Biomedical Application, Patent Repository, Clinical Trial and Regulatory Updates on Hydrogel: An Extensive Review
Abstract
:1. Introduction
2. Classification of Hydrogel-Based System
3. Polymers Used for Fabricating Hydrogel
4. Properties of Hydrogel
4.1. Swelling
4.2. Mechanical Properties
4.3. Porosity and Permeation
4.4. Crosslinking
5. Method of Preparation
6. Applications of Hydrogel
6.1. Cosmetics Applications
6.2. Wound Dressings
6.3. Drug Delivery
Injectable Hydrogels for Disease Treatment
6.4. Tissue Engineering and Regenerative Medicine
6.5. Other Applications
6.5.1. Hydrogel Machines
6.5.2. Biosensor
6.5.3. Actuator
6.5.4. Coatings
6.5.5. Optics
6.5.6. Hydrogel Electronics
7. Regulatory Aspects of Hydrogel and Its Components
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Chirani, N.; Gritsch, L.; Motta, F.L.; Fare, S. History and Applications of Hydrogels. J. Biomed. Sci. 2015, 4. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.-H.; Kuo, C.-Y.; Chen, S.-H.; Mao, S.-H.; Chang, C.-Y.; Shalumon, K.T.; Chen, J.-P. Thermosensitive Injectable Hydrogel for Simultaneous Intraperitoneal Delivery of Doxorubicin and Prevention of Peritoneal Adhesion. Int. J. Mol. Sci. 2018, 19, 1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wichterle, O.; Lím, D. Hydrophilic Gels for Biological Use. Nat. Cell Biol. 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Zhou, L.; Fan, R.; Tong, A.; Li, X.; Gao, X.; Mei, L.; Zhang, X.; You, C.; Guo, G. Enhanced antitumor effects by docetaxel/LL37-loaded thermosensitive hydrogel nanoparticles in peritoneal carcinomatosis of colorectal cancer. Int. J. Nanomed. 2015, 10, 7291–7305. [Google Scholar] [CrossRef] [Green Version]
- Fan, D.-Y.; Tian, Y.; Liu, Z.-J. Injectable Hydrogels for Localized Cancer Therapy. Front. Chem. 2019, 7. [Google Scholar] [CrossRef]
- Hasan, A.M.A.; Abdel-Raouf, M.E.-S. Cellulose-Based Superabsorbent Hydrogels; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Stebe, K.J.; Lin, S.-Y. Dynamic surface tension and surfactant mass transfer kinetics: Measurement Techniques and analysis. Handbook Surf. Interfaces Mater. 2001, 55–106. [Google Scholar]
- Nagam, S.P.; Naga Jyothi, A.; Poojitha, J.; Aruna, S.; Nadendla, R.R. A Comprehensive review on hydrogels. Int. J. Curr. Pharm. Rev. Res. 2016, 8, 19–23. [Google Scholar]
- Caló, E.; Khutoryanskiy, V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Russo, E.; Villa, C. Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Cheng, Y.; Tong, J.; Zhang, L.; Wei, Y.; Tian, M. Recent advances in thermo-sensitive hydrogels for drug delivery. J. Mater. Chem. B 2021, 9, 2979–2992. [Google Scholar] [CrossRef]
- Okay, O. Hydrogel Sensors and Actuators; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 978-3-540-75644-6. [Google Scholar]
- Gerlach, G.; Arndt, K. Hydrogel sensors and actuators volume. In Springer Series on Chemical Sensors and Biosensors; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 9783540756446. [Google Scholar]
- Byju, A.G.; Kulkarni, A.; Gundiah, N. Mechanics of gelatin and elastin based hydrogels as tissue engineered constructs. In Proceedings of the 13th International Conference on Fracture, ICF 2013, Beijing, China, 16–21 June 2013. [Google Scholar]
- Oyen, M.L. Mechanical characterisation of hydrogel materials. Int. Mater. Rev. 2013, 59, 44–59. [Google Scholar] [CrossRef]
- Hua, J.; Ng, P.F.; Fei, B. High-strength hydrogels: Microstructure design, characterization and applications. J. Polym. Sci. Part B Polym. Phys. 2018, 56, 1325–1335. [Google Scholar] [CrossRef] [Green Version]
- Salerno, A.; Borzacchiello, R.; Netti, P.A. Pore structure and swelling behavior of porous hydrogels prepared via a thermal reverse-casting technique. J. Appl. Polym. Sci. 2011, 122, 3651–3660. [Google Scholar] [CrossRef]
- Siboro, S.A.; Anugrah, D.S.; Ramesh, K.; Park, S.-H.; Kim, H.-R.; Lim, K.T. Tunable porosity of covalently crosslinked alginate-based hydrogels and its significance in drug release behavior. Carbohydr. Polym. 2021, 260, 117779. [Google Scholar] [CrossRef]
- Dong, L.C.; Hoffman, A.S.; Yan, Q. Dextran permeation through poly(N-isopropylacrylamide) Hydrogels. J. Biomater. Sci. Polym. Ed. 1994, 5, 473–484. [Google Scholar] [CrossRef]
- ASTM. F2450-10: Standard Guide for Assessing Microstructure of Polymeric Scaffolds for Use in Tissue-Engineered Medical Products. In ASTM Book of Standards; ASTM: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Fathima, N.N.; Dhathathreyan, A.; Ramasami, T. Mercury Intrusion Porosimetry, Nitrogen Adsorption, and Scanning Electron Microscopy Analysis of Pores in Skin. Biomacromolecules 2002, 3, 899–904. [Google Scholar] [CrossRef]
- Hay, J.; Laity, P. Observations of water migration during thermoporometry studies of cellulose films. Polymers 2000, 41, 6171–6180. [Google Scholar] [CrossRef]
- Landry, M.R. Thermoporometry by differential scanning calorimetry: Experimental considerations and applications. Thermochim. Acta 2005, 433, 27–50. [Google Scholar] [CrossRef]
- Yamamoto, T.; Endo, A.; Inagi, Y.; Ohmori, T.; Nakaiwa, M. Evaluation of thermoporometry for characterization of mesoporous materials. J. Colloid Interface Sci. 2005, 284, 614–620. [Google Scholar] [CrossRef]
- Maitra, J.; Shukla, V.K. Cross-linking in Hydrogels—A Review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar] [CrossRef]
- Weber, L.M.; Lopez, C.G.; Anseth, K.S. Effects of PEG hydrogel crosslinking density on protein diffusion and encapsulated islet survival and function. J. Biomed. Mater. Res. Part A 2009, 90A, 720–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, H.-W.; Huang, D.-M.; Chang, W.-H.; Huang, R.N.; Hsu, J.-C. Evaluation of gelatin hydrogel crosslinked with various crosslinking agents as bioadhesives:In vitro study. J. Biomed. Mater. Res. 1999, 46, 520–530. [Google Scholar] [CrossRef]
- Parhi, R. Cross-Linked Hydrogel for Pharmaceutical Applications: A Review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Gulrez, H.S.K.; Al-Assaf, S.O.G. Hydrogels: Methods of Preparation, Characterisation and Applications. In Progress in Molecular and Environmental Bioengineering—From Analysis and Modeling to Technology Applications; IntechOpen: London, UK, 2011. [Google Scholar]
- Bahram, M.; Mohseni, N.; Moghtader, M. An Introduction to Hydrogels and Some Recent Applications; InTech Open: Rijeka, Croatia, 2016. [Google Scholar]
- Aswathy, S.; Narendrakumar, U.; Manjubala, I. Commercial hydrogels for biomedical applications. Heliyon 2020, 6, e03719. [Google Scholar] [CrossRef] [PubMed]
- Verdier-Sévrain, S.; Bonté, F. Skin hydration: A review on its molecular mechanisms. J. Cosmet. Dermatol. 2007, 6, 75–82. [Google Scholar] [CrossRef]
- Mitura, S.; Sionkowska, A.; Jaiswal, A.K. Biopolymers for hydrogels in cosmetics: Review. J. Mater. Sci. Mater. Med. 2020, 31, 1–14. [Google Scholar] [CrossRef]
- Herndon, D.N.; Barrow, R.E.; Rutan, R.L.; Rutan, T.C.; Desai, M.H.; Abston, S. A Comparison of Conservative Versus Early Excision. Ann. Surg. 1989, 209, 547–553. [Google Scholar] [CrossRef]
- Francesko, A.; Petkova, P.; Tzanov, T. Hydrogel Dressings for Advanced Wound Management. Curr. Med. Chem. 2018, 25, 5782–5797. [Google Scholar] [CrossRef]
- Rimmer, S. Biomedical Hydrogels: Biochemistry, Manufacture and Medical Applications; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 9781845695903. [Google Scholar]
- Gupta, A.; Kowalczuk, M.; Heaselgrave, W.; Britland, S.T.; Martin, C.; Radecka, I. The production and application of hydrogels for wound management: A review. Eur. Polym. J. 2019, 111, 134–151. [Google Scholar] [CrossRef]
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef]
- Tiwari, G.; Tiwari, R.; Sriwastawa, B.; Bhati, L.; Pandey, S.; Pandey, P.; Bannerjee, S.K. Drug delivery systems: An updated review. Int. J. Pharm. Investig. 2012, 2, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef] [Green Version]
- Ashley, G.W.; Henise, J.; Reid, R.; Santi, D.V. Hydrogel drug delivery system with predictable and tunable drug release and degradation rates. Proc. Natl. Acad. Sci. USA 2013, 110, 2318–2323. [Google Scholar] [CrossRef] [Green Version]
- Larrañeta, E.; Stewart, S.; Ervine, M.; Al-Kasasbeh, R.; Donnelly, R.F. Hydrogels for Hydrophobic Drug Delivery. Classification, Synthesis and Applications. J. Funct. Biomater. 2018, 9, 13. [Google Scholar] [CrossRef] [Green Version]
- Bindu Sri, M.; Ashok, V.; Arkendu, C. As A Review on Hydrogels as Drug Delivery in the Pharmaceutical Field. Int. J. Pharm. Chem. Sci. 2012, 1, 642–741. [Google Scholar]
- Kirschner, C.M.; Anseth, K.S. Hydrogels in healthcare: From static to dynamic material microenvironments. Acta Mater. 2013, 61, 931–944. [Google Scholar] [CrossRef] [Green Version]
- Baumann, M.D.; Kang, C.E.; Stanwick, J.C.; Wang, Y.; Kim, H.; Lapitsky, Y.; Shoichet, M.S. An injectable drug delivery platform for sustained combination therapy. J. Control. Release 2009, 138, 205–213. [Google Scholar] [CrossRef]
- Marefat Seyedlar, R.; Imani, M.; Atai, M.; Nodehi, A. Temperature-Responsive Hydrogels: Materials, Mechanisms and Biological Applications. Iran. J. Polym. Sci. Technol. Persian 2018, 31, 211–237. [Google Scholar]
- Pereira, R.F.; Bártolo, P.J. Photopolymerizable hydrogels in regenerative medicine and drug delivery. In Hot Topics in Biomaterials; Future Science Ltd.: London, UK, 2014; pp. 6–28. [Google Scholar]
- Raza, A.; Rasheed, T.; Nabeel, F.; Hayat, U.; Bilal, M.; Iqbal, H.M.N. Endogenous and Exogenous Stimuli-Responsive Drug Delivery Systems for Programmed Site-Specific Release. Molecules 2019, 24, 1117. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.Y.; Kwon, D.Y.; Kwon, J.S.; Kim, J.H.; Min, B.H.; Kim, M.S. Stimuli-Responsive Injectable In situ-Forming Hydrogels for Regenerative Medicines. Polym. Rev. 2015, 55, 407–452. [Google Scholar] [CrossRef]
- Nezhad-Mokhtari, P.; Akrami-Hasan-Kohal, M.; Ghorbani, M. An injectable chitosan-based hydrogel scaffold containing gold nanoparticles for tissue engineering applications. Int. J. Biol. Macromol. 2020, 154, 198–205. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, X.; Zhang, K.; Di, H.; Feng, L.; Li, G.; Fang, J.; Cui, L.; Chen, X.; Yin, J. Injectable in situ forming poly(l-glutamic acid) hydrogels for cartilage tissue engineering. J. Mater. Chem. B 2016, 4, 947–961. [Google Scholar] [CrossRef]
- Shu, C.; Li, R.; Yin, Y.; Yin, D.; Gu, Y.; Ding, L.; Zhong, W. Synergistic dual-targeting hydrogel improves targeting and anticancer effect of Taxol in vitro and in vivo. Chem. Commun. 2014, 50, 15423–15426. [Google Scholar] [CrossRef]
- Jin, X.; Fu, Q.; Gu, Z.; Zhang, Z.; Lv, H. Injectable corilagin/low molecular weight chitosan/PLGA-PEG-PLGA thermosensitive hydrogels for localized cancer therapy and promoting drug infiltration by modulation of tumor microenvironment. Int. J. Pharm. 2020, 589, 119772. [Google Scholar] [CrossRef]
- Luque-Michel, E.; Imbuluzqueta, E.; Sebastián, V.; Blanco-Prieto, M.J. Clinical advances of nanocarrier-based cancer therapy and diagnostics. Expert Opin. Drug Deliv. 2017, 14, 75–92. [Google Scholar] [CrossRef] [Green Version]
- Perche, F.; Biswas, S.; Torchilin, V.P. Stimuli-Sensitive Polymeric Nanomedicines for Cancer Imaging and Therapy. In Handbook of Polymers for Pharmaceutical Technologies; Wiley: Hoboken, NJ, USA, 2015; pp. 311–344. [Google Scholar]
- Moreira, A.F.; Dias, D.R.; Costa, E.C.; Correia, I.J. Thermo- and pH-responsive nano-in-micro particles for combinatorial drug delivery to cancer cells. Eur. J. Pharm. Sci. 2017, 104, 42–51. [Google Scholar] [CrossRef]
- Ma, J.; Li, X.; Bao, Y. Advances in cellulose-based superabsorbent hydrogels. RSC Adv. 2015, 5, 59745–59757. [Google Scholar] [CrossRef]
- Ahsan, A.; Farooq, M.A.; Parveen, A. Thermosensitive Chitosan-Based Injectable Hydrogel as an Efficient Anticancer Drug Carrier. ACS Omega 2020, 5, 20450–20460. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Alami-Milani, M.; Geranmayeh, M.H.; Barar, J.; Erfan-Niya, H.; Omidi, Y. Dual thermo-and pH-sensitive injectable hydrogels of chitosan/(poly(N-isopropylacrylamide-co-itaconic acid)) for doxorubicin delivery in breast cancer. Int. J. Biol. Macromol. 2019, 128, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, M.; Yang, X.; Wang, Y.; Yu, L.; Sun, J.; Ding, J. Injectable hydrogels for the sustained delivery of a HER2-targeted antibody for preventing local relapse of HER2+ breast cancer after breast-conserving surgery. Theranostics 2019, 9, 6080–6098. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. pH-responsive self-healing injectable hydrogel based on N -carboxyethyl chitosan for hepatocellular carcinoma therapy. Acta Biomater. 2017, 58, 168–180. [Google Scholar] [CrossRef]
- Le, T.M.D.; Jung, B.-K.; Li, Y.; Duong, H.T.T.; Nguyen, T.L.; Hong, J.W.; Yun, C.-O.; Lee, D.S. Physically crosslinked injectable hydrogels for long-term delivery of oncolytic adenoviruses for cancer treatment. Biomater. Sci. 2019, 7, 4195–4207. [Google Scholar] [CrossRef]
- Jiang, Y.-W.; Gao, G.; Hu, P.; Liu, J.-B.; Guo, Y.; Zhang, X.; Yu, X.-W.; Wu, F.-G.; Lu, X. Palladium nanosheet-knotted injectable hydrogels formed via palladium–sulfur bonding for synergistic chemo-photothermal therapy. Nanoscale 2020, 12, 210–219. [Google Scholar] [CrossRef]
- Lee, A.L.Z.; Ng, V.W.L.; Gao, S.; Hedrick, J.L.; Yang, Y.Y. Injectable Biodegradable Hydrogels from Vitamin D-Functionalized Polycarbonates for the Delivery of Avastin with Enhanced Therapeutic Efficiency against Metastatic Colorectal Cancer. Biomacromolecules 2015, 16, 465–475. [Google Scholar] [CrossRef]
- Bubpamala, T.; Viravaidya-Pasuwat, K.; Pholpabu, P. Injectable Poly(ethylene glycol) Hydrogels Cross-Linked by Metal–Phenolic Complex and Albumin for Controlled Drug Release. ACS Omega 2020, 5, 19437–19445. [Google Scholar] [CrossRef]
- Lee, A.L.Z.; Ng, V.W.L.; Gao, S.; Hedrick, J.L.; Yang, Y.Y. Injectable Hydrogels from Triblock Copolymers of Vitamin E-Functionalized Polycarbonate and Poly(ethylene glycol) for Subcutaneous Delivery of Antibodies for Cancer Therapy. Adv. Funct. Mater. 2014, 24, 1538–1550. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Ma, P.X.; Guo, B.; Du, Y.; Han, X. pH-responsive injectable hydrogels with mucosal adhesiveness based on chitosan-grafted-dihydrocaffeic acid and oxidized pullulan for localized drug delivery. J. Colloid Interface Sci. 2019, 536, 224–234. [Google Scholar] [CrossRef]
- Silva, E.A.; Mooney, D. Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. J. Thromb. Haemost. 2007, 5, 590–598. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, F.; Long, L.; Kong, Q.; Luo, R.; Wang, Y. Dual-responsive injectable hydrogels encapsulating drug-loaded micelles for on-demand antimicrobial activity and accelerated wound healing. J. Control. Release 2020, 324, 204–217. [Google Scholar] [CrossRef]
- Fletcher, N.A.; Babcock, L.R.; Murray, E.A.; Krebs, M.D. Controlled delivery of antibodies from injectable hydrogels. Mater. Sci. Eng. C 2016, 59, 801–806. [Google Scholar] [CrossRef]
- Ren, Y.; Zhao, X.; Liang, X.; Ma, P.X.; Guo, B. Injectable hydrogel based on quaternized chitosan, gelatin and dopamine as localized drug delivery system to treat Parkinson’s disease. Int. J. Biol. Macromol. 2017, 105, 1079–1087. [Google Scholar] [CrossRef]
- Xing, L.; Sun, J.; Tan, H.; Yuan, G.; Li, J.; Jia, Y.; Xiong, D.; Chen, G.; Lai, J.; Ling, Z.; et al. Covalently polysaccharide-based alginate/chitosan hydrogel embedded alginate microspheres for BSA encapsulation and soft tissue engineering. Int. J. Biol. Macromol. 2019, 127, 340–348. [Google Scholar] [CrossRef]
- Dromel, P.C.; Singh, D.; Christoff-Tempesta, T.; Martheswaran, M.T.; Alexander-Katz, A.; Spector, M.; Young, M. Controlling Growth Factor Diffusion by Modulating Water Content in Injectable Hydrogels. Tissue Eng. Part A 2021, 27, 714–723. [Google Scholar] [CrossRef]
- Verbeke, C.; Gordo, S.; Schubert, D.A.; Lewin, S.A.; Desai, R.M.; Dobbins, J.; Wucherpfennig, K.W.; Mooney, D.J. Multicomponent Injectable Hydrogels for Antigen-Specific Tolerogenic Immune Modulation. Adv. Heal. Mater. 2017, 6. [Google Scholar] [CrossRef] [Green Version]
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 2018, 183, 185–199. [Google Scholar] [CrossRef]
- Wang, K.; Mitra, R.N.; Zheng, M.; Han, Z. Nanoceria-loaded injectable hydrogels for potential age-related macular degeneration treatment. J. Biomed. Mater. Res. Part A 2018, 106, 2795–2804. [Google Scholar] [CrossRef]
- Ungerleider, J.; Johnson, T.; Rao, N.; Christman, K. Fabrication and characterization of injectable hydrogels derived from decellularized skeletal and cardiac muscle. Methods 2015, 84, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Zhao, J.; Chen, Y.M.; Zhang, P.; Zhang, Q. Self-healing polysaccharide-based hydrogels as injectable carriers for neural stem cells. Sci. Rep. 2016, 6, 37841. [Google Scholar] [CrossRef] [Green Version]
- Von Lospichl, B.; Hemmati-Sadeghi, S.; Dey, P.; Dehne, T.; Haag, R.; Sittinger, M.; Ringe, J.; Gradzielski, M. Injectable hydrogels for treatment of osteoarthritis—A rheological study. Colloids Surf. B Biointerfaces 2017, 159, 477–483. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Xu, Y.; Ma, P.X.; Guo, B. Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chem. Eng. J. 2019, 362, 548–560. [Google Scholar] [CrossRef]
- Dong, Y.; Rodrigues, M.; Li, X.; Kwon, S.H.; Kosaric, N.; Khong, S.; Gao, Y.; Wang, W.; Gurtner, G.C. Injectable and Tunable Gelatin Hydrogels Enhance Stem Cell Retention and Improve Cutaneous Wound Healing. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Feng, G.; Zha, Z.; Huang, Y.; Li, J.; Wang, Y.; Ke, W.; Chen, H.; Liu, L.; Song, Y.; Ge, Z. Sustained and Bioresponsive Two-Stage Delivery of Therapeutic miRNA via Polyplex Micelle-Loaded Injectable Hydrogels for Inhibition of Intervertebral Disc Fibrosis. Adv. Heal. Mater. 2018, 7, e1800623. [Google Scholar] [CrossRef]
- Shaghiera, A.D.; Widiyanti, P.; Yusuf, H. Synthesis and Characterization of Injectable Hydrogels with Varying Collagen–Chitosan–Thymosin β4 Composition for Myocardial Infarction Therapy. J. Funct. Biomater. 2018, 9, 33. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Zhao, X.; Chen, X.; Wei, Y.; Du, W.; Wang, Y.; Liu, L.; Zhao, W.; Han, Z.; Kong, D.; et al. Enhanced Therapeutic Effects of Mesenchymal Stem Cell-Derived Exosomes with an Injectable Hydrogel for Hindlimb Ischemia Treatment. ACS Appl. Mater. Interfaces 2018, 10, 30081–30091. [Google Scholar] [CrossRef]
- Xie, B.; Jin, L.; Luo, Z.; Yu, J.; Shi, S.; Zhang, Z.; Shen, M.; Chen, H.; Li, X.; Song, Z. An injectable thermosensitive polymeric hydrogel for sustained release of Avastin® to treat posterior segment disease. Int. J. Pharm. 2015, 490, 375–383. [Google Scholar] [CrossRef]
- Steele, A.N.; Stapleton, L.M.; Farry, J.; Lucian, H.J.; Paulsen, M.J.; Eskandari, A.; Hironaka, C.E.; Thakore, A.D.; Wang, H.; Yu, A.C.; et al. A Biocompatible Therapeutic Catheter-Deliverable Hydrogel for In Situ Tissue Engineering. Adv. Heal. Mater. 2019, 8. [Google Scholar] [CrossRef]
- Lü, S.; Gao, C.; Xu, X.; Bai, X.; Duan, H.; Gao, N.; Feng, C.; Xiong, Y.; Liu, M. Injectable and Self-Healing Carbohydrate-Based Hydrogel for Cell Encapsulation. ACS Appl. Mater. Interfaces 2015, 7, 13029–13037. [Google Scholar] [CrossRef]
- Vong, L.B.; Bui, T.Q.; Tomita, T.; Sakamoto, H.; Hiramatsu, Y.; Nagasaki, Y. Novel angiogenesis therapeutics by redox injectable hydrogel - Regulation of local nitric oxide generation for effective cardiovascular therapy. Biomaterials 2018, 167, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Cascone, S.; Lamberti, G. Hydrogel-based commercial products for biomedical applications: A review. Int. J. Pharm. 2020, 573, 118803. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; He, C.; Xiao, C.; Li, G.; Chen, X. Injectable glycopolypeptide hydrogels as biomimetic scaffolds for cartilage tissue engineering. Biomaterials. 2015, 51, 238–249. [Google Scholar] [CrossRef]
- Cheung, H.K.; Han, T.T.Y.; Marecak, D.M.; Watkins, J.F.; Amsden, B.G.; Flynn, L.E. Composite hydrogel scaffolds incorporating decellularized adipose tissue for soft tissue engineering with adipose-derived stem cells. Biomaterials 2014, 35, 1914–1923. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.F.C.; Yan, J.; Han, T.T.Y.; Marecak, D.M.; Amsden, B.G.; Flynn, L.E. Effect of decellularized adipose tissue particle size and cell density on adipose-derived stem cell proliferation and adipogenic differentiation in composite methacrylated chondroitin sulphate hydrogels. Biomed. Mater. 2015, 10, 045010. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Yao, F.; Hao, T.; Fang, W.; Ye, L.; Zhang, Y.; Wang, Y.; Li, J.; Wang, C. Development of Electrically Conductive Double-Network Hydrogels via One-Step Facile Strategy for Cardiac Tissue Engineering. Adv. Heal. Mater. 2016, 5, 474–488. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardiol. Sci. Pract. 2013, 2013, 316–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaterji, S.; Kwon, I.K.; Park, K. Smart polymeric gels: Redefining the limits of biomedical devices. Prog. Polym. Sci. 2007, 32, 1083–1122. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Liu, J.; Lin, S.; Zhao, X. Hydrogel machines. Mater. Today 2020, 36, 102–124. [Google Scholar] [CrossRef]
- Calvert, P. Hydrogels for Soft Machines. Adv. Mater. 2009, 21, 743–756. [Google Scholar] [CrossRef]
- Yuk, H.; Zhang, T.; Lin, S.; Parada, G.A.; Zhao, X. Tough bonding of hydrogels to diverse non-porous surfaces. Nat. Mater. 2016, 15, 190–196. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Sycks, D.; Chan, H.F.; Lin, S.; Lopez, G.P.; Guilak, F.; Leong, K.W.; Zhao, X. 3D Printing: 3D Printing of Highly Stretchable and Tough Hydrogels into Complex, Cellularized Structures (Adv. Mater. 27/2015). Adv. Mater. 2015, 27, 4034. [Google Scholar] [CrossRef]
- Buenger, D.; Topuz, F.; Groll, J. Hydrogels in sensing applications. Prog. Polym. Sci. 2012, 37, 1678–1719. [Google Scholar] [CrossRef]
- Pinelli, F.; Magagnin, L.; Rossi, F. Progress in hydrogels for sensing applications: A review. Mater. Today Chem. 2020, 17, 100317. [Google Scholar] [CrossRef]
- Shin, J.; Braun, P.V.; Lee, W. Fast response photonic crystal pH sensor based on templated photo-polymerized hydrogel inverse opal. Sens. Actuators B Chem. 2010, 150, 183–190. [Google Scholar] [CrossRef]
- Huber, J.E.; Fleck, N.A.; Ashby, M.F. The selection of mechanical actuators based on performance indices. In Royal Society A: Mathematical, Physical and Engineering Sciences; The Royal Society: London, UK, 1997; Volume 453, pp. 2185–2205. [Google Scholar]
- Keplinger, C.; Sun, J.-Y.; Foo, C.C.; Rothemund, P.; Whitesides, G.M.; Suo, Z. Stretchable, Transparent, Ionic Conductors. Science 2013, 341, 984–987. [Google Scholar] [CrossRef] [Green Version]
- Fox, A.J.S.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Heal. A Multidiscip. Approach 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Yu, B.; Wang, C.; Ju, Y.M.; West, L.; Harmon, J.; Moussy, Y.; Moussy, F. Use of hydrogel coating to improve the performance of implanted glucose sensors. Biosens. Bioelectron. 2008, 23, 1278–1284. [Google Scholar] [CrossRef]
- Kurokawa, T.; Furukawa, H.; Wang, W.; Tanaka, Y.; Gong, J.P. Formation of a strong hydrogel–porous solid interface via the double-network principle. Acta Biomater. 2010, 6, 1353–1359. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Fang, Z.; Cao, Y.; Du, H.; Wu, H.; Beuerman, R.; Chan-Park, M.B.; Duan, H.; Xu, R. High Refractive Index Inorganic–Organic Interpenetrating Polymer Network (IPN) Hydrogel Nanocomposite toward Artificial Cornea Implants. ACS Macro Lett. 2012, 1, 876–881. [Google Scholar] [CrossRef]
- Choi, M.; Humar, M.; Kim, S.; Yun, S.-H. Step-Index Optical Fiber Made of Biocompatible Hydrogels. Adv. Mater. 2015, 27, 4081–4086. [Google Scholar] [CrossRef]
- Chung, K.; Wallace, J.; Kim, S.-Y.; Kalyanasundaram, S.; Andalman, A.S.; Davidson, T.J.; Mirzabekov, J.J.; Zalocusky, K.A.; Mattis, J.; Denisin, A.; et al. Structural and molecular interrogation of intact biological systems. Nat. Cell Biol. 2013, 497, 332–337. [Google Scholar] [CrossRef]
- Farandos, N.M.; Yetisen, A.K.; Monteiro, M.J.; Lowe, C.R.; Yun, S.H. Contact lens sensors in ocular diagnostics. Adv. Healthcare Mater. 2015, 4, 792–810. [Google Scholar] [CrossRef]
- Nicolson, P.C.; Vogt, J. Soft contact lens polymers: An evolution. Biomaterials 2001, 22, 3273–3283. [Google Scholar] [CrossRef]
- Su, G.; Zhou, T.; Zhang, Y.; Liu, X.; Zhang, A. Microdynamics mechanism of D2O absorption of the poly(2-hydroxyethyl methacrylate)-based contact lens hydrogel studied by two-dimensional correlation ATR-FTIR spectroscopy. Soft Matter 2016, 12, 1145–1157. [Google Scholar] [CrossRef] [PubMed]
- Muncan, J.; Mileusnić, I.; Rosić, J. Šakota; Vasic-Milovanovic, A.; Matija, L. Water Properties of Soft Contact Lenses: A Comparative Near-Infrared Study of Two Hydrogel Materials. Int. J. Polym. Sci. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wolffsohn, J.; Mroczkowska, S.; Hunt, O.A.; Bilkhu, P.S.; Drew, T.; Sheppard, A. Crossover Evaluation of Silicone Hydrogel Daily Disposable Contact Lenses. Optom. Vis. Sci. 2015, 92, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-J.; Wu, H.; Hu, Y.; Young, M.; Wang, H.; Lynch, D.; Xu, F.; Cong, H.; Cheng, G. Ionic Conductivity of Polyelectrolyte Hydrogels. ACS Appl. Mater. Interfaces 2018, 10, 5845–5852. [Google Scholar] [CrossRef] [PubMed]
- Dvir, T.; Timko, B.; Brigham, M.; Naik, S.R.; Karajanagi, S.S.; Levy, O.; Jin, H.; Parker, K.K.; Langer, R.; Kohane, D.S. Nanowired three-dimensional cardiac patches. Nat. Nanotechnol. 2011, 6, 720–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, S.R.; Jung, S.M.; Zalabany, M.; Kim, K.; Zorlutuna, P.; Kim, S.B.; Nikkhah, M.; Khabiry, M.; Azize, M.; Kong, J.; et al. Carbon-Nanotube-Embedded Hydrogel Sheets for Engineering Cardiac Constructs and Bioactuators. ACS Nano 2013, 7, 2369–2380. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.; Yu, G.; Zhai, D.; Lee, H.R.; Zhao, W.; Liu, N.; Wang, H.; Tee, C.K.; Shi, Y.; Cui, Y.; et al. Hierarchical nanostructured conducting polymer hydrogel with high electrochemical activity. Proc. Natl. Acad. Sci. USA 2012, 109, 9287–9292. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Ma, C.; Peng, L.; Yu, G. Conductive “Smart” Hybrid Hydrogels with PNIPAM and Nanostructured Conductive Polymers. Adv. Funct. Mater. 2015, 25, 1219–1225. [Google Scholar] [CrossRef]
- Know Your Dressings: Hydrogels. Available online: https://dermarite.com/know-your-dressings-hydrogels/ (accessed on 12 June 2021).
- INTRASITE◊ GEL. Available online: https://www.smith-nephew.com/key-products/advanced-wound-management/intrasite-gel (accessed on 12 June 2021).
- Hydrogel Based Product. Available online: https://www.lohmann-rauscher.com/en/searchresults/?id=2324&L=1&q=hydrogel+based+product&op= (accessed on 12 June 2021).
- Neoheal®. Hydrogel Dressing for Wound Management. Available online: https://kikgel.com.pl/en/products/neoheal/ (accessed on 12 June 2021).
- Woun’Dres® Collagen Hydrogel. Available online: https://www.coloplast.us/woundres-collagen-hydrogel-1-en-us.aspx (accessed on 12 June 2021).
- Safe n Simple™. Available online: www.sns-medical.com (accessed on 19 June 2021).
- Collagen Hydrogel for Face Profi Derm Professional. Available online: https://www.cosmeticsbulgaria.com/en/product/collagen-hydrogel-for-face-profi-derm-professional/ (accessed on 19 June 2021).
- Advanced Génifique Light Pearl Hydrogel Melting 360 Eye Mask. Available online: https://www.lancome-usa.com/skincare/advanced-genifique-light-pearl-hydrogel-melting-360-eye-mask/LAN390.html (accessed on 19 June 2021).
- Advanced Génifique Hydrogel Melting Sheet Mask. Available online: https://www.lancome-usa.com/skincare/advanced-genifique-hydrogel-melting-sheet-mask/LAN192.html (accessed on 19 June 2021).
- Moira Cosmetics. Available online: https://www.moirabeauty.com/search (accessed on 19 June 2021).
- Silicone Hydrogel Contact Lens (Daily). Available online: https://www.miacare.com/weben/html/product/show.aspx?num=27 (accessed on 19 June 2021).
- CONFiDENCE (Daily)—Black/Brown/Violet. Available online: https://www.miacare.com/weben/html/product/show.aspx?num=29 (accessed on 28 June 2021).
- Charcoal Hydrogel Under Eye Masks. Available online: https://www.elfcosmetics.com/charcoal-hydrogel-under-eye-masks/500011.html (accessed on 28 June 2021).
- Introducing the Breakthrough of Blue Cut Technology in Cntact Lenses. Available online: https://maxvuevision.com/v2/ (accessed on 28 June 2021).
- Seven RX. Available online: https://markennovy.com/our_products/seven-rx/ (accessed on 28 June 2021).
- CLINICAL RESOURCE: A Case Study Using ActivHeal® Hydrogel and ActivHeal® Hydrocolloid to Promote Autolytic Debridement of an Acute Wound – Hydrocolloid. Available online: http://www.activheal.com/?s=hydrogel (accessed on 28 June 2021).
- NU-GEL™ Hydrogel with Alginate. Available online: https://www.kciuk.co.uk/healthcare-professionals/uk-product-catalog/catalog/nu-gel-hydrogel-with-alginate (accessed on 28 June 2021).
- The only FDA-Approved, Once-Yearly CPP Treatment. Available online: https://www.supprelinla.com/patients/what-is-supprelin-la/ (accessed on 28 June 2021).
- Cervidil. Available online: https://www.rxlist.com/cervidil-drug.htm (accessed on 28 June 2021).
- SQZgel™. Available online: https://www.researchgate.net/publication/329529474_SQZgel (accessed on 28 June 2021).
- Mebiol® Gel. Available online: https://www.cosmobiousa.com/products/mebiol-gel (accessed on 28 June 2021).
- GelrinC Overview. Available online: http://www.regentis.co.il/products.asp (accessed on 28 June 2021).
- Mebiol Gel®. Available online: https://search.cosmobio.co.jp/cosmo_search_p/search_gate2/docs/MBG_/PMW205001COS.20180608.pdf (accessed on 28 June 2021).
- HyStem™ Hyaluronic Acid Based Hydrogels for 3D Cell Culture Applications. Available online: https://www.sigmaaldrich.com/technical-documents/articles/biology/hystem-3d-hydrogels.html (accessed on 5 July 2021).
- Corning® PuraMatrix™ Peptide Hydrogel. Available online: https://www.corning.com/media/worldwide/global/documents/faq_DL_028_Corning_PuraMatrix_Peptide_Hydrogel.pdf (accessed on 5 July 2021).
- Synthetic Peptide Hydrogel. Available online: https://www.biogelx.com/technology-synthetic-peptide-hydrogel-2/ (accessed on 5 July 2021).
- SpaceOAR™ Hydrogel. Available online: https://www.bostonscientific.com/en-US/products/hydrogel-spacers/spaceoar-hydrogel.html (accessed on 5 July 2021).
- Bulkamid: For the Treatment of Stress Urinary Incontinence. Available online: https://bulkamid.com/# (accessed on 5 July 2021).
- VersaGel®/Symphony® 3D Cell Culture Platform by Cypre Inc. Available online: https://www.selectscience.net/products/versagel--+-symphony---3d-cell-culture-platform/?prodID=210830 (accessed on 5 July 2021).
- Hydrogel Cathetor. Available online: https://www.medtronic.com/covidien/en-us/search.html#q=hydrogel%20cathetor (accessed on 5 July 2021).
- Bolt™ 12%, Bis-Tris, 1.0 mm, Mini Protein Gel, 12-well. Available online: https://www.thermofisher.com/order/catalog/product/NW00122BOX?SID=srch-srp-NW00122BOX#/NW00122BOX?SID=srch-srp-NW00122BOX (accessed on 5 July 2021).
- Catoira, M.C.; González-Payo, J.; Fusaro, L.; Ramella, M.; Boccafoschi, F. Natural hydrogels R&D process: Technical and regulatory aspects for industrial implementation. J. Mater. Sci. Mater. Med. 2020, 31. [Google Scholar] [CrossRef]
- Donawa, M.E. Regulation of novel biomedical hydrogel products. In Biomedical Hydrogels; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- RULES OF PROCEDURE. Available online: https://ec.europa.eu/health/sites/default/files/scientific_committees/docs/rules_procedure_2016_en.pdf. (accessed on 20 August 2021).
- Inactive Ingredient Search for Approved Drug Products. Available online: https://www.accessdata.fda.gov/scripts/cder/iig/index.cfm (accessed on 20 August 2021).
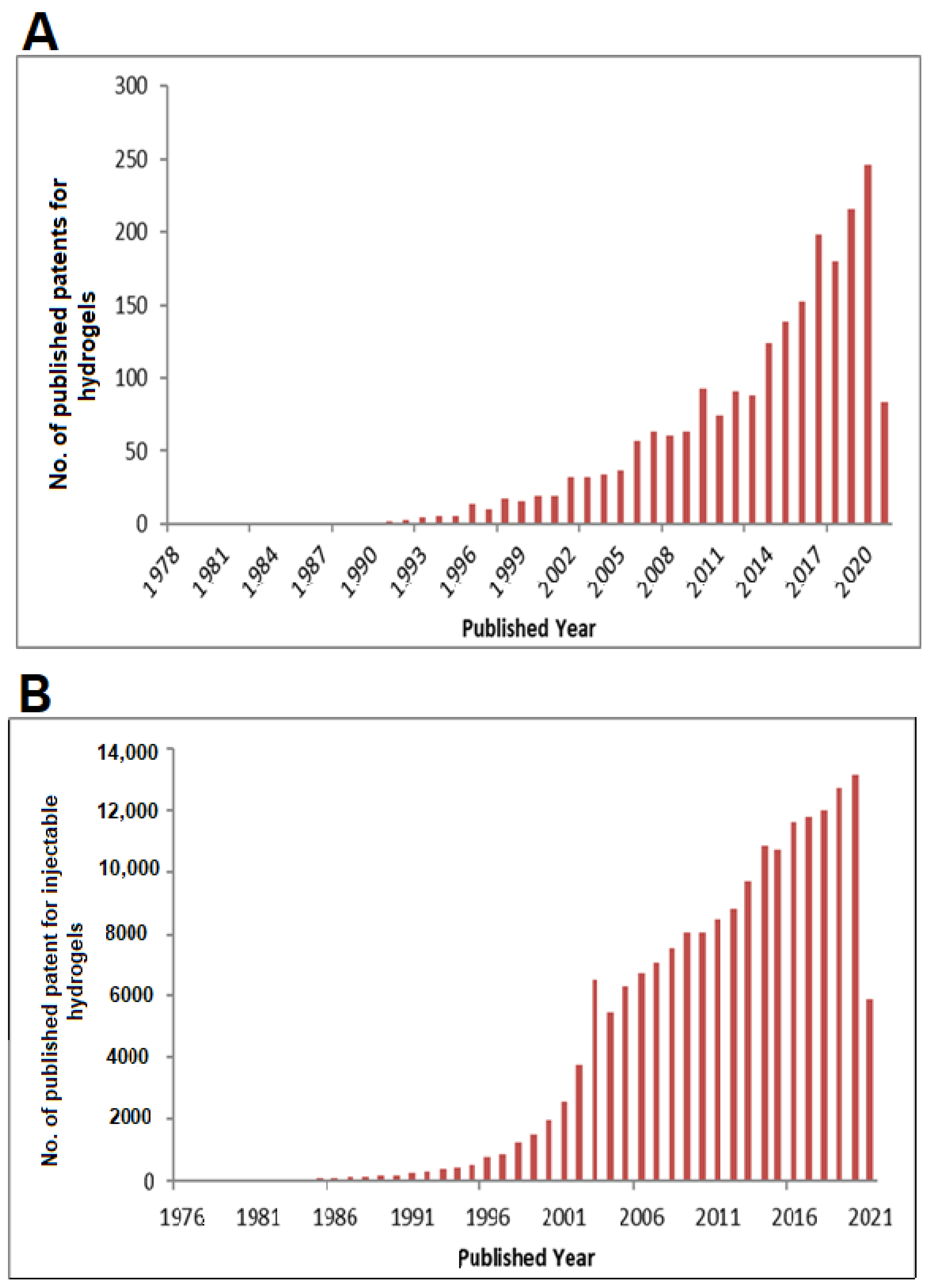
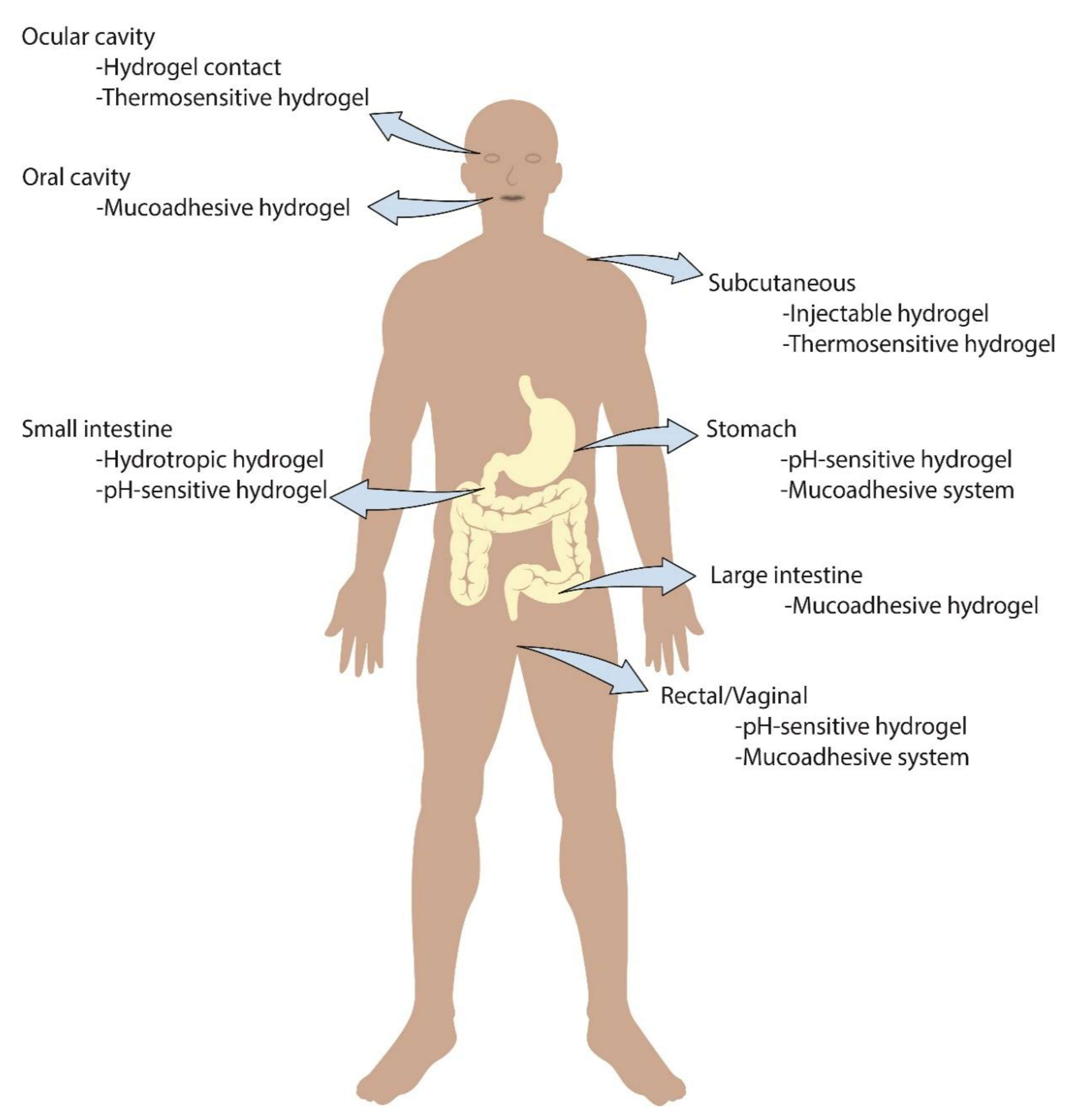
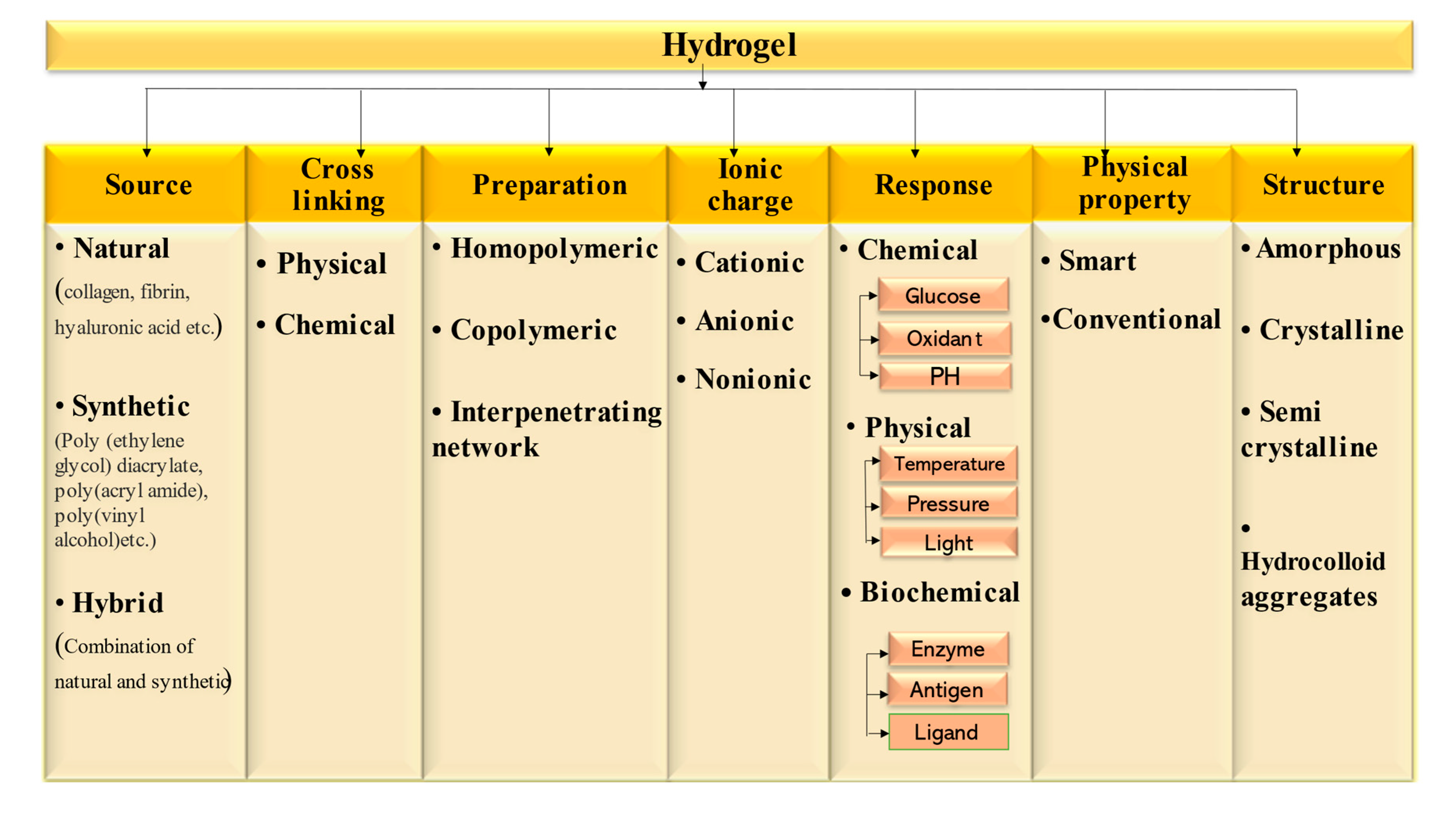


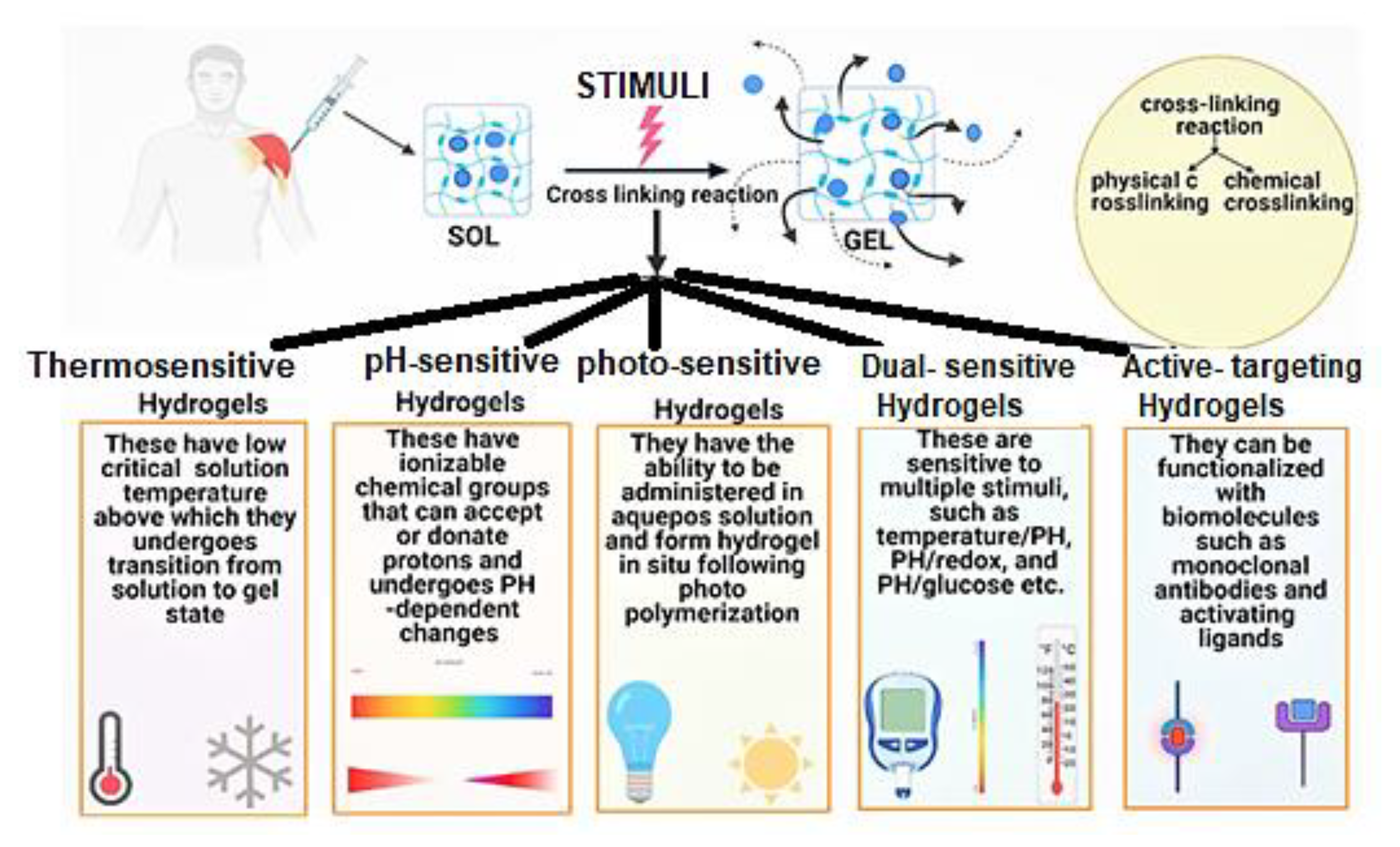
| Natural Polymer | Synthetic Polymer | Hybrid Polymer |
|---|---|---|
| Hyaluronic acid | PEG-PLA-PEG | P (PEG-co-peptides) |
| Pectin | PEG-PLGA-PEG | P-(HPMA-g-peptide) |
| Alginic acid | PEG-PCL-PEG | P (PLGA-co-serine) |
| Carrageenan | PLA-PEG-PLA | Alginate-g-(PEO-PPO-PEO) |
| Chondroitin sulphate | PHB | HA-g-NIPAAm |
| Dextrin sulphate | P (PEG/PBO terephthalate) | Collagen-n-acryl ate |
| Chitosan | Polyurethane | Alginate-acryl ate |
| Polylysine | Polyimide | |
| Collagen (and gelatine) | Polyvinylpyrrolidone | |
| Carboxy methylchitin | Polyvinyl alcohol | |
| Fibrin and silk fibroin | Polyacrylate | |
| Dextrin | Polythene oxide | |
| Pullulan | Polymethacrylate | |
| Agarose Elastin Glycosaminoglycans Decellularized Hydrogels |
| S. No. | Patent No./Country | Title | Disease/Problem | Details |
|---|---|---|---|---|
| 1 | US10799696B2 United States | Polymer formulations for nasolacrimal stimulation | Dry eye | The hydrogel formulation (prepared by a UV crosslinking process) permits electrical stimulation of the lacrimal gland, nasal or sinus tissue to ↑ production of tear and to treat dry eye |
| 2 | US20200085733A1 United States | Hypotonic hydrogel formulations for enhanced transport of active agents at mucosal surfaces | Administered into vagina or colorectum for diagnostic, prophylactic and therapeutic purpose | An aqueous polymeric hydrogel (poloxamers) used as a barrier by forming plug and/or used for the delivery to a mucosal/epithelial surface for therapeutic, preventive, diagnostic or nutraceutical purpose |
| 3 | US20200114010A1 United States | Non-injectable hydrogel formulations for smart release | The formulation contains anti-inflammatories, anti-infectives, or other therapeutic, prophylactic, or diagnostic agents that can be administered orally to produce desire action | A non-injectable formulation/formulation for instillation, with self-assembling hydrogels designed of gelators, in the form of capsules, tablets, oral suspensions, rectal or vaginal suppositories, enemas, and inserts |
| 4 | US20180023049A1 United States | Synthetic peptide hydrogel formulations for use as extracellular matrix | Cell culture experimentation | Synthetic peptide hydrogel solutions having a pH level of about 3.5/˂ and having a tonicity within an isotonic osmolality range |
| 5 | US20200360281A1 United States | A thermo-responsive hydrogel for intertumoral administration as a treatment in solid tumor cancers | Intra tumoral treatment of solid cancer | Injectable thermo-responsive hydrogel forming a chitosan and genipin interpenetrating scaffold by crosslinking can effectively incorporate chemotherapeutic drugs without any loss of thermo-responsiveness |
| 6 | WO2019067406A1 WIPO (PCT) | Biomimetic, moldable, self-assembled cellulose silica-based trimeric hydrogels and their use as viscosity modifying carriers in industrial applications | Use as low-cost and safe carriers and aqueous viscosity modifiers in various industrial and medical applications | A moldable, cellulose silica-based hydrogels which is fully scalable |
| 7 | US20190127726A1 United States | Delivering enzyme using an injectable hydrogel depot | To deliver enzymes | A delivery system for carrying an injectable enzyme hydrogel formulation consisting of an enzyme together with other components |
| 8 | WO2017152112A2 WIPO (PCT) | Hydrogel systems for skeletal interfacial tissue regeneration applied to epiphyseal growth plate repair | It can be applied through numerous different modalities depending on the nature of the physical injury | Biomaterials, systems, and methods for guiding regeneration of an epiphyseal growth plate or similar interfacial tissue structures |
| 9 | JP6293254B2 Japan | Silicone hydrogel lens with crosslinked hydrophilic coating | Contact lens | Coated silicone hydrogel contact lens containing a surface coating of silicone hydrogel and a non-silicone hydrogel which is a crosslinked polymer consisting of one or more cross-linkable materials and a crosslinked carboxyl-containing polymer material |
| 10 | CN105209016B China | Biocompatible hydrogel polymer matrices for cell delivery | A solid support that is beneficial for cell viability and functionality | Biocompatible hydrogel polymer matrices, bioabsorbable and releases cells at the target site, thus permitting controlled delivery |
| 11 | US20200299627A1 United States | Crosslinked hydrogel compositions for regulating states of encapsulated cancer cells | Method of regulating the state of cancer cells | A system composed of a crosslinked poly alkylene glycol based hydrogel, systems comprising a number of cancer cells in contact with the culture media and encapsulated inside the hydrogel, and the method of making and using the same |
| 12 | JP2020514500A Japan | Antibacterial polymer and antibacterial hydrogel | Antimicrobial polymers and antimicrobial hydrogels | An antimicrobial hydrogel containing a substituted C5-C15 alkyl; a polyethyleneimine-alkyl-polyethylene glycol methacrylate implant ratio ranging from 1:1:1 to 1:20:20 and a method of forming the same, providing a device having a surface coating the antimicrobial hydrogel as well |
| 13 | EP2801377B1 European Patent Office | Hydrogel comprising cells for local release of growth factors to mediate motor recovery after stroke | Hydrogel comprising cells that provide a sustained release of brain derived neurotrophic factor (BDNF) for improving recovery of a mammal after cerebral ischemia | Method of administering a therapeutically effective amount of BDNF to the infarct cavity in the mammalian brain for the treatment of cerebral ischemia |
| 14 | US20190282699A1 United States | Thiolated hyaluronan-based hydrogels crosslinked using oxidized glutathione | Hydrogel comprising the therapeutic agent, carboxymethylated hyaluronan and thiolated gelatin | Methods, compositions and kits linking to hyaluronan based matrices with oxidized glutathione as a crosslinking agent |
| 15 | WO2021019562A2 WIPO (PCT) | Bioengineered formulation, process for preparing and implementations thereof | Bioengineered formulation for corneal applications | A bioengineered formulation consisting of a modified collagen peptide and hyaluronic acid further, encompassing stem cells/exosomes or combinations therefrom |
| 16 | US10632070B2 United States | Hydrogel toxin-absorbing or binding nanoparticles | For ↓ or neutralizing the effect of a toxin, or for treating or preventing an infection by a microbe that produces a toxin, in a subject | Polymeric hydrogel formulation infused with a toxin-absorbing or binding nanoparticle |
| 17 | CN105979969B China | Topical compositions and methods of using the same | A topical pharmaceutical composition | A topical composition comprises a nitric oxide releasing active pharmaceutical ingredient mixed with a hydrophilic and a hydrophobic composition, in which the nitric oxide releasing active pharmaceutical ingredient encompasses a diazeniumdiolate (A nitric oxide releasing compound of a functional group) |
| 18 | JP6309458B2 Japan | Silicone hydrogel comprising N-vinylamide and hydroxyalkyl (meth) acrylate or (meth) acrylamide | Silicone hydrogel soft contact lenses provide improved oxygen permeability compared to soft lenses made from non-silicone materials | A silicone hydrogel comprising n-vinylamide and hydroxyalkyl (meth) acrylate/(meth) acrylamide |
| 19 | US10620456B2 United States | Increased stiffness center optic in soft contact lenses for astigmatism correction | Contact lenses for the correction of astigmatism | Contact lenses having a higher stiffness in the central optic zone for the correction of astigmatic refractive errors as well as possible higher order aberrations created by corneal geometry |
| 20 | JP6143269B2 Japan | Self-assembled composite ultra-small peptide polymer hydrogel | Topical agents for wound healing, as well as for delivering pharmaceuticals and other bioactive agents/components | A method for fabricating composite hydrogels, as implants/injectables that encourage tissue regeneration and as topical agents for wound healing to deliver pharmaceuticals and other bioactive agents components |
| 21 | US9937254B2 United States | Water-soluble supramolecular complexes | The complexes are useful in a variety of pharmaceutical and cosmetic products and may be combined with an effective amount of a cosmetic, medicament, or diagnostic in a solid dosage form | Water-soluble supramolecular complexes formed when combined with water, form a transparent thermo-reversible hydrogel/solution that may be repeatedly hydrated and dehydrated for sparely soluble and insoluble pharmaceutical agents, exhibits ↑ gelling efficiency, ↑ solubility and/or stability |
| 22 | EP2708224A1 European Patent Office | Biocompatible hydrogel polymer formulations for the controlled delivery of biomolecules | A biocompatible, bioabsorbable hydrogel polymer that releases the therapeutic agent at a target site, avoiding systemic exposure in a controlled delivery | The kits including at least one nucleophilic compound/monomer unit, minimum one electrophilic compound/monomer unit, and at least one drug. Further, the therapeutic agent such as a protein or other biomolecule is capable of gelling in vivo |
| 23 | JP2017527422A Japan | Composite materials for tissue repair | Composite materials and methods which restore lost soft tissue volume and promote soft tissue regeneration | A structural framework composite having a polymeric fiber component covalently bonded to a hydrogel material possessing ↑ properties |
| 24 | US20190343761A1 United States | Antibiotic formulations for lower back pain | Injectable, thermo gelling hydrogel formulations to relieve and/or treat low back pain | A thermosensitive hydrogel, consisting of an effective amount of an antibiotic, a radio-contrast agent, and at least 1 pharmaceutically acceptable excipient |
| 25 | US20170360912A1 United States | Chitosan-based hydrogel and applications thereof. | Chitosan-based hydrogel for medical and cosmetic treatments | flowable formulation and becomes a gel after a gelation time (depending on temperature) just immediately after preparation, containing chitosan, 0.4 M of sodium hydrogen carbonate (SHC), and a weak base different from the SHC |
| 26 | US10842743B2 United States | Modified hyaluronic acid hydrogels and proteins for the time-controlled release of biologic agents | Composition as a liquid capable of in situ formation of a hyaluronic acid-based hydrogel for treating a subject suffering from tumor(s) | Discloses the hyaluronic acid-based hydrogels, solutions for preparing same, and methods relating to this. It includes properties such as extended release, self-resorption of drug, and/or ↓ degradation, denaturation, and/or functional inactivation of active agents |
| 27 | US9211107B2 United States | Ruggedized ultrasound hydrogel insert | A ruggedized hydrogelproduct suitable for use in medical applications where sterile components are required | It contains a gel component, water for hydrating it, and minimum one free radical absorber component that has the capacity to absorb free radicals produced during the sterilization of the hydrogel through a high-energy sterilization procedure and can survive the effects of high-energy sterilization procedures, without substantial structural degradation |
| 28 | TWI558414B Taiwan | Thermosensitive injectable hydrogel for drug delivery | Heat-sensitive injectable hydrogels for drug delivery for delivering anti-cancer drugs | A heat-sensitive injectable hydrogel system based on hyaluronic acid and a copolymer of polyethylene oxide and poly oxypropylene, (having a gel formation temperature of 30 °C to 37 °C), providing an efficient drug delivery system that ↑ the therapeutic efficacy of the drug |
| 29 | JP6066237B2 Japan | Antibacterial ophthalmic contact lenses | Antibacterial ophthalmic devices made of hydrogel and epsilon polylysine (εPLL) | It comprises a hydrogel and at least 5μg εPLL bonded non-covalently to the hydrogel, the contact lens and the packaging solutions |
| 30 | EP3151872B1 European Patent Office | Wound dressing | A stimuli responsive wound dressing application against a wound site of a human or animal body | A wound dressing containing a lyophilized hyaluronic acid hydrogel and a number of implanted devices within hydrogel, each device includes chitosan and hypromellose where the formulation absorbs water and/or exudates and maintain a moist wound site which encourages angiogenesis and wound healing |
| 31 | EP3708167A1 European Patent Office | Immunomodulating treatments of body cavities | A combination medicaments for use in treatment of a cancer of an internal body cavity including urinary tract cancer, meant for local administration in a thermo-reversible hydrogel composition | A biocompatible hydrogel composition incorporating the combination of at least 2 immunomodulatory agents, where one or more of the therapeutic agents are embedded inside, and slowly released from it |
| 32 | WO2019221559A1 WIPO (PCT) | Microneedle adhesive patch based on hydrogel formulation | A microneedle patch that can be utilized for transdermal drug delivery to promote wound regeneration shows brilliant tissue adhesion, biocompatibility, and biodegradability | It comprises a 1st hydrogel layer with mussel adhesive protein and hyaluronic acid and 2nd hydrogel layer with silk fibroin, and a method for manufacturing it |
| 33 | WO2020036526A1 WIPO (PCT) | A biphasic hydrogel formulation and methods of production and use thereof | Creates an environment that relieves or encourages the healing process for the treatment of insect bites, erythema, pruritus, sunburn, acne, dry skin or callus | A hydrogel patch where a biphasic formulation is organized that encompassing a liquid layer externally and an elastic hydrogel in which the water formed on the surface of the elastic gel is physically cooling the skin by evaporation and give a 1st boost of the drug directly when placing on the skin |
| 34 | US20200246472A1 United States | Hydrogel-forming composition for controlled release | Drug delivery systems (injectable biogel) | Peptide hydrogelators capable of forming hydrogels as carriers of active ingredients/biological materials and act as sustained/controlled release systems |
| 35 | AU2015374022B2 Australia | Polyfunctional radical scavenger hydrogel formulation | Providing extended protection of the extracellular space within a wound site | The polyfunctional radical scavenger hydrogel formulation, A portion of the 1st radical scavenger included with the formulation and/or 2nd radical scavenger included within the formulation either in dissolved, suspended and/or bonded to a polymer of the hydrogel |
| 36 | US10471181B2 United States | Fiber-hydrogel composite surgical meshes for tissue repair | A surgical scaffold device for reducing foreign body response, managing tissue-materials interface, and improving the integration of the surgical mesh with the surrounding tissue of a subject | It disclose a composition and methods for a hydrogel/ nanofiber-hydrogel composite integrated with a surgical scaffold or mesh |
| Type of Hydrogel | Disease | Formulation | Study Outcome | Status | Clinical Trial Identifier |
|---|---|---|---|---|---|
| Hydroxyethyl cellulose hydrogel | Knee pain by osteoarthritis | Injection | NA | On-going | NCT04061733 |
| Polyacrylamide | Knee pain by osteoarthritis | Intra-articular injection | Clinical examination reported a transition from −7, meaning worse to 7, better on a scale of −1 to 7. | Completed | NCT03060421 |
| Polyacrylamide hydrogel and hyaluronic acid | Knee pain by osteoarthritis | Intra-articular injection | NA | On-going | NCT02763956 |
| Polyacrylonitrile hydrogel | Degenerative disc disease | Intra-discal | NA | On-going | NCT02763956 |
| Hydroxyethylcellulose hydrogel | Knee pain by osteoarthritis | Intra-articular injection | NA | On-going | NCT04061733 |
| Extracellular matrix hydrogel | Heart failure | ||||
| Alginate hydrogel | Heart failure | Intra-myocardial injection | Improved maximum oxygen uptake | Completed | NCT01311791 |
| Renal cells gelatin hydrogel | Kidney disease | Injection | Improved levels of creatinine, proteinuria, GFR | Completed | NCT02525263 |
| Renal cells gelatin hydrogel | Congenital chronic kidney disease | Injection | NA | On-going | NCT04115345 |
| Human amniotic epithelial cells hydrogel | Asherman’s syndrome | Intra-uterine injection | NA | On-going | NCT03223454 |
| Cardiac stem cells gelatin hydrogel | Ischemic cardiomyopathy | Intra-myocardial injection | Improved ventricular dysfunction | Completed | NCT00981006 |
| Radiopaque Hydrogel | Pancreatic cancer | Injection | NA | On-going | NCT03307564 |
| Biosentry Hydrogel | Pneumothorax risk after Lung biopsy procedures | Tract plug | NA | On-going | NCT02224924 |
| TraceIT hydrogel | Oropharyngeal cancer | Injection | NA | On-going | NCT03713021 |
| TraceIT hydrogel | Rectal cancer | Transperineal injection | NA | On-going | NCT03258541 |
| SpaceOAR hydrogel (PEG) | Prevention of radiation exposure to rectum in radiation therapy | Injection | Reduced adverse effects and limited radiation exposure observed in subjects | Completed | NCT01538628 |
| SpaceOAR hydrogel | Image Guided Intensity Modulated Radiotherapy for prostate cancer | Injection | Reduced rectal toxicity was observed following radiation therapy | Completed | NCT02212548 |
| TracelT hydrogel | Bladder cancer radiation therapy | Injection | Helped in locating bladder tumor during imaging process | Completed | NCT03125226 |
| VentriGel | Myocardial infarction/heart failure | Trans-endocardial injection | Parameters such as ejection fraction, end-diastolic volume and end-systolic volume were improved in myocardial infarction patients. | Completed | NCT02305602 |
| Gut Guarding Gel (alginate with calcium lactate) | Endoscopic Submucosal Dissection | Sub-mucosal injection | It enhanced the mucosa formation and reduced bleeding/tissue injury following endoscopy | Completed | NCT03321396 |
| Polyacrylamide hydrogel | urinary incontinence | Transurethral injection | The bladder retention volume was monitored and successful voiding was observed | Completed | NCT02776423 |
| Polyacrylamide hydrogel and botox | urinary incontinence | Midurethral injection | Micturitions per day increased and relief from urinary incontinence observed | Completed | NCT02815046 |
| Polyacrylamide hydrogel | Anal incontinence | Transanal injection | Reduced Wexner scores were observed after treatment | Completed | NCT02550899 |
| OTX-TKI (polyethylene glycol hydrogel with tyrosine kinase inhibitor) | Age-related Macular Degeneration | Intravitreal injection | NA | On-going | NCT03630315 |
| Hydrogel | Active Ingredient | Type of Disease | In Vitro Cell Line | In Vivo Model | Conclusion | Reference |
|---|---|---|---|---|---|---|
| Thermosensitive chitosan-based | Disulfiram(DSF) | Cancer | Human HCC cell lines (SMMC-7721 cells) | - | A novel injectable sustained formulation for anticancer drugs aimed at the delivery of DSF for long-term cancer treatment | [61] |
| Dual thermo-and pH-sensitive injectable hydrogels of chitosan/(poly(N-isopropylacrylamide-co-itaconic acid) | Doxorubicin | Breast cancer | MCF-7 cells | - | Cytocompatible and exert no/negligible cytotoxicity on MCF-7 cells and has the potential for local therapy of breast cancer | [62] |
| pH-sensitive poly(lactic acid-co-glycolic acid)-b-poly(ethylene glycol)-b-poly(lactic acid-co-glycolic acid) (PLGA-PEG-PLGA) triblock copolymers | Herceptin | Breast cancer | - | SK-BR-3 tumor bearing mice | Great potential for preventing the relapse of HER2+ breast tumors after breast-conserving surgery with ↑ therapeutic efficacy, ↓ side effects and ↑ patient compliance | [63] |
| pH-sensitive injectable-polysaccharide-based self-healing hydrogels | Doxorubicin | Hepatocellular carcinoma | HepG2(release of drug from hydrogel) L929 cells (Cytotoxicity test of the hydrogel) | - | Self-healing property with high drug-loading ratio could prolong their lifetime during implantation and provide the benefit of nominally invasive surgery | [64] |
| Dual pH- and temperature-responsive physically crosslinked injectable hydrogel | Cancer | Oncolytic adenoviruses | - | Human xenograft tumor models | Exhibited ↑ and long-term antitumor therapeutic effects in tumor models and might have potential for long-term cancer treatment | [65] |
| Novel palladium nanosheet (Pd NS)-based chemo-photothermalhydrogel (Pd Gel) | Palladium and doxorubicin | Cancer | - | Mouse | A novel anticancer strategy that allows the release of doxorubicin more precisely, eliminate tumor more efficiently and inhibit tumor metastasis more persistently | [66] |
| ABA triblock copolymers of vitamin D-functionalized polycarbonate and poly(ethylene glycol), that is, VDm-PEG-VDm were synthesized and employed to form physically crosslinked injectable hydrogels | Bevacizumab; Avastin | Cancer | HCT116 xenograft mouse models | Injection of the hydrogel was effective to show antimetastatic activity as that of 4× weekly injections of Avastin thus ↓ the injection frequency and may ↑ patient compliance to treat metastatic cancer | [67] | |
| pH-responsive injectable hydrogels made of a supramolecular cross-link network | doxorubicin | Cancer | L929 mouse fibroblasts | - | Showed biocompatibility, controlled release profiles and tunable properties which show a ↑ potential as a drug-releasing material for localized treatments | [68] |
| Triblock Copolymers of Vitamin E-Functionalized Polycarbonate and Poly(ethylene glycol) | Herceptin | Breast cancer | Human breast cancer cell lines (antitumor specificity and efficacy) | BT474 tumor-bearing mice- (biocompatibility and biodegradability) | ↑ potential for use in subcutaneous and sustained delivery of antibodies to ↑ therapeutic efficacy and/or ↑ patient compliance as compared to intravenous and subcutaneous delivery of Herceptin in solution form | [69] |
| pH-responsive injectable hydrogels with mucosal adhesiveness based on chitosan-grafted-dihydrocaffeic acid and oxidized pullulan | Doxorubicin | Colon tumor | Colon tumor cells (HCT116 cells) | - | Showed good drug release, effectively killing colon tumor cells, ideal candidates for development of colon cancer drug delivery carriers /mucoadhesive drug delivery systems | [70] |
| Alginate hydrogel system | Angiogenesis with vascular endothelial growth factor (VEGF) | Cardiovascular diseases | Human microvascular dermal endothelial cells | Act as a new generation of therapeutic delivery vehicle by combining long-term in vivo therapeutic advantages with minimal invasion to treat cardiovascular diseases | [71] | |
| Dual-responsive (pH and ROS) injectable hydrogels encapsulating drug-loaded micelles | Amikacin, andNaproxen | Wound healing | SD male rats | Possess good biocompatibility with efficient antibacterial and anti-inflammatory action, ↑ the healing process and promising to be applied topically against various microbial infections | [72] | |
| Alginate–chitosan hydrogels | IgG model antibodies and Fab antibody fragments | Applications in drug delivery and regenerative medicine | - | - | Offers controlled delivery of antibodies and antibody fragments and will be promising formulation for several applications in drug delivery and regenerative medicine | [73] |
| Dopamine-based and polydopamine crosslinked injectable hydrogels | Dopamine and metronidazole | Parkinson’s disease | - | mouse L929 fibroblast cells | Can be used as long-term, localized, sustained release injectable system for dopamine as well as anti-inflammatory drugs to treat Parkinson | [74] |
| Covalently crosslinked composite hydrogel embedded with microspheres | Soft tissue engineering | - | - | Can be exploited as a potential opportunity to use this injectable composite gel scaffold in protein delivery and soft tissue engineering applications | [75] | |
| Gelatin-hydroxyphenyl propionic acid (Gtn-HPA) and hyaluronic acid-tyramine (HA-Tyr)-based hydrogels | Human epidermal growth factor (hEGF) | Ophthalmic applications | Hydrodynamic model, giving a normalized diffusion and release of hEGF and provide the most suitable explanation for the measured solute diffusion coefficient | [76] | ||
| Porous alginate gels | Peptide antigen | Immunotherapies | - | Nonobese diabetic mouse model of type 1 diabetes | A noninflammatory biomaterial system can generate antigen-specific, that may enable the development of new therapies to treat transplant rejection/autoimmune diseases | [77] |
| Self-healing injectable micelle/hydrogel composites quaternized chitosan (QCS) solution and benzaldehyde-terminated poly(ethylene oxide)-b-poly(propylene oxide)-b-poly(ethylene oxide) (PEO99-b-PPO65-b-PEO99, Pluronic® F127 (PF127)) (PF127-CHO) solution | Curcumin | Wound dressing for joints skin wound healing | Female Kunming mice | Self-healing antibacterial adhesive hydrogels with good mechanical property offer significant promise as dressing materials for joints skin wound healing | [78] | |
| Alginate-gelatin injectable hydrogel | Oligochitosan coated cerium oxide nanoparticles | Age-related macular degeneration | Human retinal pigment epithlium-19 (ARPE-19) and umbilical endothelium | - | Biocompatible and have ↑ potential in protecting cells from angiogenesis, apoptosis, and production of proinflammatory cytokines with controlled drug release | [79] |
| Decellularized injectable cardiac and skeletal muscle extracellular matrix hydrogel | - | Potential scaffolds for tissue regeneration and/or repair for treating myocardial infarction, heart failure and peripheral artery disease | - | - | Tissue specific biomaterial therapies with minimal invasion | [80] |
| Polysaccharide-based hydrogels(N-carboxyethyl chitosan and oxidized sodium alginate) | Neural stem cells delivery | Neurological disorders | Neural stem cells | - | Neural stem cells transplantation and management of neurological diseases | [81] |
| Non-degradable dendritic polyglycerol sulfate (dPGS) hydrogel | Dendritic polyglycerol sulfate | Osteoarthritis | - | - | Formulation having good viscoelastic properties and has the benefit of being much less easily displaced from its injection site | [82] |
| Conductive anti-oxidant hydrogels (N-carboxyethyl chitosan and oxidized hyaluronic acid-graft-aniline tetramer | Amoxicillin | Wound dressing | C2C12 myoblast cells (Cytocompatibility) Escherichia coli and Staphylococcus aureus (Antibacterial activity) | Male Kunming mice | Have good antibacterial, biodegradation, electroactive and free radical scavenging property to efficiently prevent the wound infection and can be designed as an electroactive injectable hydrogel with promising applications | [83] |
| Injectable poly(ethylene glycol) (PEG)–gelatin hydrogel | Murine adipose-derived stem cells | Wound Healing and tissue regeneration | - | Murine wound healing model | Significantly ↑ cell retention, ↑ angiogenesis, and ↑ wound closure and can be used for regulating stem cell behaviors in 3D culture, delivering cells for wound healing and other tissue regeneration applications | [84] |
| Polyplex Micelle-Loaded Injectable Hydrogels | MicroRNA-29 | Intervertebral disc degeneration(IDD) | Rabbits (therapeutic efficacy on fibrosis Inhibition) Sprague-Dawley rats (In vivo delivery analysis) | Successfully stop the expression of matrix metalloproteinases, prevent the fibrosis process and reverse IDD in animal models | [85] | |
| Collagen–chitosan-based hydrogel | Thymosin β4, (a 43-amino acid peptide) | Myocardial Infarction | Monolayers of BHK-21 | Stimulate angiogenesis and epicardial heart cell migration can be considered as a carrier of other negatively charged active biomolecules and thus shows numerous applications | [86] | |
| Chitosan hydrogel | Human placenta-derived mesenchymal stem cell -derived exosomes | Hindlimb Ischemia | - | Murine model | Can ↑ the retention and stability of exosomes and further ↑ the therapeutic effects that may facilitate the development of easy and effective approaches for assessing and enhancing the therapeutic effects of stem cell-derived exosomes | [87] |
| Sustained release, thermosensitive polymeric [poly(lactic acid-co-glycolic acid)-poly(ethylene glycol)-poly(lactic acid-co-glycolic acid) (PLGA-PEG-PLGA)]hydrogel | Avastin® | Posterior segment disorders | - | Rat | A promising candidate for ocular drug delivery of Avastin® through intravitreal injection | [88] |
| Catheter-injectable hydrogel utilizing a polymer–nanoparticle crosslinking mechanism | - | Various therapeutic applications | Wistar rats | Biocompatible, cell-signaling and can be differentially released with distinct elution profiles, allowing precise control over drug delivery | [89] | |
| Self-healing hydrogel based on chondroitin sulfate multiple aldehyde and N-succinyl-chitosan | Cells encapsulated in the hydrogel | Cell carrier and in tissue engineering | Rat model | Shows biodegradability, produced ↓ inflammatory response and having potential application as a cell carrier and in tissue engineering. | [90] | |
| Physiological temperature-responsive controllable NO-releasing redox injectable hydrogel | Nitric oxide(NO) | Cardiovascular diseases | - | Mice | Significantly ↑ the angiogenesis and new blood vessels formation by regulating the sustained release of NO and redox equilibrium in animal model. It has a ↑ potential in preventing and treating diseases | [91] |
| S.No. | Product | Product Manufactured by/Marketed by | Type of Hydrogel | Active Component | Indications | Reference |
|---|---|---|---|---|---|---|
| 1 | AquaDerm™ | DermaRite | Hydrogel sheet | 2-Acrylamido-2 methyl-1 propane sulfonic acid sodium, Propylene Glycol, Poly (ethylene glycol) dimethacrylate, 2-Hydroxy-2-methylpropiophenone with 38–55% water | Minor burns, pressure ulcers and radiation tissue damage | [124] |
| 2 | DermaSynTM | Amorphous hydrogel | Acute/chronic partial and full thickness wounds/ulcers having minimal exudate | |||
| 3 | DermaGauze™ | Hydrogel impregnated gauze dressing | Acrylate polymer | Acute/chronic partial and full thickness wounds having minimal exudate and wounds with tunneling or sinus tracts | ||
| 4 | DermaSyn/Ag™ | Water-based antibacterial silver Wound gel | Silver | Venous ulcers, tissue trauma, pressure ulcers, diabetic ulcers, surgical incisions Thermal burns, etc. | ||
| 5 | Intrasite® GEL | Smith and Nephew | Hydrogel | Carboxymethyl cellulose and propylene glycol | Ease gentle, effective autolytic debridement to prepare the wound bed in all types of wounds | [125] |
| 6 | Suprasorb® G | Lohmann and Rauscher Global | Hydrogel film | Water (70%), acrylic polymers based on a taurate derivative, polyethylene, phenoxyethanol, transparent polyethylene carrier film | Used for the management of the first and second degree burns, dry fractures, ulcer of the lower leg, pressure ulcer, etc. | [126] |
| 7 | Neoheal® | Kikgel | Hydrogel sheet | Water (90%), polyvinylopyrrolidone, polyethylene glycol and agar, crosslinked by a beam of electrons. | Burns, ulcerations, bedsores and all types of skin damages where humid medium is favourable | [127] |
| 8 | Woun’Dres® Collagen Hydrogel | Coloplast | Collagen Hydrogel | Polymers such as carbomer and collagen | Dry wounds and eschar | [128] |
| 9 | Purilon® | Water, calcium alginate and sodium carboxymethyl cellulose | First and second degree burns, leg ulcers, pressure ulcers, non-infected diabetic foot ulcers | |||
| 10 | Simpurity® | Safe n’Simple | Absorbent hydrogel sheets | water, polyethylene oxide, polyvinyl alcohol, acrylate, polyurethane | Wounds with minimal to no exudate, skin burns and dry scabs | [129] |
| 11 | SimpurityHydroGel® | Impregnated Gauze Wound Dressings | First and second degree burns, pressure sores and leg ulcers | |||
| 12 | ProfiDerm® | Dr. Derm Professional | Hydrocollagen face gel | Sea collagen and hyaluronic acid | Nourishes, hydrates, soothes skin, helps to regenerate the skin of the face, ↑ the elasticity and tones the tissues | [130] |
| 13 | Advanced génifique light pearl hydrogel melting 360 eye mask | Lancome Paris | Hydrogel eye mask | ↓ the appearance of undereye bags, puffy eyes, undereye circles and rejuvenate the eye area | [131] | |
| 14 | Advanced génifique hydrogel melting sheet mask | Hydrogel sheet mask | Water, glycerine, polyacrylate-13, bifidus extract | Moisturized face skin, and make it radiant, smoother, shiny and healthy | [132] | |
| 15 | Water bomb hydrogel mask | Moria | Hydrogel mask | Sodium polyacrylate, glycerine, cellulose gum, water | Restore hydration at a deeper level, soothesand rejuvenate the skin | [133] |
| 16 | EautraSil™ | Miacare™ | Silicone Hydrogel Contact Lens | Hyaluronic Acid and Sodium Alginate | Effectively prevent hypoxia-related complications (corneal neovascularization, redness, and corneal epithelium-aging) | [134] |
| 17 | Confidence | Silicone Hydrogel Contact Lens with Dot Matrix Colour Printing Technology | Hyaluronic Acid and Sodium Alginate | Long lasting comfort | [135] | |
| 18 | Charcoal Hydrogel under eye mask | ELF cosmetics | Hydrogel mask | Charcoal Powder, Green Tea Extract, Lavender Extract | Under eye skin protection and rejuvenation | [136] |
| 19 | SEVEN RX® | Mark’ennovy | Hydrogel lens | Bioinspired silicone hydrogel lens | Short sight and long sight | [137,138] |
| 20 | Gentle 59 | Mark’ennovy | Bio-inspired hydrogel lens | Short sight and long sight | [137] | |
| 21 | MaxvueHiToric® | Maxvue vision | Silicone hydrogel | Hyaluronic Acid | Astigmatism | [137] |
| 22 | ACTIVHEAL® HYDROGEL | Advanced medical solution Ltd. | Amorphous gel | A primary wound dressing contains 85% water | Dry and sloughy wounds with zero to low exudate such as pressure ulcers, leg ulcers, cavity wounds, graft at donor sites, post op surgical wounds, lacerations and abrasions | [139] |
| 23 | Nu-Gel® Hydrogel with Alginate | Systagenix wound management | Hydrogel with alginate | Alginate | Helps in management of chronic wounds through all stages of healing. Manage dry, encrusted and necrotic, sloughy, granulating andepithelialising wounds. | [140] |
| 24 | SUPPRELIN® LA | Endo Pharmaceuticals, USA | Implant | Histrelin acetate | Treatment of children having central precocious puberty | [141] |
| 25 | Cervidil® | Ferring Pharmaceuticals, Inc. | Cervidil (dinoprostone) Vaginal Insert | crosslinked polyethylene oxide/urethane polymer, dinoprostone | Initiation and/or continuation of cervical maturement in pregnant women | [142] |
| 26 | SQZgel™ | MacroMed | Controlled-release oral tablets | chitosan and polyethylene glycol | Hypertension | [143] |
| 27 | Mebiol® Gel | Cosmo Bio co ltd | Thermoreversible hydrogel | poly(N-isopropylacrylamide) and poly(ethylene glycol) | High transparency for cell observation, stem cell culture, cell implantation, organ/tissue regeneration, drug delivery, and non-cell culture applications | [144] |
| 28 | Gelrin C™ | Regentis Biomaterial Ltd. | Photo crosslinked hydrogel | polyethylene glycol and human fibrinogen protein | projected for the reparation of focal defects in cartilage and/or osteochondral defects | [145] |
| 29 | Mebiol® Gel | Cosmo Bio | Thermoreversible Hydrogel | Poly (N- isopropylacrylamide) and POLY glycol ethylene | Cell implantation, organ and tissue regeneration, stem cell culture, drug delivery, and non-cell culture applications | [146] |
| 30 | HyStem® Hydrogel | ESI BIO | Hyaluronic acid UV light-controlled system | hyaluronic acid | 3D cell culture for tissue engineering purposes and 3D printing applications | [147] |
| 31 | Corning® PuraMatrix™ | Corning Incorporated Life sciences | Peptide hydrogel | - | 3D cell culture used for stem cell proliferation, tumor cell migration and invasion, and in vivo analysis of tissue regeneration | [148] |
| 32 | Biogelx™ | Bioglex Ltd. | Simple, short self-assembling peptides | - | Create an optimal environment for the culture of a variety of cell types, deliver synthetic yet biologically-relevant alternatives to animal-derived 3D matrices for example matrigel and collagen | [149] |
| 33 | SpaceOAR® | Boston scientific | Absorbable Injectable hydrogel | - | Imaging of cancerous cells and protecting healthy cells from radiation induced damage | [150] |
| 34 | Bulkamid® | Contura International | Soft injectable, transparent, hydrophilic gel | Synthetic polyacrylamide and water | Stress urinary incontinence | [151] |
| 35 | Symphony® | Cypre’s | Stimuli–responsive hydrogels as sensors | 3D photolithographic instrument | Animal cell culture, 3D imaging, iPSCs, ESc cell lines, etc. | [152] |
| 36 | Valleylab™ | Medtronic | Covalent anchored coatings | Chitosan-hyaluronic acid-based hydrogel catheter | Postoperative adhesion | [153] |
| 37 | Bolt Bis-Tris Plus Gels | Thermo Fisher | Bioassay matrices(gel) | Polyacrylamide gels | Western blot transfer and analysis | [154] |
| 38 | Tadpole™ | Nervena® | Hydrogel electrode | Ionically conductive hydrogels | Use in Parotidectomy and otologic Surgery |
| Common Name | USP-NF Name | Preferred Substance Name (USFDA) | EP Name | CAS No. | Maximum Potency per Unit Topical Dose(as per USFDA) | Relevant Physico-Chemical Properties | GRAS Listed |
|---|---|---|---|---|---|---|---|
| Alginate | Alginic acid, sodium alginate | Sodium alginate | Alginate, sodium | 9005-38-3 | 0.25% w/w | Dissolves in water and forms a viscous solution | ✓ |
| Collagen | Gelatin | Type II Collagen | Collagen | 9007-34-5 | 8% w/w | ✓ | |
| Gelatin | Gelatin | Gelatin | Gelatin | 9000-70-8 | 350 mg | ✓ | |
| PEG 200 | Polyethylene glycol 200 | Polyethylene glycol 200 | Polyethylene glycol 200 | 112,607 | 39% w/w | Viscosity 3.9–4.8 mPas at 98 °C | ✓ |
| PEG 1000 | Polyethylene glycol 1000 | Polyethylene glycol 1000 | Polyethylene glycol 1000 | 25,322,683 | 0.5% w/w | Viscosity 16–19 mPas at 98 °C | |
| PEG 1600 | Polyethylene glycol 1600 | Polyethylene glycol 1600 | Polyethylene glycol 1600 | 25,322,683 | 29.7% w/w | Viscosity 28–36 mPas at 98 °C | ✓ |
| PEG 300 | Polyethylene glycol 300 | Polyethylene glycol 300 | Polyethylene glycol 300 | 25,322,683 | 57% w/w | Viscosity 5.4–6.4 mPas at 98 °C | ✓ |
| PEG 400 | Polyethylene glycol 400 | Polyethylene glycol 400 | Polyethylene glycol 400 | 25,322,683 | 99% w/v | Viscosity 6.8–8 mPas at 98 °C | ✓ |
| Polyacrylic acid | Poly(acrylic acid) | Polyacrylic acid | Polyacrylate | 9003-01-4 | 196 mg | Viscosity 50–200 mPas at 20 °C | ✓ |
| Polyvinyl alcohol | Polyvinyl alcohol | Polyvinyl alcohol | Polyvinyl alcohol | 9002-89-5 | 140 mg | ✓ | |
| Polyacrylamide | Polyacrylamide | Polyacrylamide | Polyacrylamide | 9003-05-8 | 5% w/w | ✓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohapatra, S.; Mirza, M.A.; Hilles, A.R.; Zakir, F.; Gomes, A.C.; Ansari, M.J.; Iqbal, Z.; Mahmood, S. Biomedical Application, Patent Repository, Clinical Trial and Regulatory Updates on Hydrogel: An Extensive Review. Gels 2021, 7, 207. https://doi.org/10.3390/gels7040207
Mohapatra S, Mirza MA, Hilles AR, Zakir F, Gomes AC, Ansari MJ, Iqbal Z, Mahmood S. Biomedical Application, Patent Repository, Clinical Trial and Regulatory Updates on Hydrogel: An Extensive Review. Gels. 2021; 7(4):207. https://doi.org/10.3390/gels7040207
Chicago/Turabian StyleMohapatra, Sradhanjali, Mohd. Aamir Mirza, Ayah Rebhi Hilles, Foziyah Zakir, Andreia Castro Gomes, Mohammad Javed Ansari, Zeenat Iqbal, and Syed Mahmood. 2021. "Biomedical Application, Patent Repository, Clinical Trial and Regulatory Updates on Hydrogel: An Extensive Review" Gels 7, no. 4: 207. https://doi.org/10.3390/gels7040207
APA StyleMohapatra, S., Mirza, M. A., Hilles, A. R., Zakir, F., Gomes, A. C., Ansari, M. J., Iqbal, Z., & Mahmood, S. (2021). Biomedical Application, Patent Repository, Clinical Trial and Regulatory Updates on Hydrogel: An Extensive Review. Gels, 7(4), 207. https://doi.org/10.3390/gels7040207








