Biocompatible Chitosan-Based Hydrogels for Bioabsorbable Wound Dressings
Abstract
:1. Introduction
2. Experimental Investigation
2.1. Materials
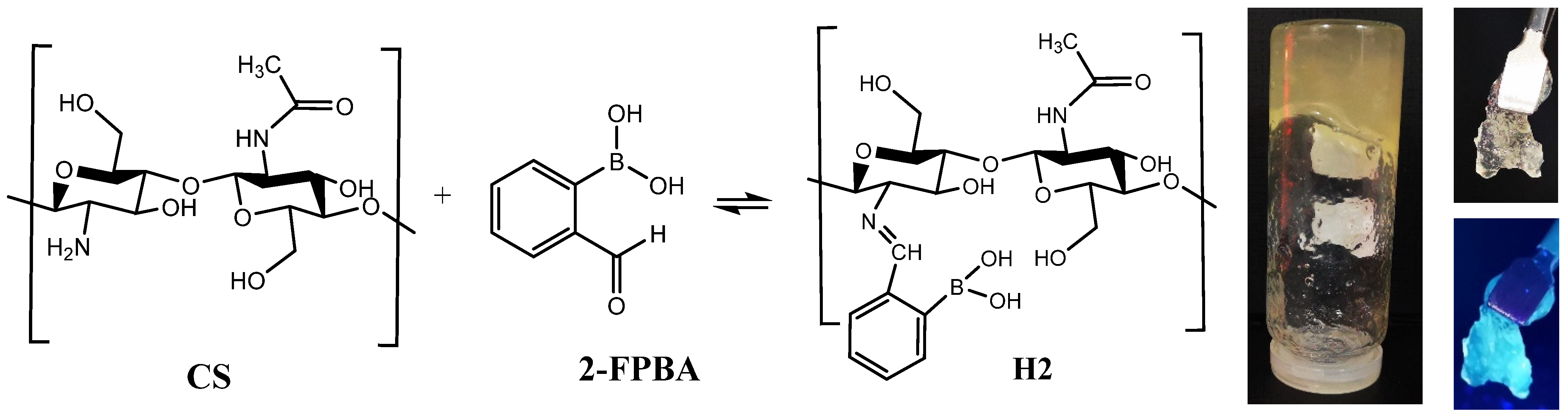
2.2. Synthesis of the Hydrogel
2.3. Equipment and Methods
2.4. Enzymatic Biodegradation Tests
3. Results and Discussion
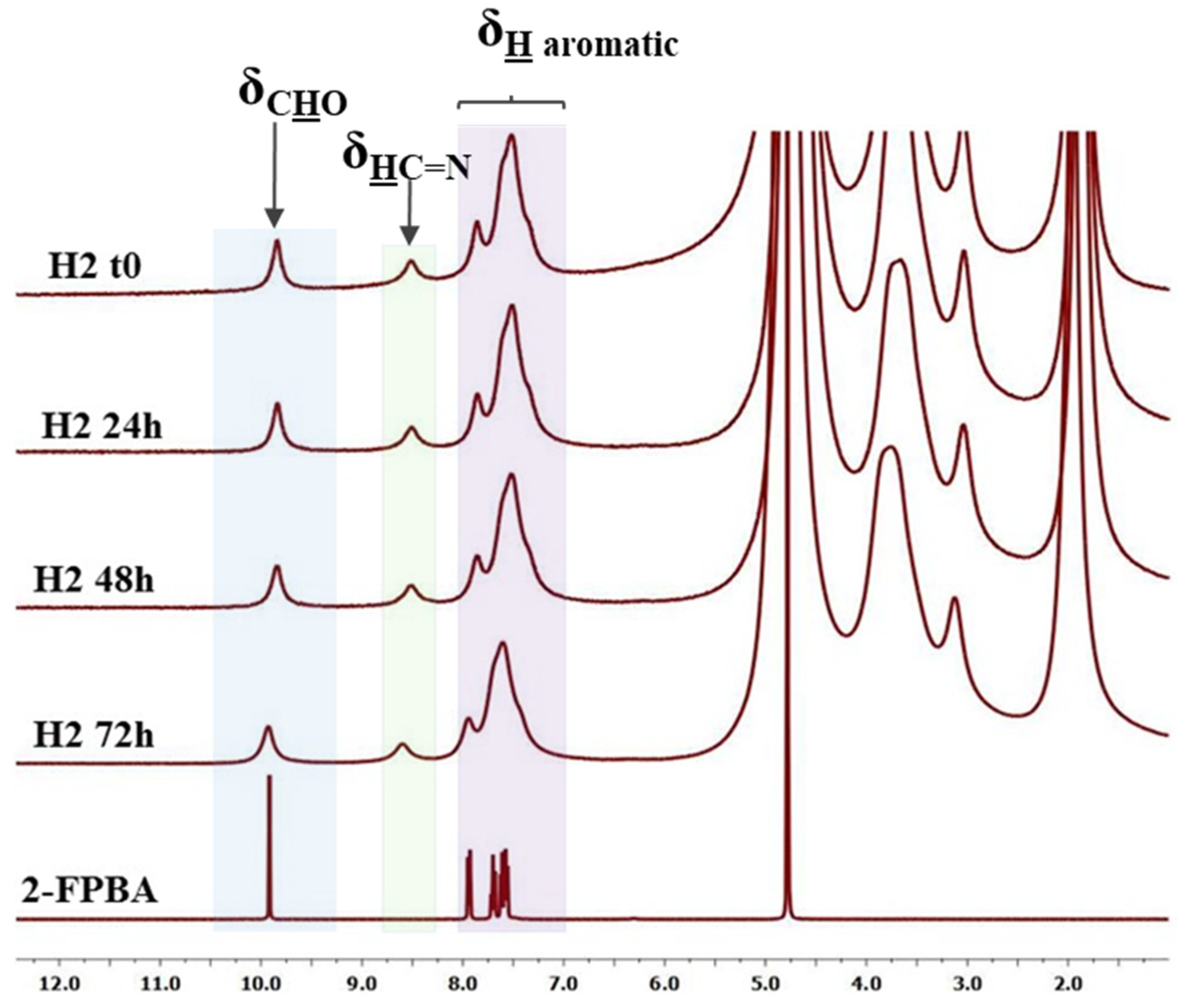
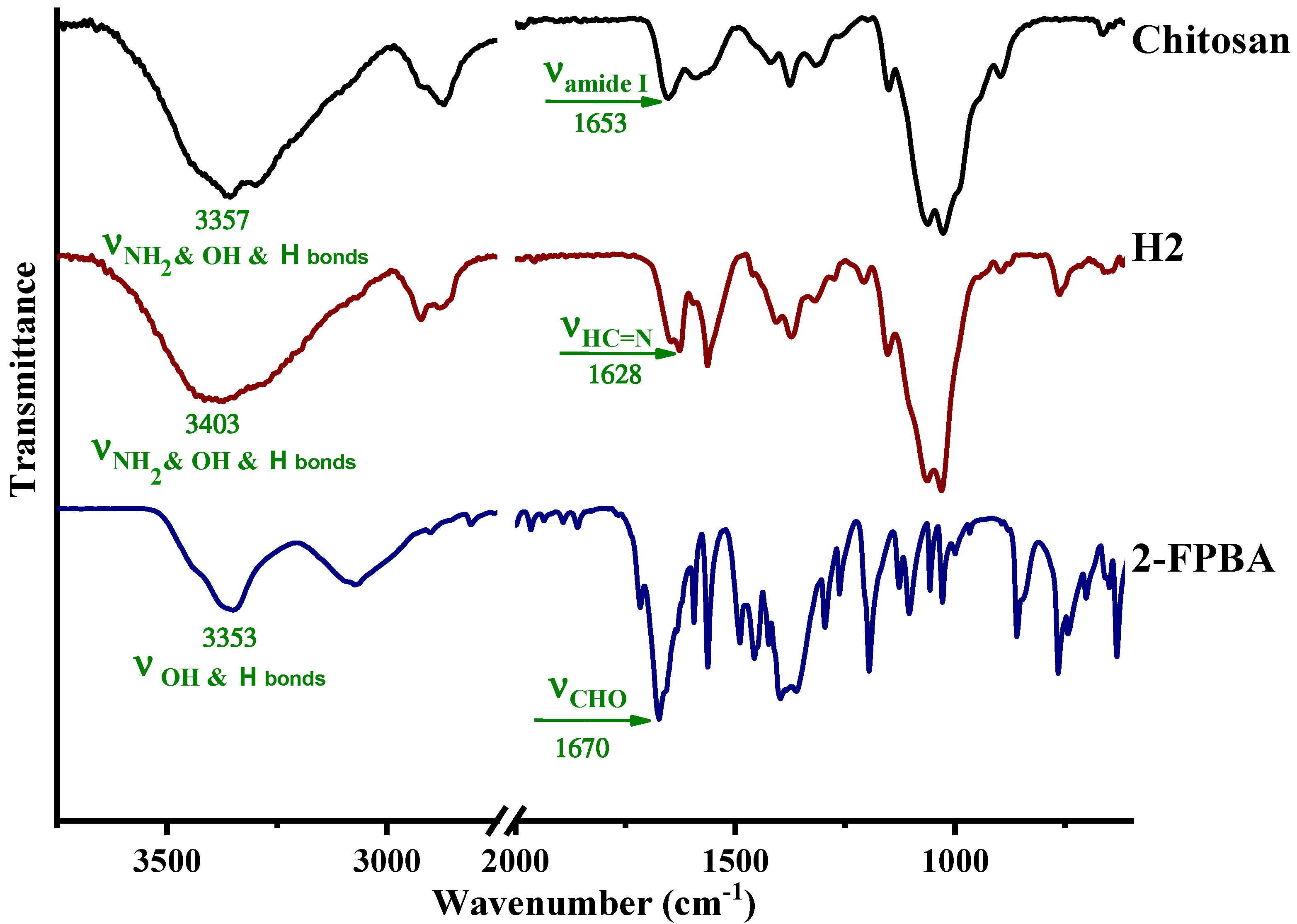
3.1. Structural and Supramolecular Characterization
3.2. In Vitro Biologic Properties
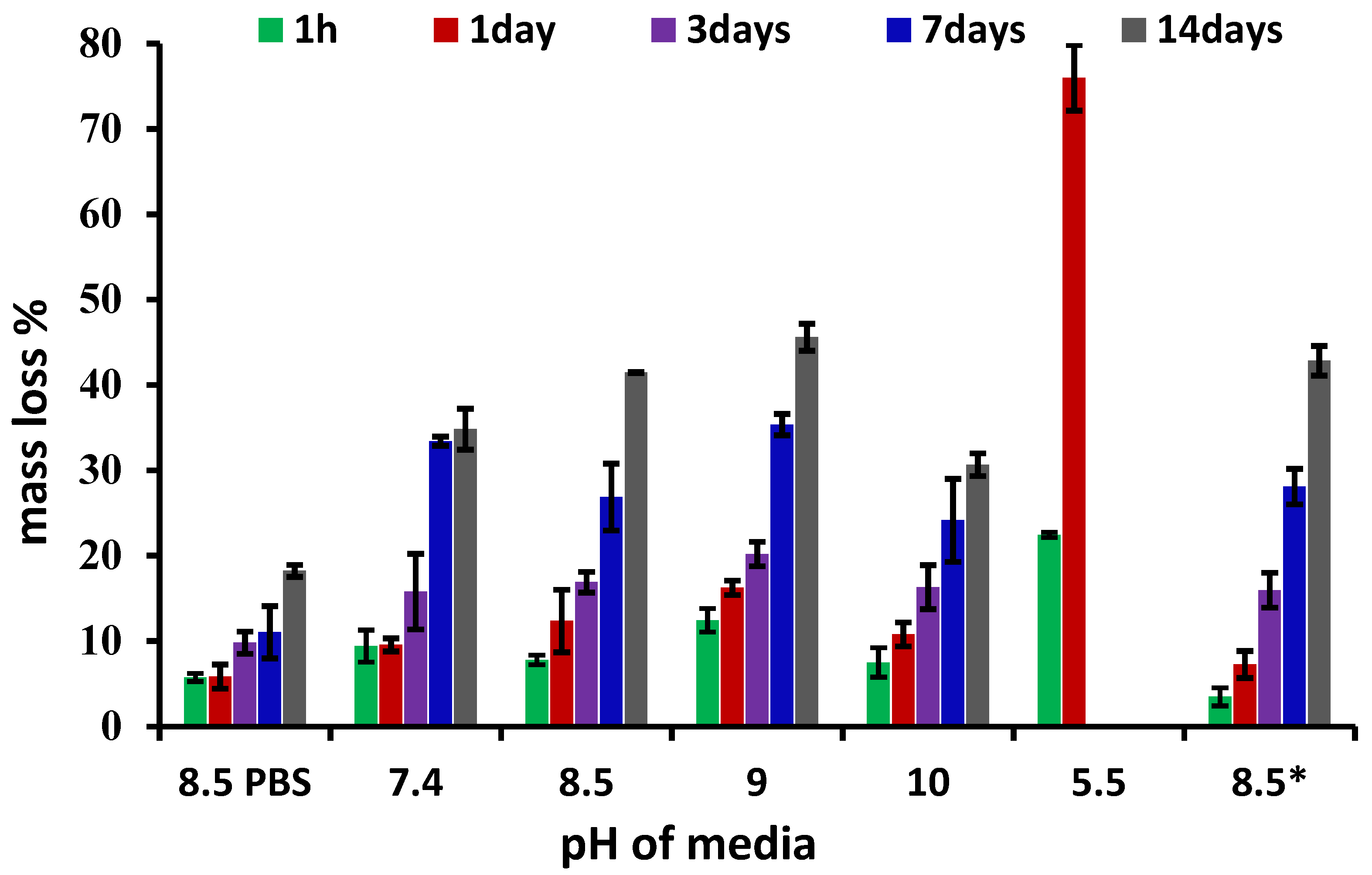
3.3. Biodegradation Investigation
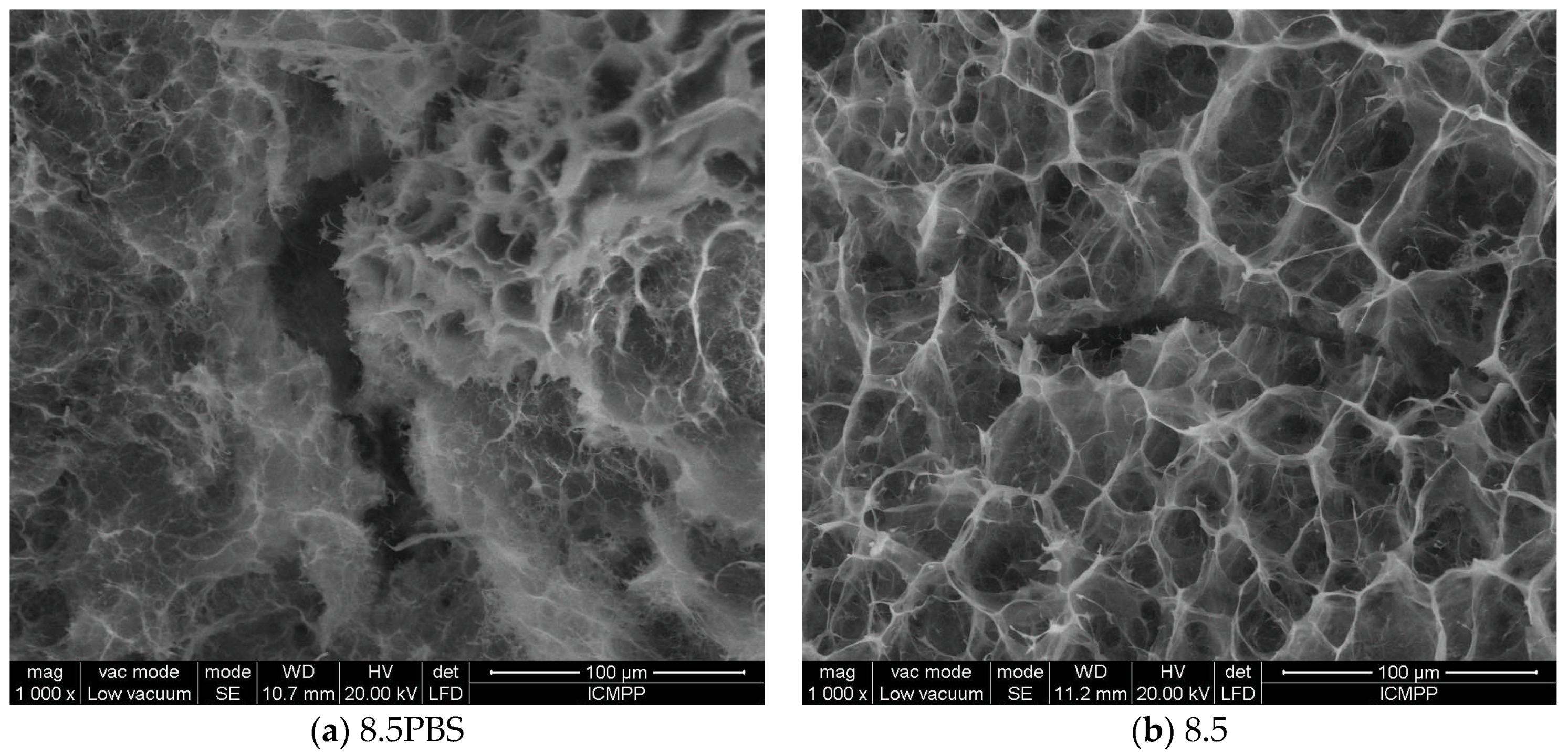
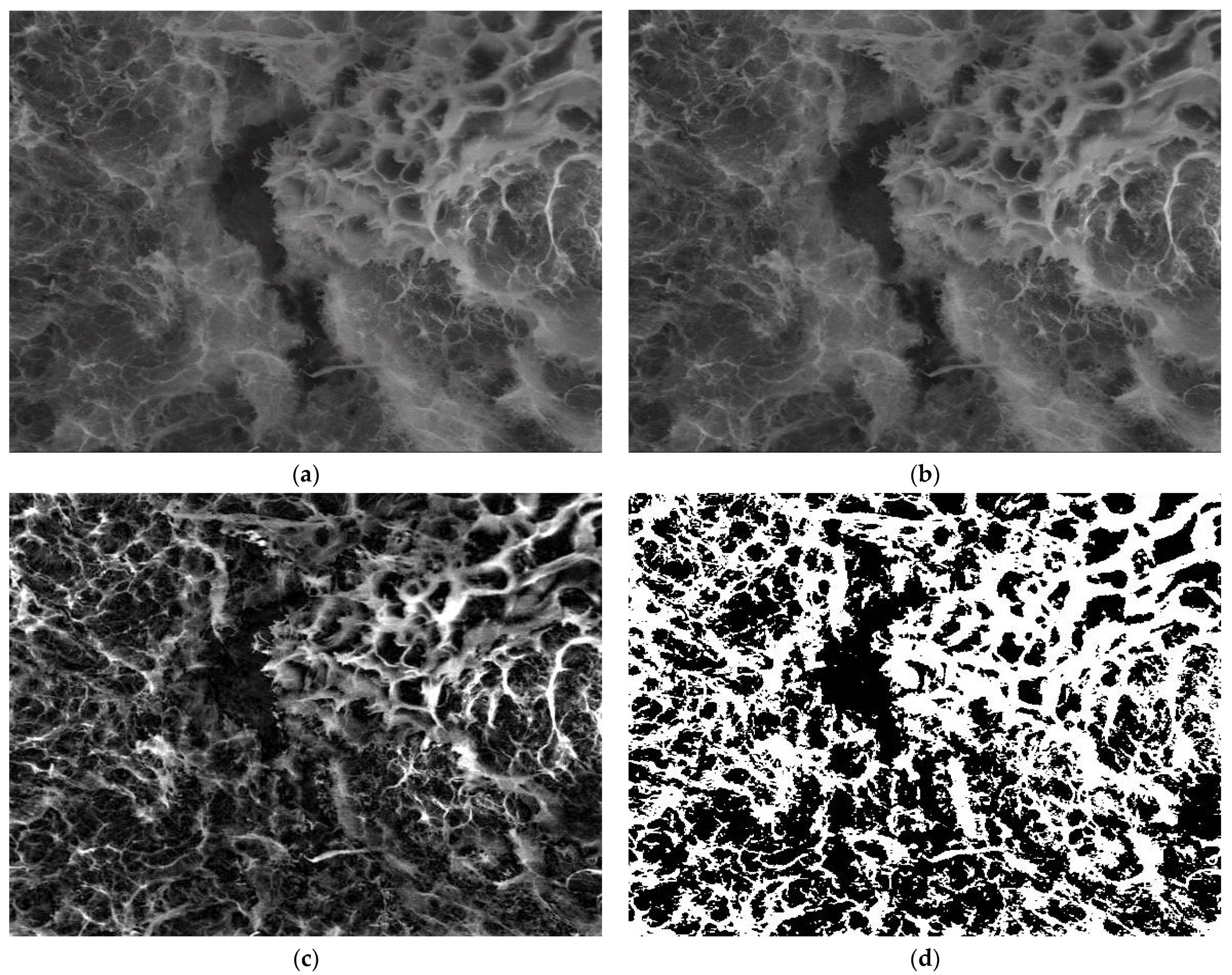
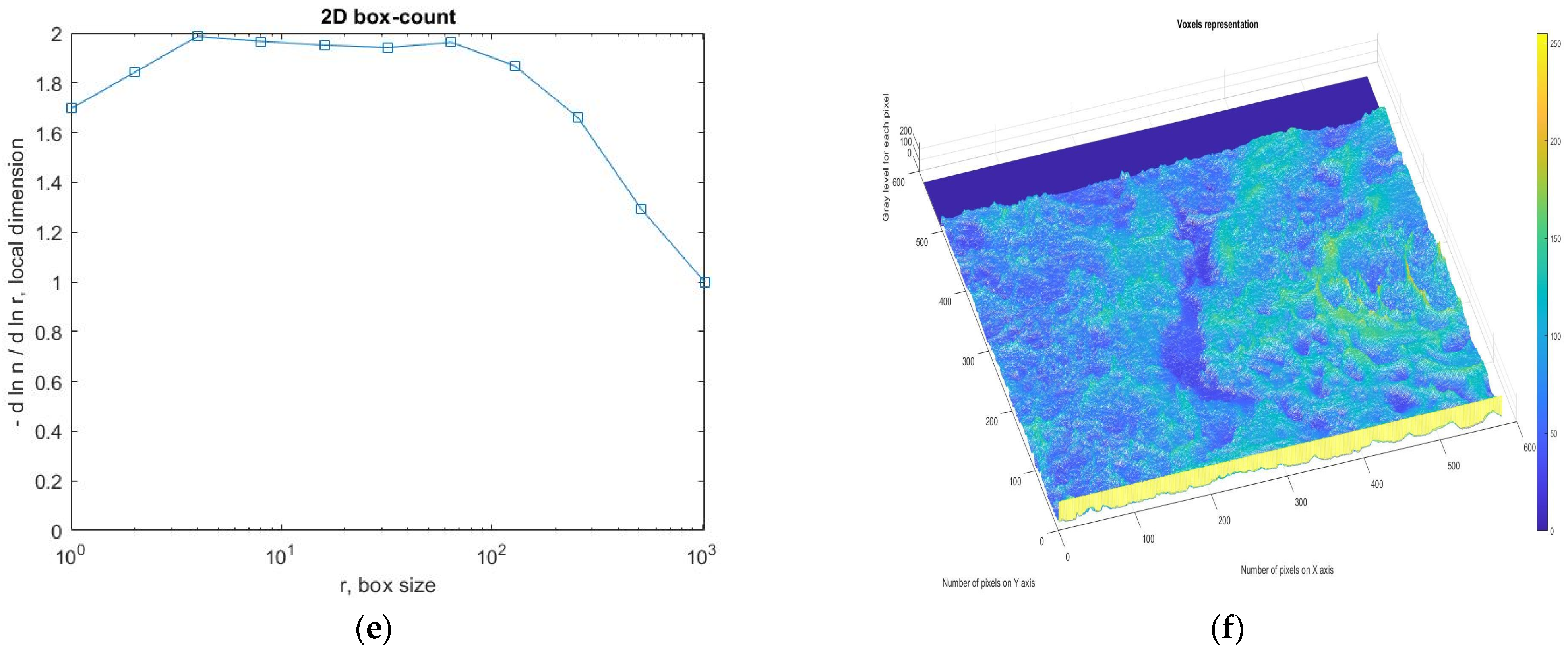
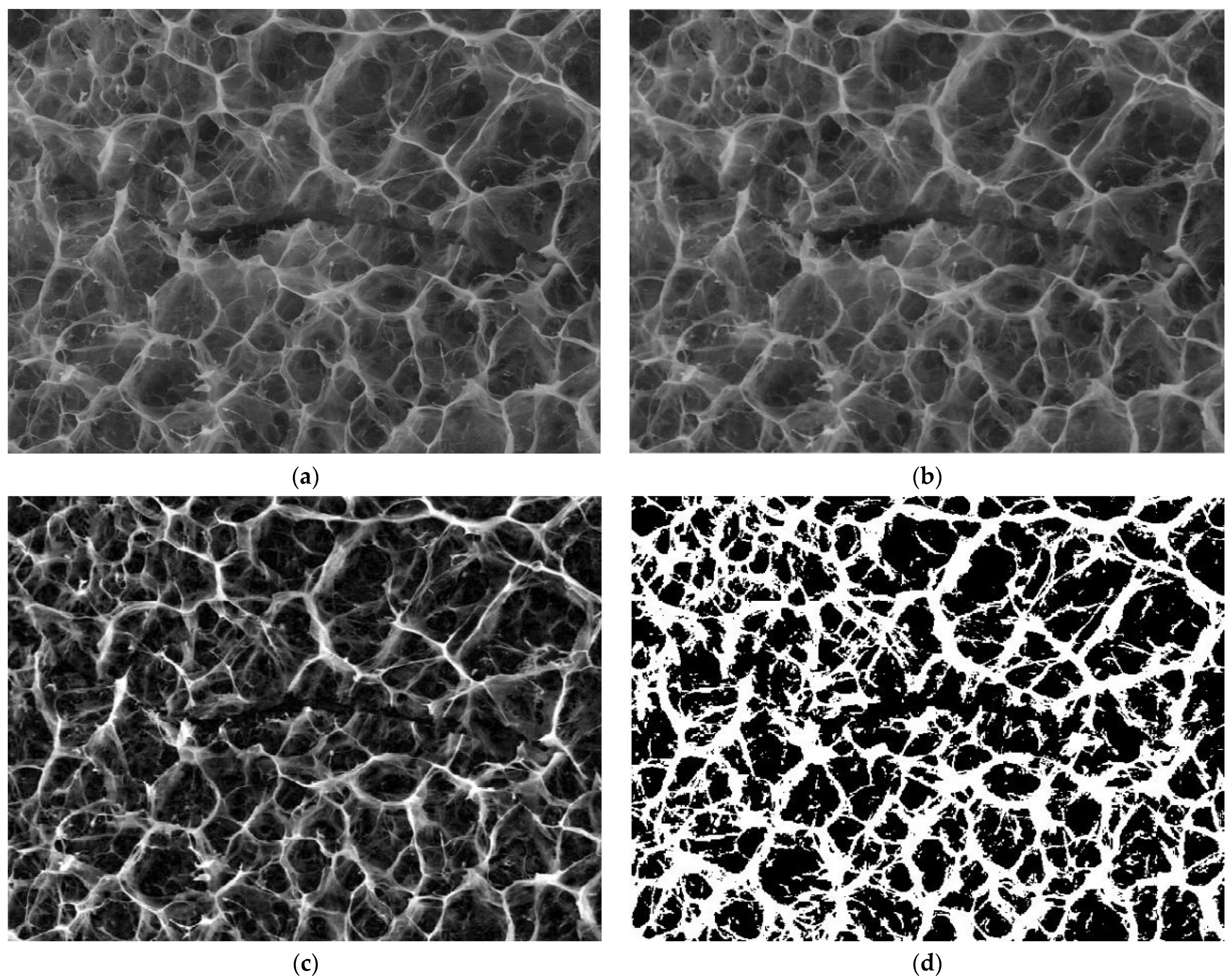
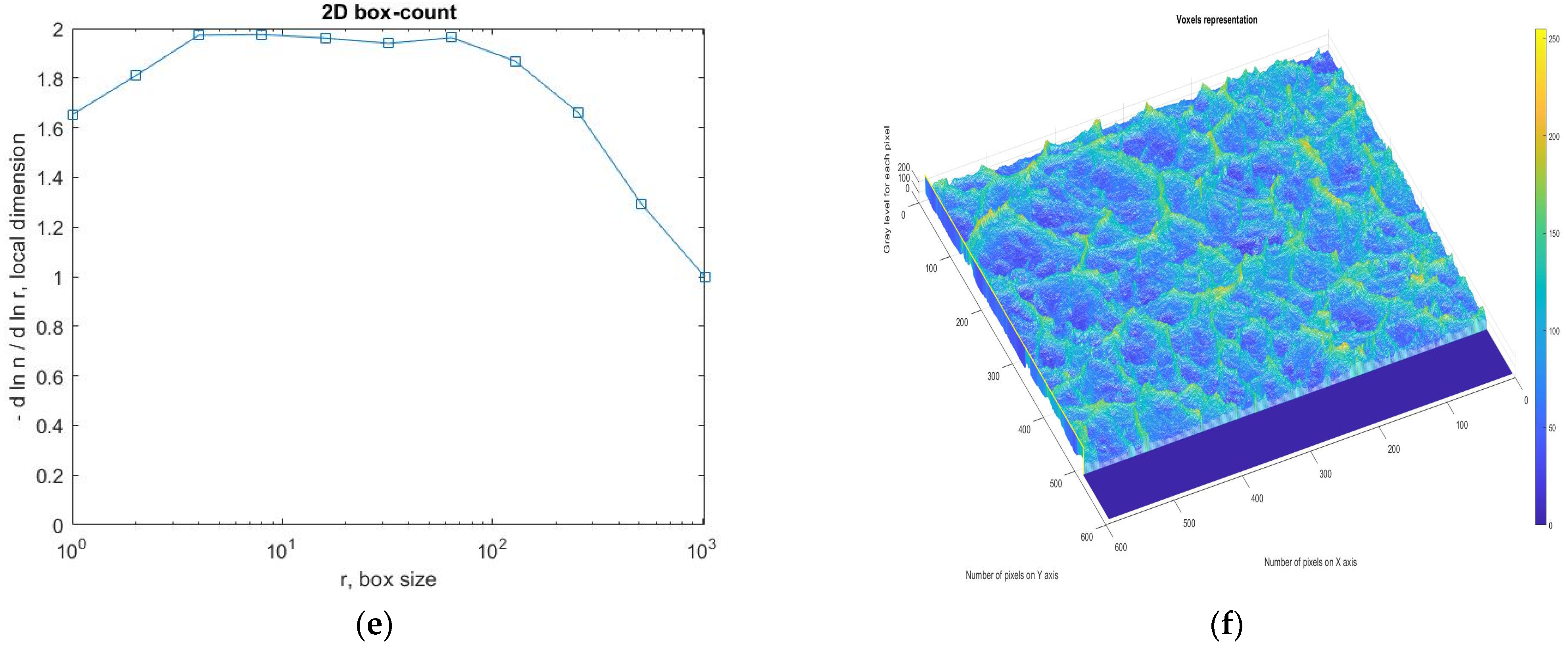
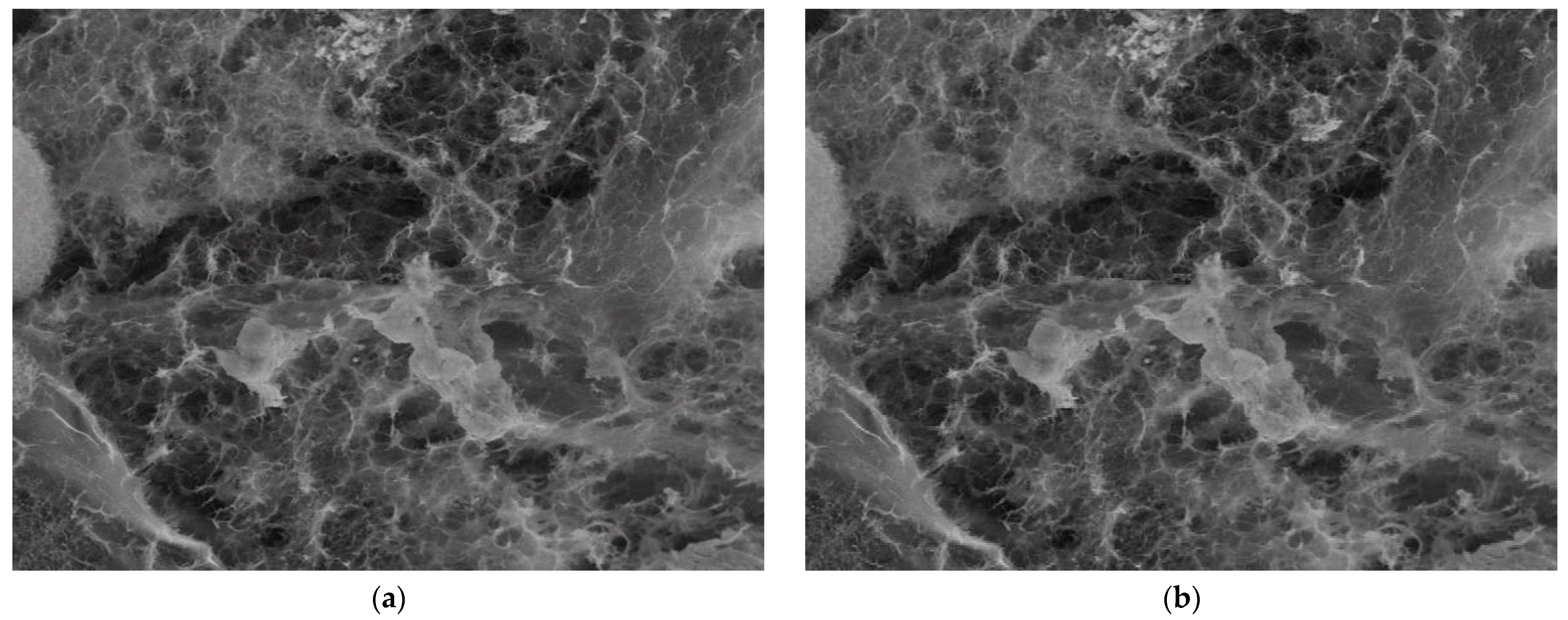
3.4. Application Results of Fractal and Lacunar Analysis Algorithms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Shpichka, A.; Butnaru, D.; Bezrukov, E.A.; Sukhanov, R.B.; Atala, A.; Burdukovskii, V.; Zhang, Y.; Timashev, P. Skin tissue regeneration for burn injury. Stem Cell Res. Ther. 2019, 10, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, A.H.; Mathieu, L.; Maibach, H.I. Acute chemical skin injuries in the United States: A review. Crit. Rev. Toxicol. 2018, 48, 540–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sani, H.; Lenghoven, D. Permanent physical disfigurement and disability due to burns injury: Case series. Int. J. Hum. Health Sci. 2020, 4, 319–321. [Google Scholar] [CrossRef]
- Yazarlu, O.; Iranshahi, M.; Kashani, H.R.K.; Reshadat, S.; Habtemariam, S.; Iranshahy, M.; Hasanpour, M. Perspective on the application of medicinal plants and natural products in wound healing: A mechanistic review. Pharmacol. Res. 2021, 174, 105841. [Google Scholar] [CrossRef] [PubMed]
- Ghandforoushan, P.; Golafshan, N.; Kadumudi, F.B.; Castilho, M.; Dolatshahi-Pirouz, A.; Orive, G. Injectable and adhesive hydrogels for dealing with wounds. Expert Opin. Biol. Ther. 2022; accepted/in press. [Google Scholar] [CrossRef] [PubMed]
- Anisiei, A.; Oancea, F.; Marin, L. Electrospinning of chitosan-based nanofibers: From design to prospective applications. Rev. Chem. Eng. 2020, 3, 325–336. [Google Scholar] [CrossRef]
- Kamińska, M.; Cybulska, A.; Skonieczna-Żydecka, K.; Augustyniuk, K.; Grochans, E.; Karakiewicz, B. Effectiveness of Hydrocolloid Dressings for Treating Pressure Ulcers in Adult Patients: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 7881. [Google Scholar] [CrossRef]
- Fan, F.; Saha, S.; Hanjaya-Putra, D. Biomimetic Hydrogels to Promote Wound Healing. Front. Bioeng. Biotechnol. 2021, 9. [Google Scholar] [CrossRef]
- Savencu, I.; Iurian, S.; Porfire, A.; Bogdan, C.; Tomuță, I. Review of advances in polymeric wound dressing films. React. Funct. Polym. 2021, 168, 105059. [Google Scholar] [CrossRef]
- Duceac, I.A.; Verestiuc, L.; Dimitriu, C.D.; Maier, V.; Coseri, S. Design and Preparation of New Multifunctional Hydrogels Based on Chitosan/Acrylic Polymers for Drug Delivery and Wound Dressing Applications. Polymers 2020, 12, 1473. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, P.B.S.; de Oliveira, W.F.; Silva, P.M.D.S.; Correia, M.T.D.S.; Kennedy, J.F.; Coelho, L.C.B.B. Skincare application of medicinal plant polysaccharides—A review. Carbohydr. Polym. 2021, 277, 118824. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef] [Green Version]
- Alven, S.; Aderibigbe, B.A. Chitosan and Cellulose-Based Hydrogels for Wound Management. Int. J. Mol. Sci. 2020, 21, 9656. [Google Scholar] [CrossRef] [PubMed]
- Golmohammadi, R.; Najar-Peerayeh, S.; Moghadam, T.T.; Hosseini, S.M.J. Synergistic Antibacterial Activity and Wound Healing Properties of Selenium-Chitosan-Mupirocin Nanohybrid System: An in Vivo Study on Rat Diabetic Staphylococcus aureus Wound Infection Model. Sci. Rep. 2020, 10, 2854. [Google Scholar] [CrossRef] [Green Version]
- Ono, S.; Imai, R.; Ida, Y.; Shibata, D.; Komiya, T.; Matsumura, H. Increased wound pH as an indicator of local wound infection in second degree burns. Burns 2015, 41, 820–824. [Google Scholar] [CrossRef]
- Andreica, B.-I.; Ailincai, D.; Sandu, A.-I.; Marin, L. Amphiphilic chitosan-g-poly(trimethylene carbonate)—A new approach for biomaterials design. Int. J. Biol. Macromol. 2021, 193, 414–424. [Google Scholar] [CrossRef]
- Marin, L.; Ailincai, D.; Mares, M.; Paslaru, E.; Cristea, M.; Nica, V.; Simionescu, B.C. Imino-chitosan biopolymeric films. Obtaining, self-assembling, surface and antimicrobial properties. Carbohydr. Polym. 2015, 117, 762–770. [Google Scholar] [CrossRef]
- Ailincai, D.; Marin, L.; Morariu, S.; Mares, M.; Bostanaru, A.-C.; Pinteala, M.; Simionescu, B.C.; Barboiu, M. Dual crosslinked iminoboronate-chitosan hydrogels with strong antifungal activity against Candida planktonic yeasts and biofilms. Carbohydr. Polym. 2016, 152, 306–316. [Google Scholar] [CrossRef]
- Lungu, R.; Anisiei, A.; Rosca, I.; Sandu, A.-I.; Ailincai, D.; Marin, L. Double functionalization of chitosan based nanofibers towards biomaterials for wound healing. React. Funct. Polym. 2021, 167, 105028. [Google Scholar] [CrossRef]
- Anisiei, A.; Bostanaru, A.-C.; Mares, M.; Marin, L. Imination of chitosan nanofibers in a heterogeneous system. Synthesis optimization and impact on fiber morphology. Cellul. Chem. Technol. 2021, 55, 785–793. [Google Scholar] [CrossRef]
- Anisiei, A.; Rosca, I.; Sandu, A.-I.; Bele, A.; Cheng, X.; Marin, L. Imination of Microporous Chitosan Fibers—A Route to Biomaterials with “On Demand” Antimicrobial Activity and Biodegradation for Wound Dressings. Pharmaceutics 2022, 14, 117. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, S.; Prabakaran, P.; Prasad, E.; Satapathy, S. Augmenting Photoinduced Charge Transport in a Single-Component Gel System: Controlled In Situ Gel-Crystal Transformation at Room Temperature. Chem.—A Eur. J. 2018, 24, 6217–6230. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, S.; Prasad, E. Charge Transfer Modulated Self-Assembly in Poly(aryl ether) Dendron Derivatives with Improved Stability and Transport Characteristics. ACS Appl. Mater. Interfaces 2016, 8, 26176–26189. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk-Woźniak, A.; Cyrański, M.K.; Frączak, B.T.; Lewandowska, A.; Madura, I.D.; Sporzyński, A. Imino- and aminomethylphenylboronic acids: Stabilizing effect of hydrogen bonds. Tetrahedron 2012, 68, 3761–3767. [Google Scholar] [CrossRef]
- Iftime, M.-M.; Morariu, S.; Marin, L. Salicyl-imine-chitosan hydrogels: Supramolecular architecturing as a cross-linking method toward multifunctional hydrogels. Carbohydr. Polym. 2017, 165, 39–50. [Google Scholar] [CrossRef]
- Bratskaya, S.; Privar, Y.; Skatova, A.; Slobodyuk, A.; Kantemirova, E.; Pestov, A. Carboxyalkylchitosan-based hy-drogels with “imine clip”: Enhanced stability and amino acids-induced disassembly under physiological condi-tions. Carbohydr. Polym. 2021, 274, 118618. [Google Scholar] [CrossRef]
- Bratskaya, S.; Skatova, A.; Privar, Y.; Boroda, A.; Kantemirova, E.; Maiorova, M.; Pestov, A. Stimuli-Responsive Dual Cross-Linked N-Carboxyethylchitosan Hydrogels with Tunable Dissolution Rate. Gels 2021, 7, 188. [Google Scholar] [CrossRef]
- Dierking, I.; Al-Zangana, S. Lyotropic Liquid Crystal Phases from Anisotropic Nanomaterials. Nanomaterials 2017, 7, 305. [Google Scholar] [CrossRef]
- Marin, L.; Popa, M.; Anisiei, A.; Irimiciuc, S.-A.; Agop, M.; Petrescu, T.-C.; Vasincu, D.; Himiniuc, L. A Theoretical Model for Release Dynamics of an Antifungal Agent Covalently Bonded to the Chitosan. Molecules 2021, 26, 2089. [Google Scholar] [CrossRef]
- Rippke, F.; Berardesca, E.; Weber, T.M. pH and Microbial Infections. Immunodefic. Skin 2018, 54, 87–94. [Google Scholar]
- Kuo, S.-H.; Shen, C.-F.; Cheng, C.-M. Role of pH Value in Clinically Relevant Diagnosis. Diagnosis 2020, 10, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karperien, A.L.; Jelinek, H.F. Box-Counting Fractal Analysis: A Primer for the Clinician. In Springer Series in Computational Neuroscience; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2016; pp. 13–43. [Google Scholar]
- Karperien, A.; Jelinek, H.F.; Miloševic, N.T. Reviewing Lacunarity Analysis and Classification of Microglia in Neuroscience. In Proceedings of the 8th European Conference on Mathematical and Theoretical Biology, Kraków, Poland, 28 June–2 July 2011. [Google Scholar]
- Nichita, M.V.; Paun, M.A.; Paun, V.A.; Paun, V.P. Fractal analysis of brain glial cells. Fractals dimension and lacunarity. Univ. Politeh. Buchar. Sci. Bull. Ser. A Appl. Math. Phys. 2019, 81, 273–284. [Google Scholar]
- Bordescu, D.; Paun, M.A.; Paun, V.A.; Paun, V.P. Fractal analysis of Neuroimagistic. Lacunarity degree, a precious indicator in the detection of Alzheimer’s disease. Univ. Politeh. Buchar. Sci. Bull. Ser. A Appl. Math. Phys. 2018, 80, 309–320. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lungu, R.; Paun, M.-A.; Peptanariu, D.; Ailincai, D.; Marin, L.; Nichita, M.-V.; Paun, V.-A.; Paun, V.-P. Biocompatible Chitosan-Based Hydrogels for Bioabsorbable Wound Dressings. Gels 2022, 8, 107. https://doi.org/10.3390/gels8020107
Lungu R, Paun M-A, Peptanariu D, Ailincai D, Marin L, Nichita M-V, Paun V-A, Paun V-P. Biocompatible Chitosan-Based Hydrogels for Bioabsorbable Wound Dressings. Gels. 2022; 8(2):107. https://doi.org/10.3390/gels8020107
Chicago/Turabian StyleLungu, Ramona, Maria-Alexandra Paun, Dragos Peptanariu, Daniela Ailincai, Luminita Marin, Mihai-Virgil Nichita, Vladimir-Alexandru Paun, and Viorel-Puiu Paun. 2022. "Biocompatible Chitosan-Based Hydrogels for Bioabsorbable Wound Dressings" Gels 8, no. 2: 107. https://doi.org/10.3390/gels8020107
APA StyleLungu, R., Paun, M.-A., Peptanariu, D., Ailincai, D., Marin, L., Nichita, M.-V., Paun, V.-A., & Paun, V.-P. (2022). Biocompatible Chitosan-Based Hydrogels for Bioabsorbable Wound Dressings. Gels, 8(2), 107. https://doi.org/10.3390/gels8020107









