Stimuli Responsive Poly(Vinyl Caprolactam) Gels for Biomedical Applications
Abstract
:1. Introduction
1.1. Stimuli Responsive Gels
1.2. Importance of Thermoresponsive Poly(Vinyl Caprolactam)(PNVCL)
1.3. Biocompatibility of PNVCL
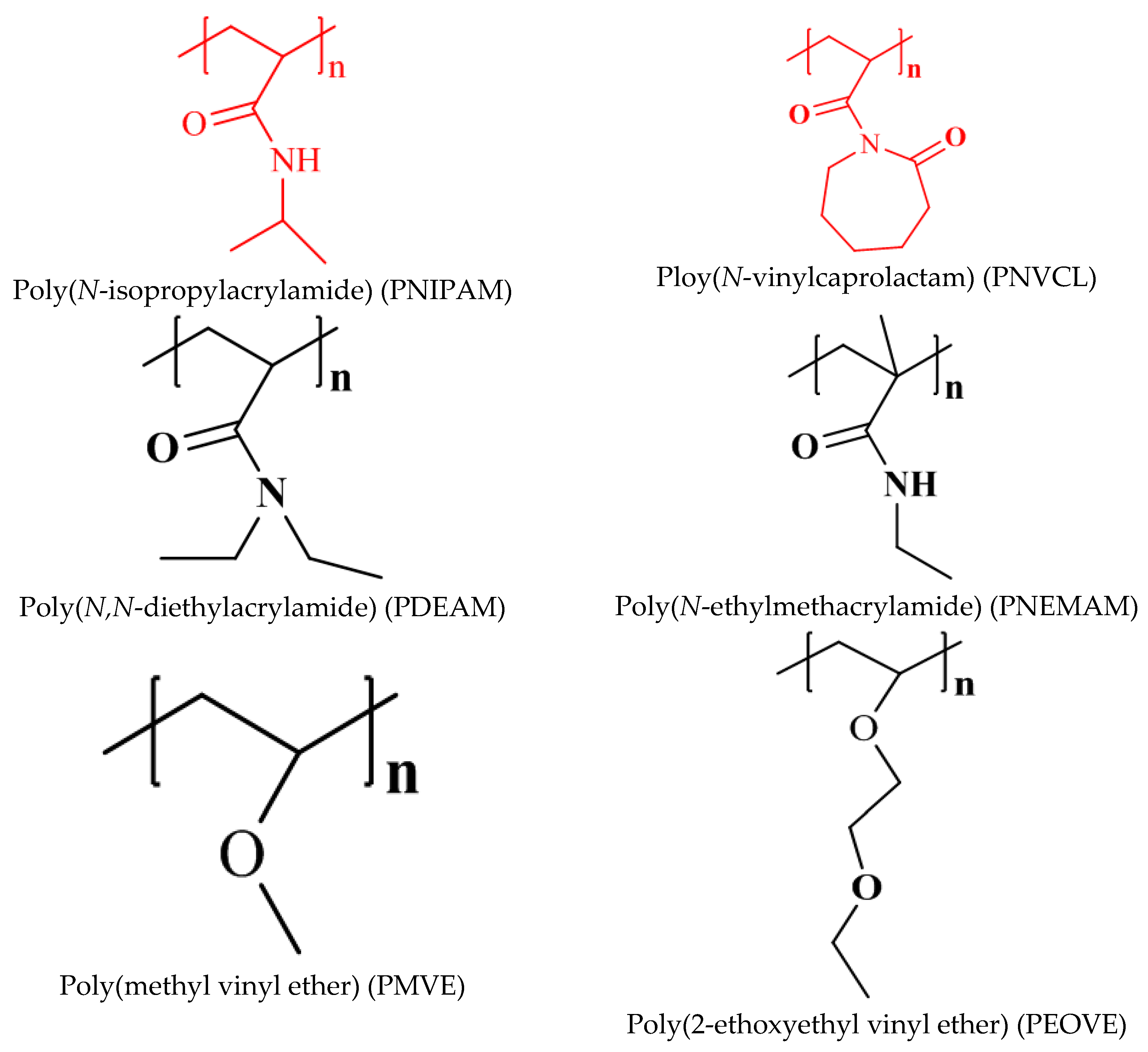
2. Stimuli Responsive Poly(Vinyl Caprolactam) Gels
2.1. Macro Hydrogels
2.2. Micro or Nanogels
3. Biomedical Applications
3.1. Drug Delivery and Antibacterial Applications

| Gel Composition | Responsiveness | Release Carriers | Application | Reference | |
|---|---|---|---|---|---|
| P(NVCL-co-SIA) | thermo | Hydrogel | Far | Controlled release | [73] |
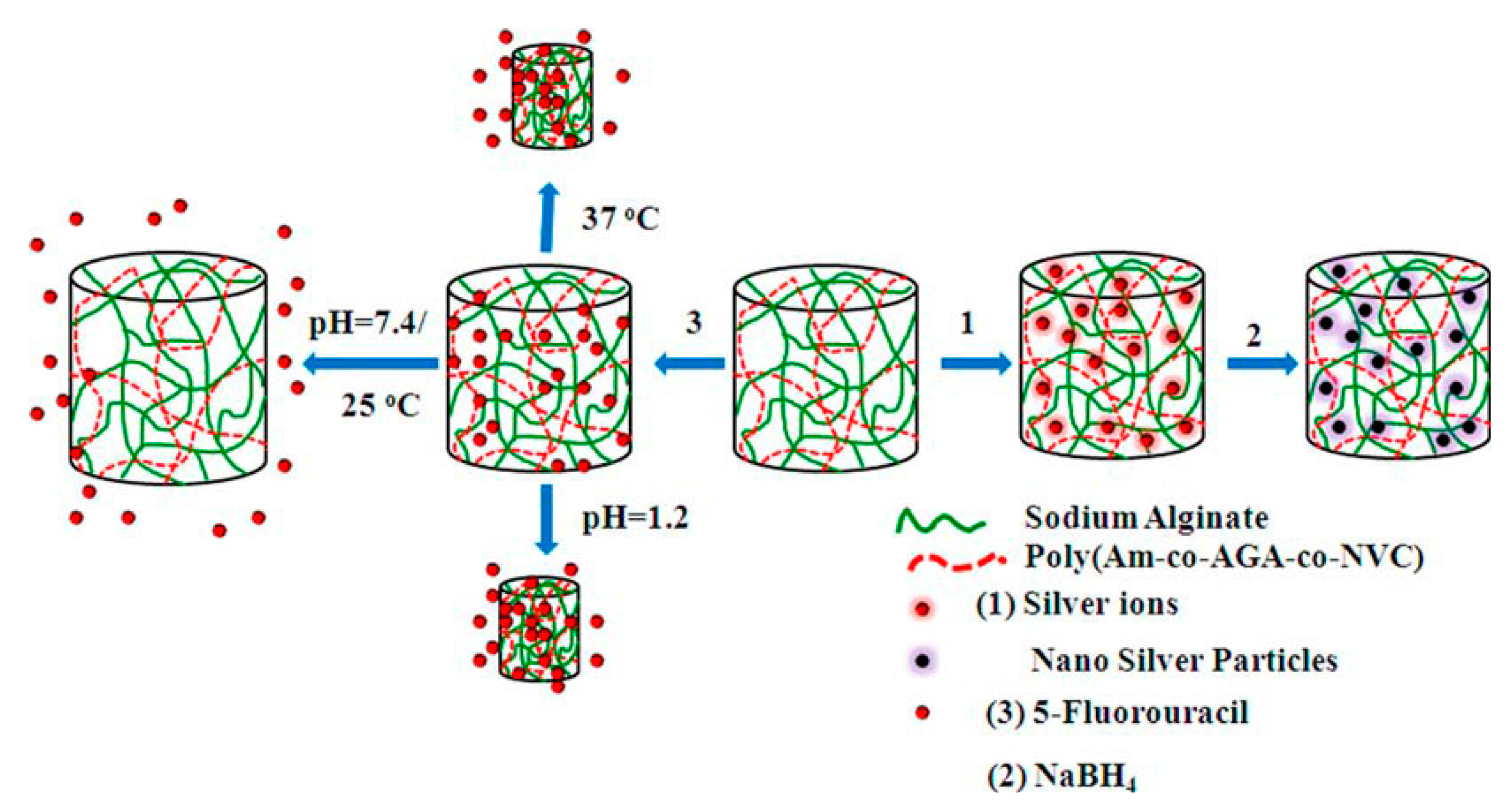
| P(AAm-co-NVCL-co-AGA)/SAlg | pH/thermo | Composite hydrogel | 5-FU/Ag nano | Controlled release/Antibacterial | [74] |
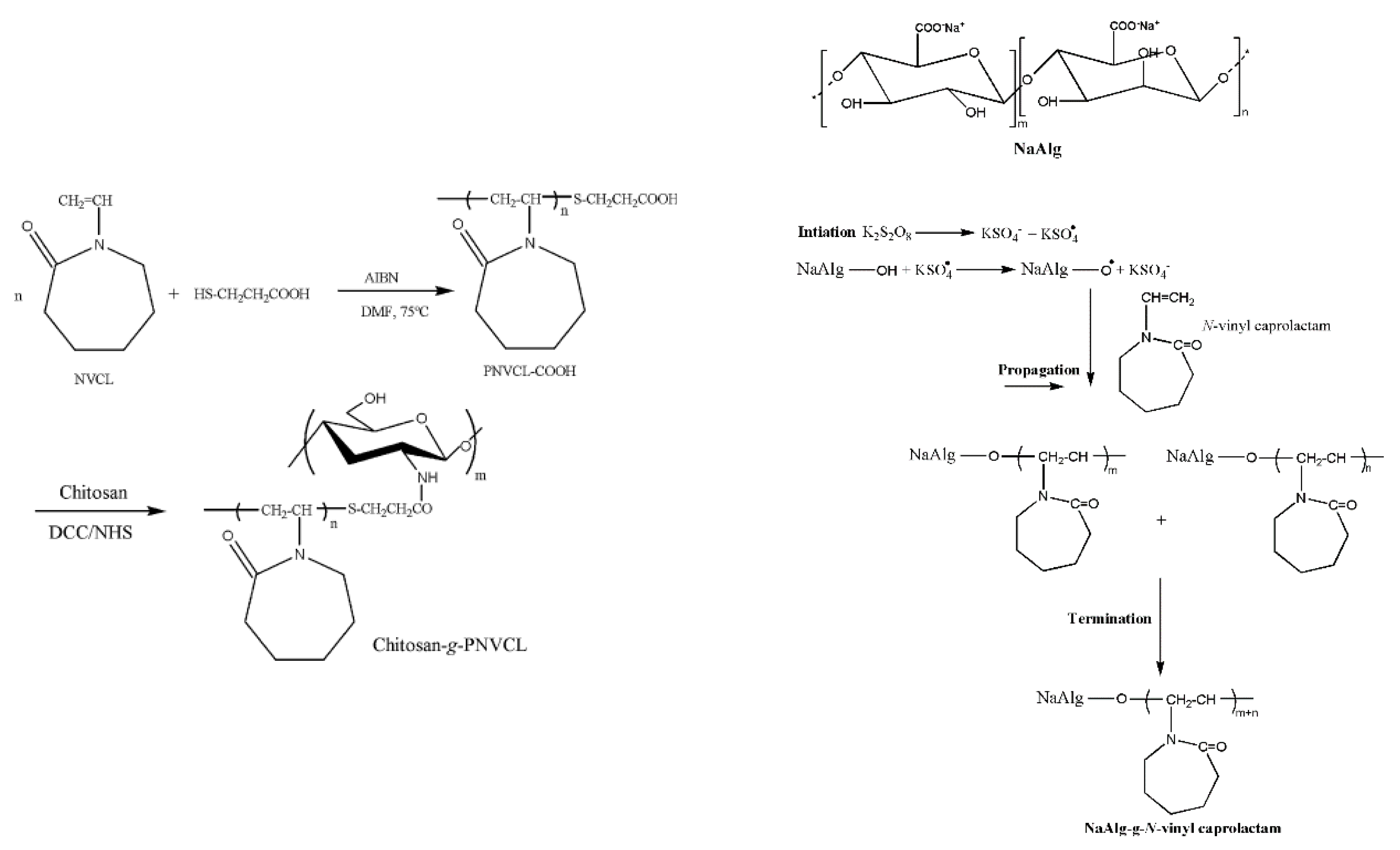
| SAlg-g-PNVCL | pH/thermo | Microgel | 5-FU | Controlled release | [75] |
| SAlg-g-PNVCL | pH/thermo | Gel beads | 5-FU | Controlled release | [76] |
| CS-g-PNVCL | pH/thermo | Gel beads | Ket | Controlled release | [77] |
| PNVCL-g-PEO | thermo | Gel particles | Nad/Prop/Ket/SA | Controlled release | [78] |
| PNVCL-co-GMA | thermo | Hybrid microgel | Ag | antibacterial | [79] |
| P(NVCL-co-UA) | pH/thermo | Microgel | Dox | Controlled release | [80] |
| P(NVCL-PMAA) | pH/thermo/reduction | Microgel | Dox | Tumor targeted drug delivery | [81] |
| P(NVCL-co-AGA) | pH/thermo | Nanogel | 5-FU | Controlled release | [85] |
| PVOH-b-PNVCL | pH/thermo/reduction | Nanogel | NR | Tumor targeted drug delivery | [86] |
| P(NVCL-co-HEMA) | thermo | Nanogel | CUR | Targeted drug delivery | [87] |
| P(NVCL-co-PEGMA)/VP/2MBA | pH/thermo | Nanogel | 5-FU | Controlled release | [88] |
| PNVCL/Dex-MA | pH/thermo/Enzyme | Nanogel | dextranase | Enzymatic degradation | [89] |
| Fib-g-PNVCL | Thermo | Nanogel | 5-FU/Meg | Tumor targeted drug delivery | [90] |
| HPCL-click-PNVCL | Thermo | Films | --- | controlled drug delivery | [91] |
| PVCL-NH2/PMAA | Thermo/pH | Gel cubes, capsules | ---- | controlled drug delivery | [92] |
| PNVCL | thermo | Hydrogel | Bacteria and fungi | Medicine | [93] |
| PNVCL-CaAlg | pH/thermo | Hydrogel | Enzymes and cells | Medicine | [94] |
| PNVCL-CaAlg | pH/thermo | Hydrogel | Protease | Biotechnology/medicine | [95] |
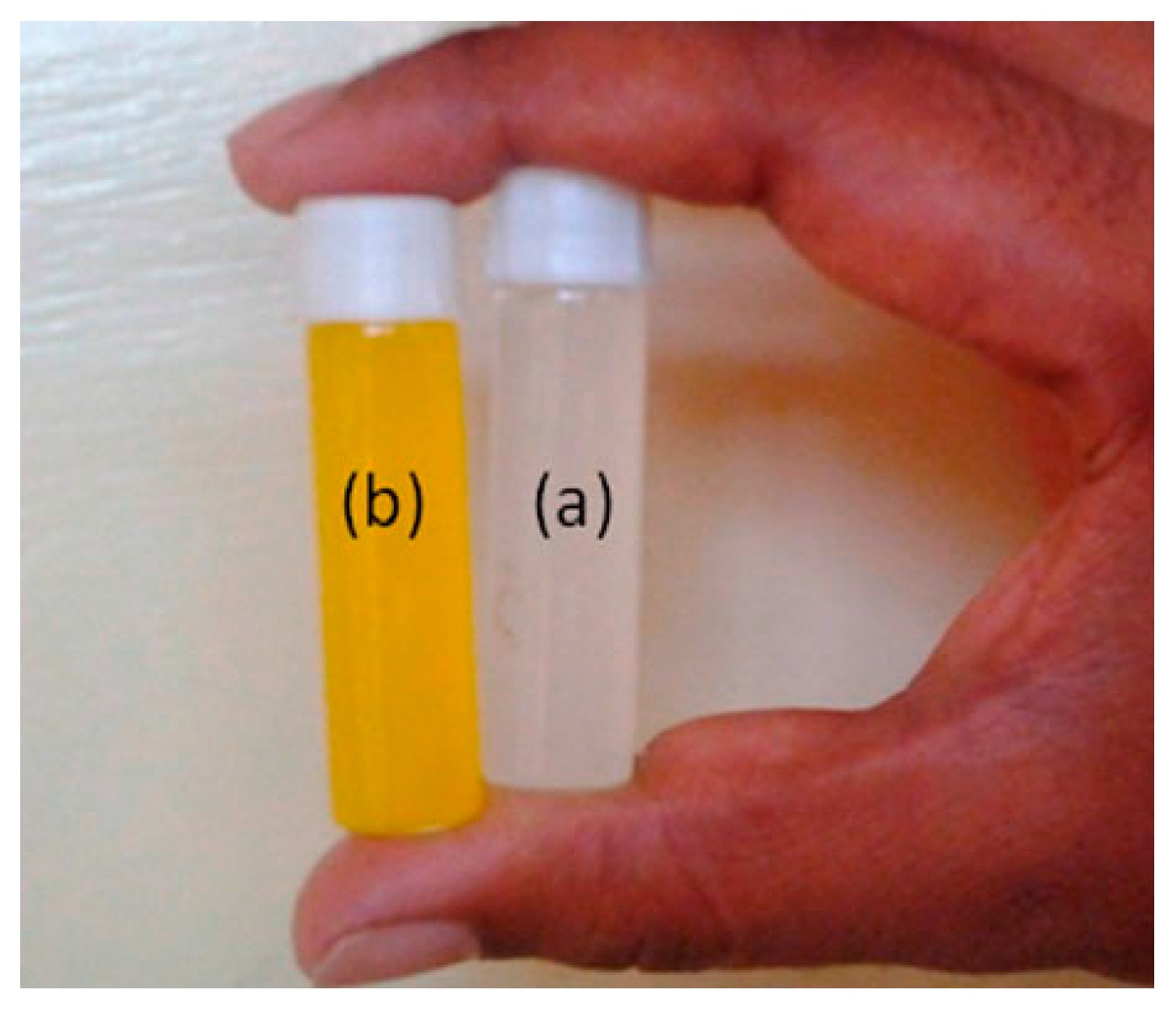
3.2. Enzyme/Cell Immobilization
4. Conclusions and Future Outlooks
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Monomers | Crosslinkrs |
| NVCL: N-vinyl caprolactam | BISVP: 3,3’-(ethane-1,1-diyl)bis(1-vinyl-2-pyrrolidone) |
| NIPAM: N-isopropyl acrylamide | DMD: N,N-diallyl-N,N-dimethyl ammonium chloride |
| DEAM: N,N-diethyl acrylamide | MBA: N, N’-Methylenebisacrylamide |
| MVE: methyl vinyl ether | EBVP: Ethylidene-bis-3(N-vinyl-2-pyrrolidone) |
| NEMAM: N-ethylmethacrylamide | EGDMA: Ethylene glycol dimethacrylate |
| EOVE: 2-ethoxyethyl vinyl ether | GA: Glutaraldehyde |
| NVIBAM: N-vinylisobutyramide | TPP: Tripolyphosphate |
| VP: vinyl pyrrolidine | Polymers |
| AAm: acrylamide | PAA: Poly(acrylamide) |
| DEAEMA: 2-(diethylamino)ethyl methacrylate | PEO: polyethylene oxide |
| AcrNEP: N-acryloyl-N’-ethyl piperazine | SAlg: Sodium Alginate |
| AMA: Allyl methacrylate | CS: Chitosan |
| IA: Itaconic acid | PVOH: Polyvinyl alcohol |
| AAc:Acrylic acid | Fib: Fibrinogen |
| MAAc: Methacrylic acid | PNVCL: Poly(N-vinyl caprolactam) |
| DMAm: N,N-dimethylacrylamide | PNIPAM: poly(N-isopropylacrylamide) |
| Vim: Vinyl imadazole | Intiators |
| AAEM: Acetoacetoxy metthacrylate | AIBN: Azobis(isobutyronitrile) |
| 3MDG: 3-o-methacryloyl-1,2:5,6-di-o-isopropylidene-α-d-glucofuranose | ABIN: 2,2’-azobis(2-methylpropoionate) |
| AGA: Acrylamidoglycolic acid | KPS: Potassium persulphate |
| NaA: Sodium acrylate | APS: Ammonium persulphate |
| C11EO12:PEO macromonomer | Surfactants |
| UA: Undecenoic acid | SDS: Sodium dodecyl sulphate |
| 2MBA: 2-methacryloyloxybenzoic acid | DPB: N-dodecylpyridinium bromide |
| Dx-MA: Dextran mehacrylate | |
| Drugs | Others |
| 5-FU: 5-Fluorouracil | NaBH4: Sodium borohydride |
| Far: Farmazine | GSH: Glutathione |
| Dox: Doxorubicin | LCST: Lowest critical Solution temperature |
| Ket: Ketoprofen | LLS: Laser light scattering studies |
| CUR: Curcumin | DLS: Dynamic light scattering studies |
| Meg:Megestrol acetate | IPN: Interpenetrating Polymer Network |
| Nad: Nadolol | MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| Prop: Propranolol | RAFT: Reversible addition–fragmentation chain transfer |
| SA: Salicylic acid |
References
- Langer, R.; Peppas, N.A. Advances in biomaterials, drug delivery, and bionanotechnology. AIChE J. 2003, 49, 2990–3006. [Google Scholar] [CrossRef]
- Gil, E.S.; Hudson, S.M. Stimuli responsive polymers and their conjugates. Prog. Polym. Sci. 2004, 9, 1173–1222. [Google Scholar] [CrossRef]
- Enas, M.A. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar]
- Tanaka, T. Collapse of gels and the critical end point. Phys. Rev. Lett. 1978, 40, 820–824. [Google Scholar] [CrossRef]
- Ashley, S. Artificial muscles. Sci. Am. 2003, 289, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Mather, P.T. Responsive materials: Soft answers for hard problems. Nat. Mater. 2007, 6, 93–94. [Google Scholar] [CrossRef] [PubMed]
- Peteu, S.F. Responsive Materials Configured for Micro and Nano actuation. J. Intell. Mater. Syst. Struct. 2007, 18, 147–152. [Google Scholar] [CrossRef]
- Kashyap, N.; Kumar, N.; Kumar, M. Hydrogels for pharmaceutical and biomedical applications. Crit. Rev. Ther. Drug Carr. Syst. 2005, 22, 107–149. [Google Scholar] [CrossRef]
- Stamatialis, D.F.; Papenburg, B.J.; Gironés, M.; Saiful, S.; Bettahalli, S.N.M. Wessling, Medical applications of membranes: Drug delivery, artificial organs and tissue engineering. J. Membr. Sci. 2008, 308, 1–34. [Google Scholar] [CrossRef]
- Roy, D.; Brooks, W.L.A.; Sumerlin, B.S. New directions in thermoresponsive polymers. Chem. Soc. Rev. 2013, 42, 7214–7243. [Google Scholar] [CrossRef] [PubMed]
- Gibson, M.I.; Reilly, R.K. To aggregate, or not to aggregate? Considerations in the design and application of polymeric thermally-responsive nanoparticles. Chem. Soc. Rev. 2013, 42, 7204–7213. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive terpolymers based on methacrylate monomers: Effect of architecture and composition. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 775–783. [Google Scholar] [CrossRef]
- Li, Z.; Wang, F.; Roy, S.; Sen, C.K.; Guan, J. Injectable, highly flexible, and thermosensitive hydrogels capable of delivering superoxide dismutase. Biomacromolecules 2009, 10, 3306–3316. [Google Scholar] [CrossRef] [PubMed]
- Hacker, M.C.; Klouda, L.; Ma, B.B.; Kretlow, J.D.; Mikos, A.G. Synthesis and characterization of injectable, thermally and chemically gelable, amphiphilic poly(N-isopropylacrylamide)-based macromers. Biomacromolecules 2008, 9, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Xi, J.; Zhao, X.; Tang, X. Deswelling Comparison of Temperature-Sensitive Poly(N-isopropylacrylamide) Microgels Containing Functional -OH Groups with Different Hydrophilic Long Side Chains. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 3575–3583. [Google Scholar] [CrossRef]
- Ito, D.; Kubota, K. Solution properties and thermal behavior of poly(N-n-propylacrylamide) in water. Macromolecules 1997, 30, 7828–7834. [Google Scholar] [CrossRef]
- Ono, Y.; Shikata, T. Hydration and Dynamic Behavior of Poly(N-isopropylacrylamide)s in Aqueous Solution: A Sharp Phase Transition at the Lower Critical Solution Temperature. J. Am. Chem. Soc. 2006, 128, 10030–10031. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Cheng, S.-X.; Zhang, X.-Z.; Zhuo, R.-X. Thermo-sensitive polymeric micelles based on poly(N-isopropylacrylamide) as drug carriers. Prog. Polym. Sci. 2009, 34, 893–910. [Google Scholar] [CrossRef]
- Rzaev, Z.M.O.; Dinçer, S.; Pişkin, E. Functional copolymers of N-isopropylacrylamide for bioengineering applications. Prog. Polym. Sci. 2007, 32, 534–595. [Google Scholar] [CrossRef]
- Qin, S.; Geng, Y.; Discher, D.E.; Yang, S. Temperature-Controlled Assembly and Release from Polymer Vesicles of Poly(ethylene oxide)-blockpoly(N-isopropylacrylamide). Adv. Mater. 2006, 18, 2905–2909. [Google Scholar] [CrossRef]
- Li, W.; Zhao, H.; Qian, W.; Li, H.; Zhang, L.; Ye, Z.; Zhang, G.; Xia, M.; Li, J.; Gao, J.; et al. Chemotherapy for gastric cancer by finely tailoring anti-Her2 anchored dual targeting immunomicelles. Biomaterials 2012, 33, 5349–5362. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Hillmyer, M.A.; Lodge, T.P. Micellization and Micelliar Aggregation of Poly(ethylene-alt-propylene)-b-poly(ethyleneoxide)-b-poly(N-isopropylacrylamide) Triblock Terpolymers in Water. Macromolecules 2011, 44, 1635–1641. [Google Scholar] [CrossRef]
- Zhou, C.; Hillmyer, M.A.; Lodge, T.P. Efficient Formation of Multicompartment Hydrogels by Stepwise Self-Assembly of Thermoresponsive ABC Triblock Terpolymers. J. Am. Chem. Soc. 2012, 134, 10365–10368. [Google Scholar] [CrossRef] [PubMed]
- Mi, L.; Xue, H.; Li, Y.; Jiang, S.A. Thermoresponsive Antimicrobial Wound Dressing Hydrogel Based on a Cationic Betaine Ester. Adv. Funct. Mater. 2011, 21, 4028–4034. [Google Scholar] [CrossRef]
- Patenaude, M.; Hoare, T. Injectable, Mixed Natural-Synthetic Polymer Hydrogels with Modular Properties. Biomacromolecules 2012, 13, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Gehan, H.; Fillaud, L.; Chehimi, M.M.; Aubard, J.; Hohenau, A.; Felidj, N.; Mangeney, C. Thermo-induced Electromagnetic Coupling in Gold/Polymer Hybrid Plasmonic Structures Probed by Surface-Enhanced Raman Scattering. ACS Nano 2010, 4, 6491–6500. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Sukhishvili, S.A. Temperature-Induced Swelling and Small Molecule Release with Hydrogen-Bonded Multilayers of Block Copolymer Micelles. ACS Nano 2009, 3, 3595–3605. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Cambre, J.N.; Sumerlin, B.S. Future perspectives and recent advances in stimuli-responsive materials. Prog. Polym. Sci. 2010, 35, 278–301. [Google Scholar] [CrossRef]
- Lackey, C.A.; Press, O.W.; Hoffman, A.S.; Stayton, P.S. A. Biomimetic pH-Responsive Polymer Directs Endosomal Release and Intracellular Delivery of an Endocytosed Antibody Complex. Bioconjugate Chem. 2002, 13, 996–1001. [Google Scholar] [CrossRef]
- Sugihara, S.; Yamashita, K.; Matsuzuka, K.; Ikeda, I.; Maeda, Y. Transformation of Living Cationic Polymerization of Vinyl Ethers to RAFT Polymerization Mediated by a Carboxylic RAFT Agent. Macromolecules (Washington DC USA) 2012, 45, 794–804. [Google Scholar] [CrossRef]
- Meeussen, F.; Nies, E.; Bergmans, H.; Verbrugghe, S.; Goethals, E.; Du Prez, F. Phase behaviour of poly (N-vinyl caprolactam) in water. Polymer 2000, 41, 8597–8602. [Google Scholar] [CrossRef]
- Sun, S.; Wu, P. Infrared Spectroscopic Insight into Hydration Behavior of Poly(N-vinylcaprolactam) in Water. J. Phys. Chem. B 2011, 115, 11609–11618. [Google Scholar] [CrossRef] [PubMed]
- Jiří, S. NMR investigations of phase transition in aqueous polymer solutions and gels. Curr. Opin. Colloid Interface Sci. 2009, 14, 184–191. [Google Scholar]
- Kermagoret, A.; Fustin, C.-A.; Bourguignon, M.; Detrembleur, C.; Jerome, C.; Debuigne, A. One-pot controlled synthesis of double thermoresponsive N-vinylcaprolactam-based copolymers with tunable LCSTs. Polym. Chem. 2013, 4, 2575–2583. [Google Scholar] [CrossRef]
- Schild, H.G.; Tirrell, D.A. Microcalorimetric determination of lower critical solution temperature in aqueous polymer solutions. J. Phys. Chem. 1990, 94, 4352–4356. [Google Scholar] [CrossRef]
- Kirsh, Y.E. Water Soluble Poly-N-Vinylamides; John Wiley & Sons: Chichester, UK, 1998. [Google Scholar]
- Chilkoti, A.; Dreher, M.R.; Meyer, D.E.; Raucher, D. Targeted Drug Delivery by Thermally Responsive Polymers. Adv. Drug Deliv. 2002, 54, 613–630. [Google Scholar] [CrossRef]
- Vihola, H. Studies on Thermosensitive Poly(N-vinylcaprolactam) Based Polymers for Pharmaceutical Applications; University of Helsinki: Helsinki, Finland, 2007. [Google Scholar]
- Shtanko, N.I.; Lequieu, W.; Goethals, E.J.; Prez, F.E. pH- and Thermoresponsive Properties of Poly(N-vinylcaprolactam-co-acrylic acid) Copolymers. Polym. Int. 2003, 52, 1605–1610. [Google Scholar] [CrossRef]
- Makhaeva, E.E.; Tenhu, H.; Khoklov, A.R. Conformational Changes of Poly(vinylcaprolactam) Macromolecules and Their Complexes with Ionic Surfactants in Aqueous Solution. Macromolecules 1998, 31, 6112–6118. [Google Scholar] [CrossRef]
- Lau, A.C.W.; Wu, C. Thermally Sensitive and Biocompatible Poly(Nvinylcaprolactam): Synthesis and Characterization of High Molar Mass Linear Chains. Macromolecules 1999, 32, 581–584. [Google Scholar] [CrossRef]
- Tager, A.A.; Safronov, A.P.; Berezyuk, E.A.; Galaev, I.Yu. Lower Critical Solution Temperature and Hydrophobic Hydration in Aqueous Polymer Solutions. Colloid Polym. Sci. 1994, 272, 1234–1239. [Google Scholar] [CrossRef]
- Vihola, H.; Laukkanen, A.; Valtola, L.; Tenhu, H.; Hirvonen. Cytotoxicity of thermosensitive polymers poly(N-isopropyl acrylamide), poly(N-vinyl caprolactam) and amphiphilically modified poly(N-vinyl caprolactam). J. Biomater. 2005, 26, 3055–3064. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Liu, F.; Kozlovskaya, V.; Palchak, Z.; Kharlampieva, E. Thermoresponsive Micelles from Double LCST-Poly(3- methyl-N-vinylcaprolactam) Block Copolymers for Cancer Therapy. ACS Macro Lett. 2015, 4, 308–311. [Google Scholar] [CrossRef]
- Nakayama, M.; Okano, T.; Miyazaki, T.; Kohori, F.; Sakai, K.; Yokoyama, M. Molecular design of biodegradable polymeric micelles for temperature-responsive drug release. J. Controll. Release 2006, 115, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, J.; Nakayama, M.; Okano, T. Temperature-responsive polymeric micelles for optimizing drug targeting to solid tumors. J. Controlled Release 2014, 193, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Kozlovskaya, V.; Medipelli, S.; Xue, B.; Ahmad, F.; Saeed, M.; Cropek, D.; Kharlampieva, E. Temperature-Sensitive Polymersomes for Controlled Delivery of Anticancer Drugs. Chem. Mater. 2015, 27, 7945–7956. [Google Scholar] [CrossRef]
- Prabaharan, M.; Grailer, J.J.; Steeber, D.A.; Gong, S. Thermosensitive micelles based on folate-conjugated poly(N-vinylcaprolactam)-block-poly(ethylene glycol) for tumor targeted drug delivery. Macromol. Biosci. 2009, 9, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Kozlovskaya, V.; Cox, C.P.; Wang, Y.; Saeed, M.; Kharlampieva, E. Synthesis and self-assembly of thermosensitive double-hydrophilic poly(Nvinylcaprolactam)-b-poly(N-vinyl-2-pyrrolidone) diblock copolymers. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 2725–2737. [Google Scholar] [CrossRef]
- Elenu, E.M.; Le, T.M.T.; Sergei, G.S.; Alexei, R.K. Thermoshrinking behavior of poly(vinylcapro1actam) gels in aqueous solution. Macromol. Chem. Phys. 1996, 197, 1973–1982. [Google Scholar]
- Volodymyr, B.; Sven, R. Monitoring of the Gelation Process on a Radical Chain Crosslinking Reaction Based on N-Vinylcaprolactam by Using Dynamic Light Scattering, Macromol. Chem. Phys. 2004, 205, 724–730. [Google Scholar]
- Leslie, D.M.; Jeffrey, A.H. Tensile Properties of Poly(N-vinyl caprolactam) Gels; National Aeronautics and Space Administration Langley Research Center Hampton: Virginia, USA, 2004; pp. 1–10. [Google Scholar]
- Mamytbekov, G.; Bouchal, K.; Ilavsky, M. Phase transition in swollen gels 26. Effect of charge concentration on temperature dependence of swelling and mechanical behavior of poly(N-vinlycaprolactam) gels. Eur. Polym. J. 1999, 35, 1925–1933. [Google Scholar] [CrossRef]
- Ilavský, M.; Mamytbekov, G.; Sedláková, Z.; Hanyková, L.; Dušek, K. Phase transition in swollen gels 29. Temperature dependences of swelling and mechanical behaviour of poly(N-vinylcaprolactam-co-1-vinyl-2-pyrrolidone) gels in water. Polym. Bull. 2001, 46, 99–106. [Google Scholar]
- Váček, J.; Radecki, M.; Zhigunov, A.; Šťastná, J.; Valentová, H.; Sedláková, Z. Structures and interactions in collapsed hydrogels of thermoresponsive interpenetrating polymer networks. Colloids Polym. Sci. 2015, 293, 709–720. [Google Scholar]
- Vyshivannaya, O.V.; Laptinskaya, T.V.; Makhaeva, E.E.; Khokhlov, A.R. Dynamic Light Scattering in Semi_Interpenetrating Polymer Networks Based on Polyacrylamide and Poly(N_vinylcaprolactam). Polym. Sci. Ser. A 2012, 54, 693–706. [Google Scholar] [CrossRef]
- Cakal, E.; Cavus, S. Novel Poly(N-vinylcaprolactam-co-2-(diethylamino)ethyl methacrylate) Gels: Characterization and Detailed Investigation on Their Stimuli-Sensitive Behaviors and Network Structure. Ind. Eng. Chem. Res. 2010, 49, 1741–11751. [Google Scholar] [CrossRef]
- Roshan Deen, G.; Lim, E.K.; Hao Mah, C.; Heng, K.M. New Cationic Linear Copolymers and Hydrogels of N-Vinyl Caprolactam and N-Acryloyl-N′-ethyl Piperazine: Synthesis, Reactivity, Influence of External Stimuli on the LCST and Swelling Properties. Ind. Eng. Chem. Res. 2012, 51, 13354–13365. [Google Scholar] [CrossRef]
- Selva, C.; Elc, C. Synthesis and Characterization of Novel Poly(N-vinylcaprolactam-coitaconic Acid) Gels and Analysis of pH and Temperature Sensitivity. Ind. Eng. Chem. Res. 2012, 51, 1218–1226. [Google Scholar]
- Gaballa, H.A.; Geever, L.M.; Killion, J.A.; Higginbotham, C.L. Synthesis and characterization of physically crosslinked N-vinylcaprolactam, acrylic acid, methacrylic acid, and N,N-dimethylacrylamide hydrogels. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1555–1564. [Google Scholar] [CrossRef]
- Zavgorodnya, O.; Kozlovskaya, V.; Liang, X.; Kothalawala, N.; Catledge, S.A.; Dass, A.; Kharlampieva, E. Temperature-responsive properties of poly(N-vinylcaprolactam) multilayer hydrogels in the presence of Hofmeister anions. Mater. Res. Express 2014, 1. [Google Scholar] [CrossRef]
- Sanna, R.; Fortunati, E.; Alzari, V.; Nuvoli, D.; Terenzi, A.; Casula, M.F.; Kenny, J.M.; Mariani, A. Poly(N-vinylcaprolactam) nanocomposites containing nanocrystalline cellulose: A green approach to thermoresponsive hydrogels. Cellulose 2013, 20, 2393–2402. [Google Scholar] [CrossRef]
- Gao, Y.; Au-Yeung, S.C.F.; Wu, C. Interaction between Surfactant and Poly(N-vinylcaprolactam) Microgels. Macromolecules 1999, 32, 674–3677. [Google Scholar] [CrossRef]
- Boyko, V.; Richter, S.; Grillo, I.; Geissler, E. Structure of Thermosensitive Poly(Nvinylcaprolactam-co-N-vinylpyrrolidone) Microgels. Macromolecules 2005, 38, 5266–5270. [Google Scholar] [CrossRef]
- Laukkanen, A.; Hietala, S.; Maunu, S.L.; Tenhu, H. Poly(N-vinylcaprolactam) Microgel Particles Grafted with Amphiphilic Chains. Macromolecules 2000, 33, 8703–8708. [Google Scholar] [CrossRef]
- Yibing, G.; Steve, C.F.A.Y.; Chi, W. Interaction between surfactant and poly(N-vinyl caprolactam) microgels. Macromolecules 1999, 32, 3674–3677. [Google Scholar]
- Imaz, A.; Forcada, J. N-Vinylcaprolactam-Based Microgels: Synthesis and Characterization. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 2510–2524. [Google Scholar] [CrossRef]
- Pich, A.; Tessier, A.; Boyko, V.; Lu, Y.; Adler, H.P. Synthesis and Characterization of Poly(vinylcaprolactam)-Based Microgels Exhibiting Temperature and pH-Sensitive Properties. Macromolecules 2006, 39, 7701–7707. [Google Scholar] [CrossRef]
- Imaz, A.; Forcada, J. N-Vinylcaprolactam-Based Microgels: Effect of the Concentration and Type of Cross-linker. J. Polym. Sci. A Polym. Chem. 2008, 46, 2766–2775. [Google Scholar] [CrossRef]
- Imaz, A.; Forcada, J. Optimized buffered polymerizations to produce N-vinylcaprolactam-based Microgels. Eur. Polym. J. 2009, 45, 3164–3175. [Google Scholar] [CrossRef]
- Peng, S.; Wu, C. Ca2+-induced Thermoreversible and Controllable Complexation of Poly(N-vinylcaprolactam-co-sodium acrylate) Microgels in Water. J. Phys. Chem. B 2001, 105, 2331–2335. [Google Scholar] [CrossRef]
- Peng, S.; Wu, C. Ca+2-induced complexation between thermally sensitive spherical poly(N-vinyl-caprolactam-co-sodium acrylate) microgels and linear gelatin chains in water. Polymer 2001, 42, 7343–7347. [Google Scholar] [CrossRef]
- Iskakov, R.M.; Sedinkin, S.V.; Mamytbekov, G.K.; Batyrbekov, E.O.; Bekturov, E.A.; Zhubanov, B.A. Controlled Release of Farmazin from Thermosensitive Gels Based on Poly(N-vinylcaprolactam). Russ J. Appl. Chem. 2004, 7, 339–341. [Google Scholar] [CrossRef]
- Rama Subba Reddy, P.; Madhusudana Rao, K.; Krishna Rao, K.S.V.; Schichpanov, Y.; Ha, C.S. Synthesis of Alginate Based Silver Nanocomposite Hydrogels for Biomedical Applications. Macromol. Res. 2014, 8, 832–842. [Google Scholar] [CrossRef]
- Madhusudana-Rao, K.; Krishna-Rao, K.S.V.; Sudhakar, P.; Chowdoji-Rao, K.; Subha, M.C.S. Synthesis and Characterization of biodegradable Poly (Vinyl caprolactam) grafted on to sodium alginate and its microgels for controlled release studies of an anticancer drug. J. Appl. Pharm. Sci. 2013, 3, 061–069. [Google Scholar]
- Swamy, B.Y.; Chang, J.H.; Ahn, H.; Lee, W.K.; Chung, I. Thermoresponsive N-vinyl caprolactam grafted sodium alginate hydrogel beads for the controlled release of an anticancer drug. Cellulose 2013, 20, 1261–1273. [Google Scholar] [CrossRef]
- Prabaharan, M.; Grailer, J.J.; Steeber, D.A.; Gong, S. Stimuli-responsive chitosan-graftpoly(N-vinylcaprolactam) as promising material for controlled hydrophobic drug delivery. Macromol.Biosci. 2008, 8, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Vihola, H.; Laukkanen, A.; Tenhu, H.; Hirvonen, J. Drug Release Characteristics of Physically Cross-Linked Thermosensitive Poly(N-vinylcaprolactam) Hydrogel Particles. J. Pharm. Sci. 2008, 97, 4783–4793. [Google Scholar] [CrossRef] [PubMed]
- Hantzschel, N.; Hund, R.D.; Hund, H.; Schrinner, M.; Lück, C.; Pich, A. Hybrid Microgels with Antibacterial Properties. Macromol. Biosci. 2009, 9, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.; Gao, S.; Wang, W.; Zhang, M.; Zhang, Q.; Wang, C.; Li, C.; Kong, D. Temperature/pH dual responsive microgels of crosslinked poly(N-vinylcaprolactam-co-undecenoic acid) as biocompatible materials for controlled release of doxorubicin. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, J.; Chang, B.; Sun, Y.; Yang, W. Poly(N-vinylcaprolactam)-Based Biodegradable Multiresponsive Microgels for Drug Delivery. Biomacromolecules 2013, 14, 3034–3046. [Google Scholar] [CrossRef] [PubMed]
- Malmsten, M. Soft drug delivery systems. Soft Matter 2006, 2, 760–769. [Google Scholar] [CrossRef]
- Napier, M.E.; Desimone, J.M. Nanoparticle drug delivery platform. Polym. Rev. 2007, 47, 321–327. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol. Rev. 2001, 53, 283–318. [Google Scholar] [PubMed]
- Madhusudana Rao, K.; Mallikarjuna, B.; Krishna Rao, K.S.V.; Siraj, S.; Chowdoji Rao, K.; Subha, M.C.S. Novel thermo/pH sensitive nanogels composed from poly(N-vinylcaprolactam) for controlled release of an anticancer drug. Colloids Surf. B 2013, 102, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Sudhakara, K.; Madhusudana Rao, K.; Subha, M.C.S.; Chowdoji Rao, K.; RotimiSadiku, E. Temperature-responsive poly(N-vinylcaprolactam-co-hydroxyethyl methacrylate) nanogels for controlled release studies of curcumin. Des. Monomers Polym. 2015, 18, 705–713. [Google Scholar] [CrossRef]
- Gonalez-Ayon, M.A.; Adriana Sanudo-Barajas, J.; Picos-Corrales, L.A.; Licea-Claverie, A. PNVCL-PEGMA nanohydrogels with tailored transition temperature for controlled release of 5-Fluorouracil. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 2662–2672. [Google Scholar] [CrossRef]
- Liu, J.; Detrembleur, C.; Hurtgen, M.; Debuigne, A.; Pauw-Gillet, M.C.; Mornet, S.; Duguet, E.; Jérome, C. Reversibly-crosslinked Thermo- and Redox-responsive Nanogels for Controlled Drug Release. Polym. Chem. 2014, 5, 77–88. [Google Scholar] [CrossRef]
- Aguirre, G.; Ramos, J.; Forcada, J. Synthesis of new enzymatically degradable thermo-responsive nanogels. Soft Matter 2013, 9, 261–270. [Google Scholar] [CrossRef]
- Rejinold, N.S.; Baby, T.; Chennazhi, K.P.; Jayakumar, R. Multi Drug Loaded Thermo-Responsive Fibrinogen-graft-Poly(N-vinyl Caprolactam) Nanogels for Breast Cancer Drug Delivery. J. Biomed. Nanotechnol. 2015, 11, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Li, M.; Zhang, B.; Neoh, K.G.; Kang, E.T. Hyperbranched polycaprolactone-clickpoly(N-vinylcaprolactam) amphiphilic copolymers and their applications as temperature-responsive membranes. J. Mater. Chem. B 2014, 2, 814–825. [Google Scholar] [CrossRef]
- Liang, X.; Kozlovskaya, V.; Chen, Y.; Zavgorodnya, O.; Kharlampieva, E. Thermosensitive Multilayer Hydrogels of Poly(N-vinylcaprolactam) as Nanothin Films and Shaped Capsules. Chem. Mater. 2012, 24, 3707–3719. [Google Scholar] [CrossRef] [PubMed]
- Donova, M.V.; Kuzkina, I.F.; Arinbasarova, A.Y.; Pashkin, I.I.; Markvicheva, E.E.; Baklashova, T.G.; Sukhodolskaya, G.V.; Fokina, V.V.; Kirsh, Y.E.; Koshcheyenko, K.A.; et al. Poly(N-vinylcaprolactam) gel. A novel matrix for entrapment of microorganism. Biotechnol. Tech. 1993, 7, 415–422. [Google Scholar] [CrossRef]
- Kuptsova, S.V.; Mareeva, T.Y.; Vikhrov, A.A.; Dugina, T.N.; Strukova, S.M.; Belokon, Y.N.; Kochetkov, K.A.; Baranova, E.N.; Zubov, V.P.; Poncelet, D.; et al. Immobilized enzymes and cells in poly(N-vinyl caprolactam)-based hydrogels. Appl. Biochem. Biotechnol. 2000, 88, 145–157. [Google Scholar]
- Markvicheva, E.A.; Bronin, A.S.; Kudryavtseva, N.E.; Rumsh, L.D.; Kirsh, Y.E.; Zubov, V.P. Immobilization of proteases in composite hydrogel based in poly(N-vinylcaprolactam). Biotechnol. Tech. 1994, 8, 143–148. [Google Scholar] [CrossRef]
- Wan, D.; Zhou, Q.; Pu, H.; Yang, G. Controlled radical polymerization of N-vinylcaprolactam mediated by xanthate or dithiocarbamate. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3756–3765. [Google Scholar] [CrossRef]
- Beija, M.; Marty, J.-D.; Destarac, M. Thermoresponsive poly(N-vinyl caprolactam)-coated gold nanoparticles: Sharp reversible response and easy tenability. Chem. Commun. 2011, 47, 2826–2828. [Google Scholar] [CrossRef] [PubMed]
- Marie, H.; Ji, L.; Antoine, D.; Christine, J.; Christophe, D. Synthesis of thermo-responsive poly(N-vinylcaprolactam)-containing block copolymers by cobalt-mediated radical polymerization. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 400–408. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, K.M.; Rao, K.S.V.K.; Ha, C.-S. Stimuli Responsive Poly(Vinyl Caprolactam) Gels for Biomedical Applications. Gels 2016, 2, 6. https://doi.org/10.3390/gels2010006
Rao KM, Rao KSVK, Ha C-S. Stimuli Responsive Poly(Vinyl Caprolactam) Gels for Biomedical Applications. Gels. 2016; 2(1):6. https://doi.org/10.3390/gels2010006
Chicago/Turabian StyleRao, Kummara Madhusudana, Kummari Subba Venkata Krishna Rao, and Chang-Sik Ha. 2016. "Stimuli Responsive Poly(Vinyl Caprolactam) Gels for Biomedical Applications" Gels 2, no. 1: 6. https://doi.org/10.3390/gels2010006
APA StyleRao, K. M., Rao, K. S. V. K., & Ha, C.-S. (2016). Stimuli Responsive Poly(Vinyl Caprolactam) Gels for Biomedical Applications. Gels, 2(1), 6. https://doi.org/10.3390/gels2010006









