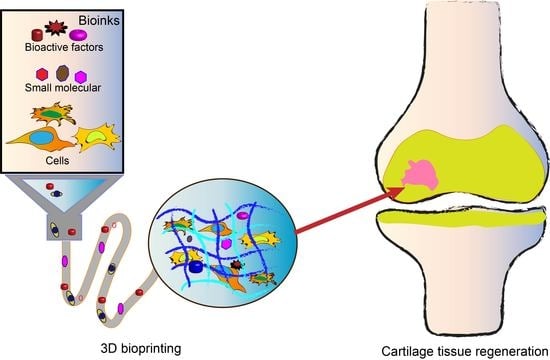
3D Bioprinting of Hydrogels for Cartilage Tissue Engineering
Abstract
:1. Introduction
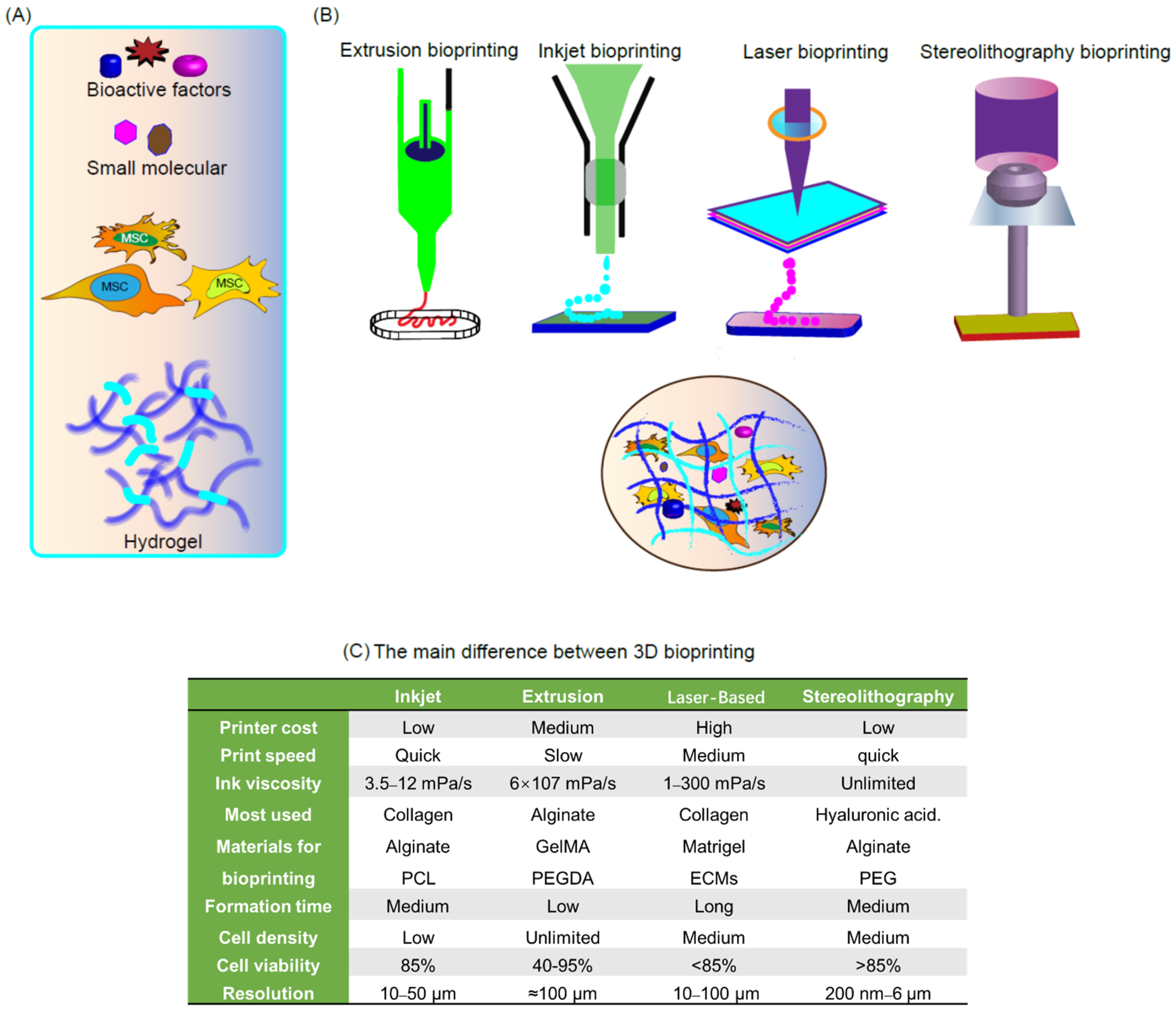
2. Bioprinting Technologies
2.1. Inkjet-Based 3D Printing
2.2. Micro-Extrusion Bioprinting
2.3. Laser-Based 3D Printing
2.4. Stereolithography-Based 3D Bioprinting
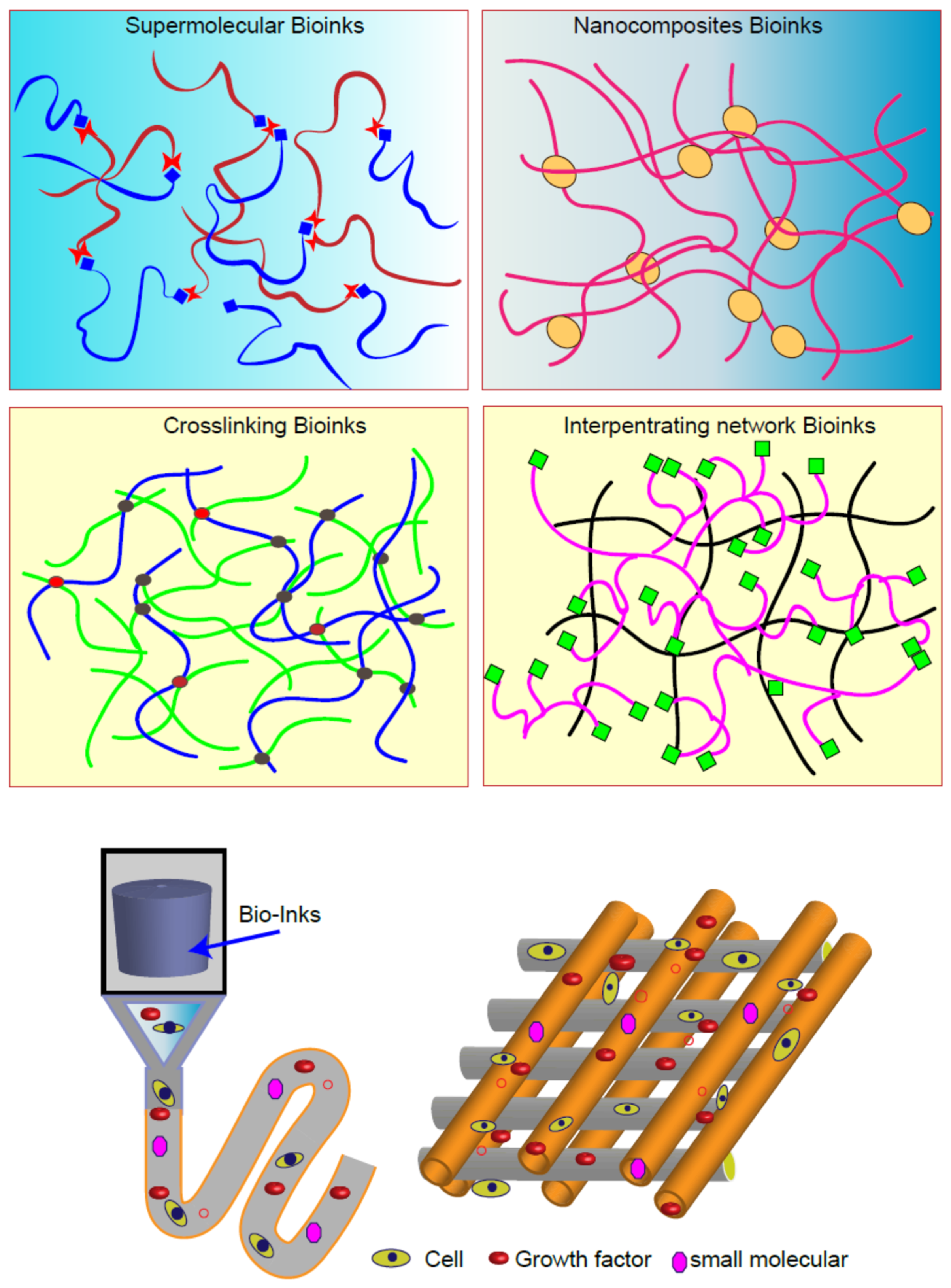
3. Bioinks for 3D Bioprinting
3.1. Scaffold Materials
3.2. Cell Sourcing
3.3. Growth Factors
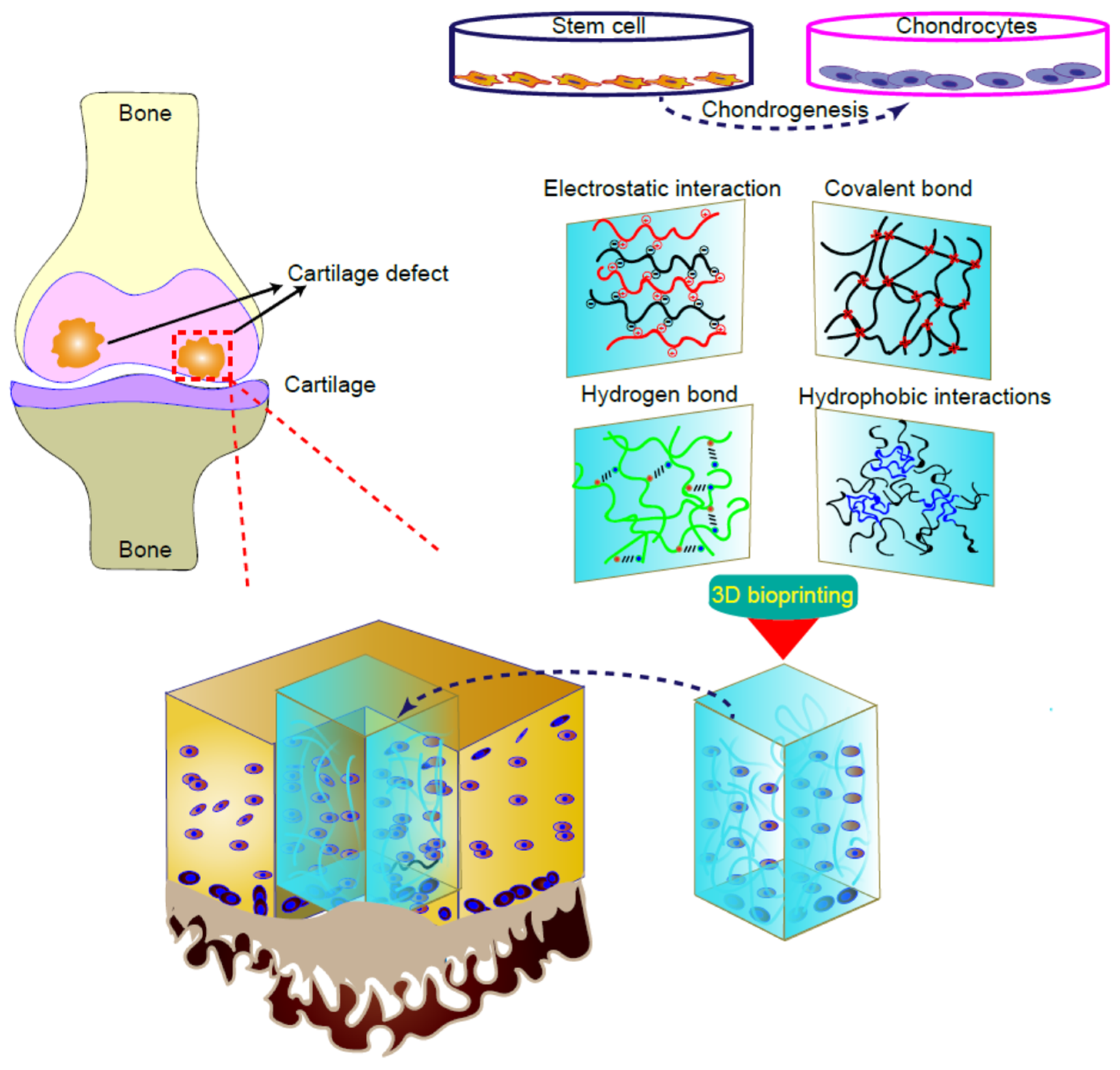
4. 3D Bioprinted Cartilage Tissues
5. Outlook
5.1. Advanced Developments in 3D Bioprinting Technology
5.2. 3D Bioprinting for Cartilage Tissue Engineering
5.3. Challenges and Limitations of 3D Bioprinting in Clinical Transplantation Applications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef] [PubMed]
- Thayer, P.; Martinez, H.; Gatenholm, E. History and Trends of 3D Bioprinting. Methods Mol. Biol. 2020, 2140, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Derby, B. Inkjet Printing of Functional and Structural Materials: Fluid Property Requirements, Feature Stability, and Resolution. Annu. Rev. Mater. Res. 2010, 40, 395–414. [Google Scholar] [CrossRef]
- Unagolla, J.M.; Jayasuriya, A.C. Hydrogel-based 3D bioprinting: A comprehensive review on cell-laden hydrogels, bioink formulations, and future perspectives. Appl. Mater. Today 2020, 18, 100479. [Google Scholar] [CrossRef] [PubMed]
- Chimene, D.; Lennox, K.K.; Kaunas, R.R.; Gaharwar, A.K. Advanced Bioinks for 3D Printing: A Materials Science Perspective. Ann. Biomed. Eng. 2016, 44, 2090–2102. [Google Scholar] [CrossRef] [PubMed]
- Szymański, T.; Mieloch, A.A.; Richter, M.; Trzeciak, T.; Florek, E.; Rybka, J.D.; Giersig, M. Utilization of Carbon Nanotubes in Manufacturing of 3D Cartilage and Bone Scaffolds. Materials 2020, 13, 4039. [Google Scholar] [CrossRef]
- Kumar, A.; IA, I.M.; Han, S.S. 3D printable carboxylated cellulose nanocrystal-reinforced hydrogel inks for tissue engineering. Biofabrication 2020, 12, 025029. [Google Scholar] [CrossRef]
- Huang, J.; Liang, Y.; Jia, Z.; Chen, J.; Duan, L.; Liu, W.; Zhu, F.; Liang, Q.; Zhu, W.; You, W.; et al. Development of Magnetic Nanocomposite Hydrogel with Potential Cartilage Tissue Engineering. ACS Omega 2018, 3, 6182–6189. [Google Scholar] [CrossRef]
- Huang, J.; Liu, W.; Liang, Y.; Li, L.; Duan, L.; Chen, J.; Zhu, F.; Lai, Y.; Zhu, W.; You, W.; et al. Preparation and biocompatibility of diphasic magnetic nanocomposite scaffold. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 87, 70–77. [Google Scholar] [CrossRef]
- Huang, J.; Liang, Y.; Huang, Z.; Zhao, P.; Liang, Q.; Liu, Y.; Duan, L.; Liu, W.; Zhu, F.; Bian, L.; et al. Magnetic Enhancement of Chondrogenic Differentiation of Mesenchymal Stem Cells. ACS Biomater. Sci. Eng. 2019, 5, 2200–2207. [Google Scholar] [CrossRef]
- Huang, J.; Liang, Y.; Huang, Z.; Xiong, J.; Wang, D. Preparation, Characterization, and Biological Testing of Novel Magnetic Nanocomposite Hydrogels. ACS Omega 2020, 5, 9733–9743. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Jia, Z.; Liang, Y.; Huang, Z.; Rong, Z.; Xiong, J.; Wang, D. Pulse electromagnetic fields enhance the repair of rabbit articular cartilage defects with magnetic nano-hydrogel. RSC Adv. 2020, 10, 541–550. [Google Scholar] [CrossRef] [Green Version]
- Noh, I.; Kim, N.; Tran, H.N.; Lee, J.; Lee, C. 3D printable hyaluronic acid-based hydrogel for its potential application as a bioink in tissue engineering. Biomater. Res. 2019, 23, 3. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhou, D.; Chen, J.; Zhang, X.; Li, X.; Zhao, W.; Xu, T. Biomaterials Based on Marine Resources for 3D Bioprinting Applications. Mar. Drugs 2019, 17, 555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rastogi, P.; Kandasubramanian, B. Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication 2019, 11, 042001. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Hou, W.; Bai, S. Gelatin-Based Hydrogels for Organ 3D Bioprinting. Polymers 2017, 9, 401. [Google Scholar] [CrossRef] [Green Version]
- De Melo, B.A.G.; Jodat, Y.A.; Cruz, E.M.; Benincasa, J.C.; Shin, S.R.; Porcionatto, M.A. Strategies to use fibrinogen as bioink for 3D bioprinting fibrin-based soft and hard tissues. Acta Biomater. 2020, 117, 60–76. [Google Scholar] [CrossRef]
- Marques, C.F.; Diogo, G.S.; Pina, S.; Oliveira, J.M.; Silva, T.H.; Reis, R.L. Collagen-based bioinks for hard tissue engineering applications: A comprehensive review. J. Mater. Sci. Mater. Med. 2019, 30, 32. [Google Scholar] [CrossRef]
- Hauptstein, J.; Böck, T.; Bartolf-Kopp, M.; Forster, L.; Stahlhut, P.; Nadernezhad, A.; Blahetek, G.; Zernecke-Madsen, A.; Detsch, R.; Jüngst, T.; et al. Hyaluronic Acid-Based Bioink Composition Enabling 3D Bioprinting and Improving Quality of Deposited Cartilaginous Extracellular Matrix. Adv. Healthc. Mater. 2020, 9, e2000737. [Google Scholar] [CrossRef]
- Turner, P.R.; Murray, E.; McAdam, C.J.; McConnell, M.A.; Cabral, J.D. Peptide Chitosan/Dextran Core/Shell Vascularized 3D Constructs for Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 32328–32339. [Google Scholar] [CrossRef]
- Abaci, A.; Guvendiren, M. Designing Decellularized Extracellular Matrix-Based Bioinks for 3D Bioprinting. Adv. Healthc. Mater. 2020, 9, e2000734. [Google Scholar] [CrossRef] [PubMed]
- Xin, S.; Chimene, D.; Garza, J.E.; Gaharwar, A.K.; Alge, D.L. Clickable PEG hydrogel microspheres as building blocks for 3D bioprinting. Biomater. Sci. 2019, 7, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.D.; Wright, N.M. Polyethylene glycol hydrogel spinal sealant (DuraSeal Spinal Sealant) as an adjunct to sutured dural repair in the spine: Results of a prospective, multicenter, randomized controlled study. Spine 2011, 36, 1906–1912. [Google Scholar] [CrossRef] [PubMed]
- Abdollahiyan, P.; Oroojalian, F.; Mokhtarzadeh, A.; de la Guardia, M. Hydrogel-Based 3D Bioprinting for Bone and Cartilage Tissue Engineering. Biotechnol. J. 2020, 15, e2000095. [Google Scholar] [CrossRef]
- Li, X.; Liang, Y.; Xu, X.; Xiong, J.; Ouyang, K.; Duan, L.; Wang, D. Cell-to-Cell Culture Inhibits Dedifferentiation of Chondrocytes and Induces Differentiation of Human Umbilical Cord-Derived Mesenchymal Stem Cells. BioMed Res. Int. 2019, 2019, 5871698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Duan, L.; Liang, Y.; Zhu, W.; Xiong, J.; Wang, D. Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Contribute to Chondrogenesis in Coculture with Chondrocytes. BioMed Res. Int. 2016, 2016, 3827057. [Google Scholar] [CrossRef]
- Colle, J.; Blondeel, P.; de Bruyne, A.; Bochar, S.; Tytgat, L.; Vercruysse, C.; van Vlierberghe, S.; Dubruel, P.; Declercq, H. Bioprinting predifferentiated adipose-derived mesenchymal stem cell spheroids with methacrylated gelatin ink for adipose tissue engineering. J. Mater. Sci. Mater. Med. 2020, 31, 36. [Google Scholar] [CrossRef]
- Lach, M.S.; Wroblewska, J.; Kulcenty, K.; Richter, M.; Trzeciak, T.; Suchorska, W.M. Chondrogenic Differentiation of Pluripotent Stem Cells under Controllable Serum-Free Conditions. Int. J. Mol. Sci. 2019, 20, 2711. [Google Scholar] [CrossRef] [Green Version]
- Harrell, C.R.; Gazdic, M.; Fellabaum, C.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Therapeutic Potential of Amniotic Fluid Derived Mesenchymal Stem Cells Based on their Differentiation Capacity and Immunomodulatory Properties. Curr. Stem Cell Res. Ther. 2019, 14, 327–336. [Google Scholar] [CrossRef]
- Jia, Z.; Liu, Q.; Liang, Y.; Li, X.; Xu, X.; Ouyang, K.; Xiong, J.; Wang, D.; Duan, L. Repair of articular cartilage defects with intra-articular injection of autologous rabbit synovial fluid-derived mesenchymal stem cells. J. Transl. Med. 2018, 16, 123. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Liang, Y.; Li, X.; Ouyang, K.; Wang, M.; Cao, T.; Li, W.; Liu, J.; Xiong, J.; Li, B.; et al. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials 2021, 269, 120539. [Google Scholar] [CrossRef]
- Barkholt, L.; Flory, E.; Jekerle, V.; Lucas-Samuel, S.; Ahnert, P.; Bisset, L.; Büscher, D.; Fibbe, W.; Foussat, A.; Kwa, M.; et al. Risk of tumorigenicity in mesenchymal stromal cell-based therapies--bridging scientific observations and regulatory viewpoints. Cytotherapy 2013, 15, 753–759. [Google Scholar] [CrossRef]
- Badran, Z.; Abdallah, M.N.; Torres, J.; Tamimi, F. Platelet concentrates for bone regeneration: Current evidence and future challenges. Platelets 2018, 29, 105–112. [Google Scholar] [CrossRef]
- Cai, G.; Liu, W.; He, Y.; Huang, J.; Duan, L.; Xiong, J.; Liu, L.; Wang, D. Recent advances in kartogenin for cartilage regeneration. J. Drug Target. 2019, 27, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Li, C.; Ke, C.J.; Sun, J.S.; Lin, F.H. Kartogenin Enhances Chondrogenic Differentiation of MSCs in 3D Tri-Copolymer Scaffolds and the Self-Designed Bioreactor System. Biomolecules 2021, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Cheng, P.; Sang, S.; Xiang, C.; An, Y.; Wei, X.; Shen, Z.; Zhang, Y.; Li, P. Mesenchymal stem cells loaded on 3D-printed gradient poly(ε-caprolactone)/methacrylated alginate composite scaffolds for cartilage tissue engineering. Regen. Biomaterials 2021, 8, rbab019. [Google Scholar] [CrossRef] [PubMed]
- Kesti, M.; Eberhardt, C.; Pagliccia, G.; Kenkel, D.; Grande, D.; Boss, A.; Zenobi-Wong, M. Bioprinting Complex Cartilaginous Structures with Clinically Compliant Biomaterials. Adv. Funct. Mater. 2015, 25, 7406–7417. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Wang, F.; Chen, C.; Gong, X.; Yin, L.; Yang, L. Engineering zonal cartilage through bioprinting collagen type II hydrogel constructs with biomimetic chondrocyte density gradient. BMC Musculoskelet. Disord. 2016, 17, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daly, A.C.; Critchley, S.E.; Rencsok, E.M.; Kelly, D.J. A comparison of different bioinks for 3D bioprinting of fibrocartilage and hyaline cartilage. Biofabrication 2016, 8, 045002. [Google Scholar] [CrossRef]
- Levato, R.; Webb, W.R.; Otto, I.A.; Mensinga, A.; Zhang, Y.; van Rijen, M.; van Weeren, R.; Khan, I.M.; Malda, J. The bio in the ink: Cartilage regeneration with bioprintable hydrogels and articular cartilage-derived progenitor cells. Acta Biomater. 2017, 61, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Rathan, S.; Dejob, L.; Schipani, R.; Haffner, B.; Möbius, M.E.; Kelly, D.J. Fiber Reinforced Cartilage ECM Functionalized Bioinks for Functional Cartilage Tissue Engineering. Adv. Healthc. Mater. 2019, 8, e1801501. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Luo, C.; Zhai, C.; Li, Z.; Zhang, Y.; Yuan, T.; Dong, S.; Zhang, J.; Fan, W. Crosslinker-free silk/decellularized extracellular matrix porous bioink for 3D bioprinting-based cartilage tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111388. [Google Scholar] [CrossRef]
- Xu, T.; Binder, K.W.; Albanna, M.Z.; Dice, D.; Zhao, W.; Yoo, J.J.; Atala, A. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication 2013, 5, 015001. [Google Scholar] [CrossRef]
- Daly, A.C.; Kelly, D.J. Biofabrication of spatially organised tissues by directing the growth of cellular spheroids within 3D printed polymeric microchambers. Biomaterials 2019, 197, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.; Shim, J.-H.; Jang, J.; Kim, S.-W.; Cho, D.-W. An additive manufacturing-based PCL–alginate–chondrocyte bioprinted scaffold for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2015, 9, 1286–1297. [Google Scholar] [CrossRef] [PubMed]
- Galarraga, J.H.; Kwon, M.Y.; Burdick, J.A. 3D bioprinting via an in situ crosslinking technique towards engineering cartilage tissue. Sci. Rep. 2019, 9, 19987. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Hägg, D.A.; Forsman, A.; Ekholm, J.; Nimkingratana, P.; Brantsing, C.; Kalogeropoulos, T.; Zaunz, S.; Concaro, S.; Brittberg, M.; et al. Cartilage Tissue Engineering by the 3D Bioprinting of iPS Cells in a Nanocellulose/Alginate Bioink. Sci. Rep. 2017, 7, 658. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xu, Y.; Liu, Y.; Wang, Z.; Li, Y.; Jiang, G.; Mo, X.; Zhou, G. Three-dimensional printed electrospun fiber-based scaffold for cartilage regeneration. Mater. Des. 2019, 179, 107886. [Google Scholar] [CrossRef]
- Mouser, V.H.; Abbadessa, A.; Levato, R.; Hennink, W.E.; Vermonden, T.; Gawlitta, D.; Malda, J. Development of a thermosensitive HAMA-containing bio-ink for the fabrication of composite cartilage repair constructs. Biofabrication 2017, 9, 015026. [Google Scholar] [CrossRef]
- Stichler, S.; Böck, T.; Paxton, N.; Bertlein, S.; Levato, R.; Schill, V.; Smolan, W.; Malda, J.; Teßmar, J.; Blunk, T.; et al. Double printing of hyaluronic acid/poly(glycidol) hybrid hydrogels with poly(ε-caprolactone) for MSC chondrogenesis. Biofabrication 2017, 9, 044108. [Google Scholar] [CrossRef]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hägg, D.; Gatenholm, P. 3D Bioprinting Human Chondrocytes with Nanocellulose-Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef]
- Shie, M.Y.; Chang, W.C.; Wei, L.J.; Huang, Y.H.; Chen, C.H.; Shih, C.T.; Chen, Y.W.; Shen, Y.F. 3D Printing of Cytocompatible Water-Based Light-Cured Polyurethane with Hyaluronic Acid for Cartilage Tissue Engineering Applications. Materials 2017, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, L.K.; Huebner, P.; Fisher, M.B.; Spang, J.T.; Starly, B.; Shirwaiker, R.A. 3D-Bioprinting of Polylactic Acid (PLA) Nanofiber-Alginate Hydrogel Bioink Containing Human Adipose-Derived Stem Cells. ACS Biomater. Sci. Eng. 2016, 2, 1732–1742. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Xu, Y.; Gu, Y.; Yao, Q.; Li, J.; Wang, L. IGF-1-releasing PLGA nanoparticles modified 3D printed PCL scaffolds for cartilage tissue engineering. Drug Deliv. 2020, 27, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; You, Y.; Jiang, W.; Wang, B.; Wu, Q.; Dai, K. 3D bioprinting dual-factor releasing and gradient-structured constructs ready to implant for anisotropic cartilage regeneration. Sci. Adv. 2020, 6, eaay1422. [Google Scholar] [CrossRef]
- Cheng, Z.; Xigong, L.; Weiyi, D.; Jingen, H.; Shuo, W.; Xiangjin, L.; Junsong, W. Potential use of 3D-printed graphene oxide scaffold for construction of the cartilage layer. J. Nanobiotechnol. 2020, 18, 97. [Google Scholar] [CrossRef]
- Huang, J.; Huang, Z.; Liang, Y.; Yuan, W.; Bian, L.; Duan, L.; Rong, Z.; Xiong, J.; Wang, D.; Xia, J. 3D printed gelatin/hydroxyapatite scaffolds for stem cell chondrogenic differentiation and articular cartilage repair. Biomater. Sci. 2021, 9, 2620–2630. [Google Scholar] [CrossRef]
- Singh, Y.P.; Bandyopadhyay, A.; Mandal, B.B. 3D Bioprinting Using Cross-Linker-Free Silk-Gelatin Bioink for Cartilage Tissue Engineering. ACS Appl. Mater. Interfaces 2019, 11, 33684–33696. [Google Scholar] [CrossRef]
- Li, C.; Wang, K.; Zhou, X.; Li, T.; Xu, Y.; Qiang, L.; Peng, M.; Xu, Y.; Xie, L.; He, C.; et al. Controllable fabrication of hydroxybutyl chitosan/oxidized chondroitin sulfate hydrogels by 3D bioprinting technique for cartilage tissue engineering. Biomed. Mater. 2019, 14, 025006. [Google Scholar] [CrossRef]
- Yang, X.; Lu, Z.; Wu, H.; Li, W.; Zheng, L.; Zhao, J. Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 83, 195–201. [Google Scholar] [CrossRef]
- Shi, W.; Sun, M.; Hu, X.; Ren, B.; Cheng, J.; Li, C.; Duan, X.; Fu, X.; Zhang, J.; Chen, H.; et al. Structurally and Functionally Optimized Silk-Fibroin–Gelatin Scaffold Using 3D Printing to Repair Cartilage Injury In Vitro and In Vivo. Adv. Mater. 2017, 29, 1701089. [Google Scholar] [CrossRef]
- Park, J.Y.; Choi, J.C.; Shim, J.H.; Lee, J.S.; Park, H.; Kim, S.W.; Doh, J.; Cho, D.W. A comparative study on collagen type I and hyaluronic acid dependent cell behavior for osteochondral tissue bioprinting. Biofabrication 2014, 6, 035004. [Google Scholar] [CrossRef]
- Little, C.J.; Kulyk, W.M.; Chen, X. The Effect of Chondroitin Sulphate and Hyaluronic Acid on Chondrocytes Cultured within a Fibrin-Alginate Hydrogel. J. Funct. Biomater. 2014, 5, 197–210. [Google Scholar] [CrossRef]
- Rhee, S.; Puetzer, J.L.; Mason, B.N.; Reinhart-King, C.A.; Bonassar, L.J. 3D Bioprinting of Spatially Heterogeneous Collagen Constructs for Cartilage Tissue Engineering. ACS Biomater. Sci. Eng. 2016, 2, 1800–1805. [Google Scholar] [CrossRef]
- Schuurman, W.; Khristov, V.; Pot, M.W.; van Weeren, P.R.; Dhert, W.J.; Malda, J. Bioprinting of hybrid tissue constructs with tailorable mechanical properties. Biofabrication 2011, 3, 021001. [Google Scholar] [CrossRef]
- Mohan, T.; Maver, T.; Štiglic, A.D.; Stana-Kleinschek, K.; Kargl, R. 6-3D bioprinting of polysaccharides and their derivatives: From characterization to application. In Fundamental Biomaterials: Polymers; Thomas, S., Balakrishnan, P., Sreekala, M.S., Eds.; Woodhead Publishing: John Solston, UK, 2018; pp. 105–141. [Google Scholar]
- Du, Y.; Liu, H.; Yang, Q.; Wang, S.; Wang, J.; Ma, J.; Noh, I.; Mikos, A.G.; Zhang, S. Selective laser sintering scaffold with hierarchical architecture and gradient composition for osteochondral repair in rabbits. Biomaterials 2017, 137, 37–48. [Google Scholar] [CrossRef]
- Hong, S.; Sycks, D.; Chan, H.F.; Lin, S.; Lopez, G.P.; Guilak, F.; Leong, K.W.; Zhao, X. 3D Printing: 3D Printing of Highly Stretchable and Tough Hydrogels into Complex, Cellularized Structures. Adv. Mater. 2015, 27, 4034. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xiong, J.; Yang, L.; Zhang, J.; Sun, S.; Liang, Y. Cell-free exosome-laden scaffolds for tissue repair. Nanoscale 2021, 13, 8740–8750. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Xu, X.; Xu, L.; Chen, H.; Li, X.; Alahdal, M.; Xiao, Y.; Liang, Y.; Xia, J. Exosome-mediated drug delivery for cell-free therapy of osteoarthritis. Curr. Med. Chem. 2020, 27. [Google Scholar] [CrossRef]
- Duan, L.; Xu, L.; Xu, X.; Qin, Z.; Zhou, X.; Xiao, Y.; Liang, Y.; Xia, J. Exosome-mediated delivery of gene vectors for gene therapy. Nanoscale 2021, 13, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Duan, L.; Lu, J.; Xia, J. Engineering exosomes for targeted drug delivery. Theranostics 2021, 11, 3183–3195. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xu, X.; Li, X.; Xiong, J.; Li, B.; Duan, L.; Wang, D.; Xia, J. Chondrocyte-Targeted MicroRNA Delivery by Engineered Exosomes toward a Cell-Free Osteoarthritis Therapy. ACS Appl. Mater. Interfaces 2020, 12, 36938–36947. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, G.; Garcia, J.; Amir, J. 3D Bioprinting: New Directions in Articular Cartilage Tissue Engineering. ACS Biomater. Sci. Eng. 2017, 3, 2657–2668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conoscenti, G.; Schneider, T.; Stoelzel, K.; Carfì Pavia, F.; Brucato, V.; Goegele, C.; La Carrubba, V.; Schulze-Tanzil, G. PLLA scaffolds produced by thermally induced phase separation (TIPS) allow human chondrocyte growth and extracellular matrix formation dependent on pore size. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 449–459. [Google Scholar] [CrossRef]
| Biomateria | Bioprinting Method | Cell Type | Function | Ref. |
|---|---|---|---|---|
| Polycaprolactone/Alginate | Extrusion-based | Chondrocyte | Cartilage regeneration | [36] |
| Polysaccharides/gellan/alginate/BioCartilage | Extrusion-based | Chondrocyte | Supports proliferation of chondrocytes | [37] |
| Collagen type II hydrogel | Extrusion-based | Chondrocyte | Promote biomimetic chondrocyte density gradient and formation of type II collagen hydrogel structure | [38] |
| Gelatin | Extrusion-based | MSCs | Maintain MSC viability (~80%), development of hyaline-like and fibrocartilage-like tissue | [39] |
| Gelatin | Extrusion-based | Chondrocytes, chondroprogenitor cells, MSCs | Supports the synthesis of new cartilage in stratified co-culture | [40] |
| Cartilage extracellular matrix (cECM)/alginate | Extrusion-based | MSCs | Prmote COL 2 and ACAN expression and cartilage formation | [41] |
| ECM/silk fibroin | Extrusion-based | BMSCs | Supports the controlled release of cartilage growth factors and enhances the formation of cartilage | [42] |
| Fibrinogen/fibrin | Inkjet printing | Rabbit chondrocytes | Enhance mechanical properties and cartilage ECM | [43] |
| PCL/GelMA | Inkjet printing | MSCs and chondrocytes | Native-like collagen anisotropies | [44] |
| PCL-alginate | Extrusion | Chondrocytes | Promotes the formation of cartilage tissue and type II collagen | [45] |
| Polysaccharides, Gellan, Alginate | Extrusion | Chondrocytes | Enhances deposition of cartilage matrix proteins | [37] |
| Norbornene-modified hyaluronic acid (NorHA) | Extrusion | MSCs | Induces chondrogenesis and cartilage formation | [46] |
| Nanocellulose/Alginate | Extrusion | Induced pluripotent stem cells (iPSCs) | Induces chondrogenic and cartilage production | [47] |
| Gelatin, PLGA | Extrusion | Chondrocytes | Promotes cartilage regeneration and maintenance of cartilage tissue shape in vivo | [48] |
| HAMA/pHPMA-lac/PEG | Extrusion-based | Equine chondrocytes | Promotes cartilage-like tissue formation | [49] |
| PG-HA, allyl-functional PGs | Extrusion-based | Human and equine MSCs | Promotes chondrogenic differentiation | [50] |
| Nanocellulose-Alginate | Extrusion-based | Chondrocytes | Maintain cell viability of 73% to 86% | [51] |
| Polyurethane, Hyaluronic Acid | Extrusion-based | Wharton’s jelly mesenchymal stem cells | High cytocompatibility | [52] |
| PLA/Alginate hydrogel | Extrusion-based | Human adipose-derived stem cells | Exhibits high levels of cell proliferation; promotes ECM secretion and chondrogenic differentiation | [53] |
| PLGA/PDA/PCL | Fused Deposition Modeling | Chondrocytes and rBMSCs | Continuous IGF-1 release and better cartilage formation ability | [54] |
| PLGA/Hydrogel/PCL | Extrusion-based | BMSCs | Dual-factor releasing and gradient-structured | [55] |
| Graphene oxide (GO)/chitosan/collagen type-I | Inkjet printing | Chondrocyte | Cartilage-matrix regeneration | [56] |
| Gelatin/hydroxyapatite | Microextrusion | hUCB-MSCs | Articular cartilage repairs | [57] |
| Silk/Gelatin | Extrusion-based | Chondrocytes | Biocompatibility | [58] |
| Hydroxybutyl chitosan/oxidized chondroitin sulfate | Inkjet | Human adipose-derived stem cells | Multifunctional cell delivery hydrogels for the cartilage repair | [59] |
| Collagen/alginate | Extrusion-based | Chondrocytes | Inhibit chondrocytes dedifferentiation and miantain the phenotype | [60] |
| Silk/Fibroin/Gelatin | Extrusion-based | BMSCs | Inhibited the dedifferentiation of chondrocytes and maintained the phenotype. | [61] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Xiong, J.; Wang, D.; Zhang, J.; Yang, L.; Sun, S.; Liang, Y. 3D Bioprinting of Hydrogels for Cartilage Tissue Engineering. Gels 2021, 7, 144. https://doi.org/10.3390/gels7030144
Huang J, Xiong J, Wang D, Zhang J, Yang L, Sun S, Liang Y. 3D Bioprinting of Hydrogels for Cartilage Tissue Engineering. Gels. 2021; 7(3):144. https://doi.org/10.3390/gels7030144
Chicago/Turabian StyleHuang, Jianghong, Jianyi Xiong, Daping Wang, Jun Zhang, Lei Yang, Shuqing Sun, and Yujie Liang. 2021. "3D Bioprinting of Hydrogels for Cartilage Tissue Engineering" Gels 7, no. 3: 144. https://doi.org/10.3390/gels7030144
APA StyleHuang, J., Xiong, J., Wang, D., Zhang, J., Yang, L., Sun, S., & Liang, Y. (2021). 3D Bioprinting of Hydrogels for Cartilage Tissue Engineering. Gels, 7(3), 144. https://doi.org/10.3390/gels7030144