
Abstract
1. Introduction
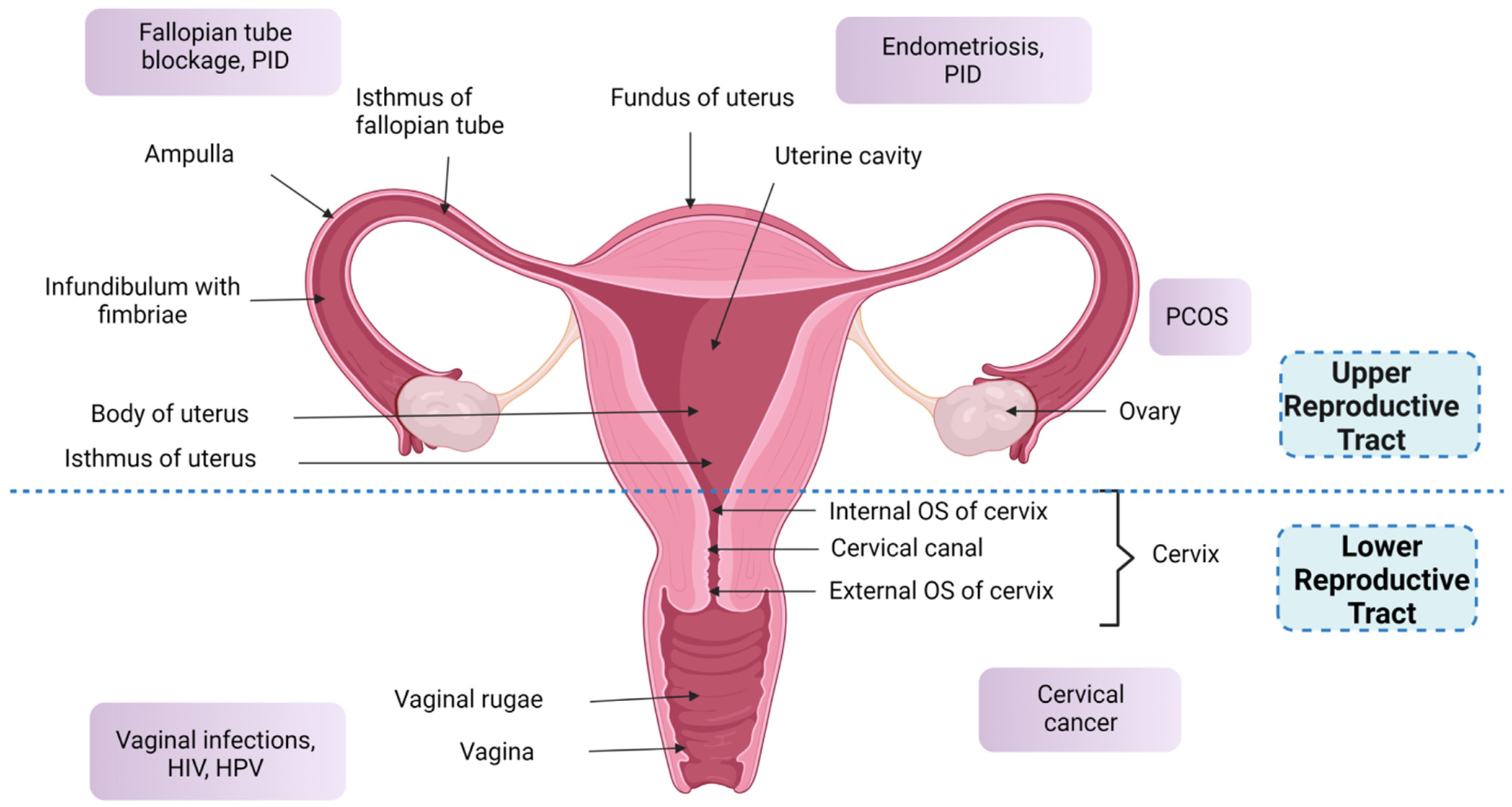
2. Anatomical and Physiological Features of the Female Reproductive Tract
| Site | Dimensions and Features | Functions | Associated Diseases | References | |
|---|---|---|---|---|---|
| Upper reproductive tract | Uterus | 7.5 cm length; 5 cm width; comprises fundus, body, and isthmus; uterine wall comprises endometrium (epithelial cells), myometrium (smooth muscle cells), and perimetrium (connective tissue) | Implants, nourishes, and protects the embryo; receptors for sex steroids present in endometrium | Adenomyosis; Endometriosis; Luteal phase defect; Uterus fibroids | [21,22,23,24] |
| Fallopian tubes | 7–14 cm length; lumen is 0.1–1 mm in uterus–isthmus junction, 1–2 mm in isthmus–ampullary junction, and 1 cm in ampulla–infundibulum junction; comprises isthmus, ampulla, and infundibulum; tube wall composed of endosapinx (innermost mucosal layer), myosalpinx (middle muscular layer), and external serosa | Ciliated cells mobilise gametes and embryo; secretory cells nourish oocyte and embryo | Tubular blockage | [25,26] | |
| Ovaries | 2.5–3.5 cm length; 2 cm width; comprises internal medulla and external cortex consisting of follicles and stroma | Release oocytes, estrogen, and progesterone | Polycystic ovarian syndrome | [26,27] | |
| Lower reproductive tract | Cervix | 3–4 cm length; 2.5 cm width; comprises endocervix (columnar cells) and ectocervix (squamous epithelium cells); 20–60 mL/day of cervical mucus secretion pH 7.0; Mucus composition: 95–99% water, 1–4% enzymes/ proteins/ mucin | Acts as passage for sperm; supports foetus till birth faciliates childbirth; defensive roles against pathogens due to mucin and immunoglobulins | HPV infection; cervical cancer | [28,29,30] |
| Vagina | 7–15 cm length; 2.1–4.5 cm width; daily vaginal secretions ≈6 mL and 0.5–0.75 mL present at any given time;wider near the cervix; variable surface area of 50–600 cm2; transverse folds (rugae) present in vaginal wall; pH 3.8–4.2; vaginal wall comprises 30–100 µm thick mucus layer, layer of epithelium cells, lamina propria, muscular layer, and tunica adventitia | Rugae increases vaginal surface area and causes vaginal extension during coitus and childbirth; passage for menstrual flow and childbirth; acidic pH has defensive role against pathogens | Vaginal infection, atrophy, and lesions | [3,31] |
| Site | Physiological Condition | pH | Wall Thickness (mm) | Mucus | Microbial Content | Microbial Diversity | References | |
|---|---|---|---|---|---|---|---|---|
| Volume or Mass (Daily) | Viscosity | |||||||
| Uterus (Endometrium) | Follicular (proliferative) | 7.22 | 4.0–10.0 | - | - | - | - | [26,32] |
| Ovulatory | 7.35 | 10.0–11.0 | 1.5 mL | - | - | - | [26,32] | |
| Luteal (secretory) | 7.0–7.8 | 4.0–6.0 | - | - | - | - | [23,33,34] | |
| Cervix | Follicular | <7.0 | - | 20–60 mg | High | - | - | [32,35,36] |
| Ovulatory | 7.0 | - | 700 mg | Low | - | - | [35,36,37] | |
| Luteal | <7.0 | - | 20–60 mg | High | - | - | [32,35,38] | |
| Vagina | Pre-puberty | 7.0 | ˂0.15 | - | - | Low | High | [39,40] |
| Follicular | 4.0–6.0 | 0.075–1.0 | 4.14 g | High | High | Low | [6,26,31,41] | |
| Ovulatory | 3.8–4.2 | 0.15–2.0 | 5.88 g | Low | High | Low | [6,31,37,39,40] | |
| Luteal | 3.8–4.2 | ˂0.15 | 4.11 g | High | High | Low | [6,26,31,37] | |
| Menopause | 6.0–7.5 | 0.11–0.15 | <2.94 g | - | Low | High | [31,39,40,42,43] | |
2.1. Upper Reproductive Tract
- Uterus
- Fallopian tubes
- Ovaries
2.2. Lower Reproductive Tract
- Cervix
- Vagina
3. Vaginal Drug Delivery Systems: History and Present Therapeutics
3.1. Drug Absorption from the Lower and Upper Female Reproductive Tract
3.1.1. Physiological Factors Affecting Drug Absorption
3.1.2. Physicochemical Properties of Drug and Excipients Affecting Absorption
3.2. Gynaecological Conditions and Their Management Using Conventional Dosage Forms
| Dosage Form | Formulation Features | Advantages | Disadvantages | Active Agents | References |
|---|---|---|---|---|---|
| Insert/ Tablet/ Capsule/ Pessary | Rod/conical/wedge- shaped, disintegrates/dissolves, releasing drug locally in the vaginal cavity | Ease of administration and retrieval when use is undesirable, fast/slow dissolving, user-friendly, increased drug stability in tablet and capsule formulation, economical | Vaginal leakage, reduced drug residence time, diminished drug stability in pessaries | Oestrogen, Dinoprostone, Clotrimazole mucoadhesive tablets, Lactobacillus plantarum capsule | [37,86] |
| Gel | Formed by chemical bonding or physical entanglement between the polymeric chains | Ease of application, adequate spreading across the vaginal mucosa, and enhanced patient comfort | Poor drug retention | Metronidazole, Dinoprostone | [3,6] |
| Ointment | Drug dissolved in aqueous phase and mixed in oil phase | High acceptability, easy administration | Leakage, multiple administrations required to attain maximum therapeutic benefit | Terameprocol | [14,72,89] |
| Sponge | Solid porous structure with dispersed gas in solid matrix | Ability to load higher drug amount, drug released under the pressure exerted by movements of FRT | Mucosal irritation | Nonoxynol-9 | [3,90] |
| Cream | Biphasic system, dissolved in internal phase and dispersed in external phase | High acceptability, prolonged vaginal drug residence time | Leakage, multiple administrations required to attain maximum therapeutic benefit | Clindamycin | [14,49,72,91,92] |
| Cervical patch | Bilaminar sheet of bioadhesive layer containing drug and backing layer | Reduced drug exposure to surrounding vaginal tissue | Limited rate of drug load | 5-fluorouracil | [59] |
| Vaginal ring | Circular devices with controlled drug release pattern where initial burst release is followed by steady-state drug release | Controlled and sustained drug release profile, reduced exposure of drug to adjacent tissue, no leakage problems, economical, applicator not required, better patient compliance | Uncomfortable, limited rate of drug load and drug molecules, higher drug waste, irregular drug distribution | Clotrimazole, dapivirine | [14,37,49,59,93,94] |
| Vaginal film | Fast/slow-dissolving polymeric film, which dissolves on vaginal mucosa | Good drug retention in vagina, fast/sustained release, no disturbance to normal vaginal microbiome, not messy, compatible with various drugs, better stability of drug | Inconvenience of administration | Dapivirine, itraconazole | [14,95,96] |
- Vaginal infections
- Cervical ripening, labour induction, and childbirth
- Prophylaxis of HIV
- Atrophic vaginitis (AV)
- Pelvic inflammatory disease (PID)
- Endometriosis
- Contraceptives
- Infertility
- Polycystic ovarian syndrome (PCOS) and ovarian cancer (OC)
- Cervical cancer
| Active Drug | Brand Name® | Dosage Form | Indication | Manufacturer | Reference |
|---|---|---|---|---|---|
| Oestradiol | Vagifem | Tablet | Atrophic vaginitis | Novo Nordisk Health Care AG | [131] |
| Dinoprostone | Prostin E2, | Tablet | Cervical ripening and labour induction | Pfizer | [104] |
| Dinoprostone | Cervidil | Insert | Cervical ripening and labour induction | Forest Laboratories | [49] |
| Misoprostol | Misodel | Insert | Labour induction | Ferring Pharmaceuticals | [132] |
| Progesterone | Endometrin | Insert | Assists embryo transplantation | Ferring Pharmaceuticals | [133] |
| Oestradiol | Imvexxy | Inserts | Atrophic vagina | Therapeutics MD | [88] |
| Clotrimazole | Gino-Canesten | Cream | Vulovaginal candidiasis | Bayer | [72] |
| Sertaconazole | Sertopic | Cream | Vulovaginal candidiasis | CPH | [72] |
| Clindamycin | Dalacin V | Cream | Antibacterial | Pfizer | [72] |
| Z. multiflora | Leucorex | Cream | Trichomoniasis | Barijessence | [134] |
| Oestriol | Ovestin | Cream | Oestrogen hormone supplement | Aspen | [72] |
| Etonogestrel/ Ethinyloestradiol | Nuvaring | Ring | Endometriosis, cervical cancer | Organon | [3,24,59,94] |
| Progesterone | Progering | Ring | Release progesterone | Laboratorios Andrómaco | [94] |
| Oestradiol | Estring | Ring | Oestrogen replacement therapy, cervical cancer | Pfizer | [3,59] |
| Nonoxyl-9 | Today | Sponge | Spermicide | Almatica Pharma, Inc. | [3] |
| Progesterone | Crinone | Gel | Assisted reproductive procedures | Merck | [135] |
| Nonoxynol-9 | Vaginal Contraceptive Film | Film | Spermicide | Apothecus | [3] |
| Lactobacilli gasser and Lactobacilli rhamnosus | EcoVag | Capsule | Bacterial vaginosis | HÄLSA Pharma GmbH | [45] |
| Progesterone | Utrogestran | Capsule | Luteal phase support | Laboratories Besins International | [136] |
3.3. Current and Emerging Trends in the Treatment of Gynaecological Conditions
4. Sol–Gel Platform Technology in Vaginal Drug Delivery System
4.1. Features and Use of Vaginal Sol–Gel Formulations
| Indication | API | Drug Form | Stimuli-Sensitive and Mucoadhesive Polymers (w/v) | Gelation Trigger | Gelation Mechanism | Comments | References |
|---|---|---|---|---|---|---|---|
| Bacterial vaginosis | Metronidazole | Free drug | 20% poloxamer 407 and 10% poloxamer 188 | Temperature | Swelling due to polymeric crosslinking | Increased prolonged curative rate with sol–gel (80%) compared to conventional gel (47.4%) | [177] |
| Clotrimazole | Free drug | 15% poloxamer 407, 15% and/or 20% poloxamer 188, and 0.2% w/v polycarbophil | Temperature | Micelle formation | Antifungal effect for 10 days; reduced toxicity to epithelium cells of human cervix | [97,158] | |
| Secnidazole | Aerosol foam | 0.45% carbopol 940 with 0.35% HPMC K4 M and 0.35% carbopol 940 with 0.35% HPC | pH | Hydrogen bonding | Less than 50% of drug released by 8 h, indicating controlled drug release | [188] | |
| Secnidazole | Free drug | 20% poloxamer 407, 1% poloxamer 188, and 1 or 2.5% chitosan | Temperature | Micelle formation | Approximately 1–2-fold increase in mucoadhesiveness with chitosan | [11] | |
| Clindamycin | Free drug | 1% gellan gum and 1% HPMC | Ion | Polymeric crosslinking | Good gelling capacity; good mucoadhesion and adequate inhibition of microbial growth | [50,189] | |
| Voriconazole | Drug- hydroxypropyl β-cyclodextrin inclusion complex | Poloxamer 407, poloxamer 188 HPMC, HEC, polycarbophil, and carrageenan | Temperature | Formation of closely packed micelles in aqueous medium | Increased vaginal tissue uptake by the use of cyclodextrin and sustained drug release for 8 h using in situ gel in female Wistar rats compared to conventional formulation | [176] | |
| Amphotericin B | Drug- Hydroxypropyl ϒ-cyclodextrin complex | 25% poloxamer-based multiblock copolymers | pH and temperature | Hydrogen bonding | Toxicity reduced by complexation; dissolution controlled drug release rate; prolonged drug release observed at pH 7.4 and pH 9.0 | [172] | |
| Herpes simplex virus (HSV) infection | Acyclovir | Nanoparticle | 18% poloxamer 407 | pH and temperature | Polymeric crosslinking | Drug’s therapeutic level achieved with 10 times smaller amount of drug; relative bioavailability increased twice compared to suspension dosage form of pure drug | [174,190] |
| Infertility | Fetilty-Promoting intrauterine infusion liquid (FPL) | Icariin extracted from Epimedium, safflower, and motherwort | 19% poloxamer 407, 2.5% poloxamer 188, and 0.3% HPMC | Temperature | Hydrogen bonding | Uterus and ovarian indices significantly increased in the rats receiving the sol–gel formulation compared to control group; oestradiol levels increased after day 7 to day 22 | [191] |
| Sildenafil citrate | Free drug | 15% poloxamer 407 and 1% HEC | Temperature | Entanglement and condensed micelle packing at increased polymer concentration | Sol–gel transition temperature reduced by addition of HEC; increased endometrial thickness as well as uterine flow with reduced dosing length compared to vaginal suppositories | [192] | |
| Pre- exposure prophylaxis of HIV | Raltegravir + efaviren (RAL + EFV) | Nanoparticles | 20% poloxamer 407 and 1% poloxamer 188 | Temperature | Hydrogen bonding | Inhibitory concentration of RAL + EFV–NPs less than the solution form; sol–gel proved an efficient delivery vehicle of NPs | [13,193] |
| Tenofovir | Microsphere | α,β-glycerophosphate (GP), chitosan, sodium alginate | Temperature | Electrostatic interaction between polymers | Viscosity of chitosan–GP complex strengthened by sodium alginate; initial burst release (30%) in the first 30 min followed by cumulative release (87.82%) after 24 hrs | [194] | |
| Contraceptive | Nonoxynol-9 | Free drug | 18% poloxamer 407 and 1% or 6% poloxamer | Temperature | Micelle formation | Increased vaginal residence time compared to solution form; rapid hydrogel erosion and drug release | [11,182] |
| Intrauterine device insertion for contraception | Lidocaine | Free drug | 18% poloxamer 407, 5% poloxamer 188, and 0.3% gellan gum | Temperature and ionic strength | Hydrogen bonding between the polymers | Better acceptance and pain management by sol–gel formulation compared to conventional gel | [193] |
| Hormone replacement therapy, preterm birth | Progesterone | Free drug | 5% glycol chitin | Temperature | Hydrophobic interaction | No significant effect on gel property by viscosity reduction after dilution by vaginal fluid but not recommended in presence of semen; prolonged vaginal residence time and controlled drug release | [187,195] |
| Cervical cancer | Doxorubicin | Free drug | 7% glycol chitin | Temperature | Hydrophobic interaction | Initial 20% burst release followed by sustained release for 13 days | [186,195] |
| Source | Polymers | Role/Feature | References |
|---|---|---|---|
| Plant | Cellulose derivatives e.g., HPMC, HPC, HEC, MC, EC | Thermo responsive gelation; Mucoadhesive; non-biodegradable | |
| Pectin | Mucoadhesive | ||
| Alginate | Biocompatible; biodegradable; anionic; ion-responsive gelation | ||
| Carrageenan | Mucoadhesive; antimicrobial and antiviral activity | [50,196] | |
| Animal | Chitosan | Polycationic copolymer; Mucoadhesive; biocompatible; biodegradable; antibacterial activity | [37,50] |
| Gelatin | Biocompatible; biodegradable; | [50] | |
| Hyaluronic acid | Negatively charged | [37] | |
| Microbial | Gellan gum | Ion-responsive gelation | [50] |
| Xanthan gum | Form physical gel | [50] | |
| Synthetic | Poloxamers | Non-ionic triblock copolymer; amphiphilic; multi-stimuli responsive gelation | [37,50,197] |
| Polyacrylates | Viscosity affected by formulation pH | [37] | |
| Polyethylene glycol | Water soluble | [50] | |
| Polyvinylpyrrolidone | Linear; water soluble | [50] |
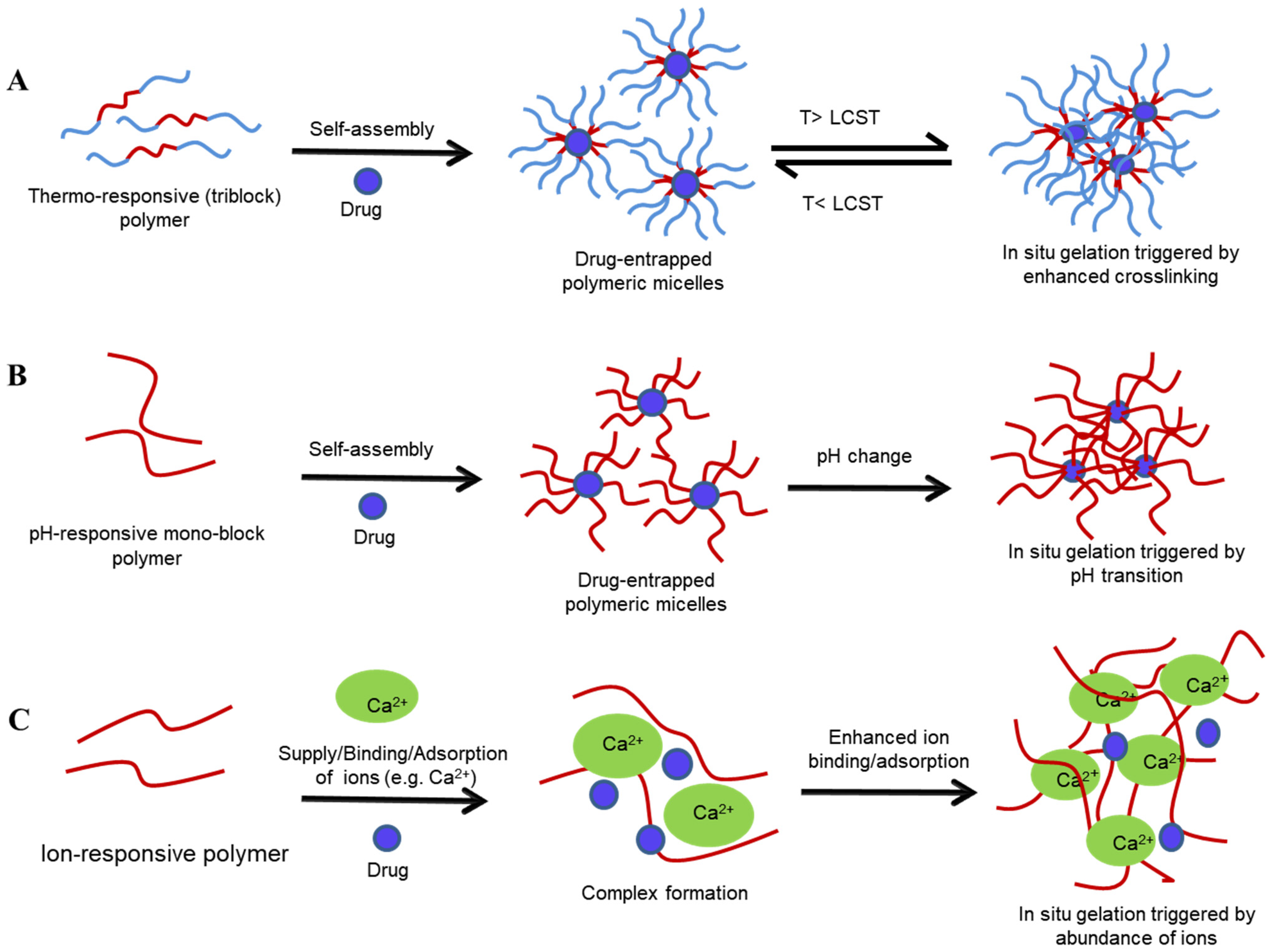
4.2. In Situ Sol-to-Gel Phase Transition Stimuli
4.2.1. Thermoresponsive Gelation
- Poloxamers
- Cellulose derivatives
- Gelatin
4.2.2. pH Sensitive Sol–Gel Systems
- Chitosan
- Polyacrylates (PA)
4.2.3. Ion-Sensitive Sol–Gel Systems
- Gellan gum
- Alginate
- Pectin
5. Applicators for Intravaginal Administration of Dosage Forms
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Caramella, C.M.; Ferrari, F. Recent Advances in the Development of In Situ Gelling Drug Delivery Systems for Non-Parenteral Administration Routes. Pharmaceutics 2020, 12, 859. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Choudhury, H.; Abdul-Aziz, A.; Bhattamisra, S.K.; Gorain, B.; Carine, T.; Wee Toong, T.; Yi, N.J.; Win Yi, L. Promising Drug Delivery Approaches to Treat Microbial Infections in the Vagina: A Recent Update. Polymers 2021, 13, 26. [Google Scholar] [CrossRef]
- Neves, J.D.; de Oliveira, R.P.; de Oliveira, A.P.; Rodrigues, F.; Sarmento, B. Vaginal mucosa and drug delivery. In Mucoadhesive Materials and Drug Delivery Systems, 1st ed.; Wiley: Chichester, UK, 2014; pp. 99–132. [Google Scholar]
- Wong, T.W.; Dhanawat, M.; Rathbone, M.J. Vaginal drug delivery: Strategies and concerns in polymeric nanoparticle development. Expert Opin. Drug Deliv. 2014, 11, 1419–1434. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.A.; Panda, A.K.; Asif, S.; Verma, D.; Talegaonkar, S.; Manzoor, N.; Khan, A.; Ahmed, F.J.; Dudeja, M.; Iqbal, Z. A vaginal drug delivery model. Drug Deliv. 2016, 23, 3123–3134. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.T.; Brown, M.B. Polymeric gels for intravaginal drug delivery. J. Control. Release 2018, 270, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Caramella, C.M.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Sandri, G. Mucoadhesive and thermogelling systems for vaginal drug delivery. Adv. Drug Deliv. Rev. 2015, 92, 39–52. [Google Scholar] [CrossRef]
- Matanović, M.R.; Kristl, J.; Grabnar, P.A. Thermoresponsive polymers: Insights into decisive hydrogel characteristics, mechanisms of gelation, and promising biomedical applications. Int. J. Pharm. 2014, 472, 262–275. [Google Scholar] [CrossRef]
- Alexander, A.A.; Khan, J.; Giri, T.K.; Tripathi, D.K.; Saraf, S.; Saraf, S. Advancement in stimuli triggered in situ gelling delivery for local and systemic route. Expert Opin. Drug Deliver. 2012, 9, 1573–1592. [Google Scholar] [CrossRef]
- Jalalvandi, E.; Shavandi, A. In situ-forming and pH-responsive hydrogel based on chitosan for vaginal delivery of therapeutic agents. J. Mater. Sci. Mater. Med. 2018, 29, 158. [Google Scholar] [CrossRef]
- Argenta, D.F.; Bernardo, B.D.C.; Chamorro, A.F.; Matos, P.R.; Caon, T. Thermosensitive hydrogels for vaginal delivery of secnidazole as an approach to overcome the systemic side-effects of oral preparations. Eur. J. Pharm. Sci. 2021, 159, 105722. [Google Scholar] [CrossRef]
- Mennini, N.; Casella, G.; Cirri, M.; Maestrelli, F.; Mura, P. Development of cyclodextrin hydrogels for vaginal delivery of dehydroepiandrosterone. J. Pharm. Pharmacol. 2016, 68, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Date, A.A.; Shibata, A.; Goede, M.; Sanford, B.; la Bruzzo, K.; Belshan, M.; Destache, C.J. Development and evaluation of a thermosensitive vaginal gel containing raltegravir+ efavirenz loaded nanoparticles for HIV prophylaxis. Antiviral Res. 2012, 96, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Antimisiaris, S.G.; Mourtas, S. Recent advances on anti-HIV vaginal delivery systems development. Adv. Drug Deliv. Rev. 2015, 92, 123–145. [Google Scholar] [CrossRef]
- Dorr, M.L.; Pierson, R.C.; Daggy, J.; Quinney, S.K.; Haas, D.M. Buccal versus vaginal misoprostol for term induction of labor: A retrospective cohort study. Am. J. Perinatol. 2019, 36, 765. [Google Scholar] [CrossRef] [PubMed]
- Tosti, C.; Biscione, A.; Morgante, G.; Bifulco, G.; Luisi, S.; Petraglia, F. Hormonal therapy for endometriosis: From molecular research to bedside. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 209, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Elad, D.; Jaffa, A.J.; Grisaru, D. Biomechanics of early life in the female reproductive tract. Physiology 2020, 35, 134–143. [Google Scholar] [CrossRef]
- Zierden, H.C.; Ortiz, J.I.; DeLong, K.; Yu, J.; Li, G.; Dimitrion, P.; Bensouda, S.; Laney, V.; Bailey, A.; Anders, N.M. Enhanced drug delivery to the reproductive tract using nanomedicine reveals therapeutic options for prevention of preterm birth. Sci. Transl. Med. 2021, 13. [Google Scholar] [CrossRef]
- Das Neves, J.; Notario-Pérez, F.; Sarmento, B. Women-specific routes of administration for drugs: A critical overview. Adv. Drug Deliv. Rev. 2021, 176, 113865. [Google Scholar] [CrossRef]
- Smoleński, M.; Karolewicz, B.; Gołkowska, A.M.; Nartowski, K.P.; Małolepsza-Jarmołowska, K. Emulsion-Based Multicompartment Vaginal Drug Carriers: From Nanoemulsions to Nanoemulgels. Int. J. Mol. Sci. 2021, 22, 6455. [Google Scholar] [CrossRef]
- Aplin, J. Uterus—Endometrium. In Encyclopedia of Reproduction, 2nd ed.; Skinner, M.K., Ed.; Academic Press: New York, NY, USA, 2018; pp. 326–332. [Google Scholar]
- Ellis, H. Anatomy of the uterus. Anaesth. Intensive Care Med. 2011, 12, 99–101. [Google Scholar] [CrossRef]
- Myers, K.M.; Elad, D. Biomechanics of the human uterus. WIREs Syst. Biol. Med. 2017, 9, e1388. [Google Scholar] [CrossRef] [PubMed]
- Friend, D.R. Drug delivery for the treatment of endometriosis and uterine fibroids. Drug Deliv. Transl. Res. 2017, 7, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Bahathiq, A.O.; Ledger, W.L. Historical Background and Functional Anatomy; Cambridge University: Cambridge, UK, 2010. [Google Scholar]
- Szmelskyj, I.; Aquilina, L.; Szmelskyj, A.O. Chapter 2—Anatomy and physiology of the reproductive system: Prerequirements for conception. An Integrated Approach to Treatment and Management. In Acupuncture for IVF and Assisted Reproduction; Szmelskyj, I., Aquilina, L., Szmelskyj, A.O., Eds.; Churchill Livingstone: London, UK, 2015; pp. 23–58. [Google Scholar]
- Graziottin, A.; Gambini, D. Anatomy and physiology of genital organs—women. Handb. Clin. Neurol. 2015, 130, 39–60. [Google Scholar] [PubMed]
- Curlin, M.; Bursac, D. Cervical mucus: From biochemical structure to clinical implications. Front. Biosci. 2013, 5, 507–515. [Google Scholar] [CrossRef]
- Herfs, M.; Vargas, S.O.; Yamamoto, Y.; Howitt, B.E.; Nucci, M.R.; Hornick, J.L.; Mckeon, F.D.; Xian, W.; Crum, C.P. A novel blueprint for ‘top down’differentiation defines the cervical squamocolumnar junction during development, reproductive life, and neoplasia. J. Pathol. 2013, 229, 460–468. [Google Scholar] [CrossRef]
- Yang, E.J.; Quick, M.C.; Hanamornroongruang, S.; Lai, K.; Doyle, L.A.; McKeon, F.D.; Xian, W.; Crum, C.P.; Herfs, M. Microanatomy of the cervical and anorectal squamocolumnar junctions: A proposed model for anatomical differences in HPV-related cancer risk. Mod. Pathol. 2015, 28, 994–1000. [Google Scholar] [CrossRef]
- Taurin, S.; Almomen, A.A.; Pollak, T.; Kim, S.J.; Maxwell, J.; Peterson, C.M.; Owen, S.C.; Janát-Amsbury, M.M. Thermosensitive hydrogels a versatile concept adapted to vaginal drug delivery. J. Drug Target. 2018, 26, 533–550. [Google Scholar] [CrossRef]
- Ng, K.Y.B.; Mingels, R.; Morgan, H.; Macklon, N.; Cheong, Y. In vivo oxygen, temperature and pH dynamics in the female reproductive tract and their importance in human conception: A systematic review. Hum. Reprod. Update 2018, 24, 15–34. [Google Scholar] [CrossRef]
- Ding, N.; He, Y.; Qi, Y.; Zhang, H.; Xu, J.; Lei, J.; Yuan, L.; Ma, L.; Xue, H.; Jin, Z. Endometrial T2 values and thickness measured during the spontaneous menstrual cycle: Potential imaging biomarker related to female physiological hormones. Chin. J. Acad. Radiol. 2021, 4, 98–104. [Google Scholar] [CrossRef]
- Lykke, M.R.; Becher, N.; Haahr, T.; Boedtkjer, E.; Jensen, J.S.; Uldbjerg, N. Vaginal, Cervical and Uterine pH in Women with Normal and Abnormal Vaginal Microbiota. Pathogens 2021, 10, 90. [Google Scholar] [CrossRef]
- Martyn, F.; McAuliffe, F.; Wingfield, M. The role of the cervix in fertility: Is it time for a reappraisal? Hum. Reprod. 2014, 29, 2092–2098. [Google Scholar] [CrossRef]
- Taherali, F.; Varum, F.; Basit, A.W. A slippery slope: On the origin, role and physiology of mucus. Adv. Drug Deliv. Rev. 2018, 124, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Lalan, M.S.; Patel, V.N.; Misra, A. Polymers in vaginal drug delivery: Recent advancements. In Applications of Polymers in Drug Delivery, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 281–303. [Google Scholar]
- Nakano, F.Y.; Leão, R.D.B.F.; Esteves, S.C. Insights into the role of cervical mucus and vaginal pH in unexplained infertility. MedicalExpress 2015, 2, 1–8. [Google Scholar] [CrossRef]
- Justin-Temu, M.; Damian, F.; Kinget, R.; Mooter, G.V.D. Intravaginal gels as drug delivery systems. J. Womens Health 2004, 13, 834–844. [Google Scholar] [CrossRef]
- Muhleisen, A.L.; Herbst-Kralovetz, M.M. Menopause and the vaginal microbiome. Maturitas 2016, 91, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Godha, K.; Tucker, K.M.; Biehl, C.; Archer, D.F.; Mirkin, S. Human vaginal pH and microbiota: An update. Gynecol. Endocrinol. 2018, 34, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.A.; Rodriguez, A.C.; Gage, J.C.; Herrero, R.; Hildesheim, A.; Wacholder, S.; Burk, R.; Schiffman, M. A large, population-based study of age-related associations between vaginal pH and human papillomavirus infection. BMC Infect. Dis. 2012, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Tietz, K. Vaginal and Intrauterine Delivery Systems. In In Vitro Drug Release Testing of Special Dosage Forms; John Wiley & Sons Ltd.: New York, NY, USA, 2019; pp. 177–209. [Google Scholar]
- Furst, T.; Piette, M.; Lechanteur, A.; Evrard, B.; Piel, G. Mucoadhesive cellulosic derivative sponges as drug delivery system for vaginal application. Eur. J. Pharm. Biopharm. 2015, 95, 128–135. [Google Scholar] [CrossRef]
- Marcotte, H.; Krogh Andersen, K.; Lin, Y.; Zuo, F.; Zeng, Z.; Larsson, P.G.; Brandsborg, E.; Brønstad, G.; Hammarström, L. Characterization and complete genome sequences of L. rhamnosus DSM 14870 and L. gasseri DSM 14869 contained in the EcoVag® probiotic vaginal capsules. Microbiol. Res. 2017, 205, 88–98. [Google Scholar] [CrossRef]
- Leyva-Gómez, G.; Prado-Audelo, D.; María, L.; Ortega-Peña, S.; Mendoza-Muñoz, N.; Urbán-Morlán, Z.; González-Torres, M.; Carmen, G.-D.; Figueroa-González, G.; Reyes-Hernández, O.D. Modifications in vaginal microbiota and their influence on drug release: Challenges and opportunities. Pharmaceutics 2019, 11, 217. [Google Scholar] [CrossRef]
- Vigani, B.; Faccendini, A.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Grisoli, P.; Ferrari, F. Development of a mucoadhesive in situ gelling formulation for the delivery of Lactobacillus gasseri into vaginal cavity. Pharmaceutics 2019, 11, 511. [Google Scholar] [CrossRef]
- Kaambo, E.; Africa, C.; Chambuso, R.; Passmore, J.-A.S. Vaginal microbiomes associated with aerobic vaginitis and bacterial vaginosis. Front. Public Health 2018, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnürch, A.; Hornof, M. Intravaginal Drug Delivery Systems: Design, Challenges, and Solutions. Am. J. Adv. Drug Deliv. 2003, 1, 241–254. [Google Scholar] [CrossRef]
- Osmałek, T.; Froelich, A.; Jadach, B.; Tatarek, A.; Gadziński, P.; Falana, A.; Gralińska, K.; Ekert, M.; Puri, V.; Wrotyńska-Barczyńska, J. Recent Advances in Polymer-Based Vaginal Drug Delivery Systems. Pharmaceutics 2021, 13, 884. [Google Scholar] [CrossRef]
- Hussain, A.; Ahsan, F. The vagina as a route for systemic drug delivery. J. Control. Release 2005, 103, 301–313. [Google Scholar] [CrossRef]
- Machado, R.M.; Palmeira-de-Oliveira, A.; Gaspar, C.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R. Studies and methodologies on vaginal drug permeation. Adv. Drug Deliv. Rev. 2015, 92, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Sassi, A.B.; McCullough, K.D.; Cost, M.R.; Hillier, S.L.; Rohan, L.C. Permeability of tritiated water through human cervical and vaginal tissue. J. Pharm. Sci. 2004, 93, 2009–2016. [Google Scholar] [CrossRef] [PubMed]
- Laksitorini, M.; Prasasty, V.D.; Kiptoo, P.K.; Siahaan, T.J. Pathways and progress in improving drug delivery through the intestinal mucosa and blood-brain barriers. Ther. Deliv. 2014, 5, 1143–1163. [Google Scholar] [CrossRef] [PubMed]
- Cicinelli, E.; Rubini, G.; De Ziegler, D.; Barba, B.; Pinto, V.; Di Stefano, M.G.; Mele, M. Absorption and preferential vagina-to-uterus distribution after vaginal administration of 99mTc-pertechnetate in postmenopausal women. Fertil. Steril. 2001, 76, 1108–1112. [Google Scholar] [CrossRef]
- Warren, M. Vaginal progesterone and the vaginal first-pass effect. Climacteric 2018, 21, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Dhande, R.; Thakkar, H. Development of intravaginal rod insert bearing liposomal raloxifene hydrochloride and Leuprolide acetate as a potential carrier for uterine targeting. J. Pharm. Pharmacol. 2021, 73, 653–663. [Google Scholar] [CrossRef]
- das Neves, J.; Araújo, F.; Andrade, F.; Amiji, M.; Bahia, M.F.; Sarmento, B. Biodistribution and pharmacokinetics of dapivirine-loaded nanoparticles after vaginal delivery in mice. Pharm. Res. 2014, 31, 1834–1845. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Guan, Y.; Ding, J.; Ma, C.; Xie, Z. Vaginal drug delivery approaches for localized management of cervical cancer. Adv. Drug Deliv. Rev. 2021, 174, 114–126. [Google Scholar] [CrossRef]
- Gomaa, E.; Lila, A.S.A.; Hasan, A.A.; Fakhr-eldin, S.G. Preparation and characterization of intravaginal vardenafil suppositories targeting a complementary treatment to boost in vitro fertilization process. Eur. J. Pharm. Sci. 2018, 111, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Lechanteur, A.; das Neves, J.; Sarmento, B. The role of mucus in cell-based models used to screen mucosal drug delivery. Adv. Drug Deliv. Rev. 2018, 124, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Vanić, Ž.; Škalko-Basnet, N. Nanopharmaceuticals for improved topical vaginal therapy: Can they deliver? Eur. J. Pharm. Sci. 2013, 50, 29–41. [Google Scholar] [CrossRef]
- Sharma, P.; Srivastava, P.; Seth, A.; Tripathi, P.N.; Banerjee, A.G.; Shrivastava, S.K. Comprehensive review of mechanisms of pathogenesis involved in Alzheimer’s disease and potential therapeutic strategies. Prog. Neurobiol. 2019, 174, 53–89. [Google Scholar] [CrossRef]
- Ensign, L.M.; Cone, R.; Hanes, J. Nanoparticle-based drug delivery to the vagina: A review. J. Control. Release 2014, 190, 500–514. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.A.; Beasley, D.E.; Dunn, R.R.; Archie, E.A. Lactobacilli dominance and vaginal pH: Why is the human vaginal microbiome unique? Front. Microbiol. 2016, 7, 1936. [Google Scholar] [CrossRef]
- Klatt, N.R.; Cheu, R.; Birse, K.; Zevin, A.S.; Perner, M.; Noël-Romas, L.; Grobler, A.; Westmacott, G.; Xie, I.Y.; Butler, J. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science 2017, 356, 938–945. [Google Scholar] [CrossRef]
- Xu, J.; Bian, G.; Zheng, M.; Lu, G.; Chan, W.Y.; Li, W.; Yang, K.; Chen, Z.J.; Du, Y. Fertility factors affect the vaginal microbiome in women of reproductive age. Am. J. Reprod. Immunol. 2020, 83, e13220. [Google Scholar] [CrossRef]
- Huang, B.; Fettweis, J.M.; Brooks, J.P.; Jefferson, K.K.; Buck, G.A. The changing landscape of the vaginal microbiome. Clin. Lab. Med. 2014, 34, 747–761. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.S.; Ellaiah, P.; Sudhahar, D. Novel approaches in vaginal drug delivery systems for local and systemic treatments. J. Pharm. Res. 2010, 3, 675–680. [Google Scholar]
- Urbán-Morlán, Z.; Serrano-Mora, L.E.; Martínez-Acevedo, L.; Leyva-Gómez, G.; Mendoza-Muñoz, N.; Quintanar-Guerrero, D. New developments in intrauterine drug delivery systems and devices. In Drug Delivery Devices and Therapeutic Systems; Elsevier: Amsterdam, The Netherlands, 2021; pp. 601–622. [Google Scholar]
- Aka-Any-Grah, A.; Bouchemal, K.; Koffi, A.; Agnely, F.; Zhang, M.; Djabourov, M.; Ponchel, G. Formulation of mucoadhesive vaginal hydrogels insensitive to dilution with vaginal fluids. Eur. J. Pharm. Biopharm. 2010, 76, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.M.; Palmeira-de-Oliveira, A.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R. Vaginal semisolid products: Technological performance considering physiologic parameters. Eur. J. Pharm. Sci. 2017, 109, 556–568. [Google Scholar] [CrossRef]
- Sofi, H.S.; Abdal-Hay, A.; Ivanovski, S.; Zhang, Y.S.; Sheikh, F.A. Electrospun nanofibers for the delivery of active drugs through nasal, oral and vaginal mucosa: Current status and future perspectives. Mater. Sci. Eng. C 2020, 111, 110756. [Google Scholar] [CrossRef]
- Araújo, F.; Martins, C.; Azevedo, C.; Sarmento, B. Chemical modification of drug molecules as strategy to reduce interactions with mucus. Adv. Drug Deliv. Rev. 2018, 124, 98–106. [Google Scholar] [CrossRef]
- Devadasu, V.R.; Deb, P.K.; Maheshwari, R.; Sharma, P.; Tekade, R.K. Physicochemical, pharmaceutical, and biological considerations in GIT absorption of drugs. In Dosage Form Design Considerations; Academic Press: Cambridge, MA, USA, 2018; pp. 149–178. [Google Scholar]
- Mathias, N.R.; Hussain, M.A. Non-invasive Systemic Drug Delivery: Developability Considerations for Alternate Routes of Administration. J. Pharm. Sci. 2010, 99, 1–20. [Google Scholar] [CrossRef]
- Talegaonkar, S.; Iqbal, Z. In vitro/in vivo performance of different complexes of itraconazole used in the treatment of vaginal candidiasis. Braz. J. Pharm. Sci. 2012, 48, 759–772. [Google Scholar]
- Jalalvandi, E.; Jafari, H.; Amorim, C.A.; Petri, D.F.S.; Nie, L.; Shavandi, A. Vaginal Administration of Contraceptives. Sci. Pharm. 2021, 89, 3. [Google Scholar] [CrossRef]
- Faisal, W.; Soliman, G.M.; Hamdan, A.M. Enhanced skin deposition and delivery of voriconazole using ethosomal preparations. J. Liposome Res. 2018, 28, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, V.; Putty, S.; Mathes, C.; Murowchick, J.B.; Youan, B.B.C. Evaluation of degradation kinetics and physicochemical stability of tenofovir. Drug Test. Anal. 2015, 7, 207–213. [Google Scholar] [CrossRef]
- Acharya, P.C.; Fernandes, C.; Suares, D.; Shetty, S.; Tekade, R.K. Solubility and solubilization approaches in pharmaceutical product development. In Dosage Form Design Considerations; Elsevier: Amsterdam, The Netherlands, 2018; pp. 513–547. [Google Scholar]
- Passos, J.S.; Martino, L.C.D.; Dartora, V.F.C.; Araujo, G.L.B.D.; Ishida, K.; Lopes, L.B. Development, skin targeting and antifungal efficacy of topical lipid nanoparticles containing itraconazole. Eur. J. Pharm. Sci. 2020, 149, 105296. [Google Scholar] [CrossRef] [PubMed]
- Van Eyk, A.; Van der Bijl, P.; Moll, L. Physicochemical characteristics of molecules and their diffusion across human vaginal mucosa. Eur. J. Inflamm. 2008, 6, 65–71. [Google Scholar] [CrossRef]
- Major, I.; McConville, C. Vaginal drug delivery for the localised treatment of cervical cancer. Drug Deliv. Transl. Res. 2017, 7, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Ensign, L.M.; Hoen, T.E.; Maisel, K.; Cone, R.A.; Hanes, J.S. Enhanced vaginal drug delivery through the use of hypotonic formulations that induce fluid uptake. Biomaterials 2013, 34, 6922–6929. [Google Scholar] [CrossRef]
- Tomás, M.; Palmeira-de-Oliveira, A.; Simões, S.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R. Bacterial vaginosis: Standard treatments and alternative strategies. Int. J. Pharm. 2020, 587, 119659. [Google Scholar] [CrossRef]
- Stewart, L.M.; Holman, C.D.A.J.; Hart, R.; Bulsara, M.K.; Preen, D.B.; Finn, J.C. In vitro fertilization and breast cancer: Is there cause for concern? Fertil. Steril. 2012, 98, 334–340. [Google Scholar] [CrossRef][Green Version]
- Liu, J.H.; Bernick, B.; Mirkin, S. Estradiol softgel inserts for the treatment of VVA symptoms: An expert opinion. Expert Opin. Drug Deliv. 2020, 17, 1573–1581. [Google Scholar] [CrossRef]
- Johal, H.S.; Garg, T.; Rath, G.; Goyal, A.K. Advanced topical drug delivery system for the management of vaginal candidiasis. Drug Deliv. 2016, 23, 550–563. [Google Scholar] [CrossRef]
- Shaker, D.S.; Ismail, S.; Hamed, S.; El-Shishtawy, E.M. Butoconazole nitrate vaginal sponge: Drug release and antifungal efficacy. J. Drug Deliv. Sci. Technol. 2018, 48, 274–287. [Google Scholar] [CrossRef]
- Ravel, J.; Moreno, I.; Simón, C. Bacterial vaginosis and its association with infertility, endometritis, and pelvic inflammatory disease. Am. J. Obstet. Gynecol. 2021, 224, 251–257. [Google Scholar] [CrossRef]
- Simões, A.; Veiga, F.; Vitorino, C.; Figueiras, A. A Tutorial for Developing a Topical Cream Formulation Based on the Quality by Design Approach. J. Pharm. Sci. 2018, 107, 2653–2662. [Google Scholar] [CrossRef] [PubMed]
- Tiboni, M.; Campana, R.; Frangipani, E.; Casettari, L. 3D printed clotrimazole intravaginal ring for the treatment of recurrent vaginal candidiasis. Int. J. Pharm. 2021, 596, 120290. [Google Scholar] [CrossRef]
- Brache, V.; Faundes, A. Contraceptive vaginal rings: A review. Contraception 2010, 82, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Z.; Dilnawaz, F. Nanocarriers for vaginal drug delivery. Recent Pat. Drug Deliv. Formul. 2019, 13, 3–15. [Google Scholar] [CrossRef]
- Machado, R.M.; Palmeira-De-Oliveira, A.; Martinez-De-Oliveira, J.; Palmeira-De-Oliveira, R. Vaginal Films for Drug Delivery. J. Pharm. Sci. 2013, 102, 2069–2081. [Google Scholar] [CrossRef]
- Palmeira-de-Oliveira, R.; Palmeira-de-Oliveira, A.; Martinez-de-Oliveira, J. New strategies for local treatment of vaginal infections. Adv. Drug Deliv. Rev. 2015, 92, 105–122. [Google Scholar] [CrossRef]
- Coudray, M.S.; Madhivanan, P. Bacterial vaginosis—A brief synopsis of the literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 245, 143–148. [Google Scholar] [CrossRef]
- Vazquez, F.; Fernández-Blázquez, A.; García, B. Vaginosis. Vaginal microbiota. Enferm. Infecc. Microbiol. Clin. 2019, 37, 592–601. [Google Scholar] [CrossRef]
- Denning, D.W.; Kneale, M.; Sobel, J.D.; Rautemaa-Richardson, R. Global burden of recurrent vulvovaginal candidiasis: A systematic review. Lancet Infect. Dis. 2018, 18, e339–e347. [Google Scholar] [CrossRef]
- Haas, D.M.; Daggy, J.; Flannery, K.M.; Dorr, M.L.; Bonsack, C.; Bhamidipalli, S.S.; Pierson, R.C.; Lathrop, A.; Towns, R.; Ngo, N.; et al. A comparison of vaginal versus buccal misoprostol for cervical ripening in women for labor induction at term (the IMPROVE trial): A triple-masked randomized controlled trial. Am. J. Obstet. Gynecol. 2019, 221, 259.e1–259.e16. [Google Scholar] [CrossRef] [PubMed]
- Pierce, S.; Bakker, R.; Myers, D.A.; Edwards, R.K. Clinical insights for cervical ripening and labor induction using prostaglandins. AJP Rep. 2018, 8, e307. [Google Scholar] [CrossRef]
- Gomez, H.B.; Hoffman, M.K.; Caplan, R.; Ruhstaller, K.; Young, M.H.H.; Sciscione, A.C. Buccal vs vaginal misoprostol combined with Foley catheter for cervical ripening at term (the BEGIN trial): A randomized controlled trial. Am. J. Obstet. Gynecol. 2021, 224, 524.e1–524.e8. [Google Scholar] [CrossRef]
- Abdelaziz, A.; Mahmoud, A.A.; Ellaithy, M.I.; Abees, S.H. Pre-induction cervical ripening using two different dinoprostone vaginal preparations: A randomized clinical trial of tablets and slow release retrievable insert. Taiwan. J. Obstet. Gynecol. 2018, 57, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Global HIV & AIDS Statistics—2020 Fact Sheet. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 28 May 2021).
- Traore, Y.L.; Chen, Y.; Ho, E.A. Current state of microbicide development. Clin. Pharmacol. Ther. 2018, 104, 1074–1081. [Google Scholar] [CrossRef]
- Coutinho, C.; Sarmento, B.; das Neves, J. Targeted microbicides for preventing sexual HIV transmission. J. Control. Release 2017, 266, 119–128. [Google Scholar] [CrossRef]
- Mahalingam, A.; Jay, J.I.; Langheinrich, K.; Shukair, S.; McRaven, M.D.; Rohan, L.C.; Herold, B.C.; Hope, T.J.; Kiser, P.F. Inhibition of the transport of HIV in vitro using a pH-responsive synthetic mucin-like polymer system. Biomaterials 2011, 32, 8343–8355. [Google Scholar] [CrossRef]
- Fernández-Romero, J.A.; Teleshova, N.; Zydowsky, T.M.; Robbiani, M. Preclinical assessments of vaginal microbicide candidate safety and efficacy. Adv. Drug Deliv. Rev. 2015, 92, 27–38. [Google Scholar] [CrossRef]
- Alexander, N.J.; Baker, E.; Kaptein, M.; Karck, U.; Miller, L.; Zampaglione, E. Why consider vaginal drug administration? Fertil. Steril. 2004, 82, 1–12. [Google Scholar] [CrossRef]
- Tai, F.-W.; Chang, C.Y.-Y.; Chiang, J.-H.; Lin, W.-C.; Wan, L. Association of pelvic inflammatory disease with risk of endometriosis: A nationwide cohort study involving 141,460 individuals. J. Clin. Med. 2018, 7, 379. [Google Scholar] [CrossRef] [PubMed]
- Yagur, Y.; Weitzner, O.; Tiosano, L.B.; Paitan, Y.; Katzir, M.; Schonman, R.; Klein, Z.; Miller, N. Characteristics of Pelvic Inflammatory Disease caused by Sexually transmitted disease–An epidemiologic Study. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102176. [Google Scholar] [CrossRef] [PubMed]
- Falconer, H.; Yin, L.; Salehi, S.; Altman, D. Association between pelvic inflammatory disease and subsequent salpingectomy on the risk for ovarian cancer. Eur. J. Cancer 2021, 145, 38–43. [Google Scholar] [CrossRef]
- Pathak, M.; Coombes, A.G.A.; Ryu, B.; Cabot, P.J.; Turner, M.S.; Palmer, C.; Wang, D.; Steadman, K.J. Sustained Simultaneous Delivery of Metronidazole and Doxycycline From Polycaprolactone Matrices Designed for Intravaginal Treatment of Pelvic Inflammatory Disease. J. Pharm. Sci. 2018, 107, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef]
- Garzon, S.; Laganà, A.S.; Barra, F.; Casarin, J.; Cromi, A.; Raffaelli, R.; Uccella, S.; Franchi, M.; Ghezzi, F.; Ferrero, S. Novel drug delivery methods for improving efficacy of endometriosis treatments. Expert Opin. Drug Deliv. 2021, 18, 355–367. [Google Scholar] [CrossRef]
- Cicinelli, E. Intravaginal oestrogen and progestin administration: Advantages and disadvantages. Best Pract. Res. Clin. Obstet. Gynaecol. 2008, 22, 391–405. [Google Scholar] [CrossRef]
- Godin, R.; Marcoux, V. Vaginally Administered Danazol: An Overlooked Option in the Treatment of Rectovaginal Endometriosis? J. Obstet. Gynaecol. Can. 2015, 37, 1098–1103. [Google Scholar] [CrossRef]
- Mirkin, S.; Simon, J.A.; Liu, J.H.; Archer, D.F.; Castro, P.D.; Graham, S.; Bernick, B.; Komm, B. Evaluation of endometrial progesterone receptor expression after 12 weeks of exposure to a low-dose vaginal estradiol insert. Menopause 2021, 28, 998. [Google Scholar] [CrossRef]
- Casado-Espada, N.M.; de Alarcón, R.; de la Iglesia-Larrad, J.I.; Bote-Bonaechea, B.; Montejo, Á.L. Hormonal contraceptives, female sexual dysfunction, and managing strategies: A review. J. Clin. Med. 2019, 8, 908. [Google Scholar] [CrossRef]
- Sivasankaran, S.; Jonnalagadda, S. Advances in controlled release hormonal technologies for contraception: A review of existing devices, underlying mechanisms, and future directions. J. Control. Release 2021, 330, 797–811. [Google Scholar] [CrossRef]
- Bahamondes, L.; Bahamondes, M.V. New and emerging contraceptives: A state-of-the-art review. Int. J. Womens Health 2014, 6, 221. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Coppola, J.S. A New Vaginal pH Regulator for Hormone-Free, On-Demand Contraception. Nurs. Womens Health 2021, 25, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-X.; Chen, S.-R.; Su, P.-P.; Huang, F.-H.; Shi, Y.-C.; Shi, Q.-Y.; Lin, S. Using mesenchymal stem cells to treat female infertility: An update on female reproductive diseases. Stem Cells Int. 2019, 2019, 9071720. [Google Scholar] [CrossRef] [PubMed]
- Christen, M.; Schertz, J.C.; Arriagada, P.; Keitel, J.; Müller, H. The redesigned follitropin α pen injector for infertility treatment. Expert Opin. Drug Deliv. 2011, 8, 833–839. [Google Scholar] [CrossRef]
- Saini, N.; Sodhi, R.K.; Bajaj, L.; Pandey, R.S.; Jain, U.K.; Katare, O.P.; Madan, J. Intravaginal administration of metformin hydrochloride loaded cationic niosomes amalgamated with thermosensitive gel for the treatment of polycystic ovary syndrome: In vitro and in vivo studies. Colloids Surf. B 2016, 144, 161–169. [Google Scholar] [CrossRef]
- Sam, S.; Ehrmann, D.A. Metformin therapy for the reproductive and metabolic consequences of polycystic ovary syndrome. Diabetologia 2017, 60, 1656–1661. [Google Scholar] [CrossRef]
- Weiss, J.M.; Tauchert, S.; Ludwig, A.K.; Diedrich, K. Treatment strategies in PCOS patients. Reprod. Biomed. Online 2005, 10, 67–74. [Google Scholar] [CrossRef]
- Briden, L.; Shirin, S.; Prior, J.C. The central role of ovulatory disturbances in the etiology of androgenic polycystic ovary syndrome (PCOS)—Evidence for treatment with cyclic progesterone. Drug Discov. Today Dis. Models 2020, 32, 71–82. [Google Scholar] [CrossRef]
- Federico, C.; Sun, J.; Muz, B.; Alhallak, K.; Cosper, P.F.; Muhammad, N.; Jeske, A.; Hinger, A.; Markovina, S.; Grigsby, P. Localized delivery of cisplatin to cervical cancer improves its therapeutic efficacy and minimizes its side effect profile. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 1483–1494. [Google Scholar] [CrossRef]
- Pickar, J.; Amadio, J.; Bernick, B.; Mirkin, S. Pharmacokinetic studies of solubilized estradiol given vaginally in a novel softgel capsule. Climacteric 2016, 19, 181–187. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bolla, D.; Weissleder, S.V.; Radan, A.P.; Gasparri, M.L.; Raio, L.; Müller, M.; Surbek, D. Misoprostol vaginal insert versus misoprostol vaginal tablets for the induction of labour: A cohort study. BMC Pregnancy Childbirth 2018, 18, 149. [Google Scholar] [CrossRef] [PubMed]
- Peet, M.M.; Agrahari, V.; Anderson, S.M.; Hanif, H.; Singh, O.N.; Thurman, A.R.; Doncel, G.F.; Clark, M.R. Topical Inserts: A Versatile Delivery Form for HIV Prevention. Pharmaceutics 2019, 11, 374. [Google Scholar] [CrossRef] [PubMed]
- Küng, E.; Fürnkranz, U.; Walochnik, J. Chemotherapeutic options for the treatment of human trichomoniasis. Int. J. Antimicrob. Agents 2019, 53, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Velázquez, N.S.; Turino, L.N.; Luna, J.A.; Mengatto, L.N. Progesterone loaded thermosensitive hydrogel for vaginal application: Formulation and in vitro comparison with commercial product. Saudi Pharm. J. 2019, 27, 1096–1106. [Google Scholar] [CrossRef]
- Child, T.; Leonard, S.A.; Evans, J.S.; Lass, A. Systematic review of the clinical efficacy of vaginal progesterone for luteal phase support in assisted reproductive technology cycles. Reprod. Biomed. Online 2018, 36, 630–645. [Google Scholar] [CrossRef]
- Notario-Pérez, F.; Cazorla-Luna, R.; Martín-Illana, A.; Ruiz-Caro, R.; Tamayo, A.; Rubio, J.; Veiga, M.-D. Optimization of tenofovir release from mucoadhesive vaginal tablets by polymer combination to prevent sexual transmission of HIV. Carbohydr. Polym. 2018, 179, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.A.; Pickar, J.H.; Shadiack, A.M.; Warrier, B.; Graham, S.; Bernick, B.; Mirkin, S. Physical characteristics and properties of estradiol softgel vaginal inserts. Menopause 2020, 27, 150. [Google Scholar] [CrossRef]
- Blakney, A.K.; Ball, C.; Krogstad, E.A.; Woodrow, K.A. Electrospun fibers for vaginal anti-HIV drug delivery. Antiviral Res. 2013, 100, S9–S16. [Google Scholar] [CrossRef] [PubMed]
- Blakney, A.K.; Jiang, Y.; Woodrow, K.A. Application of electrospun fibers for female reproductive health. Drug Deliv. Transl. Res. 2017, 7, 796–804. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Y.; Sun, S.; Khan, A.R.; Ji, J.; Yang, M.; Zhai, G. Recent advances in electrospun for drug delivery purpose. J. Drug Target. 2019, 27, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Zong, M.-H.; Linhardt, R.J.; Feng, K.; Wu, H. Electrospinning: A novel nano-encapsulation approach for bioactive compounds. Trends Food Sci. Technol. 2017, 70, 56–68. [Google Scholar] [CrossRef]
- Katouzian, I.; Jafari, S.M. Nano-encapsulation as a promising approach for targeted delivery and controlled release of vitamins. Trends Food Sci. Technol. 2016, 53, 34–48. [Google Scholar] [CrossRef]
- Ye, C.; Chi, H. A review of recent progress in drug and protein encapsulation: Approaches, applications and challenges. Mater. Sci. Eng. C 2018, 83, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Paulo, F.; Santos, L. Design of experiments for microencapsulation applications: A review. Mater. Sci. Eng. C 2017, 77, 1327–1340. [Google Scholar] [CrossRef]
- Otto, D.P.; Otto, A.; De Villiers, M.M. Differences in physicochemical properties to consider in the design, evaluation and choice between microparticles and nanoparticles for drug delivery. Expert Opin. Drug Deliv. 2015, 12, 763–777. [Google Scholar] [CrossRef] [PubMed]
- Martín-Villena, M.; Fernández-Campos, F.; Calpena-Campmany, A.; Bozal-de Febrer, N.; Ruiz-Martínez, M.; Clares-Naveros, B. Novel microparticulate systems for the vaginal delivery of nystatin: Development and characterization. Carbohydr. Polym. 2013, 94, 1–11. [Google Scholar] [CrossRef]
- Moreno, M.A.; Gómez-Mascaraque, L.G.; Arias, M.; Zampini, I.C.; Sayago, J.E.; Ramos, L.L.P.; Schmeda-Hirschmann, G.; López-Rubio, A.; Isla, M.I. Electrosprayed chitosan microcapsules as delivery vehicles for vaginal phytoformulations. Carbohydr. Polym. 2018, 201, 425–437. [Google Scholar] [CrossRef]
- Laelorspoen, N.; Wongsasulak, S.; Yoovidhya, T.; Devahastin, S. Microencapsulation of Lactobacillus acidophilus in zein–alginate core–shell microcapsules via electrospraying. J. Funct. Foods 2014, 7, 342–349. [Google Scholar] [CrossRef]
- El-Hammadi, M.M.; Arias, J.L. Nanomedicine for vaginal drug delivery. In Theory and Applications of Nonparenteral Nanomedicines; Elsevier: Amsterdam, The Netherlands, 2021; pp. 235–257. [Google Scholar]
- El-Hammadi, M.M.; Arias, J.L. Nanotechnology for vaginal drug delivery and targeting. In Nanoengineered Biomaterials for Advanced Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2020; pp. 647–682. [Google Scholar]
- Sharma, P.; Garg, S. Pure drug and polymer based nanotechnologies for the improved solubility, stability, bioavailability and targeting of anti-HIV drugs. Adv. Drug Deliv. Rev. 2010, 62, 491–502. [Google Scholar] [CrossRef]
- Joye, I.J.; McClements, D.J. Biopolymer-based nanoparticles and microparticles: Fabrication, characterization, and application. Curr. Opin. Colloid Interface Sci. 2014, 19, 417–427. [Google Scholar] [CrossRef]
- Leyva-Gómez, G.; Piñón-Segundo, E.; Mendoza-Muñoz, N.; Zambrano-Zaragoza, M.L.; Mendoza-Elvira, S.; Quintanar-Guerrero, D. Approaches in polymeric nanoparticles for vaginal drug delivery: A review of the state of the art. Int. J. Mol. Sci. 2018, 19, 1549. [Google Scholar] [CrossRef] [PubMed]
- Netsomboon, K.; Bernkop-Schnürch, A. Mucoadhesive vs. mucopenetrating particulate drug delivery. Eur. J. Pharm. Biopharm. 2016, 98, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yu, T.; Wang, Y.Y.; Lai, S.K.; Zeng, Q.; Miao, B.; Tang, B.C.; Simons, B.W.; Ensign, L.M.; Liu, G. Vaginal Delivery of Paclitaxel via Nanoparticles with Non-Mucoadhesive Surfaces Suppresses Cervical Tumor Growth. Adv. Healthc. Mater. 2014, 3, 1044–1052. [Google Scholar] [CrossRef]
- Jeong, B.; Kim, S.W.; Bae, Y.H. Thermosensitive sol–gel reversible hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 154–162. [Google Scholar] [CrossRef]
- Agrawal, M.; Saraf, S.; Saraf, S.; Dubey, S.K.; Puri, A.; Gupta, U.; Kesharwani, P.; Ravichandiran, V.; Kumar, P.; Naidu, V.G.M.; et al. Stimuli-responsive In situ gelling system for nose-to-brain drug delivery. J. Control. Release 2020, 327, 235–265. [Google Scholar] [CrossRef]
- Bazban-Shotorbani, S.; Hasani-Sadrabadi, M.M.; Karkhaneh, A.; Serpooshan, V.; Jacob, K.I.; Moshaverinia, A.; Mahmoudi, M. Revisiting structure-property relationship of pH-responsive polymers for drug delivery applications. J. Control. Release 2017, 253, 46–63. [Google Scholar] [CrossRef]
- Bahram, M.; Mohseni, N.; Moghtader, M. An introduction to hydrogels and some recent applications. In Emerging Concepts in Analysis and Applications of Hydrogels; IntechOpen: London, UK, 2016. [Google Scholar]
- Ruel-Gariepy, E.; Leroux, J.-C. In situ-forming hydrogels—review of temperature-sensitive systems. Eur. J. Pharm. Biopharm. 2004, 58, 409–426. [Google Scholar] [CrossRef]
- da Silva, J.B.; Cook, M.T.; Bruschi, M.L. Thermoresponsive systems composed of poloxamer 407 and HPMC or NaCMC: Mechanical, rheological and sol-gel transition analysis. Carbohydr. Polym. 2020, 240, 116268. [Google Scholar] [CrossRef]
- Sarwal, A.; Singh, G.; Singh, S.; Singh, K.; Sinha, V. Novel and effectual delivery of an antifungal agent for the treatment of persistent vulvovaginal candidiasis. J. Pharm. Investig. 2019, 49, 135–147. [Google Scholar] [CrossRef]
- Enggi, C.K.; Isa, H.T.; Sulistiawati, S.; Ardika, K.A.R.; Wijaya, S.; Asri, R.M.; Mardikasari, S.A.; Donnelly, R.F.; Permana, A.D. Development of thermosensitive and mucoadhesive gels of cabotegravir for enhanced permeation and retention profiles in vaginal tissue: A proof of concept study. Int. J. Pharm. 2021, 609, 121182. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.G.; Lee, S.-E.; Kang, B.-S.; Ng, C.L.; Davaa, E.; Park, J.-S. Thermosensitive and mucoadhesive sol-gel composites of paclitaxel/dimethyl-β-cyclodextrin for buccal delivery. PLoS ONE 2014, 9, e109090. [Google Scholar] [CrossRef] [PubMed]
- Araujo, V.H.S.; de Souza, M.P.C.; Carvalho, G.C.; Duarte, J.L.; Chorilli, M. Chitosan-based systems aimed at local application for vaginal infections. Carbohydr. Polym. 2021, 261, 117919. [Google Scholar] [CrossRef] [PubMed]
- Zierden, H.C.; Josyula, A.; Shapiro, R.L.; Hsueh, H.T.; Hanes, J.; Ensign, L.M. Avoiding a Sticky Situation: Bypassing the Mucus Barrier for Improved Local Drug Delivery. Trends Mol. Med. 2021, 27, 436–450. [Google Scholar] [CrossRef]
- Lacey, C.; Woodhall, S.; Qi, Z.; Sawant, S.; Cowen, M.; McCormack, S.; Jiang, S. Unacceptable side-effects associated with a hyperosmolar vaginal microbicide in a phase 1 trial. Int. J. STD AIDS 2010, 21, 714–717. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, X.; Gan, Y.; Yu, M. Engineering nanoparticles to overcome the mucus barrier for drug delivery: Design, evaluation and state-of-the-art. Med. Drug Discov. 2021, 12, 100110. [Google Scholar] [CrossRef]
- Tuğcu-Demiröz, F.; Saar, S.; Kara, A.A.; Yıldız, A.; Tunçel, E.; Acartürk, F. Development and characterization of chitosan nanoparticles loaded nanofiber hybrid system for vaginal controlled release of benzydamine. Eur. J. Pharm. Sci. 2021, 161, 105801. [Google Scholar] [CrossRef]
- Halder, J.; Pradhan, D.; Kar, B.; Ghosh, G.; Rath, G. Nanotherapeutics approaches to overcome P-glycoprotein-mediated multi-drug resistance in cancer. Nanomed. Nanotechnol. Biol. Med. 2021, 40, 102494. [Google Scholar] [CrossRef]
- Kim, Y.-T.; Shin, B.-K.; Garripelli, V.K.; Kim, J.-K.; Davaa, E.; Jo, S.; Park, J.-S. A thermosensitive vaginal gel formulation with HPγCD for the pH-dependent release and solubilization of amphotericin B. Eur. J. Pharm. Sci. 2010, 41, 399–406. [Google Scholar] [CrossRef]
- Chindamo, G.; Sapino, S.; Peira, E.; Chirio, D.; Gallarate, M. Recent Advances in Nanosystems and Strategies for Vaginal Delivery of Antimicrobials. Nanomaterials 2021, 11, 311. [Google Scholar] [CrossRef]
- Ramyadevi, D.; Rajan, K.S.; Vedhahari, B.N.; Ruckmani, K.; Subramanian, N. Heterogeneous polymer composite nanoparticles loaded in situ gel for controlled release intra-vaginal therapy of genital herpes. Colloids Surf. B 2016, 146, 260–270. [Google Scholar] [CrossRef]
- Iqbal, R.; Qureshi, O.S.; Yousaf, A.M.; Raza, S.A.; Sarwar, H.S.; Shahnaz, G.; Saleem, U.; Sohail, M.F. Enhanced solubility and biopharmaceutical performance of atorvastatin and metformin via electrospun polyvinylpyrrolidone-hyaluronic acid composite nanoparticles. Eur. J. Pharm. Sci. 2021, 161, 105817. [Google Scholar] [CrossRef] [PubMed]
- Deshkar, S.S.; Palve, V.K. Formulation and development of thermosensitive cyclodextrin-based in situ gel of voriconazole for vaginal delivery. J. Drug Deliv. Sci. Technol. 2019, 49, 277–285. [Google Scholar] [CrossRef]
- Shaaban, O.M.; Fetih, G.N.; Abdellah, N.H.; Ismail, S.; Ibrahim, M.A.; Ibrahim, E.S.A. Pilot randomized trial for treatment of bacterial vaginosis using in situ forming metronidazole vaginal gel. J. Obstet. Gynaecol. Res. 2011, 37, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Nguyen, P.K.; Cabral, H.J.; Diez-Barroso, R.; Derry, P.J.; Kanahara, S.M.; Kumar, V.A. Development of peptide inhibitors of HIV transmission. Bioact. Mater. 2016, 1, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, R.P.; Pillay, V.; Choonara, Y.E.; du Toit, L.C.; Ndesendo, V.M.; Bawa, P.; Cooppan, S. A review of multi-responsive membranous systems for rate-modulated drug delivery. AAPS PharmSciTech 2010, 11, 441–459. [Google Scholar] [CrossRef]
- Navath, R.S.; Menjoge, A.R.; Dai, H.; Romero, R.; Kannan, S.; Kannan, R.M. Injectable PAMAM dendrimer–PEG hydrogels for the treatment of genital infections: Formulation and in vitro and in vivo evaluation. Mol. Pharm. 2011, 8, 1209–1223. [Google Scholar] [CrossRef]
- Nie, L.; Zou, P.; Dong, J.; Sun, M.; Ding, P.; Han, Y.; Ji, C.; Zhou, Q.; Yuan, H.; Suo, J. Injectable vaginal hydrogels as a multi-drug carrier for contraception. Appl. Sci. 2019, 9, 1638. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, F.; Feng, L.; Yang, L.; Chen, L.; Wei, G.; Lu, W. In vivo retention of poloxamer-based in situ hydrogels for vaginal application in mouse and rat models. Acta Pharm. Sin. B 2017, 7, 502–509. [Google Scholar] [CrossRef]
- das Neves, J.; Nunes, R.; Rodrigues, F.; Sarmento, B. Nanomedicine in the development of anti-HIV microbicides. Adv. Drug Deliv. Rev. 2016, 103, 57–75. [Google Scholar] [CrossRef]
- Lakshmi, Y.S.; Kumar, P.; Kishore, G.; Bhaskar, C.; Kondapi, A.K. Triple combination MPT vaginal microbicide using curcumin and efavirenz loaded lactoferrin nanoparticles. Sci. Rep. 2016, 6, 25479. [Google Scholar] [CrossRef]
- Mesquita, L.; Galante, J.; Nunes, R.; Sarmento, B.; das Neves, J. Pharmaceutical vehicles for vaginal and rectal administration of anti-HIV microbicide nanosystems. Pharmaceutics 2019, 11, 145. [Google Scholar] [CrossRef]
- Li, Z.; Cho, S.; Kwon, I.C.; Janát-Amsbury, M.M.; Huh, K.M. Preparation and characterization of glycol chitin as a new thermogelling polymer for biomedical applications. Carbohydr. Polym. 2013, 92, 2267–2275. [Google Scholar] [CrossRef] [PubMed]
- Almomen, A.; Cho, S.; Yang, C.-H.; Li, Z.; Jarboe, E.A.; Peterson, C.M.; Huh, K.M.; Janát-Amsbury, M.M. Thermosensitive progesterone hydrogel: A safe and effective new formulation for vaginal application. Pharm. Res. 2015, 32, 2266–2279. [Google Scholar] [CrossRef] [PubMed]
- Arun Karthick, R.; Ramya Devi, D.; Vedha Hari, B.N. Investigation of sustained release mucoadhesive in-situ gel system of Secnidazole for the persistent treatment of vaginal infections. J. Drug Deliv. Sci. Technol. 2018, 43, 362–368. [Google Scholar] [CrossRef]
- Patel, P.; Patel, P. Formulation and evaluation of clindamycin HCL in situ gel for vaginal application. Int. J. Pharm. Investig. 2015, 5, 50. [Google Scholar] [CrossRef]
- Vandenhaute, M.; Schelfhout, J.; Van Vlierberghe, S.; Mendes, E.; Dubruel, P. Cross-linkable, thermo-responsive Pluronic® building blocks for biomedical applications: Synthesis and physico-chemical evaluation. Eur. Polym. J. 2014, 53, 126–138. [Google Scholar] [CrossRef]
- Lu, C.; Liu, M.; Fu, H.; Zhang, W.; Peng, G.; Zhang, Y.; Cao, H.; Luo, L. Novel thermosensitive in situ gel based on poloxamer for uterus delivery. Eur. J. Pharm. Sci. 2015, 77, 24–28. [Google Scholar] [CrossRef]
- Soliman, G.M.; Fetih, G.; Abbas, A.M. Thermosensitive bioadhesive gels for the vaginal delivery of sildenafil citrate: In vitro characterization and clinical evaluation in women using clomiphene citrate for induction of ovulation. Drug Dev. Ind. Pharm. 2017, 43, 399–408. [Google Scholar] [CrossRef]
- Abd Ellah, N.H.; Abouelmagd, S.A.; Abbas, A.M.; Shaaban, O.M.; Hassanein, K.M.A. Dual-responsive lidocaine in situ gel reduces pain of intrauterine device insertion. Int. J. Pharm. 2018, 538, 279–286. [Google Scholar] [CrossRef]
- Yang, T.-T.; Cheng, Y.-Z.; Qin, M.; Wang, Y.-H.; Yu, H.-L.; Wang, A.-L.; Zhang, W.-F. Thermosensitive chitosan hydrogels containing polymeric microspheres for vaginal drug delivery. Biomed. Res. Int. 2017, 2017, 3564060. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Tang, Y.; Wang, Q.; Li, Y.; Yang, J.; Du, Y.; Kennedy, J.F. Rheological behaviour of chitin in NaOH/urea aqueous solution. Carbohydr. Polym. 2011, 83, 1128–1133. [Google Scholar] [CrossRef]
- Dos Santos, A.M.; Carvalho, S.G.; Araujo, V.H.S.; Carvalho, G.C.; Gremião, M.P.D.; Chorilli, M. Recent advances in hydrogels as strategy for drug delivery intended to vaginal infections. Int. J. Pharm. 2020, 590, 119867. [Google Scholar] [CrossRef] [PubMed]
- Akash, M.S.H.; Rehman, K. Recent progress in biomedical applications of Pluronic (PF127): Pharmaceutical perspectives. J. Control. Release 2015, 209, 120–138. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, C.; Fernandes, S.N.; Godinho, M.H.; Borges, J.P.; Soares, P.I. Functional stimuli-responsive gels: Hydrogels and microgels. Gels 2018, 4, 54. [Google Scholar] [CrossRef]
- Pandey, P.; Cabot, P.J.; Wallwork, B.; Panizza, B.J.; Parekh, H.S. Formulation, functional evaluation and ex vivo performance of thermoresponsive soluble gels-A platform for therapeutic delivery to mucosal sinus tissue. Eur. J. Pharm. Sci. 2017, 96, 499–507. [Google Scholar] [CrossRef]
- Russo, E.; Villa, C. Poloxamer hydrogels for biomedical applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef]
- Ci, L.; Huang, Z.; Liu, Y.; Liu, Z.; Wei, G.; Lu, W. Amino-functionalized poloxamer 407 with both mucoadhesive and thermosensitive properties: Preparation, characterization and application in a vaginal drug delivery system. Acta Pharm. Sin. B 2017, 7, 593–602. [Google Scholar] [CrossRef]
- Barros, S.C.; da Silva, A.A.; Costa, D.B.; Cesarino, I.; Costa, C.M.; Lanceros-Méndez, S.; Pawlicka, A.; Silva, M.M. Thermo-sensitive chitosan–cellulose derivative hydrogels: Swelling behaviour and morphologic studies. Cellulose 2014, 21, 4531–4544. [Google Scholar] [CrossRef]
- Klouda, L. Thermoresponsive hydrogels in biomedical applications: A seven-year update. Eur. J. Pharm. Biopharm. 2015, 97, 338–349. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.-L. Review of Applications and Future Prospects of Stimuli-Responsive Hydrogel Based on Thermo-Responsive Biopolymers in Drug Delivery Systems. Polymers 2021, 13, 2086. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Chen, D.; Yu, H.; Sun, K.; Liu, W.; Wang, H. Dual thermoresponsive and pH-responsive self-assembled micellar nanogel for anticancer drug delivery. Drug Deliv. 2014, 21, 258–264. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, J.; Zhang, Z.; Chen, W.; Hu, Q.; Wang, Y. Convenient one-step approach based on stimuli-responsive sol-gel transition properties to directly build chitosan-alginate core-shell beads. Food Hydrocoll. 2019, 87, 253–259. [Google Scholar] [CrossRef]
- Cirri, M.; Maestrelli, F.; Scuota, S.; Bazzucchi, V.; Mura, P. Development and microbiological evaluation of chitosan and chitosan-alginate microspheres for vaginal administration of metronidazole. Int. J. Pharm. 2021, 598, 120375. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Tenreiro, C.; Diez-Bueno, L.; Concheiro, A.; Torres-Labandeira, J.J.; Alvarez-Lorenzo, C. Cyclodextrin/carbopol micro-scale interpenetrating networks (ms-IPNs) for drug delivery. J. Control. Release 2007, 123, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Migliozzi, S.; Angeli, P.; Mazzei, L. Gelation kinetics of non-aqueous Carbopol dispersions. Colloids Surf. B 2019, 577, 84–95. [Google Scholar] [CrossRef]
- Singh, V.K.; Anis, A.; Banerjee, I.; Pramanik, K.; Bhattacharya, M.K.; Pal, K. Preparation and characterization of novel carbopol based bigels for topical delivery of metronidazole for the treatment of bacterial vaginosis. Mater. Sci. Eng. C 2014, 44, 151–158. [Google Scholar] [CrossRef]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate gel particles–A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef]
- Mishra, R.; Soni, K.; Mehta, T. Mucoadhesive vaginal film of fluconazole using cross-linked chitosan and pectin. J. Therm. Anal. Calorim. 2017, 130, 1683–1695. [Google Scholar] [CrossRef]
- Bakke, A.J.; Zaveri, T.; Higgins, M.J.; Ziegler, G.R.; Hayes, J.E. Design aspects of vaginal applicators that influence acceptance among target users. Sci. Rep. 2021, 11, 9802. [Google Scholar] [CrossRef]
- Montesino, M.; Labrie, F.; Archer, D.F.; Zerhouni, J.; Côté, I.; Lavoie, L.; Beauregard, A.; Martel, C.; Vaillancourt, M.; Moyneur, E. Evaluation of the acceptability of intravaginal prasterone ovule administration using an applicator. Gynecol. Endocrinol. 2016, 32, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Omar, R.F.; Trottier, S.; Brousseau, G.; Ouellet, C.; Danylo, A.; Ong, T.; Bergeron, M.G. Universal vaginal applicator for the uniform distribution of vaginal gel and cream formulations: A magnetic resonance imaging study. J. Obstet. Gynaecol. Can. 2014, 36, 42–50. [Google Scholar] [CrossRef]
- Brache, V.; Cohen, J.A.; Cochon, L.; Alvarez, F. Evaluating the clinical safety of three vaginal applicators: A pilot study conducted in the Dominican Republic. Contraception 2006, 73, 72–77. [Google Scholar] [CrossRef]
- Brunner, H.; Theodor, R.A. Multiple use applicator for vaginal tablets/vaginal inserts: Compliance verification and suitability studies. BMC Womens Health 2020, 20, 235. [Google Scholar] [CrossRef]


| Applicator Type | Dimensions (mm) | Features | Advantages | Disadvantages | Product Examples | Reference |
|---|---|---|---|---|---|---|
| Single use | 114 × 12.7 with a tapered, rounded tip | Comprises plunger, barrel, and cap fabricated from PP and a piston inside the barrel made of non-latex rubber; pre-filled or manual filling | Reduced cost due to bulk production | Higher plastic waste | KY-gel; Canesten® cream | [217,218,219] |
| Multiple use | 114.5 × 11.3 | Comprises barrel and plunger fabricated from PE | Can be refilled and reusable, reducing packaging, storage, and transportation costs | Sanitary concerns | Ovestin® intravaginal cream | [215,218,219] |
| Single-use squeeze tube | 105 × 29 tube, plus 5-mm-wide applicator tip | Single-piece device fabricated from PE | Pre-filled, cost-effective | Cannot be filled manually | Norden-Pac applicator | [218] |
| Multiple pores | - | Presence of PE-fabricated membrane around the reservoir, infused with drug product and with perforations | Covers entire vaginal mucosa immediately after application; uniform drug delivery; pre-filled; biodegradable | High manufacturing cost | Universal vaginal applicator | [217] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thapa, R.; Gurung, S.; Parat, M.-O.; Parekh, H.S.; Pandey, P. Application of Sol–Gels for Treatment of Gynaecological Conditions—Physiological Perspectives and Emerging Concepts in Intravaginal Drug Delivery. Gels 2022, 8, 99. https://doi.org/10.3390/gels8020099
Thapa R, Gurung S, Parat M-O, Parekh HS, Pandey P. Application of Sol–Gels for Treatment of Gynaecological Conditions—Physiological Perspectives and Emerging Concepts in Intravaginal Drug Delivery. Gels. 2022; 8(2):99. https://doi.org/10.3390/gels8020099
Chicago/Turabian StyleThapa, Ritu, Shila Gurung, Marie-Odile Parat, Harendra S. Parekh, and Preeti Pandey. 2022. "Application of Sol–Gels for Treatment of Gynaecological Conditions—Physiological Perspectives and Emerging Concepts in Intravaginal Drug Delivery" Gels 8, no. 2: 99. https://doi.org/10.3390/gels8020099
APA StyleThapa, R., Gurung, S., Parat, M.-O., Parekh, H. S., & Pandey, P. (2022). Application of Sol–Gels for Treatment of Gynaecological Conditions—Physiological Perspectives and Emerging Concepts in Intravaginal Drug Delivery. Gels, 8(2), 99. https://doi.org/10.3390/gels8020099








