Site-Specific Vesicular Drug Delivery System for Skin Cancer: A Novel Approach for Targeting
Abstract
:1. Introduction
1.1. Skin Cancer and Skin Cancer Prevalence Statistics
1.2. History of Treatments and Limitations of Convention Dosage Form for Skin Cancer Treatment
2. Methodology
3. Critical Biological Barriers for Skin Cancer Therapeutics
4. Nanocarriers against Skin Cancer
5. Vesicular Drug Delivery for Skin Cancer Treatment
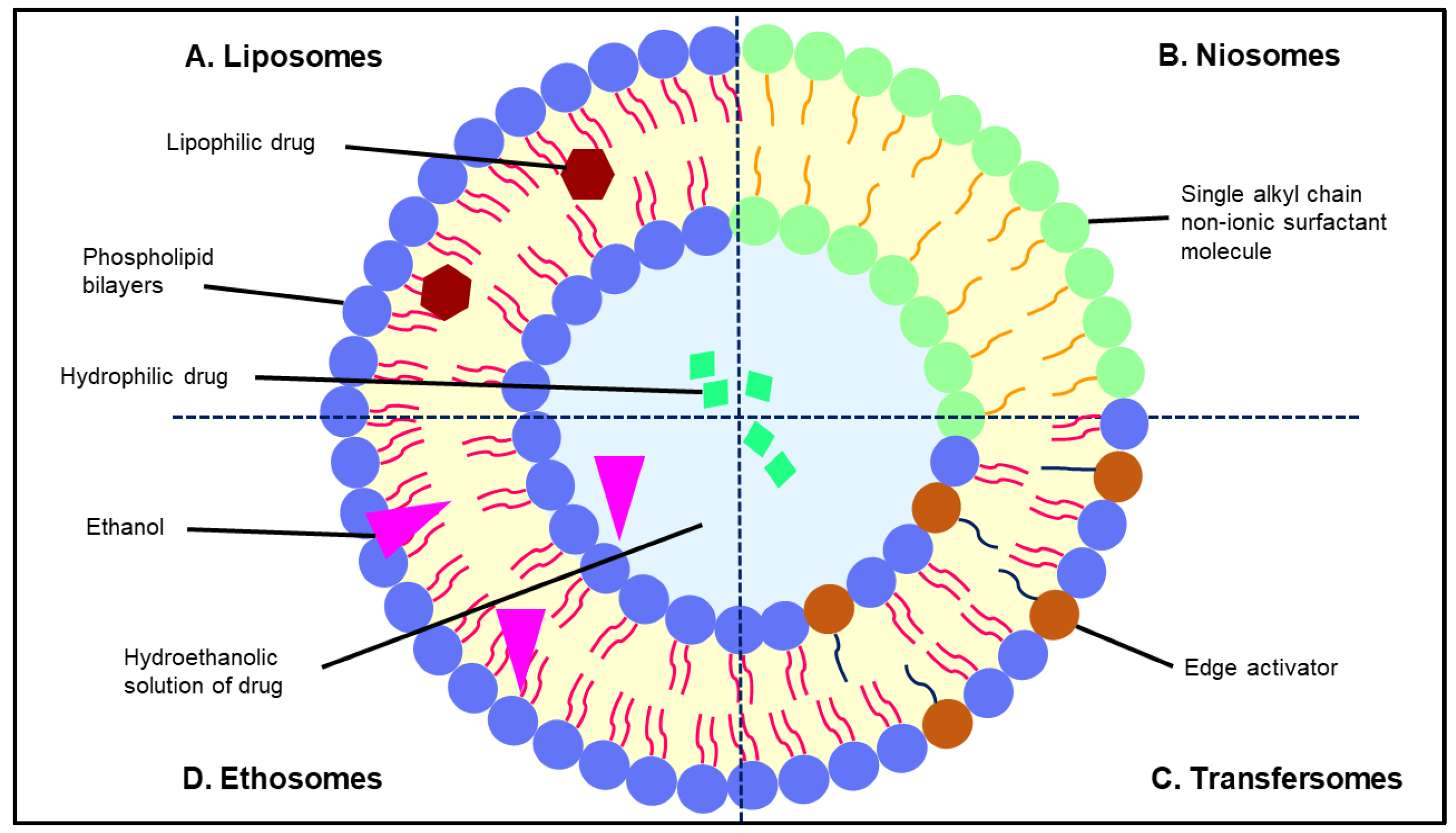
5.1. Liposomes
5.2. Niosomes
5.3. Transfersomes
5.4. Ethosomes
6. Beneficial Aspects of Vesicular Drug Delivery over Another Nanocarrier in Treatment of Skin Cancer
6.1. Liposomes
6.2. Niosomes
6.3. Transfersomes
6.4. Ethosomes
7. Safety Concern of Vesicular Drug Delivery for Skin Cancer Treatment and Its Clinical Aspects against Skin Cancer
8. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yousef, H.; Sharma, S. Anatomy, Skin (Integument), Epidermis; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Akhtar, N.; Khan, R.A. Liposomal systems as viable drug delivery technology for skin cancer sites with an outlook on lipid-based delivery vehicles and diagnostic imaging inputs for skin conditions’. Prog. Lipid Res. 2016, 64, 192–230. [Google Scholar] [CrossRef]
- Khazaei, Z.; Ghorat, F.; Jarrahi, A.M.; Adineh, H.A.; Sohrabivafa, M.; Goodarzi, E. Global incidence and mortality of skin cancer by histological subtype and its relationship with the human development index (HDI); An ecology study in 2018. World Cancer Res. J. 2019, 6, e1265. [Google Scholar]
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Ultraviolet radiation and skin cancer. Int. J. Dermatol. 2010, 49, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Radiation: Ultraviolet (UV) Radiation and Skin Cancer. Available online: https://www.who.int/news-room/q-a-detail/radiation-ultraviolet-(uv)-radiation-and-skin-cancer (accessed on 2 January 2021).
- Affandi, A.M. Skin cancer: 13-year experience at the Department of Dermatology, Hospital Kuala Lumpur, Malaysia. J. Glob. Oncol. 2018, 4, 79s. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Malaysia|Source: Globocan 2020; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Guy, G.P.; Machlin, S.R.; Ekwueme, D.U.; Yabroff, K.R. Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007–2011. Am. J. Prev. Med. 2015, 48, 183–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linares, M.A.; Zakaria, A.; Nizran, P. Skin Cancer. Prim. Care Clin. Off. Pract. 2015, 42, 645–659. [Google Scholar] [CrossRef]
- Simões, M.C.F.; Sousa, J.J.S.; Pais, A.A.C.C. Skin cancer and new treatment perspectives: A review. Cancer Lett. 2015, 357, 8–42. [Google Scholar] [CrossRef]
- Losquadro, W.D. Anatomy of the skin and the pathogenesis of nonmelanoma skin cancer. Facial Plast. Surg. Clin. N. Am. 2017, 25, 283–289. [Google Scholar] [CrossRef]
- Krishnan, V.; Mitragotri, S. Nanoparticles for topical drug delivery: Potential for skin cancer treatment. Adv. Drug Deliv. Rev. 2020, 153, 87–108. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Non-Melanoma Skin Cancer|Source: Globocan 2020; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Didona, D.; Paolino, G.; Bottoni, U.; Cantisani, C. Non melanoma skin cancer pathogenesis overview. Biomedicines 2018, 6, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cameron, M.C.; Lee, E.; Hibler, B.P.; Barker, C.A.; Mori, S.; Cordova, M.; Nehal, K.S.; Rossi, A.M. Basal cell carcinoma: Epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J. Am. Acad. Dermatol. 2019, 80, 303–317. [Google Scholar] [CrossRef] [Green Version]
- Fania, L.; Didona, D.; Morese, R.; Campana, I.; Coco, V.; Di Pietro, F.R.; Ricci, F.; Pallotta, S.; Candi, E.; Abeni, D.; et al. Basal cell carcinoma: From pathophysiology to novel therapeutic approaches. Biomedicines 2020, 8, 449. [Google Scholar] [CrossRef]
- Skin Cancer Foundation. Melanoma Overview. Available online: https://www.skincancer.org/skin-cancer-information/melanoma/ (accessed on 16 January 2021).
- Orthaber, K.; Pristovnik, M.; Skok, K.; Perić, B.; Maver, U. Skin cancer and its treatment: Novel treatment approaches with emphasis on nanotechnology. J. Nanomater. 2017, 2017, 20. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. World|Source: Globocan 2020; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Oliveria, S.A.; Saraiya, M.; Geller, A.C.; Heneghan, M.K.; Jorgensen, C. Sun exposure and risk of melanoma. Arch. Dis. Child. 2006, 91, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Kato, J.; Horimoto, K.; Sato, S.; Minowa, T.; Uhara, H. Dermoscopy of melanoma and non-melanoma skin cancers. Front. Med. 2019, 6, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismail, M.; Khan, S.; Khan, F.; Noor, S.; Sajid, H.; Yar, S.; Rasheed, I. Prevalence and significance of potential drug-drug interactions among cancer patients receiving chemotherapy. BMC Cancer 2020, 20, 335. [Google Scholar] [CrossRef] [Green Version]
- Xiao, H.; Noble, G.T.; Stefanick, J.F.; Qi, R.; Kiziltepe, T.; Jing, X.; Bilgicer, B. Photosensitive Pt(IV)-azide prodrug-loaded nanoparticles exhibit controlled drug release and enhanced efficacy in vivo. J. Control Release 2014, 173, 11–17. [Google Scholar] [CrossRef]
- Mou, Q.; Ma, Y.; Zhu, X.; Yan, D. A small molecule nanodrug consisting of amphiphilic targeting ligand-chemotherapy drug conjugate for targeted cancer therapy. J. Control Release 2016, 230, 34–44. [Google Scholar] [CrossRef]
- Buajordet, I.; Ebbesen, J.; Erikssen, J.; Brørs, O.; Hilberg, T. Fatal adverse drug events: The paradox of drug treatment. J. Intern. Med. 2001, 250, 327–341. [Google Scholar] [CrossRef]
- Neville, J.A.; Welch, E.; Leffell, D.J. Management of nonmelanoma skin cancer in 2007. Nat. Clin. Pract. Oncol. 2007, 4, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, A.; Geisse, J.K. Medical therapies for non-melanoma skin cancer. Clin. Dermatol. 2004, 22, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Marks, R.; Gebauer, K.; Shumack, S.; Amies, M.; Bryden, J.; Fox, T.L.; Owens, M.L. Imiquimod 5% cream in the treatment of superficial basal cell carcinoma: Results of a multicenter 6-week dose-response trial. J. Am. Acad. Dermatol. 2001, 44, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Shumack, S.; Robinson, J.; Kossard, S.; Golitz, L.; Greenway, H.; Schroeter, A.; Andres, K.; Amies, M.; Owens, M. Efficacy of topical 5% imiquimod cream for the treatment of nodular basal cell carcinoma. Arch. Dermatol. 2002, 138, 1165–1171. [Google Scholar] [CrossRef]
- Chua, B.; Jackson, J.E.; Lin, C.; Veness, M.J. Radiotherapy for early non-melanoma skin cancer. Oral Oncol. 2019, 98, 96–101. [Google Scholar] [CrossRef]
- Chen, E.L.A.; Srivastava, D.; Nijhawan, R.I. Mohs micrographic surgery: Development, technique, and applications in cutaneous malignancies. Semin. Plast. Surg. 2018, 32, 60–68. [Google Scholar] [CrossRef]
- Wain, R.A.J.; Tehrani, H. Reconstructive outcomes of Mohs surgery compared with conventional excision: A 13-month prospective study. Br. J. Plast. Surg. 2015, 68, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Downes, R.N.; Walker, N.P.J.; Collin, J.R.O. Micrographic (MOHS’) surgery in the management of periocular basal cell epitheliomas. Eye 1990, 4, 160–168. [Google Scholar] [CrossRef]
- Gorzelanny, C.; Mess, C.; Schneider, S.W.; Huck, V.; Brandner, J.M. Skin barriers in dermal drug delivery: Which barriers have to be overcome and how can we measure them? Pharmaceutics 2020, 12, 684. [Google Scholar] [CrossRef]
- Lee, A.Y. Molecular mechanism of epidermal barrier dysfunction as primary abnormalities. Int. J. Mol. Sci. 2020, 21, 1194. [Google Scholar] [CrossRef] [Green Version]
- Yokouchi, M.; Kubo, A. Maintenance of tight junction barrier integrity in cell turnover and skin diseases. Exp. Dermatol. 2018, 27, 876–883. [Google Scholar] [CrossRef] [Green Version]
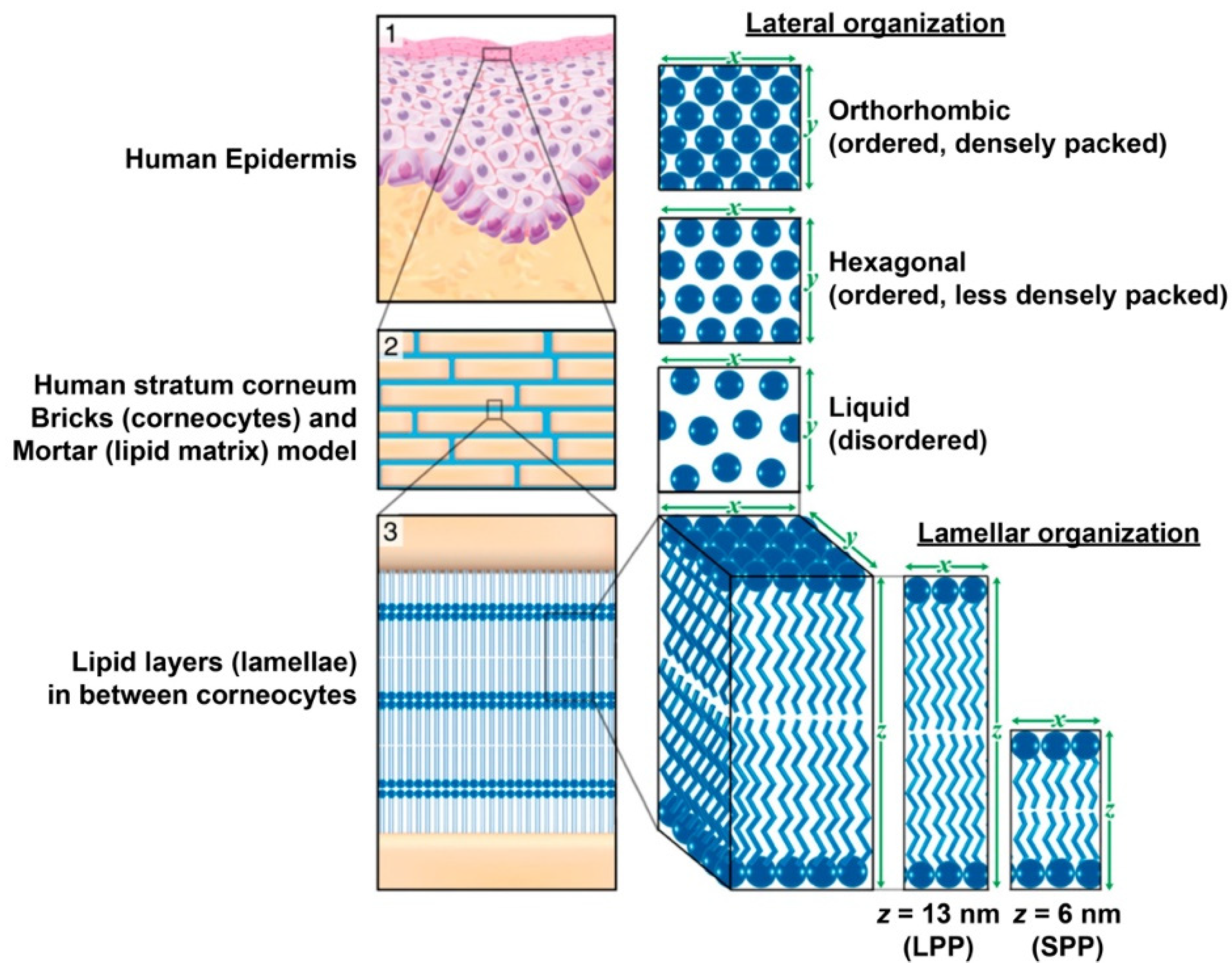
- Matsui, T.; Amagai, M. Dissecting the formation, structure and barrier function of the stratum corneum. Int. Immunol. 2015, 27, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Bouwstra, J.A.; Gooris, G.S.; van der Spek, J.A.; Bras, W. Structural investigations of human stratum corneum by small-angle X-ray scattering. J. Investig. Dermatol. 1991, 97, 1005–1012. [Google Scholar] [CrossRef] [Green Version]
- Bouwstra, J.A.; Gooris, G.S.; Bras, W.; Downing, D.T. Lipid organization in pig stratum corneum. J. Lipid Res. 1995, 36, 685–695. [Google Scholar] [CrossRef]
- Rancan, F.; Giulbudagian, M.; Jurisch, J.; Blume-Peytavi, U.; Calderón, M.; Vogt, A. Drug delivery across intact and disrupted skin barrier: Identification of cell populations interacting with penetrated thermoresponsive nanogels. Eur. J. Pharm. Biopharm. 2017, 116, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, N.; Rosenthal, R.; Furuse, M.; Moll, I.; Fromm, M.; Brandner, J.M. Contribution of tight junction proteins to ion, macromolecule, and water barrier in keratinocytes. J. Investig. Dermatol. 2013, 133, 1161–1169. [Google Scholar] [CrossRef] [Green Version]
- Brandner, J.M.; Zorn-Kruppa, M.; Yoshida, T.; Moll, I.; Beck, L.A.; De Benedetto, A. Epidermal tight junctions in health and disease. Tissue Barriers 2015, 3, e974451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, J.W.; Kloepper, J.; Langan, E.A.; Kim, Y.; Yeo, J.; Kim, M.J.; Hsi, T.C.; Rose, C.; Yoon, G.S.; Lee, S.J.; et al. A guide to studying human hair follicle cycling in vivo. J. Investig. Dermatol. 2016, 136, 34–44. [Google Scholar] [CrossRef] [Green Version]
- Zorn-Kruppa, M.; Vidal-y-Sy, S.; Houdek, P.; Wladykowski, E.; Grzybowski, S.; Gruber, R.; Gorzelanny, C.; Harcup, J.; Schneider, S.W.; Majumdar, A.; et al. Tight Junction barriers in human hair follicles—Role of claudin-1. Sci. Rep. 2018, 8, 12800. [Google Scholar] [CrossRef] [Green Version]
- Petrofsky, J.S. Control of skin Blood Flow. In Textbook of Aging Skin; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1091–1104. [Google Scholar]
- Severino, P.; Fangueiro, J.F.; Ferreira, S.V.; Basso, R.; Chaud, M.V.; Santana, M.H.A.; Rosmaninho, A.; Souto, E.B. Nanoemulsions and nanoparticles for non-melanoma skin cancer: Effects of lipid materials. Clin. Transl. Oncol. 2013, 15, 417–424. [Google Scholar] [CrossRef]
- Prabhakar, U.; Maeda, H.; Jain, R.K.; Sevick-Muraca, E.M.; Zamboni, W.; Farokhzad, O.C.; Barry, S.T.; Gabizon, A.; Grodzinski, P.; Blakey, D.C. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013, 73, 2412–2417. [Google Scholar] [CrossRef] [Green Version]
- Maeda, H.; Greish, K.; Fang, J. The EPR effect and polymeric drugs: A paradigm shift for cancer chemotherapy in the 21st century. Adv. Polym. Sci. 2006, 193, 103–121. [Google Scholar] [CrossRef]
- Giacone, D.V.; Dartora, V.F.M.C.; de Matos, J.K.R.; Passos, J.S.; Miranda, D.A.G.; de Oliveira, E.A.; Silveira, E.R.; Costa-Lotufo, L.V.; Maria-Engler, S.S.; Lopes, L.B. Effect of nanoemulsion modification with chitosan and sodium alginate on the topical delivery and efficacy of the cytotoxic agent piplartine in 2D and 3D skin cancer models. Int. J. Biol. Macromol. 2020, 165, 1055–1065. [Google Scholar] [CrossRef]
- Fofaria, N.M.; Qhattal, H.S.S.; Liu, X.; Srivastava, S.K. Nanoemulsion formulations for anti-cancer agent piplartine—Characterization, toxicological, pharmacokinetics and efficacy studies. Int. J. Pharm. 2016, 498, 12–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Entezar-Almahdi, E.; Mohammadi-Samani, S.; Tayebi, L.; Farjadian, F. Recent advances in designing 5-Fluorouracil delivery systems: A stepping stone in the safe treatment of colorectal cancer. Int. J. Nanomed. 2020, 15, 5445–5458. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Ahmad, R.; Mohammed Buheazaha, T.; Salman AlHomoud, H.; Al-Nasif, H.A.; Sarafroz, M. A comparative ex vivo permeation evaluation of a novel 5-Fluorocuracil nanoemulsion-gel by topically applied in the different excised rat, goat, and cow skin. Saudi J. Biol. Sci. 2020, 27, 1024–1040. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.; Kashaw, S.K.; Sau, S.; Kushwah, V.; Jain, S.; Agrawal, R.K.; Iyer, A.K. pH responsive 5-Fluorouracil loaded biocompatible nanogels for topical chemotherapy of aggressive melanoma. Colloids Surf. B Biointerfaces 2019, 174, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.; Kashaw, S.K.; Sau, S.; Iyer, A.K. Stumuli-responsive bio-hybrid nanogels: An emerging platform in medicinal arena. Glob. J. Nanomed. 2017, 1, 555564. [Google Scholar]
- Zhang, J.; Liu, P.; Zhang, Z.; Han, J.; Yang, X.; Wang, A.; Zhang, X. Apatinib-loaded nanoparticles inhibit tumor growth and angiogenesis in a model of melanoma. Biochem. Biophys. Res. Commun. 2020, 521, 296–302. [Google Scholar] [CrossRef]
- Nanofiber—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/materials-science/nanofibers (accessed on 7 November 2021).
- Janani, I.; Lakra, R.; Kiran, M.S.; Korrapati, P.S. Selectivity and sensitivity of molybdenum oxide-polycaprolactone nanofiber composites on skin cancer: Preliminary in-vitro and in-vivo implications. J. Trace Elem. Med. Biol. 2018, 49, 60–71. [Google Scholar] [CrossRef]
- Suneet, K.; De, T.; Rangarajan, A.; Jain, S. Magnetic nanofibers based bandage for skin cancer treatment: A non-invasive hyperthermia therapy. Cancer Rep. 2020, 3, e1281. [Google Scholar] [CrossRef] [PubMed]
- Dianzani, C.; Zara, G.P.; Maina, G.; Pettazzoni, P.; Pizzimenti, S.; Rossi, F.; Gigliotti, C.L.; Ciamporcero, E.S.; Daga, M.; Barrera, G. Drug delivery nanoparticles in skin cancers. BioMed Res. Int. 2014, 2014, 895986. [Google Scholar] [CrossRef]
- Labala, S.; Mandapalli, P.K.; Kurumaddali, A.; Venuganti, V.V.K. Layer-by-layer polymer coated gold nanoparticles for topical delivery of imatinib mesylate to treat melanoma. Mol. Pharm. 2015, 12, 878–888. [Google Scholar] [CrossRef]
- Rao, Y.F.; Chen, W.; Liang, X.G.; Huang, Y.Z.; Miao, J.; Liu, L.; Lou, Y.; Zhang, X.G.; Wang, B.; Tang, R.K.; et al. Epirubicin-loaded superparamagnetic iron-oxide nanoparticles for transdermal delivery: Cancer therapy by circumventing the skin barrier. Small 2015, 11, 239–247. [Google Scholar] [CrossRef]
- Bayón-Cordero, L.; Alkorta, I.; Arana, L. Application of solid lipid nanoparticles to improve the efficiency of anticancer drugs. Nanomaterials 2019, 9, 474. [Google Scholar] [CrossRef] [Green Version]
- Khallaf, R.A.; Salem, H.F.; Abdelbary, A. 5-Fluorouracil shell-enriched solid lipid nanoparticles (SLN) for effective skin carcinoma treatment. Drug Deliv. 2016, 23, 3452–3460. [Google Scholar] [CrossRef] [Green Version]
- Geetha, T.; Kapila, M.; Prakash, O.; Deol, P.K.; Kakkar, V.; Kaur, I.P. Sesamol-loaded solid lipid nanoparticles for treatment of skin cancer. J. Drug Target. 2015, 23, 159–169. [Google Scholar] [CrossRef]
- Hua, S. Lipid-based nano-delivery systems for skin delivery of drugs and bioactives. Front. Pharmacol. 2015, 6, 219. [Google Scholar] [CrossRef]
- Jain, S.; Jain, V.; Mahajan, S.C. Lipid based vesicular drug delivery systems. Adv. Pharm. 2014, 2014, 574673. [Google Scholar] [CrossRef] [Green Version]
- Saeed, M.; Zalba, S.; Seynhaeve, A.L.B.; Debets, R.; Ten Hagen, T.L.M. Liposomes targeted to MHC-restricted antigen improve drug delivery and antimelanoma response. Int. J. Nanomed. 2019, 14, 2069–2089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamichhane, N.; Udayakumar, T.S.; D’Souza, W.D.; Simone, C.B.; Raghavan, S.R.; Polf, J.; Mahmood, J. Liposomes: Clinical applications and potential for image-guided drug delivery. Molecules 2018, 23, 288. [Google Scholar] [CrossRef] [Green Version]
- Rata, D.M.; Cadinoiu, A.N.; Atanase, L.I.; Popa, M.; Mihai, C.T.; Solcan, C.; Ochiuz, L.; Vochita, G. Topical formulations containing aptamer-functionalized nanocapsules loaded with 5-fluorouracil—An innovative concept for the skin cancer therapy. Mater. Sci. Eng. C 2021, 119, 111591. [Google Scholar] [CrossRef] [PubMed]
- Choi, F.D.; Kraus, C.N.; Elsensohn, A.N.; Carley, S.K.; Lehmer, L.M.; Nguyen, R.T.; Linden, K.G.; Shiu, J. Programmed cell death 1 protein and programmed death-ligand 1 inhibitors in the treatment of nonmelanoma skin cancer: A systematic review. J. Am. Acad. Dermatol. 2020, 82, 440–459. [Google Scholar] [CrossRef]
- Capanema, N.S.V.; Mansur, A.A.P.; Carvalho, S.M.; Carvalho, I.C.; Chagas, P.; de Oliveira, L.C.A.; Mansur, H.S. Bioengineered carboxymethyl cellulose-doxorubicin prodrug hydrogels for topical chemotherapy of melanoma skin cancer. Carbohydr. Polym. 2018, 195, 401–412. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, Y.; Wang, L.; Liu, W.; Xiao, L.; Lin, Q.; Gong, T.; Sun, X.; He, Q.; Zhang, Z.; et al. Improved melanoma suppression with target-delivered TRAIL and Paclitaxel by a multifunctional nanocarrier. J. Control Release 2020, 325, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Petrilli, R.; Eloy, J.O.; Saggioro, F.P.; Chesca, D.L.; de Souza, M.C.; Dias, M.V.S.; DaSilva, L.L.P.; Lee, R.J.; Lopez, R.F.V. Skin cancer treatment effectiveness is improved by iontophoresis of EGFR-targeted liposomes containing 5-FU compared with subcutaneous injection. J. Control Release 2018, 283, 151–162. [Google Scholar] [CrossRef]
- Zou, L.; Ding, W.; Zhang, Y.; Cheng, S.; Li, F.; Ruan, R.; Wei, P.; Qiu, B. Peptide-modified vemurafenib-loaded liposomes for targeted inhibition of melanoma via the skin. Biomaterials 2018, 182, 1800334. [Google Scholar] [CrossRef] [PubMed]
- Dorrani, M.; Garbuzenko, O.B.; Minko, T.; Michniak-Kohn, B. Development of edge-activated liposomes for siRNA delivery to human basal epidermis for melanoma therapy. J. Control Release 2016, 228, 150–158. [Google Scholar] [CrossRef]
- Yeo, P.L.; Lim, C.L.; Chye, S.M.; Ling, A.P.K.; Koh, R.Y. Niosomes: A review of their structure, properties, methods of preparation, and medical applications. Asian Biomed. 2017, 11, 301–313. [Google Scholar] [CrossRef] [Green Version]
- Ge, X.; Wei, M.; He, S.; Yuan, W.-E. Advances of non-ionic surfactant vesicles (niosomes) and their application in drug delivery. Pharmaceutics 2019, 11, 55. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Sui, M.; Fan, W. Nanoparticles for tumor targeted therapies and their pharmacokinetics. Curr. Drug Metab. 2010, 11, 129–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paolino, D.; Cosco, D.; Muzzalupo, R.; Trapasso, E.; Picci, N.; Fresta, M. Innovative bola-surfactant niosomes as topical delivery systems of 5-fluorouracil for the treatment of skin cancer. Int. J. Pharm. 2008, 353, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B.A. Design of drug delivery systems containing artemisinin and its derivatives. Molecules 2017, 22, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dwivedi, A.; Mazumder, A.; du Plessis, L.; du Preez, J.L.; Haynes, R.K.; du Plessis, J. In vitro anti-cancer effects of artemisone nano-vesicular formulations on melanoma cells. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 2041–2050. [Google Scholar] [CrossRef]
- Mohamed, H.B.; El-Shanawany, S.M.; Hamad, M.A.; Elsabahy, M. Niosomes: A strategy toward prevention of clinically significant drug incompatibilities. Sci. Rep. 2017, 7, 6340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhasin, B.; Londhe, V.Y. An overview of transferosomal drug delivery. Int. J. Pharm. Sci. Res. 2018, 9, 2175–2184. [Google Scholar] [CrossRef]
- Opatha, S.A.T.; Titapiwatanakun, V.; Chutoprapat, R. Transfersomes: A promising nanoencapsulation technique for transdermal drug delivery. Pharmaceutics 2020, 12, 855. [Google Scholar] [CrossRef]
- Lei, W.; Yu, C.; Lin, H.; Zhou, X. Development of tacrolimus-loaded transfersomes for deeper skin penetration enhancement and therapeutic effect improvement in vivo. Asian J. Pharm. Sci. 2013, 8, 336–345. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Pandit, J.; Sultana, Y.; Sultana, S.; Ali, A.; Aqil, M.; Chauhan, M. Novel carbopol-based transfersomal gel of 5-fluorouracil for skin cancer treatment: In vitro characterization and in vivo study. Drug Deliv. 2015, 22, 795–802. [Google Scholar] [CrossRef]
- Pandey, A.; Mittal, A.; Chauhan, N.; Alam, S. Role of surfactants as penetration enhancer in transdermal drug delivery system. J. Mol. Pharm. Org. Process Res. 2014, 2, 2–7. [Google Scholar] [CrossRef]
- Chen, M.; Shamim, M.A.; Shahid, A.; Yeung, S.; Andresen, B.T.; Wang, J.; Nekkanti, V.; Meyskens, F.L.; Kelly, K.M.; Huang, Y. Topical delivery of carvedilol loaded nano-transfersomes for skin cancer chemoprevention. Pharmaceutics 2020, 12, 1151. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raahulan, S.; Sanapalli, B.K.R.; Karri, V.V.S.R. Paclitaxel loaded transfersomal vesicular drug delivery for the treatment of melanoma skin cancers. Int. J. Res. Pharm. Sci. 2019, 10, 2891–2897. [Google Scholar] [CrossRef]
- Godic, A.; Poljšak, B.; Adamic, M.; Dahmane, R. The role of antioxidants in skin cancer prevention and treatment. Oxid. Med. Cell. Longev. 2014, 2014, 860479. [Google Scholar] [CrossRef] [PubMed]
- Caddeo, C.; Manca, M.L.; Peris, J.E.; Usach, I.; Diez-Sales, O.; Matos, M.; Fernàndez-Busquets, X.; Fadda, A.M.; Manconi, M. Tocopherol-loaded transfersomes: In vitro antioxidant activity and efficacy in skin regeneration. Int. J. Pharm. 2018, 551, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Kassab, K.; El Fadeel, D.A.; Fadel, M. Topical photodynamic therapy using transfersomal aluminum phthalocyanine tetrasulfonate: In vitro and in vivo study. Lasers Med. Sci. 2013, 28, 1353–1361. [Google Scholar] [CrossRef]
- Montanari, J.; Perez, A.P.; Di Salvo, F.; Diz, V.; Barnadas, R.; Dicelio, L.; Doctorovich, F.; Morilla, M.J.; Romero, E.L. Photodynamic ultradeformable liposomes: Design and characterization. Int. J. Pharm. 2007, 330, 183–194. [Google Scholar] [CrossRef]
- Verma, P.; Pathak, K. Therapeutic and cosmeceutical potential of ethosomes: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 274–282. [Google Scholar] [CrossRef] [Green Version]
- Garg, V.; Singh, H.; Bimbrawh, S.; Singh, S.K.; Gulati, M.; Vaidya, Y.; Kaur, P. Ethosomes and transfersomes: Principles, perspectives and practices. Curr. Drug Deliv. 2016, 14, 613–633. [Google Scholar] [CrossRef]
- Eskolaky, E.B.; Ardjmand, M.; Akbarzadeh, A. Evaluation of anti-cancer properties of pegylated ethosomal paclitaxel on human melanoma cell line SKMEL-3. Trop. J. Pharm. Res. 2015, 14, 1421–1425. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Du, L.; Li, Y.; Fu, G.; Jin, Y. Improved anti-melanoma effect of a transdermal mitoxantrone ethosome gel. Biomed. Pharmacother. 2015, 73, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Lin, L.; Choi, Y.; Michniak-Kohn, B. Development and in-vitro evaluation of co-loaded berberine chloride and evodiamine ethosomes for treatment of melanoma. Int. J. Pharm. 2020, 581, 119278. [Google Scholar] [CrossRef] [PubMed]
- Nasr, S.; Rady, M.; Gomaa, I.; Syrovet, T.; Simmet, T.; Fayad, W.; Abdel-Kader, M. Ethosomes and lipid-coated chitosan nanocarriers for skin delivery of a chlorophyll derivative: A potential treatment of squamous cell carcinoma by photodynamic therapy. Int. J. Pharm. 2019, 568, 118528. [Google Scholar] [CrossRef] [PubMed]
- Moolakkadath, T.; Aqil, M.; Ahad, A.; Imam, S.S.; Praveen, A.; Sultana, Y.; Mujeeb, M.; Iqbal, Z. Fisetin loaded binary ethosomes for management of skin cancer by dermal application on UV exposed mice. Int. J. Pharm. 2019, 560, 78–91. [Google Scholar] [CrossRef]
- Kollipara, R.K.; Tallapaneni, V.; Sanapalli, B.K.R.; Vinoth Kumar, G.; Karri, V.V.S.R. Curcumin loaded ethosomal vesicular drug delivery system for the treatment of melanoma skin cancer. Res. J. Pharm. Technol. 2019, 12, 1783–1792. [Google Scholar] [CrossRef]
- Khan, N.R.; Wong, T.W. 5-Fluorouracil ethosomes—Skin deposition and melanoma permeation synergism with microwave. Artif. Cells Nanomed. Biotechnol. 2018, 46, 568–577. [Google Scholar] [CrossRef] [Green Version]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [Green Version]
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart nanoparticles for drug delivery application: Development of versatile nanocarrier platforms in biotechnology and nanomedicine. J. Nanomater. 2019, 2019, 3702518. [Google Scholar] [CrossRef]
- Sunisha, K.; Kaushal, P.M.; Shyam, B.S.; Suman, J. Ethosomes—A promising way for transdermal drug delivery. Int. J. Pharm. Sci. Res. 2015, 6, 3663–3670. [Google Scholar]
- Yingchoncharoen, P.; Kalinowski, D.S.; Richardson, D.R. Lipid-based drug delivery systems in cancer therapy: What is available and what is yet to come. Pharmacol. Rev. 2016, 68, 701–787. [Google Scholar] [CrossRef] [Green Version]
- Gharbavi, M.; Amani, J.; Kheiri-Manjili, H.; Danafar, H.; Sharafi, A. Niosome: A promising nanocarrier for natural drug delivery through blood-brain barrier. Adv. Pharmacol. Sci. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.; Abu Lila, A.S.; Shimizu, T.; Alaaeldin, E.; Hussein, A.; Sarhan, H.A.; Szebeni, J.; Ishida, T. PEGylated liposomes: Immunological responses. Sci. Technol. Adv. Mater. 2019, 20, 710–724. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.; Patel, N.; Shah, M.K.; Khatri, P.; Vora, N. Recent advances in lipid-based vesicles and particulate carriers for topical and transdermal application. J. Pharm. Sci. 2017, 106, 423–445. [Google Scholar] [CrossRef] [PubMed]
- Calienni, M.N.; Febres-Molina, C.; Llovera, R.E.; Zevallos-Delgado, C.; Tuttolomondo, M.E.; Paolino, D.; Fresta, M.; Barazorda-Ccahuana, H.L.; Gómez, B.; del Valle Alonso, S.; et al. Nanoformulation for potential topical delivery of Vismodegib in skin cancer treatment. Int. J. Pharm. 2019, 565, 108–122. [Google Scholar] [CrossRef] [PubMed]


| Objectives | Type of Nanocarriers | Polymer Used | Drug | Cell Line/Animal Model | Outcomes | Source |
|---|---|---|---|---|---|---|
| To compare the effect of sodium alginate and chitosan on NE in terms of penetration-enhancing effects. | Nano emulsion | Chitosan or sodium alginate | Piplartine (piperlongumine) | 2D cell cultures of melanoma cells (SK-MEL-28) | Chitosan- and alginate-modified NE enhanced skin penetration and higher cytotoxic effect of piplartine. | [50] |
| To prepare, optimize, and compare the effects of 5-FU–NE and carbopol based 5-FU–NE-Gel on melanoma cell lines and determine the retention and penetration of 5-FU using cow, goat, and rat skin models. | Nano emulsions Nano emulsion gel | Carbopol 934 | 5-FU | Melanoma cancer cell lines (SK-MEL-5-type) Swiss albino rat full-thickness skins Ear pinna skin from goat and cow | Demonstrated smallest globule size, viscosity, refractive index, and polydispersity index value with maximum droplet size uniformity and optimum zeta potential. Moreover, 5-FU–NE3-Gel and optimized-5-FU–NE3 showed significantly higher cytotoxic effect and permeation than 5-FU-S. | [53] |
| To engineer 5-FU encapsulated biodegradable chitosan nanogels for topical chemotherapy. | Nanogel | Chitosan Pluronic F-127 | 5-FU | Human keratinocyte (HaCaT) cell line Swiss albino male mice (DMBA induced melanoma mice model) | The engineered 5-FU-loaded, pH-responsive, and biocompatible nanogel provides immediate burst release, followed by slow and sustained drug release in the acidic melanoma tumor microenvironment with reduced side effects. | [54] |
| To study Apatinib-loaded NP on the inhibition of tumor growth and angiogenesis in melanoma model. | Synthetic polymeric nanoparticle | PLGA | Apatinib | Tumor B16 cells Mouse melanoma model | Drug-loaded nanoparticles reduced the growth of tumor cells with a high cytotoxic effect on tumor B16 cells. | [56] |
| To overcome the potential challenge through a nanofibrous scaffold by localizing MoO3 nanoparticles. | Nanofiber | Polycaprolactone | Molybdenum trioxide | Zebra fish | Enhanced targeted delivery of anticancer drug to treat skin cancer. | [58] |
| To treat skin cancer non-invasively using an external alternating current (AC) magnetic field-induced hyperthermia. | Nanofiber | Polycaprolactone | Iron Oxide | Hela cells and BALB/c mice | Skin cancer was treated by confirming the PCL-Fe3O4 nanofibrous-based bandages are sole and compelling. | [59] |
| To study the effect of layer-by-layer polymer-coated gold nanoparticles (AuNP) for topical delivery of imatinib mesylate (IM) in the treatment of melanoma. | Gold nanoparticles | Anionic poly(styrenesulfonate), cationic polyethylene imine | Imatinib mesylate | B16F10 melanoma cells porcine ear skin | Metal nanoparticles showed enhanced skin permeation and cytotoxicity against melanoma cells. | [61] |
| To investigate the use of superparamagnetic iron oxide NP as transdermal drug delivery carrier for epirubicin (EPI) in the treatment of skin cancer. | Superparamagnetic iron oxide nanoparticles | Epirubicin | WM266 melanoma cells | Improve skin permeation by using external magnetic force, and pH-responsive drug-release pattern allows the targeted delivery. | [62] | |
| To evaluate the ability of SLN to deliver 5-FU via the skin. | Solid lipid nanoparticle | Lecithin, poloxamer 188 | 5-FU | BALB/c (Bagg albino) mice | SLN formula can penetrate lipophilic membranes to a greater extent than the free drug and enhance the effects of the drug. | [64] |
| To investigate the use of sesamol-loaded SLN in a topical cream for the treatment of skin cancer. | Solid lipid nanoparticle | Glyceryl monostearate | Sesamol | Molt-4 and HL-60 cancer cell lines LACA mice | The onset of tumors was delayed when they were treated with sesamol and SLN, due to apoptotic cell death. | [65] |
| Objectives | Type of Nanocarriers | Polymer Used | Drug | Cell Line/ Animal Model | Outcomes | Source |
|---|---|---|---|---|---|---|
| To develop a liposomal melanoma target-delivery system that co-delivers tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and paclitaxel (PTX) against melanoma. | Liposomes | Soybean lecithin (S100), cholesterol, DSPE-PEG2000 | TRAIL and Paclitaxel | B16F10 (mouse melanoma cell line) MCF-7 cells (human breast cancer cell line) Female C57 BL/6 mice | Liposomes improved stability and drug release profile along with selective delivery to tumorous cells. Significantly improved drug biodistribution and anticancer efficiency in tumor-bearing mice. | [73] |
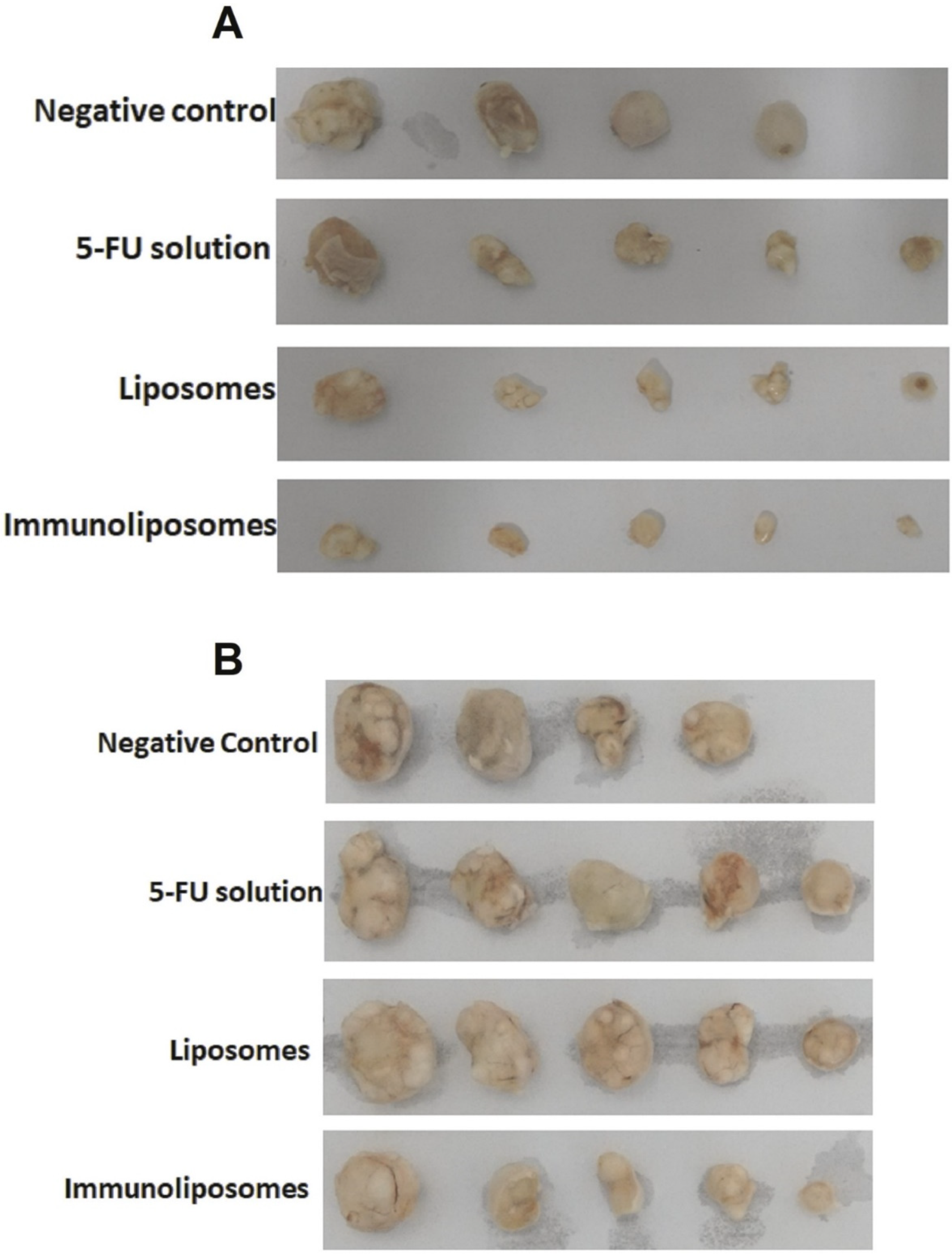
| To develop an EGFR-targeted immunoliposome loaded with 5-FU to allow co-administration of the antibody and the chemotherapeutic agent and achieve selective delivery to SCC. | Liposomes | DSPC, cholesterol, DSPE-PEG-Mal | 5-FU, cetuximab | SCC xenograft animal model EGFR-positive SCC cells Porcine ear skin | The absorption and penetration of immunoliposomes were higher compared to liposomes. Immunoliposomes had smaller tumors after iontophoresis administration compared to 5-FU solution. | [74] |
| To develop a peptide-modified vemurafenib-loaded liposome for the targeted inhibition of subcutaneous melanoma via the skin. | Liposomes | DSPE-PEG-NHS, cholesterol, lecithin | Vemurafenib | Human A375 melanoma cells Murine B16F10 melanoma cells Human umbilical vein endothelial cells (HUVEC) Male BALB/c mice (Bagg albino mouse) | Liposomes were successfully internalized by A375 cells with selective inhibition of cancer cells by Vem. Liposomes showed desired antitumor ability at a lower concentration. | [75] |
| To develop a topical siRNA delivery system that can permeate through the stratum corneum and viable epidermis and efficiently deposit therapeutic levels of siRNA to the basal epidermis/upper dermis where melanoma cells reside. | Liposomes | DOTAP | BRAF siRNA | Human cadaver skin UACC-903 melanoma cells | Liposomes with an 8:1 ratio of DOTAP:NaChol and complexed with siRNA at 16:1 showed the most effective skin permeation rate and significant deposition at upper dermis with higher internalized by melanoma cells. | [76] |
| To investigate the use of niosomes as topical delivery systems for the treatment of skin cancer with 5-FU. | Niosomes | Cholesterol, α,ω-hexadecyl-bis-(1-aza-18-crown-6), Span 80 | 5-FU | SKMEL-26 (human melanoma cell) HaCaT (human epidermal keratinocytes) | Niosomes increased percutaneous permeation (8-fold) and anticancer activity. | [80] |
| To study the anti-melanoma activity of artemisone in niosomal formulation. | Niosomes | Span 60, cholesterol | Artemisone | A-375 (human malignant melanoma cell) HaCaT (human epidermal keratinocytes) | Niosomes increase anticancer activity with negligible toxicity against normal skin. | [82] |
| To investigate improving skin absorption of 5-FU for treatment of actinic keratosis and non-melanoma skin cancer. | Transfersome | PC, Tween-80, Span-80 | 5-FU | Dorsal skin of mice (Swiss albino male mice) | Transfersomal gel showed better entrapment and drug deposition. | [87] |
| To study skin cancer prevention by using carvedilol loaded transfersomes. | Transfersome | SPC, HEPC, DSPC, Tween-80, sodium cholate | Carvedilol | Porcine ear skin Mouse epidermal cell line 3D Human Reconstituted Skin Model | Drug permeation for transfersome was lower than a free drug with a photoprotective effect. | [89] |
| To study on treatment of melanoma skin cancer by using paclitaxel loaded transfersomes. | Transfersome | PC, Span-80 | Paclitaxel | Transfersome showed the highest entrapment efficacy and the highest percentage of drug released. | [91] | |
| To study on tocopherol-loaded transfersome for evaluation of antioxidant and skin regenerative properties. | Transfersome | Soy PC, alpha-tocopherol acetate, Tween-20, Tween-40, Tween-60, Tween-80 | Alpha-tocopherol | One-day old pigs’ dorsal skin Human epidermal keratinocytes Mouse embryonic fibroblast | Transfersome using Tween-80 showed the highest entrapment efficiency and smallest vesicle size with antioxidant effect. | [93] |
| To investigate topical photodynamic therapy by using transfersomal AlPcS4. | Transfersome | PC, Sodium deoxycholate | AlPcS4 | Baby hamster kidney (BHK)-21 fibroblasts cell line BALB/c mice’s dorsal skin | AlPcS4-loaded transfersome showed better uptake into the skin and deeper penetration. | [94] |
| To study and feature ethosome particles containing Paclitaxel® and nano-drug is compared the efficacy to the free drug on the cell line of human melanoma SK-MEL-3. | Ethosome | Polyethylene glycol, cholesterol | Paclitaxel | SK-MEL-3 (Human melanoma cell) | PEGylated ethosomes increased the encapsulation efficiency of drug loading and decrease the cell viability of tumor cells. | [98] |
| To improve the anti-melanoma effect of a transdermal mitoxantrone ethosome gel. | Ethosome | Gel, soybean phospholipid | Mitoxantrone | B16 melanoma cells BALB/c nude nice | Improve permeability and cytotoxic effect of MTO with ethosome. Calreticulin expression was improved by the MTO ethosome gel on B16 melanoma cells. | [99] |
| To modify an anti-melanoma function of novel topical. | Ethosome | Propylene glycol, soybean lecithin, cholesterol | Berberine chloride, evodiamine | B16 melanoma cell | Improved skin permeability and drug delivery. Anti-melanoma effects were improved on B16 melanoma cells. | [100] |
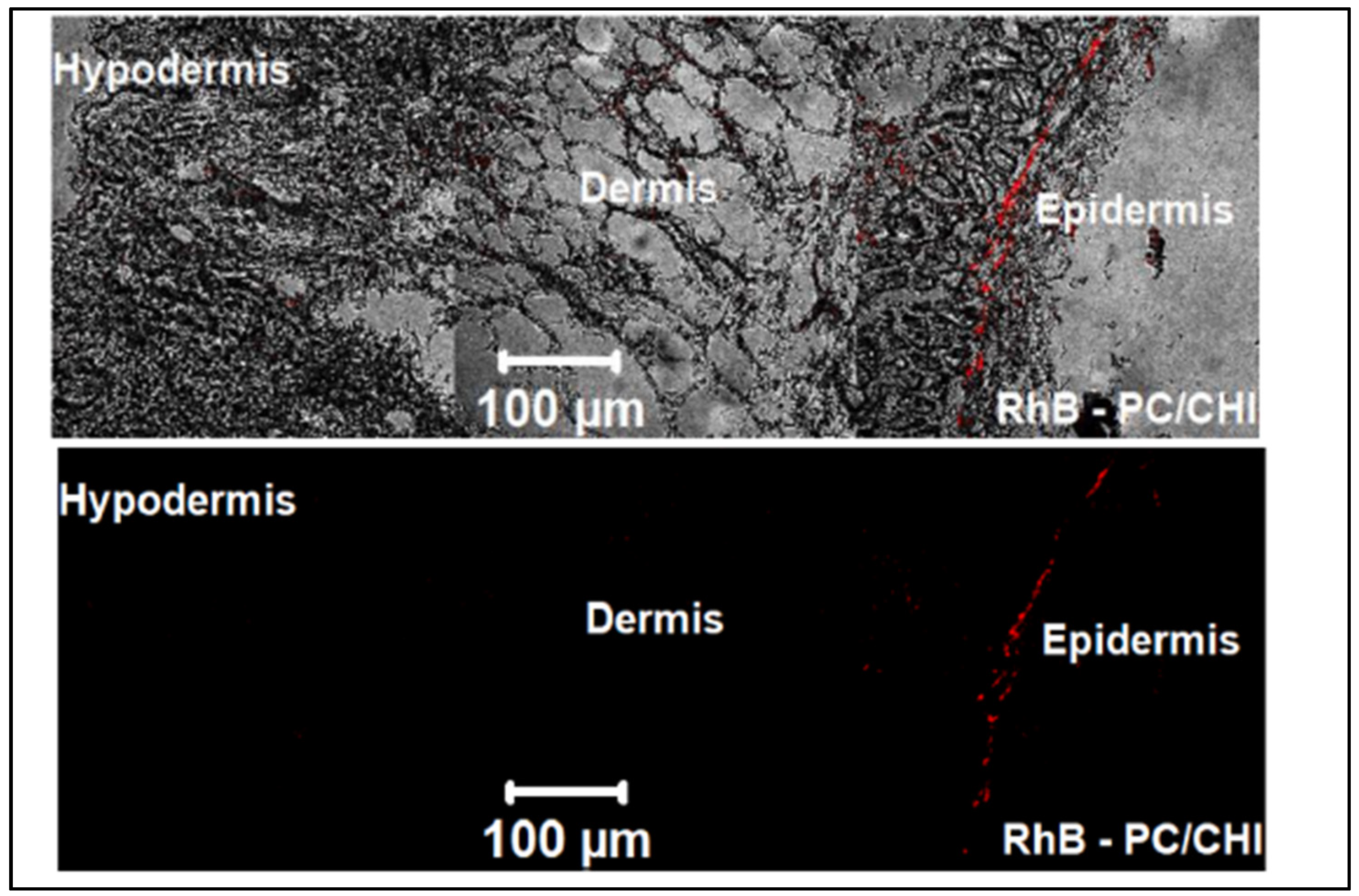
| To investigate FE-CHI loaded in both PC/CHI nanocarrier and ethosomes comparing their skin delivery applications and PDT effect. | Ethosome | Polyhydroxyethylmethacrylate, soya lecithin | Ferrous chlorophyll | A431 human epithelial squamous carcinoma cell, The skin of albino mice | The entrapment efficiency of EVO and BBR of the ethosomes formulation enhanced with decrease the levels of TNF-α and IL-1α in ethosome gel treated mice. | [101] |
| To assess the efficacy of binary ethosomes containing fisetin formulation for skin cancer management in models of the animal. | Ethosome | Propylene glycol, phospholipid, diethyl ester | Fisetin | Swiss albino mice | Mice skin treated with ethosome gel showed an increase in AUC0-8 and C skin max with decreased levels of TNF-α and IL-1α. | [102] |
| To study the treatment of skin melanoma in formulating and evaluating the curcumin-loaded ethosomes to enhance the solubility and permeability for skin melanomas’ treatment. | Ethosome | Polystyrene, cholesterol, soya lecithin | Curcumin | Rat dorsal ear skin | Ethosome gel containing Curcumin showed better release and drug deposition. Curcumin-loaded ethosome gel allows retention of curcumin in the deeper skin to completely eradicate the melanoma cells. | [103] |
| To promote penetration of the skin and/or deposition of 5-FU in vitro and in vivo. | Unilamellar Ethosome | Soya phosphotidylcholine | 5-FU | SKMEL-28 human melanoma cell Male Sprague Dawley rat | The combination of microwave and ethosome demonstrated the significant cytotoxicity effect on SKMEL-28 cells with increased retention in the skin. | [104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandey, M.; Choudhury, H.; Gorain, B.; Tiong, S.Q.; Wong, G.Y.S.; Chan, K.X.; They, X.; Chieu, W.S. Site-Specific Vesicular Drug Delivery System for Skin Cancer: A Novel Approach for Targeting. Gels 2021, 7, 218. https://doi.org/10.3390/gels7040218
Pandey M, Choudhury H, Gorain B, Tiong SQ, Wong GYS, Chan KX, They X, Chieu WS. Site-Specific Vesicular Drug Delivery System for Skin Cancer: A Novel Approach for Targeting. Gels. 2021; 7(4):218. https://doi.org/10.3390/gels7040218
Chicago/Turabian StylePandey, Manisha, Hira Choudhury, Bapi Gorain, Shao Qin Tiong, Grace Yee Seen Wong, Kai Xin Chan, Xuan They, and Wei Shen Chieu. 2021. "Site-Specific Vesicular Drug Delivery System for Skin Cancer: A Novel Approach for Targeting" Gels 7, no. 4: 218. https://doi.org/10.3390/gels7040218
APA StylePandey, M., Choudhury, H., Gorain, B., Tiong, S. Q., Wong, G. Y. S., Chan, K. X., They, X., & Chieu, W. S. (2021). Site-Specific Vesicular Drug Delivery System for Skin Cancer: A Novel Approach for Targeting. Gels, 7(4), 218. https://doi.org/10.3390/gels7040218






