Do Polymeric Nanoparticles Really Enhance the Bioavailability of Oral Drugs? A Quantitative Answer Using Meta-Analysis
Abstract
:1. Introduction
2. Methodology
2.1. Data Mining
2.2. Inclusion Data and Its Criteria
2.3. Meta-Analysis
- (a)
- Synthetic polymeric material;
- (b)
- Natural polymeric material.
3. Results and Discussion
4. Conclusions
Funding
Conflicts of Interest
References
- Hua, S. Advances in Oral Drug Delivery for Regional Targeting in the Gastrointestinal Tract—Influence of Physiological, Pathophysiological and Pharmaceutical Factors. Front. Pharmacol. 2020, 11, 524. [Google Scholar] [CrossRef] [PubMed]
- Homayun, B.; Lin, X.; Choi, H.J. Challenges and Recent Progress in Oral Drug Delivery Systems for Biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parodi, A.; Buzaeva, P.; Nigovora, D.; Baldin, A.; Kostyushev, D.; Chulanov, V.; Savvateeva, L.V.; Zamyatnin, A.A., Jr. Nanomedicine for increasing the oral bioavailability of cancer treatments. J. Nanobiotechnol. 2021, 19, 354. [Google Scholar]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.A.; Firlak, M.; Berrow, S.R.; Halcovitch, N.R.; Baldock, S.J.; Yousafzai, B.M.; Hathout, R.M.; Hardy, J.G. Electrochemically Enhanced Drug Delivery Using Polypyrrole Films. Materials 2018, 11, 1123. [Google Scholar] [CrossRef] [Green Version]
- Nasser, N.; Hathout, R.M.; Abd-Allah, H.; Sammour, O.A. Enhancement of oral bioavailability of drugs using lipid-based carriers: A meta-analysis study. Drug Dev. Ind. Pharm. 2020, 46, 2105–2110. [Google Scholar]
- Hathout, R.M.; Omran, M.K. Gelatin-based particulate systems in ocular drug delivery. Pharm. Dev. Technol. 2016, 21, 379–386. [Google Scholar] [CrossRef]
- Safwat, S.; Ishak, R.A.; Hathout, R.M.; Mortada, N.D. Statins anticancer targeted delivery systems: Re-purposing an old molecule. J. Pharm. Pharmacol. 2017, 69, 613–624. [Google Scholar] [CrossRef] [Green Version]
- Abd-algaleel, S.A.; Metwally, A.A.; Abdel-Bar, H.M.; Kassem, D.H.; Hathout, R.M. Synchronizing In Silico, In Vitro, and In Vivo Studies for the Successful Nose to Brain Delivery of an Anticancer Molecule. Mol. Pharm. 2021, 18, 3763–3776. [Google Scholar] [CrossRef]
- Des Rieux, A.; Fievez, V.; Garinot, M.; Schneider, Y.J.; Préat, V. Nanoparticles as potential oral delivery systems of proteins and vaccines: A mechanistic approach. J. Control. Release 2006, 116, 1–27. [Google Scholar] [CrossRef]
- Hathout, R.M.; Metwally, A.A. Gelatin Nanoparticles. Methods Mol. Biol. 2019, 2000, 71–78. [Google Scholar] [PubMed]
- Hathout, R.M.; Abdelhamid, S.G.; El-Housseiny, G.S.; Metwally, A.A. Comparing cefotaxime and ceftriaxone in combating meningitis through nose-to-brain delivery using bio/chemoinformatics tools. Sci. Rep. 2020, 10, 21250. [Google Scholar] [CrossRef] [PubMed]
- Hathout, R.M.; Mahmoud, O.A.; Ali, D.S.; Mamdouh, M.; Metwally, A.A. Modeling Drugs-PLGA Nanoparticles Interactions Using Gaussian Processes: Pharmaceutics Informatics Approach. J. Clust. Sci. 2021. [Google Scholar] [CrossRef]
- Kassem, D.H.; Kamal, M.M. Therapeutic efficacy of umbilical cord-derived stem cells for diabetes mellitus: A meta-analysis study. Stem Cell Res. Ther. 2020, 11, 484. [Google Scholar] [CrossRef] [PubMed]
- Elmeligy, S.; Hathout, R.M.; Khalifa, S.A.M.; El-Seedi, H.R.; Farag, M.A. Pharmaceutical manipulation of citrus flavonoids towards improvement of its bioavailability and stability. A mini review and a meta-analysis study. Food Biosci. 2021, 44, 101428. [Google Scholar] [CrossRef]
- Burns, P.B.; Rohrich, R.J.; Chung, K.C. The levels of evidence and their role in evidence-based medicine. Plast. Reconstr. Surg. 2011, 128, 305–310. [Google Scholar] [CrossRef] [Green Version]
- Fong, S.Y.; Brandl, M.; Bauer-Brandl, A. Phospholipid-based solid drug formulations for oral bioavailability enhancement: A meta-analysis. Eur. J. Pharm. Sci. 2015, 80, 89–110. [Google Scholar] [CrossRef]
- Hathout, R.M.; Metwally, A.A. Towards better modeling of drug-loading in solid lipid nanoparticles: Molecular dynamics, docking experiments and Gaussian Processes machine learning. Eur. J. Pharm. Biopharm. 2016, 108, 262–268. [Google Scholar] [CrossRef]
- Metwally, A.A.; Hathout, R.M. Computer-Assisted Drug Formulation Design: Novel Approach in Drug Delivery. Mol. Pharm. 2015, 12, 2800–2810. [Google Scholar] [CrossRef]
- Metwally, A.A.; El-Ahmady, S.H.; Hathout, R.M. Selecting optimum protein nano-carriers for natural polyphenols using chemoinformatics tools. Phytomedicine 2016, 23, 1764–1770. [Google Scholar] [CrossRef]
- Mills, E.J.; Bansback, N.; Ghement, I.; Thorlund, K.; Kelly, S.; Puhan, M.A.; Wright, J. Multiple treatment comparison meta-analyses: A step forward into complexity. Clin. Epidemiol. 2011, 3, 193–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hathout, R.M. Particulate Systems in the Enhancement of the Antiglaucomatous Drug Pharmacodynamics: A Meta-Analysis Study. ACS Omega 2019, 4, 21909–21913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgen, M.; Bloom, C.; Beyerinck, R.; Bello, A.; Song, W.; Wilkinson, K.; Steenwyk, R.; Shamblin, S. Polymeric Nanoparticles for Increased Oral Bioavailability and Rapid Absorption Using Celecoxib as a Model of a Low-Solubility, High-Permeability Drug. Pharm. Res. 2012, 29, 427–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dian, L.; Yu, E.; Chen, X.; Wen, X.; Zhang, Z.; Qin, L.; Wang, Q.; Li, G.; Wu, C. Enhancing oral bioavailability of quercetin using novel soluplus polymeric micelles. Nanoscale Res. Lett. 2014, 9, 684. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Jiang, T.T.; Yuan, Z.X.; Lu, Y. Self-Assembled Casein Nanoparticles Loading Triptolide for the Enhancement of Oral Bioavailability. Nat. Prod. Commun. 2020, 15, 1934578X20948352. [Google Scholar] [CrossRef]
- Hedaya, M.; Bandarkar, F.; Nada, A. In vitro and in vivo Evaluation of Ibuprofen Nanosuspensions for Enhanced Oral Bioavailability. Med Princ. Pract. 2021, 30, 361–368. [Google Scholar] [CrossRef]
- Penalva, R.; Esparza, I.; Larraneta, E.; Gonz+ílez-Navarro, C.J.; Gamazo, C.; Irache, J.M. Zein-Based Nanoparticles Improve the Oral Bioavailability of Resveratrol and Its Anti-inflammatory Effects in a Mouse Model of Endotoxic Shock. J. Agric. Food Chem. 2015, 63, 5603–5611. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Tao, Q.; Zou, Y.; Zhang, F.; Guo, M.; Wang, Y.; Wang, H.; Zhou, Q.; Yu, S. PLGA Nanoparticles Improve the Oral Bioavailability of Curcumin in Rats: Characterizations and Mechanisms. J. Agric. Food Chem. 2011, 59, 9280–9289. [Google Scholar] [CrossRef]
- Hasija, R.; Chaurasia, S.; Gupta, S. Assessment of Polymeric Nanoparticles to Enhance Oral Bioavailability and Antioxidant Activity of Resveratrol. Indian J. Pharm. Sci. 2021, 83, 1114–1128. [Google Scholar] [CrossRef]
- Alshetaili, A.S.; Ansari, M.J.; Anwer, M.K.; Ganaie, M.A.; Iqbal, M.; Alshahrani, S.M.; Alalaiwe, A.S.; Alsulays, B.B.; Alshehri, S.; Sultan, A.S. Enhanced Oral Bioavailability of Ibrutinib Encapsulated Poly (Lactic-co-Glycolic Acid) Nanoparticles: Pharmacokinetic Evaluation in Rats. Curr. Pharm. Anal. 2019, 15, 661–668. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, X.; Li, J.; Shen, Q. The comparison of different daidzein-PLGA nanoparticles in increasing its oral bioavailability. Int. J. Nanomed. 2012, 7, 559–570. [Google Scholar]
- Peng, W.; Jiang, X.Y.; Zhu, Y.; Omari-Siaw, E.; Deng, W.W.; Yu, J.N.; Xu, X.M.; Zhang, W.M. Oral delivery of capsaicin using MPEG-PCL nanoparticles. Acta Pharmacol. Sin. 2015, 36, 139–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, C.; Wang, Z.; Jiang, C.; Kong, M.; Zhou, X.; Li, Y.; Cheng, X.; Chen, X. Chitosan/o-carboxymethyl chitosan nanoparticles for efficient and safe oral anticancer drug delivery: In vitro and in vivo evaluation. Int. J. Pharm. 2013, 457, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.; Lopez-Jimenez, F.; Boyd, J.; D’Amico, F.; Durant, N.H.; Hlatky, M.A.; Howard, G.; Kirley, K.; Masi, C.; Powell-Wiley, T.M.; et al. Methodological Standards for Meta-Analyses and Qualitative Systematic Reviews of Cardiac Prevention and Treatment Studies: A Scientific Statement From the American Heart Association. Circulation 2017, 136, e172–e194. [Google Scholar] [CrossRef]
- Greenland, S.; Senn, S.J.; Rothman, K.J.; Carlin, J.B.; Poole, C.; Goodman, S.N.; Altman, D.G. Statistical tests, P values, confidence intervals, and power: A guide to misinterpretations. Eur. J. Epidemiol. 2016, 31, 337–350. [Google Scholar] [CrossRef] [Green Version]
- Zlowodzki, M.; Poolman, R.W.; Kerkhoffs, G.M.; Tornetta, P.; Bhandari, M.; On behalf of the International Evidence-Based Orthopedic Surgery Working Group. How to interpret a meta-analysis and judge its value as a guide for clinical practice. Acta Orthop. 2007, 78, 598–609. [Google Scholar] [CrossRef]
- Patsopoulos, N.A.; Evangelou, E.; Ioannidis, J.P. Sensitivity of between-study heterogeneity in meta-analysis: Proposed metrics and empirical evaluation. Int. J. Epidemiol. 2008, 37, 1148–1157. [Google Scholar] [CrossRef] [Green Version]
- Corr, S.C.; Gahan, C.C.G.M.; Hill, C. M-cells: Origin, morphology and role in mucosal immunity and microbial pathogenesis. FEMS Immunol. Med. Microbiol. 2008, 52, 2–12. [Google Scholar] [CrossRef] [Green Version]
- Buda, A.; Sands, C.; Jepson, M.A. Use of fluorescence imaging to investigate the structure and function of intestinal M cells. Adv. Drug Deliv. Rev. 2005, 57, 123–134. [Google Scholar] [CrossRef]
- Hathout, R.M.; El-Ahmady, S.H.; Metwally, A.A. Curcumin or bisdemethoxycurcumin for nose-to-brain treatment of Alzheimer disease? A bio/chemo-informatics case study. Nat. Prod. Res. 2018, 32, 2873–2881. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Schäfer, T.; Schwarz, M.A. The Meaningfulness of Effect Sizes in Psychological Research: Differences Between Sub-Disciplines and the Impact of Potential Biases. Front. Psychol. 2019, 10, 813. [Google Scholar] [CrossRef] [Green Version]
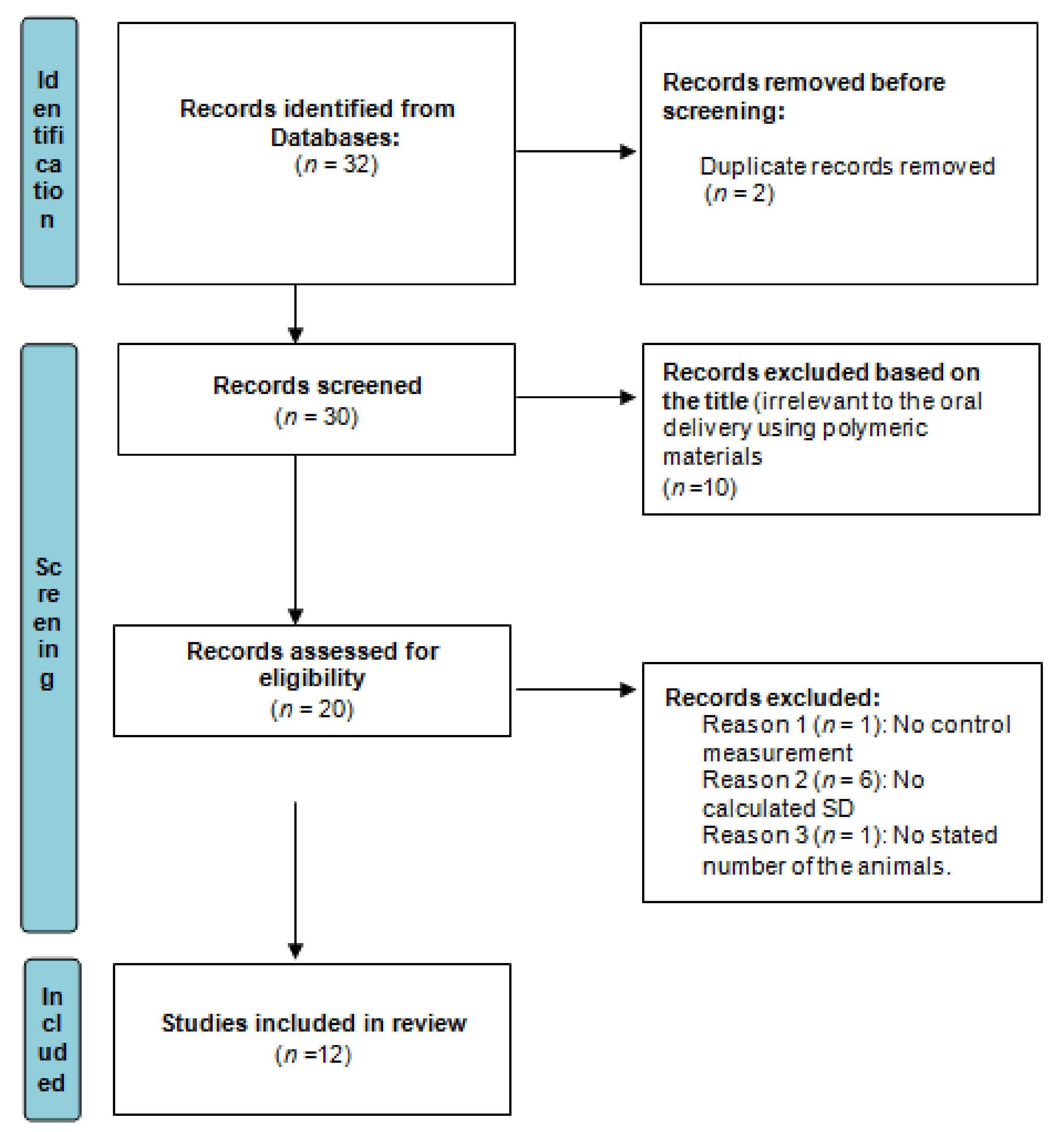
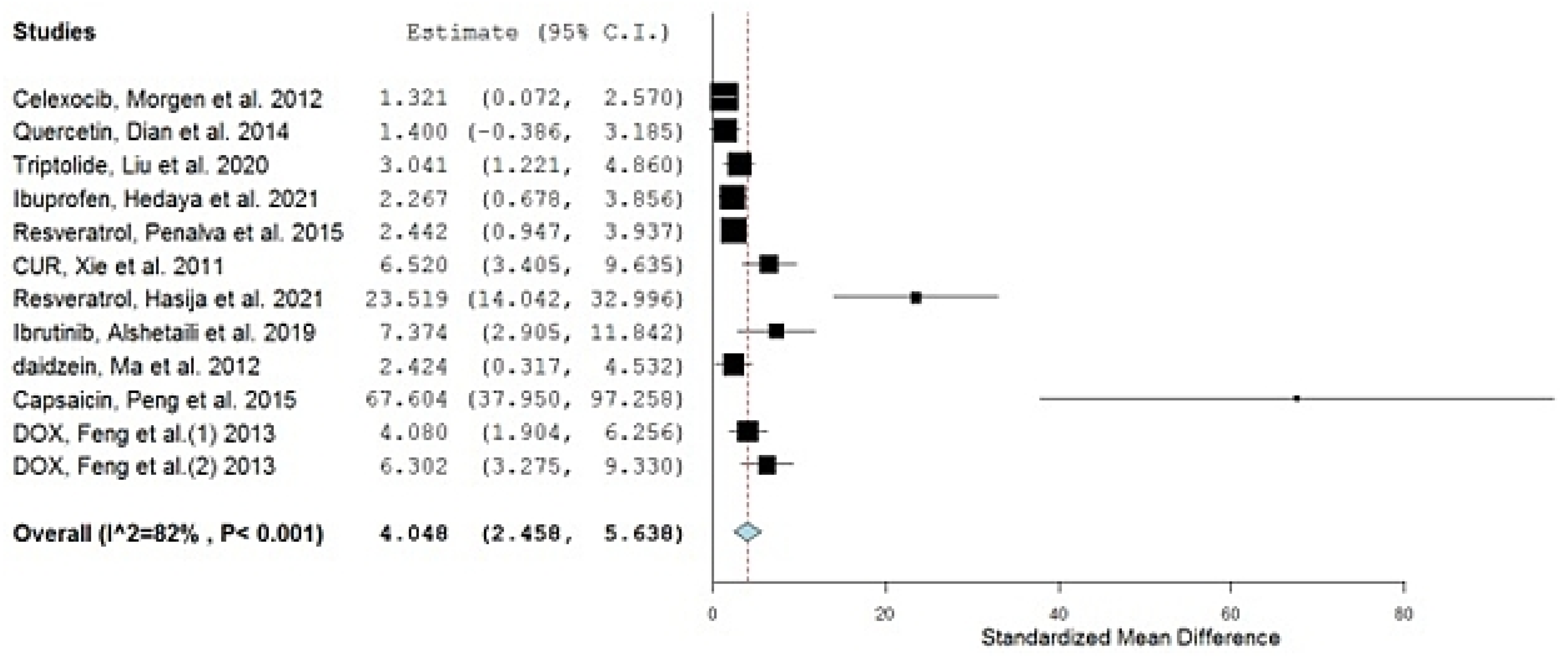
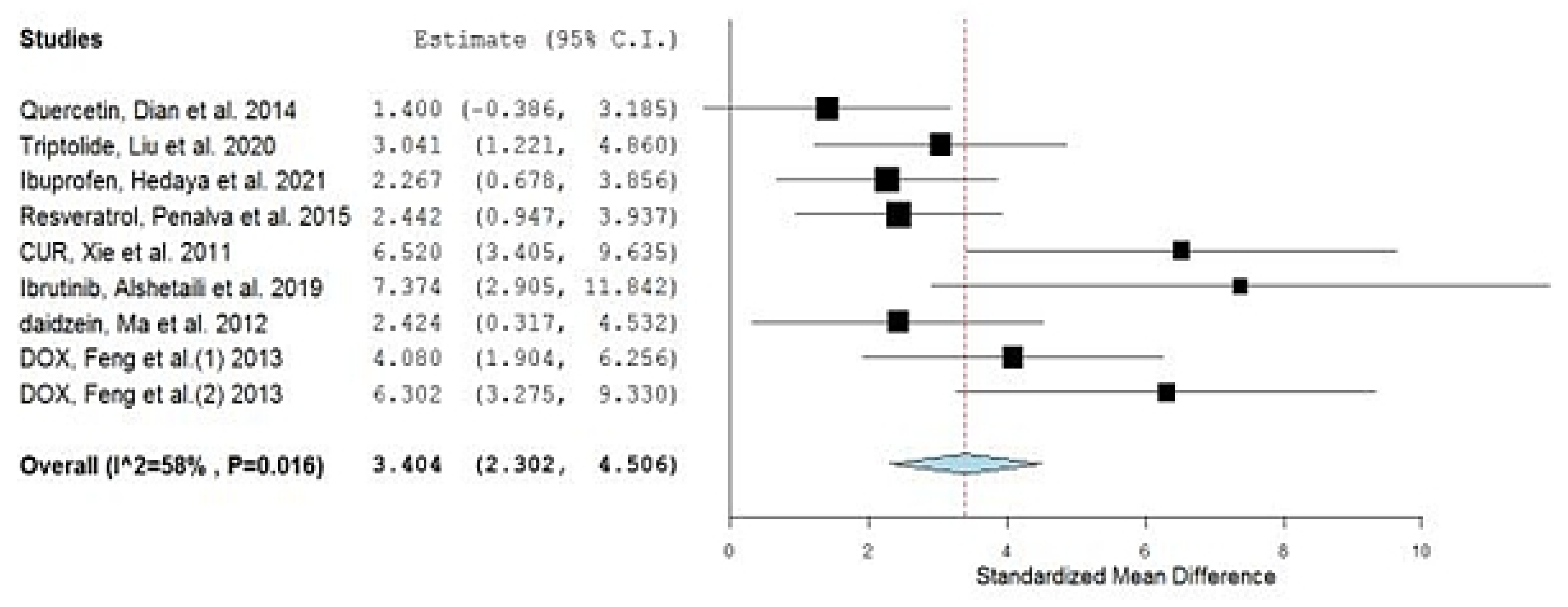
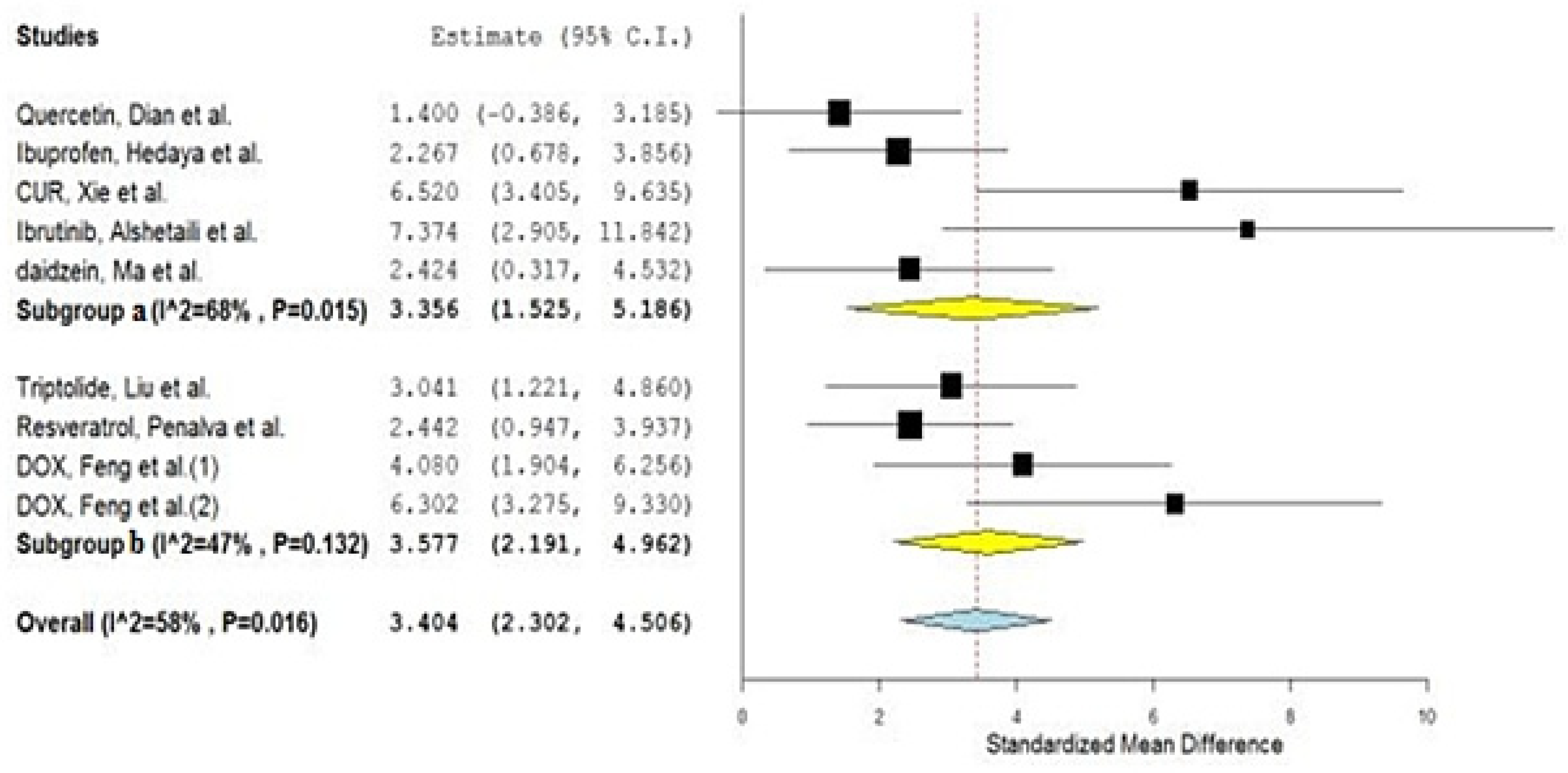
| No. | Drug | Year of Study | Group A Number of Animals | Group A Drug in NP Mean AUC (ng·h/mL) | Group AAUC SD | Group B Number of Animals | Group B Drug in Conventional Formulation Mean AUC (ng·h/mL) | Group BAUC SD | SMD | Lower C.I. | Upper C.I. | Type of Nano Carriers * | Type of Used Animals | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Celexocib, Morgen et al. | 2012 | 6 | 2031 | 1250 | 6 | 698 | 414 | 1.321 | 0.072 | 2.570 | Ethyl cellulose NPs a | Dogs | [23] |
| 2 | Quercetin, Dian et al. | 2014 | 3 | 107,840 | 54,000 | 3 | 37,680 | 16,800 | 1.400 | −0.386 | 3.185 | Solupulus PMs a | Dogs | [24] |
| 3 | Triptolide, Liu et al. | 2020 | 5 | 28,000 | 9000 | 5 | 6500 | 700 | 3.041 | 1.221 | 4.860 | Casein Nanoparticles b | Rats | [25] |
| 4 | Ibuprofen, Hedaya et al. | 2021 | 5 | 207,000 | 37,900 | 5 | 114,300 | 35,900 | 2.267 | 0.678 | 3.856 | PVP NPs a | Rabbits | [26] |
| 5 | Resveratrol, Penalva et al. | 2015 | 6 | 5170 | 2610 | 6 | 280 | 130 | 2.442 | 0.947 | 3.937 | Zein NPs b | Rats | [27] |
| 6 | CUR, Xie et al. | 2011 | 5 | 34,433 | 5533 | 5 | 6117 | 350 | 6.520 | 3.405 | 9.635 | PLGA NPs a | Rats | [28] |
| 7 | Resveratrol, Hasija et al. | 2021 | 6 | 3057 | 128 | 6 | 750 | 1 | 23.519 | 14.042 | 32.996 | Eudragit® E100 a | Rats | [29] |
| 8 | Ibrutinib, Alshetaili et al. | 2019 | 3 | 2292 | 263 | 3 | 545 | 48 | 7.374 | 2.905 | 11.842 | PLGA NPs a | Rats | [30] |
| 9 | Daidzein, Ma et al. | 2012 | 3 | 16,900 | 6930 | 3 | 1910 | 810 | 2.424 | 0.317 | 4.532 | PLGA NPs a | Rats | [31] |
| 10 | Capsaicin, Peng et al. | 2015 | 5 | 13,849 | 186 | 5 | 2324 | 113 | 67.604 | 37.950 | 97.258 | MPEG-PCL NPs a | Rats | [32] |
| 11 | DOX, Feng et al. | 2013 | 5 | 2101 | 404 | 5 | 574 | 255 | 4.080 | 1.904 | 6.256 | Chitosan b | Rats | [33] |
| 12 | DOX, Feng et al. | 2013 | 5 | 3720 | 584 | 5 | 574 | 255 | 6.302 | 3.275 | 9.330 | CS/CMC a | Rats | [33] |
| Study Names | Weights |
|---|---|
| Celexocib, Morgen et al. | 11.365% |
| Quercetin, Dian et al. | 10.590% |
| Triptolide, Liu et al. | 10.535% |
| Ibuprofen, Hedaya et al. | 10.893% |
| Resveratrol, Penalva et al. | 11.031% |
| CUR, Xie et al. | 8.320% |
| Resveratrol, Hasija et al. | 2.288% |
| Ibrutinib, Alshetaili et al. | 6.219% |
| daidzein, Ma et al. | 10.062% |
| Capsaicin, Peng et al. | 0.281% |
| DOX, Feng et al. (1) | 9.946% |
| DOX, Feng et al. (2) | 8.469% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hathout, R.M. Do Polymeric Nanoparticles Really Enhance the Bioavailability of Oral Drugs? A Quantitative Answer Using Meta-Analysis. Gels 2022, 8, 119. https://doi.org/10.3390/gels8020119
Hathout RM. Do Polymeric Nanoparticles Really Enhance the Bioavailability of Oral Drugs? A Quantitative Answer Using Meta-Analysis. Gels. 2022; 8(2):119. https://doi.org/10.3390/gels8020119
Chicago/Turabian StyleHathout, Rania M. 2022. "Do Polymeric Nanoparticles Really Enhance the Bioavailability of Oral Drugs? A Quantitative Answer Using Meta-Analysis" Gels 8, no. 2: 119. https://doi.org/10.3390/gels8020119
APA StyleHathout, R. M. (2022). Do Polymeric Nanoparticles Really Enhance the Bioavailability of Oral Drugs? A Quantitative Answer Using Meta-Analysis. Gels, 8(2), 119. https://doi.org/10.3390/gels8020119






