Fractal Structure in Silica and Composites Aerogels
Abstract
:1. Introduction
2. Literature Results Synthesis
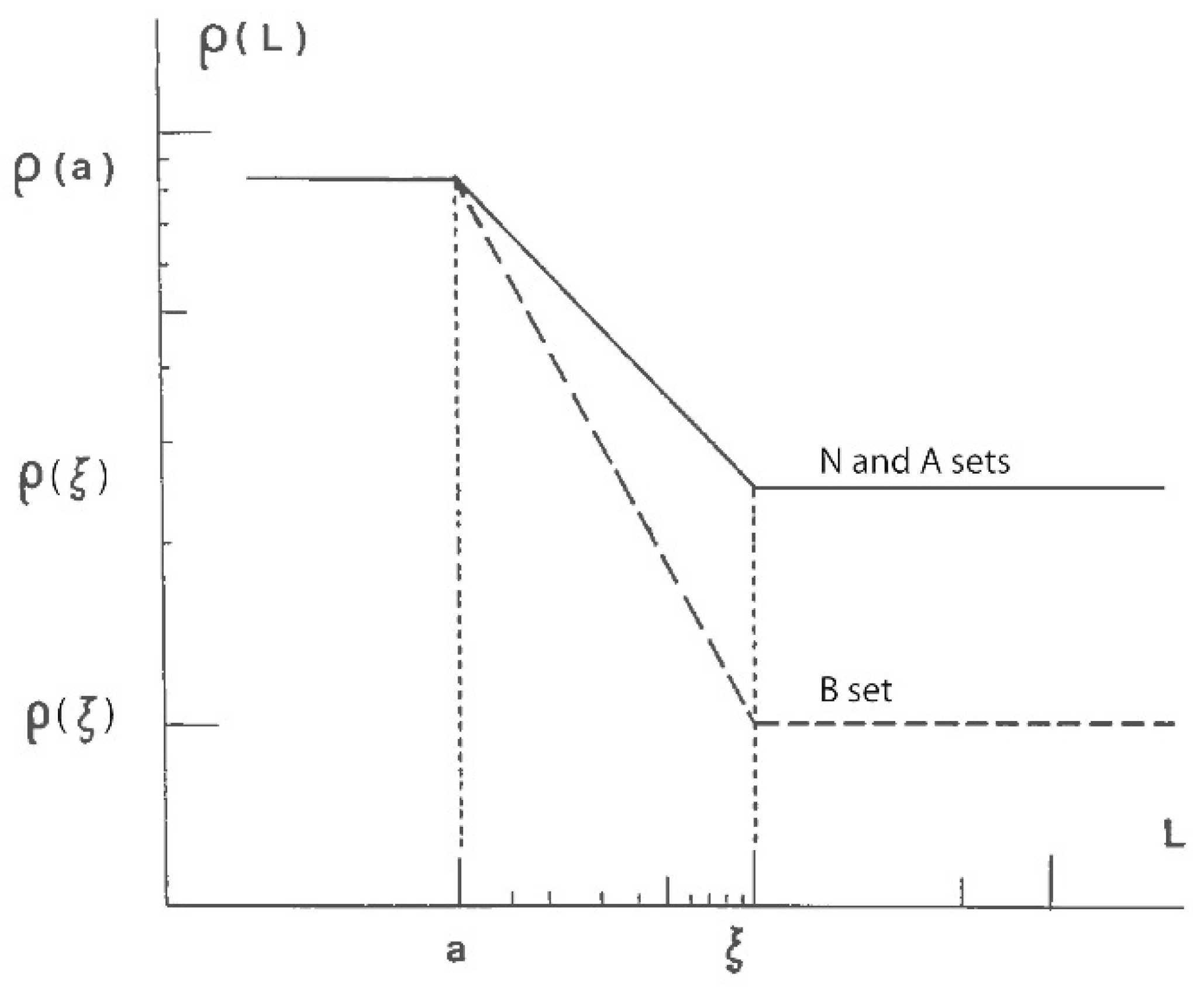
2.1. Fractal Geometry as Obtained by SANS, SAXS and USAXS Measurements
2.2. Aerogels: Fractal Structure Materials?
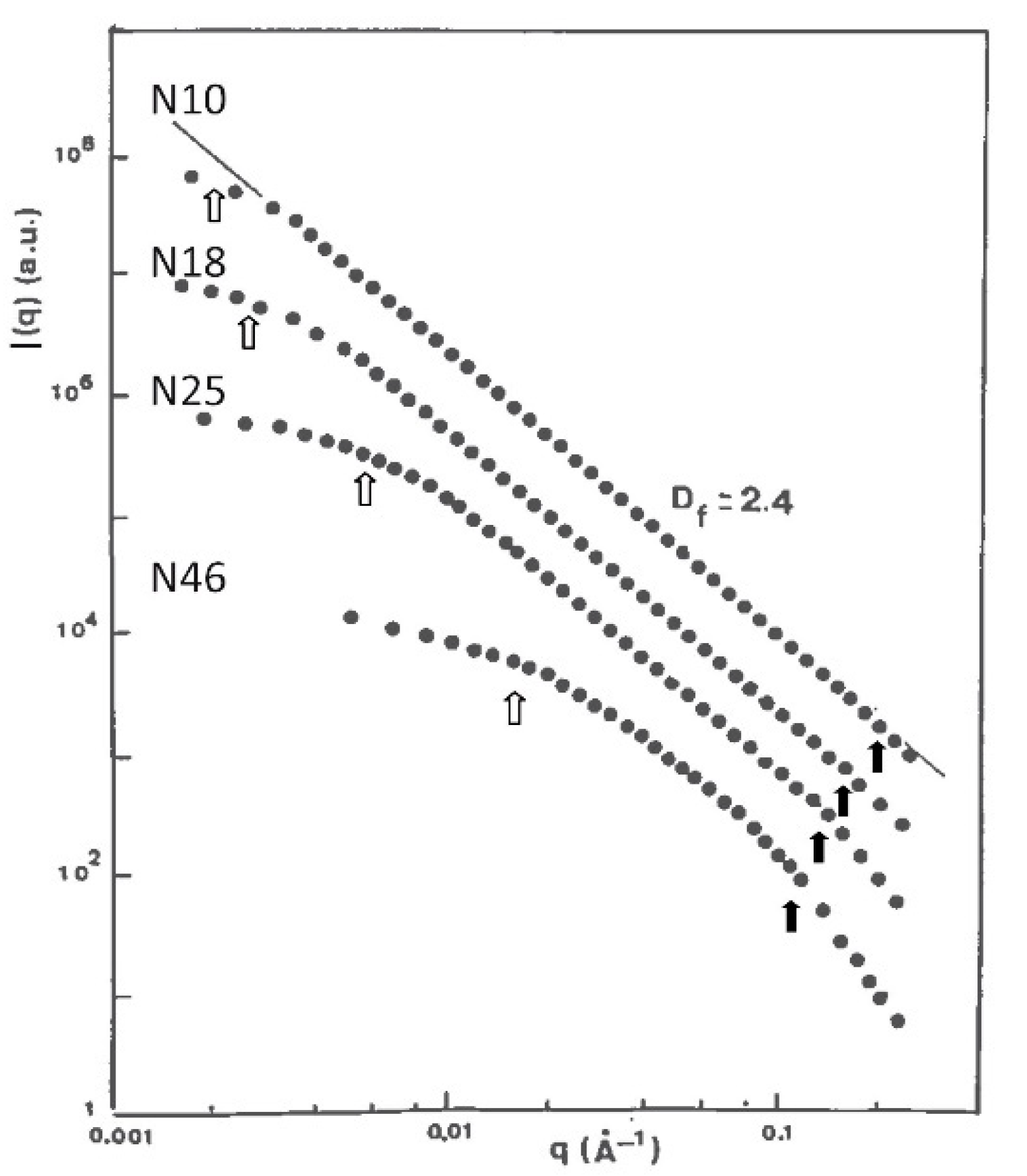
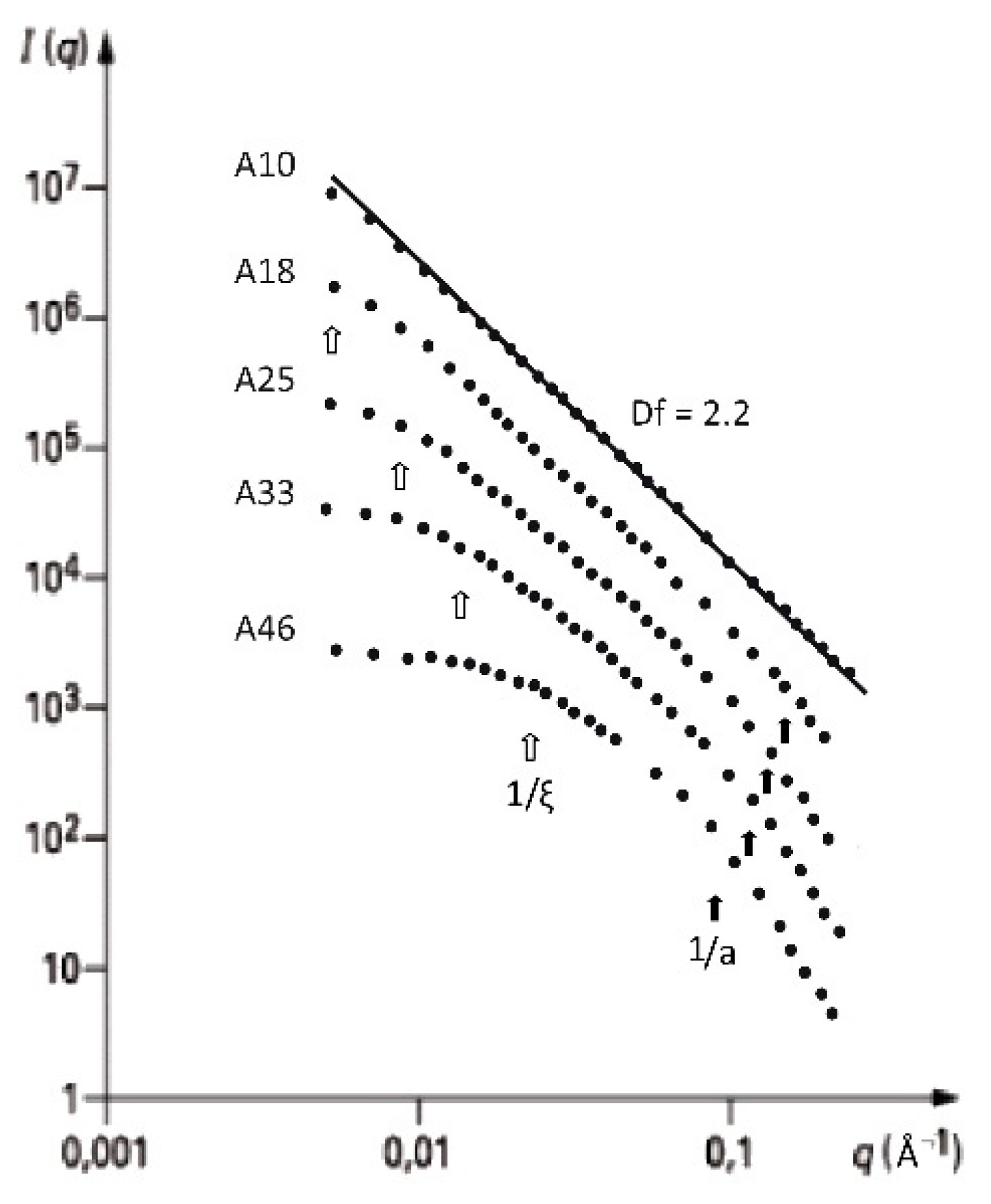
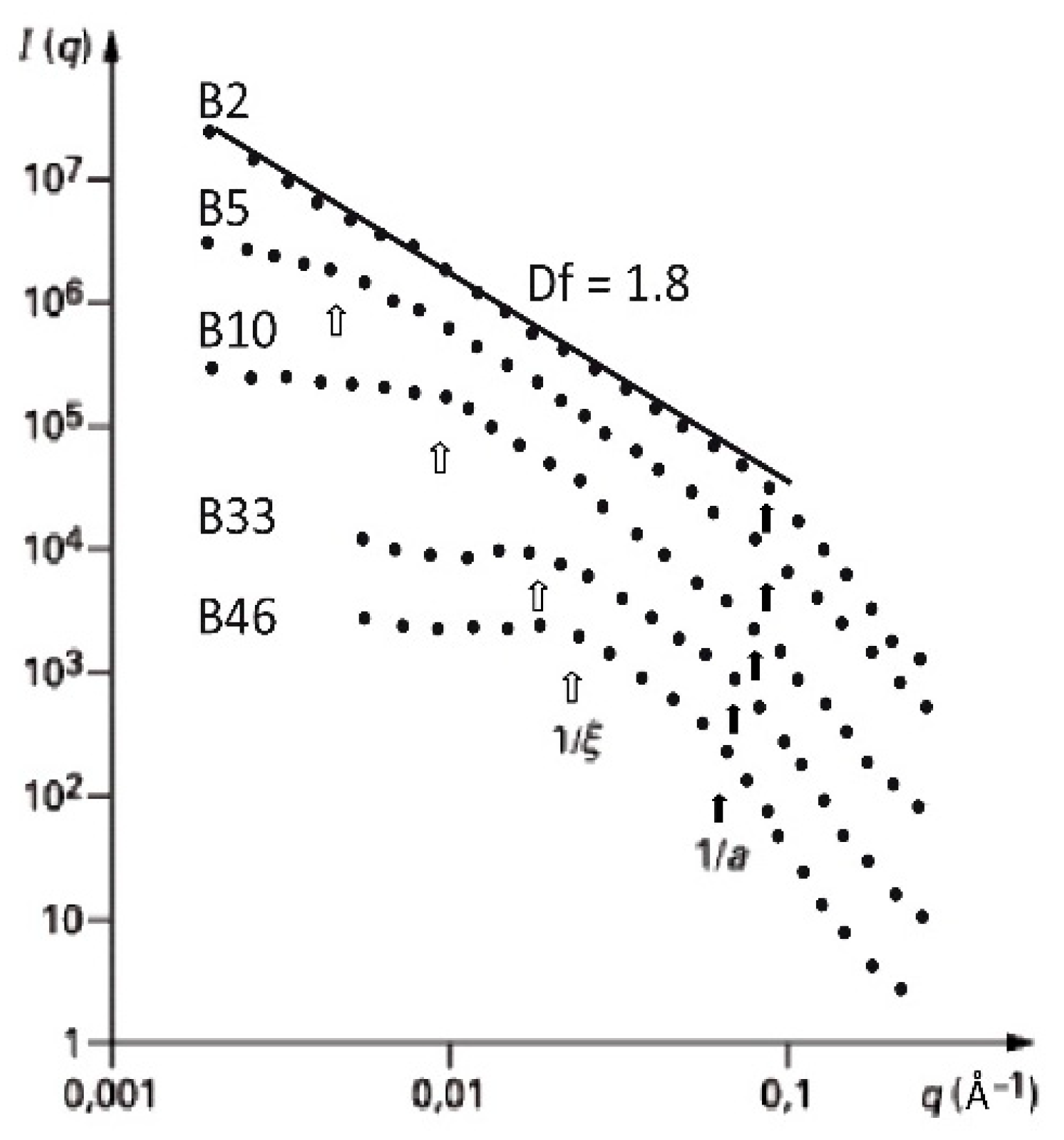
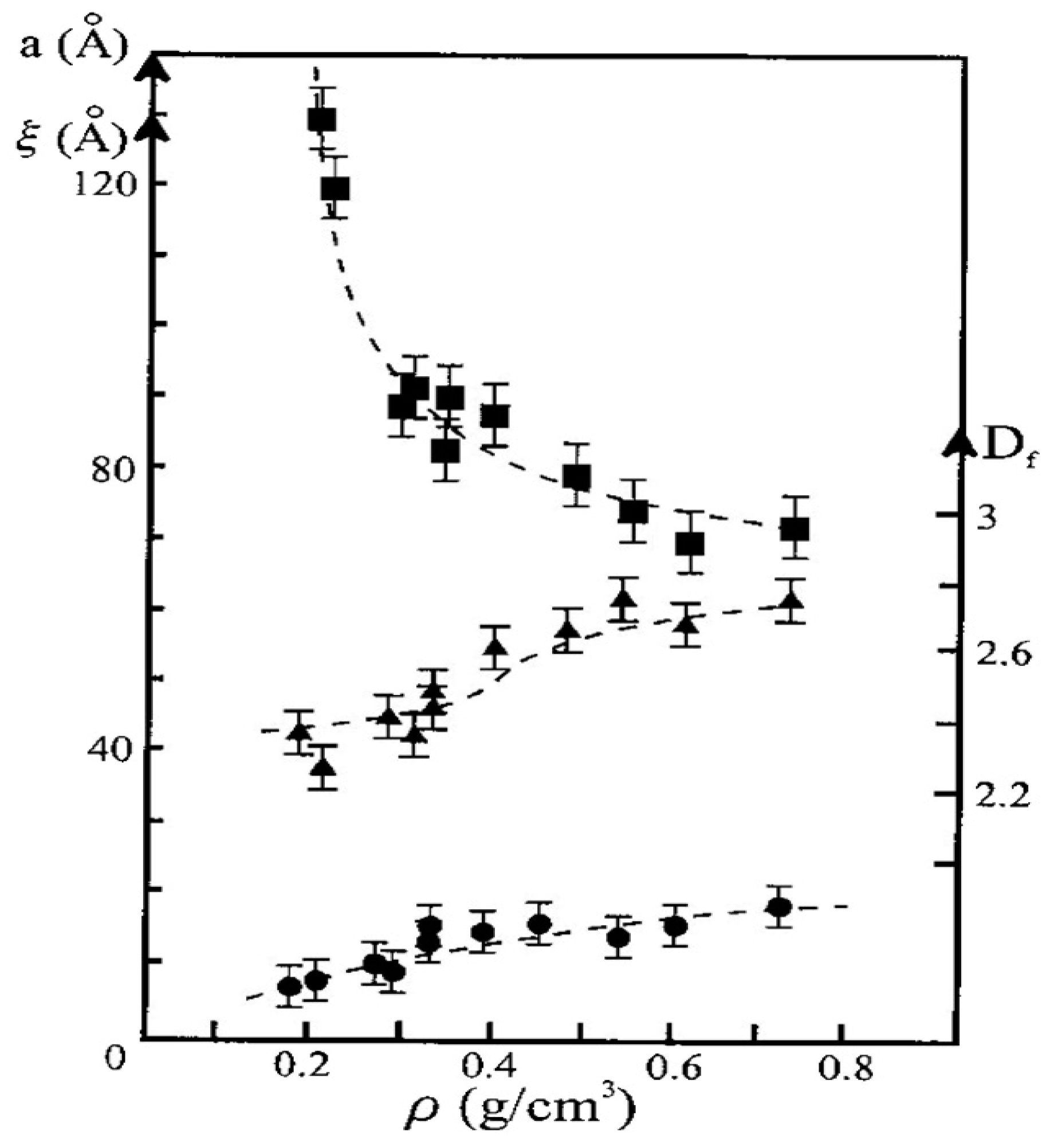
2.3. Influence of the Alkoxide Content and pH on Fractal Features
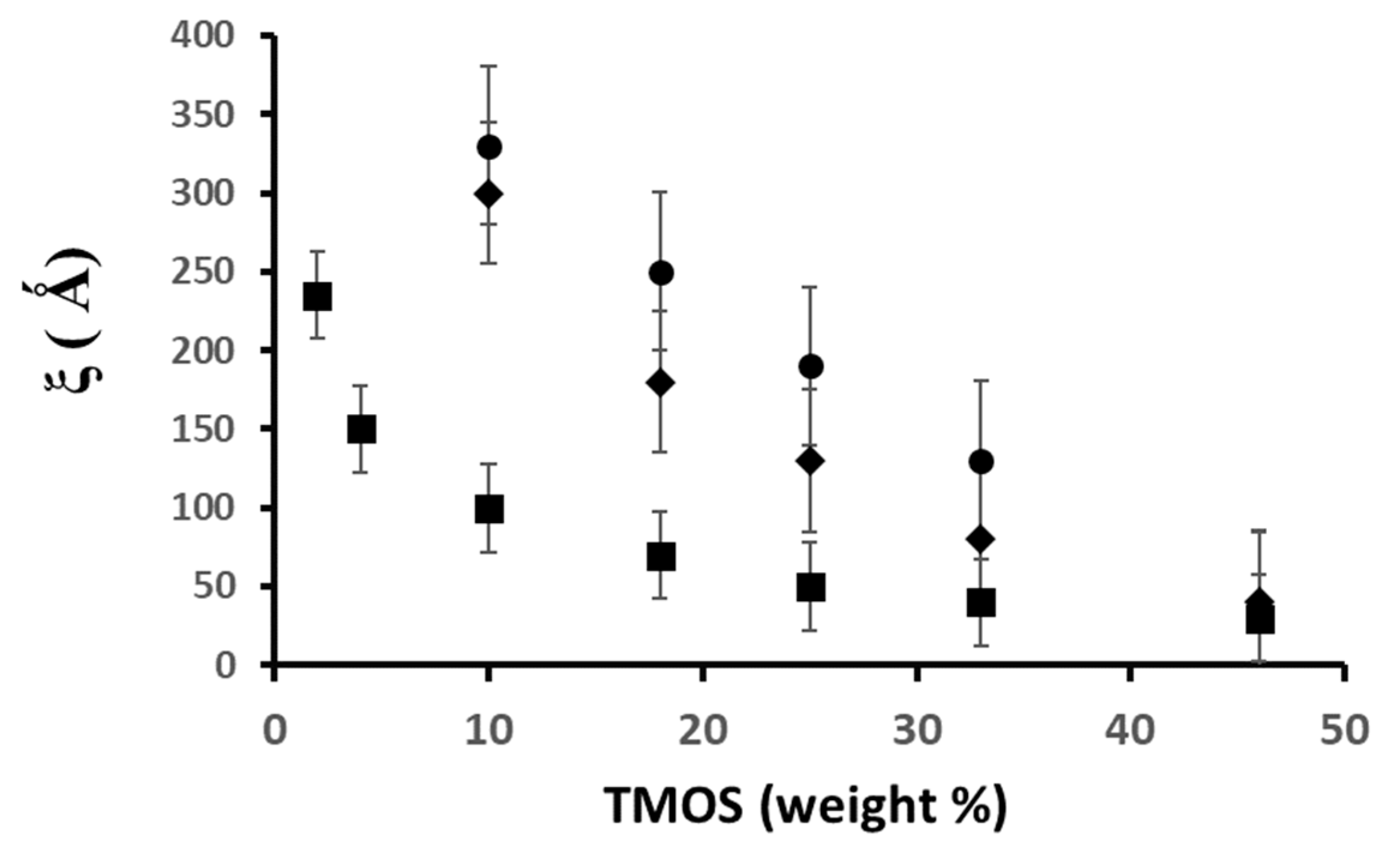
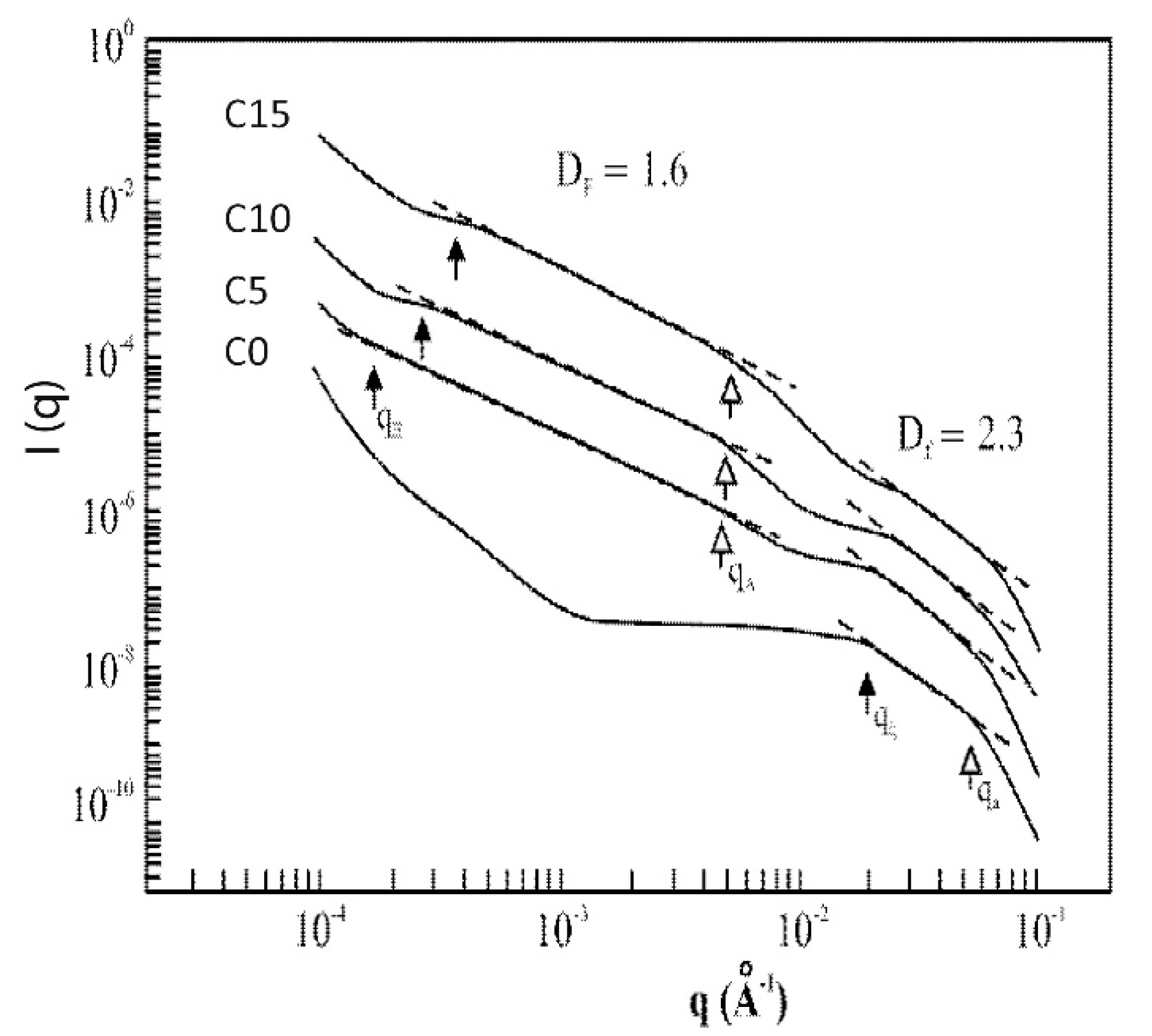
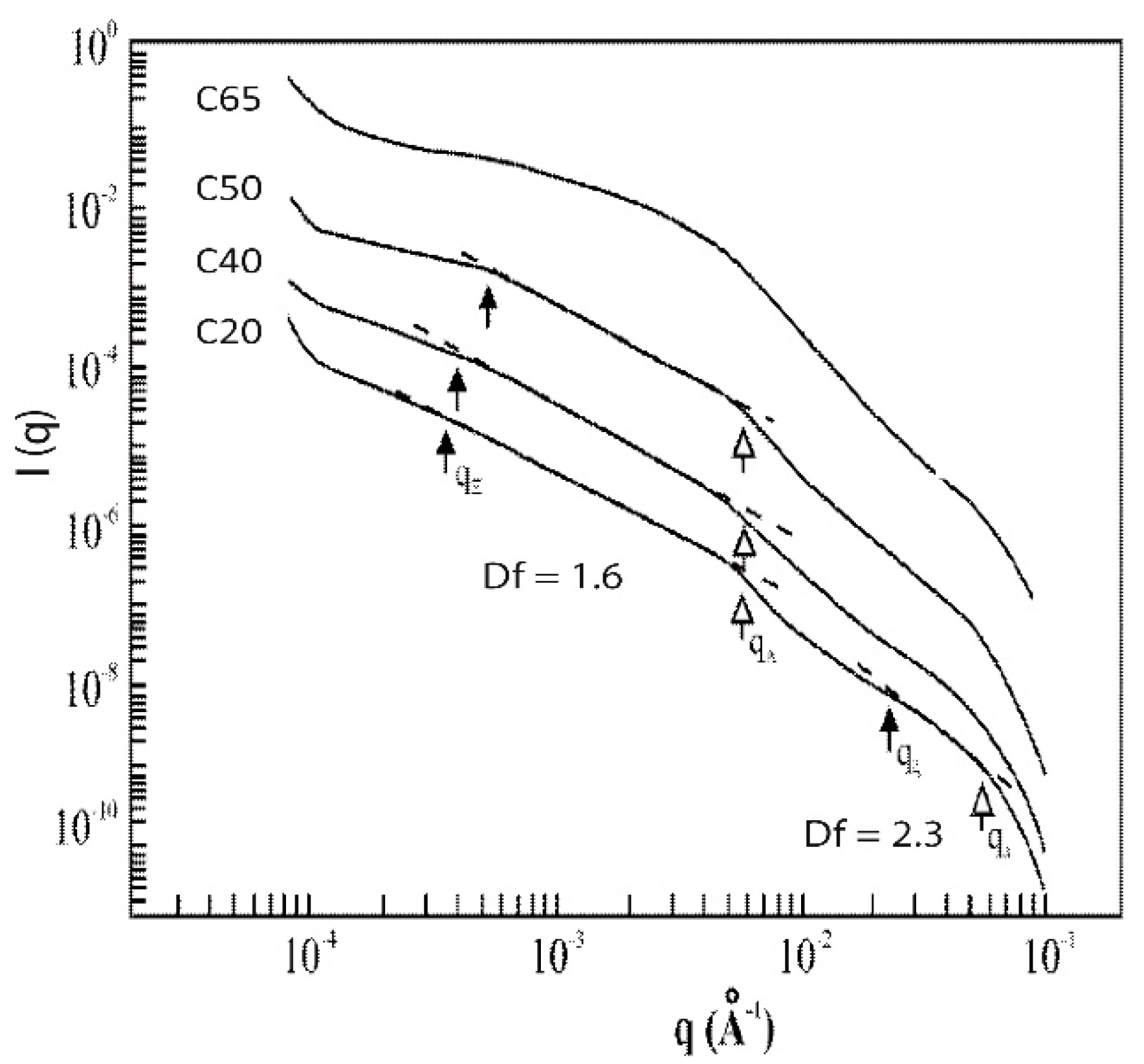
2.4. Influence of the Addition of Silica Particles on the Fractal Features
2.5. Influence of the Aerogel Sintering on Fractal Features
2.6. Influence of the Compaction Process on Fractal Features
3. Discussion
4. Conclusions
Funding
Conflicts of Interest
References
- Gross, J.; Fricke, J. Ultrasonic velocity measurements in silica, carbon and organic aerogels. J. Non Cryst. Solids 1992, 145, 217–222. [Google Scholar] [CrossRef]
- Nicolaon, G.A.; Teichner, S.J. New preparation process for silica xerogels and aerogels and their textural properties. Bull. Soc. Chim. 1968, 5, 1900–1906. [Google Scholar]
- Koebel, M.M.; Rigacci, A.; Achard, P. Aerogel-based thermal superinsulation: An overview. J. Sol-Gel Sci. Technol. 2012, 63, 315–339. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, D.W.; Keefer, K.D. Structure of Random Porous Materials: Silica Aerogel. Phys. Rev. Lett. 1986, 56, 2199–2202. [Google Scholar] [CrossRef] [PubMed]
- Brinker, J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press Inc.: San Diego, CA, USA, 1990. [Google Scholar]
- Woignier, T.; Phalippou, J.; Despetis, F.; Calas-Etienne, S. Aerogel Processing. In Handbook of Sol-Gel Science and Technology; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2016; pp. 1–27. [Google Scholar]
- Scherer, G.W. Sintering of Low Density Glasses: I. Theory. J. Am. Ceram. Soc. 1977, 60, 236–239. [Google Scholar] [CrossRef]
- Pirard, R.; Blacher, S.; Brouers, F.; Pirard, J.-P. Interpretation of mercury porosimetry applied to aerogels. J. Mater. Res. 1995, 10, 2114–2119. [Google Scholar] [CrossRef]
- Jones, S.M.; Sakamoto, J. Applications of Aerogels in Space Exploration. In Aerogels Handbook; Aegerter, M.A., Leventis, N., Koebel, M.M., Eds.; Springer: New York, NY, USA, 2011; pp. 721–746. [Google Scholar]
- Maleki, H. Recent advances in aerogels for environmental remediation applications: A review. Chem. Eng. J. 2016, 300, 98–118. [Google Scholar] [CrossRef]
- Yin, W.; Rubenstein, D.A. Biomedical Applications of Aerogels. In Aerogels Handbook; Aegerter, M.A., Leventis, N., Koebel, M.M., Eds.; Springer: New York, NY, USA, 2011; pp. 681–694. [Google Scholar]
- De Marco, I.; Miranda, S.; Riemma, S.; Iannone, R. LCA of starch aerogels for biomedical applications. Chem. Eng. Trans. 2016, 49, 319–324. [Google Scholar]
- Woignier, T.; Prassas, M.; Duffours, L. Sintering of aerogels for glass synthesis. J. Sol Gel Sci. Technol. 2018, 90, 76–86. [Google Scholar] [CrossRef] [Green Version]
- Phalippou, J.; Dieudonné, P.; Faivre, A.; Woignier, T. Aerogel Sintering: From Optical Glasses to Nuclear Waste Containment; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2016; pp. 1–21. [Google Scholar]
- Reynes, J.; Woignier, T.; Phalippou, J. Permeability measurement in composite aerogels: Application to nuclear waste storage. J. Non Cryst. Solids 2001, 285, 323–327. [Google Scholar] [CrossRef]
- Tsou, P. Silica aerogel captures cosmic dust intact. J. Non-Cryst. Solids 1995, 186, 415–427. [Google Scholar] [CrossRef]
- Forest, L.; Gibiat, V.; Hooley, A. Impedance matching and acoustic absorption in granular layers of silica aerogels. J. Non Cryst. Solids 2001, 285, 230–235. [Google Scholar] [CrossRef]
- Gross, J.; Reichenauer, G.; Fricke, J. Mechanical properties of SiO2aerogels. J. Phys. D Appl. Phys. 1988, 21, 1447–1451. [Google Scholar] [CrossRef]
- Lemay, J.; Tillotson, T.; Hrubesh, L.; Pekala, R. Microstructural Dependence 0f Aerogel Mechanical Properties. MRS Proc. 1990, 180, 321–328. [Google Scholar] [CrossRef]
- Woignier, T.; Primera, J.; Alaoui, A.; Despetis, F.; Calas-Etienne, S.; Faivre, A.; Duffours, L.; Levelut, C.; Etienne, P. Techniques for characterizing the mechanical properties of aerogels. J. Sol-Gel Sci. Technol. 2019, 93, 6–27. [Google Scholar] [CrossRef]
- Calemczuk, R.; De Goer, A.M.; Salce, B.; Maynard, R.; Zarembowitch, A. Low-Temperature Properties of Silica Aerogels. EPL Europhys. Lett. 1987, 3, 1205–1211. [Google Scholar] [CrossRef]
- Nagahara, H.; Suginouchi, T.; Hashimoto, M. P1M-8 Acoustic Properties of Nanofoam and its Applied Air-Borne Ultrasonic Transducers. In Proceedings of the 2007 IEEE Ultrasonics Symposium Proceedings, Institute of Electrical and Electronics Engineers (IEEE). Vancouver, BC, Canada, 2–6 October 2006; Volume 3, pp. 1541–1544. [Google Scholar]
- Gao, T.; Gustavsen, A.; Jelle, B.P.; He, J. Synthesis and Characterization of Aerogel Glass Materials for Window Glazing Applications. In Ceramic Engineering and Science Proceedings; Wiley: Hoboken, NJ, USA, 2015; pp. 141–150. [Google Scholar]
- Fricke, J. Aerogels and their applications. J. Non Cryst. Solids 1992, 147–148, 356–362. [Google Scholar] [CrossRef]
- Leventis, N. Three-Dimensional Core-Shell Superstructures: Mechanically Strong Aerogels. Chemin 2007, 38, 874–884. [Google Scholar] [CrossRef]
- Tillotson, T.; Hrubesh, L. Transparent ultralow-density silica aerogels prepared by a two-step sol-gel process. J. Non Cryst. Solids 1992, 145, 44–50. [Google Scholar] [CrossRef] [Green Version]
- Woignier, T.; Añez, L.; Calas-Etienne, S.; Primera, J. Gas slippage in fractal porous material. J. Nat. Gas Sci. Eng. 2018, 57, 11–20. [Google Scholar] [CrossRef]
- Añez, L.; Calas-Etienne, S.; Primera, J.; Woignier, T. Gas and liquid permeability in nano composites gels: Comparison of Knudsen and Klinkenberg correction factors. Microporous Mesoporous Mater. 2014, 200, 79–85. [Google Scholar] [CrossRef]
- Guild, M.D.; García-Chocano, V.M.; Sánchez-Dehesa, J.; Martin, T.P.; Calvo, D.C.; Orris, G.J. On the use of aerogel as a soft acoustic metamaterial for airborne sound. Phys. Rev. Appl. 2016, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, D.W.; Richter, D.; Farago, B.; Frick, B.; Brinker, C.J. Dynamics of weakly connected solids: Silica aerogels. Phys. Rev. Lett. 1990, 64, 2316–2319. [Google Scholar] [CrossRef] [PubMed]
- Marlière, C.; Despetis, F.; Etienne, P.; Woignier, T. Very large-scale stucture in sintered silica aerogels as evidenced by AFM and USAXS experiments. J. Non Cryst. Solids 2001, 285, 148–153. [Google Scholar] [CrossRef]
- Woignier, T.; Phalippou, J.; Vacher, R.; Pelous, J.; Courtens, E. Different kinds of fractal structures in silica aerogels. J. Non Cryst. Solids 1990, 121, 198–201. [Google Scholar] [CrossRef]
- Craievich, A.F.; Aegerter, M.; Dos Santos, D.; Woignier, T.; Zarzycki, J. A SAXS study of silica aerogels. J. Non-Crystalline Solids 1986, 86, 394–406. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, D.W.; Olivier, B.J.; Ashley, C.S.; Richter, D.; Farago, B.; Frick, B.; Hrubesh, L.; van Bommel, M.J.; Long, S.; Krueger, S. Structure and topology of silica aerogels. J. Non Cryst. Solids 1992, 145, 105–112. [Google Scholar] [CrossRef]
- Wang, J.; Shen, J.; Zhou, B.; Wu, X. SAXS investigation of silica aerogels derived from teos. Nanostruct. Mater. 1996, 7, 699–708. [Google Scholar] [CrossRef]
- Vacher, R.; Woignier, T.; Pelous, J.; Courtens, E. Structure and self-similarity of silica aerogels. Phys. Rev. B 1988, 37, 6500–6503. [Google Scholar] [CrossRef]
- Foret, M.; Pelous, J.; Vacher, R.; Marignan, J. SANS and SAXS investigations of silica aerogels: Crossover from fractal structure to short-range packing. J. Non Cryst. Solids 1992, 145, 133–135. [Google Scholar] [CrossRef]
- Emmerling, A.; Fricke, J. Small angle scattering and the structure of aerogels. J. Non Cryst. Solids 1992, 145, 113–120. [Google Scholar] [CrossRef]
- Dietler, G.; Aubert, C.; Cannell, D.S.; Wiltzius, P. Gelation of Colloidal Silica. Phys. Rev. Lett. 1986, 57, 3117–3120. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.; Anderson, J.; Di Gregorio, J.; Smith, D.; Beaucage, G. Structural analysis of silica aerogels. J. Non Cryst. Solids 1995, 186, 142–148. [Google Scholar] [CrossRef]
- Vacher, R.; Woignier, T.; Phalippou, .; Pelous, J.; Courtens, E. Fractal structure of base catalyzed and densified silica aerogels. J. Non Cryst. Solids 1988, 106, 161–165. [Google Scholar] [CrossRef]
- Breßler, I.; Kohlbrecher, J.; Thünemann, A.F. SASfit: A tool for small-angle scattering data analysis using a library of analytical expressions. J. Appl. Crystallogr. 2015, 48, 1587–1598. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, J. Experimental Methods for Studying Fractal Aggregates. In On Growth and Form; NATO ASI Series (Series E: Applied Sciences); Stanley, H.E., Ostrowsky, N., Eds.; Springer: Dordrecht, The Netherlands, 1917; Volume 100. [Google Scholar] [CrossRef]
- Freltof, T.; Kjems, K.; Sinha, S.K. Power-law correlations and finite-size effects in silica particle aggregates studied by small-angle neutron scattering. Phys. Rev. B 1986, 33, 269–275. [Google Scholar] [CrossRef]
- Meakin, P. Formation of fractal clusters and networks by irreversible diffusion-limited aggregation. Phys. Rev. Lett. 1983, 51, 1116–1122. [Google Scholar] [CrossRef]
- Halsey, T.C.; Honda, K.; Duplantier, B. Multifractal dimensions for branched growth. J. Stat. Phys. 1996, 85, 681–743. [Google Scholar] [CrossRef] [Green Version]
- Toki, M.; Miyashita, S.; Takeuchi, T.; Kanbe, S.; Kochi, A. A large-size silica glass produced by a new sol-gel process. J. Non Cryst. Solids 1988, 100, 479–482. [Google Scholar] [CrossRef]
- Marlière, C.; Woignier, T.; Dieudonné, P.; Primera, J.; Lamy, M.; Phalippou, J. Two fractal structures in aerogel. J. Non Cryst. Solids 2001, 285, 175–180. [Google Scholar] [CrossRef]
- Woignier, T.; Duffours, L. Densification and Strengthening of Aerogels by Sintering Heat Treatments or Plastic Compression. Gels 2018, 4, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woignier, T.; Alaoui, A.; Primera, J.; Scherer, G.W. Structural effect on the plastic behavior in highly porous glasses. Key Eng. Mater. 2010, 423, 15–24. [Google Scholar] [CrossRef]
- Scherer, G.W.; Smith, D.M.; Qiu, X.; Anderson, J.M. Compression of aerogels. J. Non-Cryst. Solids 1995, 186, 316–320. [Google Scholar] [CrossRef]
- Woignier, T.; Phalippou, J. Skeletal density of silica aerogels. J. Non Cryst. Solids 1987, 93, 17–21. [Google Scholar] [CrossRef]
- Woignier, T.; Reynes, J.; Hafidi Alaoui, A.; Beurroies, I.; Phalippou, J. Different kinds of fractal structures in aerogels: Relationships with the mechanical properties. J. Non Cryst. Solids 1998, 241, 45–52. [Google Scholar] [CrossRef]
- Woignier, T.; Despetis, F.; Etienne, P.; Alaoui, A.; Duffours, L.; Phalippou, J. Evolution of the Mechanical Properties During the Gel–Glass Process. In Handbook of Sol-Gel Science and Technology; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2016; pp. 1–25. [Google Scholar]
- Woignier, T.; Primera, J.; Alaoui, A.; Etienne, P.; Despestis, F.; Calas-Etienne, S. Mechanical Properties and Brittle Behavior of Silica Aerogels. Gels 2015, 1, 256–275. [Google Scholar] [CrossRef] [Green Version]
- Aegerter, M.A.; Leventis, N.; Koebel, M.M. Aerogels Handbook; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Gui, J.-Y.; Zhou, B.; Zhong, Y.-H.; Du, A.; Shen, J. Fabrication of gradient density SiO2 aerogel. J. Sol Gel Sci. Technol. 2011, 58, 470–475. [Google Scholar] [CrossRef]
- Amaral-Labat, G.; Szczurek, A.; Fierro, V.; Masson, E.; Pizzi, A.; Celzard, A. Impact of depressurizing rate on the porosity of aerogels. Microporous Mesoporous Mater. 2012, 152, 240–245. [Google Scholar] [CrossRef]
- Esquivias, L.; Piñero, M.; Morales-Flórez, V.; De La Rosa-Fox, N. Aerogels Synthesis by Sonocatalysis: Sonogels. In Aerogels Handbook; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2011; pp. 419–445. [Google Scholar]
- Perdigoto, M.; Martins, R.; Rocha, N.; Quina, M.; Gando-Ferreira, L.; Patrício, R. Application of hydrophobic silica based aerogels and xerogels for removal of toxic organic compounds from aqueous solutions. J Colloid Interface Sci. 2012, 380, 134–140. [Google Scholar] [CrossRef]
- Alatalo, S.-M.; Pileidis, F.; Mäkilä, E.; Sevilla, M.; Repo, E.; Salonen, J.; Sillanpää, M.; Titirici, M.-M. Versatile Cellulose-Based Carbon Aerogel for the Removal of Both Cationic and Anionic Metal Contaminants from Water. ACS Appl. Mater. Interfaces 2015, 7, 25875–25883. [Google Scholar] [CrossRef]
- Karatum, O.; Steiner III, S.A.; Griffin, J.S.; Shi, W.; Plata, D.L. Flexible, mechanically durable aerogel composites for oil capture and recovery. ACS Appl Mater Interfaces 2015, 8, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Leventis, N.; Chidambareswarapattar, C.; Bang, A.; Sotiriou-Leventis, C. Cocoon-in-Web-Like Superhydrophobic Aerogels from Hydrophilic Polyurea and Use in Environmental Remediation. ACS Appl. Mater. Interfaces 2014, 6, 6872–6882. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, J.P.; Vadodariya, N.; Nataraj, S.K.; Meena, R. Chitosan-Based Aerogel Membrane for Robust Oil-in-Water Emulsion Separation. ACS Appl. Mater. Interfaces 2015, 7, 24957–24962. [Google Scholar] [CrossRef] [PubMed]
- Matias, T.; Marques, J.; Quina, M.J.; Gando-Ferreira, L.; Valente, A.J.; Portugal, A.; Durães, L. Silica-based aerogels as ad-sorbents for phenol-derivative compounds. Colloids Surf. Physicochem. Eng Asp. 2015, 480, 260–269. [Google Scholar] [CrossRef] [Green Version]
- Santos, A.; Ajbary, M.; Kherbeche, A.; Piñero, M.; De La Rosa-Fox, N.; Esquivias, L.; Santos, A.; Rosa-Fox, N. Fast CO2 sequestration by aerogel composites. J. Sol Gel Sci. Technol. 2007, 45, 291–297. [Google Scholar] [CrossRef]
- Morales-Flórez, V.; Santos, A.; Esquivias, L.; Santos, A. Recent insights into xerogel and aerogel mineral composites for CO2 mineral sequestration. J. Sol-Gel Sci. Technol. 2010, 59, 417–423. [Google Scholar] [CrossRef] [Green Version]
- Jeon, D.-H.; Min, B.-G.; Oh, J.G.; Nah, C.; Park, S.-J. Influence of Nitrogen moieties on CO2capture of Carbon Aerogel. Carbon Lett. 2015, 16, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Alhwaige, A.A.; Ishida, H.; Qutubuddin, S. Carbon Aerogels with Excellent CO2 Adsorption Capacity Synthesized from Clay-Reinforced Biobased Chitosan-Polybenzoxazine Nanocomposites. ACS Sustain. Chem. Eng. 2016, 4, 1286–1295. [Google Scholar] [CrossRef]
- Begag, R.; Krutka, H.; Dong, W.; Mihalcik, D.; Rhine, W.; Gould, G.; Baldic, J.; Nahass, P. Superhydrophobic amine functionalized aerogels as sorbents for CO2capture. Greenh. Gases Sci. Technol. 2013, 3, 30–39. [Google Scholar] [CrossRef]
- Garrido, R.; Silvestre, J.D.; Flores-Colen, I. Economic and Energy Life Cycle Assessment of aerogel-based thermal renders. J. Clean. Prod. 2017, 151, 537–545. [Google Scholar] [CrossRef]
- De Guinoa, A.S.; Zambrana-Vasquez, D.; Alcalde, A.; Corradini, M.; Zabalza, I. Environmental assessment of a nano-technological aerogel-based panel for building insulation. J. Clean. Prod. 2017, 161, 1404–1415. [Google Scholar] [CrossRef] [Green Version]
- Pinto, I.; Silvestre, J.D.; De Brito, J.; Júlio, M.D.F. Environmental impact of the subcritical production of silica aerogels. J. Clean. Prod. 2020, 252, 119696. [Google Scholar] [CrossRef]
- Masera, G.; Wakili, K.G.; Stahl, T.; Brunner, S.; Galliano, R.; Monticelli, C.; Aliprandi, S.; Zanelli, A.; Elesawy, A. Development of a Super-insulating, Aerogel-based Textile Wallpaper for the Indoor Energy Retrofit of Existing Residential Buildings. Procedia Eng. 2017, 180, 1139–1149. [Google Scholar] [CrossRef] [Green Version]
- Karatum, O.; Bhuiya, M.H.; Carroll, M.K.; Anderson, A.M.; Plata, D.L. Life Cycle Assessment of Aerogel Manufacture on Small and Large Scales: Weighing the Use of Advanced Materials in Oil Spill Remediation. J. Ind. Ecol. 2018, 22, 1365–1377. [Google Scholar] [CrossRef]
- Bhuiya, M.H.; Anderson, A.M.; Carroll, M.K.; Bruno, B.A.; Ventrella, J.L.; Silberman, B.; Keramati, B. Preparation of Monolithic Silica Aerogel for Fenestration Applications: Scaling up, Reducing Cycle Time, and Improving Performance. Ind. Eng. Chem. Res. 2016, 55, 6971–6981. [Google Scholar] [CrossRef]


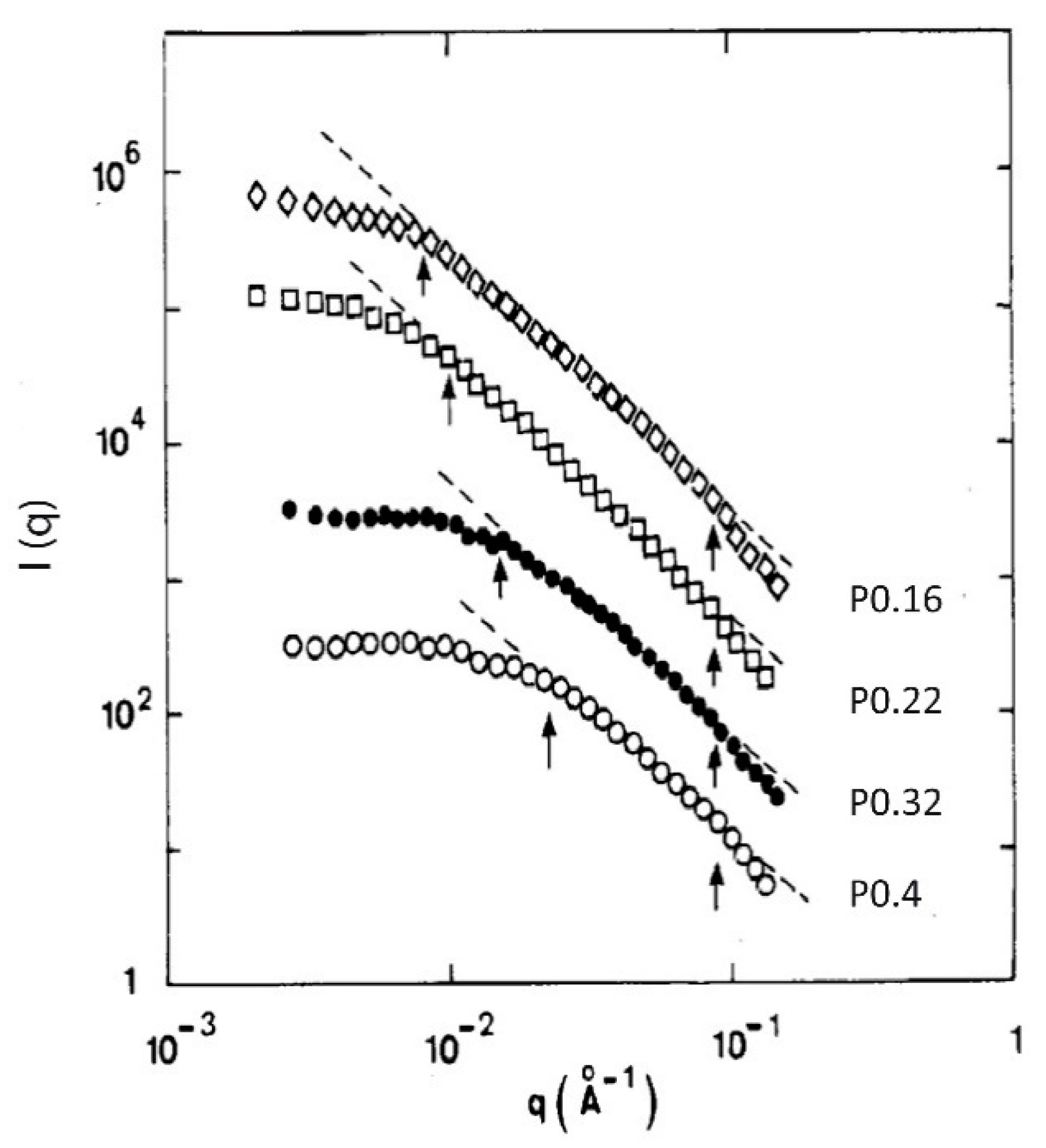
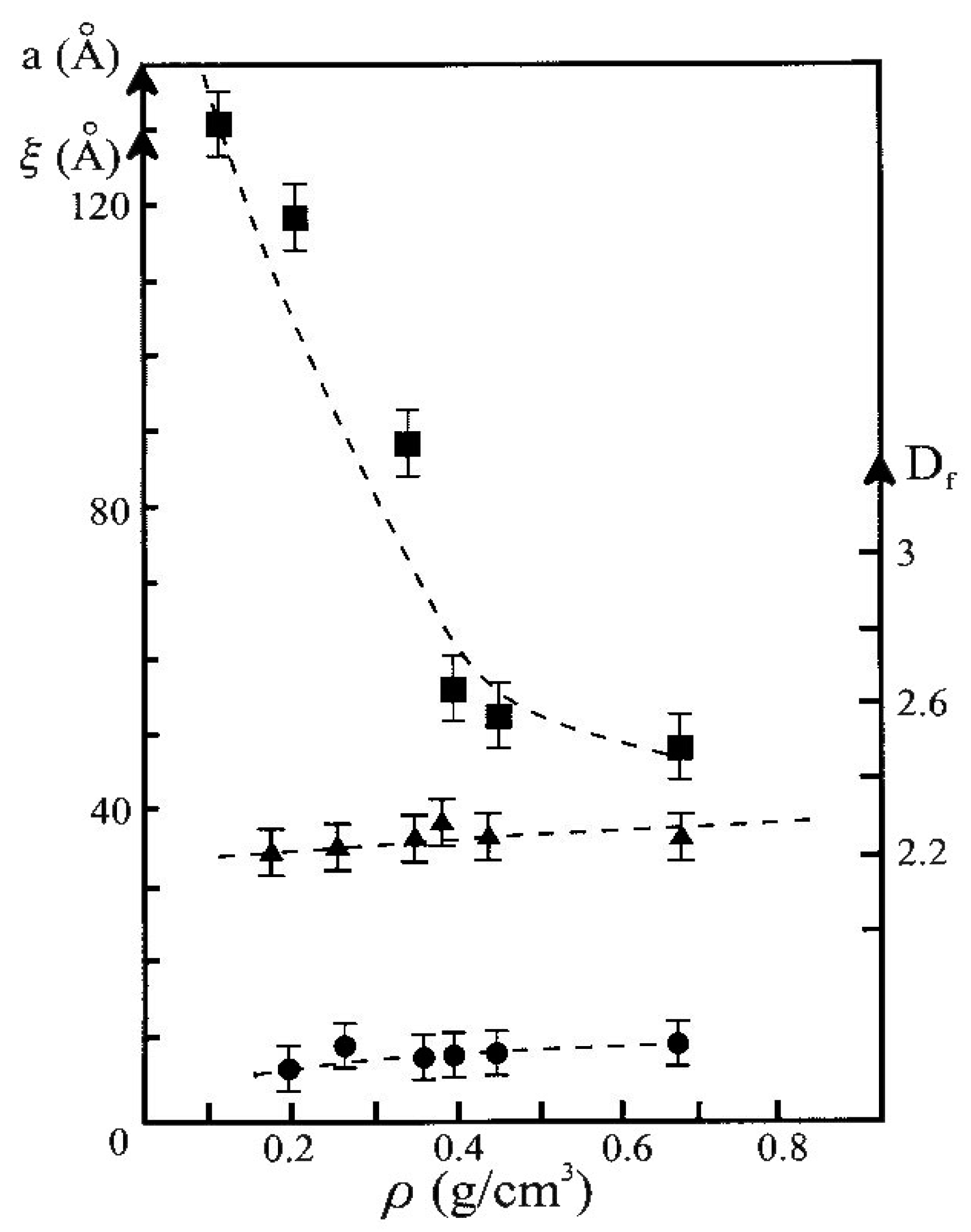
| TMOS (vol. %) | Bulk Density (g·cm−3) B Set | Bulk Density (g·cm−3) N Set | Bulk Density (g·cm−3) A Set |
|---|---|---|---|
| 46 | 0.22 ± 0.01 | 0.31 ± 0.01 | 0.42 ± 0.01 |
| 33 | 0.17 ± 0.01 | 0.22 ± 0.01 | 0.36 ± 0.01 |
| 25 | 0.12 ± 0.01 | 0.16 ± 0.01 | 0.3 ± 0.01 |
| 18 | 0.09 ± 0.01 | 0.12 ± 0.01 | 0.21 ± 0.01 |
| 10 | 0.05 ± 0.01 | 0.06 ± 0.01 | 0.11 ± 0.01 |
| 5 | 0.03 ± 0.01 | ||
| 2 | 0.02 ±0.01 |
| Synthesis Process | Df | ξ(Å) | a (Å) |
|---|---|---|---|
| Acid set | 2.4 ± 0.1 | 40–320 | ≈10 |
| Base set | 1.8 ± 0.1 | 40–250 | 15–20 |
| Neutral set | 2.2 ± 0.1 | 40–310 | ≈10 |
| Composite set | 2.3 and 1.6 ± 0.1 | 50–5000 | ≈10 and ≈100 |
| Sintered set | 2.3–2.7 ± 0.1 | 70–130 | ≈10–20 |
| Compressed set | 2.3 ± 0.1 | 50–130 | ≈10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woignier, T.; Primera, J.; Alaoui, A.; Dieudonne, P.; Duffours, L.; Beurroies, I.; Calas-Etienne, S.; Despestis, F.; Faivre, A.; Etienne, P. Fractal Structure in Silica and Composites Aerogels. Gels 2021, 7, 1. https://doi.org/10.3390/gels7010001
Woignier T, Primera J, Alaoui A, Dieudonne P, Duffours L, Beurroies I, Calas-Etienne S, Despestis F, Faivre A, Etienne P. Fractal Structure in Silica and Composites Aerogels. Gels. 2021; 7(1):1. https://doi.org/10.3390/gels7010001
Chicago/Turabian StyleWoignier, Thierry, Juan Primera, Adil Alaoui, Philippe Dieudonne, Laurent Duffours, Isabelle Beurroies, Sylvie Calas-Etienne, Florence Despestis, Annelise Faivre, and Pascal Etienne. 2021. "Fractal Structure in Silica and Composites Aerogels" Gels 7, no. 1: 1. https://doi.org/10.3390/gels7010001
APA StyleWoignier, T., Primera, J., Alaoui, A., Dieudonne, P., Duffours, L., Beurroies, I., Calas-Etienne, S., Despestis, F., Faivre, A., & Etienne, P. (2021). Fractal Structure in Silica and Composites Aerogels. Gels, 7(1), 1. https://doi.org/10.3390/gels7010001






