Tailoring Electronic Structures via Ce/C Co-Doping and Oxygen Vacancy in TiO2 Aerogels for Enhanced Solar Fuel Production
Abstract
1. Introduction
2. Results and Discussion
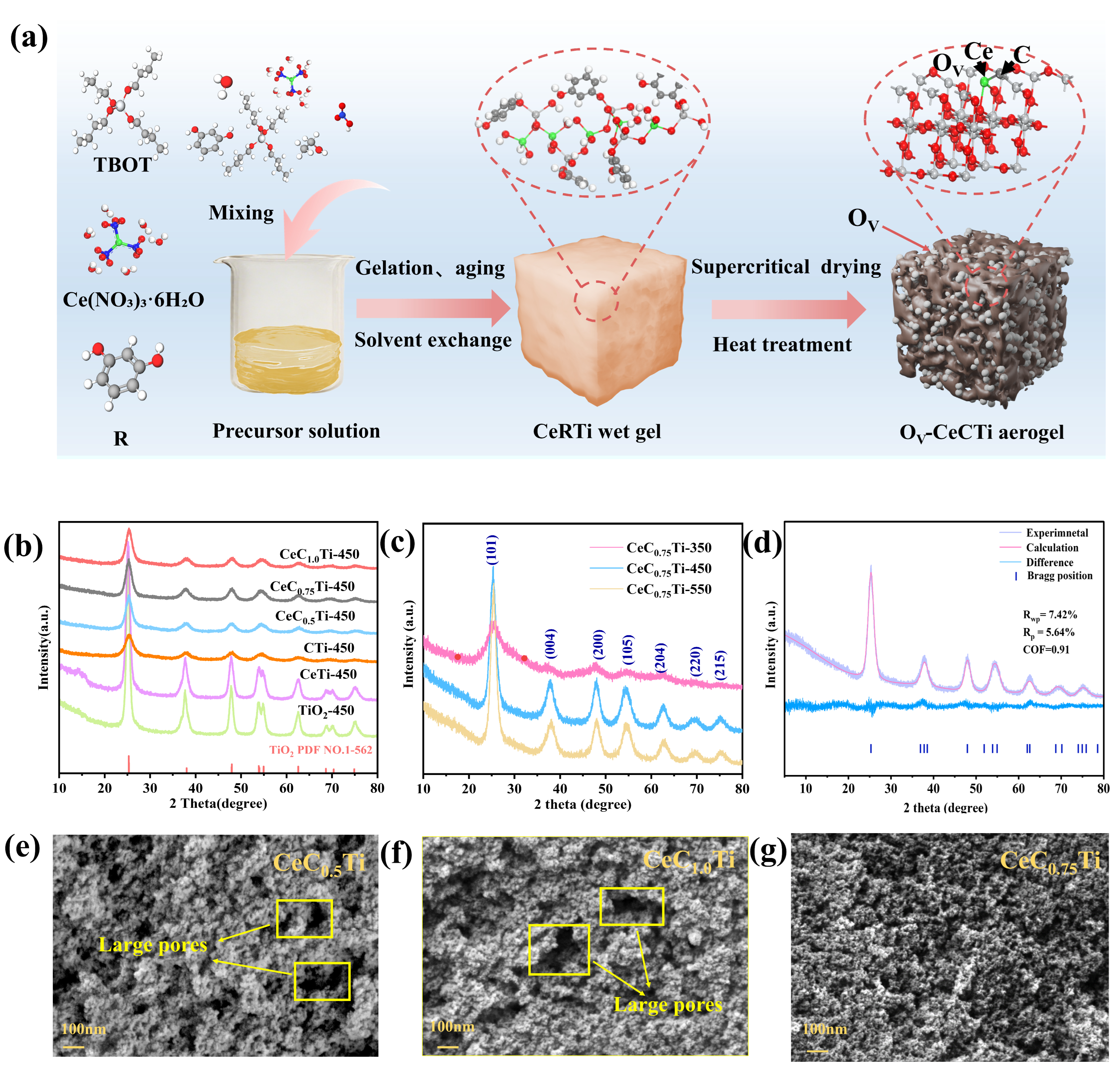
2.1. Chemical Composition and Structural Analysis
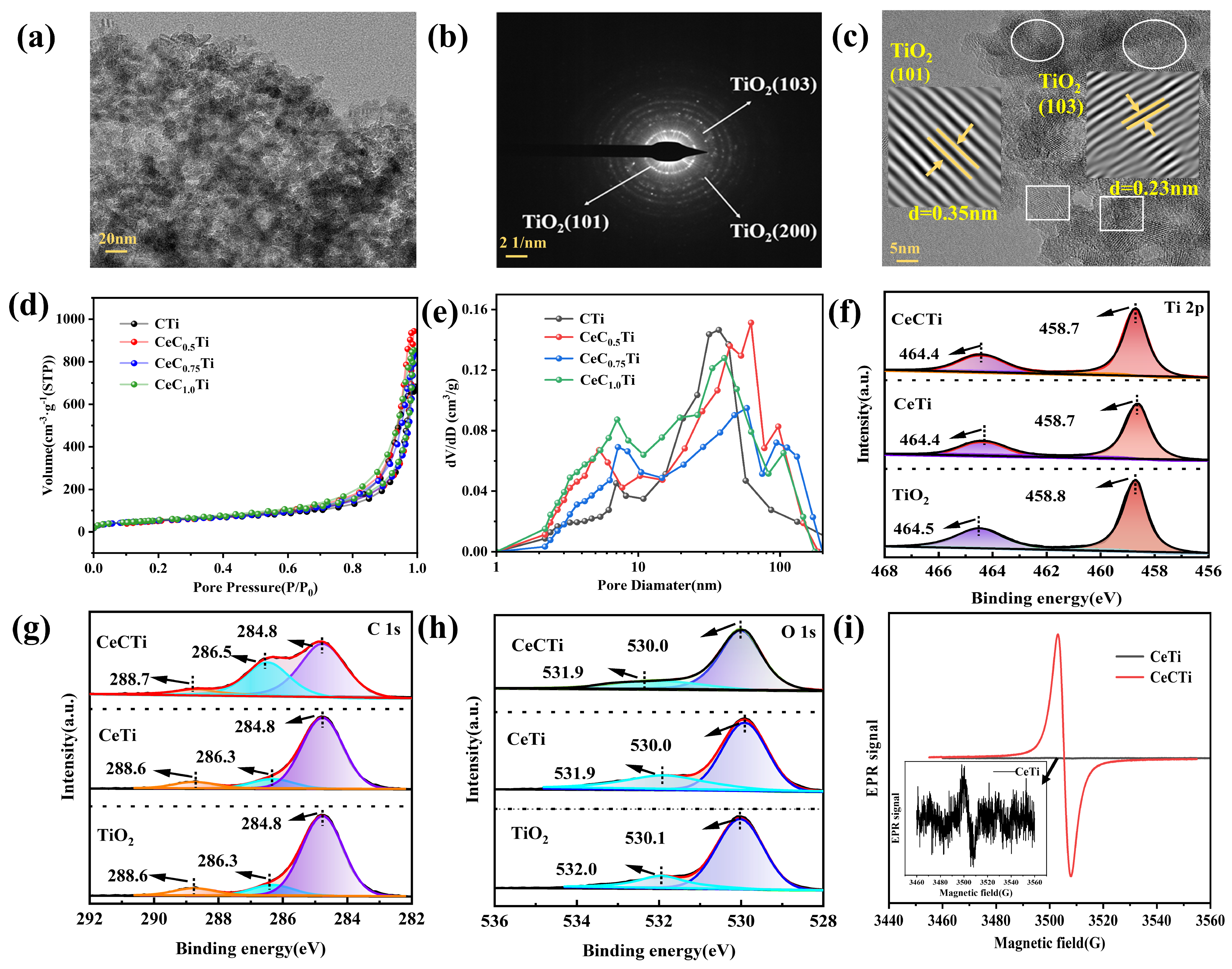
2.2. Multiscale Structure Analysis
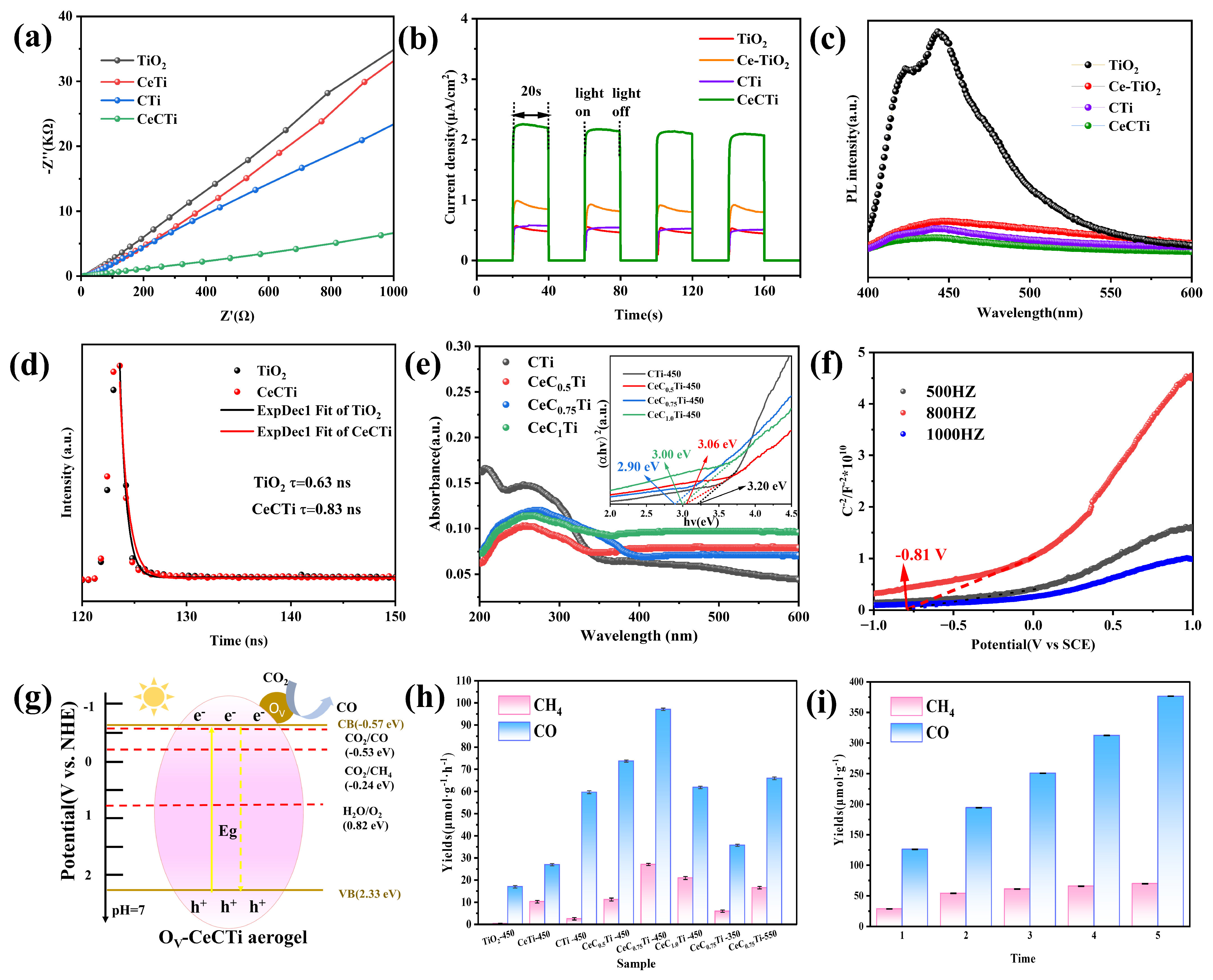
2.3. Photoelectrochemical Properties and Reduction Performance
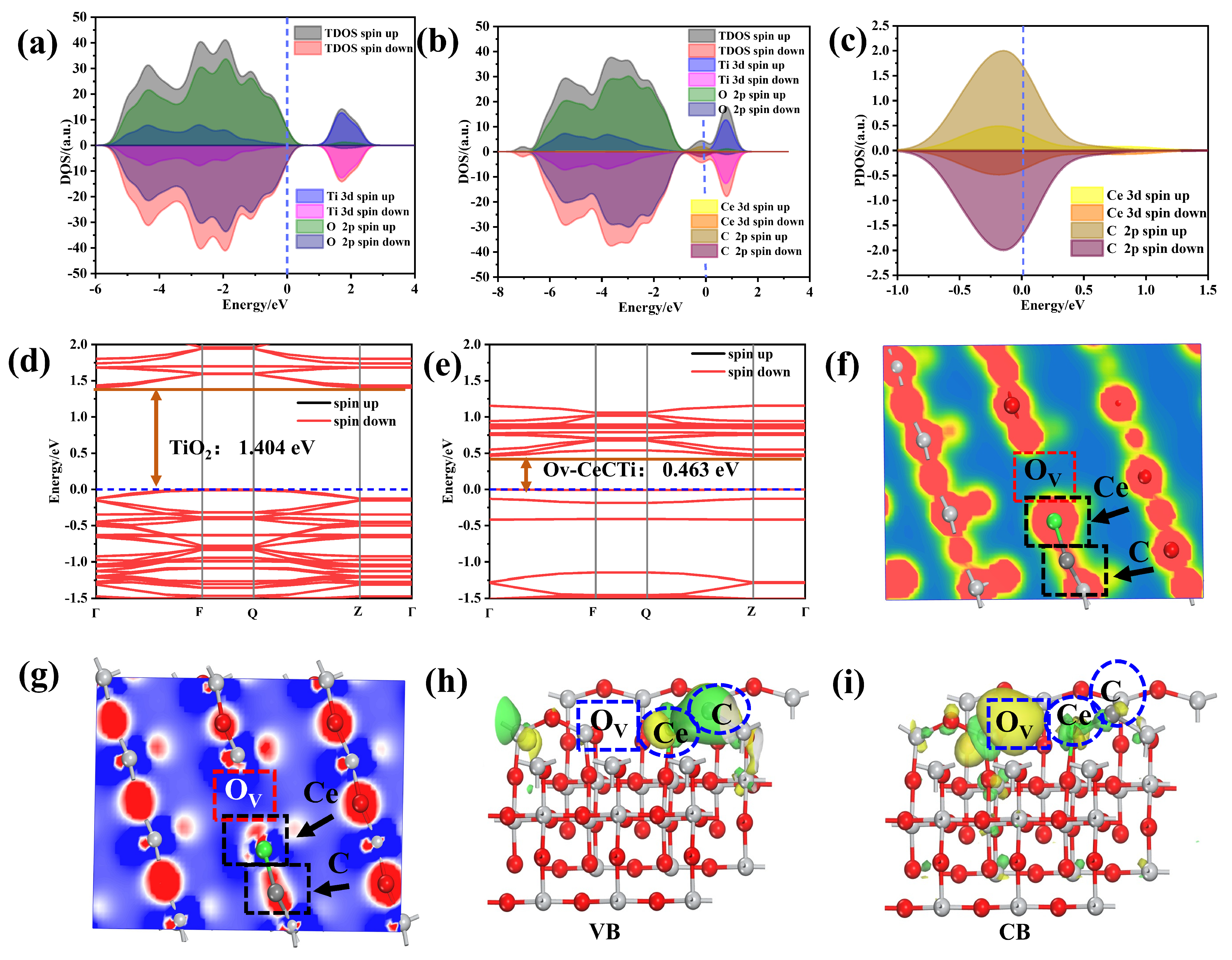
2.4. DFT Calculation
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Method
4.2.1. Synthesis of the CeCTi Aerogels
4.2.2. Characterizations
4.2.3. Photoactivity Test
4.2.4. Theoretical Calculations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CO2 | Carbon Dioxide |
| DFT | Density Functional Theory |
References
- Rhimi, B.; Zhou, M.; Yan, Z.; Cai, X.; Jiang, Z. Cu-Based Materials for Enhanced C2+ Product Selectivity in Photo-/Electro-Catalytic CO2 Reduction: Challenges and Prospects. Nano-Micro Lett. 2024, 16, 2150–5551. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, D.; Yang, J.; Duan, L.; Zhang, P.; Gao, M.; He, J.; Gu, Y.; Lan, K.; Zhang, J.; et al. One-Dimensional Single-Crystal Mesoporous TiO2 Supported CuW6O24 Clusters as Photocatalytic Cascade Nanoreactor for Boosting Reduction of CO2 to CH4. Adv. Mater. 2024, 36, 2409188. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xu, H.; Yin, K.; Li, Z.; Fan, J.; Wu, X.; Wu, Z. Modulating charge transfer for photo-driven N2O reduction via electronegativity differences between Cu and support. Appl. Catal. B-Environ. 2025, 377, 125528. [Google Scholar] [CrossRef]
- Hao, X.; Wu, J.; Cai, X.; Li, K.; Song, C.; Guo, X. Plasmonic Ag-decorated GaN for efficient photothermal CO2 conversion. Appl. Catal. B-Environ. 2025, 373, 125366. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Zhong, C.; He, C.; Khan, M.; Liu, D.; Wang, J.; Yang, R.; Kan, M.; Wang, L.; et al. High-Loading Cu Single-Atom Engineering on g-C3N4 for Visible-Light CO2 Photoreduction. Small 2025, 21, 2503390. [Google Scholar] [CrossRef]
- Hu, C.; Cao, J.; Jia, X.; Sun, H.; Lin, H.; Chen, S. Difunctional Ni2P decorated novel Z-scheme BiVO4/g-C3N4 heterojunction for achieving highly efficient CO2 reduction and tetracycline oxidation. Appl. Catal. B-Environ. 2023, 337, 122957. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, S.; La, P.; Zhao, D.; Ji, Z.; Wang, X. 3D-printing advanced ZIF-67@ aluminum phosphate/Al2O3 ceramic catalyst by aluminum phosphate-assisted surface bonding. Mater. Sci. Addit. Manuf. 2025, 4, 025220037. [Google Scholar] [CrossRef]
- Si, Y.; Li, Y.; Cheng, M.; Ren, Y.; Zhou, J.; Sun, Z.; Guan, J.; Liu, M.; Duan, L.; Li, N. Synergistic dual-oxygen-vacancy design boosts photothermal CO2 reduction into ethylene. Nano Energy 2025, 138, 110838. [Google Scholar] [CrossRef]
- Chang, W.; Wang, H.; Wu, T.; Zhang, S.; Li, Y.; Wang, J.; Yang, Y.; Wang, L. Interfacial lattice matched sub-2 nm RuO2 on rutile TiO2 nanorod for visible light driven CO2 conversion to CH4. J. Mater. Sci. Technol. 2025, 235, 28–36. [Google Scholar] [CrossRef]
- Lundberg, D.J.; Parviz, D.; Kim, H.; Lebowitz, M.; Lu, R.X.; Strano, M.S. Universal Kinetic Mechanism Describing CO2 Photoreductive Yield and Selectivity for Semiconducting Nanoparticle Photocatalysts. J. Am. Chem. Soc. 2022, 144, 13623–13633. [Google Scholar] [CrossRef]
- Xu, X.; Labidi, A.; Luo, T.; Gao, T.; Nuraje, N.; Zvereva, I.; Wang, C. In situ fabrication of TiO2 nanoparticles/2D porphyrin metal–organic frameworks for enhancing the photoreduction of CO2 to CO. J. Mater. Chem. A 2025, 13, 11389–11395. [Google Scholar] [CrossRef]
- Ban, C.; Wang, Y.; Feng, Y.; Zhu, Z.; Duan, Y.; Ma, J.; Zhang, X.; Liu, X.; Zhou, K.; Zou, H.; et al. Photochromic single atom Ag/TiO2 catalysts for selective CO2 reduction to CH4. Energy Environ. Sci. 2024, 17, 518–530. [Google Scholar] [CrossRef]
- Li, Q.; Wang, H.; Zhang, M.; Li, G.; Chen, J.; Jia, H. Suppressive Strong Metal-Support Interactions on Ruthenium/TiO2 Promote Light-Driven Photothermal CO2 Reduction with Methane. Angew. Chem. Int. Ed. 2023, 62, e202300129. [Google Scholar] [CrossRef]
- Pu, W.; Zhou, Y.; Song, X.; Meng, Z.; Niu, J.; Li, Y.; Duan, Y.; Carabineiro, S.A.C.; Wang, W.; Dong, F. Modulating free radical types in ZnSn(OH)6 through orbital rehybridization by interstitial C-doping for deep oxidation of toluene. J. Mater. Sci. Technol. 2026, 246, 247–255. [Google Scholar] [CrossRef]
- Xu, M.; Li, R.; Wang, W.; Jia, T.; Jia, W.; Wu, Y.; Zhang, C.; Wang, W.; Zhang, L. The promotion of CO2 reduction by light-induced magnetism in plasmonic metal nanoparticles. Appl. Catal. B-Environ. 2025, 378, 125570. [Google Scholar] [CrossRef]
- Qi, C.; Min, P.; Zhou, X.; Jin, M.; Sun, X.; Wu, J.; Liu, Y.; Zhang, H.; Yu, Z. Multifunctional Asymmetric Bilayer Aerogels for Highly Efficient Electromagnetic Interference Shielding with Ultrahigh Electromagnetic Wave Absorption. Nano-Micro Lett. 2025, 17, 291. [Google Scholar] [CrossRef]
- Yu, G.; Shao, G.; Xu, Z.; Chen, Y.; Huang, X. Hierarchical interface-engineered magnetic graphene-SiCN aerogels via a stepwise confinement strategy for low-frequency and broadband microwave absorption. Adv. Ceram. 2025, 14, 9221187. [Google Scholar] [CrossRef]
- Katona, G.; Sipos, B.; Csóka, I. Advancements in the Field of Protein-Based Hydrogels: Main Types, Characteristics, and Their Applications. Gels 2025, 11, 306. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, M.; Ji, D.; Ma, C.; Yuan, B.; Feng, R.; Tan, J.; Yang, B. Resin-free aramid honeycombs with extraordinary microwave absorption, thermal insulation, flame retardant and mechanical performance. J. Mater. Sci. Technol. 2025, 233, 132–143. [Google Scholar] [CrossRef]
- Singh, A.N.; Nam, K.W. Gel-Based Self-Powered Nanogenerators: Materials, Mechanisms, and Emerging Opportunities. Gels 2025, 11, 451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhi, W.; Duan, J.; Zhang, L.; Liang, F.; Ma, C. Mechanically robust SiC aerogel with both electromagnetic absorption and pollutant adsorption via microtube/nanowire structure design. Adv. Ceram. 2025, 14, 9221181. [Google Scholar] [CrossRef]
- Li, Y.; Xue, Y.; Gao, X.; Wang, L.; Liu, X.; Wang, Z.; Shen, S. Cayanamide Group Functionalized Crystalline Carbon Nitride Aerogel for Efficient CO2 Photoreduction. Adv. Funct. Mater. 2024, 34, 2312634. [Google Scholar] [CrossRef]
- Saure, L.M.; Kohlmann, N.; Qiu, H.Y.; Shetty, S.; Nia, A.S.; Ravishankar, N.; Feng, X.; Szameit, A.; Kienle, L.; Adelung, R.; et al. Hybrid Aeromaterials for Enhanced and Rapid Volumetric Photothermal Response. ACS Nano 2023, 17, 22444–22455. [Google Scholar] [CrossRef]
- Rosa, D.; Abbasova, N.; Fazi, M. Photocatalytic Activity of Iron-Doped Titania Coupled with Biochar for Wastewater Treatment. Chem. Eng. Trans. 2025, 118, 230–240. [Google Scholar] [CrossRef]
- Zhang, C.; Fang, J.; Xu, X.; Zhang, M.; Han, Z.; Liao, J. Al2O3/MgO-doped, CaO-based adsorbents for CO2 capture: A performance study. Ann. N. Y. Acad. Sci. 2025, 1552, 388–400. [Google Scholar] [CrossRef]
- Xia, Y.; Man, J.; Wu, X.; Huang, S.; Lu, A.; Shen, X.; Cui, S.; Chen, X.; Fu, G. Oxygen-vacancy-assisted construction of Ce-TiO2 aerogel for efficiently boosting photocatalytic CO2 reduction without any sacrifice agent. Ceram. Int. 2023, 49, 6100–6112. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.; Zhang, J.; Zeng, F.; Bao, J.; Liu, L.; Tian, G. Cocatalyst Embedded Ce-BDC-CeO2 S-Scheme Heterojunction Hollowed-Out Octahedrons With Rich Defects for Efficient CO2 Photoreduction. Small 2024, 20, 2406487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hai, C.; Zhu, Z.; Zhuo, X.; Sun, Y.; Dong, S.; Ma, L.; He, X.; Xu, Q.; Zhou, Y. Accelerating the adsorption kinetics of layer-structured TiO2·H2O adsorbents for selectively separating Li+ from low-grade brines via Ce doping. Chem. Eng. J. 2025, 511, 162298. [Google Scholar] [CrossRef]
- Qi, D.; Wang, J.; Zhang, J.; Su, K.; Xu, J.; Lv, W.; Wang, Y.; Zhang, Z.; Xiao, Y. Improving Generation Performance of 1O2 by Atomic Doping for Efficient Catalytic Advanced Oxidation Processes. Adv. Funct. Mater. 2024, 34, 2406470. [Google Scholar] [CrossRef]
- Wang, J.; Hao, Q.; Yang, R.; Niu, X.; Wang, R.; Yang, L.; Huang, Q.; Ye, J.; Yang, H.; Wu, Y. A dual S-scheme heterojunction SrTiO3/SrCO3/C-doped TiO2 as H2 production photocatalyst and its charge transfer mechanism. Appl. Catal. B-Environ. 2024, 35, 124232. [Google Scholar] [CrossRef]
- Tang, X.; Yu, A.; Yang, Q.; Yuan, H.; Wang, Z.; Xie, J.; Zhou, L.; Guo, Y.; Ma, D.; Dai, S. Significance of Epitaxial Growth of PtO2 on Rutile TiO2 for Pt/TiO2 Catalysts. J. Am. Chem. Soc. 2024, 146, 3764–3772. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Y.; Ding, S.; Yin, Z.; Liu, Y.; Hu, Z. Unprecedented stability for photocatalytic selective oxidation of NO achieved by targeted construction of extraction-electron-surface and capture-hole-subsurface sites. Appl. Catal. B-Environ. 2025, 378, 125577. [Google Scholar] [CrossRef]
- Kubiak, A. A scalable IoT-controlled photocatalytic system for pharmaceutical removal in real wastewater treatment applications. Chem. Eng. J. 2025, 518, 164709. [Google Scholar] [CrossRef]
- Usuki, S.; Morita, M.; Takada, T.; Jiang, T.; Taki, N.; Uesaka, Y.; Togawa, H.; Maeda, K.; Katsumata, K.; Liu, S.; et al. Dual-function ZnO/CeO2 photocatalyst for simultaneous methane decomposition and CO2 adsorption at room temperature. Chem. Eng. J. 2025, 509, 161408. [Google Scholar] [CrossRef]
- Khan, A.; Le Pivert, M.; Ranjbari, A.; Dragoe, D.; Bahena-Uribe, D.; Colbeau Justin, C.; Herrero, C.; Rutkowska-Zbik, D.; Deschamps, J.; Remita, H. Cu-Based MOF/TiO2 Composite Nanomaterials for Photocatalytic Hydrogen Generation and the Role of Copper. Adv. Funct. Mater. 2025, 25, 2501736. [Google Scholar] [CrossRef]
- Maqbool, Q.; Favoni, O.; Wicht, T.; Lasemi, N.; Sabbatini, S.; Stöger-Pollach, M.; Ruello, M.L.; Tittarelli, F.; Rupprechter, G. Highly Stable Self-Cleaning Paints Based on Waste-Valorized PNC-Doped TiO2 Nanoparticles. ACS Catal. 2024, 14, 4820–4834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, F.; Ding, X.; Feng, M.; Sun, L.; Lu, J.; Xue, X.; Zhu, X. Interface engineering in 2D/0D/2D COF@TiO2/MXene S-scheme heterojunction: Decipher charge pathways for antibiotic removal and H2O2 production. Chem. Eng. J. 2025, 508, 160891. [Google Scholar] [CrossRef]
- Hao, C.; Qiu, Z.; Yang, Z.; Hu, G.; Chang, F.; Zhou, Y.; Wågberg, T.; An, X.G.; Ma, Y. Highly Efficient Photo-Assisted Degradation of Waste Plasticizers over Atomically Dispersed Cobalt Sites. Small 2025, 21, e06342. [Google Scholar] [CrossRef]
- Liu, H.; Sun, F.; Li, X.; Ma, Q.; Liu, G.; Yu, H.; Yu, W.; Dong, X.; Su, Z. g-C3N4/TiO2/ZnIn2S4 graphene aerogel photocatalysts with double S-scheme heterostructure for improving photocatalytic multifunctional performances. Compos. Part B-Eng. 2023, 259, 110746. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, T.; Liang, W.; Bai, P.; Zheng, H.; Lei, Y.; Hu, Z.; Xie, T. Promoted solar-driven dry reforming of methane with Pt/mesoporous-TiO2 photo-thermal synergistic catalyst: Performance and mechanism study. Energ. Convers. Manag. 2022, 258, 115496. [Google Scholar] [CrossRef]
- Tang, Y.; Ruan, J.; Xin, Y.; Ren, X.; Yuan, H.; Liu, X.; Chen, Q. Organic acid etching strategy for synthesis of ZIF-8@ZIF-67-Derived hollow Co@C/C rods with excellent electromagnetic wave absorption in C-band. Chem. Eng. J. 2025, 511, 161999. [Google Scholar] [CrossRef]
- Deng, R.; Liu, X.; Huang, J.; Wang, Y.; Chen, D.; Wang, C.; Lai, K.; Mao, C.; Qian, G.; Zheng, Y.; et al. The Lattice Defects of Nanofilm Introduced by N Doping Promotes Radical Oxygen Species Production for Rapid Photocatalytic Elimination of Contact Infection. Adv. Funct. Mater. 2025, 35, 2500256. [Google Scholar] [CrossRef]
- Gupta, S.; Kwak, Y.; Raj, R.P.; Selvam, P. Selvam, Ytterbium–nitrogen co-doped ordered mesoporous TiO2: An innovative hetero-phase photocatalyst for harnessing solar energy in green hydrogen production. J. Mater. Chem. A 2024, 12, 6906–6927. [Google Scholar] [CrossRef]
- Liao, L.; Xie, G.; Xie, X.; Zhang, N. Advances in Modulating the Activity and Selectivity of Photocatalytic CO2 Reduction to Multicarbon Products. J. Phys. Chem. C 2023, 127, 2766–2781. [Google Scholar] [CrossRef]
- Garcia-Mulero, A.; Asiri, A.M.; Albero, J.; Primo, A.; Garcia, H. All-carbon microporous graphitic photocatalyst-promoted reduction of CO2 to CO in the absence of metals or dopant elements. Nanoscale 2022, 14, 11575–11582. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Xu, Q.; Wang, X.; Luo, C.; Zhao, M.; Ma, Q.; Yu, H.; Yu, W.; Dong, X. Multi-Electric Field-Enhanced CuInS2-Modified TiO2(Anatase) /TiO2(Rutile) /PVDF Nanofiber Membrane for Multifunctional Piezo-Photocatalysis. Adv. Funct. Mater. 2025, 35, e05795. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, Y.; Chen, M.; Zhang, H.; Yu, J.; Jiang, T.; Wu, M. A Photo-Assisted Zinc-Air Battery with MoS2/Oxygen Vacancies Rich TiO2 Heterojunction Photocathode. Small 2024, 20, 2408627. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, H.; Yue, C.; He, L.; Li, H.; Zhang, H.; Yang, S.; Ma, T. Photocatalytic degradation by TiO2-conjugated/coordination polymer heterojunction: Preparation, mechanisms, and prospects. Appl. Catal. B-Environ. 2024, 344, 123605. [Google Scholar] [CrossRef]
- Guo, L.; Chen, W.; Wang, C.; Dong, B. Application of electrochemically assisted synthesis of MOFs-derived phosphides as catalyst for CH4-CO2 reforming. Int. J. Electrochem. Sci. 2023, 18, 26–32. [Google Scholar] [CrossRef]
- Dimitriou, C.; Belles, L.; Boukos, N.; Deligiannakis, Y. {TiO2/TiO2(B)} Quantum Dot Hybrids: A Comprehensible Route toward High-Performance [>0.1 mol gr−1 h−1] Photocatalytic H2 Production from H2O. ACS Catal. 2024, 14, 17919–17934. [Google Scholar] [CrossRef]
- Yao, C.; Song, Q.; Meng, L.; Zhong, H.; Cao, W.; Li, H.; Sun, C.; Pang, S.; Zhang, L. Interfacial polarization and lattice hydrogenation enable accelerated aluminum combustion with hydrogen-rich fluoropolymers. Chem. Eng. J. 2025, 521, 167068. [Google Scholar] [CrossRef]
- Hu, L.; Deng, J.J.; Lin, Y.; Liang, Q.; Ge, B.; Weng, Q.; Bai, Y.; Li, Y.; Deng, Y.; Chen, G.; et al. Restructuring Electrolyte Solvation by a Versatile Diluent Toward Beyond 99.9% Coulombic Efficiency of Sodium Plating/Stripping at Ultralow Temperatures. Adv. Mater. 2024, 36, 2312161. [Google Scholar] [CrossRef]
- Lei, B.; Cui, W.; Chen, P.; Chen, L.; Li, J.; Dong, F. C-Doping Induced Oxygen-Vacancy in WO3 Nanosheets for CO2 Activation and Photoreduction. ACS Catal. 2022, 12, 9670–9678. [Google Scholar] [CrossRef]
- Lv, F.; Zhang, W.; He, L.; Bai, X.; Song, Y.; Zhao, Y. 3D porous flower-like CoAl2O4 to boost the photocatalytic CO2 reduction reaction. J. Mater. Chem. A 2023, 11, 2826–2835. [Google Scholar] [CrossRef]
- Shen, Y.; Ren, C.; Zheng, L.; Xu, X.; Long, R.; Zhang, W.; Yang, Y.; Zhang, Y.; Yao, Y.; Chi, H.; et al. Room-temperature photosynthesis of propane from CO2 with Cu single atoms on vacancy-rich TiO2. Nat. Commun. 2023, 14, 1117. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; He, H.; Tao, M.; Muhammad, Y.; Gong, W.; Liu, Q.; Zhao, Z.; Zhao, Z. Chloroplast-inspired microenvironment engineering of inverse opal structured IO-TiO2/Chl/IL for highly efficient CO2 photolytic reduction to CH4. Chem. Eng. J. 2023, 464, 142685. [Google Scholar] [CrossRef]
- Xue, J.; Jia, X.; Sun, Z.; Li, H.; Shen, Q.; Liu, X.; Jia, H.; Zhu, Y. Selective CO2 photoreduction to C2 hydrocarbon via synergy between metastable ordered oxygen vacancies and hydrogen spillover over TiO2 nanobelts. Appl. Catal. B-Environ. 2024, 342, 123372. [Google Scholar] [CrossRef]
- Zhang, Y.; Niu, C.; Liu, J.; Caruso, R.; Zhang, X. A highly durable AgOx cluster/mesoporous TiO2 photocatalyst with synergistic effects induced superior H2 evolution and CO2 reduction. J. Mater. Chem. A 2023, 11, 25910–25917. [Google Scholar] [CrossRef]
- Yang, L.; Du, J.; Deng, J.; Sulaiman, N.H.M.; Feng, X.; Liu, C.; Zhou, X. Defective Nb2C MXene Cocatalyst on TiO2 Microsphere for Enhanced Photocatalytic CO2 Conversion to Methane. Small 2024, 20, 2307007. [Google Scholar] [CrossRef]
- Lin, J.; Chen, Y.; Kan, L.; Yu, X.; Tian, G. Defect-mediated hierarchical tubular CoSe2@TiO2 heterostructure photocatalysts for boosted CO2 photoreduction. J. Mater. Chem. A 2025, 13, 17102–17111. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, X.; Liu, X.; Gao, T.; Pinna, N.; Wang, Y. Copper Doping Enhances the Activity and Selectivity of Atomically Precise Ag44 Nanoclusters for Photocatalytic CO2 Reduction. Adv. Funct. Mater. 2025, e03708. [Google Scholar] [CrossRef]
- Zou, Z.; Wang, Y.; Zhang, T.; Lu, J.; Turkevich, V.; Yu, Y.; Xu, J.; Lin, H.; Li, Y.; Wang, L. Hollow p-n heterostructures with vacancy engineering to enhance Z-scheme photocatalytic CO2 reduction. Chem. Eng. J. 2025, 520, 165595. [Google Scholar] [CrossRef]
- Xu, X.; Su, B.; Wang, S.; Xing, W.; Hung, S.; Pan, Z.; Fang, Y.; Zhang, G.; Zhang, H.; Wang, X. CO2 Photoreduction by H2O: Cooperative Catalysis of Palladium Species on Poly(triazine imide) Crystals. Angew. Chem. Int. Edit. 2025, 64, e202512386. [Google Scholar] [CrossRef]
- Gao, Y.B.; Zhang, M.; Fan, Z.; Yang, D.; Xu, M.; Zhao, X.; Song, Z.; Wang, W.; Mao, Y. In-situ generation of S-scheme heterojunction via A-Site defects reconstruction perovskite oxide for efficient CO2 photoreduction. Nano Energy 2025, 141, 111132. [Google Scholar] [CrossRef]
- Geng, W.; Xiong, Y.; Chen, C.; Ning, S.; Xiong, Z.; Deng, S.; Tan, Y.; Song, X.; Pan, M.; Mayor, M.; et al. Ternary Metalation in a Copper-Covalent Organic Framework for Tandem Photocatalytic CO2 Reduction with High Selectivity. Angew. Chem. Int. Edit. 2025, 64, e2505546. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, Q.; Li, T.; He, Y.; Peng, W.; Wang, C. Photo-thermal synergistic catalytic carbon dioxide reduction on WN-WO3/In2O3 catalyst with controllable product selectivity. Chem. Eng. J. 2025, 519, 165094. [Google Scholar] [CrossRef]
- Zhang, L.; Xuan, Y.; Wang, Q.; Zhao, D.; Liu, X. Synergistic Effect of Photochromism and Vacancy Engineering within Z-Scheme Doped BiOBr-BaTiO3 Heterostructures Promoting CO2 Photocatalysis. Adv. Funct. Mater. 2025, e14966. [Google Scholar] [CrossRef]
- Song, W.; Chong, K.; Qi, G.; Xiao, Y.; Chen, G.; Li, B.; Tang, Y.; Zhang, X.; Yao, Y.; Lin, Z.; et al. Unraveling the Transformation from Type-II to Z-Scheme in Perovskite-Based Heterostructures for Enhanced Photocatalytic CO2 Reduction. J. Am. Chem. Soc. 2024, 146, 3303–3314. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhang, K.; Ouyang, Y.; Wang, K.; Zhang, Y.; Chen, X.; Zhao, J.; Wang, Q.; Shen, H.-M.; Yang, Y.-F.; et al. Substituent Polarity-Regulated Interfacial Polarization in Bismuth Oxyhalide/Metalloporphyrin Composites for Enhanced Charge Transfer and CO2 Photoreduction. ACS Catal. 2025, 16, 646–657. [Google Scholar] [CrossRef]
| Sample | BET Specific Surface Area (m2·g−1) | BJH Adsorption Average Pore Size (nm) | Pore Volume (cm3·g−1) |
|---|---|---|---|
| CTi | 191.27 | 24.04 | 1.06 |
| CeC0.5Ti | 186.54 | 28.54 | 1.46 |
| CeC0.75Ti | 188.81 | 26.28 | 1.29 |
| CeC1.0Ti | 195.73 | 25.07 | 1.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Guan, J.; Wang, W.; Wu, X.; Xia, Y.; Shi, B.; Liu, S.; Xu, L.; Zhang, R.; Sun, Y.; Lin, Y. Tailoring Electronic Structures via Ce/C Co-Doping and Oxygen Vacancy in TiO2 Aerogels for Enhanced Solar Fuel Production. Gels 2026, 12, 128. https://doi.org/10.3390/gels12020128
Guan J, Wang W, Wu X, Xia Y, Shi B, Liu S, Xu L, Zhang R, Sun Y, Lin Y. Tailoring Electronic Structures via Ce/C Co-Doping and Oxygen Vacancy in TiO2 Aerogels for Enhanced Solar Fuel Production. Gels. 2026; 12(2):128. https://doi.org/10.3390/gels12020128
Chicago/Turabian StyleGuan, Jiahan, Wei Wang, Xiaodong Wu, Yu Xia, Bingyan Shi, Shibei Liu, Lijie Xu, Ruiyang Zhang, Yunlong Sun, and Yuqian Lin. 2026. "Tailoring Electronic Structures via Ce/C Co-Doping and Oxygen Vacancy in TiO2 Aerogels for Enhanced Solar Fuel Production" Gels 12, no. 2: 128. https://doi.org/10.3390/gels12020128
APA StyleGuan, J., Wang, W., Wu, X., Xia, Y., Shi, B., Liu, S., Xu, L., Zhang, R., Sun, Y., & Lin, Y. (2026). Tailoring Electronic Structures via Ce/C Co-Doping and Oxygen Vacancy in TiO2 Aerogels for Enhanced Solar Fuel Production. Gels, 12(2), 128. https://doi.org/10.3390/gels12020128







