Hydrogels in Burn Wound Management—A Review
Abstract
:1. Introduction
2. Burn Wound Cooling
3. Hydrogels in Burn Wound Management
4. Types of Hydrogels
5. Antimicrobial Activity
6. Promotion of Wound Healing
7. Thermo-Sensitive Hydrogel
8. Clinical Application
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herman, T.F.; Bordoni, B. Wound Classification. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Oltulu, P.; Ince, B.; Kokbudak, N.; Findik, S.; Kilinc, F. Measurement of epidermis, dermis, and total skin thicknesses from six different body regions with a new ethical histometric technique. Turk. J. Plast. Surg. 2018, 26, 56. [Google Scholar] [CrossRef]
- Chopra, K.; Calva, D.; Sosin, M.D.; Tadisina, K.K.; Banda, A.; De La Cruz, C.; Chaudhry, M.R.; Legesse, T.; Drachenberg, C.B.; Manson, P.N.; et al. A comprehensive examination of topographic thickness of skin in the human face. Aesthetic Surg. J. 2015, 35, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Levy, V.; Lindon, C.; Zheng, Y.; Harfe, B.D.; Morgan, B.A. Epidermal stem cells arise from the hair follicle after wounding. FASEB J. 2007, 21, 1358–1366. [Google Scholar] [CrossRef]
- Demling, R.H. Burn injury. Acute Care 1985, 11, 119–186. [Google Scholar] [PubMed]
- Jackson, D.M. The diagnosis of the depth of burning. Br. J. Surg. 1953, 40, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.M. Second thoughts on the burn wound. J. Trauma Inj. Infect. Crit. Care 1969, 9, 839–862. [Google Scholar] [CrossRef] [PubMed]
- Demling, R.H.; Mazess, R.B.; Wolberg, W. The effect of immediate and delayed cold immersion on burn edema formation and resorption. J. Trauma Inj. Infect. Crit. Care 1979, 19, 56–60. [Google Scholar] [CrossRef]
- Raine, T.J.; London, M.D.; Robson, M.C.; Heggers, J.P. Progression of thermal injury: A morphologic study. Plast. Reconstr. Surg. 1982, 69, 491–499. [Google Scholar] [CrossRef]
- De Camara, D.L.; Raine, T.; Robson, M.C. Ultrastructural aspects of cooled thermal injury. J. Trauma Inj. Infect. Crit. Care 1981, 21, 911–919. [Google Scholar] [CrossRef]
- Wood, F.M.; Phillips, M.; Jovic, T.; Cassidy, J.T.; Cameron, P.; Edgar, D.W. Steering Committee of the Burn Registry of Australia and New Zealand (BRANZ). Water First Aid Is Beneficial In Humans Post-Burn: Evidence from a Bi-National Cohort Study. PLoS ONE 2016, 11, e0147259. [Google Scholar] [CrossRef]
- Ashman, H. Cooling of thermal burn injuries: A literature review. J. Paramed. Pract. 2018, 10, 200–204. [Google Scholar] [CrossRef]
- Wright, E.H.; Tyler, M.; Vojnovic, B.; Pleat, J.; Harris, A.; Furniss, D. Human model of burn injury that quantifies the benefit of cooling as a first aid measure. Br. J. Surg. 2019, 106, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Cuttle, L.; Kempf, M.; Liu, P.-Y.; Kravchuk, O.; Kimble, R.M. The optimal duration and delay of first aid treatment for deep partial thickness burn injuries. Burns 2010, 36, 673–679. [Google Scholar] [CrossRef]
- Hughes, A.; Almeland, S.K.; Leclerc, T.; Ogura, T.; Hayashi, M.; Mills, J.-A.; Norton, I.; Potokar, T. Recommendations for burns care in mass casualty incidents: WHO Emergency Medical Teams Technical Working Group on Burns (WHO TWGB) 2017–2020. Burns 2021, 47, 349–370. [Google Scholar] [CrossRef] [PubMed]
- Coats, T.J.; Edwards, C.; Newton, R.; Staun, E. The effect of gel burn dressings on skin temperature. Emerg. Med. J. 2002, 19, 224–225. [Google Scholar] [CrossRef]
- Strużyna, J.; Surowiecka, A.; Korzeniowski, T. Letter to the Editor on Recommendations for burns care in mass casualty incidents: WHO Emergency Medical Teams Technical Working Group on Burns (WHO TWGB) 2017–2020. Burns 2021, 47, 1929–1930. [Google Scholar] [CrossRef]
- Wichterle, O.; Lím, D. Hydrophilic Gels for Biological Use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Goodwin, N.S. European Resuscitation Council 2015 burn 1st Aid recommendations–concerns and issues for first responders. Burns 2016, 42, 1148–1150. [Google Scholar] [CrossRef]
- Walker, A.; Baumber, R.; Robson, B. Pre-hospital management of burns by the UK fire service. Emerg. Med. J. 2005, 22, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Hhauflaire-Uhoda, E.; Paquet, P.; Pierard, G.E. Dew point effect of cooled hydrogel pads on human stratum conreum biosurface. Dermatology 2008, 216, 37–39. [Google Scholar] [CrossRef]
- Holbert, M.D.; Kimble, R.M.; Chatfield, M.; Griffin, B.R. Effectiveness of a hydrogel dressing as an analgesic adjunct to first aid for the treatment of acute paediatric burn injuries: A prospective randomised controlled trial. BMJ Open 2021, 11, e039981. [Google Scholar] [CrossRef]
- Daunton, C.; Kothari, S.; Smith, L.; Steele, D. A history of materials and practices for wound management. Wound Pract. Res. 2012, 20, 174–184. [Google Scholar]
- Murphy, S.; Skardal, A.; Atala, A. Evaluation of hydrogels for bio-printing applications. Biomed. Mater. Res. 2013, 101, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Wasiak, J.; Cleland, H.; Campbell, F.; Spinks, A. Dressings for superficial and partial thickness burns. Cochrane Database Syst Rev. 2013, 2013, CD002106. [Google Scholar] [CrossRef] [PubMed]
- Madaghiele, M.; Demitri, C.; Sannino, A.; Ambrosio, L. Polymeric hydrogels for burn wound care: Advanced skin wound dressings and regenerative templates. Burns Trauma 2014, 2, 153–161. [Google Scholar] [CrossRef]
- Nalampang, K.; Panjakha, R.; Molloy, R.; Tighe, B.J. Structural effects in photopolymerized sodium AMPS hydrogels crosslinked with poly(ethylene glycol) diacrylate for use as burn dressings. J. Biomater. Sci. Polym. Ed. 2013, 24, 1291–1304. [Google Scholar] [CrossRef]
- Edwards, J. Hydrogels and their potential uses in burn wound management. Br. J. Nurs. 2010, 19, S12–S16. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Huang, Z.; Wang, X.; Chen, L.; Zhang, Y.; Zhang, L. On-Demand Dissolvable Self-Healing Hydrogel Based on Carboxymethyl Chitosan and Cellulose Nanocrystal for Deep Partial Thickness Burn Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 41076–41088. [Google Scholar] [CrossRef]
- Bankoti, K.; Rameshbabu, A.P.; Datta, S.; Goswami, P.; Roy, M.; Das, D.; Ghosh, S.K.; Das, A.K.; Mitra, A.; Pal, S.; et al. Dual Functionalized Injectable Hybrid Extracellular Matrix Hydrogel for Burn Wounds. Biomacromolecules 2021, 22, 514–533. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Handley, J.M. Adverse events associated with nonablative cutaneous visible and infrared laser treatment. J. Am. Acad. Dermatol. 2006, 55, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Cassuto, D.; Molla, J.-F.; Scrimali, L.; Sirago, P. Right-left comparison study of hydrogel pad versus transparent fluid gel in patients with dermo-cosmetic lesions undergoing non-ablative laser therapy. J. Cosmet. Laser Ther. 2009, 11, 45–51. [Google Scholar] [CrossRef]
- Stoica, A.E.; Chircov, C.; Grumezescu, A.M. Hydrogel Dressings for the Treatment of Burn Wounds: An Up-To-Date Overview. Materials 2020, 13, 2853. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhang, A.; Yuan, C.; Chen, X.; Liu, Y. Recent trends on burn wound care: Hydrogel dressings and scaffolds. Biomater. Sci. 2021, 9, 4523–4540. [Google Scholar] [CrossRef] [PubMed]
- Burd, A. Evaluating the use of hydrogel sheet dressings in comprehensive burn wound care. Ostomy Wound Manag. 2007, 53, 52–62. [Google Scholar]
- Shu, W.; Wang, Y.; Zhang, X.; Li, C.; Le, H.; Chang, F. Functional Hydrogel Dressings for Treatment of Burn Wounds. Front. Bioeng. Biotechnol. 2021, 9, 788461. [Google Scholar] [CrossRef]
- Khodja, A.N.; Mahlous, M.; Tahtat, D.; Benamer, S.; Youcef, S.L.; Chader, H.; Mouhoub, L.; Sedgelmaci, M.; Ammi, N.; Mansouri, M.B.; et al. Evaluation of healing activity of PVA/chitosan hydrogels on deep second degree burn: Pharmacological and toxicological tests. Burns 2013, 39, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Gharibi, R.; Shaker, A.; Rezapour-Lactoee, A.; Agarwal, S. Antibacterial and Biocompatible Hydrogel Dressing Based on Gelatin- and Castor-Oil-Derived Biocidal Agent. ACS Biomater. Sci. Eng. 2021, 7, 3633–3647. [Google Scholar] [CrossRef]
- Boonkaew, B.; Kempf, M.; Kimble, R.; Supaphol, P.; Cuttle, L. Antimicrobial efficacy of a novel silver hydrogel dressing compared to two common silver burn wound dressings: Acticoat™ and PolyMem Silver®. Burns 2014, 40, 89–96. [Google Scholar] [CrossRef]
- Biazar, E.; Roveimiab, Z.; Shahhosseini, G.; Khataminezhad, M.; Zafari, M.; Majdi, A. Biocompatibility Evaluation of a New Hydrogel Dressing Based on Polyvinylpyrrolidone/Polyethylene Glycol. J. Biomed. Biotechnol. 2011, 2012, 343989. [Google Scholar] [CrossRef]
- Oliveira, R.N.; Rouzé, R.; Quilty, B.; Alves, G.; Soares, G.D.A.; Thiré, R.; McGuinness, G. Mechanical properties and in vitro characterization of polyvinyl alcohol-nano-silver hydrogel wound dressings. Interface Focus 2014, 4, 20130049. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.T.S.; Lakshmanan, V.K.; Anilkumar, T.; Ramya, C.; Reshmi, P.; Unnikrishnan, A.; Nair, S.V.; Jayakumar, R. Flexible and Microporous Chitosan Hydrogel/Nano ZnO Composite Bandages for Wound Dressing: In Vitro and In Vivo Evaluation. ACS Appl. Mater. Interfaces 2012, 4, 2618–2629. [Google Scholar] [CrossRef]
- Chakavala, S.; Patel, N.; Pate, N.V.; Thakkar, V.; Patel, K.; Gandhi, T. Development and in vivo evaluation of silver sulfadiazine loaded hydrogel consisting polyvinyl alcohol and chitosan for severe burns. J. Pharm. Bioallied Sci. 2012, 4, 54–56. [Google Scholar] [CrossRef]
- Zohdi, R.M.; Zakaria, Z.A.B.; Yusof, N.; Mustapha, N.M.; Abdullah, M.N. Gelam (Melaleuca spp.) honey-based hydrogel as burn wound dressing. Evid. Based Complementary Altern. Med. 2012, 2012, 843025. [Google Scholar]
- Zhou, G.; Ruhan, A.; Ge, H.; Wang, L.; Liu, M.; Wang, B.; Su, H.; Yan, M.; Xi, Y.; Fan, Y. Research on a novel poly (vinyl alcohol)/lysine/vanillin wound dressing: Biocompatibility, bioactivity and antimicrobial activity. Burns 2014, 40, 1668–1678. [Google Scholar] [CrossRef] [PubMed]
- Grolman, J.M.; Singh, M.; Mooney, D.; Eriksson, E.; Nuutila, K. Antibiotic-Containing Agarose Hydrogel for Wound and Burn Care. J. Burn Care Res. 2019, 40, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y.; Cai, K.; Zhang, B.; Tang, S.; Zhang, W.; Liu, W. Antibacterial polysaccharide-based hydrogel dressing containing plant essential oil for burn wound healing. Burn. Trauma 2021, 9, tkab041. [Google Scholar] [CrossRef]
- Zhu, C.; Zhao, J.; Kempe, K.; Wilson, P.; Wang, J.; Velkov, T.; Li, J.; Davis, T.; Whittaker, M.; Haddleton, D.M. A Hydrogel-Based Localized Release of Colistin for Antimicrobial Treatment of Burn Wound Infection. Macromol. Biosci. 2017, 17. [Google Scholar] [CrossRef]
- Sun, G.; Zhang, X.; Shen, Y.-I.; Sebastian, R.; Dickinson, L.E.; Fox-Talbot, K.; Reinblatt, M.; Steenbergen, C.; Harmon, J.W.; Gerecht, S. Dextran hydrogel scaffolds enhance angiogenic responses and promote complete skin regeneration during burn wound healing. Proc. Natl. Acad. Sci. USA 2011, 108, 20976–20981. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Huang, S.; Hu, Z.; Yang, W.; Lan, Y.; Zhu, J.; Hancharou, A.; Guo, R.; Tang, B. In situ formed anti-inflammatory hydrogel loading plasmid DNA encoding VEGF for burn wound healing. Acta Biomater. 2019, 100, 191–201. [Google Scholar] [CrossRef]
- Natesan, S.; Zamora, D.O.; Wrice, N.L.; Baer, D.G.; Christy, R.J. Bilayer Hydrogel with Autologous Stem Cells Derived from Debrided Human Burn Skin for Improved Skin Regeneration. J. Burn Care Res. 2013, 34, 18–30. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef]
- White-Dzuro, C.G.; Burns, B.; Pollins, A.; Rector, J.A.; Assi, P.E.; Thomas, H.C.; Jackson, K.; Perdikis, G.; Al Kassis, S.; Bellan, L.M.; et al. Successful prevention of secondary burn progression using infliximab hydrogel: A murine model. Burns 2021. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Mazumder, B.; Rajkonwar, J.; Pathak, M.P.; Patowary, P.; Chattopadhyay, P. bFGF and collagen matrix hydrogel attenuates burn wound inflammation through activation of ERK and TRK pathway. Sci. Rep. 2021, 11, 3357. [Google Scholar] [CrossRef] [PubMed]
- Church, D.; Elsayed, S.; Reid, O.; Winston, B.; Lindsay, R. Burn wound infections. Clin. Microbiol. Rev. 2006, 2, 403–434. [Google Scholar] [CrossRef]
- Gomez, R.; Murray, C.K.; Hospenthal, D.R.; Cancio, L.C.; Renz, E.M.; Holcomb, J.B.; Wade, C.E.; Wolf, S. Causes of Mortality by Autopsy Findings of Combat Casualties and Civilian Patients Admitted to a Burn Unit. J. Am. Coll. Surg. 2009, 208, 348–354. [Google Scholar] [CrossRef]
- Altoparlak, U.; Erol, S.; Akcay, M.N.; Celebi, F.; Kadanali, A. The time-related changes of antimicrobial resistance patterns and predominant bacterial profiles of burn wounds and body flora of burned patients. Burns 2004, 30, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Kopecki, Z. Development of next-generation antimicrobial hydrogel dressing to combat burn wound infection. Biosci. Rep. 2021, 41. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Konieczynska, M.D.; Villa-Camacho, J.C.; Ghobril, C.; Perez-Viloria, M.; Tevis, K.M.; Blessing, W.A.; Nazarian, A.; Rodriguez, E.K.; Grinstaff, M.W. On-Demand Dissolution of a Dendritic Hydrogel-based Dressing for Second-Degree Burn Wounds through Thiol–Thioester Exchange Reaction. Angew. Chem. 2016, 128, 10138–10141. [Google Scholar] [CrossRef]
- Konop, M.; Damps, T.; Misicka, A.; Rudnicka, L. Certain Aspects of Silver and Silver Nanoparticles in Wound Care: A Minireview. J. Nanomater. 2016, 2016, 7614753. [Google Scholar] [CrossRef]
- Boonkaew, B.; Barber, P.M.; Rengpipat, S.; Supaphol, P.; Kempf, M.; He, J.; John, V.T.; Cuttle, L. Development and Characterization of a Novel, Antimicrobial, Sterile Hydrogel Dressing for Burn Wounds: Single-Step Production with Gamma Irradiation Creates Silver Nanoparticles and Radical Polymerization. J. Pharm. Sci. 2014, 103, 3244–3253. [Google Scholar] [CrossRef] [PubMed]
- Jodar, K.S.P.; Balca, V.M.; Chaud, O.M.V.; Tubino, M.; Yoshida, V.M.H.; Oliveira, J.M., Jr.; Vila, M.M.D.C. Development and Characterization of a Hydrogel Containing Silver Sulfadiazine for Antimicrobial Topical Applications. J. Pharm. Sci. 2015, 104, 2241–2254. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, H.; Yang, X.; Zhang, W.; Jiang, M.; Wen, T.; Wang, J.; Guo, R.; Liu, H. Preparation and Application of Quaternized Chitosan- and AgNPs-Base Synergistic Antibacterial Hydrogel for Burn Wound Healing. Molecules 2021, 26, 4037. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-I.; Song, H.-H.G.; Papa, A.; Burke, J.A.; Volk, S.W.; Gerecht, S. Acellular Hydrogels for Regenerative Burn Wound Healing: Translation from a Porcine Model. J. Investig. Dermatol. 2015, 135, 2519–2529. [Google Scholar] [CrossRef]
- Strużyna, J.; Pojda, Z. Zastosowania komórek macierzystych z tkanki tłuszczowej w medycynie regeneracyjnej. Chir. Plast. Oparzenia Plast. Surg. Burn. 2015, 3, 151–157. [Google Scholar] [CrossRef]
- Shukla, L.; Morrison, W.A.; Shayan, R. Adipose-derived stem cells in radiotherapy injury: A new frontier. Front Surg. 2015, 2. [Google Scholar] [CrossRef]
- Suh, A.; Pham, A.; Cress, M.J.; Pincelli, T.; TerKonda, S.P.; Bruce, A.J.; Zubair, A.C.; Wolfram, J.; Shapiro, S.A. Adipose-derived cellular and cell-derived regenerative therapies in dermatology and aesthetic rejuvenation. Ageing Res. Rev. 2019, 54, 100933. [Google Scholar] [CrossRef]
- Gimble, J.M.; Katz, A.J.; Bunnell, B. Adipose-Derived Stem Cells for Regenerative Medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef]
- Chen, A.; Zhang, L.; Chen, P.; Zhang, C.; Tang, S.; Chen, X. Comparison of the Efficacy and Safety of Cell-Assisted Lipotransfer and Platelet-Rich Plasma Assisted Lipotransfer: What Should We Expect from a Systematic Review with Meta-Analysis? Cell Transplant. 2021, 30, 963689721989607. [Google Scholar] [CrossRef]
- Xiong, S.; Yi, C.; Pu, L.L. An Overview of Principles and New Techniques for Facial Fat Grafting. Clin. Plast. Surg. 2020, 47, 7–17. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, N.A.; Syed, A.; Wong, M.; Hicks, J.; Nunez, G.; Jitianu, A.; Siler, Z.; Peterson, M. Polydopamine Antioxidant Hydrogels for Wound Healing Applications. Gels 2020, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Ou, Q.; Zhang, S.; Fu, C.; Yu, L.; Xin, P.; Gu, Z.; Cao, Z.; Wu, J.; Wang, Y. More natural more better: Triple natural anti-oxidant puerarin/ferulic acid/polydopamine incorporated hydrogel for wound healing. J. Nanobiotechnol. 2021, 19, 237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ou, Q.; Xin, P.; Yuan, Q.; Wang, Y.; Wu, J. Polydopamine/puerarin nanoparticle-incorporated hybrid hydrogels for enhanced wound healing. Biomater. Sci. 2019, 7, 4230–4236. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Hu, T.; Han, Y.; Guo, B. Mussel-inspired, antibacterial, conductive, antioxidant, injectable composite hydrogel wound dressing to promote the regeneration of infected skin. J. Colloid Interface Sci. 2019, 556, 514–528. [Google Scholar] [CrossRef]
- Yuan, Y.; Shen, S.; Fan, D. A physicochemical double cross-linked multifunctional hydrogel for dynamic burn wound healing: Shape adaptability, injectable self-healing property and enhanced adhesion. Biomaterials 2021, 276, 120838. [Google Scholar] [CrossRef]
- Pan, H.; Fan, D.; Duan, Z.; Zhu, C.; Fu, R.; Li, X. Non-stick hemostasis hydrogels as dressings with bacterial barrier activity for cutaneous wound healing. Mater. Sci. Eng. C 2019, 105, 110118. [Google Scholar] [CrossRef]
- Zhang, K.; Xue, K.; Loh, X. Thermo-Responsive Hydrogels: From Recent Progress to Biomedical Applications. Gels 2021, 7, 77. [Google Scholar] [CrossRef]
- Lei, Z.; Singh, G.; Min, Z.; Shixuan, C.; Xu, K.; Pengcheng, X.; Xueer, W.; Yinghua, C.; Lu, Z.; Lin, Z. Bone marrow-derived mesenchymal stem cells laden novel thermo-sensitive hydrogel for the management of severe skin wound healing. Mater. Sci. Eng. C 2018, 90, 159–167. [Google Scholar] [CrossRef]
- Cao, D.; Chen, X.; Cao, F.; Guo, W.; Tang, J.; Cai, C.; Cui, S.; Yang, X.; Yu, L.; Su, Y.; et al. An Intelligent Transdermal Formulation of ALA-Loaded Copolymer Thermogel with Spontaneous Asymmetry by Using Temperature-Induced Sol–Gel Transition and Gel–Sol (Suspension) Transition on Different Sides. Adv. Funct. Mater. 2021, 31, 2100349. [Google Scholar] [CrossRef]
- Wang, W.; Wat, E.; Hui, P.C.; Chan, B.; Ng, F.S.; Kan, C.W.; Wang, X.; Hu, H.; Wong, E.C.; Lau, C.B.; et al. Dual-functional transdermal drug delivery system with controllable drug loading based on thermosensitive poloxamer hydrogel for atopic dermatitis treatment. Sci. Rep. 2016, 6, 24112. [Google Scholar] [CrossRef] [PubMed]
- Djekic, L.; Krajisnik, D.; Martinovic, M.; Djordjevic, D.; Primorac, M. Characterization of gelation proces and drug release profile of thermosensitive liquid lecithin/poloxamer 407 based gels as carriers for percutaneous delivery of ibuprofen. Int. J. Pharmaceut. 2015, 490, 180–189. [Google Scholar] [CrossRef]
- Kim, Y.C.; Shin, M.D.; Hackett, S.F.; Hsueh, H.T.; Silva, R.L.; Date, A.; Han, H.; Kim, B.-J.; Xiao, A.; Kim, Y.; et al. Gelling hypotonic polymer solution for extended topical drug delivery to the eye. Nat. Biomed. Eng. 2020, 4, 1053–1062. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, J.; Li, N. A novel thermo-sensitive hydrogel-based on poly(N-isopropylacrylamide)/hyaluronic acid of ketoconazole for ophthalmic delivery. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1282–1287. [Google Scholar] [CrossRef]
- Tan, G.; Yu, S.; Li, J.; Pan, W. Development and characterization of nanostructured lipid carriers based chitosan thermosensitive hydrogel for delivery of dexamethasone. Int. J. Biol. Macromol. 2017, 103, 941–947. [Google Scholar] [CrossRef]
- Chu, K.; Chen, L.; Xu, W.; Li, H.; Zhang, Y.; Xie, W.; Zheng, J. Preparation of a Paeonol-Containing Temperature-Sensitive In Situ Gel and Its Preliminary Efficacy on Allergic Rhinitis. Int. J. Mol. Sci. 2013, 14, 6499–6515. [Google Scholar] [CrossRef] [PubMed]
- Crowe, T.; Greenlee, M.H.W.; Kanthasamy, A.; Hsu, W.H. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef]
- Abouhussein, D.M.; Khattab, A.; Bayoumi, N.A.; Mahmoud, A.F.; Sakr, T.M. Brain targeted rivastigmine mucoadhesive thermosensitive in situ gel: Optimization, in vitro evaluation, radiolabeling, in vivo pharmacokinetics and biodistribution. J. Drug Deliv. Sci. Technol. 2018, 43, 129–140. [Google Scholar] [CrossRef]
- Morelli, L.; Cappelluti, M.A.; Ricotti, L.; Lenardi, C.; Gerges, I. An Injectable System for Local and Sustained Release of Antimicrobial Agents in the Periodontal Pocket. Macromol. Biosci. 2017, 17, 1700103. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.; Seguin, J.; Destruel, P.-L.; Dumortier, G.; Maury, M.; Dhotel, H.; Bessodes, M.; Scherman, D.; Mignet, N.; Boudy, V. Cyanine derivative as a suitable marker for thermosensitive in situ gelling delivery systems: In vitro and in vivo validation of a sustained buccal drug delivery. Int. J. Pharm. 2017, 534, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Song, X.; Wen, Y.; Zhu, J.L.; Li, J. Injectable Thermoresponsive Hydrogel Formed by Alginate-g-Poly (Nisopropylacrylamide) That Releases Doxorubicin-Encapsulated Micelles as a Smart Drug Delivery System. ACS Appl. Mater. Interfaces 2017, 9, 35673–35682. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Wang, H.; Qiu, Y.-K.; Liow, S.S.; Li, Z.; Loh, X.J. PHB-Based Gels as Delivery Agents of Chemotherapeutics for the Effective Shrinkage of Tumors. Adv. Heal. Mater. 2016, 5, 2679–2685. [Google Scholar] [CrossRef]
- Jiang, Y.; Meng, X.; Wu, Z.; Qi, X. Modified chitosan thermosensitive hydrogel enables sustained and efficient anti-tumor therapy via intratumoral injection. Carbohydr. Polym. 2016, 144, 245–253. [Google Scholar] [CrossRef]
- Hang, J.; Zhang, M.; Lin, R.; Yun, S.; Du, Y.; Wang, L.; Yao, Q.; Zannettino, A.; Zhang, H. Allogeneic primary mesenchymal stem/stromal cell aggregates within poly (N-isopropylacrylamide-co-acrylic acid) hydrogel for osteochondral regeneration. Appl. Mater. Today 2020, 18, 100487. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Chang, F.; Xu, W.; Ding, J. Repair of full-thickness articular cartilage defect using stem cell-encapsulated thermogel. Mater. Sci. Eng. C 2018, 88, 79–87. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, Y.; Chen, J.; Chang, F.; Wang, J.; Ding, J.; Chen, X. Component effect of stem cell-loaded thermosensitive polypeptide hydrogels on cartilage repair. Acta Biomater. 2018, 73, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Wang, S.J.; Zhao, X.R.; Zhu, Y.F.; Yu, J.K. 3D-Printed Poly(epsilon-caprolactone) Scaffold Integrated with Cell-laden Chitosan Hydrogels for Bone Tissue Engineering. Sci. Rep. 2017, 7, 13412. [Google Scholar] [CrossRef]
- Kim, M.H.; Park, H.; Park, W.H. Effect of pH and precursor salts on in situ formation of calcium phosphate nanoparticles in methylcellulose hydrogel. Carbohydr. Polym. 2018, 191, 176–182. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Wu, Y.; Zhao, Y.; Chen, H.; Yuan, Y.; Xu, K.; Zhang, H.; Lu, Y.; Wang, J.; et al. Heparin-Poloxamer Thermosensitive Hydrogel Loaded with bFGF and NGF Enhances Peripheral Nerve Regeneration in Diabetic Rats. Biomaterials 2018, 168, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Cheng, Y.; Tong, J.; Zhang, L.; Wei, Y.; Tian, M. Recent advances in thermo-sensitive hydrogels for drug delivery. J. Mater. Chem. B 2021, 9, 2979–2992. [Google Scholar] [CrossRef]
- Huang, H.; Qi, X.; Chen, Y.; Wu, Z. Thermo-sensitive hydrogels for delivering biotherapeutic molecules: A review. Saudi Pharm. J. 2019, 27, 990–999. [Google Scholar] [CrossRef]
- Zhao, Y.-Z.; Jiang, X.; Lin, Q.; Xu, H.-L.; Huang, Y.-D.; Lu, C.-T.; Cai, J. Thermosensitive heparin-poloxamer hydrogels enhance the effects of GDNF on neuronal circuit remodeling and neuroprotection after spinal cord injury. J. Biomed. Mater. Res. Part A 2017, 105, 2816–2829. [Google Scholar] [CrossRef]
- Goertz, O.; Abels, C.; Knie, U.; May, T.; Hirsch, T.; Daigeler, A.; Steinau, H.-U.; Langer, S. Clinical Safety and Efficacy of a Novel Thermoreversible Polyhexanide-Preserved Wound Covering Gel. Eur. Surg. Res. 2010, 44, 96–101. [Google Scholar] [CrossRef]
- Kramer, A.; Dissemond, J.; Kim, S.; Willy, C.; Mayer, D.; Papke, R.; Tuchmann, F.; Assadian, O. Consensus on Wound Antisepsis: Update 2018. Ski. Pharmacol. Physiol. 2018, 31, 28–58. [Google Scholar] [CrossRef]
- Jin, J.; Chen, Z.; Xiang, Y.; Tang, T.; Zhou, H.; Hong, X.; Fan, H.; Zhang, X.; Luo, P.; Ma, B.; et al. Development of a PHMB hydrogel-modified wound scaffold dressing with antibacterial activity. Wound Repair Regen. 2020, 28, 480–492. [Google Scholar] [CrossRef]
- Hirche, C.; Almeland, S.K.; Dheansa, B.; Fuchs, P.; Governa, M.; Hoeksema, H.; Korzeniowski, T.; Lumenta, D.B.; Marinescu, S.; Martinez-Mendez, J.R.; et al. Eschar removal by bromelain based enzymatic debridement (Nexobrid®) in burns: European consensus guidelines update. Burns 2020, 46, 782–796. [Google Scholar] [CrossRef]
- Dhaliwal, K.; Lopez, N. Hydrogel dressings and their application in burn wound care. Br. J. Community Nurs. 2018, 23 (Suppl. S9), S24–S27. [Google Scholar] [CrossRef]
- Ou, K.-L.; Tzeng, Y.-S.; Chiao, H.-Y.; Chiu, H.-T.; Chen, C.-Y.; Chu, T.-S.; Huang, D.-W.; Hsu, K.-F.; Chang, C.-K.; Wang, C.-H.; et al. Clinical Performance of Hydrogel-based Dressing in Facial Burn Wounds. Ann. Plast. Surg. 2021, 86, S18–S22. [Google Scholar] [CrossRef]
- Burks, R.I. Ultrasound in wound care. Phys. Ther. 2000, 80, 1015–1017. [Google Scholar] [CrossRef]
- Klucinec, B.; Scheidler, M.; Denegar, C.; Domholdt, E.; Burgess, S. Effectiveness of Wound Care Products in the Transmission of Acoustic Energy. Phys. Ther. 2000, 80, 469–476. [Google Scholar] [CrossRef]

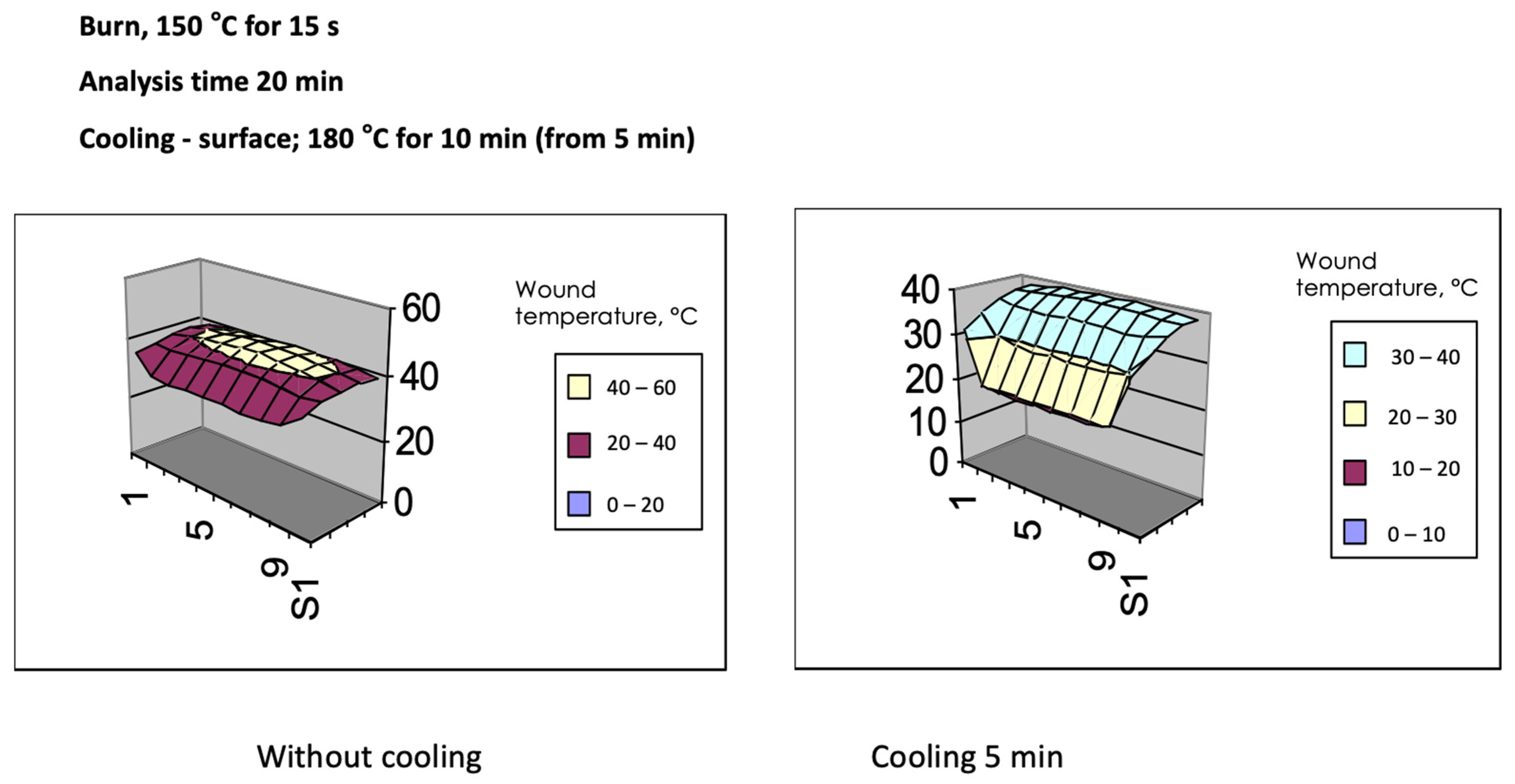
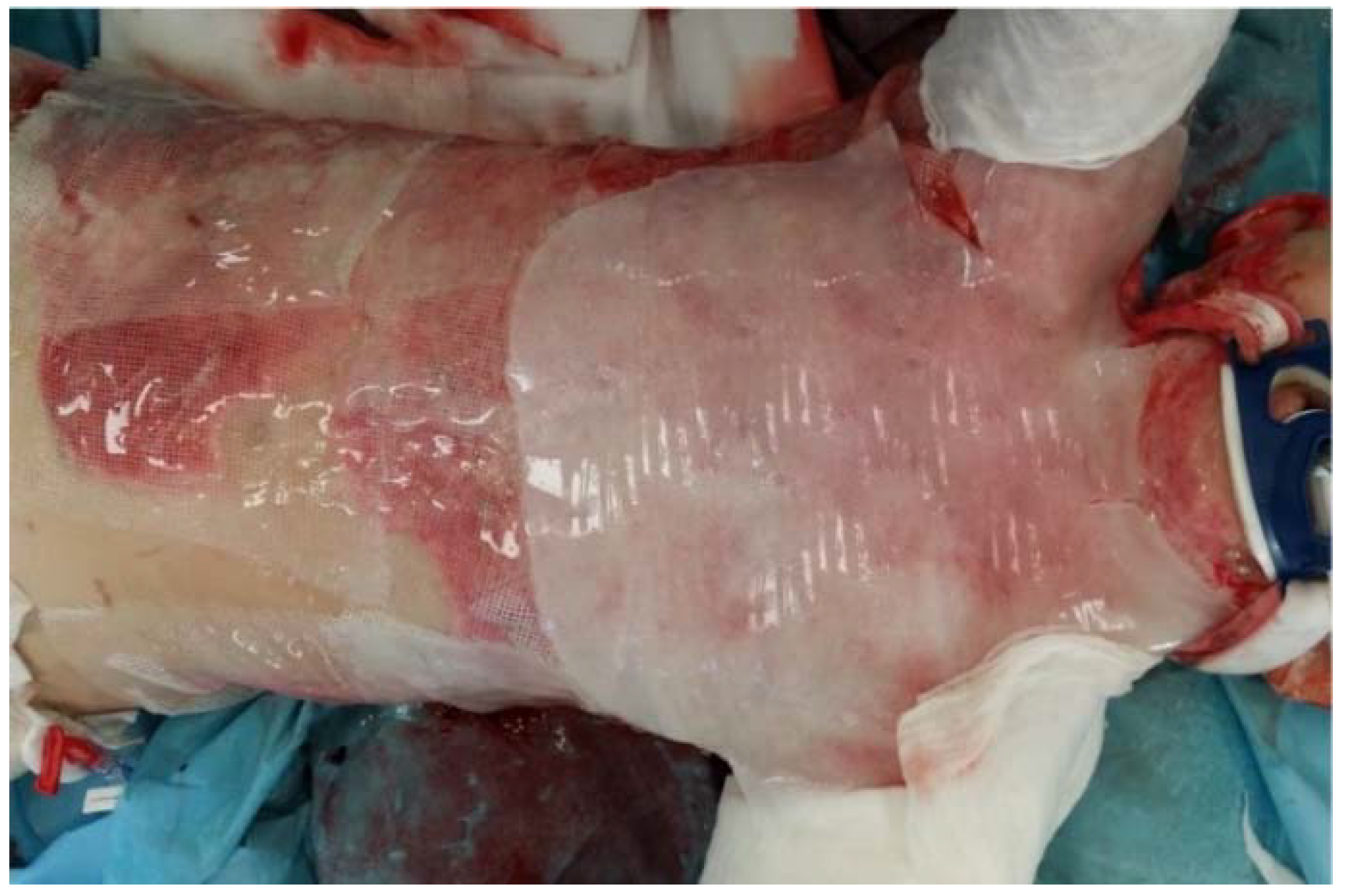



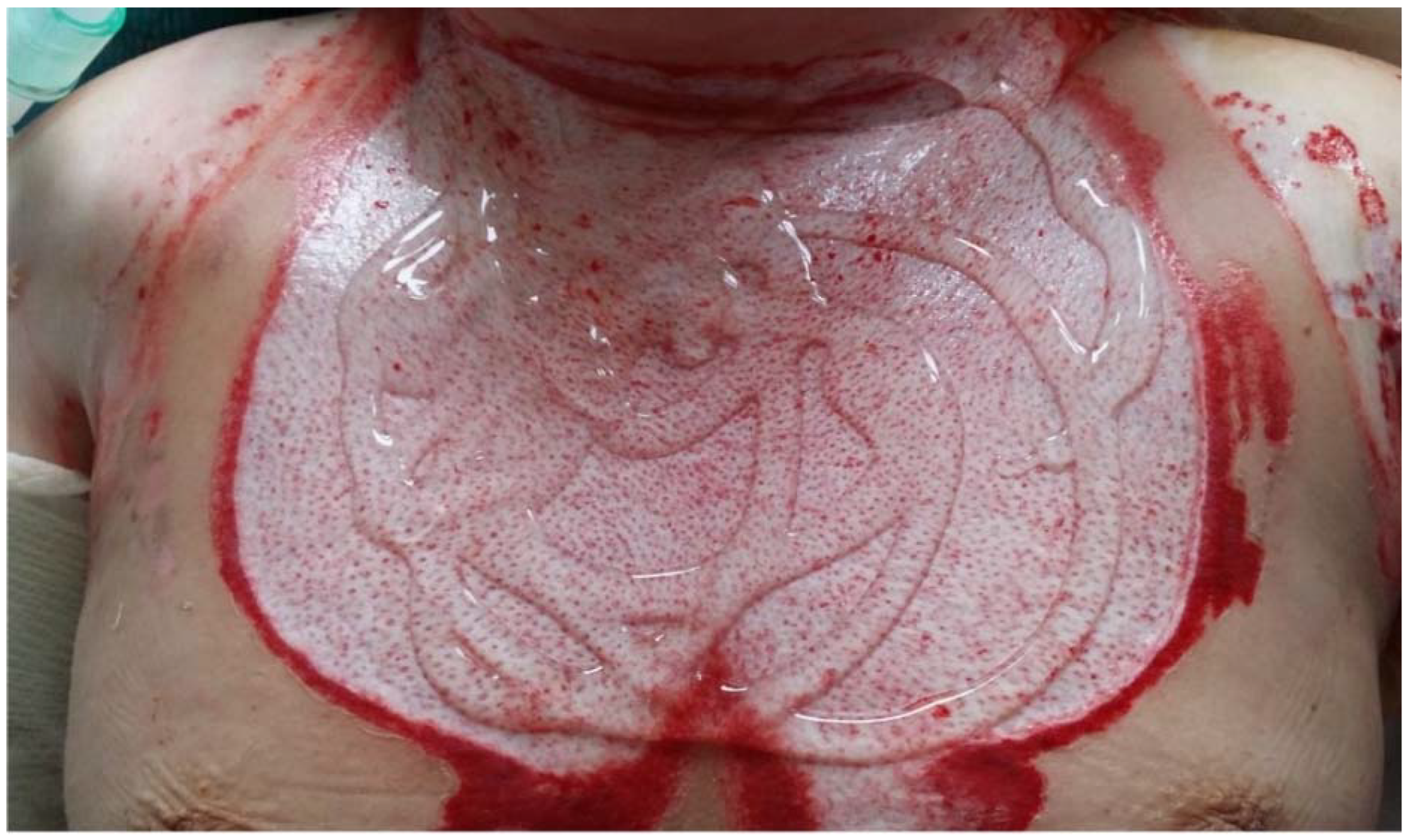
| Study | Study Type | Dressing Activity | Patients and Methods | Outcomes |
|---|---|---|---|---|
| Structural effects in photopolymerized sodium AMPS hydrogels crosslinked with poly(ethylene glycol) diacrylate for use as burn dressings [27] | Experimental | Wound healing | Hydrogel sheets were exposed to water binding, swelling and tested for cytotoxicity. | A potential for biomedical use as dressings for partial thickness burn |
| On-Demand Dissolvable Self-Healing Hydrogel Based on Carboxymethyl Chitosan and Cellulose Nanocrystal for Deep Partial Thickness Burn Wound Healing [29] | Experimental | Wound healing | An injectable hydrogel with carboxymethyl chitosan rigid rod-like dialdehyde-modified cellulose nanocrystal was administrated in a rat model after burn wound surgical debridement. | The hydrogel stimulates cell growth, is resoluble with amino acids and adjusts to the wound bed. |
| Dual Functionalized Injectable Hybrid Extracellular Matrix Hydrogel for Burn Wounds [30] | Experimental | Wound healing | An injectable hybrid decellularized crosslinked hydrogel derived from rat dermal tissue was tested for safety and efficiency. | The hydrogel containing cytokine and growth factors and was shown to be non-immunogenic and nontoxic. |
| Evaluation of healing activity of PVA/chitosan hydrogels on deep second degree burn: pharmacological and toxicological tests [38] | Experimental | Wound healing | Hydrogel containing chitosan was tested in a rat burn wound model. Toxicological test, irritation tests and histopathological analyses were performed. | Hydrogels containing chitosan accelerated wound healing at different times of the process. Had low irritation index. |
| Antimicrobial efficacy of a novel silver hydrogel dressing compared to two common silver burn wound dressings: Acticoat and PolyMem Silver [40]. | Experimental | Antimicrobial activity | Hydrogel containing 2-acrylamido-2-methylpropane sulfonic acid sodium salt with silver nanoparticles was tested for antimicrobial activity | Silver containing hydrogels inhibited growth of MSSA, Pseudomonas aeruginosa, but did not decrease VRE. Nanocrystal dressing based on polyethylene showed superior antimicrobial properties. |
| Biocompatibility evaluation of a new hydrogel dressing based on polyvinylpyrrolidone/polyethylene glycol [41] | Experimental | Wound healing and antimicrobial activity | Hydrogel samples (PEG, PVP, agar and water) were evaluated for fibroblast cytotoxicity, antifungal and antibacterial properties. | The material was nontoxic, showed good antibacterial and antifungal actions against Staphylococcus aureus, Staphylococcus epidermidis, Escherichia Coli k12 but no effect on Pseudomonas aeruginosa. |
| Mechanical properties and in vitro characterization of polyvinyl alcohol-nano-silver hydrogel wound dressings [42]. | Experimental | Antimicrobial activity | PVA-Ag hydrogels were examined for cytotoxicity, antibacterial features, swelling and drug delivery | PVA-Ag were not toxic for human fibroblast, and could be used in burn wound management. |
| Flexible and microporous chitosan hydrogel/nano ZnO composite bandages for wound dressing: in vitro and in vivo evaluation [43] | Experimental | Antimicrobial activity | Microporous chitosan hydrogen/nano zinc oxide composite bandages were evaluated for cell cytotoxicity, swelling, and antibacterial properties. Murine model of a burn wound | Dressing is not cytotoxic, improves wound healing and neovascularization. |
| Development and in vivo evaluation of silver sulfadiazine loaded hydrogel consisting polyvinyl alcohol and chitosan for severe burns [44] | Experimental | Wound healing | Murine model of a burn wound. Hydrogel containing 1% sulfadiazine tested for cytotoxicity and healing properties. | The dressing is safe and improved burn wound healing. |
| Gelam (Melaleuca spp.) honey- based hydrogel as burn wound dressing [45] | Experimental | Wound healing | A hydrogel with honey incorporated was tested on a rat model | Acceleration of wound healing and epithelialization was observed |
| Research on a novel poly (vinyl alcohol)/lysine/vanillin wound dressing: Biocompatibility, bioactivity and antimicrobial activity [46] | Experimental | Wound healing, antimicrobial activity | PVA hydrogels tested for antibacterial features and environmental scanning electron microscope (ESEM) and rat model | The dressing had antimicrobial activity and stimulated vessel formation and epithelization |
| Antibiotic-Containing Agarose Hydrogel for Wound and Burn Care [47] | Experimental | Antimicrobial activity | An agarose hydrogel with minocycline was tested for safety and antimicrobial features on a pig model. | The hydrogel showed 80% bioactivity after 7 days with much of the drug release within first 25 h. |
| Antibacterial polysaccharide-based hydrogel dressing containing plant essential oil for burn wound healing [48] | Experimental | Antimicrobial | Polysaccharide- based hydrogel with incorporated essential oils (eucalyptus, ginger and cumin) were examined regarding safety and antimicrobial activity. | Adding essential oils to hydrogels improved its antimicrobial activity against Staphylococcus aureus and Escherichia Coli. No indirect nor direct cytotoxicity was observed. |
| A Hydrogel-Based Localized Release of Colistin for Antimicrobial Treatment of Burn Wound Infection [49] | Experimental | Antimicrobial activity | A glycol chitosan/DF-PEG hydrogel loaded with colistin was tested for its safety and antimicrobial action. | Colistin was effectively released from the hydrogel and acted against Pseudomonas aeruginosa. |
| Dextran hydrogel scaffolds enhance angiogenic responses and promote complete skin regeneration during burn wound healing [50] | Experimental | Wound healing | Murine burn model applicated after early burn wound excision | The dressing improved neovascularization and wound healing |
| In situ formed anti-inflammatory hydrogel loading plasmid DNA encoding VEGF for burn wound healing [51] | Experimental | Wound healing | Hydrogel from chemically modified hyaluronic acid (HA), dextran (Dex), and β-cyclodextrin (β-CD) integrating resveratrol (Res) and vascular endothelial growth factor (VEGF) was tested on a rat model. | The novel dressing was proved to be safe and to improve wound healing. The density of CD31 and α-SMA, characteristic for new vessels, were increased. Levels of IL-1β and TNF-α in the treated wounds were similar to correctly healing wound. |
| Bilayer hydrogel with autologous stem cells derived from debrided human burn skin for improved skin regeneration [52] | Experimental | Wound healing | Human ADSCs incorporated in PEG hydrogel and applicated on full thickness burn wound rat model | ADSC/PEG hydrogels improved healing even of full thickness wounds and stimulated dermis remodeling |
| Non-stick hemostasis hydrogels as dressings with bacterial barrier activity for cutaneous wound healing [53] | Experimental | Antimicrobial activity, wound healing | A rabbit model was used to evaluate features of potentially hemostatic multifunctional hydrogel composed of poly (vinyl alcohol), human-like collagen (HLC) and sodium alginate (SA) | The hydrogels showed hemostasis, anti-protein absorption, and bacterial barrier activity. No cytotoxicity was observed. |
| Successful prevention of secondary burn progression using infliximab hydrogel: A murine model [54] | Experimental | Wound healing | Microcapillary gelatin- alginate hydrogel with infused anti-TNF α was tested for efficiency and safety in a murine model. | The novel dressing reduced depth of thermal injury and promoted wound healing by downregulation of proinflammatory cytokines. |
| bFGF and collagen matrix hydrogel attenuates burn wound inflammation through activation of ERK and TRK pathway [55] | Experimental | Wound healing | A collagen hydrogel with incorporated bFGF and silver sulfadiazine was tested in a rat model and evaluated for efficiency and safety. | The hydrogel promoted wound healing by NGF, stimulation of fibroblast proliferation, increasing neoangiogenesis. No serious cytotoxicity was observed. |
| Study | Study Type | Patients and Methods | Outcomes |
|---|---|---|---|
| Pre-hospital management of burns by the UK fire service [20] | A questionary | 62 UK fire and rescue services were questioned about first aid in burns | 76% use hydrogel dressing, while 37% would cool the wound with hydrogel |
| Effectiveness of a hydrogel dressing as an analgesic adjunct to first aid for the treatment of acute pediatric burn injuries: a prospective randomized controlled trial [22] | A prospective randomised controlled trial | Children were enrolled into two groups: intervention with inert hydrogel or control with polyvinylchloride film | No significant between-group differences in pain scores were found between 17 paediatric burn patients who received hydrogel dressings and those who received standard care |
| Evaluating the use of hydrogel sheet dressings in comprehensive burn wound care [36] | A prospective clinical observation | 50 burn wounds in 30 patients treated with hydrogel sheets. Full-thickness and partial-thickness burn wounds, as well as the donor areas were treated. | No adverse events were reported. The hydrogel dressing reduced pain, improved wound healing |
| Clinical safety and efficacy of a novel thermoreversible polyhexanide-preserved wound covering gel [104] | A randomized controlled single-center study | 44 patients, test group—hydrogel with polyhexanide, control group—ointment with sulfadiazine | There was less pain and wound staining in the test group. Hydrogels were safe and effective. |
| Clinical Performance of Hydrogel-based Dressing in Facial Burn Wounds: A Retrospective Observational Study [109] | A retrospective observational study | 21 patients with burn enrolled in the study, a hydrogel mask was used. Full epithelialization took 10.86 days | Hydrogel mask improved healing and reduced scarring in a group of patients with second-degree facial burns. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surowiecka, A.; Strużyna, J.; Winiarska, A.; Korzeniowski, T. Hydrogels in Burn Wound Management—A Review. Gels 2022, 8, 122. https://doi.org/10.3390/gels8020122
Surowiecka A, Strużyna J, Winiarska A, Korzeniowski T. Hydrogels in Burn Wound Management—A Review. Gels. 2022; 8(2):122. https://doi.org/10.3390/gels8020122
Chicago/Turabian StyleSurowiecka, Agnieszka, Jerzy Strużyna, Aleksandra Winiarska, and Tomasz Korzeniowski. 2022. "Hydrogels in Burn Wound Management—A Review" Gels 8, no. 2: 122. https://doi.org/10.3390/gels8020122
APA StyleSurowiecka, A., Strużyna, J., Winiarska, A., & Korzeniowski, T. (2022). Hydrogels in Burn Wound Management—A Review. Gels, 8(2), 122. https://doi.org/10.3390/gels8020122






