Chitosan@Carboxymethylcellulose/CuO-Co2O3 Nanoadsorbent as a Super Catalyst for the Removal of Water Pollutants
Abstract
:1. Introduction
2. Result and Discussion
2.1. Characterization
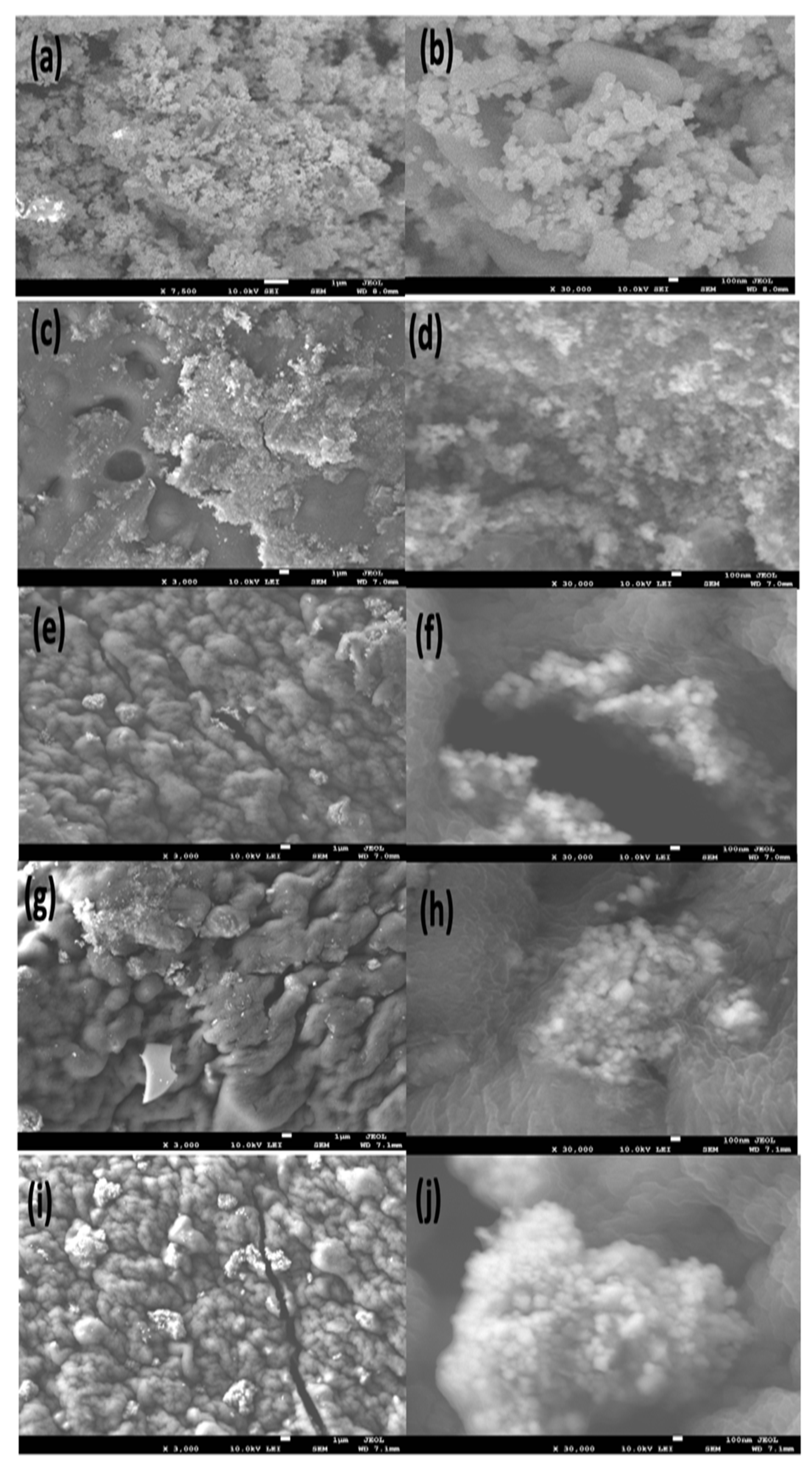
2.1.1. Field-Emission Scanning Electron Microscope (FE-SEM) Analysis
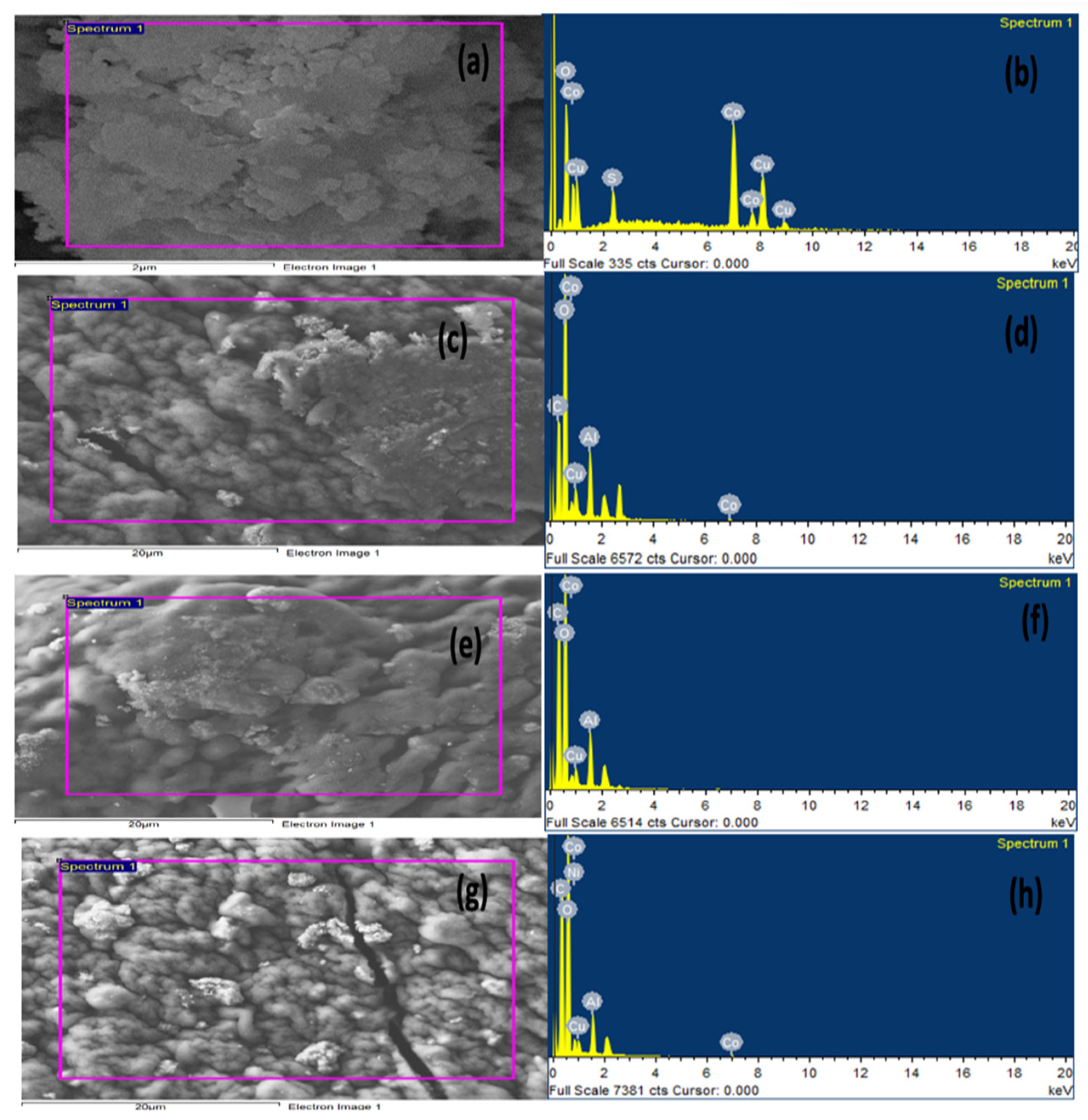
2.1.2. Energy Dispersive X-ray (EDX) Analysis
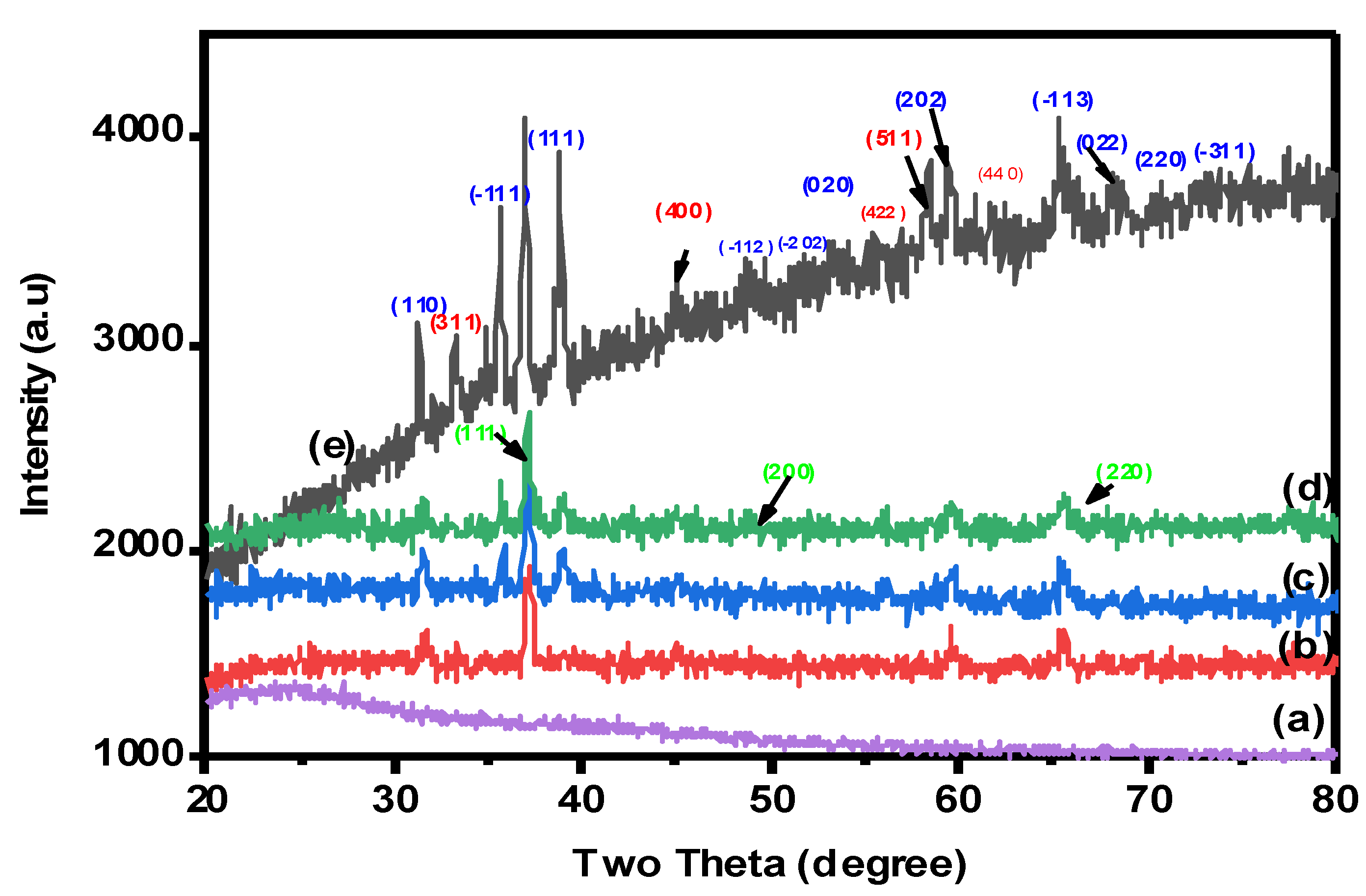
2.1.3. X-ray Diffraction (XRD) Analysis
2.1.4. FT-IR Analysis
2.2. Metal Uptake Study
2.3. Optimization of Ni(II) Adsorption
2.3.1. Effect of Initial Concentration of Ni(II) Solution
2.3.2. Effect of pH of Ni(II) Solution
2.3.3. Effect of Ni(II) Adsorption Contact Time
2.3.4. Effect of Adsorbent Dose
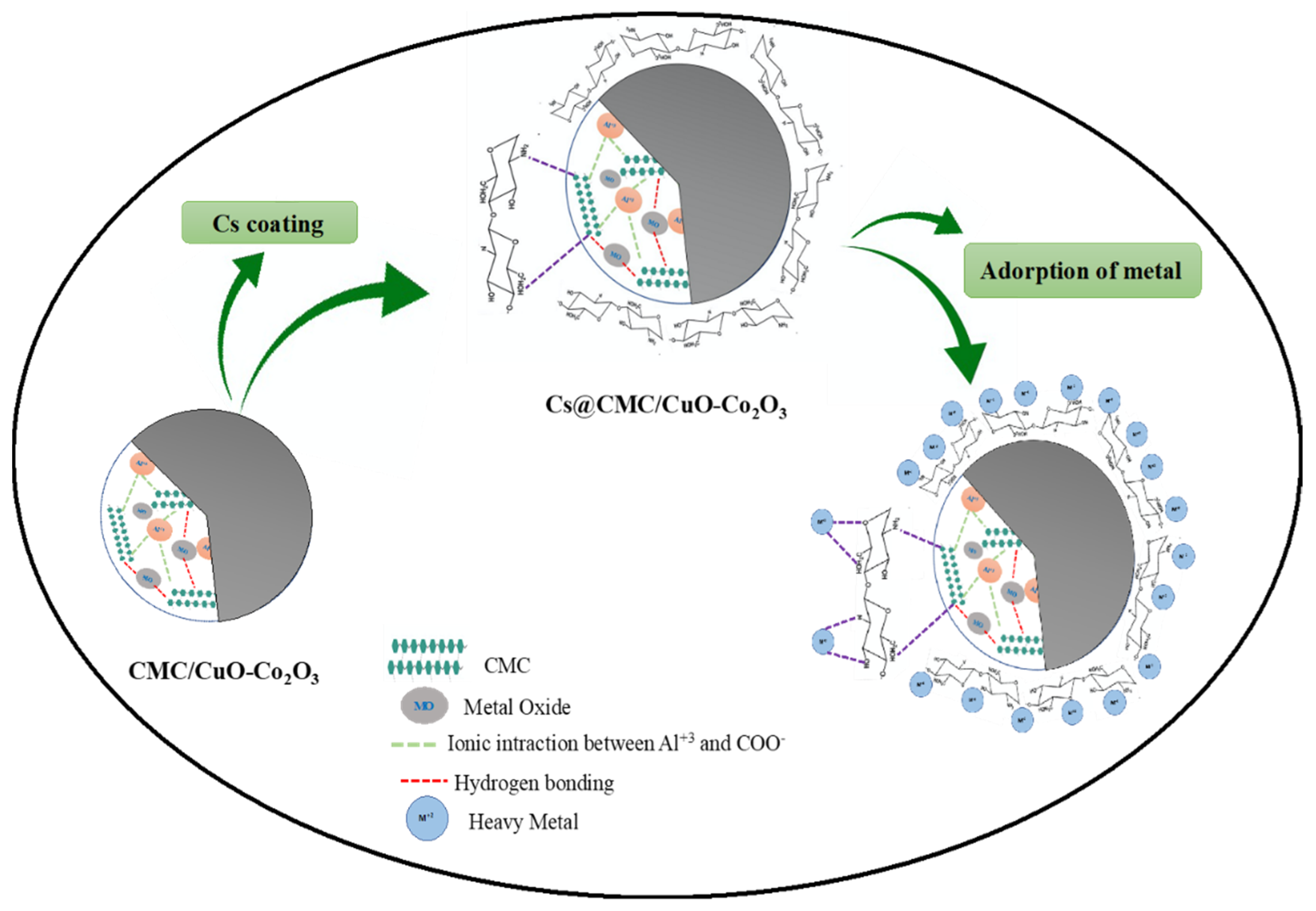
2.4. Adsorption Mechanism
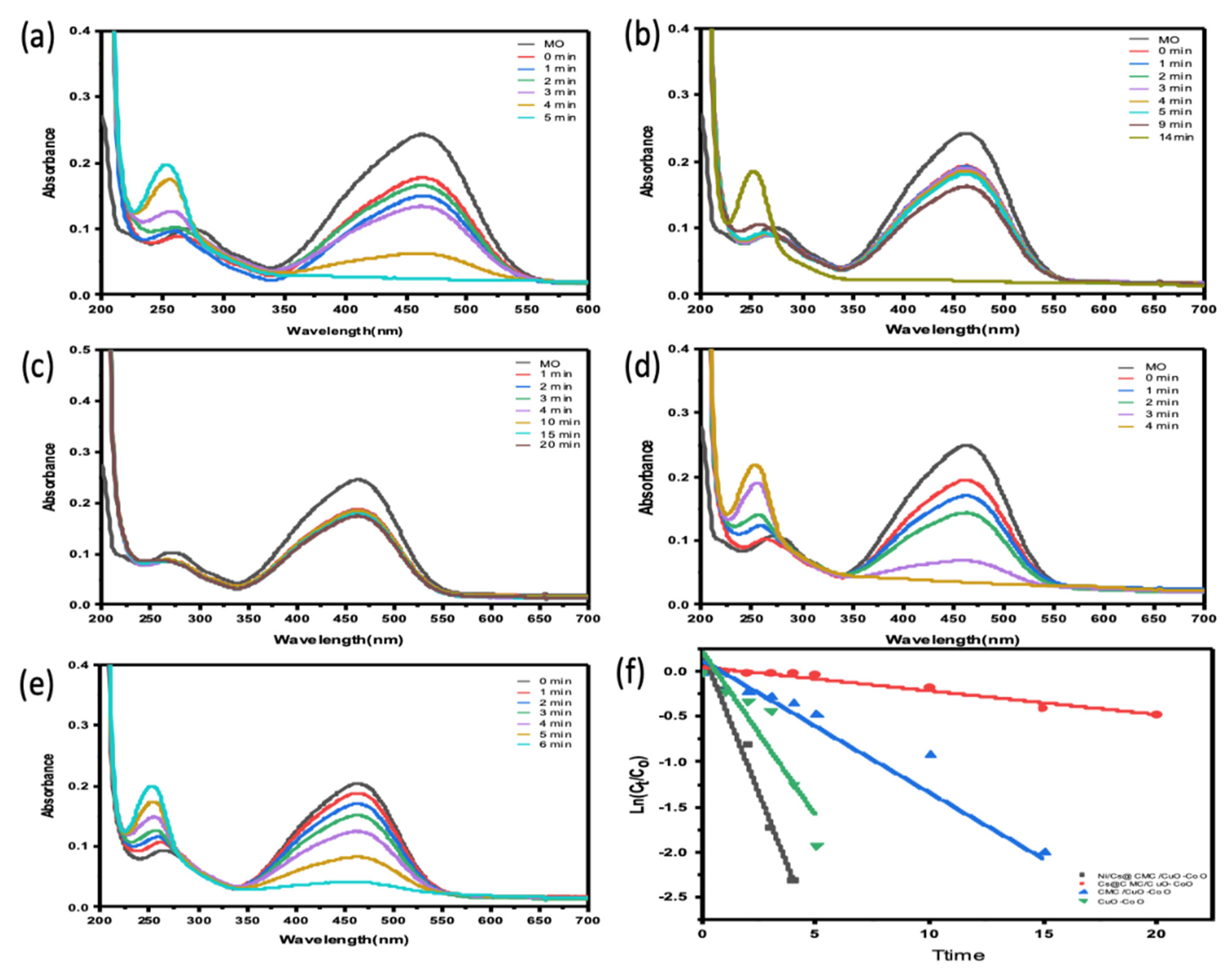
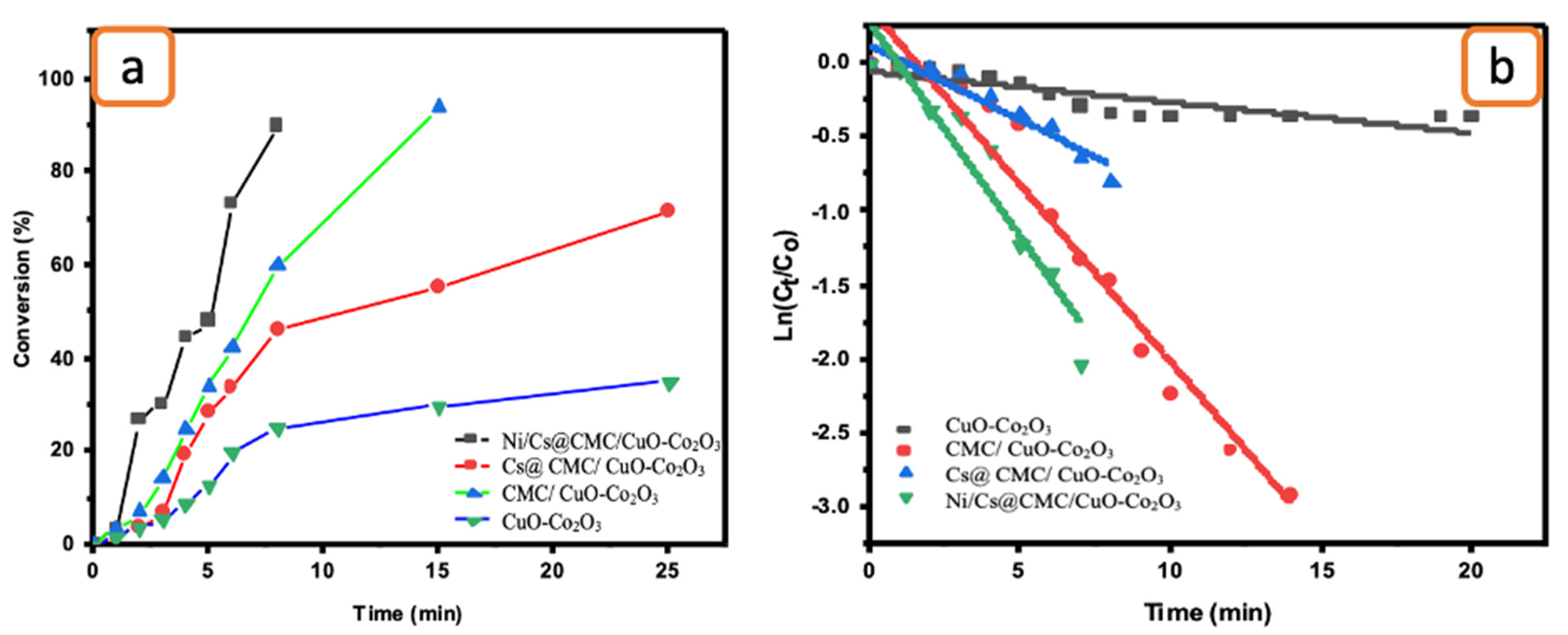
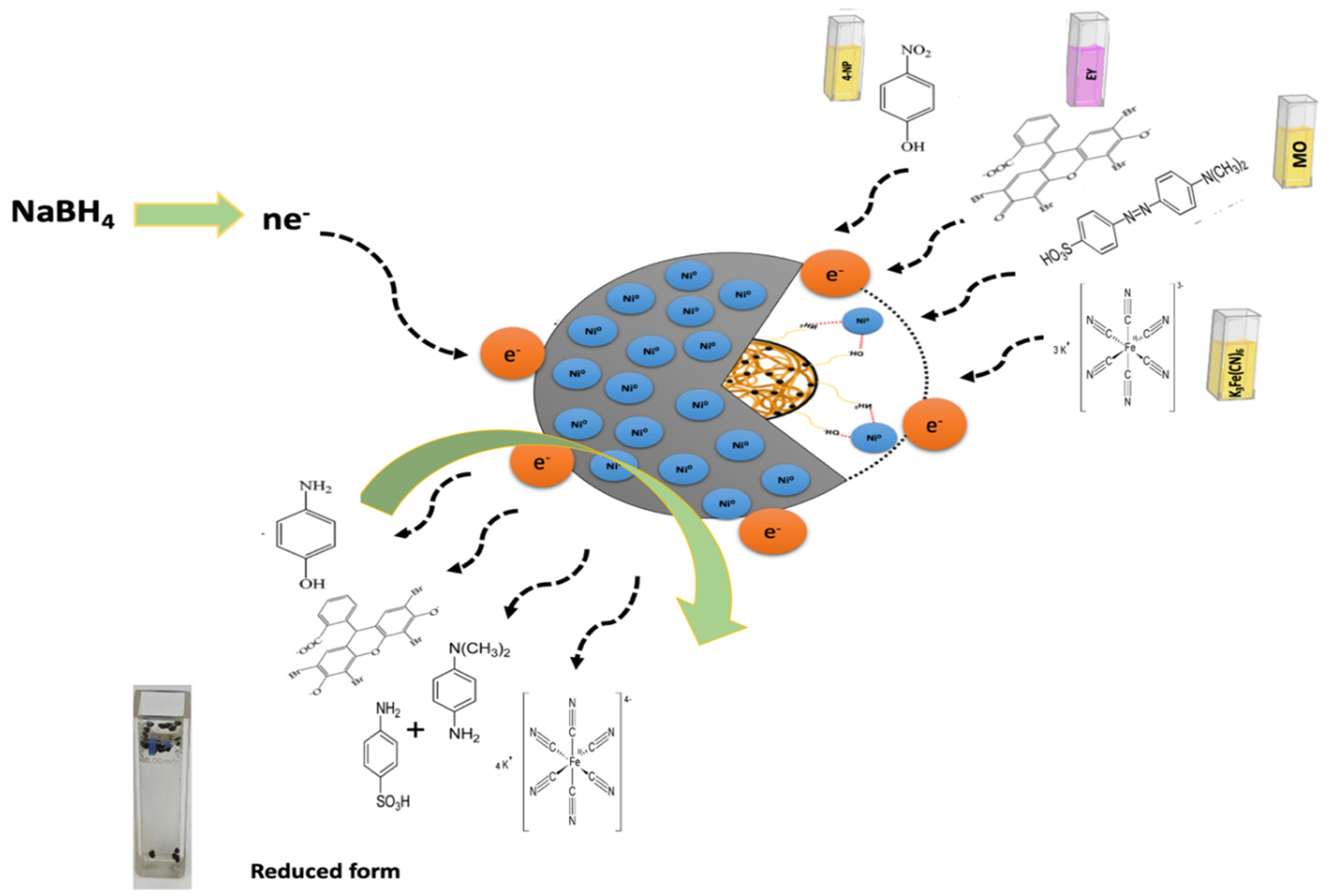
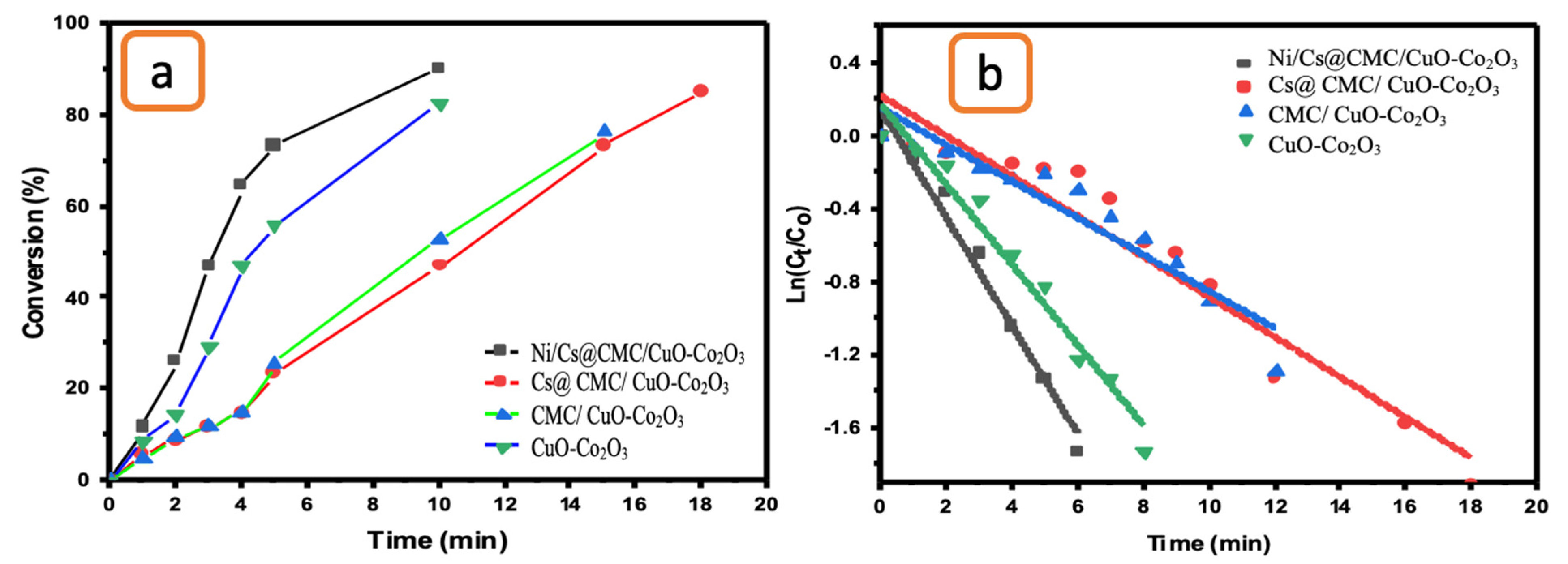
2.5. Catalytic Reduction Study
2.6. Catalytic Optimization
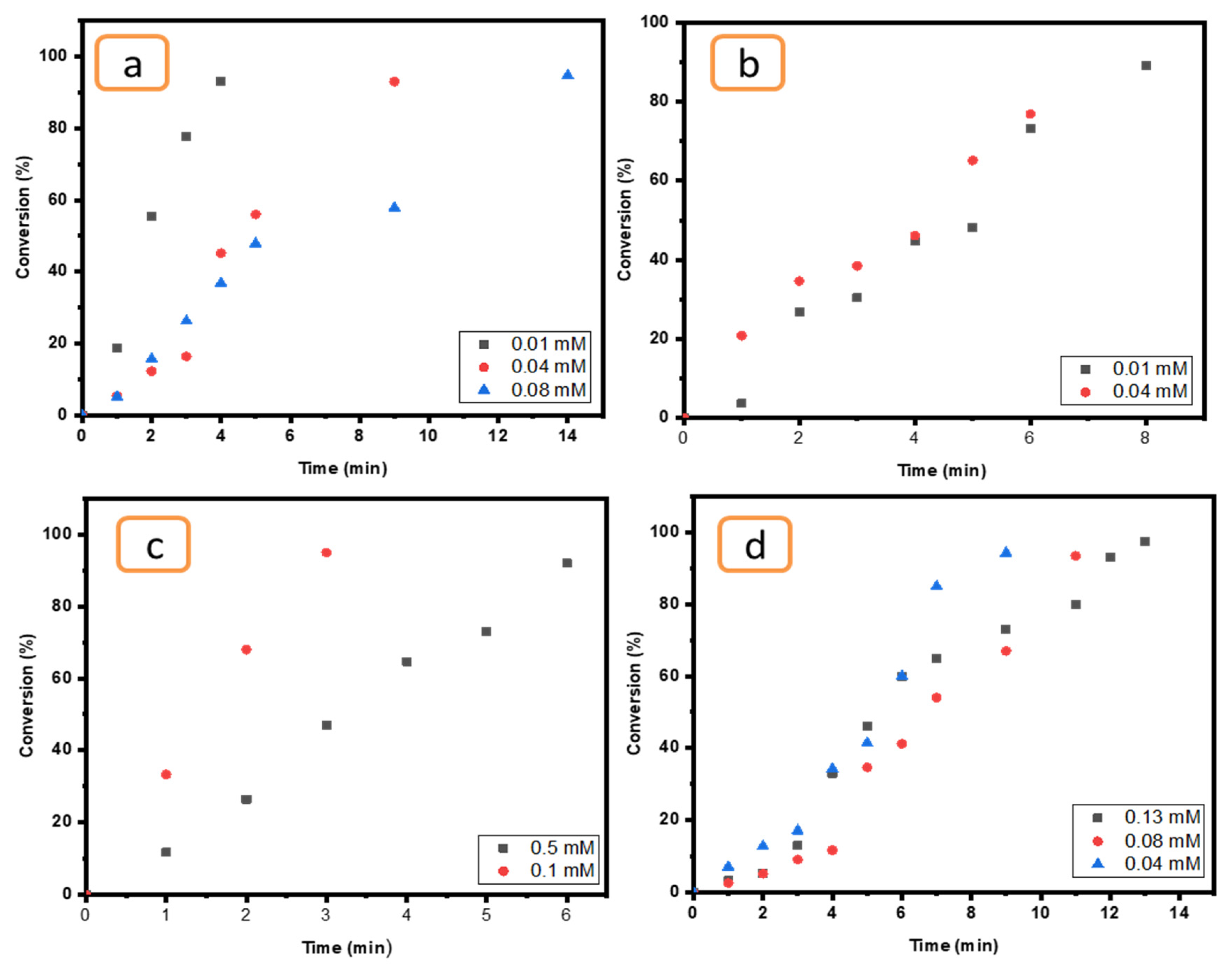
2.6.1. Effect of Contaminant Concentrations
2.6.2. Effect of NaBH4 Concentration
2.6.3. Effect of Number of Ni/Cs@CMC/CuO-Co2O3 Beads
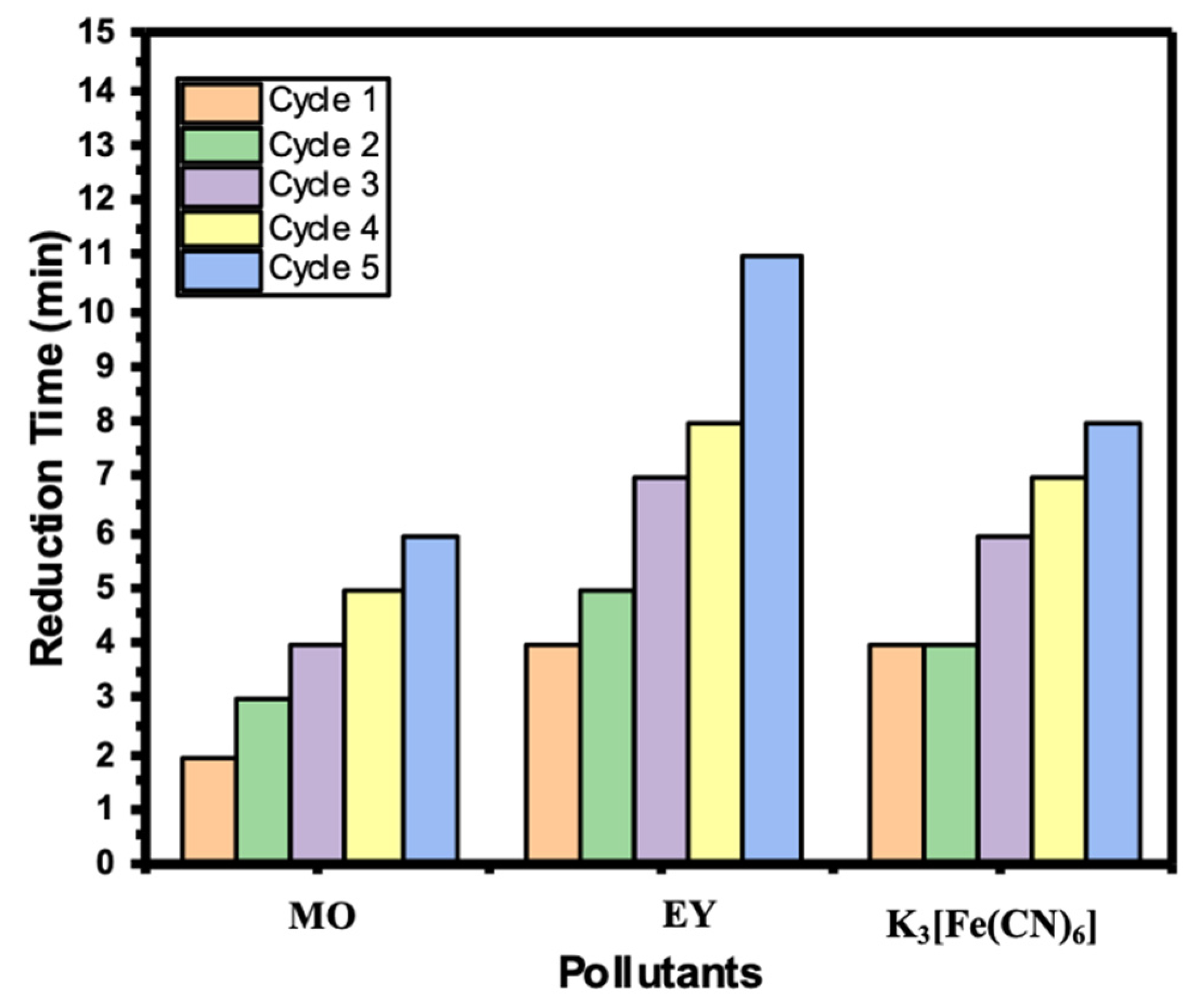
2.7. Recyclability of Ni/Cs@CMC/CuO-Co2O3 Beads
2.8. Application to Real Samples
3. Conclusions
4. Experimental
4.1. Materials
4.2. Synthesis of Nanocomposites and Beads
4.2.1. Preparation of CuO-Co2O3 Nanocomposite
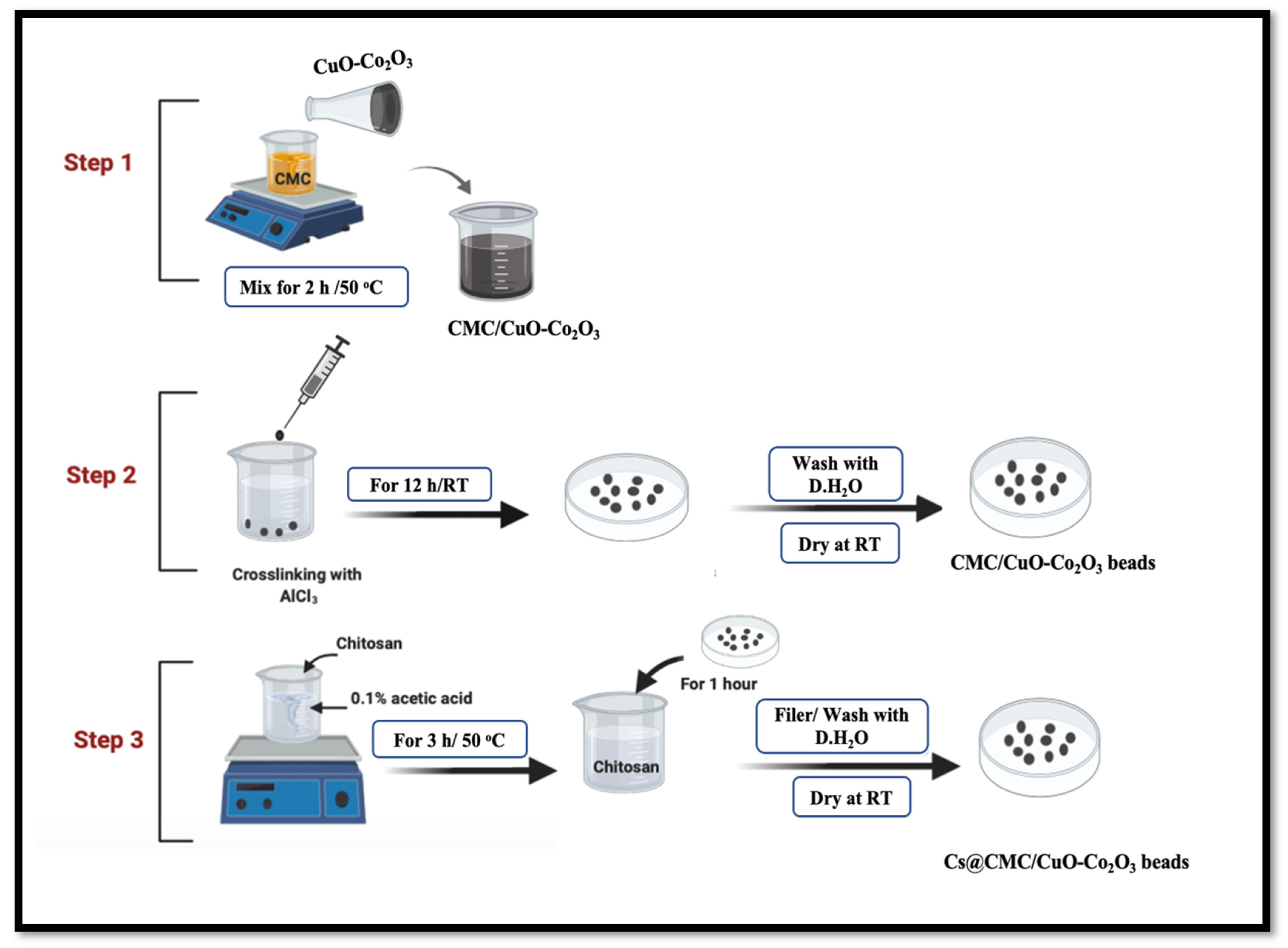
4.2.2. Synthesis of Cs@CMC/CuO-Co2O3 Beads
4.3. Metal Uptake Adsorption
4.4. Formation of Zero-Valent Nanoparticles
4.5. Catalytic Reduction Experiments
4.6. Characterization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crini, G. Non-Conventional Low-Cost Adsorbents for Dye Removal: A Review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef] [PubMed]
- Omidvar, A.; Jaleh, B.; Nasrollahzadeh, M. Preparation of the GO/Pd Nanocomposite and Its Application for the Degradation of Organic Dyes in Water. J. Colloid Interface Sci. 2017, 496, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Veerakumar, P.; Salamalai, K.; Thanasekaran, P.; Lin, K.C. Simple Preparation of Porous Carbon-Supported Ruthenium: Propitious Catalytic Activity in the Reduction of Ferrocyanate(III) and a Cationic Dye. ACS Omega 2018, 3, 12609–12621. [Google Scholar] [CrossRef] [PubMed]
- Maslamani, N.; Khan, S.B.; Danish, E.Y.; Bakhsh, E.M.; Zakeeruddin, S.M.; Asiri, A.M. Carboxymethyl Cellulose Nanocomposite Beads as Super-Efficient Catalyst for the Reduction of Organic and Inorganic Pollutants. Int. J. Biol. Macromol. 2021, 167, 101–116. [Google Scholar] [CrossRef]
- Kavak, D. Removal of Lead from Aqueous Solutions by Precipitation: Statistical Analysis and Modeling. Desalination Water Treat. 2013, 51, 1720–1726. [Google Scholar] [CrossRef]
- Trivunac, K.; Stevanovic, S. Removal of Heavy Metal Ions from Water by Complexation-Assisted Ultrafiltration. Chemosphere 2006, 64, 486–491. [Google Scholar] [CrossRef]
- Maslamani, N.; Manandhar, E.; Geremia, D.K.; Logue, B.A. ICE Concentration Linked with Extractive Stirrer (ICECLES). Anal. Chim. Acta 2016, 941, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Mende, M.; Schwarz, D.; Steinbach, C.; Boldt, R.; Schwarz, S. Simultaneous Adsorption of Heavy Metal Ions and Anions from Aqueous Solutions on Chitosan—Investigated by Spectrophotometry and SEM-EDX Analysis. Colloids Surf. A Physicochem. Eng. Asp. 2016, 510, 275–282. [Google Scholar] [CrossRef]
- Nageeb, M. Adsorption Technique for the Removal of Organic Pollutants from Water and Wastewater. Org. Pollut. Monit. Risk Treat. 2013, 7, 167–194. [Google Scholar]
- Schieder, D.; Dobias, B.; Klumpp, E.; Schwuger, M.J. Adsorption and Solubilisation of Phenols in the Hexadecyltrimethylammonium Chloride Adsorbed Layer on Quartz and Corundum. Colloids Surf. A Physicochem. Eng. Asp. 1994, 88, 103–111. [Google Scholar] [CrossRef]
- Almeida, C.A.P.; Debacher, N.A.; Downs, A.J.; Cottet, L.; Mello, C.A.D. Removal of Methylene Blue from Colored Effluents by Adsorption on Montmorillonite Clay. J. Colloid Interface Sci. 2009, 332, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Girods, P.; Dufour, A.; Fierro, V.; Rogaume, Y.; Rogaume, C.; Zoulalian, A.; Celzard, A. Activated Carbons Prepared from Wood Particleboard Wastes: Characterisation and Phenol Adsorption Capacities. J. Hazard. Mater. 2009, 166, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Aksu, Z. Biosorption of Reactive Dyes by Dried Activated Sludge: Equilibrium and Kinetic Modelling. Biochem. Eng. J. 2001, 7, 79–84. [Google Scholar] [CrossRef]
- Sun, X.; Shen, J.; Yu, D.; Ouyang, X.-K. Preparation of pH-Sensitive Fe3O4@C/Carboxymethyl Cellulose/Chitosan Composite Beads for Diclofenac Sodium Delivery. Int. J. Biol. Macromol. 2019, 127, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Bakhsh, E.M.; Ali, F.; Khan, S.B.; Marwani, H.M.; Danish, E.Y.; Asiri, A.M. Copper Nanoparticles Embedded Chitosan for Efficient Detection and Reduction of Nitroaniline. Int. J. Biol. Macromol. 2019, 131, 666–675. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Kaur, S.; Brar, S.K. Facile Fabrication and Characterization of Chitosan-Based Zinc Oxide Nanoparticles and Evaluation of Their Antimicrobial and Antibiofilm Activity. Int. Nano Lett. 2014, 4, 107. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, M.E.; Abdou, A.E.H.; Sobhy, M.E.; Fekry, N.A. Solid–Solid Crosslinking of Carboxymethyl Cellulose Nanolayer on Titanium Oxide Nanoparticles as a Novel Biocomposite for Efficient Removal of Toxic Heavy Metals from Water. Int. J. Biol. Macromol. 2017, 105, 1269–1278. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Saber-Samandari, S.; Joneidi-Yekta, H.; Mohseni, M. Adsorption of Anionic and Cationic Dyes from Aqueous Solution Using Gelatin-Based Magnetic Nanocomposite Beads Comprising Carboxylic Acid Functionalized Carbon Nanotube. Chem. Eng. J. 2017, 308, 1133–1144. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Wang, X.; Sun, Z.; Ma, J.; Wu, T.; Xing, F.; Gao, J. Porous graphene oxide/carboxymethyl cellulose monoliths, with high metal ion adsorption. Carbohydr. Polym. 2014, 101, 392–400. [Google Scholar] [CrossRef]
- Behzadi Nia, S.; Pooresmaeil, M.; Namazi, H. Carboxymethylcellulose/Layered Double Hydroxides Bio-Nanocomposite Hydrogel: A Controlled Amoxicillin Nanocarrier for Colonic Bacterial Infections Treatment. Int. J. Biol. Macromol. 2020, 155, 1401–1409. [Google Scholar]
- Maslamani, N.; Khan, S.B.; Danish, E.Y.; Bakhsh, E.M.; Zakeeruddin, S.M.; Asiri, A.M. Super Adsorption Performance of Carboxymethyl Cellulose/Copper Oxide-Nickel Oxide Nanocomposite toward the Removal of Organic and Inorganic Pollutants. Environ. Sci. Pollut. Res. 2021, 28, 38476–38496. [Google Scholar] [CrossRef] [PubMed]
- Rasoulzadeh, M.; Namazi, H. Carboxymethyl Cellulose/Graphene Oxide Bio-Nanocomposite Hydrogel Beads as Anticancer Drug Carrier Agent. Carbohydr. Polym. 2017, 168, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Crini, G. Recent Developments in Polysaccharide-Based Materials Used as Adsorbents in Wastewater Treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Vakili, M.; Deng, S.; Cagnetta, G.; Wang, W.; Meng, P.; Liu, D.; Yu, G. Regeneration of Chitosan-Based Adsorbents Used in Heavy Metal Adsorption: A Review. Sep. Purif. Technol. 2019, 224, 373–387. [Google Scholar] [CrossRef]
- Yusof, N.A.A.; Zain, N.M.; Pauzi, N. Synthesis of Chitosan/Zinc Oxide Nanoparticles Stabilized by Chitosan via Microwave Heating. Bull. Chem. React. Eng. Catal. 2019, 14, 450–458. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, A.G.; Saleh, A.S.; Elsharma, E.M.; Metwally, E.; Siyam, T. Chitosan g maleic Acid for Effective Removal of Copper and Nickel Ions from Their Solutions. Int. J. Biol. Macromol. 2019, 121, 1287–1294. [Google Scholar] [CrossRef]
- Jang, E.S.; Khan, S.B.; Seo, J.; Nam, Y.H.; Choi, W.J.; Akhtar, K.; Han, H. Synthesis and characterization of novel UV-curable polyurethane–clay nanohybrid: Influence of organically modified layered silicates on the properties of polyurethane. Prog. Organ. Coat. 2011, 71, 36–42. [Google Scholar] [CrossRef]
- Kim, D.; Jung, J.; Kim, Y.; Lee, M.; Seo, J.; Khan, S.B. Structure and thermal properties of octadecane/expanded graphite composites as shape-stabilized phase change materials. Int. J. Heat Mass Trans. 2016, 95, 735–741. [Google Scholar] [CrossRef]
- Khan, S.B.; Rahman, M.M.; Jang, E.S.; Akhtar, K.; Han, H. Special susceptive aqueous ammonia chemi-sensor: Extended applications of novel UV-curable polyurethane-clay nanohybrid. Talanta 2011, 84, 1005–1010. [Google Scholar] [CrossRef]
- Ismail, M.; Khan, M.; Akhtar, K.; Khan, M.A.; Asiri, A.M.; Khan, S.B. Biosynthesis of Silver Nanoparticles: A Colorimetric Optical Sensor for Detection of Hexavalent Chromium and Ammonia in Aqueous Solution. Available online: https://www.sciencedirect.com/science/article/abs/pii/S1386947718301279 (accessed on 19 January 2022). [CrossRef]
- Kamal, T.; Ahmad, I.; Khan, S.B.; Asiri, A.M. Bacterial cellulose as support for biopolymer stabilized catalytic cobalt nanoparticles. Int. J. Biol. Macromol. 2019, 135, 1162–1170. [Google Scholar] [CrossRef]
- Jang, E.S.; Khan, S.B.; Seo, J.; Akhtar, K.; Choi, J.; Kim, K.I.; Han, H. Synthesis and characterization of novel UV-Curable PU-Si hybrids: Influence of silica on thermal, mechanical, and water sorption properties of polyurethane acrylates. Macromol. Res. 2011, 19, 1006–1013. [Google Scholar] [CrossRef]
- Kamal, T.; Ali, N.; A Naseem, A.; B Khan, S.; M Asiri, A. Polymer nanocomposite membranes for antifouling nanofiltration. Recent. Pat. Nanotechnol. 2016, 10, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, D.; Seo, J.; Han, H.; Khan, S.B. Preparation and characterization of poly (propylene carbonate)/exfoliated graphite nanocomposite films with improved thermal stability, mechanical properties and barrier properties. Poly. Int. 2013, 62, 1386–1394. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Sharma, S.; Naushad, M.; Ghfar, A.A.; Ala’a, H.; Ahamad, T.; Sharma, N.; Stadler, F.J. Carboxymethyl cellulose structured nano-adsorbent for removal of methyl violet from aqueous solution: Isotherm and kinetic analyses. Cellulose 2020, 27, 3677–3691. [Google Scholar] [CrossRef]
- Ali, F.; Khan, S.B.; Kamal, T.; Alamry, K.A.; Asiri, A.M. Chitosan-Titanium Oxide Fibers Supported Zero-Valent Nanoparticles: Highly Efficient and Easily Retrievable Catalyst for the Removal of Organic Pollutants. Sci. Rep. 2018, 8, 6260. [Google Scholar] [CrossRef] [Green Version]
- Ali, N.; Kamal, T.; Ul-Islam, M.; Khan, A.; Shah, S.J.; Zada, A. Chitosan-coated cotton cloth supported copper nanoparticles for toxic dye reduction. Int. J. Biol. Macromol. 2018, 111, 832–838. [Google Scholar] [CrossRef]
- Rajeevgandhi, C.; Sathiyamurthy, K.; Guganathan, L.; Savithiri, S.; Bharanidharan, S.; Mohan, K. Experimental and Theoretical Investigations on the Spinel Structure of Co2O3 Nanoparticles Synthesized via Simple Co-Precipitation Method. J. Mater. Sci. Mater. Electron. 2020, 31, 16769–16779. [Google Scholar] [CrossRef]
- Barkhordari, S.; Yadollahi, M.; Namazi, H. pH Sensitive Nanocomposite Hydrogel Beads Based on Carboxymethyl Cellulose/Layered Double Hydroxide as Drug Delivery Systems. J. Polym. Res. 2014, 21, 454. [Google Scholar] [CrossRef]
- Roh, H.; Kim, Y.; Kim, Y.K.; Harbottle, D.; Lee, J.W. Amino-Functionalized Magnetic Chitosan Beads to Enhance Immobilization of Potassium Copper Hexacyanoferrate for Selective Cs+ Removal and Facile Recovery. RSC Adv. 2019, 9, 1106–1114. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.A.; Riaz-ur-Rehman; Khan, M.A. Adsorption of Chromium (III), Chromium (VI) and Silver (I) on Bentonite. Waste Manag. 1995, 15, 271–282. [Google Scholar] [CrossRef]
- Freire, T.M.; Fechine, L.M.U.D.; Queiroz, D.C.; Freire, R.M.; Denardin, J.C.; Ricardo, N.M.P.S.; Rodrigues, T.N.B.; Gondim, D.R.; Junior, I.J.S.; Fechine, P.B.A. Magnetic Porous Controlled Fe3O4–Chitosan Nanostructure: An Ecofriendly Adsorbent for Efficient Removal of Azo Dyes. Nanomaterials 2020, 10, 1194. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.V.; Dai Tran, L.; Nguyen, T.N. Preparation of chitosan/magnetite composite beads and their application for removal of Pb (II) and Ni (II) from aqueous solution. Mater. Sci. Eng. C 2010, 30, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.R.; Eagleton, L.C.; Acrivos, A.; Vermeulen, T. Pore- and Solid-Diffusion Kinetics in Fixed-Bed Adsorption under Constant-Pattern Conditions. Ind. Eng. Chem. Fundam. 1966, 5, 212–223. [Google Scholar] [CrossRef]
- Liu, X.; Hu, Q.; Fang, Z.; Zhang, X.; Zhang, B. Magnetic Chitosan Nanocomposites: A Useful Recyclable Tool for Heavy Metal Ion Removal. Langmuir 2009, 25, 3–8. [Google Scholar] [CrossRef]
- Kaur, N.; Kaur, M.; Singh, D. Fabrication of Mesoporous Nanocomposite of Graphene Oxide with Magnesium Ferrite for Efficient Sequestration of Ni (II) and Pb (II) Ions: Adsorption, Thermodynamic and Kinetic Studies. Environ. Pollut. 2019, 253, 111–119. [Google Scholar] [CrossRef]
- Vijaya, Y.; Popuri, S.R.; Boddu, V.M.; Krishnaiah, A. Modified Chitosan and Calcium Alginate Biopolymer Sorbents for Removal of Nickel (II) through Adsorption. Carbohydr. Polym. 2008, 72, 261–271. [Google Scholar] [CrossRef]
- Kong, A.; Ji, Y.; Ma, H.; Song, Y.; He, B.; Li, J. A Novel Route for the Removal of Cu(II) and Ni(II) Ions via Homogeneous Adsorption by Chitosan Solution. J. Clean. Prod. 2018, 192, 801–808. [Google Scholar] [CrossRef]
- Hao, Y.-M.; Man, C.; Hu, Z.-B. Effective removal of Cu (II) ions from aqueous solution by amino-functionalized magnetic nanoparticles. J. Hazard. Mater. 2010, 184, 392–399. [Google Scholar] [CrossRef]
- Tseng, R.-L.; Wu, P.-H.; Wu, F.-C.; Juang, R.-S. A convenient method to determine kinetic parameters of adsorption processes by nonlinear regression of pseudo-nth-order equation. Chem. Eng. J. 2014, 237, 153–161. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Jiang, D.; Yang, Y.; Huang, C.; Huang, M.; Chen, J.; Rao, T.; Ran, X. Removal of the Heavy Metal Ion Nickel (II) via an Adsorption Method Using Flower Globular Magnesium Hydroxide. J. Hazard. Mater. 2019, 373, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Tao, X.; Xu, J.; Yin, N. Novel Chitosan–MOF Composite Adsorbent for the Removal of Heavy Metal Ions. Chem. Lett. 2016, 45, 1365–1368. [Google Scholar] [CrossRef]
- Gibbs, G.; Tobin, J.M.; Guibal, E. Sorption of Acid Green 25 on Chitosan: Influence of Experimental Parameters on Uptake Kinetics and Sorption Isotherms. J. Appl. Polym. Sci. 2003, 90, 1073–1080. [Google Scholar] [CrossRef]
- Guibal, E. Interactions of Metal Ions with Chitosan-Based Sorbents: A Review. Sep. Purif. Technol. 2004, 38, 43–74. [Google Scholar] [CrossRef]
- Al-Ghamdi, Y.O.; Khan, S.A. Stabilization of zero-valent Au nanoparticles on carboxymethyl cellulose layer coated on chitosan-CBV 780 zeolite Y sheets: Assessment in the reduction of 4-nitrophenol and dyes. Cellulose 2020, 27, 8827–8841. [Google Scholar] [CrossRef]
- Amir, M.; Kurtan, U.; Baykal, A.; Sözeri, H. MnFe2O4@ PANI@ Ag heterogeneous nanocatalyst for degradation of industrial aqueous organic pollutants. J. Mater. Sci. Tech. 2016, 32, 134–141. [Google Scholar] [CrossRef]
- Vidhu, V.; Philip, D. Catalytic degradation of organic dyes using biosynthesized silver nanoparticles. Micron 2014, 56, 54–62. [Google Scholar] [CrossRef]
- Tüysüz, H.; Hwang, Y.J.; Khan, S.B.; Asiri, A.M.; Yang, P. Mesoporous Co3O4 as an Electrocatalyst for Water Oxidation. Nano Res. 2013, 6, 47–54. [Google Scholar] [CrossRef]
- Rasheed, T.; Nabeel, F.; Bilal, M.; Iqbal, H.M.N. Biogenic Synthesis and Characterization of Cobalt Oxide Nanoparticles for Catalytic Reduction of Direct Yellow-142 and Methyl Orange Dyes. Biocatal. Agric. Biotechnol. 2019, 19, 101154. [Google Scholar] [CrossRef]
- Umamaheswari, C.; Lakshmanan, A.; Nagarajan, N.S. Green Synthesis, Characterization and Catalytic Degradation Studies of Gold Nanoparticles against Congo Red and Methyl Orange. J. Photochem. Photobiol. B Biol. 2018, 178, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.S.H.M.; Khan, S.A. Stabilization of Various Zero-Valent Metal Nanoparticles on a Superabsorbent Polymer for the Removal of Dyes, Nitrophenol, and Pathogenic Bacteria. ACS Omega 2020, 5, 7379–7391. [Google Scholar] [CrossRef] [PubMed]
- Komalam, A.; Muraleegharan, L.G.; Subburaj, S.; Suseela, S.; Babu, A.; George, S. Designed Plasmonic Nanocatalysts for the Reduction of Eosin Y: Absorption and Fluorescence Study. Int. Nano Lett. 2012, 2, 26. [Google Scholar] [CrossRef] [Green Version]
- Kamal, T.; Ahmad, I.; Khan, S.B.; Ul-Islam, M.; Asiri, A.M. Microwave Assisted Synthesis and Carboxymethyl Cellulose Stabilized Copper Nanoparticles on Bacterial Cellulose Nanofibers Support for Pollutants Degradation. J. Polym. Environ. 2019, 27, 2867–2877. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, X.; Qin, Z.; Zhang, L.; Ye, Y.; Cao, M.; Gao, L.; Jiao, T. Preparation of PdNPs Doped Chitosan-Based Composite Hydrogels as Highly Efficient Catalysts for Reduction of 4-Nitrophenol. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125889. [Google Scholar] [CrossRef]
- Kamal, T.; Khan, S.B.; Asiri, A.M. Nickel Nanoparticles-Chitosan Composite Coated Cellulose Filter Paper: An Efficient and Easily Recoverable Dip-Catalyst for Pollutants Degradation. Environ. Pollut. 2016, 218, 625–633. [Google Scholar] [CrossRef]
- Nithiyanantham, U.; Ede, S.R.; Anantharaj, S.; Kundu, S. Self-Assembled NiWO4 Nanoparticles into Chain-like Aggregates on DNA Scaffold with Pronounced Catalytic and Supercapacitor Activities. Cryst. Growth Des. 2015, 15, 673–686. [Google Scholar] [CrossRef]
- Xie, R.; Wei, T.; Bai, S.; Wang, Z.; Chai, F.; Chen, L.; Zang, S. The Synthesis and Catalytic Activity of Bimetallic CuAg Nanoparticles and Their Magnetic Hybrid Composite Materials. New J. Chem. 2020, 44, 9684–9690. [Google Scholar] [CrossRef]
- Xia, Q.; Su, D.; Yang, X.; Chai, F.; Wang, C.; Jiang, J. One Pot Synthesis of Gold Hollow Nanospheres with Efficient and Reusable Catalysis. RSC Adv. 2015, 5, 58522–58527. [Google Scholar] [CrossRef]
- Maslamani, N.; Khan, S.B.; Danish, E.Y.; Bakhsh, E.M.; Akhtar, K.; Asiri, A.M. Metal nanoparticles supported chitosan coated carboxymethyl cellulose beads as a catalyst for the selective removal of 4-nitrophenol. Chemosphere 2022, 291, 133010. [Google Scholar] [CrossRef]

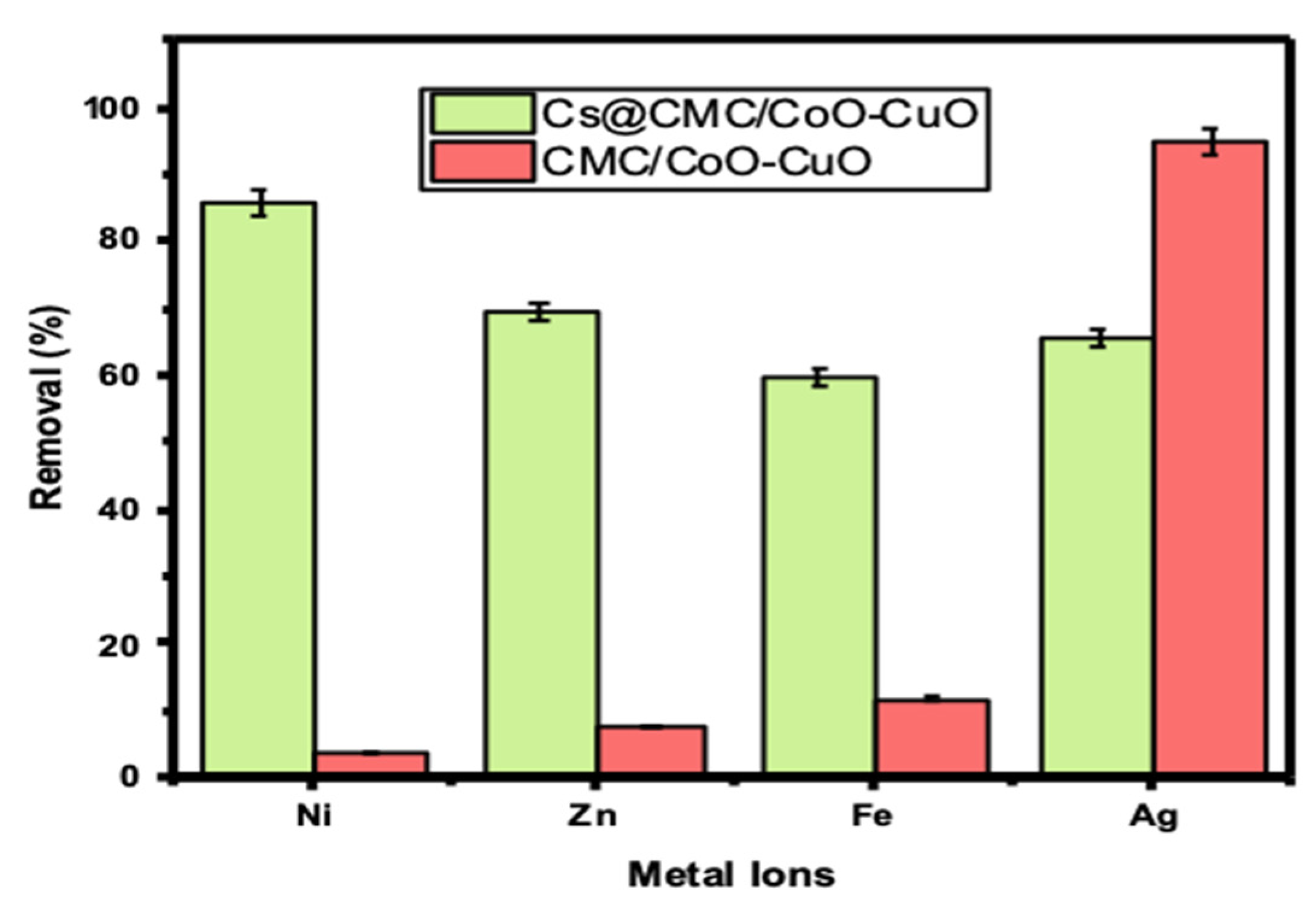




| Metal Ions | Wavelength Detection (nm) | Initial Conc. (mgL−1) | CMC/CuO-Co2O3 | Cs@CMC/CuO-Co2O3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Final Conc. (mgL−1) | qe (mgg −1) | Kd (mLg−1) | R (%) | Final Conc. (mgL−1) | qe (mgg−1) | Kd (mLg−1) | R (%) | |||
| Ni(II) | 221.648 | 5 | 4.8 | 0.2 | 41.666 | 4 | 0.697 | 4.3 | 6173.6 | 86.06 |
| Zn(II) | 213.9 | 5 | 4.6 | 0.4 | 86.956 | 8 | 1.5 | 3.5 | 2333.3 | 70 |
| Fe(II) | 248 | 5 | 4.4 | 0.6 | 136.363 | 12 | 2 | 3 | 1500 | 60 |
| Ag(I) | 243.778 | 5 | 0.23 | 5 | 20,739.1 | 95.4 | 1.7 | 3.3 | 1941.1 | 66 |
| Models | Linear Equations | Plot | |
|---|---|---|---|
| Isotherm | Langmuir | (4) | |
| Freundlich | (5) | ||
| Kinetic | Pseduo-first-order | (6) | |
| Pseduo-second-order | (7) |
| Langmuir Model | Freundlich Model | ||||||
|---|---|---|---|---|---|---|---|
| Metal Ion | R2 | b (Lmg−1) | R2 | ||||
| Ni(II) | 12.00 | 0.943 | 0.50 | 0.08 | 0.553 | 9.10 | 5.06 |
| (mg g−1) Experiment | Pseudo-First-Order | Pseudo-Second-Order | |||||
|---|---|---|---|---|---|---|---|
| Metal Ion | R2 | K1 (min−1) | mg g−1 | R2 | K2 (g mg−1min−1) | mg g−1 | |
| Ni(II) | 11.00 | 0.703 | 0.0011 | 2.72 | 0.986 | −0.222 | 4.05 |
| Adsorbent | Metal Ion | Condition | qmax (mgg−1) | Ref. |
|---|---|---|---|---|
| CS/TEOS/APTES | Ni(II) | pH 5.2, 30 °C, 30 min | 696.2 | [48] |
| Chitosan-g-maleic acid | Ni(II) | pH 8, room temperature, 60 min | 73.5 | [26] |
| Flower globular FGMH | Ni(II) | pH 6–7, 20 °C, 50 min | 248 | [52] |
| Chitosan-MOF | Ni(II) | pH 5, 20 °C, 480 min | 56 | [53] |
| Cs@CMC/CuO-Co2O3 | Ni(II) | pH 7, room temperature, 60 min | 11 | This study |
| Rate Constant K (s−1) and Adjacent R2 Value | ||||
|---|---|---|---|---|
| Compound | CuO-Co2O3 | CMC/CuO-Co2O3 | Cs@CMC/CuO-Co2O3 | Ni/Cs@CMC/CuO-Co2O3 |
| MO | 6.11 × 10−3 and 0.865 | 2.6 × 10−3 and 0.915 | 4.4 × 10−4 and 0.953 | 1.06 × 10−2 and 0.964 |
| EY | 2.95 × 10−4 and 0.989 | 3.2 × 10−3 and 0.928 | 7.71 × 10−4 and 0.968 | 4.58 × 10−3 and 0.936 |
| 4-NP | 2.7 × 10−3 and 0.855 | 2.62 × 10−3 and 0.842 | 1.7 × 10−5 and 0.824 | 4.26 × 10−3 and 0.912 |
| K3[Fe(CN)6] | 3.6 × 10−3 and 0.964 | 1.69 × 10−3 and 0.835 | 1.8 × 10−3 and 0.909 | 5.1 × 10−3 and 0.975 |
| Pollutant | Catalyst | Time (min) | Rate Constant | Ref. |
|---|---|---|---|---|
| MO | Ni/Cs@CMC/CuO-Co2O3 | 2 | 1.06 × 10−2 | This study |
| MO | TO-CoNPs | 60 | - | [60] |
| MO | AuNPs | 8 | 1.7 × 10−3 | [61] |
| MO | WBs loaded with Ni NPs | 15 | 2.37 × 10−3 | [62] |
| EY | Ni/Cs@CMC/CuO-Co2O3 | 6 | 4.58 × 10−3 | This study |
| EY | Chitosan-capped AuNPs | 50 | - | [63] |
| 4-NP | Ni/Cs@CMC/CuO-Co2O3 | 13 | 4.26 × 10−3 | This study |
| 4-NP | CMC-Cu | 35 | 9.1 × 10−4 | [64] |
| 4-NP | PdNPs doped chitosan | 15 | 3.63 × 10−3 | [65] |
| 4-NP | Ni/CS-FP | 28 | - | [66] |
| K3[Fe(CN)6] | Ni/Cs@CMC/CuO-Co2O3 | 6 | 5.1 × 10−3 | This study |
| K3[Fe(CN)6] | NiWO4 Nanoparticles | 240 | - | [67] |
| K3[Fe(CN)6] | Fe3O4-CuAg NPs | 3 | 19.3 × 10−3 | [68] |
| K3[Fe(CN)6] | CMC/CuO-NiO | 0.5 | 6.88 × 10−2 | [4] |
| Real Sample | Reduction Time (min) | Reduction % |
|---|---|---|
| Full-Fat Milk | 15 min | 65.5 |
| Orange Juice | 6 min | 91.4 |
| Pineapple Juice | 5 min | 95 |
| Apple Juice | 5 min | 97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maslamani, N.; Bakhsh, E.M.; Khan, S.B.; Danish, E.Y.; Akhtar, K.; Fagieh, T.M.; Su, X.; Asiri, A.M. Chitosan@Carboxymethylcellulose/CuO-Co2O3 Nanoadsorbent as a Super Catalyst for the Removal of Water Pollutants. Gels 2022, 8, 91. https://doi.org/10.3390/gels8020091
Maslamani N, Bakhsh EM, Khan SB, Danish EY, Akhtar K, Fagieh TM, Su X, Asiri AM. Chitosan@Carboxymethylcellulose/CuO-Co2O3 Nanoadsorbent as a Super Catalyst for the Removal of Water Pollutants. Gels. 2022; 8(2):91. https://doi.org/10.3390/gels8020091
Chicago/Turabian StyleMaslamani, Nujud, Esraa M. Bakhsh, Sher Bahadar Khan, Ekram Y. Danish, Kalsoom Akhtar, Taghreed M. Fagieh, Xintai Su, and Abdullah M. Asiri. 2022. "Chitosan@Carboxymethylcellulose/CuO-Co2O3 Nanoadsorbent as a Super Catalyst for the Removal of Water Pollutants" Gels 8, no. 2: 91. https://doi.org/10.3390/gels8020091
APA StyleMaslamani, N., Bakhsh, E. M., Khan, S. B., Danish, E. Y., Akhtar, K., Fagieh, T. M., Su, X., & Asiri, A. M. (2022). Chitosan@Carboxymethylcellulose/CuO-Co2O3 Nanoadsorbent as a Super Catalyst for the Removal of Water Pollutants. Gels, 8(2), 91. https://doi.org/10.3390/gels8020091








