Highly Stretchable and Rapid Self-Recoverable Cryogels Based on Butyl Rubber as Reusable Sorbent
Abstract
:1. Introduction
2. Results and Discussion
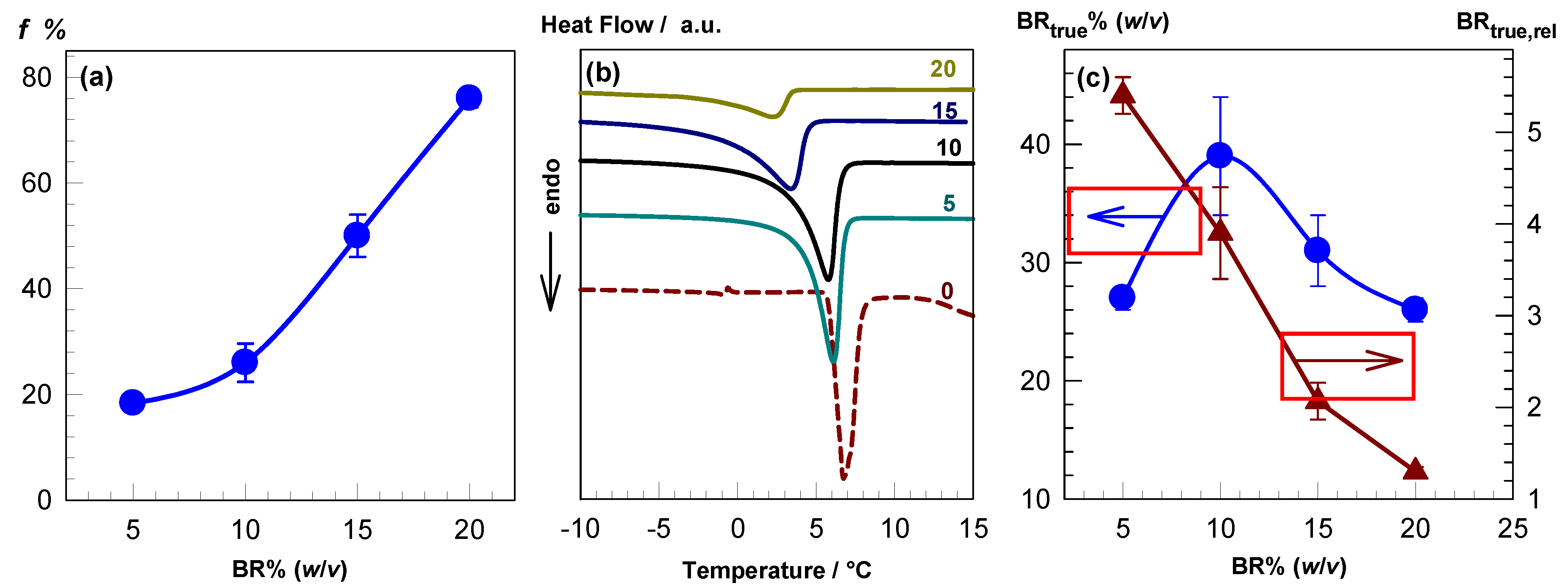
2.1. Swelling Behavior and Porosity of the Cryogels
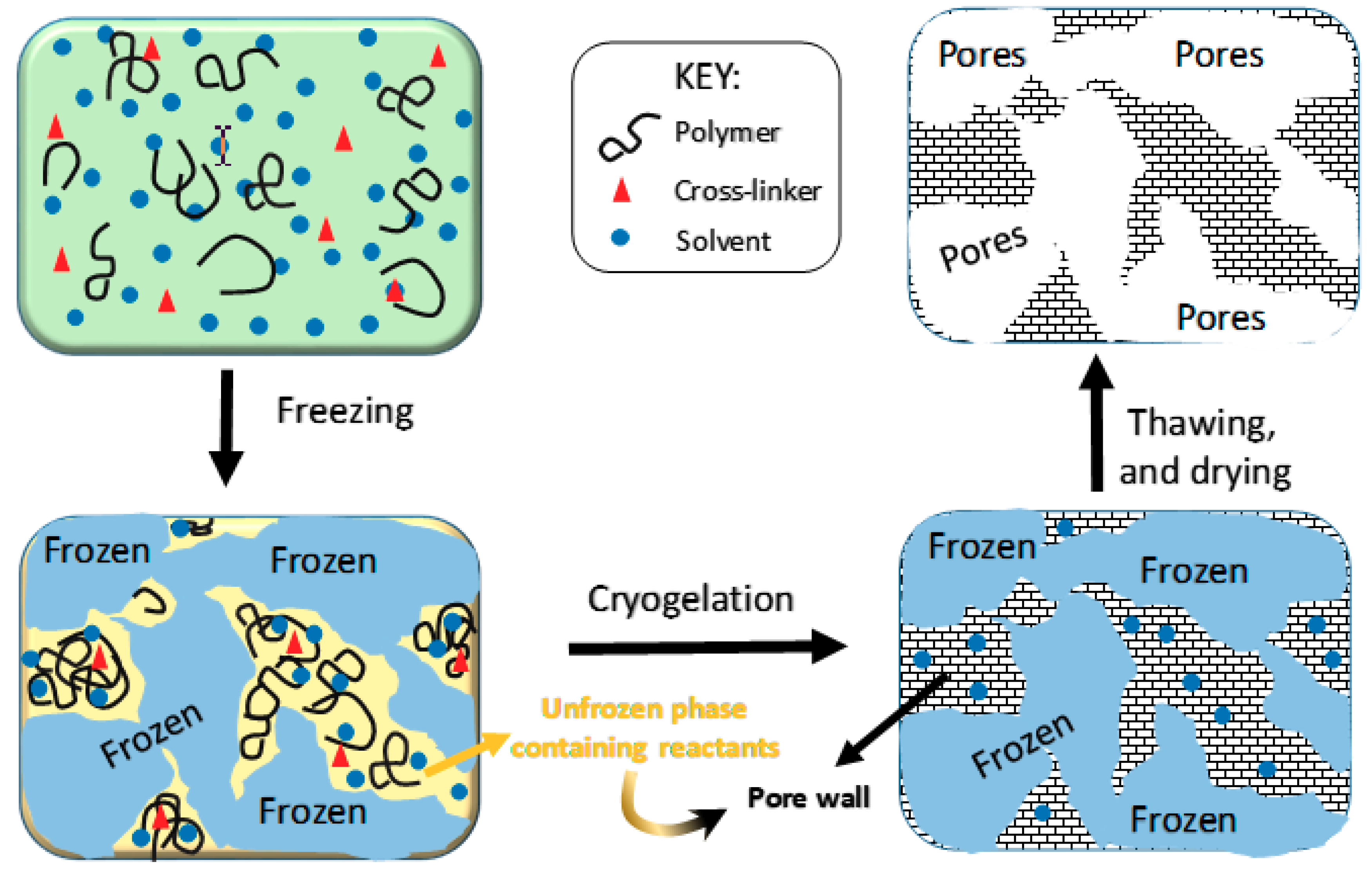
2.2. Amount of Unfrozen Solvent during Cryogelation
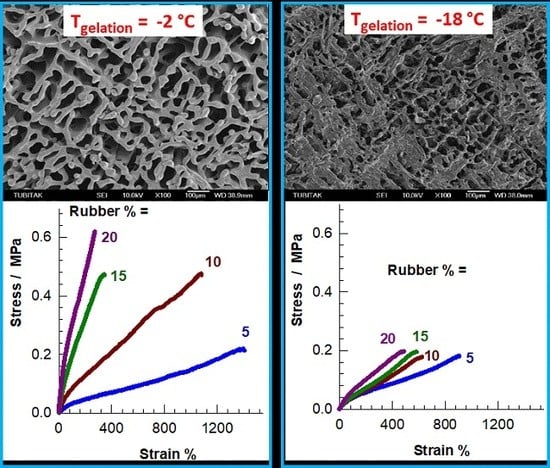
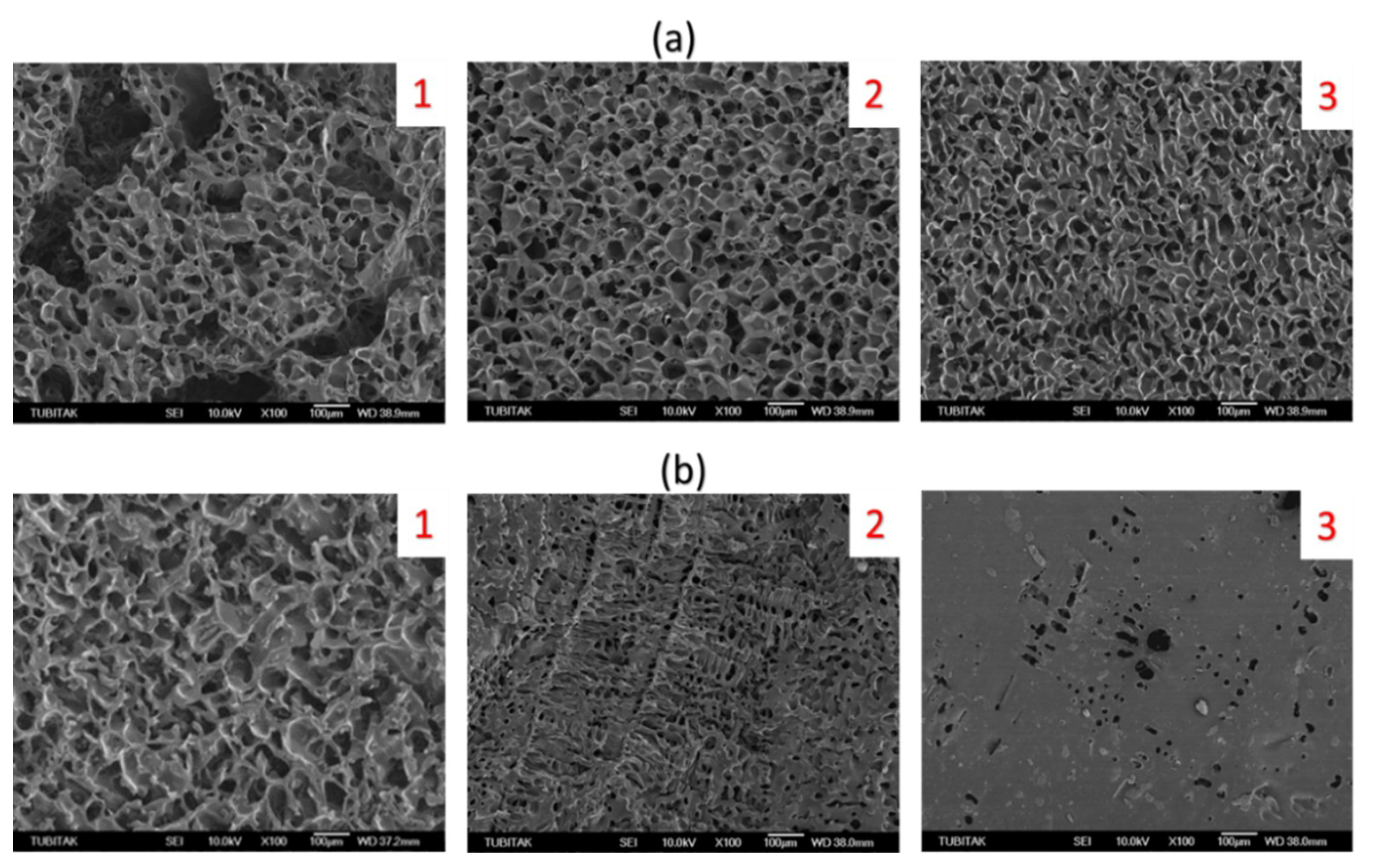
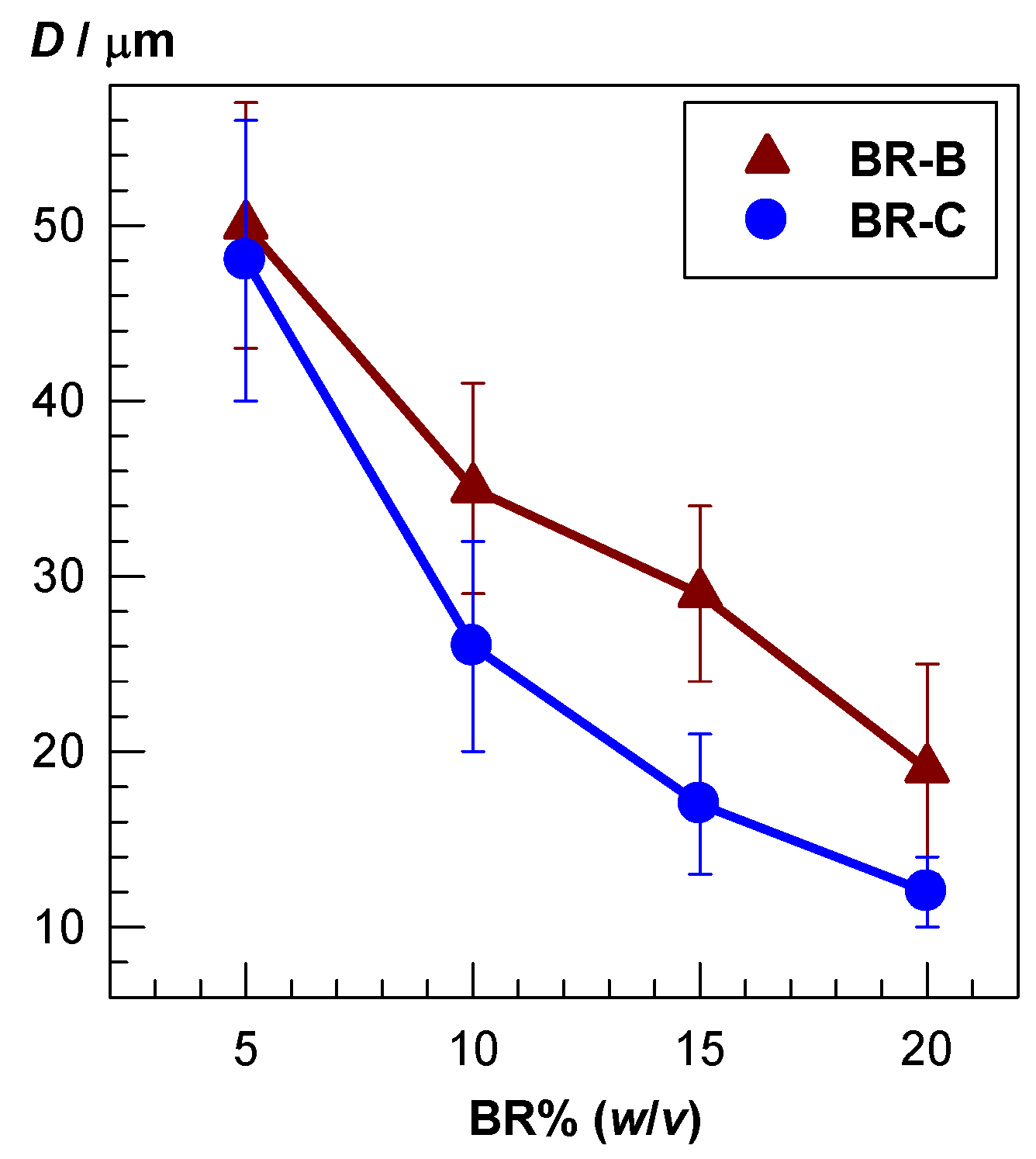
2.3. Morphological Properties of Cryogels
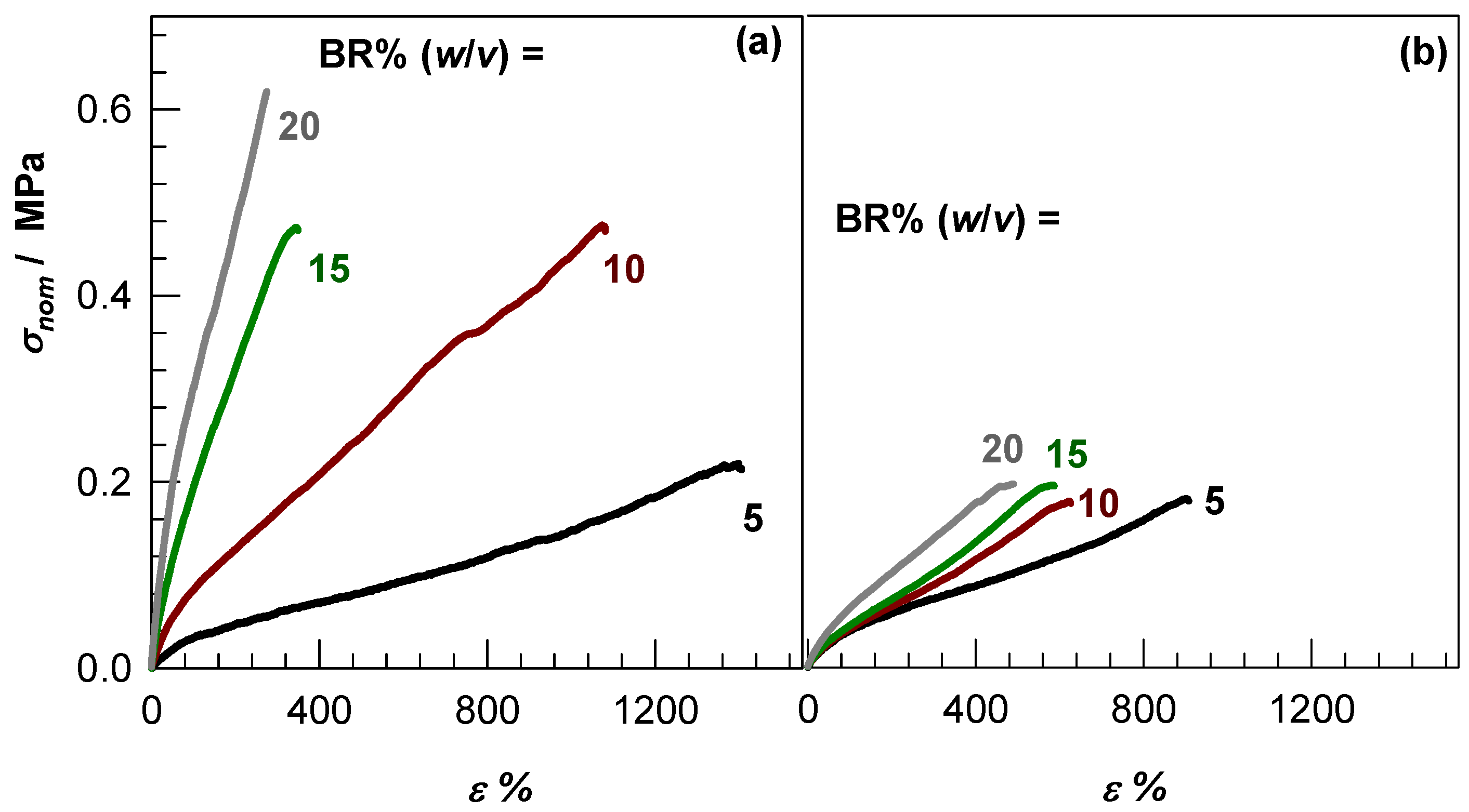
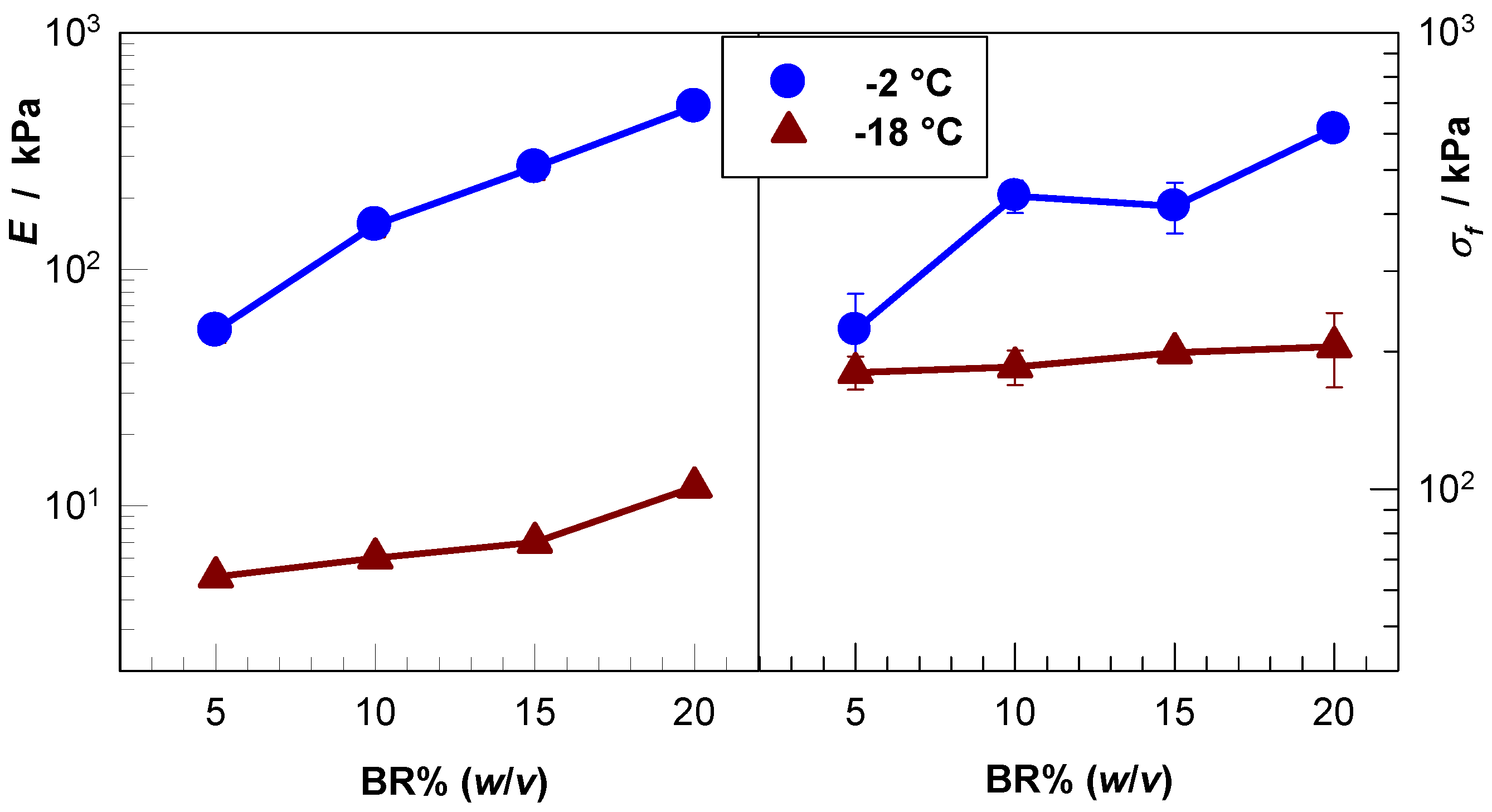
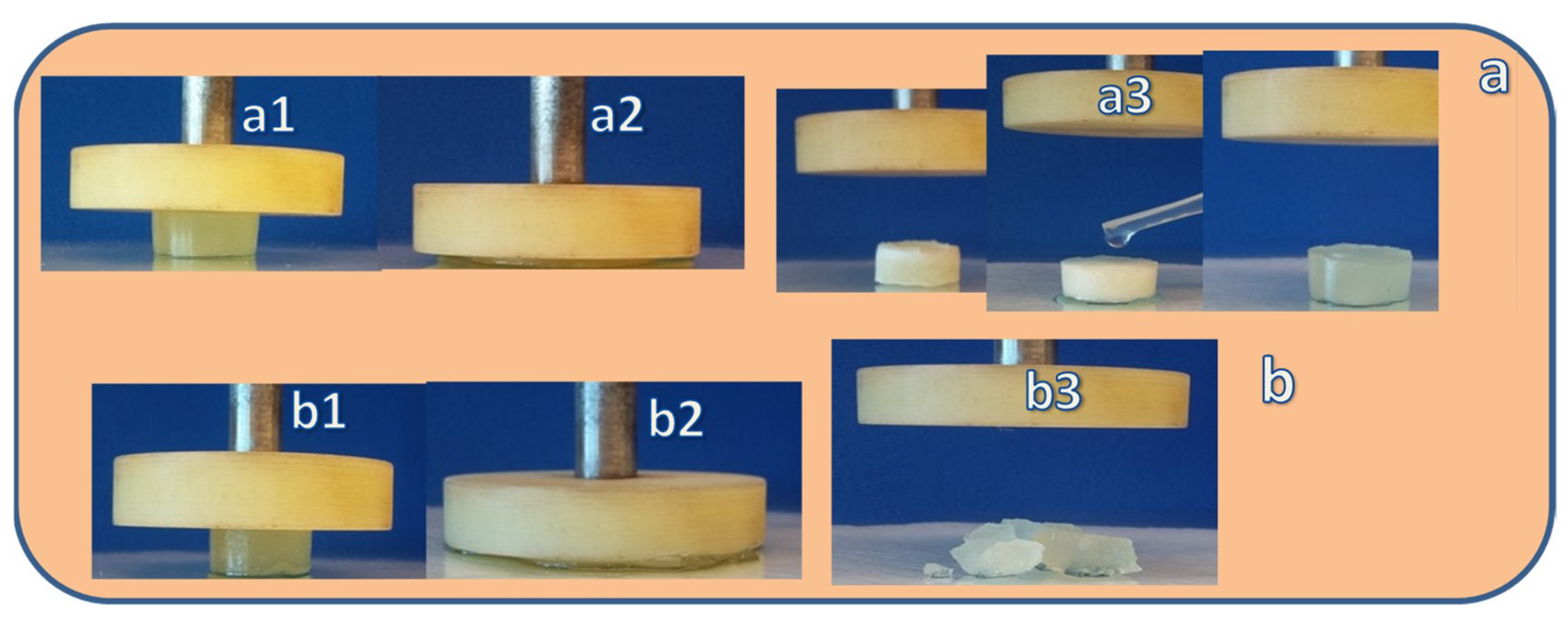
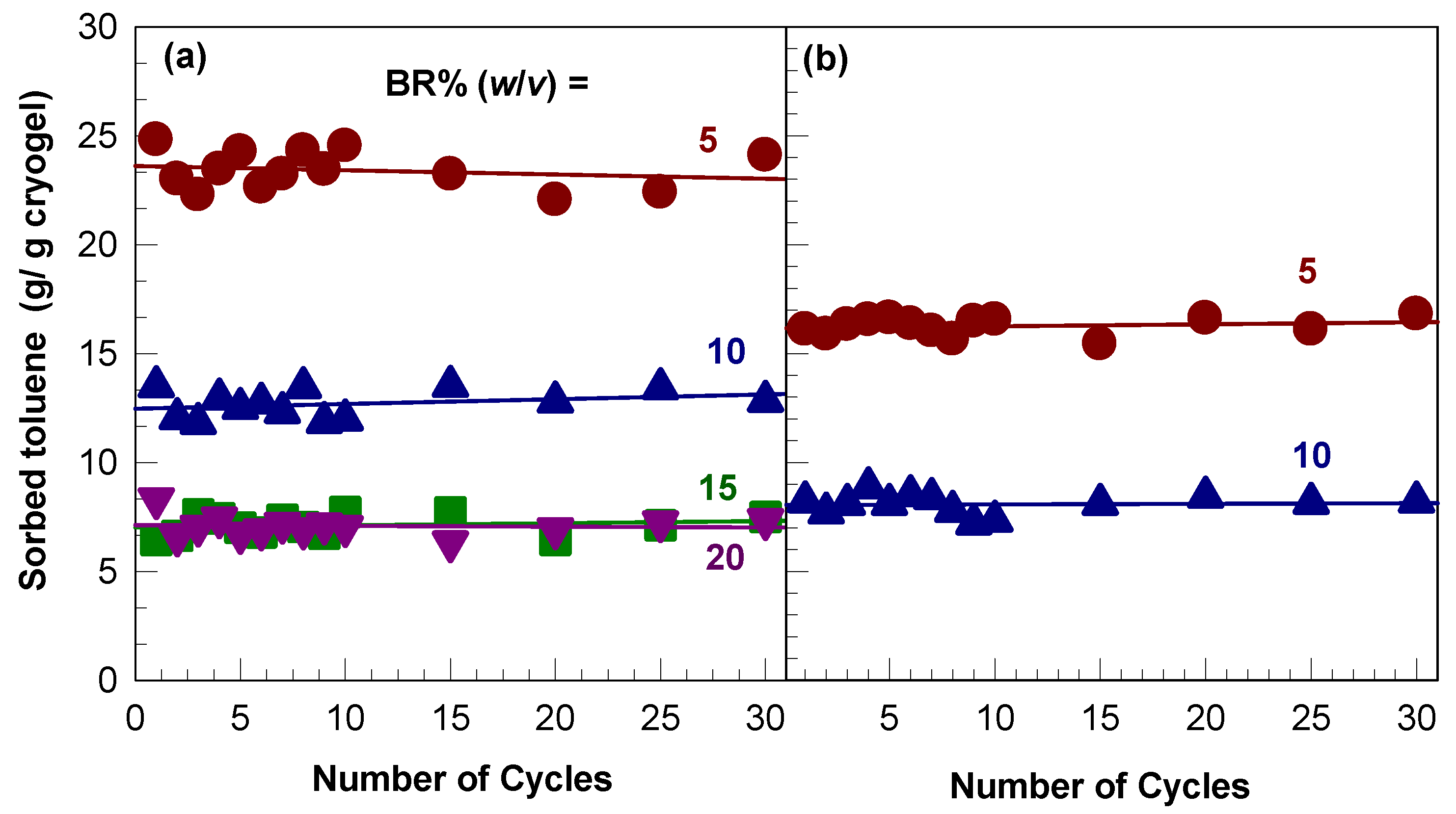
2.4. Mechanical Properties and Self-Recoverability of Cryogels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of the Cryogels
4.3. Characterization of the Cryogels
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Okay, O. General properties of hydrogels. In Hydrogel Sensors and Actuators. Springer Series on Chemical Sensors and Biosensors; Gerlach, G., Arndt, K.-F., Eds.; Springer: Berlin, Germany, 2009; Volume 6, pp. 1–14. [Google Scholar]
- Shapiro, Y.E. Structure and dynamics of hydrogels and organogels: An NMR spectroscopy approach. Prog. Polym. Sci. 2011, 36, 1184–1253. [Google Scholar] [CrossRef]
- Tan, H.; Marra, K.G. Injectable, biodegradable hydrogels for tissue engineering applications. Materials 2010, 3, 1746–1767. [Google Scholar] [CrossRef]
- Shu, X.Z.; Liu, Y.; Palumbo, F.S.; Luo, Y.; Prestwich, G.D. In situ crosslinkable hyaluronan hydrogels for tissue engineering. Biomaterials 2004, 25, 1339–1348. [Google Scholar]
- Vlierberghe, S.V.; Dubruel, P.; Schacht, E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: A review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliver. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef]
- Altunbas, A.; Lee, S.J.; Rajasekaran, S.A.; Schneider, J.P.; Pochan, D.J. Encapsulation of curcumin in self-assembling peptide hydrogels as injectable drug delivery vehicles. Biomaterials 2011, 32, 5906–5914. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X. Multi-scale multi-mechanism design of tough hydrogels: Building dissipation into stretchy networks. Soft Matter 2014, 10, 672–687. [Google Scholar] [CrossRef]
- Ahagon, A.; Gent, A.N. Threshold fracture energies for elastomers. J. Polym. Sci. Pol. Phys. 1975, 13, 1903–1911. [Google Scholar] [CrossRef]
- Brown, H.R. A model of the fracture of double network gels. Macromolecules 2007, 40, 3815–3818. [Google Scholar] [CrossRef]
- Creton, C. 50th Anniversary perspective: Networks and gels: Soft but dynamic and tough. Macromolecules 2017, 50, 8297–8316. [Google Scholar] [CrossRef]
- Okumura, Y.; Ito, K. The polyrotaxane gel: A topological gel by figure-of-eight cross-links. Adv. Mater. 2001, 13, 485–487. [Google Scholar] [CrossRef]
- Karino, T.; Shibayama, M.; Ito, K. Slide-ring gel: Topological gel with freely movable cross-links. Physica B 2006, 385, 692–696. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Nakayama, A.; Kakugo, A.; Gong, J.P.; Osada, Y.; Takai, M.; Erata, T.; Kawano, S. High mechanical strength double-network hydrogel with bacterial cellulose. Adv. Funct. Mater. 2004, 14, 1124–1128. [Google Scholar] [CrossRef]
- Haraguchi, K.; Li, H.J. Mechanical properties and structure of polymer− clay nanocomposite gels with high clay content. Macromolecules 2006, 39, 1898–1905. [Google Scholar] [CrossRef]
- Shibayama, M.; Suda, J.; Karino, T.; Okabe, S.; Takehisa, T.; Haraguchi, K. Structure and Dynamics of Poly (N-isopropylacrylamide)− Clay Nanocomposite Gels. Macromolecules 2004, 37, 9606–9612. [Google Scholar] [CrossRef]
- Tuncaboylu, D.C.; Sarı, M.; Oppermann, W.; Okay, O. Tough and self-healing hydrogels formed via hydrophobic interactions. Macromolecules 2011, 44, 4997–5005. [Google Scholar] [CrossRef]
- Tuncaboylu, D.C.; Sahin, M.; Argun, A.; Oppermann, W.; Okay, O. Dynamics and large strain behavior of self-healing hydrogels with and without surfactants. Macromolecules 2012, 45, 1991–2000. [Google Scholar] [CrossRef]
- Tanaka, T.; Fillmore, D.J. Kinetics of swelling of gels. J. Chem. Phys. 1979, 70, 1214–1218. [Google Scholar] [CrossRef]
- Okay, O. Macroporous copolymer networks. Prog. Polym. Sci. 2000, 25, 711–779. [Google Scholar] [CrossRef]
- Yang, F.; Lin, Z.; He, X.; Chen, L.; Zhang, Y. Synthesis and application of a macroporous boronate affinity monolithic column using a metal-organic gel as a porogenic template for the specific capture of glycoproteins. J. Chromatogr. A 2011, 1218, 9194–9201. [Google Scholar] [CrossRef]
- Salerno, A.; Oliviero, M.; Di Maio, E.; Iannace, S.; Netti, P.A. Design of porous polymeric scaffolds by gas foaming of heterogeneous blends. J. Mater. Sci. Mater. M 2009, 20, 2043–2051. [Google Scholar] [CrossRef] [PubMed]
- Ki, C.S.; Park, S.Y.; Kim, H.J.; Jung, H.M.; Woo, K.M.; Lee, J.W.; Park, Y.H. Development of 3-D nanofibrous fibroin scaffold with high porosity by electrospinning: Implications for bone regeneration. Biotechnol. Lett. 2008, 30, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Lozinsky, V.I. Cryogels on the basis of natural and synthetic polymers: Preparation, properties and application. Russ. Chem. Rev. 2002, 71, 489–511. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Galaev, I.Y.; Plieva, F.M.; Savina, I.N.; Jungvid, H.; Mattiasson, B. Polymeric cryogels as promising materials of biotechnological interest. Trends Biotechnol. 2003, 21, 445–451. [Google Scholar] [CrossRef]
- Okay, O.; Lozinsky, V.I. Synthesis and structure–property relationships of cryogels. Adv. Polym. Sci. 2014, 263, 103–157. [Google Scholar]
- Lozinsky, V.I.; Okay, O. Basic principles of cryotropic gelation. Adv. Polym. Sci. 2014, 263, 49–101. [Google Scholar]
- Akiba, M.; Hashim, A.S. Vulcanization and crosslinking in elastomers. Prog. Polym. Sci. 1997, 22, 475–521. [Google Scholar] [CrossRef]
- Kumar, C.S.S.R.; Nijasure, A.M. Vulcanization of rubber. Resonance 1997, 4, 55–59. [Google Scholar] [CrossRef]
- Okay, O.; Durmaz, S.; Erman, B. Solution crosslinked poly(isobutylene) gels: Synthesis and swelling behavior. Macromolecules 2000, 33, 4822–4827. [Google Scholar] [CrossRef]
- Ceylan, D.; Okay, O. Macroporous polyisobutylene gels: A novel tough organogel with superfast responsivity. Macromolecules 2007, 40, 8742–8749. [Google Scholar] [CrossRef]
- Karakutuk, I.; Okay, O. Macroporous rubber gels as reusable sorbents for the removal of oil from surface waters. React. Funct. Polym. 2010, 70, 585–595. [Google Scholar] [CrossRef]
- Ceylan, D.; Dogu, S.; Karacik, B.; Yakan, S.D.; Okay, O.S.; Okay, O. Evaluation of butyl rubber as sorbent material for the removal of oil and polycyclic aromatic hydrocarbons from seawater. Environ. Sci. Technol. 2009, 43, 3846–3852. [Google Scholar] [CrossRef] [PubMed]
- Dogu, S.; Okay, O. Tough organogels based on polyisobutylene with aligned porous structures. Polymer 2008, 49, 4626–4634. [Google Scholar] [CrossRef]
- Yılmaz, A.; Karacık, B.; Henkelmann, B.; Pfister, G.; Schramm, K.W.; Yakan, S.D.; Barlas, B.; Okay, O.S. Use of passive samplers in pollution monitoring: A numerical approach for marinas. Environ. Int. 2014, 73, 85–93. [Google Scholar] [CrossRef] [PubMed]


| Cryogel Code | Solvent | BR% (w/v) | S2Cl2% (v/w) | Tcry (°C) |
|---|---|---|---|---|
| BR-C | Cyclohexane | 5–20 | 0–20 | −2 and −18 |
| BR-B | Benzene | 5–20 | 0–20 | −18 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muslumova, S.; Yetiskin, B.; Okay, O. Highly Stretchable and Rapid Self-Recoverable Cryogels Based on Butyl Rubber as Reusable Sorbent. Gels 2019, 5, 1. https://doi.org/10.3390/gels5010001
Muslumova S, Yetiskin B, Okay O. Highly Stretchable and Rapid Self-Recoverable Cryogels Based on Butyl Rubber as Reusable Sorbent. Gels. 2019; 5(1):1. https://doi.org/10.3390/gels5010001
Chicago/Turabian StyleMuslumova, Sevil, Berkant Yetiskin, and Oguz Okay. 2019. "Highly Stretchable and Rapid Self-Recoverable Cryogels Based on Butyl Rubber as Reusable Sorbent" Gels 5, no. 1: 1. https://doi.org/10.3390/gels5010001