Novel Garlic Carbon Dot-Incorporated Starch Whey Protein Emulsion Gel for Apple Spoilage Sensing
Abstract
1. Introduction
2. Results and Discussion
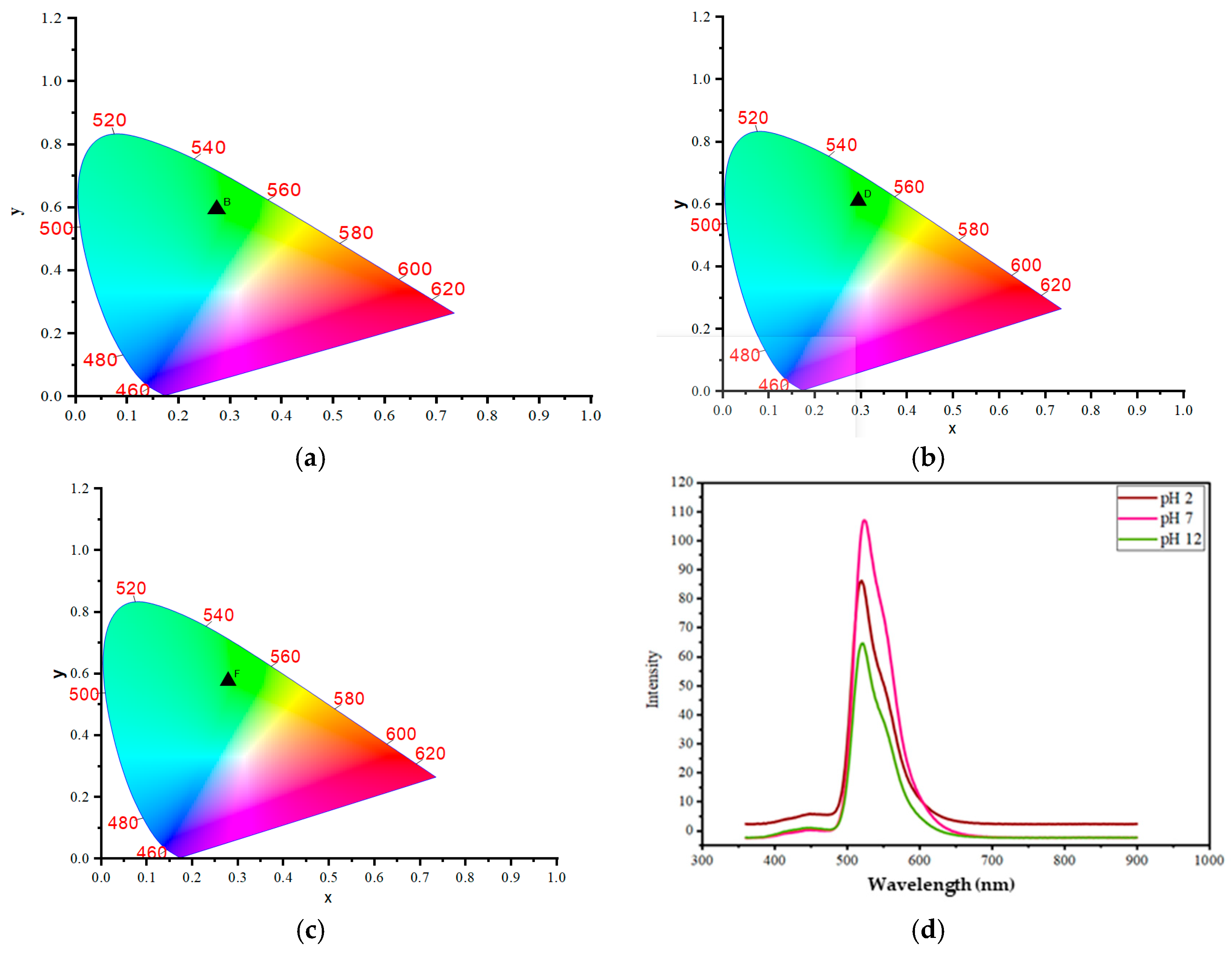
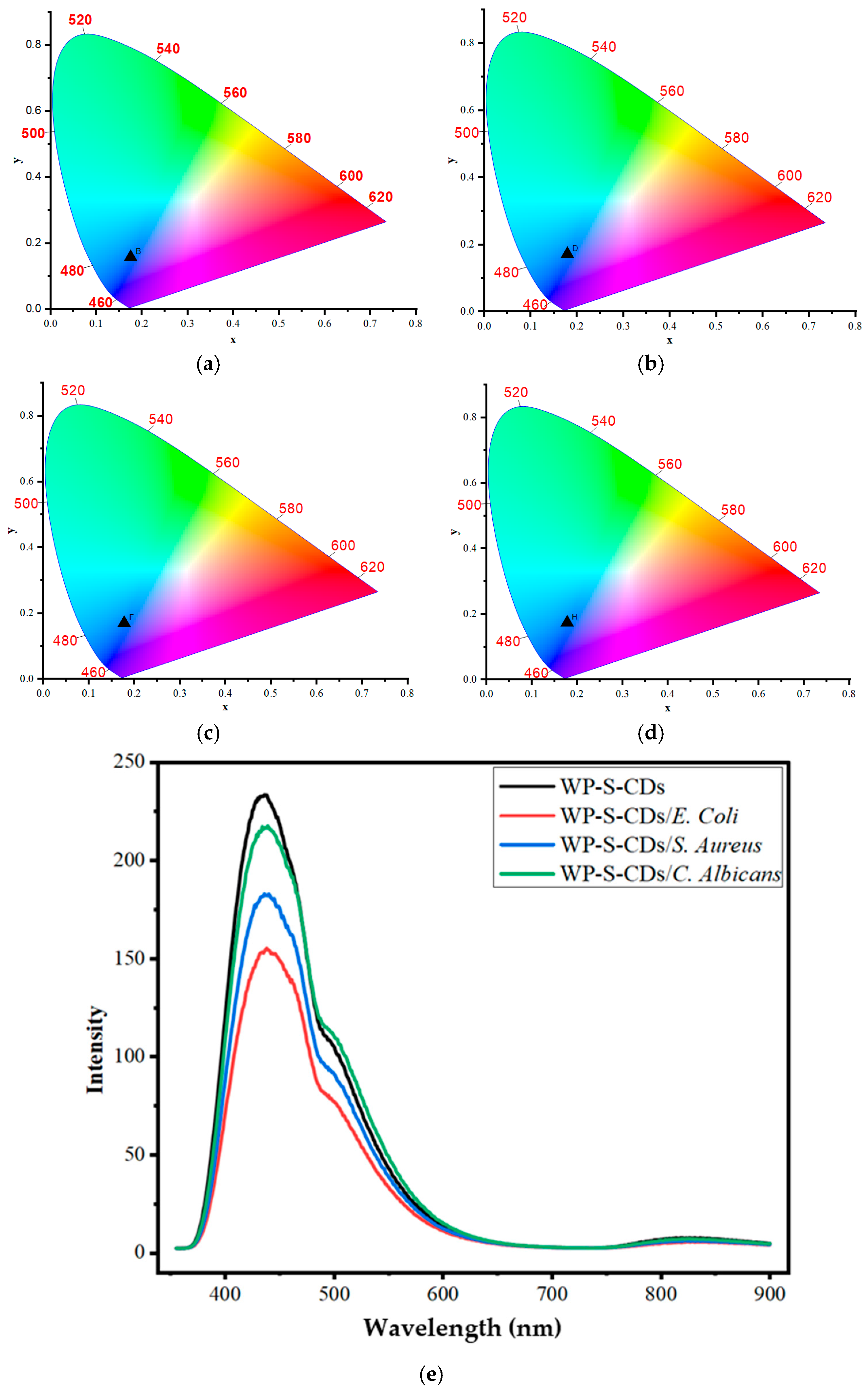
2.1. Chromaticity and Optical Properties of Garlic-Derived Carbon Dots at Different pH Values and WP-S-CDs Emulsions Before and After Contact with Different Types of Bacteria for Differentiation
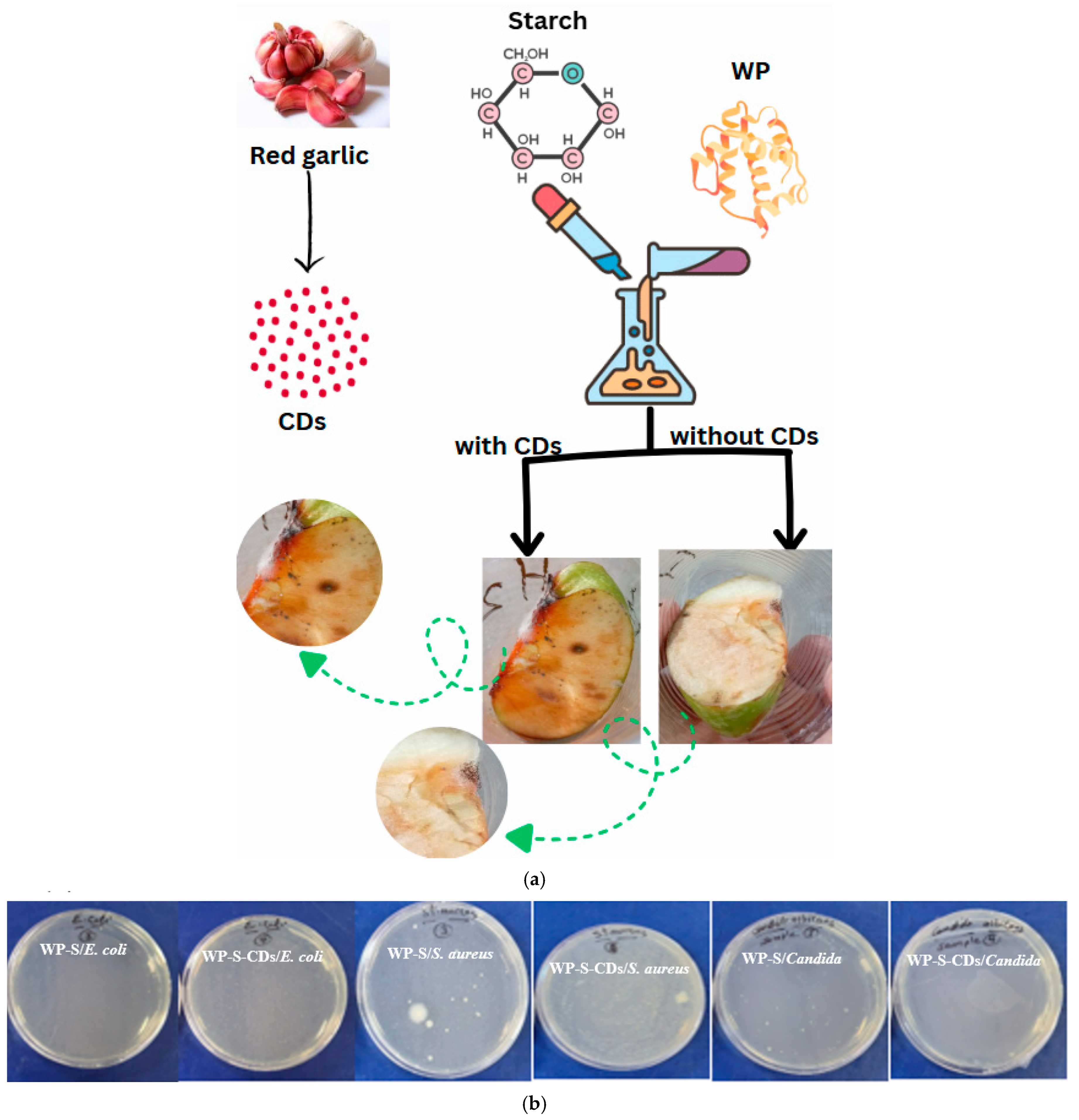
2.2. Apple Spoilage Sensing by WP-S-CDs with Antimicrobial Properties
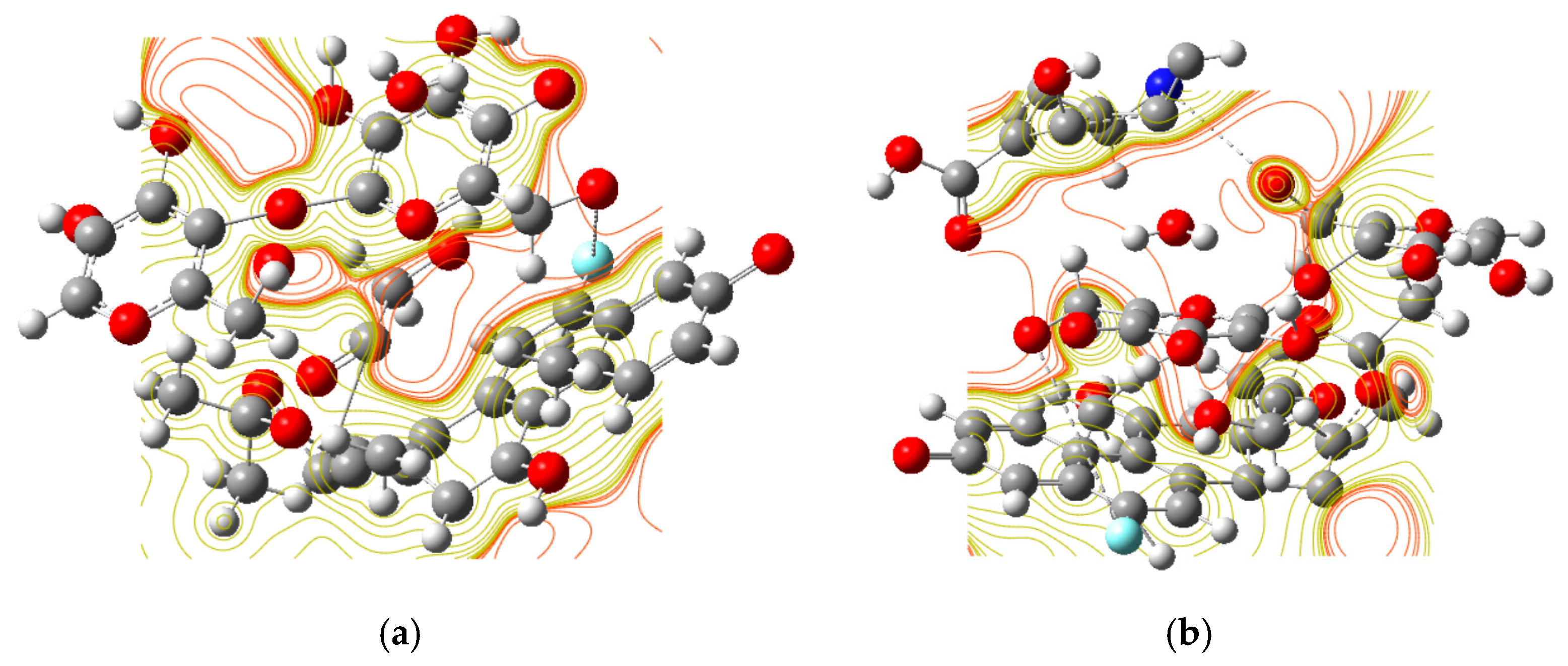
2.3. DFT Calculations with the Electrostatic Mapping
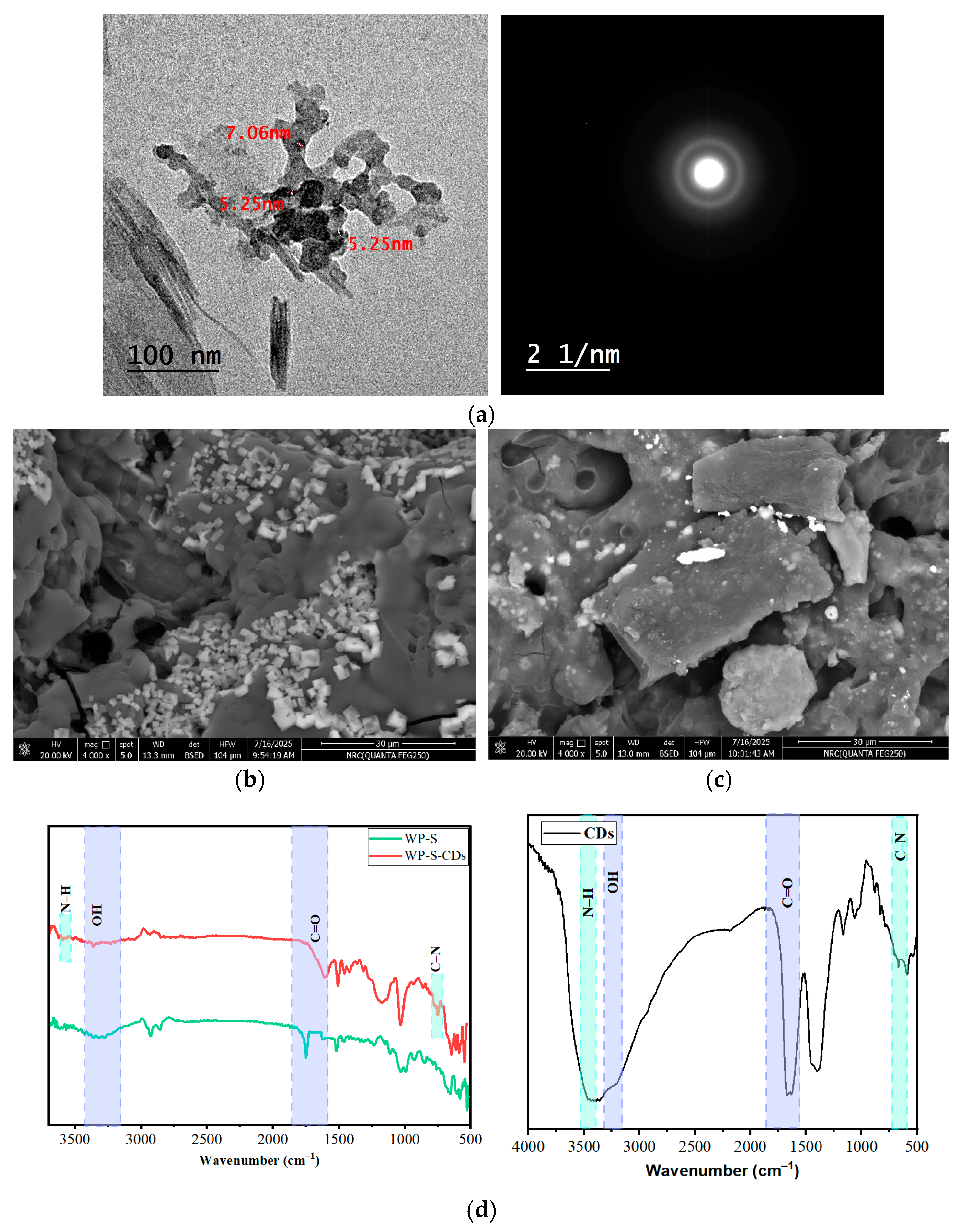
2.4. TEM Analysis for CDs, SEM and Fourier Transform Infrared Spectroscopy (FTIR) Spectra
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Carbon Dots from Red Garlic Peels
4.3. Preparation of Whey Protein–Starch Emulsions
4.4. Application of Whey Protein–Starch Emulsions Apple Spoilage Sensing
4.5. Characterization
4.5.1. Determination of the pH-Sensitivity of CDs Prepared from Red Garlic Peels
4.5.2. Morphological Observation
4.5.3. Fourier-Transform Infrared (FTIR) Spectra
4.5.4. DFT Calculations
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- López-Gálvez, F.; Gómez, P.A.; Artés, F.; Artés-Hernández, F.; Aguayo, E. Interactions between microbial food safety and environmental sustainability in the fresh produce supply chain. Foods 2021, 10, 1655. [Google Scholar] [CrossRef] [PubMed]
- Osman, S.A.; Xu, C.; Akuful, M.; Paul, E.R. Perishable food supply chain management: Challenges and the way forward. Open J. Soc. Sci. 2023, 11, 349–364. [Google Scholar] [CrossRef]
- Aworh, O.C. Food safety issues in fresh produce supply chain with particular reference to sub-Saharan Africa. Food Control 2021, 123, 107737. [Google Scholar] [CrossRef]
- Tohamy, H.-A.S. Beet root carbon dots cellulose sulfate film as a novel naked eye pH sensor for chromium and bacterial detection in tomatoes. Sci. Rep. 2025, 15, 30235. [Google Scholar] [CrossRef]
- Tohamy, H.-A.S. A novel anthocyanins hydroxyethyl cellulose film for intelligent chicken meat packaging with mechanical study, DFT calculations and molecular docking study. Sci. Rep. 2025, 15, 27311. [Google Scholar] [CrossRef]
- Saini, R.V.; Vaid, P.; Saini, N.K.; Siwal, S.S.; Gupta, V.K.; Thakur, V.K.; Saini, A.K. Recent advancements in the technologies detecting food spoiling agents. J. Funct. Biomater. 2021, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Dainty, R.H. Chemical/biochemical detection of spoilage. Int. J. Food Microbiol. 1996, 33, 19–33. [Google Scholar] [CrossRef]
- Ferone, M.; Gowen, A.; Fanning, S.; Scannell, A.G. Microbial detection and identification methods: Bench top assays to omics approaches. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3106–3129. [Google Scholar] [CrossRef]
- Gracias, K.S.; McKillip, J.L. A review of conventional detection and enumeration methods for pathogenic bacteria in food. Can. J. Microbiol. 2004, 50, 883–890. [Google Scholar] [CrossRef]
- Nisenbaum, M.; Agustinelli, S.P.; Guzmán, M.N.; Murialdo, S.E. Biospeckle laser for real-time monitoring of water and food samples: Enhancing contamination detection and quality control in field applications. Instrum. Sci. Technol. 2025, 1–35. [Google Scholar] [CrossRef]
- Ablegue, A.G.M.-D.; Zhang, M.; Guo, Q.; Rui, L. Monitoring and Shelf Life Extension of Food Products Based on Machine Learning and Deep Learning: Progress and Potential Applications. Food Rev. Int. 2025, 1–26. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, M.; Jiang, Q.; Mujumdar, A.S. Intelligent system/equipment for quality deterioration detection of fresh food: Recent advances and application. Foods 2024, 13, 1662. [Google Scholar] [CrossRef]
- Dhal, S.B.; Kar, D. Leveraging artificial intelligence and advanced food processing techniques for enhanced food safety, quality, and security: A comprehensive review. Discov. Appl. Sci. 2025, 7, 75. [Google Scholar] [CrossRef]
- Roy, L. Sensor technologies and artificial intelligence integration for real-time monitoring of food quality parameters. In Artificial Intelligence in Food Science; Elsevier: Amsterdam, The Netherlands, 2026; pp. 447–468. [Google Scholar]
- Tohamy, H.-A.S. A Novel Natural Chromogenic Visual and Luminescent Sensor Platform for Multi-Target Analysis in Strawberries and Shape Memory Applications. Foods 2025, 14, 2791. [Google Scholar] [CrossRef] [PubMed]
- Tohamy, H.-A.S. Fullerene-Functionalized Cellulosic Hydrogel Biosensor with Bacterial Turn-on Fluorescence Response Derived from Carboxymethyl Cellulose for Intelligent Food Packaging with DFT Calculations and Molecular Docking. Gels 2025, 11, 329. [Google Scholar] [CrossRef]
- Moustafa, H.; Hemida, M.H.; Nour, M.A.; Abou-Kandil, A.I. Intelligent packaging films based on two-dimensional nanomaterials for food safety and quality monitoring: Future insights and roadblocks. J. Thermoplast. Compos. Mater. 2025, 38, 1208–1230. [Google Scholar] [CrossRef]
- Sohail, M.; Sun, D.-W.; Zhu, Z. Recent developments in intelligent packaging for enhancing food quality and safety. Crit. Rev. Food Sci. Nutr. 2018, 58, 2650–2662. [Google Scholar] [CrossRef]
- Dodero, A.; Escher, A.; Bertucci, S.; Castellano, M.; Lova, P. Intelligent packaging for real-time monitoring of food-quality: Current and future developments. Appl. Sci. 2021, 11, 3532. [Google Scholar] [CrossRef]
- Azmat, F.; Imran, A.; Islam, F.; Afzaal, M.; Zahoor, T.; Akram, R.; Aggarwal, S.; Rehman, M.; Naaz, S.; Ashraf, S.; et al. Valorization of the phytochemical profile, nutritional composition, and therapeutic potentials of garlic peel: A concurrent review. Int. J. Food Prop. 2023, 26, 2642–2655. [Google Scholar] [CrossRef]
- Vanderroost, M.; Ragaert, P.; Devlieghere, F.; De Meulenaer, B. Intelligent food packaging: The next generation. Trends Food Sci. Technol. 2014, 39, 47–62. [Google Scholar] [CrossRef]
- Han, J.H.; Ho, C.H.; Rodrigues, E.T. Intelligent packaging. Innov. Food Packag. 2005, 138–155. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, W.; Xia, Y.; Xue, W.; Wu, H.; Li, Z.; Zhang, F.; Qiu, B.; Fu, C. Properties and applications of intelligent packaging indicators for food spoilage. Membranes 2022, 12, 477. [Google Scholar] [CrossRef]
- Ghoshal, G. Recent trends in active, smart, and intelligent packaging for food products. In Food packaging and preservation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 343–374. [Google Scholar]
- Luo, X.; Zaitoon, A.; Lim, L.T. A review on colorimetric indicators for monitoring product freshness in intelligent food packaging: Indicator dyes, preparation methods, and applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2489–2519. [Google Scholar] [CrossRef]
- Kamran, F.; Al Ubeed, H.; Faraz, A.; Karnpanit, W. Introduction to Vegetable Processing Wastes. In Handbook of Vegetable Processing Waste; CRC Press: Boca Raton, FL, USA, 2025; pp. 1–43. [Google Scholar]
- Ramadhani, A.M.; Nassary, E.K.; Rwehumbiza, F.B.; Massawe, B.H.; Nchimbi-Msolla, S. Potentials of synthetic biodegradable mulch for improved livelihoods on smallholder farmers: A systematic review. Front. Agron. 2024, 6, 1454060. [Google Scholar] [CrossRef]
- Nezafat, Z.; Dong, Y.; Nasrollahzadeh, M.; Shafiei, N.; Gharoubi, H.; Javanshir, S. Recent progresses in energy conversion and storage of agricultural waste-derived (carbon/nano) materials: A review. Green Chem. 2024, 26, 10687–10717. [Google Scholar] [CrossRef]
- Koiri, P.; Das, S. Agri-Food Waste Management and Treatment Approaches for Environmental Sustainability. In Environmental Engineering and Waste Management: Recent Trends and Perspectives; Springer: Berlin/Heidelberg, Germany, 2024; pp. 343–373. [Google Scholar]
- Khan, M.N.; Sial, T.A.; Ali, A.; Wahid, F. Impact of agricultural wastes on environment and possible management strategies. In Frontier Studies in Soil Science; Springer: Berlin/Heidelberg, Germany, 2024; pp. 79–108. [Google Scholar]
- Vasileiadou, A. From Organic wastes to Bioenergy, Biofuels, and value-added products for urban sustainability and circular economy: A review. Urban Sci. 2024, 8, 121. [Google Scholar] [CrossRef]
- Nasrin, T.A.A.; Matin, M.A. Valorization of vegetable wastes. Food Process. By-Prod. Their Util. 2017, 53–88. [Google Scholar] [CrossRef]
- Waste, F.P. Emerging Methods for Oil Extraction from Food Processing Waste; CRC Press: Boca Raton, FL, USA, 2025. [Google Scholar]
- Sakdaronnarong, C.; Sangjan, A.; Boonsith, S.; Kim, D.C.; Shin, H.S. Recent developments in synthesis and photocatalytic applications of carbon dots. Catalysts 2020, 10, 320. [Google Scholar] [CrossRef]
- Farshbaf, M.; Davaran, S.; Rahimi, F.; Annabi, N.; Salehi, R.; Akbarzadeh, A. Carbon quantum dots: Recent progresses on synthesis, surface modification and applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1331–1348. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Zhu, A.; Tian, Y. Functional surface engineering of C-dots for fluorescent biosensing and in vivo bioimaging. Acc. Chem. Res. 2014, 47, 20–30. [Google Scholar] [CrossRef]
- Zhao, D.L.; Chung, T.-S. Applications of carbon quantum dots (CQDs) in membrane technologies: A review. Water Res. 2018, 147, 43–49. [Google Scholar] [CrossRef]
- John, V.L.; Nair, Y.; Vinod, T. Doping and surface modification of carbon quantum dots for enhanced functionalities and related applications. Part. Part. Syst. Charact. 2021, 38, 2100170. [Google Scholar] [CrossRef]
- Tohamy, H.-A.S. Amylopectin xerogel with onion based sulfur nitrogen doped carbon quantum dots as a chemosensor for chromium and biosensor for microbial spoilage in tomatoes. Sci. Rep. 2025, 15, 32667. [Google Scholar] [CrossRef]
- Tohamy, H.-A.S. Nanoemulsion mediated approaches for wound healing. In Recent Advances in Nanomedicines Mediated Wound Healing; Elsevier: Amsterdam, The Netherlands, 2025; pp. 109–129. [Google Scholar]
- Tohamy, H.-A.S. Quantum dots as an emerging nanocarrier for drug delivery. Quantum Dot Nanocarriers Drug Deliv. 2025, 363–384. [Google Scholar] [CrossRef]
- Kandasamy, S.; Yoo, J.; Yun, J.; Kang, H.-B.; Seol, K.-H.; Kim, H.-W.; Ham, J.-S. Application of whey protein-based edible films and coatings in food industries: An updated overview. Coatings 2021, 11, 1056. [Google Scholar] [CrossRef]
- Niranjana Prabhu, T.; Prashantha, K. A review on present status and future challenges of starch based polymer films and their composites in food packaging applications. Polym. Compos. 2018, 39, 2499–2522. [Google Scholar] [CrossRef]
- Thakur, R.; Pristijono, P.; Scarlett, C.J.; Bowyer, M.; Singh, S.; Vuong, Q.V. Starch-based films: Major factors affecting their properties. Int. J. Biol. Macromol. 2019, 132, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Basumatary, I.B.; Mukherjee, A.; Katiyar, V.; Kumar, S. Biopolymer-based nanocomposite films and coatings: Recent advances in shelf-life improvement of fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2022, 62, 1912–1935. [Google Scholar] [CrossRef]
- Iqbal, S.; Ayyub, A.; Bhutto, R.A.; Rehman, W. Designing Smart and Sustainable Edible Packaging Materials from Biopolymers, Proteins, and Polysaccharides. In Green Materials for Active Food Packaging; Springer: Berlin/Heidelberg, Germany, 2025; pp. 131–196. [Google Scholar]
- Singh, G.P.; Bangar, S.P.; Yang, T.; Trif, M.; Kumar, V.; Kumar, D. Effect on the Properties of Edible Starch-Based Films by the Incorporation of Additives: A Review. Polymers 2022, 14, 1987. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chen, L.; Jin, Z.; Jiao, A. Research progress of starch-based biodegradable materials: A review. J. Mater. Sci. 2021, 56, 11187–11208. [Google Scholar] [CrossRef]
- Otoni, C.G.; Azeredo, H.M.; Mattos, B.D.; Beaumont, M.; Correa, D.S.; Rojas, O.J. The food–materials nexus: Next generation bioplastics and advanced materials from agri-food residues. Adv. Mater. 2021, 33, 2102520. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-y.; Xia, T.; Li, D.; Wang, L.-j.; Wang, Y. Hybrid biodegradable materials from starch and hydrocolloid: Fabrication, properties and applications of starch-hydrocolloid film, gel and bead. Crit. Rev. Food Sci. Nutr. 2024, 64, 12841–12859. [Google Scholar] [CrossRef]
- Shah, U.; Gani, A.; Ashwar, B.A.; Shah, A.; Ahmad, M.; Gani, A.; Wani, I.A.; Masoodi, F.A. A review of the recent advances in starch as active and nanocomposite packaging films. Cogent Food Agric. 2015, 1, 1115640. [Google Scholar] [CrossRef]
- Das, R.; Kayastha, A.M. An overview on starch processing and key enzymes. In Industrial Starch Debranching Enzymes; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–20. [Google Scholar]
- Damager, I.; Engelsen, S.B.; Blennow, A.; Lindberg Møller, B.; Motawia, M.S. First principles insight into the α-glucan structures of starch: Their synthesis, conformation, and hydration. Chem. Rev. 2010, 110, 2049–2080. [Google Scholar] [CrossRef]
- Bertoft, E. Understanding starch structure: Recent progress. Agronomy 2017, 7, 56. [Google Scholar] [CrossRef]
- Syahariza, Z.A.; Sar, S.; Hasjim, J.; Tizzotti, M.J.; Gilbert, R.G. The importance of amylose and amylopectin fine structures for starch digestibility in cooked rice grains. Food Chem. 2013, 136, 742–749. [Google Scholar] [CrossRef]
- Seung, D. Amylose in starch: Towards an understanding of biosynthesis, structure and function. New Phytol. 2020, 228, 1490–1504. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Yu, L.; Su, B.; Liu, P.; Wang, J.; Liu, H.; Chen, L. Rheological properties of starches with different amylose/amylopectin ratios. J. Cereal Sci. 2009, 49, 371–377. [Google Scholar] [CrossRef]
- Li, G.; Zhu, F. Amylopectin molecular structure in relation to physicochemical properties of quinoa starch. Carbohydr. Polym. 2017, 164, 396–402. [Google Scholar] [CrossRef]
- Qiu, Z.; Zheng, Z.; Xiao, H. Sustainable valorization of garlic byproducts: From waste to resource in the pursuit of carbon neutrality. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70151. [Google Scholar] [CrossRef]
- Vardhanabhuti, B.; Foegeding, E.A.; McGuffey, M.K.; Daubert, C.R.; Swaisgood, H.E. Gelation properties of dispersions containing polymerized and native whey protein isolate. Food Hydrocoll. 2001, 15, 165–175. [Google Scholar] [CrossRef]
- Ha, E.; Zemel, M.B. Functional properties of whey, whey components, and essential amino acids: Mechanisms underlying health benefits for active people. J. Nutr. Biochem. 2003, 14, 251–258. [Google Scholar] [CrossRef]
- Guefai, F.Z.; Martínez-Rodríguez, A.; Grindlay, G.; Mora, J.; Gras, L. Elemental bioavailability in whey protein supplements. J. Food Compos. Anal. 2022, 112, 104696. [Google Scholar] [CrossRef]
- Tohamy, H.-A.S. Novel colored hydroxypropyl methyl cellulose/magnetite carbon dots films for beef packaging with DFT calculations and molecular docking study. Sci. Rep. 2025, 15, 10337. [Google Scholar] [CrossRef]
- Tohamy, H.-A.S. Artistic anti-counterfeiting with a pH-responsive fluorescent ink using DFT and molecular electrostatic potential mapping insights. Sci. Rep. 2025, 15, 19335. [Google Scholar] [CrossRef]
- Jeevahan, J.; Chandrasekaran, M. Nanoedible films for food packaging: A review. J. Mater. Sci. 2019, 54, 12290–12318. [Google Scholar] [CrossRef]
- Debeaufort, F.; Quezada-Gallo, J.-A.; Voilley, A. Edible films and coatings: Tomorrow’s packagings: A review. Crit. Rev. Food Sci. 1998, 38, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Barlow, C.; Morgan, D. Polymer film packaging for food: An environmental assessment. Resour. Conserv. Recycl. 2013, 78, 74–80. [Google Scholar] [CrossRef]
- Marsh, K.; Bugusu, B. Food packaging—Roles, materials, and environmental issues. J. Food Sci. 2007, 72, R39–R55. [Google Scholar] [CrossRef]
- Merkus, H.G.; Meesters, G.M.; Oostra, W. Particles and Nanoparticles in Pharmaceutical Products: Design, Manufacturing, Behavior and Performance; Springer: Berlin/Heidelberg, Germany, 2018; Volume 29. [Google Scholar]
- Tipper, M.; Ward, R. Finishing of nonwoven fabrics. In Handbook of Nonwovens; Elsevier: Amsterdam, The Netherlands, 2022; pp. 471–508. [Google Scholar]
- Niaz, B.; Saeed, F.; Ahmed, A.; Imran, M.; Maan, A.A.; Khan, M.K.I.; Tufail, T.; Anjum, F.M.; Hussain, S.; Suleria, H.A.R. Lactoferrin (LF): A natural antimicrobial protein. Int. J. Food Prop. 2019, 22, 1626–1641. [Google Scholar] [CrossRef]
- Bruni, N.; Capucchio, M.T.; Biasibetti, E.; Pessione, E.; Cirrincione, S.; Giraudo, L.; Corona, A.; Dosio, F. Antimicrobial Activity of Lactoferrin-Related Peptides and Applications in Human and Veterinary Medicine. Molecules 2016, 21, 752. [Google Scholar] [CrossRef] [PubMed]
- Tohamy, H.-A.S. A two-in-one lignosulfonate carbon dots for bacterial detection and fluorescence quenching in food, pharm-ceuticals, and cultural heritage preservation. Sci. Rep. 2025, 15, 43739. [Google Scholar] [CrossRef] [PubMed]
- El-Nasharty, M.; El-Sakhawy, M.; Tohamy, H.-A.S. Temperature responsive aluminum manganese doped carbon dot sensors for enhanced electrical conductivity with DFT calculations. Sci. Rep. 2025, 15, 19754. [Google Scholar] [CrossRef] [PubMed]
| Microorganism | WP-S | WP-S-CDs |
|---|---|---|
| E. coli | 81.42% | 67.44% |
| S. aureus | 95.71% | 49.42% |
| C. albicans | 68.54% | 96.63% |
| DFT | WP-S | WP-S |
|---|---|---|
| ELUMO (eV) | 0.01180 | −0.0172 |
| EHOMO (eV) | −0.1749 | −0.1805 |
| Eg (eV) | 0.1867 | 0.1633 |
| ET (au) | −2733.35 | −3313.06 |
| μ (Debye) | 20.04 | 32.66 |
| ɳ (eV) | −0.0815 | −0.098 |
| σ (eV) | −0.1226 | −10.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Tohamy, H.-A.S. Novel Garlic Carbon Dot-Incorporated Starch Whey Protein Emulsion Gel for Apple Spoilage Sensing. Gels 2026, 12, 47. https://doi.org/10.3390/gels12010047
Tohamy H-AS. Novel Garlic Carbon Dot-Incorporated Starch Whey Protein Emulsion Gel for Apple Spoilage Sensing. Gels. 2026; 12(1):47. https://doi.org/10.3390/gels12010047
Chicago/Turabian StyleTohamy, Hebat-Allah S. 2026. "Novel Garlic Carbon Dot-Incorporated Starch Whey Protein Emulsion Gel for Apple Spoilage Sensing" Gels 12, no. 1: 47. https://doi.org/10.3390/gels12010047
APA StyleTohamy, H.-A. S. (2026). Novel Garlic Carbon Dot-Incorporated Starch Whey Protein Emulsion Gel for Apple Spoilage Sensing. Gels, 12(1), 47. https://doi.org/10.3390/gels12010047







