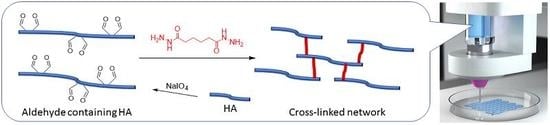
Evaluation of Hydrogels Based on Oxidized Hyaluronic Acid for Bioprinting
Abstract
1. Introduction
2. Results and Discussion
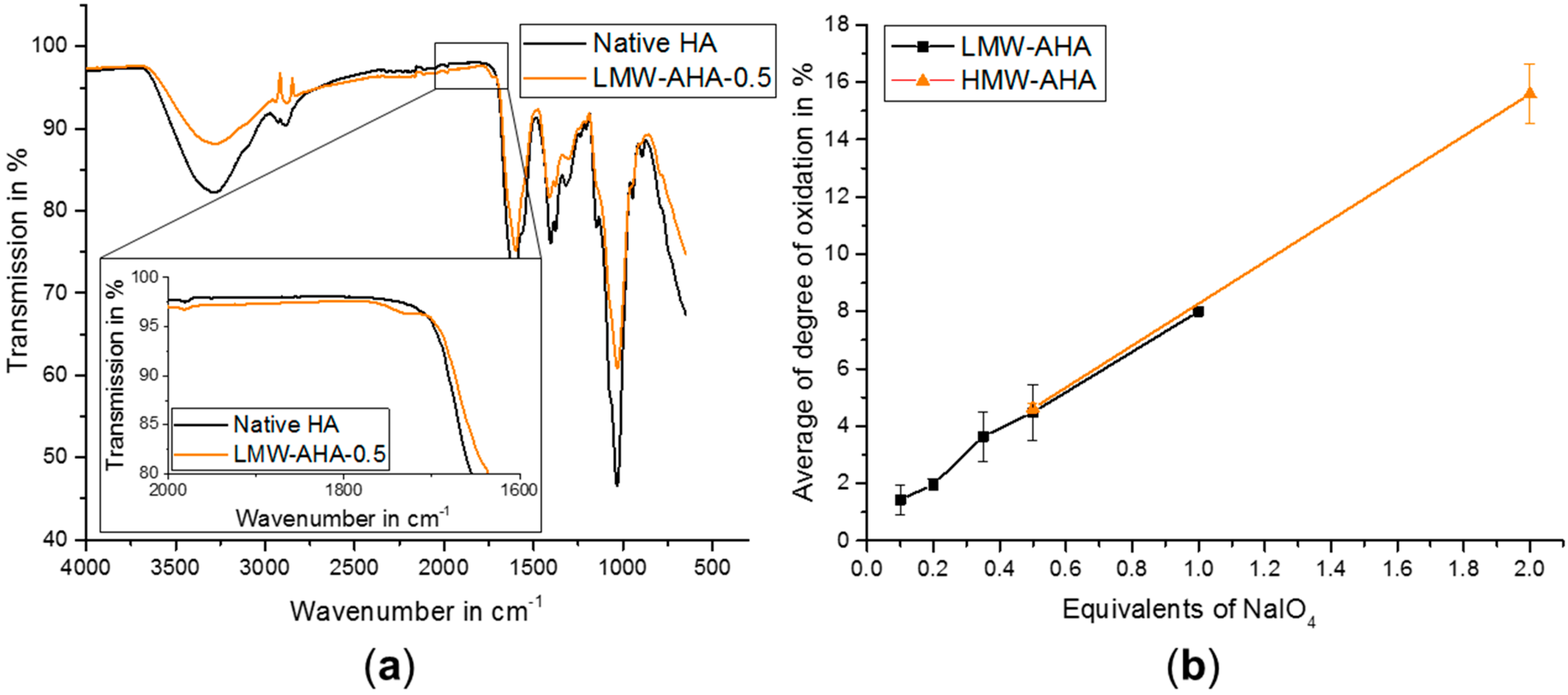
2.1. AHA Synthesis and Characterization
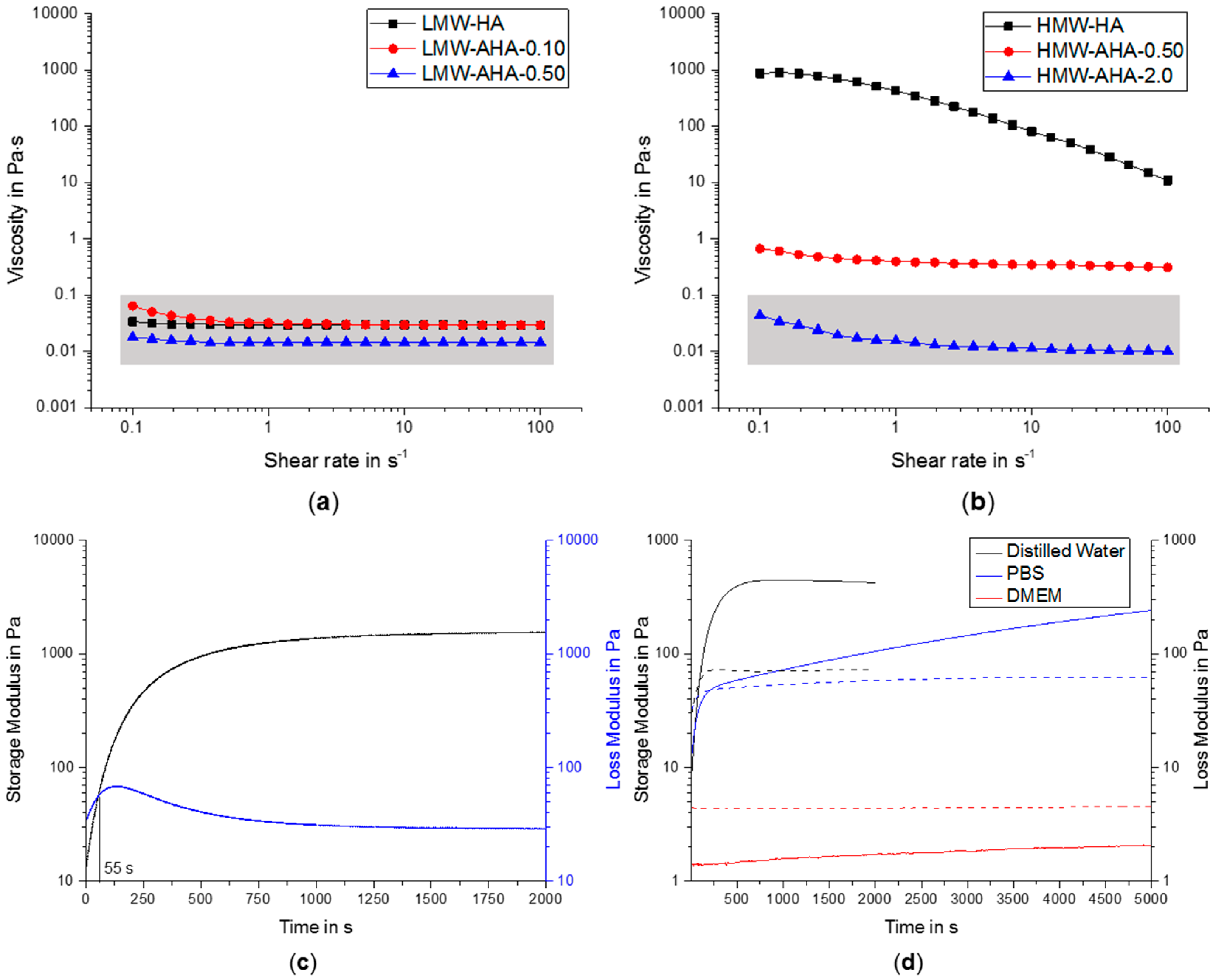
2.2. Rheology
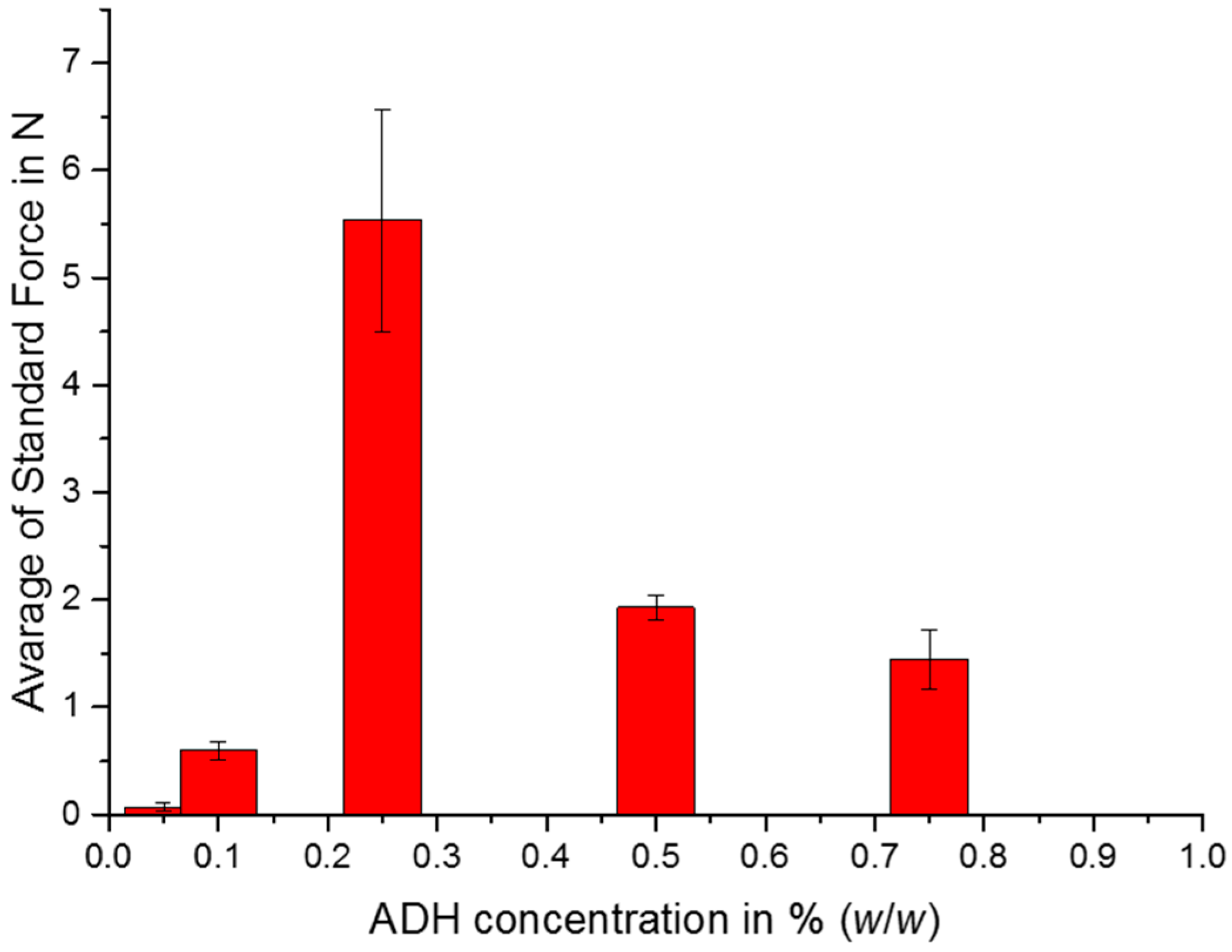
2.3. Mechanical Properties
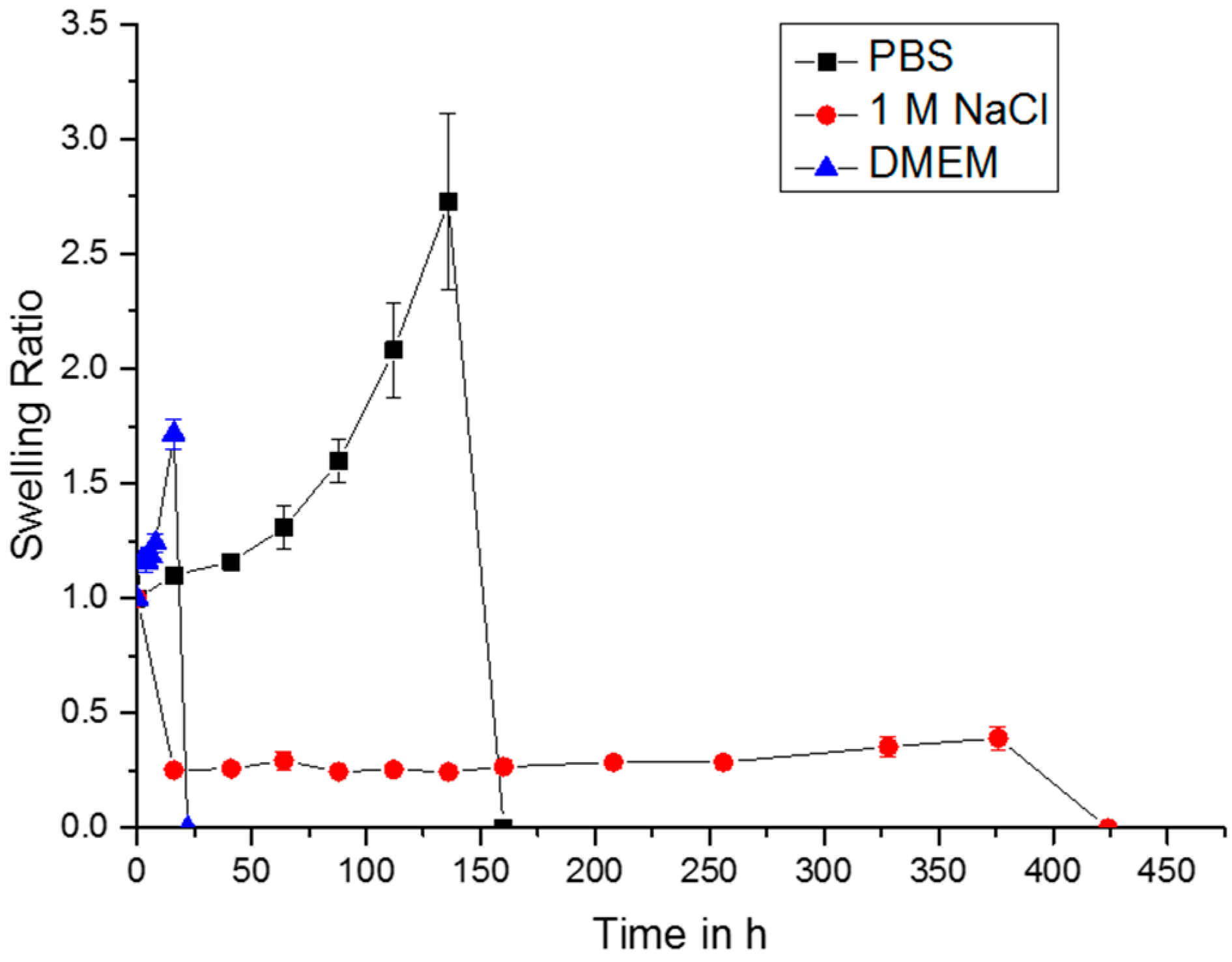
2.4. Swelling Studies
2.5. Bioprinting
3. Conclusions
4. Materials and Methods
4.1. Materials and Reagents
4.2. Synthesis of AHA
4.3. Characterization of AHA
4.4. Gelation with ADH
4.5. Rheology
4.6. Mechanical Tests
4.7. Swelling Properties
4.8. Bioprinting
Author Contributions
Funding
Conflicts of Interest
References
- Tan, H.; Chu, C.R.; Payne, K.A.; Marra, K.G. Injectable in situ forming biodegradable chitosan–hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials 2009, 30, 2499–2506. [Google Scholar] [CrossRef] [PubMed]
- Abbadessa, A.; Mouser, V.H.M.; Blokzijl, M.M.; Gawlitta, D.; Dhert, W.J.A.; Hennink, W.E.; Malda, J.; Vermonden, T. A Synthetic Thermosensitive Hydrogel for Cartilage Bioprinting and Its Biofunctionalization with Polysaccharides. Biomacromolecules 2016, 17, 2137–2147. [Google Scholar] [CrossRef] [PubMed]
- Altomare, L.; Cochis, A.; Carletta, A.; Rimondini, L.; Farè, S. Thermo-responsive methylcellulose hydrogels as temporary substrate for cell sheet biofabrication. J. Mater. Sci. Mater. Med. 2016, 27, 95. [Google Scholar] [CrossRef] [PubMed]
- Rowley, J.A.; Madlambayan, G.; Mooney, D.J. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 1999, 20, 45–53. [Google Scholar] [CrossRef]
- Hynes, R.O. The Extracellular Matrix: Not Just Pretty Fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Banerjee, R. Biopolymer-Based Hydrogels for Cartilage Tissue Engineering. Chem. Rev. 2011, 111, 4453–4474. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Jungst, T.; Smolan, W.; Schacht, K.; Scheibel, T.; Groll, J. Strategies and Molecular Design Criteria for 3D Printable Hydrogels. Chem. Rev. 2016, 116, 1496–1539. [Google Scholar] [CrossRef] [PubMed]
- Stichler, S.; Böck, T.; Paxton, N.; Bertlein, S.; Levato, R.; Schill, V.; Smolan, W.; Malda, J.; Teßmar, J.; Blunk, T.; et al. Double printing of hyaluronic acid/poly(glycidol) hybrid hydrogels with poly(ε-caprolactone) for MSC chondrogenesis. Biofabrication 2017, 9, 044108. [Google Scholar] [CrossRef] [PubMed]
- Leite, Á.J.; Sarker, B.; Zehnder, T.; Silva, R.; Mano, J.F.; Boccaccini, A.R. Bioplotting of a bioactive alginate dialdehyde-gelatin composite hydrogel containing bioactive glass nanoparticles. Biofabrication 2016, 8, 035005. [Google Scholar] [CrossRef] [PubMed]
- Kalia, J.; Raines, R.T. Hydrolytic Stability of Hydrazones and Oximes. Angew. Chem. Int. Ed. 2008, 47, 7523–7526. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Highley, C.B.; Yeh, Y.C.; Galarraga, J.H.; Uman, S.; Burdick, J.A. Three-dimensional extrusion bioprinting of single- and double-network hydrogels containing dynamic covalent crosslinks. J. Biomed. Mater. Res. A 2018, 106, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Su, W.-Y.; Chen, Y.-C.; Lin, F.-H. Injectable oxidized hyaluronic acid/adipic acid dihydrazide hydrogel for nucleus pulposus regeneration. Acta Biomater. 2010, 6, 3044–3055. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, K.A.; Potthast, A.; Christensen, B.E. Periodate oxidation of polysaccharides for modification of chemical and physical properties. Carbohydr. Res. 2010, 345, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Degner, J.; Singer, F.; Cordero, L.; Boccaccini, A.R.; Virtanen, S. Electrochemical investigations of magnesium in DMEM with biodegradable polycaprolactone coating as corrosion barrier. Appl. Surf. Sci. 2013, 282, 264–270. [Google Scholar] [CrossRef]
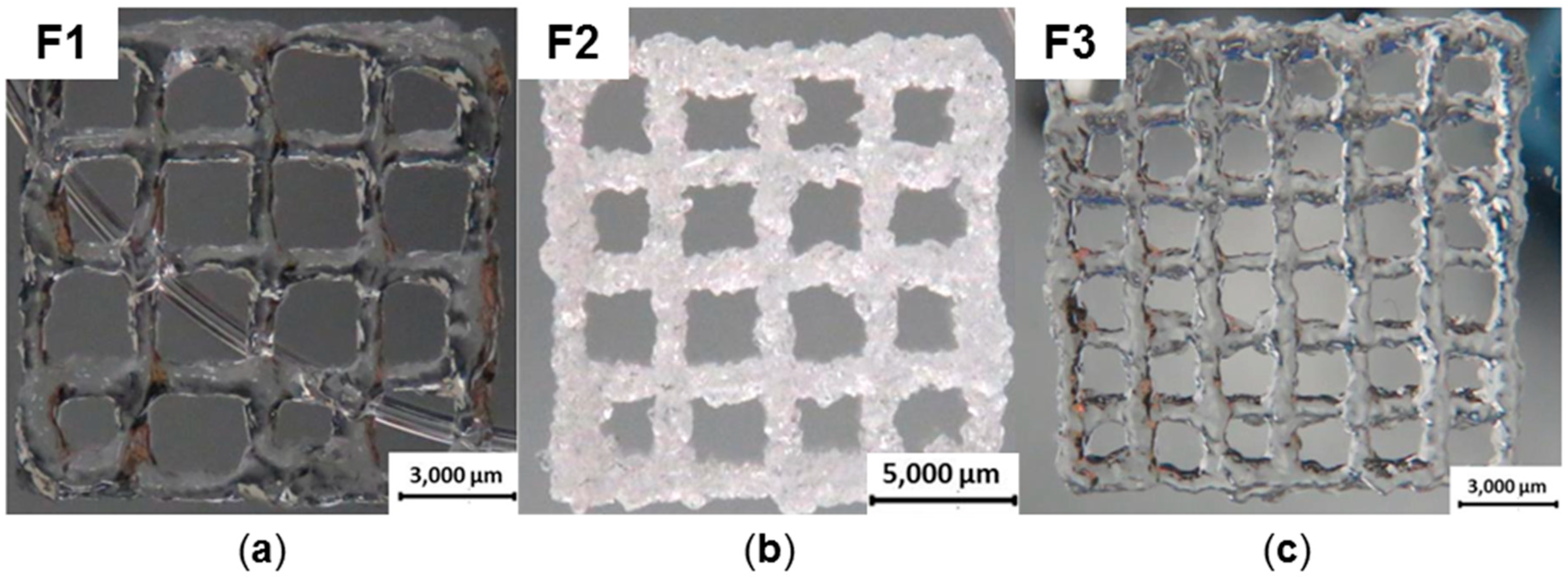
| Specimen | HMW-AHA-2.0 Concentration in % (w/w) | ADH Concentration in % (w/w) | Needle Diameter in mm | Pressure in Bar |
|---|---|---|---|---|
| F1 | 3.5 | 0.05 | 0.25 | 3.4 |
| F2 | 3.5 | 1.0 | 0.25 | 2.0 |
| F3 | 3.5 | 0.075 | 0.41 | 4.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weis, M.; Shan, J.; Kuhlmann, M.; Jungst, T.; Tessmar, J.; Groll, J. Evaluation of Hydrogels Based on Oxidized Hyaluronic Acid for Bioprinting. Gels 2018, 4, 82. https://doi.org/10.3390/gels4040082
Weis M, Shan J, Kuhlmann M, Jungst T, Tessmar J, Groll J. Evaluation of Hydrogels Based on Oxidized Hyaluronic Acid for Bioprinting. Gels. 2018; 4(4):82. https://doi.org/10.3390/gels4040082
Chicago/Turabian StyleWeis, Matthias, Junwen Shan, Matthias Kuhlmann, Tomasz Jungst, Jörg Tessmar, and Jürgen Groll. 2018. "Evaluation of Hydrogels Based on Oxidized Hyaluronic Acid for Bioprinting" Gels 4, no. 4: 82. https://doi.org/10.3390/gels4040082
APA StyleWeis, M., Shan, J., Kuhlmann, M., Jungst, T., Tessmar, J., & Groll, J. (2018). Evaluation of Hydrogels Based on Oxidized Hyaluronic Acid for Bioprinting. Gels, 4(4), 82. https://doi.org/10.3390/gels4040082