Microstructure of a Model Fresh Cheese and Bioaccessibility of Vitamin D3 Using In Vitro Digestion
Abstract
1. Introduction
2. Results and Discussion
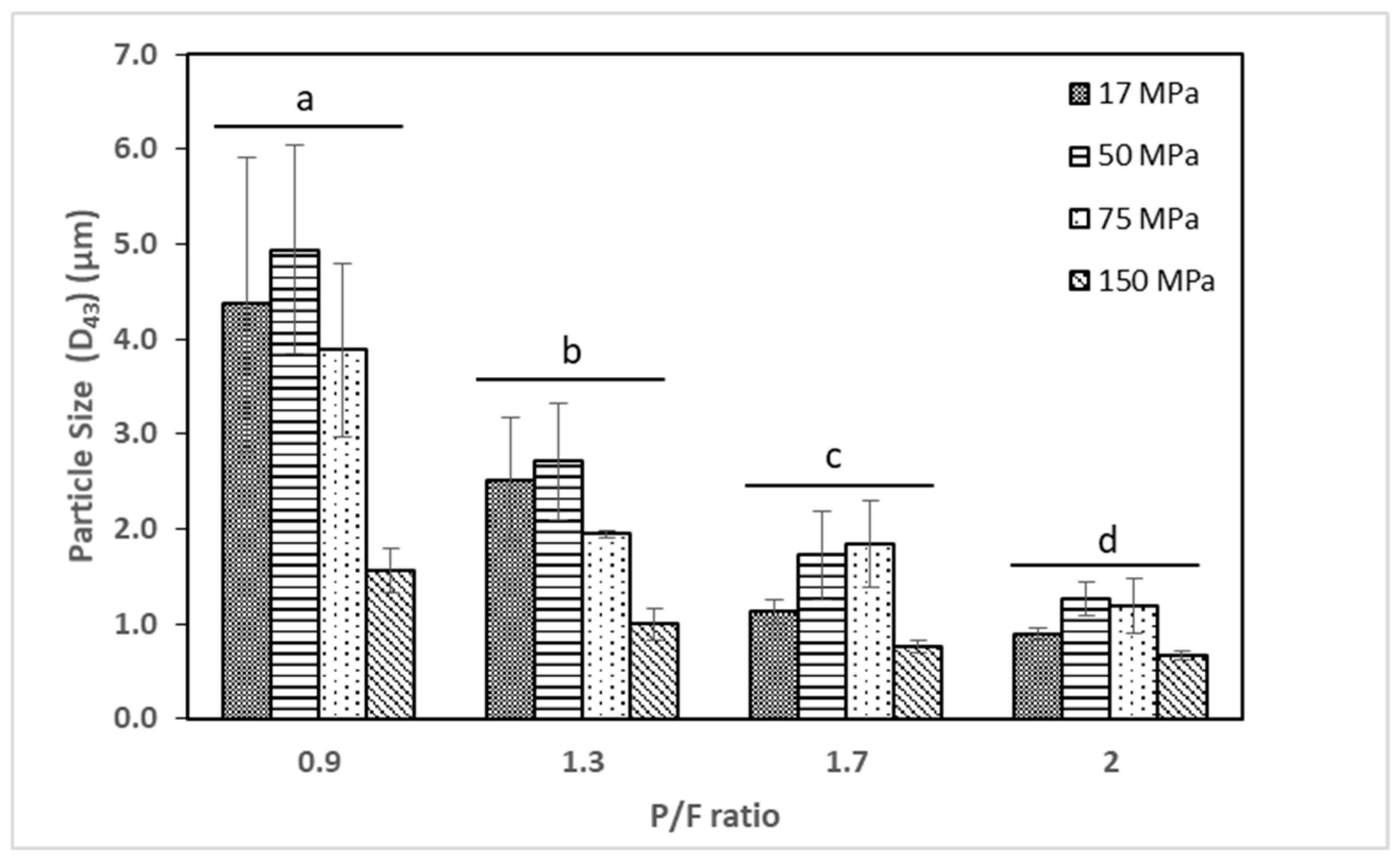
2.1. Particle Size
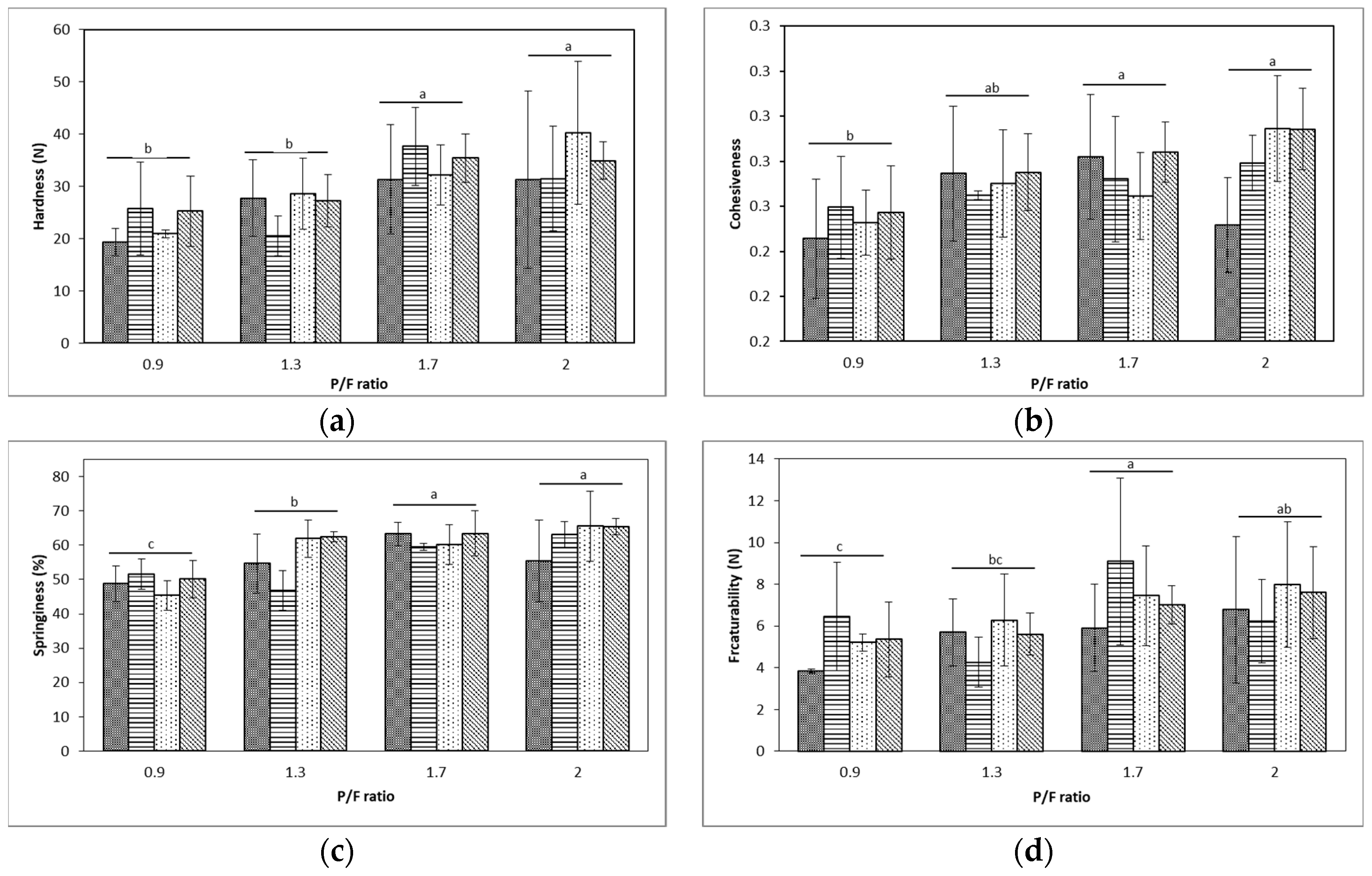
2.2. Texture Profile Analysis
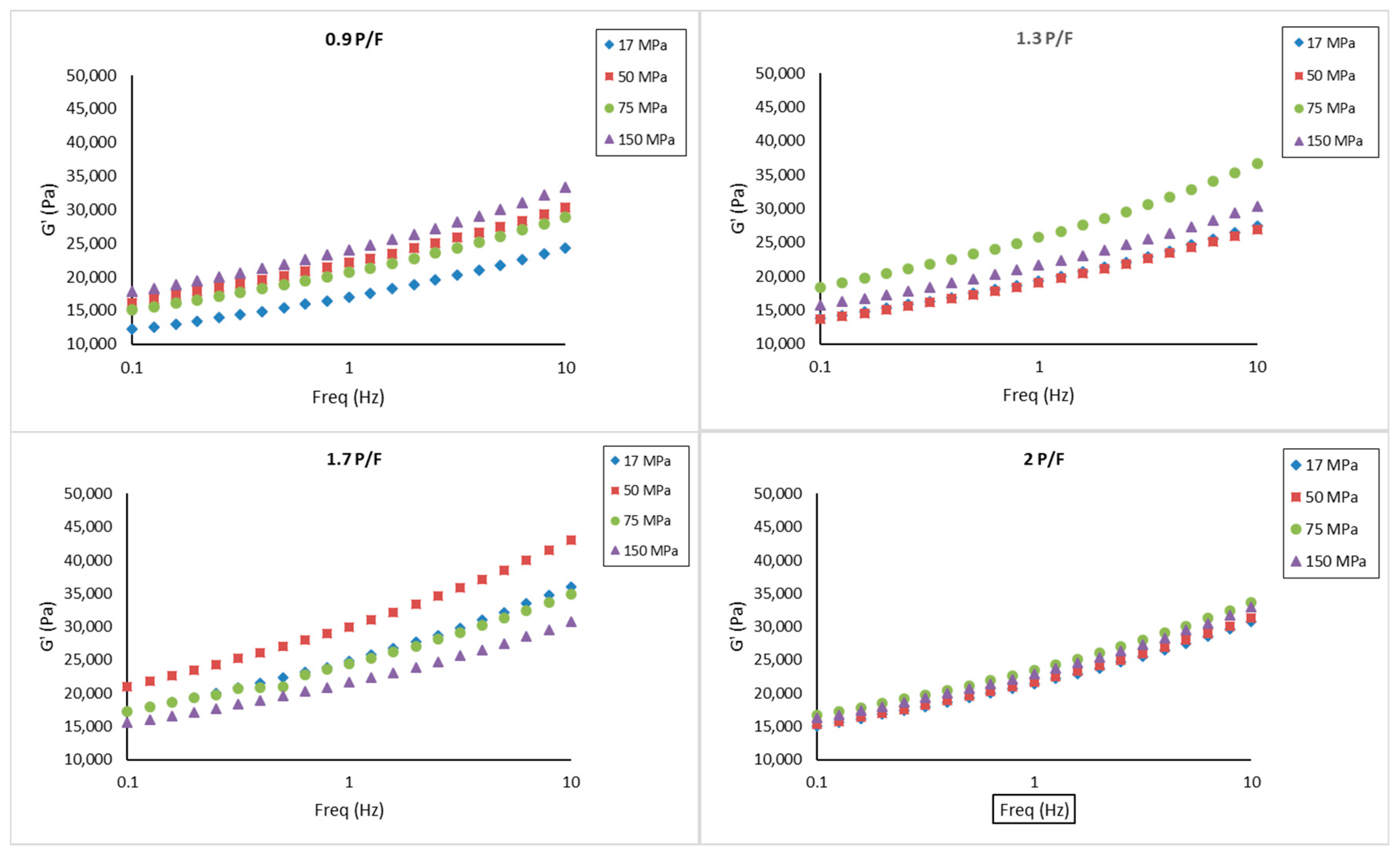
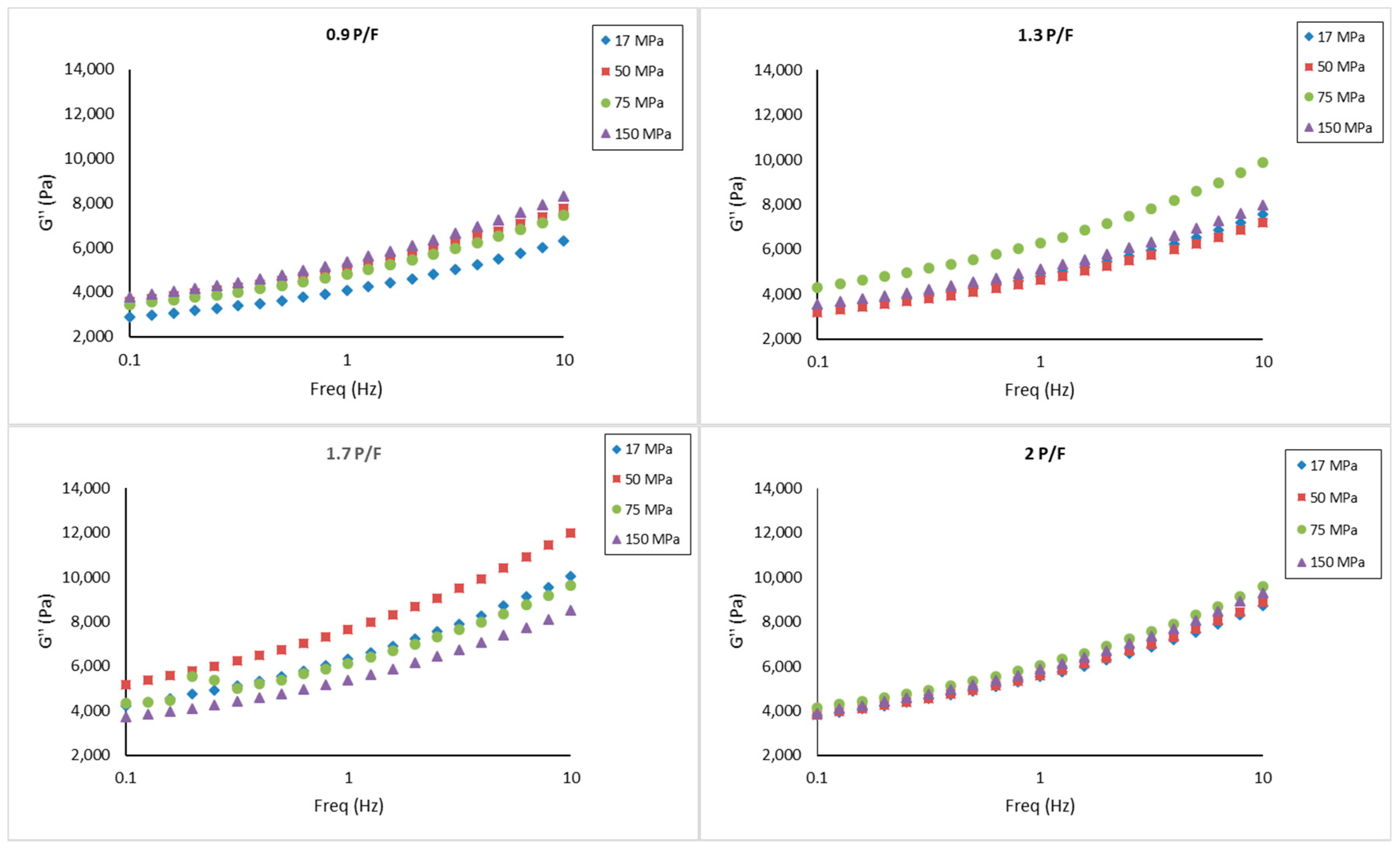
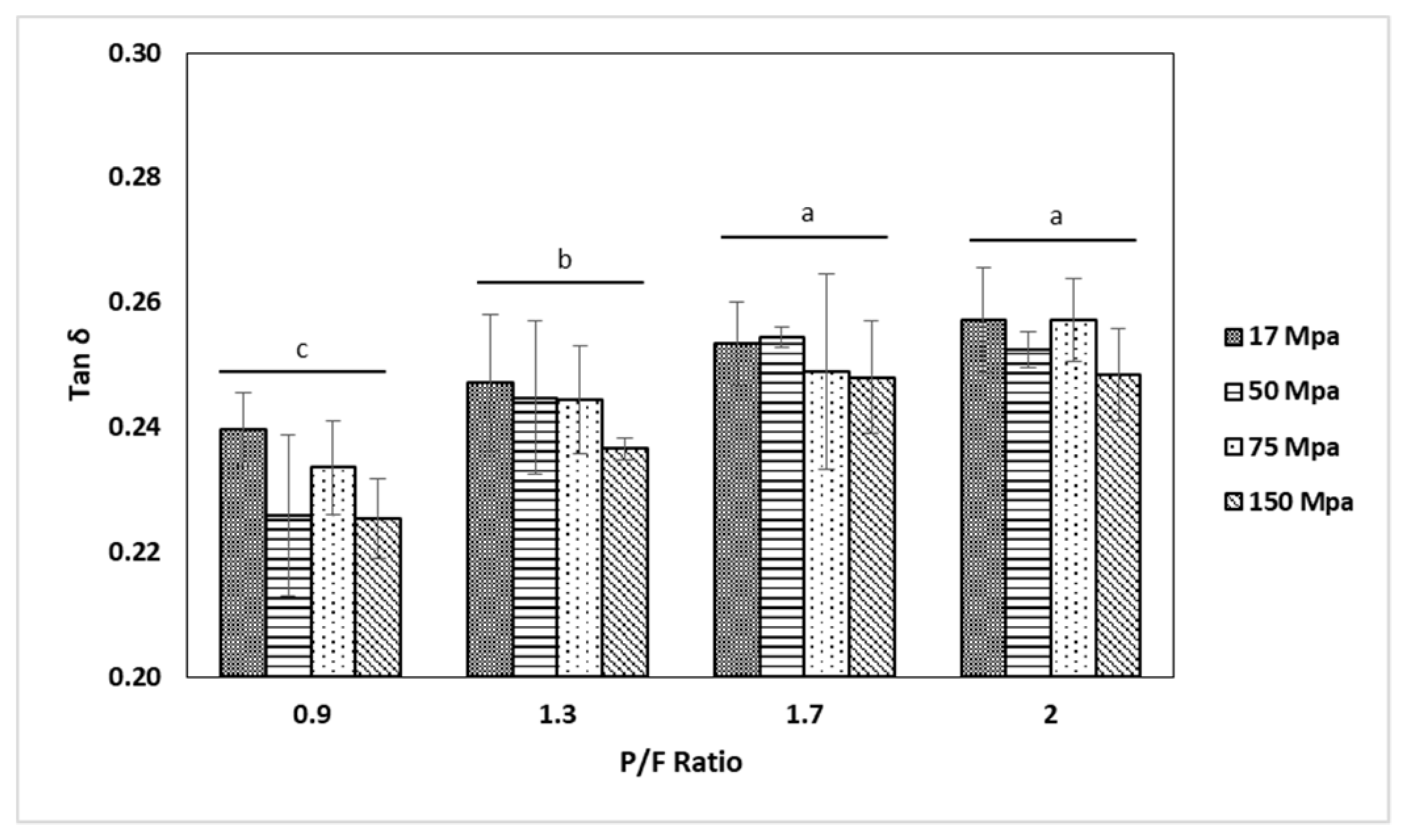
2.3. Small Amplitude Oscillatory Shear
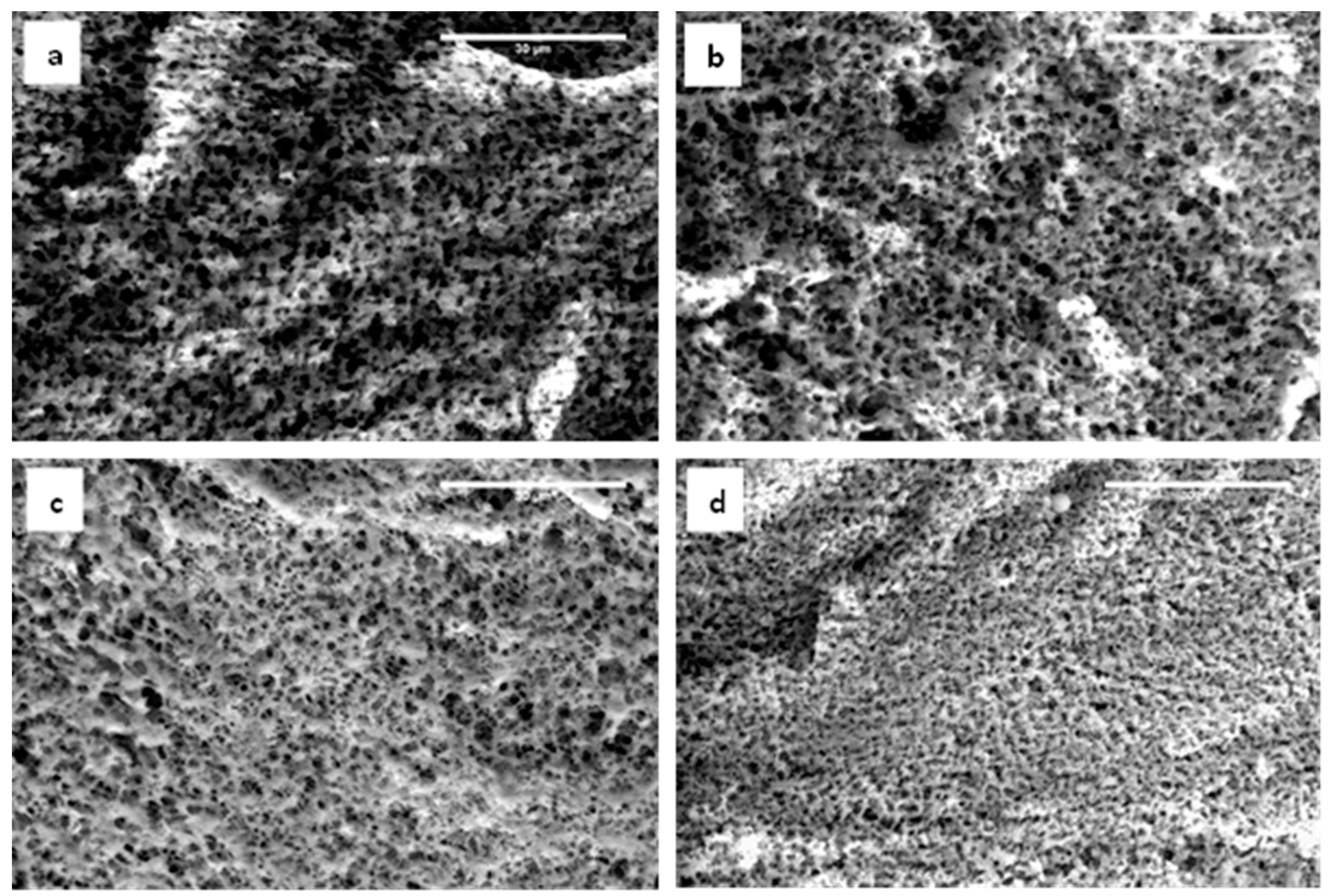
2.4. Microstructure: Porosity and Pore Size
2.5. Fortification of Model Fresh Cheese with Vitamin D3
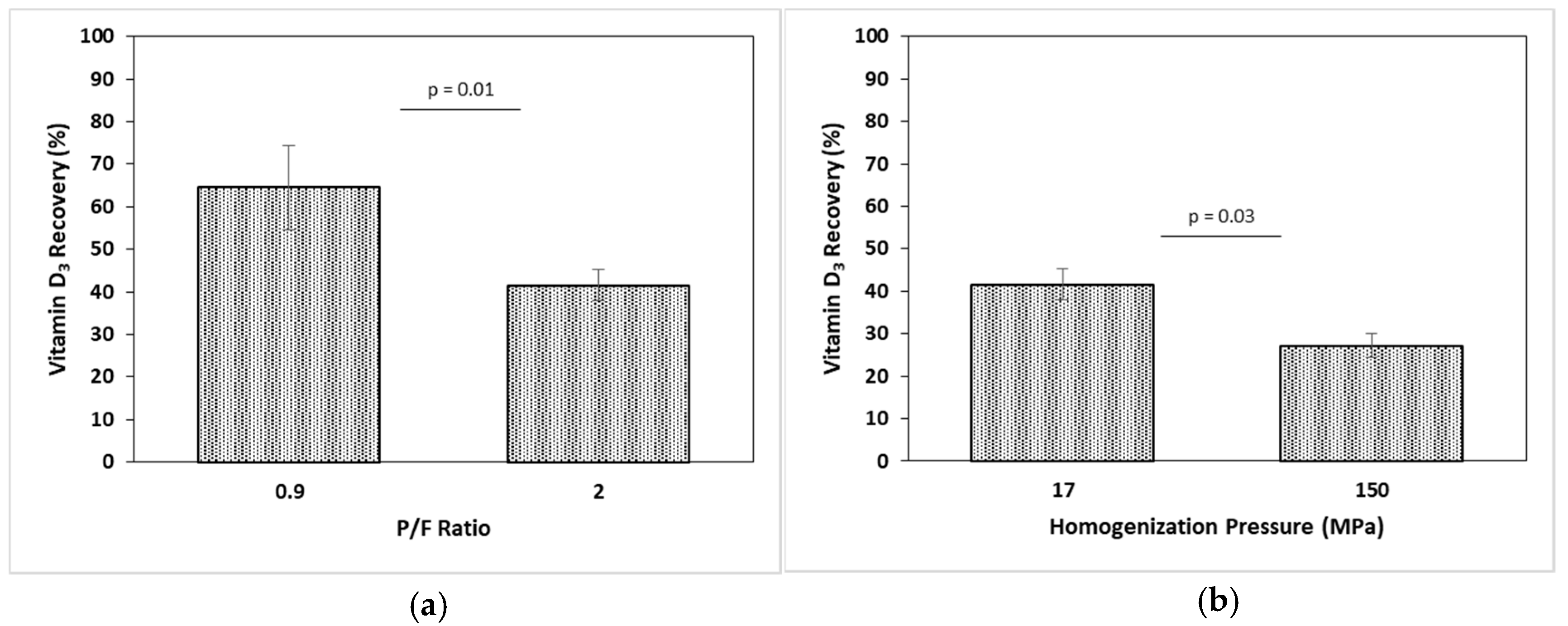
2.6. Bioaccessibility by In Vitro Digestion
3. Conclusions
4. Materials and Methods
4.1. Preparation of Model Fresh Cheese
4.2. Particle Size Analysis of Cheese Milk Emulsion
4.3. Textural and Rheological Characteristics of Model Fresh Cheese
4.3.1. Texture Profile Analysis (TPA) of Model Fresh Cheese
4.3.2. Small Amplitude Oscillatory Shear
4.4. Microstructure: Porosity and Pore Size
4.5. Vitamin D3 Fortification of Model Fresh Cheese
4.5.1. Defining Standard Concentration and Standard Curve
4.5.2. Fortification of Model Fresh Cheese with Vitamin D3
4.5.3. Traceability of Vitamin D3 in Fortified Milk and Cheese
4.5.4. Bioaccessibility of Vitamin D3 by In Vitro Digestion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aguilera, J.M.; Stanley, D.W. Microstructural Principles of Food Processing and Engineering; Aspen Publishers: Gaithersburg, MD, USA, 1999. [Google Scholar]
- Panozzo, A.; Lemmens, L.; Van Loey, A.; Manozocco, L.; Hendrickx, M.E. Microstructure and bioaccessibility of different carotenoid species as affected by high pressure homogenisation: A case study on differently coloured tomatoes. Food Chem. 2013, 141, 4094–4100. [Google Scholar] [CrossRef] [PubMed]
- Gallier, S.; Rutherfurd, S.; Moughan, P.; Singh, H. Effect of food matrix microstructure on stomach emptying rate and apparent ileal fatty acid digestibility of almond lipids. Food Funct. 2014, 5, 2410–2419. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Roque, M.J.; de Ancos, B.; Sanchez-Moreno, C.; Cano, M.P.; Elez-Martinez, P.; Martin-Belloso, O. Impact of food matrix and processing on the in vitro bioaccessibility of vitamin C, phenolic compounds, and hydrophilic antioxidant activity from fruit juice/based beverages. J. Funct. Foods 2015, 14, 33–43. [Google Scholar] [CrossRef]
- Stokes, J.R. Food Biopolymer Gels, Microgel and Nanogel Structures, Formation and Rheology. In Food Materials Science and Engineering; Bhandari, B., Roos, Y.H., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2012; pp. 151–176. [Google Scholar]
- Zuniga, R.; Troncoso, E. Improving Nutrition Throrogh the Design of Food Matrices. In Scientific, Health and Social Aspects of the Food Industry; Valdez, B., Ed.; InTech: Rijeka, Croatia, 2012; pp. 295–320. ISBN 978-953-307-916-5. [Google Scholar]
- Fernandez Farres, I.; Moakes, R.J.A.; Norton, I.T. Designing biopolymer fluid gels: A microstructural approach. Food Hydrocoll. 2014, 42, 362–372. [Google Scholar] [CrossRef]
- Remondetto, G.E.; Subirade, M. Molecular mechanisms of Fe2+-induced β-lactoglobulin cold gelation. Byopolymers 2003, 69, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, T.; Lakemond, C.M.M.; Visschers, R.W. Rheology and structure of milk protein gels. Curr. Opin. Colloid Interface Sci. 2004, 9, 298–304. [Google Scholar] [CrossRef]
- Van der Sman, R.G.M. Soft matter approaches to food structuring. Adv. Colloid Interface Sci. 2012, 176–177, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Lamothe, S.; Corbeil, M.-M.; Turgeon, S.L.; Britten, M. Influence of cheese matrix on lipid digestion in a simulated gastro-intestinal environment. Food Funct. 2012, 3, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Livney, Y.D. Milk proteins as vehicles for bioactives. Curr. Opin. Colloid Interface Sci. 2010, 15, 73–83. [Google Scholar] [CrossRef]
- Everett, D.W. Microstructure of Natural Cheeses. In Structure of Dairy Products; Tamime, A., Ed.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2007; pp. 170–209. ISBN 9780470995921. [Google Scholar]
- Hickey, C.D.; Wilkinson, M.G.; Sheehan, J.J. The influence of cheese manufacture parameters on cheese microstructure, microbial localisation and their interactions during ripening: A review. Trends Food Sci. Technol. 2015, 41, 135–148. [Google Scholar] [CrossRef]
- Lemay, A.; Paquin, P.; Lacroix, C. Influence of Microfluidization of Milk on Cheddar Cheese Composition, Color, Texture and Yield. J. Dairy Sci. 1994, 77, 2870–2879. [Google Scholar] [CrossRef]
- Escobar, D.; Clark, S.; Ganesan, V.; Repiso, V.; Waller, J.; Harte, F. High-pressure homogenization of raw and pasteurized milk modifies the yield, composition, and texture of queso fresco cheese. J. Dairy Sci. 2011, 94, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Zamora, A. Ultra-High Pressure Homogenization of Milk: Effectst of Cheese-Making; Universidad de Barcelona: Barcelona, Spain, 2009. [Google Scholar]
- Van Hekken, D.L.; Tunick, M.H.; Tomasula, P.M.; Molina, F.J.; Gardea, A. Mexican Queso Chihuahua: Rheology of fresh cheese. Int. J. Dairy Technol. 2007, 60, 5–12. [Google Scholar] [CrossRef]
- Dickinson, E. Properties of Emulsions Stabilized with Milk Proteins: Overview of Some Recent Developments. J. Dairy Sci. 1997, 80, 2607–2619. [Google Scholar] [CrossRef]
- Lee, S.-H.; Lefèvre, T.; Subirade, M.; Paquin, P. Effects of ultra-high pressure homogenization on the properties and structure of interfacial protein layer in whey protein-stabilized emulsion. Food Chem. 2009, 113, 191–195. [Google Scholar] [CrossRef]
- Fox, P.F. Cheese: Chemistry, Physics and Microbiology; Aspen Publishers Inc.: Gaithersburg, MD, USA, 1999. [Google Scholar]
- Tunick, M.H.; Van Hekken, D.L. Torsion gelometry of cheese. J. Dairy Sci. 2002, 85, 2743–2749. [Google Scholar] [CrossRef]
- Kiziloz, M.B.; Cumhur, O.; Kilic, M. Development of the structure of an imitation cheese with low protein content. Food Hydrocoll. 2009, 23, 1596–1601. [Google Scholar] [CrossRef]
- Fox, P.F.; McSweeney, P.L.H.; Cogan, T.M.; Guinee, P. Fundamentals of Cheese Science; Aspen Publishers Inc.: Gaithersburg, MD, DUA, 2000. [Google Scholar]
- Xiong, Y.L.; Aguilera, J.M.; Kinsella, J.E. Emulsified Milkfat Effects on Rheology of Acid-Induced Milk Gels. J. Food Sci. 1991, 56, 920–925. [Google Scholar] [CrossRef]
- Dimitreli, G.; Thomareis, A.S. Texture evaluation of block-type processed cheese as a function of chemical composition and in relation to its apparent viscosity. J. Food Eng. 2007, 79, 1364–1373. [Google Scholar] [CrossRef]
- Bucci, A.J.; Van Hekken, D.L.; Tunick, M.H.; Renye, J.A.; Tomasula, P.M. The effects of microfluidization on the physical, microbial, chemical, and coagulaiton properties of milk. J. Dairy Sci. 2018, 101, 6990–7001. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Mauer, K.; Hardy, J. Influence of Fat Globule Size on the Rheological Properties of a Model Acid Fresh Cheese. Food Macromol. Colloids 1995, 512–516. [Google Scholar] [CrossRef]
- Cobos, A.; Horne, D.S.; Muir, D.D. Rheological properties of acid milk gels II. Effect of composition, process and acidification conditions on products from recombined milks using the microfluidizer. Milchwissenschaft 1996, 50, 603–606. [Google Scholar]
- Cobos, A.; Horne, D.S.; Muir, D.D. Rheological properties of acid milk gels. Effect of composition, process and acidification conditions on products from recombined milks. Milchwissenschaft 1995, 50, 444–448. [Google Scholar]
- Kalab, M. Microstructure of Dairy Foods. 1. Milk Products Based on Protein. J. Dairy Sci. 1979, 62, 1352–1364. [Google Scholar] [CrossRef]
- Harwalkar, V.R.; Kalab, M. Effect of Acidulant s and Temperature on Microstructure, Firmness and Susceptibility to Syneresis of Skim Milk Gels. SEM 1981, III, 503–513. [Google Scholar]
- Berton, A.; Rouvellac, S.; Robert, B.; Rousseau, F.; Lopez, C.; Crenon, I. Effect of the size and interface composition of milk fat globules on their in vitro digestion by the human pancreatic lipase: Native versus homogenized milk fat globules. Food Hydrocoll. 2012, 29, 123–134. [Google Scholar] [CrossRef]
- Farhan, B.; Corredig, M. Milk Phospholipids: A Nanocarrier System for Delivery of Bioactive Compounds. In Lipids in Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2012; pp. 53–68. [Google Scholar]
- Liang, L.; Leung Sok Line, V.; Remondetto, G.E.; Subirade, M. In vitro release of α-tocopherol from emulsion-loaded β-lactoglobulin gels. Int. Dairy J. 2010, 20, 176–181. [Google Scholar] [CrossRef]
- Guo, Q.; Ye, A.; Lad, M.; Dalgleish, D.; Singh, H. Behaviour of whey protein emulsion gel during oral and gastric digestion: Effect of droplet size. Soft Matter 2014, 10, 4173–4183. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.H.; Lucey, J.A.; Singh, H. Rheological properties of acid milk gels as affected by the nature of the fat globule surface material and heat tretament. Int. Dairy J. 1999, 9, 537–545. [Google Scholar] [CrossRef]
- Dickinson, E. Emulsion Gels: The sructuring of soft solids with protein-stabilizaed oil droplets. Food Hydrocoll. 2012, 28, 224–241. [Google Scholar] [CrossRef]
- Tosh, S.M.; Dalgleish, D.G. The Physical Properties and Renneting Characteristics of the Synthetic Membrane of the Fat Globules of Microfluidized Milk. J. Dairy Sci. 1998, 81, 1840–1847. [Google Scholar] [CrossRef]
- Heertje, I.; Visser, J.; Smits, P. Structure Formation in acid milk gels. Food Microstruct. 1985, 4, 267–277. [Google Scholar]
- Vasbinder, A.J.; Van Mil, P.J.J.; Bot, A.; de Kruif, K.G. Acid-induced gelation of heat-treated milk studied by diffusing wave spectroscopy. Colloids Surf. B Biointerfaces 2001, 21, 245–250. [Google Scholar] [CrossRef]
- Kosikowski, F. Cheese and Fermented Milk Foods; Edwards Brothers, Inc.: Ann Arbor, MI, USA, 1977. [Google Scholar]
- Ann Augustin, M.; Clarke, P.T. Dry Milk Ingredients. In Dairy Ingredients for Food Processing; Wiley-Blackwell: Hoboken, NJ, USA, 2011; pp. 141–159. [Google Scholar]
- Siapantas, L.G.; Kosikowski, F.V. Properties of Latin-American White Cheese as Influenced by Glacial Acetic Acid. J. Dairy Sci. 1967, 50, 1589–1591. [Google Scholar] [CrossRef]
- Fox, P.F.; McSweeney, P.L.H. Cheese: An Overview. Cheese Chem. Phys. Microbiol. 2004, 1, 1–18. [Google Scholar] [CrossRef]
- CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=133 (accessed on 7 March 2019).
- Farkye, N.Y. Acid- and acid/rennet-curd cheeses part C: Acid-heat coagulated cheeses. In Cheese—Chemistry, Physics and Microbiology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 343–348. [Google Scholar]
- Phadungath, C. The mechanism and properties of acid-coagulated milk gels. Songklanakarin J. Sci. Technol. 2005, 27, 433–448. [Google Scholar]
- Kindstedt, P. American Farmstead Cheese: The Complete Guide to Making and Selling Artisan Cheeses; Chelsea Green Publishing Company: White River Junction, VT, USA, 2005. [Google Scholar]
- Tunick, M.H.; Van Hekkken, D.; Guo, L.; Tomasula, P. Chemistry of Queso Fresco. In Hispanic Foods: Chemistry and Bioactive Compounds; American Chemical Society: Washington, DC, USA, 2012; pp. 11–23. [Google Scholar]
- Olson, D.W.; White, C.H.; Richter, R.L. Effect of Pressure and Fat Content on Particle Sizes in Microfluidized Milk. J. Dairy Sci. 2004, 87, 3217–3223. [Google Scholar] [CrossRef]
- Chen, A.H.; Larkin, J.W.; Clark, C.J.; Irwin, W.E. Textural Analysis of Cheese. J. Dairy Sci. 1979, 62, 901–907. [Google Scholar] [CrossRef]
- Tunick, M.H.; Mackey, K.L.; Smith, P.W.; Holsinger, V.H. Effects of composition and storage on the texture of Mozzarella cheese. NedMilk Dairy J. 1991, 45, 117–125. [Google Scholar]
- Gutiérrez-Méndez, N.N.; Trancoso-Reyes, N.; Leal-Ramos, M.Y. Texture profile analysis of Fresh cheese and Chihuahua cheese using miniature cheese models. Tecnociencia Chihuahua 2013, 7, 65–74. [Google Scholar]
- Rogers, N.R.; McMahon, D.J.; Daubert, C.R.; Berry, T.K.; Foegeding, E.A. Rheological properties and microstructure of Cheddar cheese made with different fat contents. J. Dairy Sci. 2010, 93, 4565–4576. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.Y.; Lee, Y. Temporal Sodium Release Related to Gel Microstructural Properties –Implications for Sodium Reduction. J. Food Sci. 2014, 79, 2245–2252. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Esteve, M.J.; Frígola, A. Determination of vitamins E (α-, γ- and δ-tocopherol) and D (cholecalciferol and ergocalciferol) by liquid chromatography in milk, fruit juice and vegetable beverage. Eur. Food Res. Technol. 2011, 232, 829–836. [Google Scholar] [CrossRef]
- Kazmi, S.A.; Vieth, R.; Rousseau, D. Vitamin D3 fortification and quantification in processed dairy products. Int. Dairy J. 2007, 17, 753–759. [Google Scholar] [CrossRef]
- Wagner, D.; Sidhom, G.; Whiting, S.; Rousseau, D.; Vieth, R. The bioavailability of vitamin D from fortified cheeses and supplements is equivalent in adults. J. Nutr. 2008, 138, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Leskauskaite, D.; Jasutiene, I.N.A. Fortification of dairy products with vitamin D3. Int. J. Dairy Technol. 2016, 69, 177–183. [Google Scholar] [CrossRef]
- Miller, D.; Schricker, B.R.; Rasmussen, R.; Van Campen, D. An in vitro availability method for estimation iron availability from meals. Am. J. Clin. Nutr. 1981, 43, 2248–2256. [Google Scholar] [CrossRef] [PubMed]
- Garrett, D.A.; Failla, M.L.; Sarama, R.J. Development of an in vitro digestion method to assess carotenoid bioavailability from meals. J. Agric. Food Chem. 1999, 47, 4301–4309. [Google Scholar] [CrossRef] [PubMed]
- Etcheverry, P.; Grusak, M.; Fleige, L. Application of in vitro bioaccessibility and bioavailability methods for calcium, carotenoids, folate, iron, magnesium, polyphenols, zinc, and vitamins B6, B12, D, and E. Front. Physiol. 2012, 3, 317. [Google Scholar] [CrossRef] [PubMed]
| Sample | Cheese Milk after Microfluidization | Cheese Milk after Heat Treatment (82 °C ~ 35 min) | Model Fresh Cheese | Percentage of Vitamin D3 Retention in the Model Fresh Cheese | Vitamin D3 Recovery after In Vitro Digestion |
|---|---|---|---|---|---|
| µg/mL | µg/mL | µg/g | % | % | |
| P1R1 | 1.89 ± 0.17 a,* | 1.64 ± 0.03 a,* | 4.88 ± 0.16 a | 52 ± 1.76 | 64.51 ± 9.96 a |
| P1R4 | 1.38 ± 0.11 b,* | 1.40 ± 0.11 b,* | 4.98 ± 0.28 a | 52.8 ± 2.96 | 41.56 ± 3.63 b |
| P4R4 | 0.98 ± 0.19 c,* | 0.98 ± 0.17 c,* | 4.99 ± 0.31 a | 49.3 ± 3.07 | 27.17 ± 2.90 c |
| Pressure 1 (MPa) | Protein to Fat (P/F) ratio 2 | |||
|---|---|---|---|---|
| 0.9 | 1.3 | 1.7 | 2 | |
| 17 | P1R1 | P1R2 | P1R3 | P1R4 |
| 50 | P2R1 | P2R2 | P2R3 | P2R4 |
| 75 | P3R1 | P3R2 | P3R3 | P3R4 |
| 150 | P4R1 | P4R2 | P4R3 | P4R4 |
| Two Phase In-Vitro Digestion | Enzyme Cocktail Solution | Buffer Solution | pH Conditions | Incubation Conditions |
|---|---|---|---|---|
| Gastric Phase | 0.9% Saline Solution and 4 g/L pepsin from porcine gastric mucosa in 0.1 M Hydrochloric acid. | 5 M HCL | pH = 2.0 | Incubation with orbital shaking at 37 °C and 95 rpm for 1 h |
| Intestine Phase | 2 g/L pancreatin from porcine pancreas and 12 g/L bile extract in 0.1 M NaHCO3 | 0.9 M solution of NaHCO3, 2 M NaOH | Initial pH = 5.3 Final pH = 7.5 | Incubation in orbital shaking at 37 °C and 95 rpm for 2 h |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castaneda, N.; Lee, Y. Microstructure of a Model Fresh Cheese and Bioaccessibility of Vitamin D3 Using In Vitro Digestion. Gels 2019, 5, 16. https://doi.org/10.3390/gels5010016
Castaneda N, Lee Y. Microstructure of a Model Fresh Cheese and Bioaccessibility of Vitamin D3 Using In Vitro Digestion. Gels. 2019; 5(1):16. https://doi.org/10.3390/gels5010016
Chicago/Turabian StyleCastaneda, Nuria, and Youngsoo Lee. 2019. "Microstructure of a Model Fresh Cheese and Bioaccessibility of Vitamin D3 Using In Vitro Digestion" Gels 5, no. 1: 16. https://doi.org/10.3390/gels5010016
APA StyleCastaneda, N., & Lee, Y. (2019). Microstructure of a Model Fresh Cheese and Bioaccessibility of Vitamin D3 Using In Vitro Digestion. Gels, 5(1), 16. https://doi.org/10.3390/gels5010016




