Printing Structurally Anisotropic Biocompatible Fibrillar Hydrogel for Guided Cell Alignment
Abstract
1. Introduction
2. Results and Discussion
2.1. Preparation of a-CNF/Gelatin Ink
2.2. Properties of a-CNF/Gelatin Hydrogels
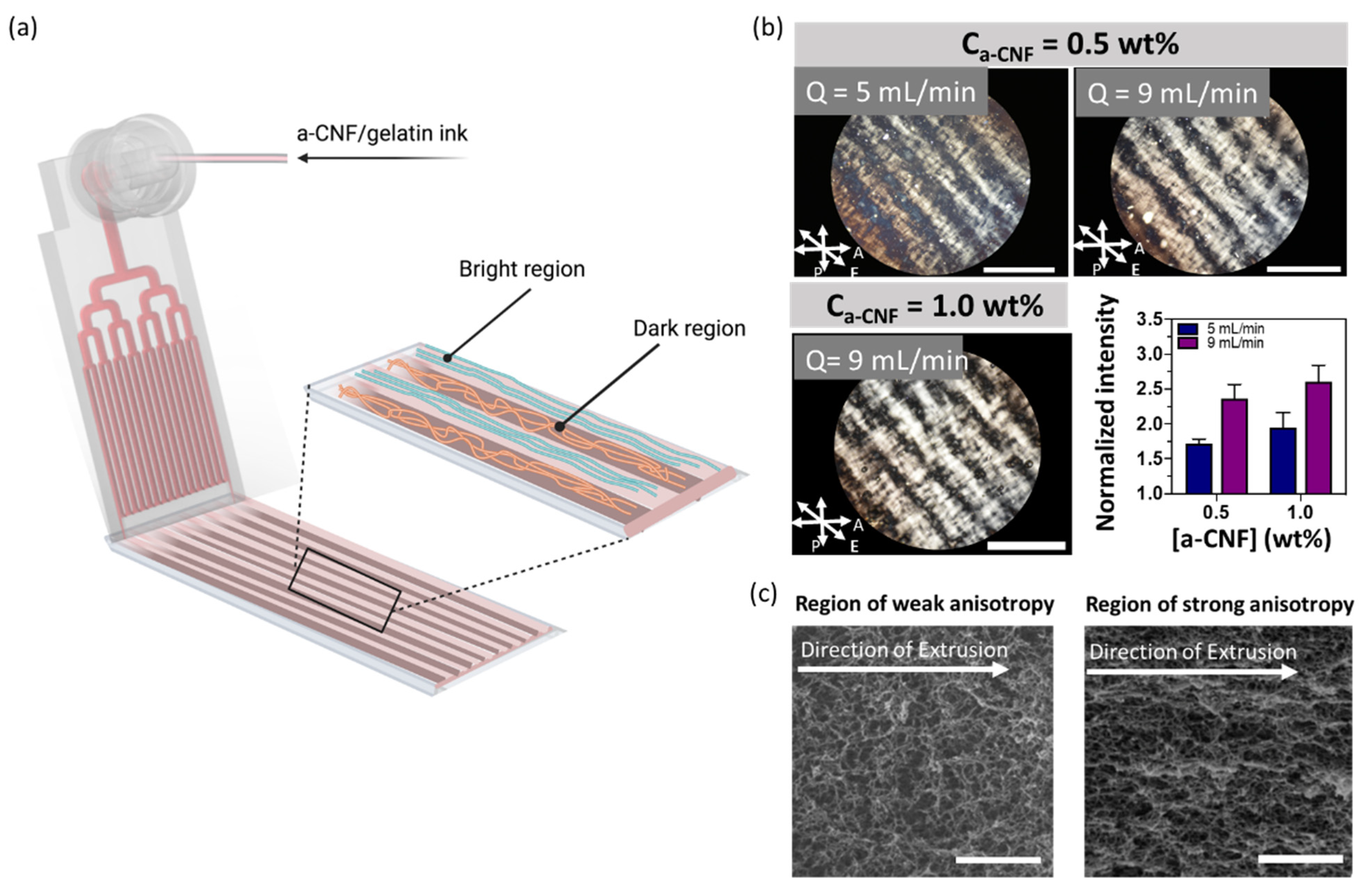
2.3. Structural Anisotropy of Extruded Hydrogel Sheets
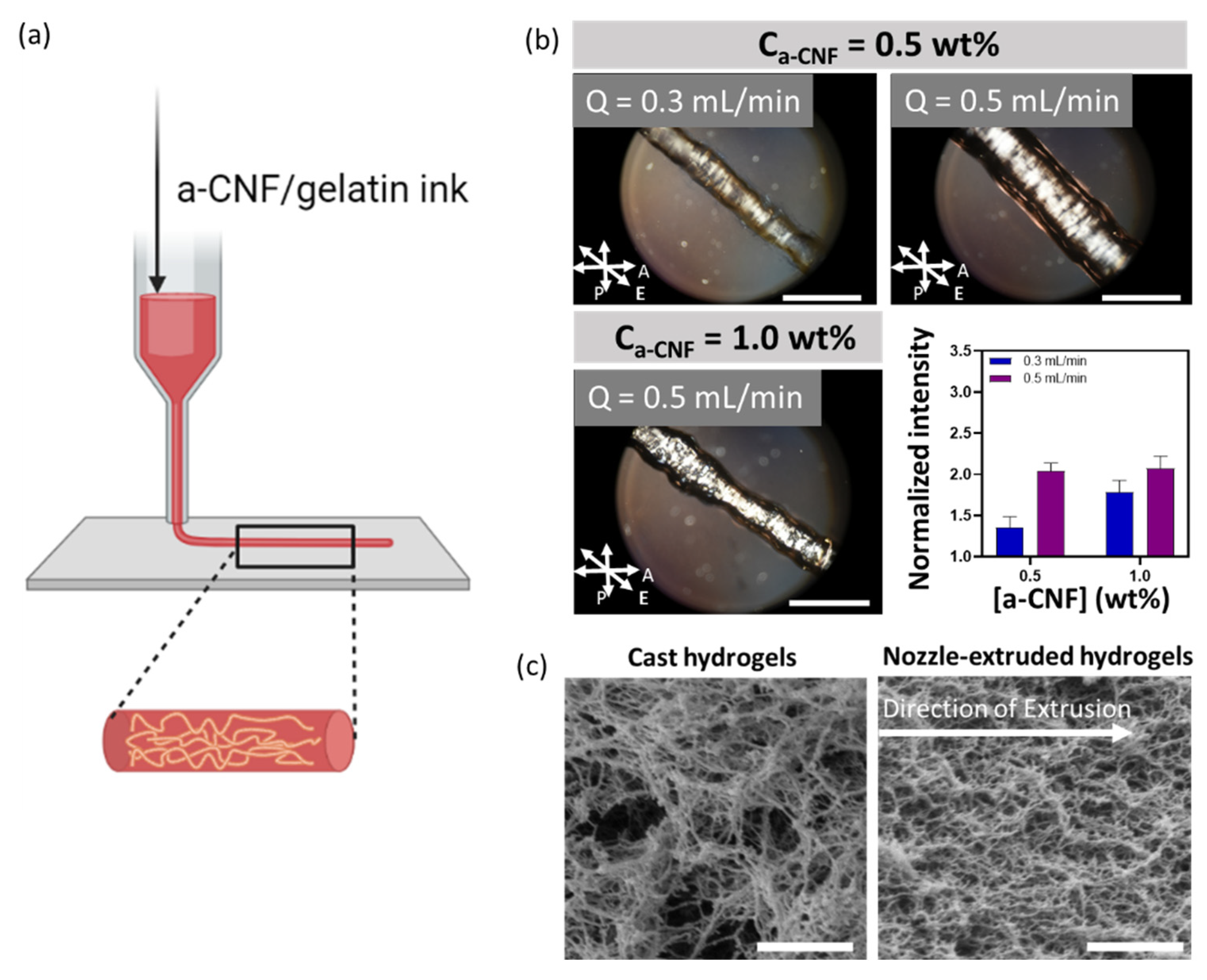
2.4. Structural Anisotropy of Extruded Hydrogel Threads
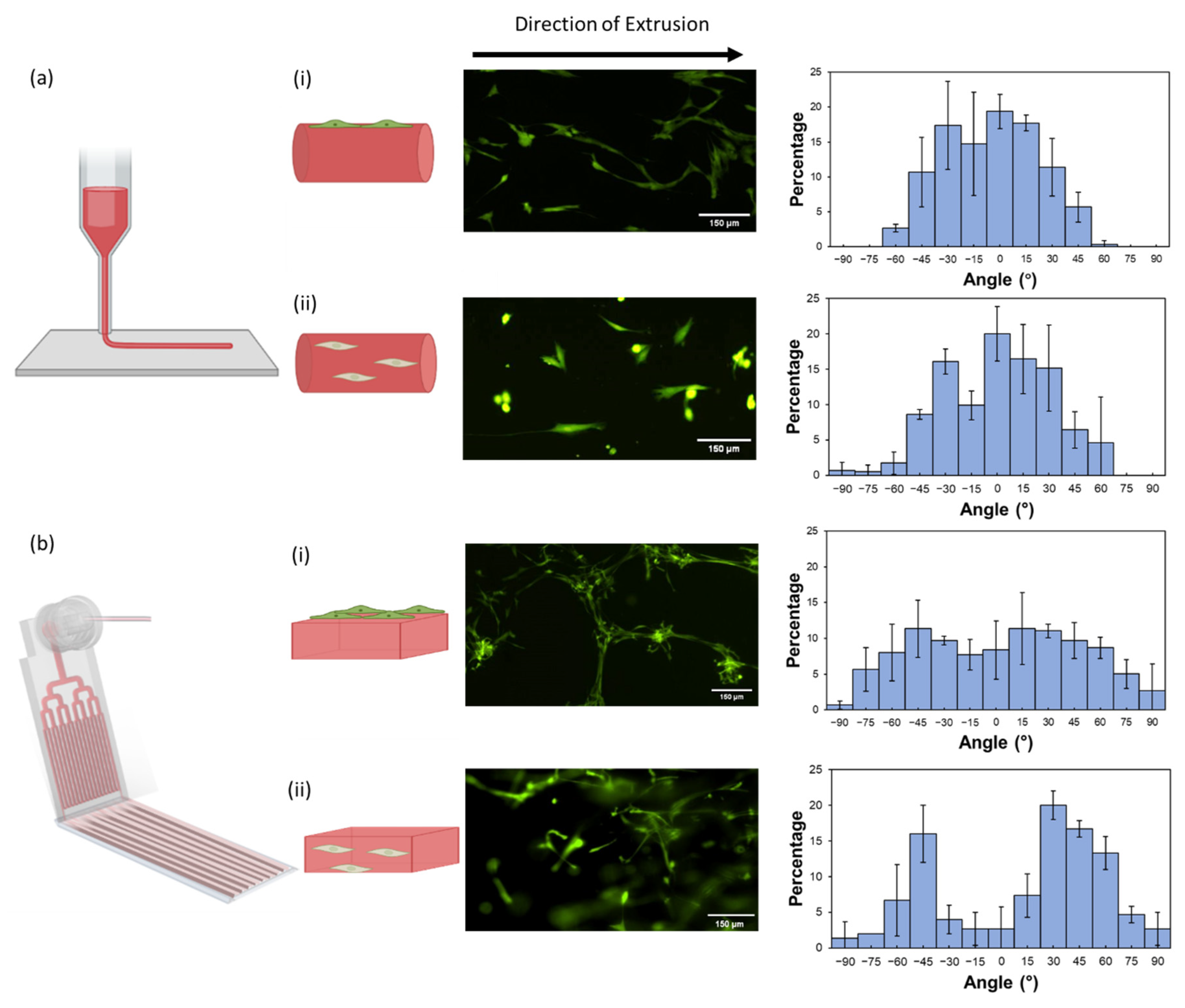
2.5. Effect of Structurally Anisotropic Hydrogels on Cell Orientation
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Modification of CNFs
4.3. Characterization of a-CNFs
4.4. Preparation and Characterization of Hydrogels
4.5. Extrusion-Based Printing of Hydrogels
4.6. Measurement of Normalized Birefringence Light Intensity
4.7. Cell Culture
4.8. Seeding HDFs on the Printed Hydrogels
4.9. Extrusion-Based Printing of Cell-Laden Hydrogels
4.10. Analysis of Cell Orientation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khuu, N.; Kheiri, S.; Kumacheva, E. Structurally anisotropic hydrogels for tissue engineering. Trends Chem. 2021, 3, 1002–1026. [Google Scholar] [CrossRef]
- Beldjilali-Labro, M.; Garcia Garcia, A.; Farhat, F.; Bedoui, F.; Grosset, J.-F.; Dufresne, M.; Legallais, C. Biomaterials in Tendon and Skeletal Muscle Tissue Engineering: Current Trends and Challenges. Materials 2018, 11, 1116. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, S.P.; Heinemeier, K.M.; Kjaer, M. Collagen homeostasis and metabolism. Metab. Influ. Risk Tendon Disord. 2016, 920, 11–25. [Google Scholar]
- Ziv, V.; Wagner, H.D.; Weiner, S. Microstructure-microhardness relations in parallel-fibered and lamellar bone. Bone 1996, 18, 417–428. [Google Scholar] [CrossRef]
- Saffitz, J.E.; Kanter, H.L.; Green, K.G.; Tolley, T.K.; Beyer, E.C. Tissue-specific determinants of anisotropic conduction velocity in canine atrial and ventricular myocardium. Circ. Res. 1994, 74, 1065–1070. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, Z.; Zhou, Y.; Zheng, S.; An, Y.; Huang, W.; He, H.; Yang, Y.; Li, S.; Chen, Y. Controlling the multiscale network structure of fibers to stimulate wound matrix rebuilding by fibroblast differentiation. ACS Appl. Mater. Interfaces 2019, 11, 28377–28386. [Google Scholar] [CrossRef]
- Huang, C.; Fu, X.; Liu, J.; Qi, Y.; Li, S.; Wang, H. The involvement of integrin β1 signaling in the migration and myofibroblastic differentiation of skin fibroblasts on anisotropic collagen-containing nanofibers. Biomaterials 2012, 33, 1791–1800. [Google Scholar] [CrossRef]
- Meek, K.M.; Knupp, C. Corneal structure and transparency. Prog. Retin. Eye Res. 2015, 49, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Brugnano, J.; Sun, S.; Vase, A.; Orwin, E. The Development of a Tissue-Engineered Cornea: Biomaterials and Culture Methods. Pediatr. Res. 2008, 63, 535–544. [Google Scholar] [CrossRef]
- Changoor, A.; Nelea, M.; Méthot, S.; Tran-Khanh, N.; Chevrier, A.; Restrepo, A.; Shive, M.S.; Hoemann, C.D.; Buschmann, M.D. Structural characteristics of the collagen network in human normal, degraded and repair articular cartilages observed in polarized light and scanning electron microscopies. Osteoarthr. Cartil. 2011, 19, 1458–1468. [Google Scholar] [CrossRef]
- Fratzl, P.; Weinkamer, R. Nature’s hierarchical materials. Prog. Mater. Sci. 2007, 52, 1263–1334. [Google Scholar] [CrossRef]
- Ueda, M.; Saito, S.; Murata, T.; Hirano, T.; Bise, R.; Kabashima, K.; Suzuki, S. Combined multiphoton imaging and biaxial tissue extension for quantitative analysis of geometric fiber organization in human reticular dermis. Sci. Rep. 2019, 9, 10644. [Google Scholar] [CrossRef]
- Ottenio, M.; Tran, D.; Ní Annaidh, A.; Gilchrist, M.D.; Bruyère, K. Strain rate and anisotropy effects on the tensile failure characteristics of human skin. J. Mech. Behav. Biomed. Mater. 2015, 41, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.A.; Johannessen, W.; Wu, J.P.; Jaw, A.; Elliott, D.M. Effect of Fiber Orientation and Strain Rate on the Nonlinear Uniaxial Tensile Material Properties of Tendon. J. Biomech. Eng. 2003, 125, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Weiner, S.; Wagner, H.D. The material bone: Structure-mechanical function relations. Annu. Rev. Mater. Sci. 1998, 28, 271–298. [Google Scholar] [CrossRef]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Hyun, J. Rheological properties of cellulose nanofiber hydrogel for high-fidelity 3D printing. Carbohydr. Polym. 2021, 263, 117976. [Google Scholar] [CrossRef]
- Shin, S.; Park, S.; Park, M.; Jeong, E.; Na, K.; Youn, H.J.; Hyun, J. Cellulose Nanofibers for the Enhancement of Printability of Low Viscosity Gelatin Derivatives. BioResources 2017, 12, 14. [Google Scholar] [CrossRef]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of Hydrogels and Aerogels Containing Nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Sanandiya, N.D.; Vasudevan, J.; Das, R.; Lim, C.T.; Fernandez, J.G. Stimuli-responsive injectable cellulose thixogel for cell encapsulation. Int. J. Biol. Macromol. 2019, 130, 1009–1017. [Google Scholar] [CrossRef]
- Apelgren, P.; Karabulut, E.; Amoroso, M.; Mantas, A.; Martínez Ávila, H.; Kölby, L.; Kondo, T.; Toriz, G.; Gatenholm, P. In Vivo Human Cartilage Formation in Three-Dimensional Bioprinted Constructs with a Novel Bacterial Nanocellulose Bioink. ACS Biomater. Sci. Eng. 2019, 5, 2482–2490. [Google Scholar] [CrossRef]
- Chen, R.-D.; Huang, C.-F.; Hsu, S.-h. Composites of waterborne polyurethane and cellulose nanofibers for 3D printing and bioapplications. Carbohydr. Polym. 2019, 212, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Oh, D.X.; Choy, S.; Nguyen, H.-L.; Cha, H.J.; Hwang, D.S. 3D cellulose nanofiber scaffold with homogeneous cell population and long-term proliferation. Cellulose 2018, 25, 7299–7314. [Google Scholar] [CrossRef]
- Prince, E.; Kumacheva, E. Design and applications of man-made biomimetic fibrillar hydrogels. Nat. Rev. Mater. 2019, 4, 99–115. [Google Scholar] [CrossRef]
- Kumbar, S.; James, R.; Nukavarapu, S.; Laurencin, C. Electrospun nanofiber scaffolds: Engineering soft tissues. Biomed. Mater. 2008, 3, 034002. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Clarkson, C.M.; Wang, L.; Liu, Y.; Lamm, M.; Pang, Z.; Zhou, Y.; Qian, J.; Tajvidi, M.; Gardner, D.J.; et al. Alignment of Cellulose Nanofibers: Harnessing Nanoscale Properties to Macroscale Benefits. ACS Nano 2021, 15, 3646–3673. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Pan, Z.-Z.; Lv, W.; Liu, B.; Wei, J.; Lv, X.; Luo, Y.; Nishihara, H.; Yang, Q.-H. A Directional Strain Sensor Based on Anisotropic Microhoneycomb Cellulose Nanofiber-Carbon Nanotube Hybrid Aerogels Prepared by Unidirectional Freeze Drying. Small 2019, 15, 1805363. [Google Scholar] [CrossRef]
- Kim, H.C.; Kim, J.W.; Zhai, L.; Kim, J. Strong and tough long cellulose fibers made by aligning cellulose nanofibers under magnetic and electric fields. Cellulose 2019, 26, 5821–5829. [Google Scholar] [CrossRef]
- Rezaei, A.; Nasirpour, A.; Fathi, M. Application of Cellulosic Nanofibers in Food Science Using Electrospinning and Its Potential Risk. Compr. Rev. Food Sci. Food Saf. 2015, 14, 269–284. [Google Scholar] [CrossRef]
- Eom, S.; Park, S.M.; Hong, H.; Kwon, J.; Oh, S.-R.; Kim, J.; Kim, D.S. Hydrogel-Assisted Electrospinning for Fabrication of a 3D Complex Tailored Nanofiber Macrostructure. ACS Appl. Mater. Interfaces 2020, 12, 51212–51224. [Google Scholar] [CrossRef]
- Huang, L.; Du, X.; Fan, S.; Yang, G.; Shao, H.; Li, D.; Cao, C.; Zhu, Y.; Zhu, M.; Zhang, Y. Bacterial cellulose nanofibers promote stress and fidelity of 3D-printed silk based hydrogel scaffold with hierarchical pores. Carbohydr. Polym. 2019, 221, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Sydney Gladman, A.; Matsumoto, E.A.; Nuzzo, R.G.; Mahadevan, L.; Lewis, J.A. Biomimetic 4D printing. Nat. Mater. 2016, 15, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Bao, C.; Hausmann, M.; Siqueira, G.; Zimmermann, T.; Kim, W.S. 3D Printed Disposable Wireless Ion Sensors with Biocompatible Cellulose Composites. Adv. Electron. Mater. 2019, 5, 1800778. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Prince, E.; Alizadehgiashi, M.; Campbell, M.; Khuu, N.; Albulescu, A.; De France, K.; Ratkov, D.; Li, Y.; Hoare, T.; Kumacheva, E. Patterning of Structurally Anisotropic Composite Hydrogel Sheets. Biomacromolecules 2018, 19, 1276–1284. [Google Scholar] [CrossRef]
- Xue, Y.; Mou, Z.; Xiao, H. Nanocellulose as a sustainable biomass material: Structure, properties, present status and future prospects in biomedical applications. Nanoscale 2017, 9, 14758–14781. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Winter, H.H.; Chambon, F. Analysis of Linear Viscoelasticity of a Crosslinking Polymer at the Gel Point. J. Rheol. 1986, 30, 367–382. [Google Scholar] [CrossRef]
- Khuu, N.; Alizadehgiashi, M.; Gevorkian, A.; Galati, E.; Yan, N.; Kumacheva, E. Temperature-Mediated Microfluidic Extrusion of Structurally Anisotropic Hydrogels. Adv. Mater. Technol. 2019, 4, 1800627. [Google Scholar] [CrossRef]
- Gevorkian, A.; Morozova, S.M.; Kheiri, S.; Khuu, N.; Chen, H.; Young, E.; Yan, N.; Kumacheva, E. Actuation of Three-Dimensional-Printed Nanocolloidal Hydrogel with Structural Anisotropy. Adv. Funct. Mater. 2021, 31, 2010743. [Google Scholar] [CrossRef]
- Alizadehgiashi, M.; Gevorkian, A.; Tebbe, M.; Seo, M.; Prince, E.; Kumacheva, E. 3D-Printed Microfluidic Devices for Materials Science. Adv. Mater. Technol. 2018, 3, 1800068. [Google Scholar] [CrossRef]
- Hansen, C.J.; Saksena, R.; Kolesky, D.B.; Vericella, J.J.; Kranz, S.J.; Muldowney, G.P.; Christensen, K.T.; Lewis, J.A. High-Throughput Printing via Microvascular Multinozzle Arrays. Adv. Mater. 2013, 25, 96–102. [Google Scholar] [CrossRef]
- Mittal, N.; Ansari, F.; Gowda, V.K.; Brouzet, C.; Chen, P.; Larsson, P.T.; Roth, S.V.; Lundell, F.; Wågberg, L.; Kotov, N.A.; et al. Multiscale Control of Nanocellulose Assembly: Transferring Remarkable Nanoscale Fibril Mechanics to Macroscale Fibers. ACS Nano 2018, 12, 6378–6388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tao, L.; Li, S.; Wei, Y. Synthesis of Multiresponsive and Dynamic Chitosan-Based Hydrogels for Controlled Release of Bioactive Molecules. Biomacromolecules 2011, 12, 2894–2901. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Lee, K.H.; Kim, Y.B.; Ambade, S.B.; Noh, S.H.; Eom, W.; Hwang, J.Y.; Lee, W.J.; Huang, J.; Han, T.H. Dynamic assembly of liquid crystalline graphene oxide gel fibers for ion transport. Sci. Adv. 2018, 4, eaau2104. [Google Scholar] [CrossRef] [PubMed]
- Trebbin, M.; Steinhauser, D.; Perlich, J.; Buffet, A.; Roth, S.V.; Zimmermann, W.; Thiele, J.; Förster, S. Anisotropic particles align perpendicular to the flow direction in narrow microchannels. Proc. Natl. Acad. Sci. 2013, 110, 6706–6711. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Phan, S.H.; Thannickal, V.J.; Prunotto, M.; Desmoulière, A.; Varga, J.; De Wever, O.; Mareel, M.; Gabbiani, G. Recent developments in myofibroblast biology: Paradigms for connective tissue remodeling. Am. J. Pathol. 2012, 180, 1340–1355. [Google Scholar] [CrossRef]
- Echave, M.C.; Domingues, R.M.A.; Gómez-Florit, M.; Pedraz, J.L.; Reis, R.L.; Orive, G.; Gomes, M.E. Biphasic Hydrogels Integrating Mineralized and Anisotropic Features for Interfacial Tissue Engineering. ACS Appl. Mater. Interfaces 2019, 11, 47771–47784. [Google Scholar] [CrossRef]
- Saito, T.; Hirota, M.; Tamura, N.; Kimura, S.; Fukuzumi, H.; Heux, L.; Isogai, A. Individualization of Nano-Sized Plant Cellulose Fibrils by Direct Surface Carboxylation Using TEMPO Catalyst under Neutral Conditions. Biomacromolecules 2009, 10, 1992–1996. [Google Scholar] [CrossRef]
- Varma, A.J.; Kulkarni, M.P. Oxidation of cellulose under controlled conditions. Polym. Degrad. Stab. 2002, 77, 25–27. [Google Scholar] [CrossRef]
- Kumari, S.; Ram, B.; Kumar, D.; Ranote, S.; Chauhan, G.S. Nanoparticles of oxidized-cellulose synthesized by green method. Mater. Sci. Energy Technol. 2018, 1, 22–28. [Google Scholar] [CrossRef]
- Kim, U.-J.; Wada, M.; Kuga, S. Solubilization of dialdehyde cellulose by hot water. Carbohydr. Polym. 2004, 56, 7–10. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Khuu, N.; Xu, F.; Kheiri, S.; Yakavets, I.; Rakhshani, F.; Morozova, S.; Kumacheva, E. Printing Structurally Anisotropic Biocompatible Fibrillar Hydrogel for Guided Cell Alignment. Gels 2022, 8, 685. https://doi.org/10.3390/gels8110685
Chen Z, Khuu N, Xu F, Kheiri S, Yakavets I, Rakhshani F, Morozova S, Kumacheva E. Printing Structurally Anisotropic Biocompatible Fibrillar Hydrogel for Guided Cell Alignment. Gels. 2022; 8(11):685. https://doi.org/10.3390/gels8110685
Chicago/Turabian StyleChen, Zhengkun, Nancy Khuu, Fei Xu, Sina Kheiri, Ilya Yakavets, Faeze Rakhshani, Sofia Morozova, and Eugenia Kumacheva. 2022. "Printing Structurally Anisotropic Biocompatible Fibrillar Hydrogel for Guided Cell Alignment" Gels 8, no. 11: 685. https://doi.org/10.3390/gels8110685
APA StyleChen, Z., Khuu, N., Xu, F., Kheiri, S., Yakavets, I., Rakhshani, F., Morozova, S., & Kumacheva, E. (2022). Printing Structurally Anisotropic Biocompatible Fibrillar Hydrogel for Guided Cell Alignment. Gels, 8(11), 685. https://doi.org/10.3390/gels8110685








