Abstract
Polymer network-based nanogels (NGs) and microgels (MGs) have emerged as highly versatile platforms for advanced drug delivery, owing to their tunable architecture, biocompatibility, and responsiveness to diverse stimuli. This review presents a comprehensive and structured analysis of NG/MGs, encompassing their classification based on polymer origin, crosslinking mechanisms, composition, charge, stimuli-responsiveness, and structural architecture. We detail synthesis strategies—including inverse microemulsion and radiation-induced polymerization—and highlight key characterization techniques essential for evaluating physicochemical and functional properties. Emphasis is placed on the design-driven applications of NG/MGs in overcoming biological barriers and enabling targeted therapies, particularly in cancer, inflammation, diabetes, and viral infections. Multifunctional NGs integrating therapeutic and diagnostic capabilities (theranostics), as well as emerging platforms for immunotherapy and personalized medicine, are critically discussed. Finally, we address translational challenges and future directions, including scalable manufacturing, regulatory considerations, and integration with smart diagnostics. This review aims to serve as a foundational resource for researchers and clinicians developing next-generation NG/MG-based therapeutics.
1. Introduction
The development of advanced drug delivery systems has become a cornerstone of modern therapeutics, aiming to enhance efficacy, reduce side effects, and enable targeted treatment. Among these systems, polymer-based NG/MGs have garnered significant attention due to their unique structural and functional properties. These soft, water-swolled for accurate correspondence, crosslinked polymeric networks—ranging from nanometer to micrometer scales—offer high drug loading capacity, tunable release kinetics, and responsiveness to environmental stimuli [1].
NGs, typically sized between 1 and 1000 nm, and MGs, which span the micrometer range, are synthesized using natural or synthetic polymers. Their internal network structure allows them to absorb large amounts of water or biological fluids without dissolving, maintaining mechanical integrity, and enabling controlled drug release [2].
Natural polymers such as chitosan [3], alginate [4,5], gelatin [6], and hyaluronic acid [7] provide excellent biocompatibility and biodegradability, while synthetic polymers like poly(N-isopropylacrylamide) (PNIPAM) [8] and polyethylene glycol (PEG) [9] offer greater control over physicochemical properties and functionalization. MGs, ranging from hundreds of nanometers to several micrometers, are better suited for localized therapies [10], tissue engineering [11], and cell encapsulation [12,13]. Both systems are built upon polymer networks whose architecture—linear, branched, or dendritic—critically influences their mechanical strength, drug loading capacity, and diffusion dynamics.
A key advantage of NG/MGs lies in their stimuli-responsive behavior [14]. These systems can undergo reversible changes in response to physical (e.g., temperature, light, magnetic field), chemical (e.g., pH, redox conditions), or biological (e.g., enzymes, biomolecules) stimuli [15]. This responsiveness enables site-specific and controlled drug release, minimizing systemic toxicity and enhancing therapeutic efficacy [16]. For example, temperature-sensitive NGs based on PNIPAM can release drugs in response to hyperthermia, while pH-sensitive gels can target acidic tumor microenvironments or inflamed tissues [8].
Recent advances have focused on engineering NG/MGs with stimuli-responsive properties (e.g., pH, temperature, redox, and enzymatic), enabling on-demand drug release and real-time biosensing [17,18]. Functionalization with targeting ligands such as folate, RGD (Arginine-Glycine-Aspartic acid) peptides [19], and transferrin has further enhanced their specificity, allowing for tumor targeting, blood-brain barrier (BBB) penetration [20], and site-specific therapy [21]. In drug delivery, NGs have demonstrated the ability to encapsulate a wide range of therapeutic agents—including anticancer drugs [22], anti-inflammatory compounds [23,24], proteins [25], and nucleic acids [26]—and release them in response to physiological cues. Controlled release profiles ranging from hours to days have been achieved through careful tuning of crosslinking density, network porosity, and polymer degradation rates [27]. MGs, on the other hand, have shown promise in tissue engineering and regenerative medicine, where their modular structure, injectability, and mechanical adaptability support cell delivery, scaffold formation, and wound healing [12]. Their ability to mimic the extracellular matrix and promote cell infiltration has opened new avenues for 3D bioprinting and implantable therapies. In vivo studies and early-phase clinical trials have confirmed the enhanced bioavailability, reduced side effects, and improved patient compliance associated with NGs-based therapies. Applications span across oncology [27], neurology [28], diabetes [29], and infectious diseases [30], with several formulations advancing toward clinical translation [31].
Looking ahead, emerging trends include the development of hybrid NGs combining organic polymers with inorganic components (e.g., gold, silica, iron oxide) for theranostics, photothermal therapy, and magnetic targeting [32]. The integration of AI-guided design, microfluidic synthesis, and personalized medicine platforms is expected to accelerate the deployment of smart NGs tailored to individual patient needs [33].
Despite these advancements, several challenges remain in the clinical translation of NG/MG technologies. Issues such as scalability, reproducibility, long-term stability, and regulatory approval must be addressed to realize their full potential. Emerging trends in green chemistry and sustainable polymer design are paving the way for eco-friendly synthesis methods, while advances in manufacturing technologies are improving scalability and consistency [34].
This review is particularly timely and significant as it addresses the growing demand for multifunctional and stimuli-responsive drug delivery platforms capable of overcoming biological barriers and achieving site-specific therapeutic action. While several reviews have discussed NG/MGs, our manuscript provides a uniquely structured classification framework, a comparative analysis of synthesis techniques, and a broad overview of biomedical applications, including cancer therapy, inflammation control, and antiviral treatments. We also highlight emerging trends such as hybrid NGs, theranostic systems, and personalized medicine, which are reshaping the future of precision therapeutics.
By integrating recent advances in polymer chemistry, nanotechnology, and biomedical engineering, this review aims to serve as a comprehensive resource for researchers and clinicians seeking to understand and apply polymer network-based NG/MGs in next-generation drug delivery systems.
2. Classification of Polymeric NG/MGs
Hydrogels are polymer networks formed by the combination of a dispersed phase (polymer chains) and a continuous phase (typically water). Depending on their particle size, they are categorized into macrogels, microgels, and nanogels. Macrogels are large, crosslinked structures that typically have a jelly-like consistency (Figure 1) [35]. In contrast, MGs and NGs are soft, smaller-scale materials formed by crosslinking polymers or biomolecules like proteins, which can create gel-like structures at microscopic or nanoscopic levels.
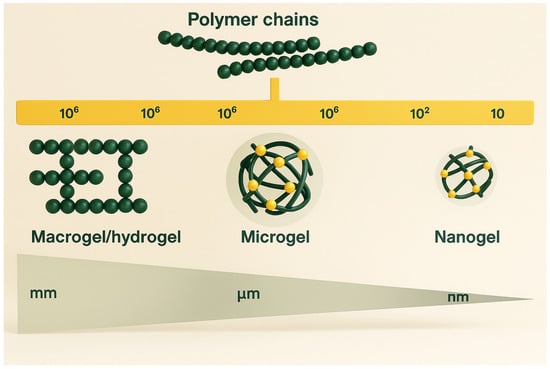
Figure 1.
Illustration depicting gel structures across multiple size scales. Redrawn and adapted with permission from Ref. [36], Elsevier 2025.
MGs generally range from 0.1 to 100 µm in diameter and are uniformly dispersed in a solvent where they swell. NGs are even smaller, typically sized between 10 and 100 nm. Unlike bulk hydrogels, MGs and NGs have a higher surface area exposed to the surrounding medium, allowing them to respond quickly to environmental stimuli such as pH, temperature, or ionic strength. This responsiveness enables them to expand or contract, a feature that solid particles lack due to their rigidity and limited interaction with solvents [37].
While NGs are often spherical, their shape can vary depending on the synthesis method and polymer architecture. For example, star-shaped NGs can be formed from polymers with multiple arms connected to a central core [38]. It’s also important to distinguish NG/MGs from polymeric micelles and surfactants. Unlike micelles, which are dynamic and undergo rapid monomer exchange, NG/MGs maintain a stable structure over time, making them more suitable for long-term applications. These properties make MGs and NGs particularly attractive for biomedical applications, including drug delivery, biosensing, and tissue engineering. Their ability to undergo reversible volume changes in response to stimuli allows for controlled release of therapeutic agents and dynamic interaction with biological environments.
Their versatility in biomedical applications—ranging from drug delivery to tissue engineering—is largely attributed to their tunable physicochemical properties. A comprehensive classification framework is essential for understanding their design, functionality, and therapeutic potential [39]. Polymer micro/NGs can be categorized based on several key criteria (Scheme 1).
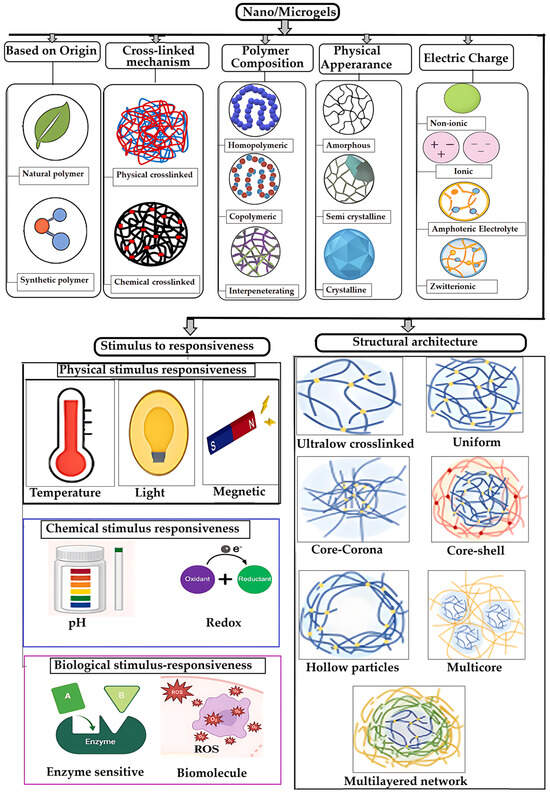
Scheme 1.
Schematic representation of various classes of polymeric NG/MGs. Redrawn and adapted with permission from [25], Elsevier 2025; [36,40], MDPI 2021.
2.1. Based on Origin of Polymers
The origin of the polymers used in NG/MGs synthesis plays a critical role in determining their physicochemical properties, biocompatibility, and suitability for biomedical applications. These polymers are broadly classified into natural, synthetic, and hybrid sources, each offering distinct advantages and limitations [41,42].
2.1.1. Natural and Hybrid Polymer NG/MGs
Natural polymer gels are made from biopolymers commonly found in living organisms, such as polysaccharides (e.g., xanthan gum, alginate, starch, chitosan), proteins (e.g., fibrin, collagen, gelatin), and polynucleotides [43,44,45,46]. These materials are especially attractive due to their biodegradability and biocompatibility, making them safer and more environmentally friendly than many synthetic alternatives. Their natural origin and functional diversity have led to growing interest in biomedical and environmental applications. To create gels with tailored structures and properties, researchers often combine gelators of different molecular weights, resulting in supramolecular hybrid hydrogels [45]. These systems can exhibit unique mechanical and functional characteristics. When natural and synthetic polymers are blended, they form hybrid hydrogels. These materials combine the advantages of both components—such as the biodegradability of natural polymers and the mechanical strength and tunability of synthetic ones. This synergy allows for better control over properties like rigidity, viscosity, and durability [47,48].
2.1.2. Synthetic Polymer NG/MGs
Synthetic polymer gels are engineered materials known for their tunable mechanical properties and controlled degradation behavior, making them suitable for a wide range of applications in biomedicine, materials science, and engineering [49,50,51]. These gels are commonly used in areas such as regenerative medicine, bioprinting, drug delivery, and energy storage. Some widely used synthetic polymers for gel formation include: Poly(ethylene glycol) (PEG) [52], Poly(vinyl alcohol) (PVA) [53], Poly(methyl methacrylate) (PMMA), Poly(hydroxyethyl methacrylate) (PHEMA) [54,55], Polyurethanes, Poly(amino acids), Poly(vinyl pyrrolidone) (PVP) [53]. These synthetic gels are often designed to mimic natural biopolymers, offering customizable features for specific applications. Many are developed as stimuli-responsive or environmentally sensitive polymers, capable of reacting to subtle changes in their surroundings. This responsiveness can manifest in various ways, such as: Sol-gel transitions, Changes in solubility, Alterations in shape or surface properties, Formation of complex gel structures [56,57]. Such dynamic behavior allows synthetic gels to adapt quickly and reversibly, making them ideal for advanced functional systems.
2.2. Based on Crosslinking Mechanism
The crosslinking mechanism is a fundamental criterion in the classification of polymeric NG/MGs, as it directly influences their structural integrity, responsiveness to environmental stimuli, and suitability for various biomedical applications. Crosslinking refers to the process by which polymer chains are interconnected to form a three-dimensional network, and it can occur through either physical or chemical interactions [58]. The nature of these interactions determines key properties such as mechanical strength, swelling behavior, degradation rate, and drug release kinetics [59]. Understanding the differences between physically and chemically crosslinked systems is essential for designing NGs tailored to specific therapeutic needs, whether for transient, reversible applications or long-term, stable drug delivery platforms.
2.2.1. Physically Crosslinked NGs
Physically crosslinked NGs are formed through non-covalent interactions such as hydrogen bonding, ionic interactions, hydrophobic forces, and van der Waals associations, which result in reversible and stimuli-responsive networks. These gels are advantageous due to their ease of synthesis, as they do not require chemical crosslinkers, thereby minimizing residual toxicity. Their dynamic nature allows them to respond to environmental changes like pH, temperature, or solvent composition, making them ideal for applications in drug delivery, diagnostics, and biomedical devices [17,18,60,61,62,63,64]. Physical gels can be fabricated in various forms, including hydrogels, cryogels, aerogels, xerogels, films, and composites, and their behavior is influenced by polymer size and dispersion medium.
2.2.2. Chemically Crosslinked NGs
In contrast, chemically crosslinked NGs are formed through covalent bonding between polymer chains, resulting in permanent, robust networks with controlled degradation and long-term stability. These gels are typically synthesized via methods such as addition or condensation polymerization, photopolymerization, and radiation-induced crosslinking, which can occur under mild conditions without added crosslinkers [65]. While chemical gels offer superior mechanical strength and are suitable for sustained release and implantable systems, they may involve more complex synthesis and potential limitations in hydrophilicity, especially when formed via ionic polymerization [17,66,67,68,69,70]. The choice between physical and chemical crosslinking significantly affects the gel’s swelling behavior, elasticity, and transport properties, and ultimately determines its suitability for specific biomedical or industrial applications.
2.3. Based on Polymeric Composition
Micro/NGs can be synthesized using various polymeric strategies, each offering distinct structural and functional attributes that influence their mechanical properties, responsiveness to stimuli, and compatibility with biological systems. These strategies are broadly categorized into homopolymeric, copolymeric, and interpenetrating polymer networks (IPNs), each with unique advantages for biomedical applications. Micro/NGs can be synthesized using various polymeric strategies, each offering distinct structural and functional attributes that influence their mechanical properties, responsiveness to stimuli, and compatibility with biological systems. These strategies are broadly categorized into homopolymeric, copolymeric, and interpenetrating polymer networks (IPNs), each with unique advantages for biomedical applications.
2.3.1. Homopolymeric NG/MGs
Homopolymeric micro- and NGs are made from a single type of monomer. Depending on how the monomers are arranged during polymerization, the resulting gel networks can vary in structure and properties. For example, block sequences group monomers into distinct segments, which may lead to phase separation within the gel. Alternating sequences arrange monomers in a regular pattern, influencing mechanical strength and swelling behavior. Random sequences, on the other hand, distribute monomers without a fixed order, resulting in more heterogeneous networks. These gels are relatively simple in design and are often chosen when uniform chemical functionality is needed, such as in basic drug delivery systems or biosensors [70].
2.3.2. Copolymeric NG/MGs
Copolymers are composed of two or more different monomers, typically including at least one hydrophilic component. This combination allows researchers to fine-tune the physical and chemical properties of the gel. By adjusting the ratio and type of monomers, scientists can control the gel’s hydrophilicity, mechanical strength, degradation rate, and responsiveness to stimuli like pH or temperature. Copolymeric NGs are especially useful in biomedical applications because they offer greater flexibility in design. They can be engineered to carry specific drugs, respond to environmental changes, or interact with biological targets, making them ideal for targeted and controlled drug delivery [15,16,71,72].
2.3.3. Multipolymer Interpenetrating Polymer Networks (IPNs)
Interpenetrating polymer networks (IPNs) are more complex structures formed by combining two or more polymer networks. Typically, one network is chemically crosslinked, while the other remains linear or loosely associated. These networks are physically intertwined but not covalently bonded. IPNs can be made from synthetic, natural, or hybrid polymers, and their unique architecture provides enhanced mechanical strength and multifunctionality. The combination of different polymers allows IPNs to respond more effectively to environmental stimuli such as changes in pH or temperature. Because of their structural complexity and adaptability, IPNs are widely used in tissue engineering and advanced drug delivery systems [11,73,74].
2.4. Based on Physical Appearance
The internal structure of micro- and NGs plays a key role in how they behave in solution and interact with biological systems. Based on their physical appearance, these gels can be categorized as amorphous, semicrystalline, or crystalline [75]. Amorphous gels lack a defined structure, making them flexible and highly responsive to stimuli. They often swell significantly when exposed to water or biological fluids. Semicrystalline gels contain both ordered and disordered regions, offering a balance between mechanical strength and flexibility. Crystalline gels have a highly ordered structure throughout, which gives them rigidity and stability but may limit their swelling and responsiveness. The choice of gel type depends on the intended application and desired performance characteristics.
2.5. Based on Electrical Charge of the Network
The electrical charge of a gel’s polymer network significantly affects its interaction with biological molecules, membranes, and external stimuli. Micro- and NGs can be neutral, ionic, amphoteric, or zwitterionic [75]. Neutral gels do not carry a net charge and are often used in applications where minimal interaction with charged species is preferred. Ionic gels can be either anionic or cationic. Anionic gels contain negatively charged groups like carboxylates or sulfates, which can bind to positively charged drugs or proteins. Cationic gels have positively charged groups such as amines, making them suitable for delivering negatively charged biomolecules like DNA or RNA.
Amphoteric gels contain both acidic and basic functional groups, allowing them to respond dynamically to changes in pH. This makes them particularly useful in environments where pH fluctuates, such as inflamed tissues or tumor sites. Zwitterionic gels are unique in that each repeating unit contains both positive and negative charges, resulting in an overall neutral charge. These gels are highly biocompatible and resistant to protein adsorption, making them ideal for implantable devices and biosensors.
2.6. Based on Responsiveness to Stimuli
Stimulus-responsive micro/NGs are designed to remain stable under normal physiological conditions but undergo controlled changes in response to specific external triggers. This property is particularly valuable in drug delivery, where NGs must circulate intact in the bloodstream and release their payload only at targeted sites. These stimuli can be physical (e.g., temperature, light, ultrasound, magnetic or electric fields, pressure), chemical (e.g., pH, redox potential), or biological (e.g., enzymes, biomolecules) [76,77] (Figure 2). The response mechanism typically involves signal detection, internal structural or chemical transformation, and a visible or functional change in the NGs [78].
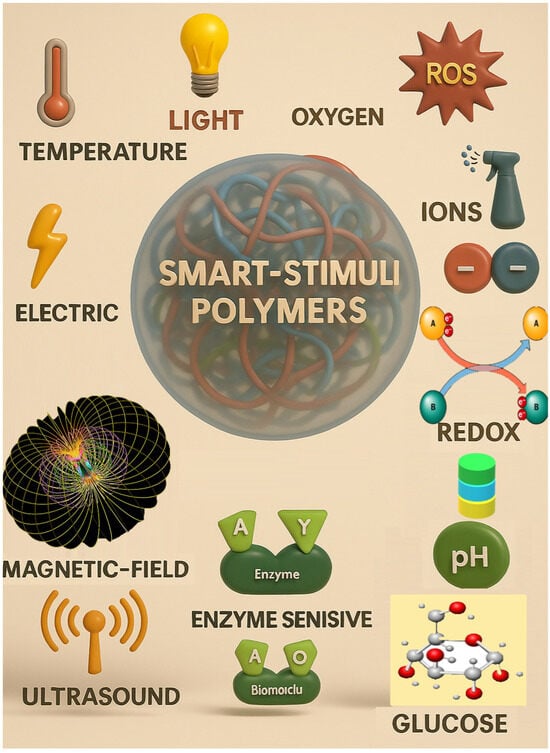
Figure 2.
Schematic representation of polymeric MG/NGs classification based on stimulus-responsiveness. Reproduced with permission from Motornov et al. [78], MDPI 2024.
2.6.1. Physical Stimulus-Responsiveness
NG/MGs can be engineered to respond to various physical stimuli, enabling precise control over drug release and therapeutic action. Temperature-responsive NGs exhibit a volume phase transition temperature (VPTT), where their size changes due to altered interactions among polymer chains and solvents. These NGs are classified based on their response direction: those with a lower critical solution temperature (LCST) shrink above a threshold, while those with an upper critical solution temperature (UCST) swell when heated. Materials like PNIPAM, PNVCL, and Pluronic copolymers are commonly used to engineer such systems [77,79,80,81,82,83,84,85,86,87]. For instance, Peng et al. developed a zwitterionic NGs (PMEDAPA-Tf@DOX) that responds to mild microwave-induced hyperthermia (~41 °C) for targeted tumor therapy [88]. Light-responsive NGs react to UV, visible, or near-infrared (NIR) light through physical changes (e.g., heat generation) or chemical transformations (e.g., bond cleavage, isomerization). These responses are often driven by functional groups in the polymer or embedded photoactive agents like metal nanoparticles [76,77,89]. Ultrasound-responsive NGs utilize mechanical or thermal effects from ultrasonic waves (20 kHz to >50 MHz) to enhance drug release and tissue penetration. Cavitation and localized heating facilitate these effects, even in hollow NGs without gas-filled microbubbles [89,90,91,92]. Magnetic field-responsive NGs incorporate magnetic nanoparticles to enable non-invasive control over drug release, imaging, and hyperthermia. Their behavior can be tuned by adjusting the field strength, making them suitable for real-time therapeutic applications [77,93,94,95]. Electro-responsive NGs contain polymers that react to electric fields through dipole orientation or redox reactions. Though less common, they hold promise for treating electrically active tissues such as nerves and muscles. For example, Ying et al. developed NGs that released antiepileptic drugs in response to abnormal brain electrical signals [96,97,98]. Lastly, pressure-responsive NGs are designed with mechano-sensitive components that deform under mechanical stress, enabling applications in cardiovascular and gastrointestinal therapies. These systems often rely on unstable complexes that undergo structural changes when compressed [99,100].
2.6.2. Chemical Stimulus-Responsive Micro/NGs
Stimulus-responsive NGs can be engineered to react to specific chemical or biological signals, enabling controlled structural changes and functional responses. Among chemical triggers, pH-responsiveness is one of the most widely studied. NGs designed with pH-sensitive polymers respond to environmental pH shifts through mechanisms such as protonation/deprotonation or cleavage of acid-labile bonds like hydrazone, imine, oxime, acetal, and ketal [101]. These systems exhibit a volume-phase transition pH (VPTpH), where the gel swells below a threshold and shrinks above it [102]. This property is particularly useful in drug delivery, as different tissues and cellular compartments exhibit distinct pH levels—for example, blood (~7.4), tumor microenvironments (lower pH), and intracellular organelles like lysosomes (~4.5–5.0) [69,95]. Redox-responsive NGs leverage physiological redox gradients, such as the difference in glutathione (GSH) concentration between intracellular (2–10 mM) and extracellular (2–20 μM) environments, or between healthy and diseased tissues [77,103]. These NGs often incorporate disulfide bonds, which cleave under reducing conditions, enabling targeted drug release in tumor cells or inflamed tissues [104]. Glucose-responsive gels typically utilize boronic acid derivatives or glucose-sensitive enzymes that interact with glucose molecules, triggering changes in the gel structure [105]. CO2-responsive gels contain functional groups like amines or carbonate linkages that react with CO2, leading to reversible changes in gel properties [105,106]. Hypoxia-responsive gels are tailored to respond to low oxygen levels, often found in tumor microenvironments. These gels release their payload selectively under hypoxic conditions, enhancing therapeutic specificity [7].
2.6.3. Biological Stimulus-Responsive Micro/NGs
Biological stimuli play a crucial role in the design of smart micro/NGs, enabling them to respond selectively to specific biochemical signals present in pathological environments. These stimuli include enzymes, biomolecules (such as glucose, ATP, and reactive oxygen species), and cellular conditions that are often dysregulated in diseases like cancer, inflammation, and infections. By incorporating biologically sensitive moieties into their polymer networks, NGs can undergo structural or chemical transformations that trigger drug release, change surface properties, or activate therapeutic functions. Enzyme-sensitive NGs are designed with substrates or analogues that can be cleaved by specific enzymes, leading to structural changes and functional activation. Enzymes such as matrix metalloproteinases (MMPs), trypsin, phosphatases, and elastases have been used to trigger responses in NGs for drug release or biosensing applications [107,108,109,110,111,112]. These systems are particularly valuable in disease contexts where enzyme expression is dysregulated. Additionally, NGs can respond to biomolecules like glucose, reactive oxygen species (ROS), and adenosine triphosphate (ATP) [113,114]. Glucose-responsive NGs can be constructed using glucose oxidase (GOx), lectins like concanavalin A (Con A), or phenylboronic acid (PBA) conjugation. These mechanisms enable glucose detection or insulin release in diabetic therapy [105,106]. ROS-responsive NGs incorporate functional groups such as diselenides, arylboronic esters, thioethers, and thioketals, which undergo bond cleavage or solubility changes upon exposure to ROS like H2O2, •OH, or 1O2 [115,116]. To enhance precision and versatility, many NGs are now designed to be dual- or multi-responsive, integrating multiple triggers such as pH and redox or temperature and light. These advanced systems allow for spatiotemporal control over drug release and degradation, broadening their potential in targeted therapy, diagnostics, and smart biomaterials [117,118,119,120,121].
2.7. Based on Structural Architecture
The internal structure of gel particles is influenced by factors such as the choice of monomers, crosslinkers, and the kinetics of particle formation, which together determine the segment density and spatial organization within the gel. Even when composed of homopolymers, these variables can lead to diverse architectural configurations. Table 1 illustrates the versatility in achieving different architectural configurations.

Table 1.
Gel Particle Architectures and Their Key Features [36].
3. Synthesis and Characterization of Micro/NGs
3.1. Synthesis Methods
MGs and NGs are synthesized through either chemical or physical crosslinking of hydrophilic polymers, each offering distinct structural and functional properties. Physical crosslinking relies on reversible, non-covalent interactions such as ionic, hydrophobic, and hydrogen bonding forces, enabling responsiveness to environmental stimuli and biodegradability—features particularly valuable for drug, protein, or cell encapsulation and release. In contrast, chemically crosslinked gels are formed via covalent bonds, providing enhanced structural stability and typically requiring crosslinking agents [131,132,133].
Effective synthesis of micro/NGs demands precise control over particle size distribution, functional group placement, and colloidal stability [131,132]. MG fabrication methods are broadly categorized into homogeneous nucleation and polymerization, emulsification, and polymer complexation. Homogeneous nucleation involves mixing water-soluble monomers, crosslinkers, and initiators in aqueous media, often via emulsion polymerization; sequential polymerization can yield core-shell structures. Emulsification techniques disperse aqueous polymer droplets in oil, followed by crosslinking using difunctional or multifunctional agents. Other methods include precipitation, inverse (mini) emulsion, inverse microemulsion, dispersion polymerization, membrane emulsification, and controlled/living radical polymerization [131,132]. Polymer complexation involves mixing oppositely charged polyelectrolytes or hydrogen-bonding polymers to form physically crosslinked networks, with factors such as hydrophobicity, molecular weight, polymer structure, solvent, and pH influencing gel formation and stability [134,135].
NGs follow similar principles but are often tailored for biomedical applications, where dispersion stability is critical. Their synthesis is generally categorized into crosslinking polymerization—using monomers and crosslinkers—and crosslinking of polymer precursors, such as amphiphilic or reactive polymers capable of self-assembly. Additional techniques include physical self-assembly in aqueous media, forming NGs through hydrogen bonding, van der Waals forces, and electrostatic interactions, and template-assisted fabrication using photolithography or micromolding, which enables precise particle design [136,137,138,139,140].
A particularly clean and efficient method involves ionizing radiation, such as gamma rays or fast electron beams, which initiate intramolecular crosslinking in water-soluble polymers without the need for monomers, surfactants, or crosslinking agents—thus eliminating purification steps [136,141]. This technique allows fine control over NGs’ properties and supports biomedical applications. However, its scalability is limited due to the complexity and cost of high-energy radiation equipment [136,141,142,143]. The synthesis methods of micro- and NGs are summarized in Table 2.

Table 2.
NG/MGs Synthesis Methods [144].
3.2. Comparative Evaluation of NG/MGs Synthesis Techniques
NG/MGs synthesis methods vary widely in their mechanisms, scalability, and compatibility with different polymer types. Among these, inverse microemulsion polymerization and radiation-induced polymerization are particularly noteworthy due to their unique advantages and limitations.
3.2.1. NGs Fabrication by Inverse Microemulsion
Inverse microemulsion polymerization confines free-radical polymerization within nanometer-sized aqueous droplets dispersed in a continuous oil phase, stabilized by surfactants and co-surfactants. These droplets act as nanoreactors, and their size—and thus NG diameter—can be tuned by adjusting the water-to-surfactant ratio (W0), surfactant hydrophilic-lipophilic balance (HLB), oil composition, and ionic strength. Water-soluble monomers such as acrylamide, N-isopropylacrylamide (NIPAM), and PEG derivatives, along with initiators, are localized in the dispersed phase. Polymerization is typically initiated thermally or via redox systems, yielding crosslinked NGs with narrow size distributions [154,155,156,157,158]. This method generally produces NGs in the range of 20–200 nm with low polydispersity, high encapsulation efficiency for hydrophilic payloads, and the ability to incorporate stimuli-responsive functionalities (e.g., pH or temperature sensitivity using NIPAM, MAA, or AMPSA) [156,157]. Recent studies have demonstrated tunable responsiveness by varying comonomer ratios and dispersed-phase volume, while environmentally friendly formulations using optimized HLB surfactants and lower processing temperatures have also been reported [156]. Classical kinetic studies of inverse miniemulsions provide further insight into rate behavior and stabilization mechanisms [155]. As a representative example, Peters et al. synthesized core–shell PNIPAM-based NGs for controlled chemotherapeutic release, achieving precise particle size and drug-loading control, though requiring prolonged dialysis to remove residual surfactants [8]. The main advantages of this technique include (i) precise size control and uniformity via droplet nanoreactors, (ii) high encapsulation efficiency for hydrophilic drugs, proteins, and nucleic acids, and (iii) mild processing conditions suitable for temperature-sensitive biomolecules [157,158].
However, limitations remain, such as (i) surfactant removal challenges requiring extensive dialysis or ultrafiltration, (ii) scale-up limitations due to microemulsion stability and phase separation, and (iii) restricted monomer scope favoring water-soluble vinyl monomers [155,156].
3.2.2. Radiation-Induced Polymerization
Radiation-induced polymerization utilizes ionizing radiation sources, including gamma rays, electron beams, or X-rays, to initiate polymerization and crosslinking processes without the need for chemical initiators. Radiolysis of water generates reactive species (e.g., hydroxyl radicals, hydrated electrons) that initiate and propagate polymer chains. The absorbed dose and dose rate determine crosslink density, NG size, and swelling behavior [159]. Because radiation simultaneously sterilizes the material, this method is highly attractive for producing clinical-grade NGs and supports continuous processing using conveyor-based electron-beam systems [160,161]. Radiation-based methods have been successfully applied to synthesize clean, injectable NGs from polymers such as PVA, PEG, PVP, chitosan, and poly(acrylic acid). Incorporation of comonomers like NIPAM or DMAEMA enables stimuli-responsive behavior [160,162]. Recent studies have reported pH-responsive pectin/PAA NGs for cancer therapy and injectable hydrogels for wound healing and tissue engineering [12,162]. As a representative example, Basak et al. demonstrated the fabrication of enzyme-responsive PEG NGs via gamma irradiation in confined aqueous droplets, achieving simultaneous crosslinking and sterilization for cardiovascular applications [9]. The main advantages of this approach include (i) initiator- and crosslinker-free synthesis, minimizing residual toxicity and enhancing biocompatibility, (ii) simultaneous sterilization, eliminating additional processing steps, and (iii) scalable, continuous manufacturing, particularly with electron-beam systems [12,161].
However, limitations include (i) dependence on specialized facilities for gamma or electron-beam irradiation, (ii) payload radiosensitivity, which may lead to degradation of proteins or nucleic acids, and (iii) polymer specificity, favoring radiation-sensitive backbones or vinyl-functionalized polymers [12,161].
3.2.3. Comparative Perspective and Design Guidance
Inverse microemulsion polymerization is preferred when precise size control (20–200 nm) and high loading of hydrophilic therapeutics are critical, provided that surfactant removal and batch processing are acceptable [157,158]. Radiation-induced polymerization is ideal when ultra-clean, initiator-free NGs and in-line sterilization are required, or when continuous electron-beam processing is available—assuming the payload can tolerate radiation exposure [12,161]. Table 3 summarizes the inverse microemulsion and radiation-induced NG/MGs fabrication routes.

Table 3.
Comparative Summary of Two NG/MGs Fabrication Routes.
3.2.4. Practical Parameter Windows
To achieve reproducible and application-specific NG properties, both inverse microemulsion polymerization and radiation-induced polymerization require careful control of key synthesis parameters. These parameters influence droplet stability, crosslinking behavior, responsiveness, and ultimately the scalability and functionality of the resulting NGs.
In inverse microemulsion polymerization, critical factors include the water-to-surfactant ratio (W0), the hydrophilic-lipophilic balance (HLB) of the selected surfactants, and the composition of the oil phase. Optimizing these variables ensures stable droplet formation and minimizes phenomena such as Ostwald ripening, which can compromise NG uniformity. Responsiveness to environmental stimuli can be finely tuned by incorporating comonomers such as N-isopropylacrylamide (NIPAM) with methacrylic acid (MAA) or 2-acrylamido-2-methylpropane sulfonic acid (AMPSA), enabling dual pH- and temperature-sensitive behavior [156,157].
In radiation-induced polymerization, the absorbed dose (kGy) and dose rate are key determinants of crosslink density and NG size. Maintaining the polymer concentration within the dilute-to-semi-dilute regime favors intramolecular crosslinking, which is essential for forming discrete NGs and avoiding undesirable bulk gelation [160,161]. This approach is particularly advantageous for synthesizing sterile, initiator-free NGs suitable for biomedical use.
A comparative overview of the scalability and application relevance of these two techniques is presented in Table 4, highlighting their respective strengths and limitations across research and industrial contexts.

Table 4.
Scalability and Application Relevance.
3.2.5. Strategic Selection Based on Application
The choice between inverse microemulsion polymerization and radiation-induced polymerization depends on the intended application, processing constraints, and regulatory considerations. For precision drug delivery, where tight size control (20–200 nm) and high encapsulation efficiency of hydrophilic therapeutics are critical, inverse microemulsion polymerization is generally preferred [157,158]. This method allows fine-tuning of NG size and responsiveness (e.g., pH or temperature sensitivity) through control of the water-to-surfactant ratio (W0), surfactant HLB, and comonomer composition, making it highly suitable for academic research and preclinical studies where batch processing and extensive purification steps are acceptable [156,158]. However, its scalability is limited by the complexity of microemulsion stabilization and the need for surfactant removal, which can impact biocompatibility [156,160].
In contrast, radiation-induced polymerization offers a cleaner, initiator-free synthesis route with built-in sterilization, making it particularly attractive for clinical translation and large-scale manufacturing [162]. This approach eliminates toxic initiators and residual surfactants, thereby improving regulatory compliance and reducing downstream purification requirements. Furthermore, the ability to integrate continuous processing using electron-beam systems enhances industrial feasibility for producing injectable hydrogels, wound dressings, and implantable biomaterials [12,162]. Nevertheless, this method requires access to specialized irradiation facilities and careful consideration of payload radiosensitivity, as sensitive biomolecules (e.g., proteins, nucleic acids) may degrade under high-energy exposure [9].
Overall, inverse microemulsion polymerization is strategically suited for customized NG systems in research and early-stage development, while radiation-induced polymerization provides a regulatory-friendly, scalable pathway for clinical-grade products, particularly in applications demanding sterility and high purity, such as injectable drug delivery systems and tissue engineering scaffolds.
3.3. Tools and Techniques for Characterizing NG/MGs
A wide array of analytical techniques is employed to investigate the size, structure, composition, behavior, and appearance of metal nanoparticle-based NG/MGs. These methods are essential for distinguishing NG/MGs from their corresponding smart NG/MGs and for understanding their physicochemical properties. Figure 3A–E illustrates representative examples of key characterization techniques used in this context.
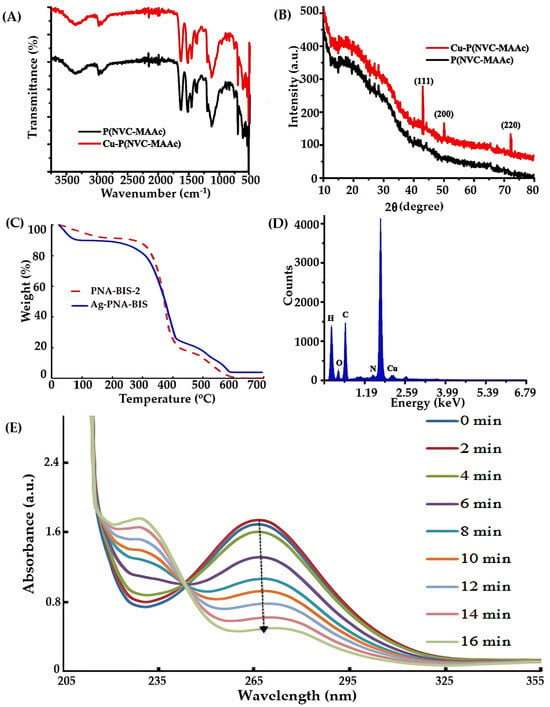
Figure 3.
Characterization techniques for NGs: (A) FTIR [165], (B) XRD [165], (C) TGA [166], (D) EDX [165], and (E) UV/Vis spectra [165]. (A,B,D,E) adopted with permission from ref. [165], Elsevier 2019 and C adopted with permission from ref. [166], Elsevier 2019.
3.3.1. Chemical Functionalities
Fourier-transform infrared spectroscopy (FTIR), shown in Figure 3A, is commonly used to identify functional groups and assess chemical interactions within the polymer matrix. It provides insights into the bonding environment and the incorporation of metal nanoparticles into the gel network. Complementary techniques such as 1H-nuclear magnetic resonance (NMR) and Raman spectroscopy (RS) are also widely applied to investigate the molecular structure and functional modifications of the organic polymer components.
X-ray diffraction (XRD), depicted in Figure 3B, is used to determine the crystalline nature of both the polymer matrix and embedded metal nanoparticles. Energy-dispersive X-ray spectroscopy (EDX) complements XRD by confirming the elemental composition and distribution of metal species within the gel. These techniques are crucial for verifying the successful incorporation and stabilization of nanoparticles in hybrid systems.
Thermal stability and decomposition behavior are assessed using thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), and differential mechanical analysis (DMA). TGA, shown in Figure 3C, is particularly useful for quantifying the metal content in hybrid MGs and evaluating their thermal degradation profiles. These thermal techniques help determine the suitability of hybrid MGs for temperature-sensitive applications.
Energy-dispersive spectroscopy (EDS), illustrated in Figure 3D, is employed to analyze the elemental composition of hybrid MGs. It provides detailed information about the distribution and concentration of metal nanoparticles within the gel matrix. Inductively coupled plasma techniques, including ICP-MS, ICP-OES, and ICP-AES, are also used to quantify the presence of noble metals and assess their uniformity across samples.
Optical techniques such as UV-Visible/NIR spectroscopy (Figure 3E) are used to monitor the volume phase transition temperature (VPTT) of MGs and hybrid systems by measuring turbidity. These methods also confirm the formation and stabilization of plasmonic nanoparticles within the gel network.
3.3.2. Morphological Characterization
Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) are essential for visualizing particle morphology. SEM provides 3D-like surface images with high depth of field, useful for examining topography and aggregation. TEM offers high-resolution 2D images of internal structures and compartments. Figure 4A illustrates TEM imaging of NGs/MGs. When combined with Energy-Dispersive X-ray Spectroscopy (EDS), SEM can also provide elemental composition data.
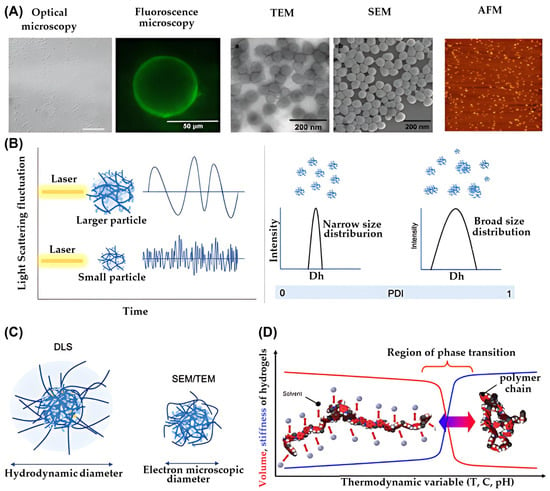
Figure 4.
(A) Representative of particle morphology characterized by different microscopy [167,168,169], reproduced with permission from ref. [154]. Springer 2016; reproduced with permission from ref. [155]. Beilstein-Institut 2015; reproduced with permission from ref. [156]. Elsevier 2015. (B) Illustration of dynamic light scattering of larger particles and smaller particles (left) and DLS profile of particles with narrow size distribution and broad size distribution (right) (C), schematic illustration presenting the measurement of particle size from DLS, SEM, and TEM and (D) the illustration presenting the interaction between polymer-polymer and polymer-solvent under specific environment, reproduced with permission from ref. [36]. Elsevier 2025.
Atomic Force Microscopy (AFM) is used to analyze surface roughness, particle height, and mechanical properties such as stiffness. It operates by scanning a sharp probe across the sample surface and measuring interaction forces. Phase imaging modes allow for the detection of chemical heterogeneity.
Fluorescence Microscopy enables visualization of the spatial distribution within NGs/MGs. It can distinguish layers or compartments using fluorescent dyes or intrinsic fluorescence, revealing complex internal structures.
3.3.3. Particle Size and Size Distribution
Dynamic Light Scattering (DLS) is a non-invasive technique that measures particle size based on light scattering caused by Brownian motion. It provides the hydrodynamic diameter, which includes both the particle and its surrounding solvent shell. This value is typically larger than the size measured by SEM or TEM, which observes particles in a collapsed or dried state. Figure 4B (left) shows DLS data for particle size, while Figure 4C compares hydrodynamic and collapsed sizes.
DLS is also used to assess size distribution and detect aggregates. The polydispersity index (PDI) derived from DLS reflects the uniformity of particle sizes. A PDI below 0.5 indicates a narrow distribution, while values above 0.7 suggest significant heterogeneity, as shown in Figure 4B (right).
3.3.4. Macroscopic Properties
Although NGs and MGs are nano-/microscale materials, they exhibit macroscopic behaviors that are observable and relevant to their practical applications. These include turbidity, swelling, viscosity, and rheological behavior.
Turbidity and Swelling Behavior
The swelling behavior of NGs/MGs is governed by interactions among polymer chains, solvent molecules, and ionic species. Figure 4D illustrates phase separation driven by polymer–polymer interactions, which promote shrinkage, and polymer–solvent interactions, which promote swelling. Temperature plays a critical role in modulating these interactions. At lower temperatures, hydrogen bonding between polymer and solvent dominates, leading to swelling. Above the lower critical solution temperature (LCST), hydrophobic interactions prevail, causing shrinkage. UV-Vis spectroscopy is used to measure turbidity by analyzing light transmittance and absorbance. DLS also helps monitor aggregation and packing density in solution. As shown in Figure 5A, increased concentration leads to tighter packing and reduced light transmission.
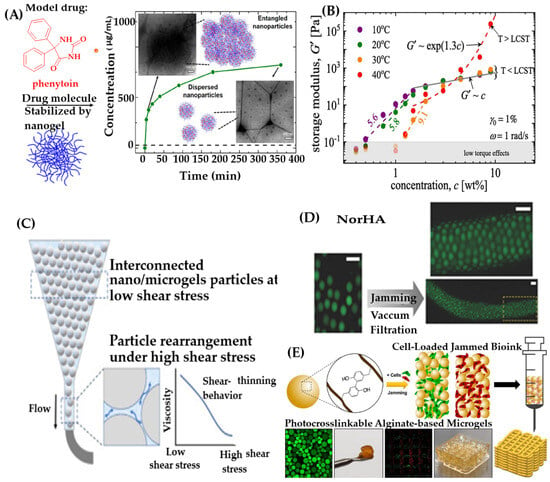
Figure 5.
(A) Effect of concentration on particle aggregation, reproduced with permission from ref. [170]. ACS 2019; (B) Concentration-dependent elastic modulus at different temperatures, reproduced with permission from ref. [171]. Elsevier 2021; (C) Shear-thinning behavior of gel-based inks for 3D printing, reproduced with permission from ref. [36]. Elsevier 2025; (D) Fluorescence images of extruded MGs, reproduced with permission from ref. [172]. Wiley 2019; (E) Schematic of cell-laden granular MG bioink for 3D bioprinting, reproduced with permission from ref. [173]. Elsevier 2023.
Rheological Properties
Rheological behavior reflects how NGs/MGs respond to mechanical stress. At low concentrations, particles are dispersed and exhibit viscous behavior. As concentration increases, physical interactions intensify, leading to viscoelastic behavior—a combination of fluid-like and solid-like properties. This transition is marked by increases in viscosity and modulus, indicating greater stiffness and resistance to deformation. Figure 5B shows that PNIPAM-based MGs exhibit a sharp increase in storage modulus above their LCST (~40 °C), especially at higher concentrations. This is due to attractive interactions and physical bonding among compact PNIPAM globules. Rheological responses such as shear-thinning and shear-thickening are critical for applications like injectable hydrogels and 3D printing. Shear-thinning facilitates smooth extrusion during printing, while shear-thickening increases resistance to flow under stress. In extrusion-based 3D printing, NG/MG-based bio-inks offer advantages over macrogels. Figure 5C illustrates shear-thinning behavior that enables precise deposition. After extrusion, gels can solidify via self-assembly or external stimuli such as temperature or light. Moreover, NG/MG-based inks address limitations of macrogels, such as restricted cell migration and nutrient exchange. The porous structure formed by loosely packed NGs/MGs facilitates efficient transport of oxygen, nutrients, and waste, supporting cell viability and function within printed scaffolds (Figure 5D,E).
3.4. Limitations of Common NG/MGs Characterization Techniques
Accurate characterization of NG/MGs is essential for understanding their physicochemical properties, optimizing formulation strategies, and ensuring reproducibility in biomedical applications. A wide array of analytical techniques is employed to assess particle size, morphology, chemical composition, and mechanical behavior. However, each method has inherent limitations that must be critically considered to avoid misinterpretation of data.
Dynamic Light Scattering (DLS) is one of the most commonly used techniques for estimating the hydrodynamic diameter of particles based on Brownian motion. While DLS is rapid and non-invasive, it is highly sensitive to the presence of aggregates. Even minor aggregation can lead to significant overestimation of particle size and polydispersity index (PDI), potentially misrepresenting the true size distribution of the sample [58,169]. Moreover, DLS cannot differentiate between single particles and loosely bound clusters, making it less reliable for heterogeneous or polydisperse systems [174]. For example, Fissan et al. [175] demonstrated that DLS failed to resolve binary nanoparticle mixtures, whereas SEM and analytical disc centrifugation (ADC) provided clear differentiation.
Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) offer high-resolution imaging of particle morphology and internal structure. However, these techniques require extensive sample preparation, including drying, staining, and exposure to vacuum conditions. Such steps can introduce artifacts like shrinkage, deformation, or aggregation, which may not reflect the native hydrated state of NGs [167,169]. Additionally, these methods provide only static snapshots and cannot capture dynamic behavior in physiological environments. The use of contrast agents or staining in TEM can further obscure fine structural details or interfere with interpretation.
To overcome these limitations, complementary techniques such as Atomic Force Microscopy (AFM), Cryo-TEM, and nanoparticle tracking analysis (NTA) are often employed. AFM enables imaging under ambient conditions without vacuum or staining, preserving hydrated morphology. Cryo-TEM allows visualization of NGs in their frozen hydrated state, minimizing structural distortion. Nevertheless, AFM is limited by its small scan area and slow imaging speed, making it less suitable for statistically representative sampling [176].
Other characterization methods also present specific challenges. Fourier Transform Infrared Spectroscopy (FTIR) is widely used to identify functional groups, but overlapping peaks and low sensitivity to minor chemical changes can hinder precise interpretation [58]. UV-Visible spectroscopy is useful for monitoring optical properties and phase transitions, yet sample turbidity and scattering can distort absorbance readings [175]. Thermogravimetric Analysis (TGA) provides insights into thermal stability and composition but requires dry samples and cannot capture behavior under physiological conditions [169]. Rheological measurements are essential for understanding viscoelastic properties, but results are influenced by concentration, temperature, and shear history, and may not directly correlate with in vivo performance [171].
In summary, no single technique can comprehensively characterize NG/MGs. A multi-technique strategy, combining complementary methods and careful experimental design, is crucial for obtaining accurate, reproducible, and physiologically relevant data.
4. Applications of Nano/Microgels (NG/MG) for Drug Delivery
4.1. Limitations, Translational Barriers, and Comparative Performance of Conventional Nanocarriers
Despite the growing interest in nanocarrier-based drug delivery systems, several translational barriers continue to hinder their clinical success [177]. Among the most widely studied nanocarriers—liposomes, dendrimers, and polymeric micelles—each presents unique advantages and limitations. Liposomes are clinically established and biocompatible, capable of encapsulating both hydrophilic and hydrophobic drugs. However, they often suffer from rapid clearance, limited long-term stability, and restricted drug loading efficiency, particularly for macromolecules like proteins. Although advanced methods such as base exchange (e.g., Doxil) have improved drug loading for certain compounds, their applicability remains narrow [178].
Dendrimers, with their hyperbranched architecture, offer precise molecular design and high drug-loading capacity. They can encapsulate small molecules and even nanoparticles [179,180,181]. Nevertheless, their complex synthesis, high cost, and potential cytotoxicity—especially due to high positive charge densities—limit their widespread use [182,183]. Cationic dendrimers and liposomes, while effective as nonviral gene delivery vectors, also raise concerns about immunogenicity and mutagenic toxicity [184,185].
Polymeric micelles, formed from amphiphilic block copolymers, are commonly used to solubilize hydrophobic drugs [186]. Despite their tunable release profiles, they often exhibit low drug loading efficiencies [187] and instability under physiological conditions, leading to premature disassembly and reduced therapeutic efficacy.
4.2. Therapeutic Versatility and Barrier-Penetrating Capabilities of Nano/Microgels (NG/MGs)
In contrast, nano/microgels (NG/MGs) offer a versatile and robust platform that addresses many of the limitations faced by traditional nanocarriers. Their high water content enables efficient encapsulation of hydrophilic biomacromolecules, such as proteins, DNA, and RNA, while their internal hydrophobic domains allow for the loading of hydrophobic drugs [188,189,190,191]. This dual-loading capability is particularly advantageous for combination therapies.
NG/MGs also exhibit stimulus-responsive behavior, enabling site-specific and controlled drug release in response to environmental cues such as pH, temperature, and redox conditions [21,192]. This responsiveness enhances therapeutic precision and minimizes systemic toxicity [193,194].
The effectiveness of NGs in drug delivery is often challenged by physiological barriers, which vary depending on the route of administration [195,196]. However, NG/MGs can be engineered to overcome physiological barriers associated with different administration routes. For instance, intravenously administered NGs must evade clearance by the kidneys, liver, and spleen to reach target tissues. Additionally, they may need to interact with or traverse biological membranes, and in some cases, cross the blood–brain barrier (BBB) to access the central nervous system (CNS) [197]. Mucosal delivery routes—such as oral, ocular, and pulmonary—require NGs to exhibit mucoadhesive or mucopenetrating properties to overcome the protective mucus layer covering epithelial surfaces [197]. In transdermal applications, NGs must penetrate the stratum corneum or release their payload effectively into the skin [20]. Several reviews have comprehensively discussed these physiological barriers and the strategies NG/MGs employ to overcome them [20,197]. Here, we highlight several representative examples demonstrating the potential of NG/MGs in overcoming such challenges.
4.2.1. Crossing the Blood–Brain Barrier
The BBB, composed of tightly joined endothelial cells, restricts the entry of most molecules into the CNS, posing a significant obstacle for treating neurological disorders [20]. However, NGs can exploit endogenous transport mechanisms such as absorptive, cell-mediated, or receptor-mediated transcytosis. Surface modifications that target specific receptors on BBB endothelial cells further enhance their ability to cross this barrier [20].
A notable example is the development of a near-infrared (NIR) responsive biomimetic NG system, ARNGs@TMZ/ICG, designed by Zhang et al. for glioblastoma multiforme (GBM) therapy [198]. This system was synthesized by crosslinking poly(deca-4,6-diynedioic acid) with pullulan, followed by encapsulation of indocyanine green (ICG) and temozolomide (TMZ). The NG core was then coated with an erythrocyte membrane functionalized with apolipoprotein E (ApoE) peptide. The ApoE modification facilitated BBB penetration and tumor cell uptake via interactions with low-density lipoprotein receptors, which are overexpressed in both U87MG brain tumor cells and BBB endothelial cells. Upon NIR irradiation, ICG generated reactive oxygen species (ROS), triggering the release of TMZ specifically within GBM lesions. This targeted delivery system significantly inhibited tumor progression in both orthotopic GBM and GBM stem cell-bearing mouse models. To address the challenge of delivering drugs across the blood–brain barrier (BBB), a specialized NG system was developed using a crosslinked network of pullulan and an oxidatively degradable polymer known as poly(deca-4,6-diynedioic acid) (PDDA) (Figure 6). This polymer remains stable under normal physiological conditions but breaks down in the presence of reactive oxygen species (ROS), which can be generated by light-activated photosensitizers.
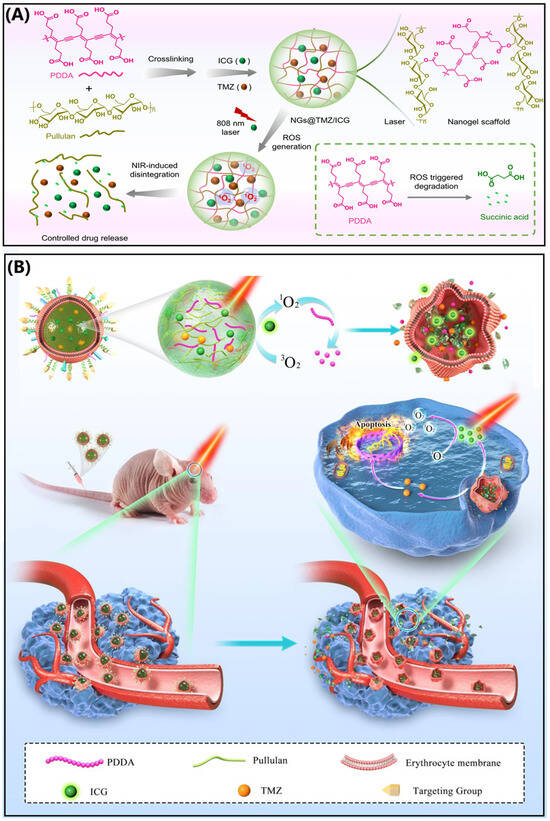
Figure 6.
Schematic of (A) NG disintegration under NIR light and (B) NIR-activatable biomimetic NGs for glioblastoma therapy. Reproduced with permission from Zhang et al. [198], Nature Communications 2022.
The NGs were loaded with two key agents: indocyanine green (ICG), a near-infrared (NIR) photosensitizer approved by the FDA, and temozolomide (TMZ), a standard chemotherapy drug for glioblastoma multiforme (GBM). This dual-loaded formulation, referred to as NGs@TMZ/ICG, was further coated with erythrocyte membranes modified with apolipoprotein E (ApoE) peptides. The ApoE decoration enhances circulation time in the bloodstream and facilitates targeted delivery to brain tumors by interacting with receptors overexpressed on both BBB endothelial cells and GBM cells. Once the NGs accumulate in the tumor site following intravenous administration, NIR light is applied externally to activate the system. The light triggers ICG to produce ROS, which causes the NG structure to degrade and release both TMZ and ICG. This controlled release mechanism promotes deeper penetration into the tumor tissue and enhances the therapeutic effect through a combination of photodynamic therapy and chemotherapy. In preclinical models, this NIR-responsive NG system demonstrated significant tumor suppression in mice with orthotopic GBM, highlighting its potential as a promising strategy for targeted brain cancer treatment.
4.2.2. Ophthalmic Drug Delivery
Ophthalmic disorders such as cataracts, infections, glaucoma, and dry eye syndrome are increasingly prevalent [199]. Although topical formulations like eye drops and ointments are commonly used, their effectiveness is limited by rapid tear turnover, blinking reflexes, and poor corneal permeability [200,201]. NGs offer a promising alternative by enabling sustained drug release, protecting drugs from enzymatic degradation, and maintaining optical clarity and comfort due to their high water content and colloidal stability [200].
An innovative approach was demonstrated by Wang et al., who developed therapeutic contact lenses embedded with NGs for sustained ophthalmic drug delivery [200]. They synthesized PSBMA-based NGs via one-step reflux-precipitation polymerization and loaded them with levofloxacin (LEV). These NGs were incorporated into hydrogel lenses using HEMA and N-vinyl-2-pyrrolidone monomer solutions through cast molding. The resulting lenses, containing 8 wt% NGs, exhibited excellent transparency, water retention, and cytocompatibility. Importantly, they provided sustained LEV release over 10 days without an initial burst, highlighting their potential as a comfortable and effective ophthalmic drug delivery platform (Figure 7).
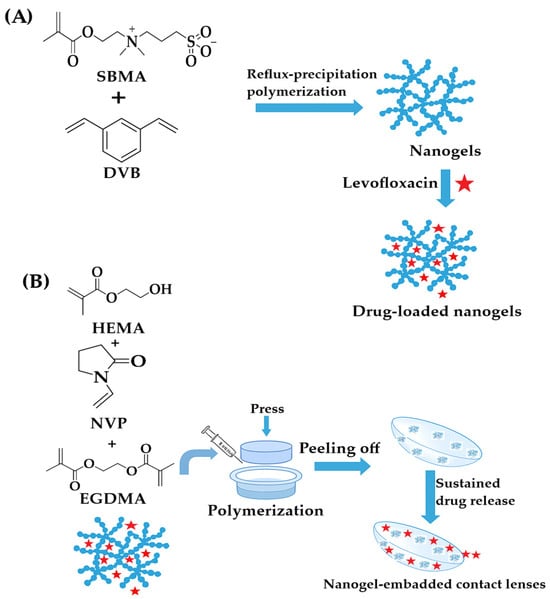
Figure 7.
(A) Reflux-precipitation polymerization of NGs and preparation of drug-loaded PSBMA NGs; (B) Fabrication of NG-embedded contact lenses, redrawn and adapted with permission from ref. [200]. MDPI 2021.
NG/MGs, with their tunable synthesis, surface modification capabilities, and diverse administration routes, offer a robust platform for overcoming physiological barriers in drug delivery. Their successful application across various disease models underscores their potential in advancing therapeutic strategies for complex conditions.
4.2.3. Cancer Therapy
Delivery of Small Molecules for Cancer Therapy Using NGs
Chemotherapy has long been a cornerstone of cancer treatment, and more recently, phototherapy has emerged as a complementary approach. Both strategies rely heavily on small molecules—chemotherapeutic agents like doxorubicin (DOX) and paclitaxel (PTX) [22,202,203,204,205,206,207,208], and photosensitizers or photothermal agents for light-based therapies [209,210]. Additionally, small-molecule inhibitors and agonists are increasingly used in cancer immunotherapy [211]. Despite their therapeutic potential, these drugs often suffer from poor bioavailability and severe side effects when administered in free form.
To address these limitations, NG-based drug delivery systems have been developed to enhance drug efficacy and reduce toxicity [211]. NGs can be administered intravenously, provided they exhibit sufficient stability, compatibility with blood components, and resistance to protein adsorption to avoid rapid clearance by the reticuloendothelial system (RES) [22,212,213,214,215].
A notable example is the hypoxia-responsive NG developed by She et al., known as HPMPC NG [22] (Figure 8). This system utilizes a zwitterionic phosphorylcholine polymer crosslinked with an azobenzene-based linker that degrades under low-oxygen conditions typical of tumor environments. Upon degradation, the NG releases DOX directly into the tumor. Its membrane-mimicking structure also enables it to cross the blood–brain barrier, allowing for effective accumulation and treatment of glioblastoma, a highly aggressive brain tumor.
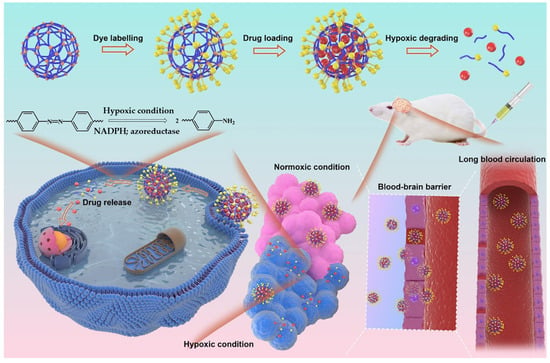
Figure 8.
Schematic of poly(phosphorylcholine)-based (HPMPC) NG with extended circulation, BBB penetration, and hypoxia-responsive drug release for glioblastoma therapy, reproduced with permission from ref. [22]. Elsevier 2021.
Beyond chemotherapy, NGs are also used to deliver photosensitizers and photothermal agents via intravenous routes [216,217]. For cancers located in mucosal tissues, such as bladder [218,219] and cervical cancer [220], NGs with mucoadhesive properties [221] are particularly beneficial. Their high viscosity allows them to adhere to mucosal surfaces, increasing local drug concentration and enhancing penetration through the mucus barrier [222]. For instance, a polypeptide-based NG (NG/HCPT) was developed using poly(L-lysine)–poly(L-phenylalanine-co-L-cystine) (PLL–P(LP-co-LC)) to deliver 10-hydroxycamptothecin (HCPT) [218] (Figure 9). The cationic nature of the polymer provided strong mucoadhesion and facilitated drug penetration. Additionally, disulfide crosslinking endowed the NG with reduction-sensitive release, enabling targeted drug delivery and enhanced antitumor activity both in vitro and in vivo.
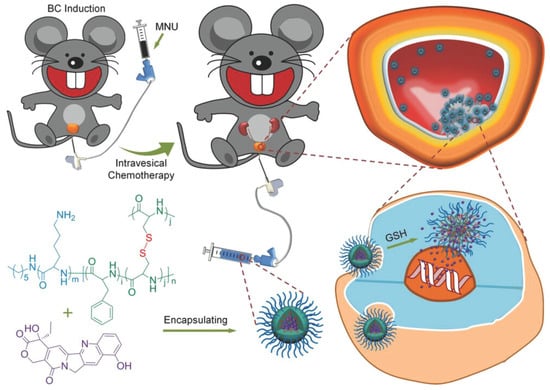
Figure 9.
Chemical structure of NG/HCPT and its metabolic pathway in vivo. NG/HCPT accumulates in tumor tissue due to mucoadhesion and cellular uptake, with GSH-triggered HCPT release, reproduced with permission from ref. [218]. Wiley 2018.
Given the complexity and heterogeneity of tumors, single-drug therapies often fall short, leading to incomplete tumor eradication and recurrence [219]. To overcome this, NGs have been designed to co-deliver multiple drugs with complementary mechanisms, creating a synergistic “drug cocktail” effect [223,224]. For example, Lu et al. developed a reduction-sensitive NG capable of simultaneously delivering DOX and HCPT [202] (Figure 10). The drugs were conjugated to a disulfide-containing crosslinker, allowing for controlled release in the reductive tumor microenvironment. This dual delivery approach enhanced DNA damage and improved therapeutic outcomes compared to monotherapy.
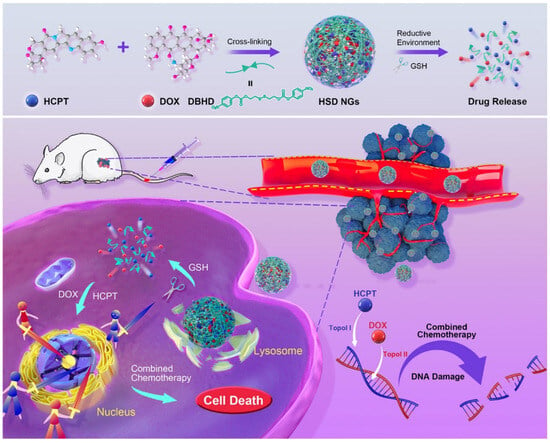
Figure 10.
Reduction-responsive chemo-capsule-based prodrug NG for enhanced chemotherapy, reproduced with permission from ref. [202]. ACS 2021.
In summary, NGs offer a highly adaptable platform for small-molecule delivery in cancer therapy. Their ability to improve drug stability, target specific tissues, and enable combination treatments makes them a promising tool for advancing cancer care.
Delivery of Biomacromolecules for Cancer Therapy Using NGs
Beyond small-molecule drugs, NGs have also been employed to deliver biomacromolecules—such as proteins and nucleic acids—for cancer treatment. These macromolecular therapeutics often offer greater specificity and reduced toxicity compared to traditional chemotherapeutics [202].
Protein drugs can act either outside or inside cells. While extracellular proteins are more commonly used clinically, intracellular proteins require delivery systems capable of crossing cell membranes, protecting the proteins from enzymatic degradation, and releasing them in the correct cellular compartment [225]. NGs, with their high water content, excellent biocompatibility, and efficient protein-loading capacity, are well-suited for this purpose [226,227,228,229,230,231,232,233,234]. For example, Chen et al. developed hyaluronic acid (HA)-based NGs to deliver two intracellular proteins: cytochrome c (CC), which induces apoptosis [226], and granzyme B (GrB), which activates or deactivates intracellular proteins to promote cell death. Encapsulation in NGs significantly enhanced the therapeutic efficacy of both proteins, with CC showing over a tenfold improvement in potency [226] (Figure 11).
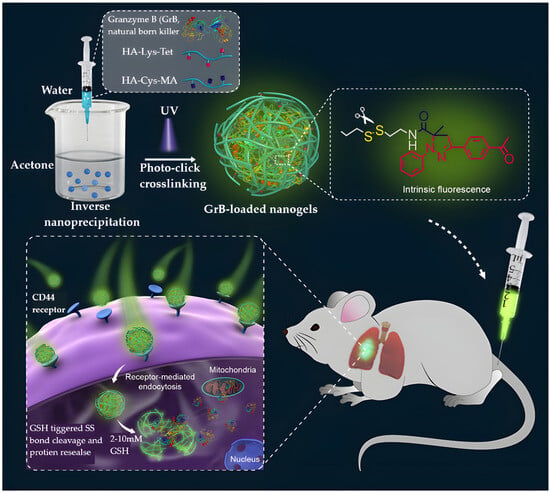
Figure 11.
Bioresponsive fluorescent HA NGs for protein encapsulation and tumor-targeted delivery via inverse nanoprecipitation and photoclick chemistry, reproduced with permission from ref. [226]. ACS 2016.
Another innovative system involved neutrophil-inspired NGs carrying two enzymes—superoxide dismutase (SOD) and chloroperoxidase (CPO)—to elevate reactive oxygen species (ROS) levels inside cancer cells [229] (Figure 12). These core–shell NGs (SCNGs) were synthesized via enzyme-mediated peptide precursor dephosphorylation. The enzymes catalyzed a cascade reaction that converted superoxide into hydrogen peroxide, which was then transformed into hypochlorous acid and singlet oxygen (·O2), effectively killing cancer cells without harming healthy tissue.
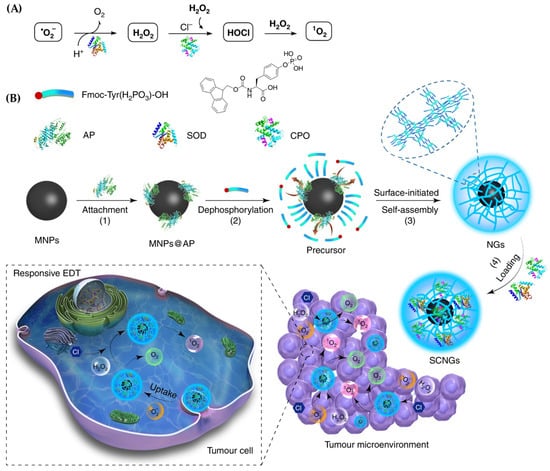
Figure 12.
Illustration of the responsive enzyme-driven therapy (EDT) mechanism and synthesis of SCNGs; (A) The therapeutic cascade involves superoxide dismutase (SOD) converting superoxide radicals (˙O2−) into hydrogen peroxide (H2O2), followed by chloroperoxidase (CPO) transforming both the generated and naturally occurring H2O2 into singlet oxygen (1O2), a potent reactive species; (B) The SCNGs are synthesized through a multi-step process: (1) functionalization of magnetic nanoparticles (MNPs) and attachment of an alkaline phosphatase (AP) trigger, (2) AP-mediated conversion of hydrophilic peptide precursors into hydrophobic gelators via dephosphorylation, (3) AP-induced self-assembly of hydrogelators around MNPs through π–π stacking and electrostatic interactions, and (4) incorporation of SOD and CPO enzymes to enable targeted and efficient EDT within the tumor microenvironment. These responsive SCNGs facilitate the transformation of endogenous reactive oxygen species (˙O2− and H2O2) into cytotoxic 1O2 via the SOD/CPO cascade, leading to selective cancer cell destruction. Reproduced with permission from ref. [229]. Nature Communications 2019.
NGs have also been widely explored for gene therapy, delivering various nucleic acids such as plasmid DNA (pDNA) [229], siRNA [235,236], nucleoside analogues [237], and microRNAs (miRNAs) [238]. These molecules can regulate gene expression, silence oncogenes, or modulate immune responses [239,240,241]. For instance, NG composed of α-lipoic acid (LA) crosslinked with ethylenediamine (ED)-modified poly(glycidyl methacrylate) (PGMA) was developed for pDNA delivery [242] (Figure 13). Inside cells, the hydrophobic LA groups were reduced to hydrophilic forms, promoting pDNA release and resulting in high cellular uptake and transfection efficiency.
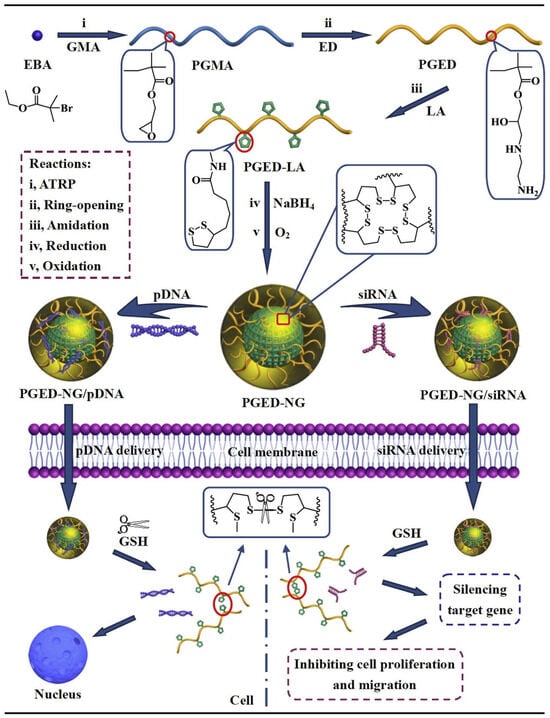
Figure 13.
Preparation of PGED-NG and its complexes with pDNA and siRNA for nucleic acid delivery [242], reproduced with permission from ref. [201]. Elsevier 2016.
In another example, siRNAs were incorporated into NGs by hybridizing with brush-like DNA-grafted polymers, forming the crosslinked structure of the gel [242]. Ding et al. used this method to deliver anti-PLK1 siRNAs, targeting the oncogene polo-like kinase 1 (PLK1) [243] (Figure 14). The NGs protected the siRNAs from degradation and enhanced cellular uptake, leading to effective gene silencing both in vitro and in vivo.
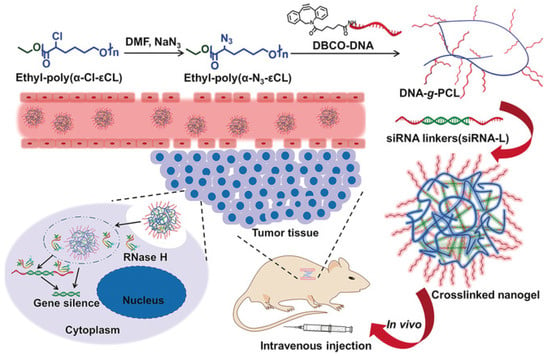
Figure 14.
Crosslinked NG formation and siRNA delivery in vivo, reproduced with permission from ref. [243]. Wiley 2018.
Additionally, miRNA-loaded NGs have been designed to modulate immune responses. Gao et al. created a virus-mimicking NG system called Vir-Gel, which encapsulated miR-155 to reprogram tumor-associated microglia and macrophages from a pro-tumor (M2) to an anti-tumor (M1) phenotype [238] (Figure 15). The NG core was coated with a red blood cell membrane and functionalized with targeting peptides to enhance cellular uptake and mimic viral infection, resulting in prolonged circulation and strong tumor inhibition.
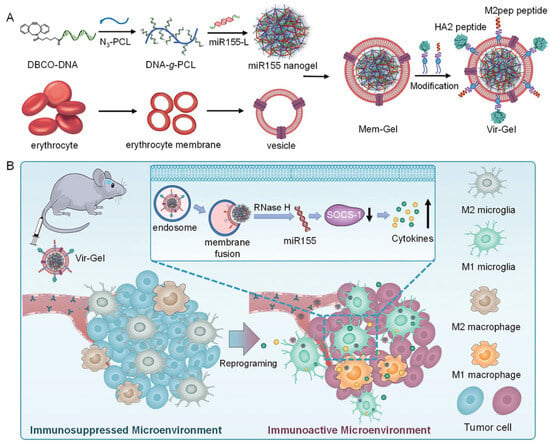
Figure 15.
Virus-mimicking nucleic acid NG (Vir-Gel) for glioblastoma therapy. (A) Construction of peptide-modified, erythrocyte membrane-coated Vir-Gel; (B) miR155-mediated immune modulation, reproduced with permission from ref. [238]. Wiley 2021.
NGs offer a powerful and flexible platform for delivering biomacromolecules in cancer therapy. Their ability to protect sensitive molecules, target specific cells, and respond to intracellular conditions makes them ideal for advancing protein- and gene-based treatments. As research progresses, the diversity of nucleic acids and proteins that can be delivered using NGs continues to expand, broadening their therapeutic potential.
NGs as Multifunctional Platforms for Cancer Therapy
Cancer remains one of the most challenging diseases due to its complexity, heterogeneity, and resistance to treatment. To improve therapeutic outcomes while minimizing side effects, researchers have developed NG platforms capable of delivering multiple therapeutic agents or combining different treatment modalities. These platforms can carry not only small molecules and biomacromolecules but also nanoparticles, enabling a wide range of combination therapies [244,245]. NGs can enhance anticancer efficacy by: (i) Co-delivering multiple drugs to boost the effectiveness of a single treatment approach [246,247,248,249], (ii) Integrating different therapeutic strategies—such as chemotherapy, photothermal therapy (PTT), photodynamic therapy (PDT), gene therapy, and magnetic therapy—into one system [234,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256]. For instance, Ding et al. developed an NG system that co-delivered polydopamine (a photothermal agent) and siRNAs targeting heat shock protein 70 (Hsp70) [257] (Figure 16). Traditional PTT requires temperatures above 50 °C, which can cause inflammation and promote metastasis [258,259,260,261,262,263,264]. By silencing Hsp70, which helps cancer cells resist heat-induced damage [265], the NG enabled effective tumor ablation at milder temperatures (42–45 °C), reducing side effects while maintaining therapeutic efficacy.
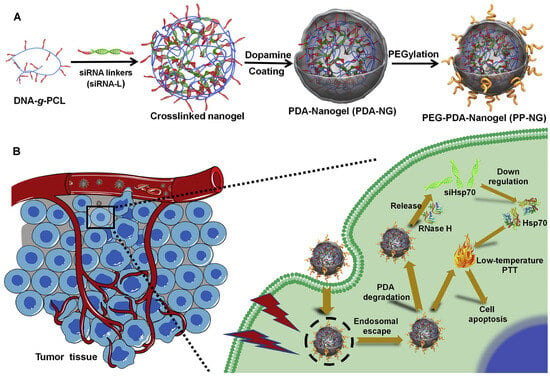
Figure 16.
Synthesis of PDA-coated nucleic acid NG and its role in siRNA-mediated low-temperature photothermal therapy. (A) PEG-PDA-NG synthesis; (B) Mechanism of siRNA-induced PTT, reproduced with permission from ref. [257]. Elsevier 2020.
Multidrug resistance in cancer is often driven by factors like drug efflux transporters (DETs) and anti-apoptotic proteins [266,267]. To address this, a smart NG named SiPING was developed using a one-step self-assembly method. It incorporated PEG–PLE, organosilica nanodots (OSiND), indocyanine green (ICG), and doxorubicin (DOX) [254] (Figure 17). This system acted like a “Trojan horse,” helping DOX evade DETs and enter cancer cells efficiently. Upon NIR light exposure, ICG degraded, triggering the release of DOX, which rapidly reached the nucleus—even in resistant cells—resulting in strong anticancer effects.
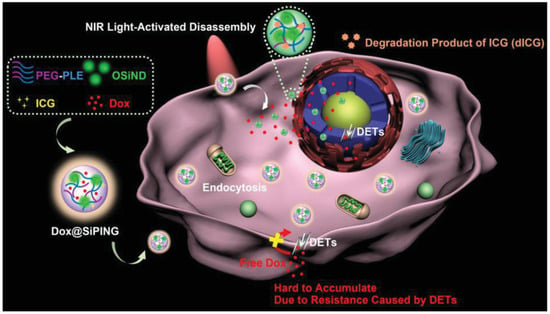
Figure 17.
Rational design of Dox-encapsulated NGs acting as ‘Trojan horses’ for enhanced cellular uptake and nuclear drug delivery to overcome MDR, reproduced with permission from ref. [254]. Wiley 2019.
In another study, a self-traceable nanoreservoir called Gem/Cip@SiPNG@HA was designed to treat bacterium-associated tumors [258] (Figure 18A). It combined gemcitabine (Gem), ciprofloxacin (Cip), and hyaluronic acid (HA) for targeted delivery. The system featured enzyme- and pH-responsive gates for controlled drug release in the tumor microenvironment. Since bacteria in tumors can deactivate Gem and cause resistance [258], co-delivery with Cip allowed simultaneous tumor inhibition and bacterial eradication. Moreover, the dead bacteria acted as immune stimulants, enhancing T cell-mediated immune responses and promoting antigen-presenting cell maturation, while Gem helped deplete immunosuppressive cells—demonstrating a novel strategy to turn tumor-associated bacteria into immunoadjuvants.
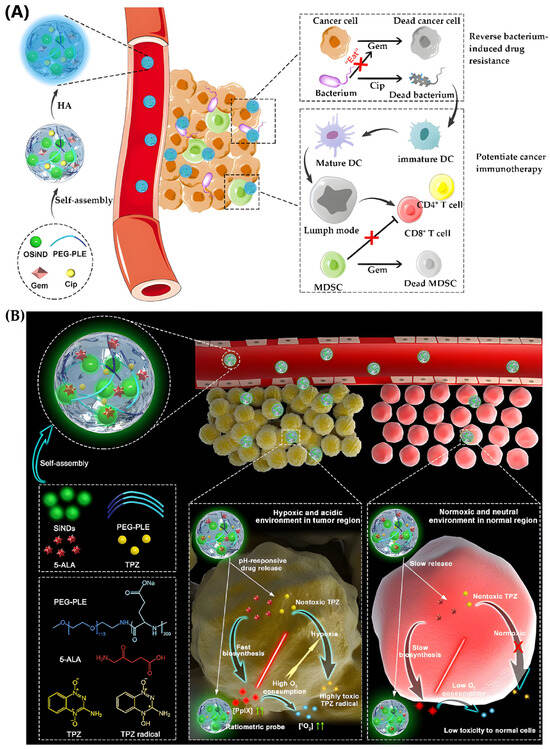
Figure 18.
NGs for cancer therapy. (A) Synthesis and mechanism of Gem/Cip@SiPNG@HA nanoreservoir for immunotherapy, reproduced with permission from ref. [258]. Wiley 2021; (B) SiPNGs as theranostic platforms, reproduced with permission from ref. [190]. Wiley 2019.
Some NGs are designed not only for therapy but also for diagnosis, making them powerful theranostic platforms. These systems can simultaneously deliver therapeutic agents and enable imaging or monitoring of disease progression [190,268,269,270,271,272,273,274]. In addition, NGs can support imaging-guided therapy, allowing real-time tracking of drug delivery and treatment response [235,275,276]. A notable example is the self-traceable NG SiPNG, developed by incorporating silicon nanodots (SiNDs), PEG–PLE, 5-aminolevulinic acid (5-ALA), and tirapazamine (TPZ) [190] (Figure 18B). SiNDs emit green fluorescence, enabling visualization of the NG. Once delivered to the tumor site, 5-ALA is converted into protoporphyrin IX (PpIX), a red-fluorescent molecule that serves both as a diagnostic probe and a photosensitizer. Upon light activation, PpIX generates singlet oxygen (1O2), consuming local oxygen and creating a hypoxic environment that enhances the cytotoxicity of TPZ. This dual-action system successfully combined imaging and therapy for precise cancer treatment.
Beyond theranostics, NGs can be engineered for precision medicine, where their structure and surface chemistry are tailored to match specific therapeutic needs. Their ability to encapsulate a wide range of materials—regardless of size, charge, or hydrophobicity—makes them ideal for customized drug delivery [191,277,278]. For instance, the Malkoch group developed dendritic NGs (DNGs) using amphiphilic block copolymers made from hydrophilic PEG and hydrophobic hyperbranched bis-MPA dendrons [191] (Figure 19A). These were functionalized with allyl groups to allow crosslinking via thiol–ene chemistry. The resulting NGs could be further modified with various thiol-containing molecules to introduce carboxyl, amine, or alkyl groups, enabling the delivery of anionic, cationic, or hydrophobic drugs, respectively. These DNGs showed controlled drug release, low toxicity to healthy cells, and efficient uptake by 3D pancreatic tumor models.
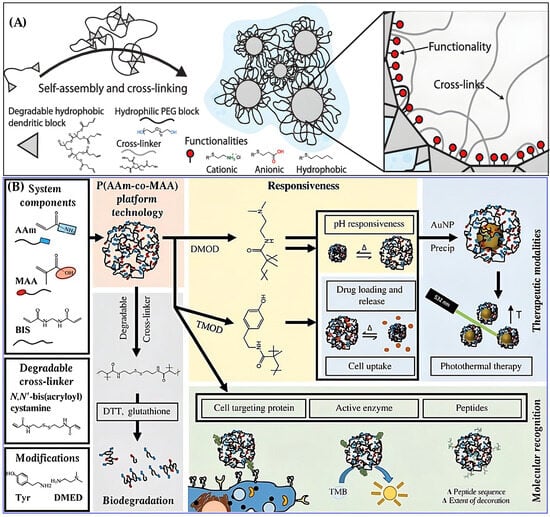
Figure 19.
NGs for cancer therapy. (A) Structure and tunability of DNGs for personalized therapy, reproduced with permission from ref. [191]. Wiley 2019; (B) Design of P(AAm-co-MAA) NG platform for precision medicine, reproduced with permission from ref. [278]. AAAS 2019.
Another example comes from the Peppas group, who created a tunable NG platform using poly(acrylamide-co-methacrylic acid) [P(AAm-co-MAA)] (Figure 19B) [278]. This system allowed: (i) Crosslinking with degradable or non-degradable linkers, enabling environment-responsive breakdown, (ii) Surface modification with small molecules or peptides, enhancing drug loading, targeting, and cellular uptake, (iii) Integration with gold nanoparticles (AuNPs) for photothermal therapy, using the NG’s reductive properties to precipitate AuNPs and enable heat generation under laser irradiation.
These features allowed the NGs to deliver multiple drugs, respond to tumor-specific stimuli, and support combination therapies—all essential for personalized cancer treatment. NGs are emerging as multifunctional platforms that bridge therapy, diagnostics, and precision medicine. Their tunable chemistry, responsiveness to biological environments, and compatibility with diverse therapeutic agents make them ideal candidates for next-generation cancer treatment strategies.
NGs as Cancer Vaccines and Synthetic Antibodies
Vaccination is a well-established method for preventing and treating diseases by stimulating the immune system with antigens derived from pathogens or their subunits [222,279]. While traditionally used against infectious diseases, vaccines are now being explored for cancer therapy [280] and other chronic conditions like metabolic syndrome [281].
In the immune response, extracellular antigens are processed by antigen-presenting cells (APCs) and presented via MHC class II molecules to activate CD4+ T cells, leading to humoral and cellular immunity [281]. However, effective cancer vaccines require activation of CD8+ cytotoxic T cells (CTLs), which involves antigen presentation through MHC class I molecules [281]. Recent advances have introduced nanoparticle-based vaccines, or nanovaccines, which offer improved delivery and immune activation [282,283]. NGs, in particular, are ideal carriers due to their ability to protect antigens from degradation, enhance uptake by APCs, enable controlled release, promote antigen presentation, and stimulate APC maturation [268,273]. NG-based cancer vaccines typically encapsulate tumor-associated antigens (TAAs), which alone may not elicit strong immune responses [284]. These are often formulated as subunit vaccines [285] or derived from tumor cell lysates. For example, carboxyl-modified cholesterol-bearing pullulan (CHP) NGs showed selective interaction with APCs and strong CTL activation due to efficient cross-presentation [279]. Targeting dendritic cells (DCs) is a key strategy in NG vaccine design, as DCs are central to T cell activation [286,287,288]. Wang et al. developed galactosyl dextran-retinol (GDR) NGs loaded with ovalbumin (OVA) as a model antigen [288] (Figure 20A,B). The galactosyl groups enabled DC targeting, while retinoic acid receptor (RAR) signaling promoted DC maturation. Additionally, the NGs induced lysosomal rupture, generating ROS that enhanced proteasome activity and MHC I antigen presentation, leading to robust anticancer immune responses.
Figure 20.
NGs as vaccines. (A) Synthesis of GDR NG and GDR/OVA nanovaccine; reproduced with permission from ref. [288]. (B) Mechanism of immune response enhancement; (C) Alg-Tat-gp100 NG for oral vaccine delivery, Elsevier 2016; reproduced with permission from ref. [241]. Elsevier 2019.
NGs can also deliver nucleic acids that encode TAAs. For instance, plasmid DNA gp100, used to treat metastatic melanoma, was encapsulated in an Alg-Tat-gp100 NG composed of alginate and Tat peptide [241] (Figure 20C). Oral delivery of this system significantly increased interferon-γ secretion and CTL activation, achieving a tumor inhibition rate of 42.5%.
Beyond vaccines, NGs can function as synthetic antibodies through molecular imprinting. These molecularly imprinted polymer (MIP) NGs are created by polymerizing around a template molecule (e.g., an antigen), which is later removed to leave specific recognition sites [289,290]. These sites allow the NGs to selectively bind target proteins, mimicking antibody behavior [291]. MIP NGs have been successfully applied in protein recognition and are being explored as antibody alternatives in cancer therapy [291] (Figure 21A,B).
Figure 21.
(A) Preparation of MIP-NGs for HSA via emulsifier-free polymerization; (B) Concept of stealth acquisition by albumin cloaking, reproduced with permission from ref. [291]. Wiley 2017.
NGs offer a versatile platform for cancer immunotherapy, functioning as both vaccines and synthetic antibodies. Their ability to deliver antigens or genetic material, target immune cells, and stimulate robust immune responses makes them promising tools for next-generation cancer treatments.
NGs for Anti-Inflammatory Therapy
NGs have emerged as promising carriers for delivering a wide range of anti-inflammatory agents, including antibiotics [292,293], nucleic acids [26], photosensitizers [26], and even bioactive polymers or metal ions [26]. Interestingly, some NGs themselves exhibit inherent anti-inflammatory properties [23,294], partly due to their hydrophilic nature, which contributes to immunomodulation [295]. In general, the more hydrophilic the NG, the stronger its ability to reduce immune responses.
Many inflammatory diseases are linked to immune system dysfunction. For example: inflammatory bowel disease (IBD) [295]. Rheumatoid arthritis (RA) [296,297] and spinal cord injury (SCI) [298,299]. To address these conditions, NGs have been developed for oral or intravenous delivery. For instance, a PEG–PEI-based NG loaded with rolipram, an anti-inflammatory drug, was designed for targeted release in SCI [300,301]. This system, called NG_Roli, effectively suppressed inflammatory markers like iNOS and Lcn2 in A1 astrocytes, leading to improved neuronal survival in treated mice.
Beyond chemotherapeutic agents, nanogels have also been employed for the delivery of nucleic acids to help mitigate inflammatory responses. For instance, Yavvari et al. developed an NG for oral delivery of PIAS1-encoded DNA to treat IBD [26]. The NG protected the DNA from degradation in the harsh gastrointestinal environment and successfully suppressed NF-κB and STAT1 signaling, reducing inflammation in a mouse colitis model. Some NGs are designed to neutralize inflammatory molecules directly. Yeo et al. created an NO-scavenging NG (NO-Scv gel) using an NO-cleavable crosslinker [302] (Figure 22A). In conditions like RA [303], IBD [304], and sepsis [305], excess NO contributes to inflammation. Upon encountering NO, the NG degraded and consumed it, outperforming dexamethasone in reducing inflammation with lower toxicity in an RA mouse model.
Figure 22.
NGs for anti-inflammatory therapy. (A) NO-scavenging and RA treatment, reproduced with permission from ref. [302]. ACS 2019; (B) PEDOT@PNIPAAm-Cur NG for sterilization, reproduced with permission from ref. [306]. Wiley 2017. (C) DNA-HCl-ZnO NG for peritonitis; reproduced with permission from ref. [307]. Elsevier 2021; (D) Polyacrylic acid NGs for biofilm eradication, reproduced with permission from ref. [293]. ACS 2019; (E) ICG&CO@G3KBPY NG for multimodal infection therapy, reproduced with permission from ref. [308]. Elsevier 2021.
NGs are also effective against bacterial-induced inflammation. They can deliver antibiotics and enhance their antimicrobial activity [24,309,310,311], or possess intrinsic antibacterial properties [312]. For example, berberine-loaded polyacrylic acid NGs modified with cationic polyelectrolytes adhered to bacterial surfaces, increasing drug concentration and improving antibacterial efficacy against E. coli and C. reinhardtii [311]. Another approach involves photothermal therapy (PTT). NG composed of PEDOT and curcumin (Cur) within a PNIPAAm network was activated by NIR laser to release Cur and kill bacteria while scavenging free radicals [306] (Figure 22B). This system effectively eliminated S. aureus and E. coli and showed strong antioxidant performance.
Besides antibiotics and photothermal agents, NGs can incorporate metal ions like Zn2+ [306] and Cu2+ [110], or cationic polymers such as PEI [309], guanidine-conjugated polymers [313], and antimicrobial polypeptides [314]. For example, Taxus baccata fruit-like polypeptide NGs (PNGs) made from poly(arginine-r-valine)-mannose and Zn2+ selectively interacted with bacterial membranes, causing membrane disruption and bacterial lysis [314]. NGs can also mimic neutrophil extracellular traps (NETs) to combat systemic infections like sepsis. Chen et al. developed a DNA-HCl-ZnO NG using kiwifruit genomic DNA crosslinked with citric acid and loaded with ZnO nanoparticles [307] (Figure 22C). This system reduced inflammatory markers (iNOS, COX2), lowered cytokine levels (IL-6, TNF-α), and decreased bacterial load in septic mice, ultimately improving survival rates.
Biofilms represent a protective mode of bacterial growth, where bacteria are embedded in a matrix of extracellular polymeric substances (EPS), including polysaccharides, proteins, lipids, and nucleic acids [315]. This matrix shields bacteria from antibiotics and immune responses, making infections harder to treat. For example, Pseudomonas aeruginosa in biofilm form can be up to 1000 times more resistant to antibiotics [316]. Once established, biofilms are difficult to eliminate and often lead to chronic inflammation [292]. To address this challenge, researchers have developed NGs capable of penetrating and disrupting biofilms [293,317,318,319,320]. One example involves polyacrylic acid NGs coated with the protease Alcalase 2.4 L FG and loaded with ciprofloxacin, a cationic antibiotic [293] (Figure 22D). The protease-modified NGs acted as hydrolases and esterases, breaking down the EPS matrix and allowing the antibiotic to reach the bacteria. This approach significantly reduced biofilm mass and bacterial density, with antibiotic efficacy enhanced by nearly 1000-fold compared to free drug treatment.
Like cancer therapy, biofilm treatment can benefit from multimodal NG platforms that combine different therapeutic agents. For instance, Cai et al. developed a carbon monoxide (CO)-enhanced NG system called ICG&CO@G3KBPY, which integrates photothermal therapy (PTT), photodynamic therapy (PDT), and gas therapy [308] (Figure 22E). This NG encapsulated indocyanine green (ICG) and MnBr(CO)5, a CO-releasing molecule, within a peptide dendrimer-based carrier. Upon NIR light exposure, the system activated PDT and PTT, while releasing CO gas to kill bacteria, reduce inflammation, and enhance ICG penetration into biofilms. This strategy proved highly effective in treating subcutaneous abscesses and urinary catheter infections, demonstrating the potential of NGs in managing biofilm-related diseases. NGs offer a powerful solution for biofilm-associated infections, combining enzymatic degradation, targeted drug delivery, and multimodal therapy. Their ability to penetrate protective bacterial matrices and modulate inflammation makes them promising candidates for future anti-infective and anti-inflammatory applications.
NGs for Antidiabetic Therapy
Diabetes mellitus is a chronic metabolic disorder characterized by elevated blood glucose levels (BGLs), which significantly impacts patients’ quality of life [308]. Insulin remains a cornerstone treatment, especially for individuals with type 1 diabetes and some with type 2 diabetes, requiring daily subcutaneous injections to regulate BGLs [308].
NGs, known for their excellent protein-loading capacity and biocompatibility, have been explored as carriers for insulin delivery [321,322,323,324]. For example, Wang et al. developed hydroxyethyl methacrylate (HEMA) NGs via emulsion polymerization for oral insulin administration [323]. In vivo studies showed that these NGs enhanced intestinal absorption of insulin, maintained glucose control for up to 12 h, and significantly improved bioavailability compared to free insulin.
Traditional insulin injections cannot fully mimic the natural secretion pattern of insulin, which includes both steady release and post-meal bursts [29,322]. To address this, researchers have designed stimuli-responsive NGs that release insulin in response to glucose levels. Li et al. created a glucose-sensitive NG, P(NIPAM-co-AAPBA-co-AANTA), composed of N-isopropylacrylamide (NIPAM), (m-acrylamidophenyl)boronic acid (AAPBA), and Nε-acrylamido-Nα-bis(carboxymethyl)-lysine (AANTA). Crosslinked with ethylene glycol dimethacrylate (EGDMA), the NG was functionalized with phenylboronic acid (PBA) and nitrilotriacetic acid (NTA) to coordinate insulin via Zn2+ ions [324] (Figure 23). Upon glucose binding, the PBA component underwent a hydrophobic-to-hydrophilic transition, triggering insulin release in a glucose-dependent manner.
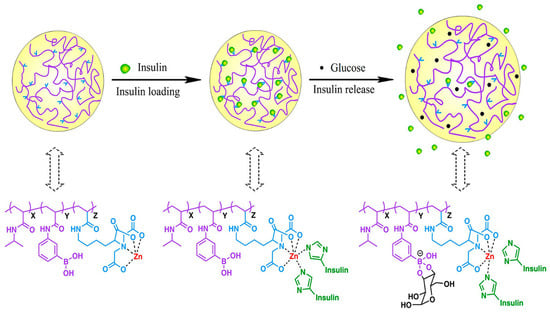
Figure 23.
NGs for diabetes treatment. Glucose-responsive insulin release from P(NIPAM-co-AAPBA-co-AANTA) NG. Reprinted with permission from Ref. [324]. © 2018 American Chemical Society.
Another approach involved using glucose oxidase (GOx). Li et al. developed a dual-responsive NG containing mPEGA, AHMDM (a glucose- and H2O2-sensitive phenylboronic ester), and QT (a thiol-based crosslinker). The NG was loaded with both GOx and insulin [321]. In the presence of glucose, GOx produced H2O2, which oxidized the phenylboronic ester, causing the NG to degrade and release insulin. This system achieved controlled “on–off” insulin release, effectively reduced blood glucose levels, and maintained normoglycemia for approximately 16 h. NGs offer a promising platform for advanced insulin delivery, especially for oral administration and glucose-responsive release. Their ability to mimic physiological insulin patterns and improve bioavailability positions them as a valuable tool in the future of diabetes management.
Anticoagulant and Thrombolytic Therapy
Blood clot formation can lead to life-threatening conditions. To address this, Xu et al. developed thrombin-responsive NGs (HV/ctNGs) for targeted delivery of recombinant hirudin variant 3 (HV), a potent thrombin inhibitor [343] (Figure 25). These NGs were synthesized using acrylamide, a thrombin-cleavable peptide crosslinker, and a clot-binding peptide. Upon encountering thrombin in clots, the NGs degraded, releasing HV to inhibit further clot formation. Notably, the system featured self-regulated release, where HV-thrombin complexes slowed further drug release, enhancing safety and prolonging circulation time. However, long-term toxicity studies are still needed.
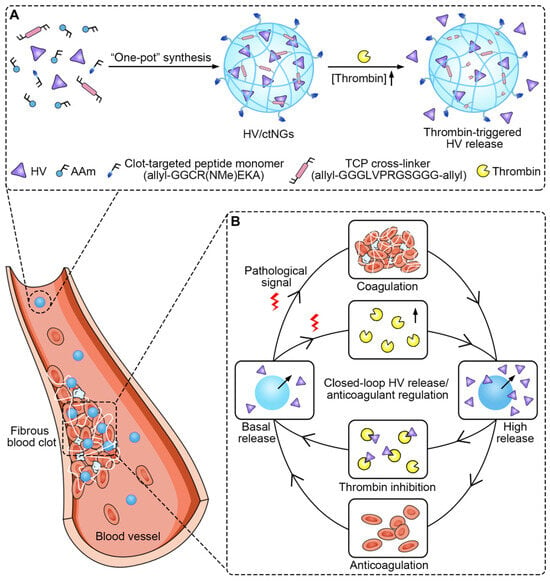
Figure 25.
(A) Synthesis and thrombin-responsiveness of HV/ctNGs; (B) their mechanism for HV delivery and anticoagulant therapy, reproduced with permission from ref. [343]. AAAS 2020.
Metabolic syndrome, which includes conditions like insulin resistance, obesity, and chronic inflammation, is a growing global health concern [351,352,353]. The gut microbiome plays a key role in its development, and recent studies suggest that NG-based immunomodulatory vaccines could help regulate immune responses and metabolic balance [281,354,355]. For example, a nasal NG vaccine containing a ghrelin–PspA fusion antigen was developed to reduce food intake and increase energy expenditure [356]. The NG enabled efficient delivery to the nasal mucosa, triggering both mucosal and systemic immune responses. In diet-induced obese mice, this vaccine led to serum IgG production and significant weight loss, highlighting its potential in obesity management.
NGs have been explored as promising tools in the context of blood transfusion. To address the challenge of blood type incompatibility, Jiang et al. created molecularly imprinted NGs (MIgels) using blood group B antigens as templates [357] (Figure 26). These MIgels selectively bound to B antigens on red blood cells (RBCs), effectively masking them and preventing agglutination during mismatched transfusions. This strategy could improve the safety and flexibility of blood transfusion practices.
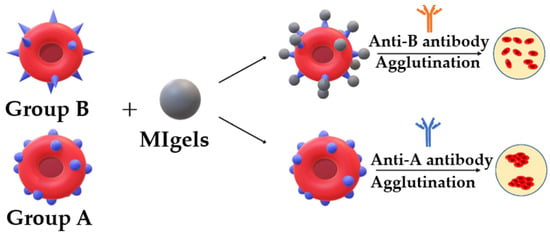
Figure 26.
Function of MIgels in selective binding with B antigen to block antibody recognition, reproduced with permission from ref. [357]. ACS 2020.
NGs continue to expand their therapeutic potential across diverse medical fields. From anticoagulation and metabolic regulation to blood compatibility and neuroimmune modulation, their versatility, responsiveness, and biocompatibility make them promising candidates for next-generation biomedical applications.
5. Emerging Innovations and Clinical Translation in NG/MGs Technologies
The field of NG/MG technologies is rapidly evolving, driven by interdisciplinary advances in polymer chemistry, nanotechnology, biomedical engineering, and computational modeling. These soft, tunable materials are increasingly recognized as intelligent platforms capable of integrating therapeutic delivery, diagnostic imaging, and real-time physiological monitoring. As research progresses, several emerging trends are shaping the future of NG-based systems.
5.1. Multifunctionality and Theranostic Applications
NGs are increasingly being recognized not merely as drug carriers but as multifunctional platforms capable of integrating therapeutic delivery, diagnostic imaging, and immunomodulation [1,358,359,360]. The evolution toward “NG platforms” reflects a broader shift in biomedical engineering, where materials are designed to perform multiple roles within a single system. For example, NGs co-loaded with multiple therapeutic agents have demonstrated synergistic effects in cancer therapy [361,362], while multifunctional imaging NGs can reduce the need for repeated or multi-agent injections by enabling simultaneous visualization across modalities [363]. Theranostic NG systems are designed to diagnose, treat, and monitor disease progression simultaneously [364,365]. By incorporating diagnostic agents, therapeutic payloads, and biosensing functionalities, theranostic NGs enable real-time feedback and dynamic treatment adjustments. [9]. For example, gadolinium-loaded NGs provide MRI visibility while delivering anticancer drugs, and dual-drug systems respond to tumor-specific stimuli such as pH or enzymatic activity. These capabilities are central to personalized medicine, where therapies are tailored to individual patient profiles and adjusted based on physiological responses [366,367].
5.2. Immunotherapy and Vaccine Delivery
A particularly promising area is the application of NGs in immunotherapy [360]. NG-based vaccines have shown superior performance compared to free antigens or adjuvants, owing to their ability to enhance antigen stability, promote uptake by antigen-presenting cells, and facilitate controlled release. Additionally, NG-based vaccines outperform traditional formulations in both efficacy and safety. These features position NGs as strong candidates for next-generation immunotherapeutic strategies [368].
5.3. Hybrid Nanogels and Functional Integration
One significant development is the rise of hybrid NGs, which combine organic polymer networks with inorganic nanomaterials. This integration enhances the functional versatility of NGs, allowing them to serve as multimodal platforms for theranostics, photothermal therapy, and magnetic targeting [369,370]. Inorganic components such as gold nanoparticles enable near-infrared imaging and localized heating for cancer ablation, while silica nanoparticles improve structural integrity and drug encapsulation [371]. Iron oxide nanoparticles contribute magnetic responsiveness and MRI contrast capabilities [372]. These hybrid systems have demonstrated promising results in co-delivering therapeutic agents while simultaneously enabling imaging and targeted delivery, thereby improving treatment precision and monitoring efficiency [365]. However, their unique physicochemical properties—such as small size, high surface area, and chemical reactivity—can also pose toxicity risks, including oxidative stress, inflammation, immunogenicity, nephrotoxicity, and neurotoxicity [373]. Recent studies have identified several key factors influencing toxicity, including particle size, shape, surface charge, and colloidal stability [373]. For example, smaller NPs tend to penetrate deeper into tissues and cells, potentially causing organ-specific accumulation and cellular damage. Additionally, surface coatings and functionalization strategies are being developed to reduce off-target effects and improve biocompatibility [374]. To address these concerns, advanced omics-based approaches—including transcriptomics, proteomics, and epigenomics—are increasingly used to understand the molecular mechanisms of NP-induced toxicity. These methods help identify biomarkers and pathways affected by NP exposure, offering mechanistic insights beyond traditional cytotoxicity assays [375]. Moreover, recent material design strategies focus on biodegradable, eco-friendly, and recyclable nanohybrids, incorporating safer components and surface modifications to minimize long-term toxicity [374]. Regulatory frameworks and standardized toxicological assessments are also evolving to ensure the safe clinical translation of these materials.
5.4. Personalized Medicine and Smart Diagnostics
The integration of NGs into personalized medicine and smart diagnostics represents another exciting frontier [376,377]. Advances in biomarker profiling, genomic analysis, and artificial intelligence are enabling the design of NGs that respond to individual patient conditions. These smart systems can adjust drug release in response to real-time biomarker levels, integrate AI-driven diagnostic platforms to interpret sensor data, and be customized to match specific disease profiles. This convergence of nanotechnology and digital health supports the vision of precision nanomedicine, where therapies are not only targeted but also adaptive, responding dynamically to the patient’s physiological state [366].
5.5. Clinical Translation Challenges and Microfluidic Solutions
Despite the promising therapeutic potential of NG/MGs, their clinical translation faces several challenges, particularly in scalability, reproducibility, and long-term stability [364,365]. Traditional batch synthesis methods often suffer from variability in particle size, crosslinking density, and drug loading efficiency, which can hinder regulatory approval and large-scale production.
5.5.1. Reproducibility and Regulatory Compliance
One promising solution is microfluidic synthesis, which enables precise control over reaction parameters such as flow rate, temperature, and mixing conditions. This approach allows for the continuous and reproducible fabrication of NGs with uniform size and composition [378,379]. Recent studies have demonstrated that microfluidic-assisted polymerization produces NGs with significantly narrower size distributions and enhanced batch-to-batch consistency compared to conventional emulsion techniques [33,379,380]. Moreover, microfluidic platforms can be integrated with real-time monitoring systems to ensure quality control during production [381,382].
5.5.2. Scalability and Manufacturing
Scalability, once considered a limitation of microfluidic systems, is being actively addressed through parallelization and modular reactor designs, enabling production at clinically relevant volumes without compromising quality [383,384,385]. Microfluidic systems support scalable manufacturing for clinical and commercial applications. These advancements collectively contribute to overcoming key barriers in the clinical translation of NG-based therapeutics.
5.5.3. Long-Term Stability
In terms of long-term stability, strategies such as lyophilization with cryoprotectants, incorporation of stabilizing agents, and surface modification with hydrophilic polymers like PEG have been shown to improve shelf-life and maintain functional integrity [386,387]. Hyaluronic acid-based MGs synthesized via microfluidic-assisted crosslinking have demonstrated sustained drug release over 30 days in vitro, with minimal structural deformation—highlighting the role of microfluidics in overcoming stability challenges [388].
6. Conclusions
6.1. Summary of Key Findings
This review has presented a detailed and structured analysis of polymer network-based NG/MGs, emphasizing their architectural diversity, synthesis techniques, and versatile applications in drug delivery and biomedical engineering. By comparing NGs/MGs, we highlighted their distinct advantages—NGs for systemic and intracellular delivery due to their small size, and MGs for localized therapies and tissue scaffolding due to their larger dimensions and mechanical tunability.
The review underscores the critical influence of polymer network parameters, such as crosslinking density and porosity, on drug encapsulation efficiency, release behavior, and mechanical properties. Stimuli-responsive systems have emerged as a transformative approach, enabling precise control over drug release and biosensing through responsiveness to environmental triggers. Functionalization strategies, including ligand conjugation, have significantly improved targeting capabilities, allowing NGs to traverse biological barriers and deliver therapeutics to challenging sites like tumors and the brain.
Importantly, the integration of hybrid NGs, multifunctional theranostic platforms, and AI-assisted diagnostics is reshaping the landscape of personalized medicine. These innovations demonstrate the growing relevance of NG/MGs in developing smart, adaptive, and patient-specific therapeutic solutions.
6.2. Outlook on Future Research Directions
To advance the clinical translation of NG/MG technologies, future research must focus on scalable and reproducible manufacturing methods, such as microfluidics and continuous flow synthesis, which are essential for industrial production and regulatory approval. Harmonizing regulatory frameworks will be crucial to facilitate the transition from bench to bedside.
The convergence of NGs with digital health platforms and biosensors opens new possibilities for real-time monitoring and dynamic treatment adjustment. Developing biodegradable and immunologically inert materials will enhance long-term safety and reduce adverse immune responses. Moreover, expanding the application of these systems into emerging therapeutic areas—such as regenerative medicine, immunotherapy, and neurodegenerative disease treatment—will significantly broaden their clinical impact.
In conclusion, this review not only consolidates current knowledge but also highlights the strategic importance of NG/MGs in shaping the future of precision medicine. By identifying key challenges and proposing actionable research directions, it serves as a valuable reference for researchers, clinicians, and developers working toward next-generation therapeutic platforms.
Author Contributions
Conceptualization: S.C.S., G.A.K.M.R.B. and J.-H.J.; Methodology: S.C.S. and N.B.; Validation: S.C.S., G.A.K.M.R.B. and J.-H.J.; Writing—Original Draft: S.C.S. and N.B.; Writing—Review and Editing: G.A.K.M.R.B. and J.-H.J.; Resources: S.C.S., G.A.K.M.R.B. and J.-H.J.; Supervision: G.A.K.M.R.B. and J.-H.J.; Funding Acquisition: J.-H.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) and the Ministry of Trade, Industry & Energy (MOTIE) of the Republic of Korea (No. RS-2025-02221568). This work was also supported by the Nuclear Safety Research Program through the Regulatory Research Management Agency for SMRs (RMAS) and the Nuclear Safety and Security Commission (NSSC) of the Republic of Korea. (No. RS-2024-00509653).
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Abbreviation | Full Term |
| 5-ALA | 5-Aminolevulinic Acid |
| APC | Antigen-Presenting Cell |
| ATRP | Atom Transfer Radical Polymerization |
| ATP | Adenosine Triphosphate |
| AuNPs | Gold Nanoparticles |
| BBB | Blood–Brain Barrier |
| CC | Cytochrome c |
| CHP | Cholesterol-bearing Pullulan |
| CLSM | Confocal Laser Scanning Microscopy |
| CMC | Critical Micelle Concentration |
| Con A | Concanavalin A |
| CPO | Chloroperoxidase |
| CPT | Camptothecin |
| CS | Chitosan |
| CTL | Cytotoxic T Lymphocyte |
| DC | Dendritic Cell |
| Dex | Dextran |
| DMA | Dynamic Mechanical Analysis |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DOX | Doxorubicin |
| DSC | Differential Scanning Calorimetry |
| DLS | Dynamic Light Scattering |
| DNA | Deoxyribonucleic Acid |
| ECM | Extracellular Matrix |
| EDX/EDS | Energy-Dispersive X-ray Spectroscopy |
| EMA | European Medicines Agency |
| EPR | Enhanced Permeability and Retention |
| FBS | Fetal Bovine Serum |
| FDA | Food and Drug Administration |
| FTIR | Fourier Transform Infrared Spectroscopy |
| GOx | Glucose Oxidase |
| GrB | Granzyme B |
| GSH | Glutathione |
| HA | Hyaluronic Acid |
| H&E | Hematoxylin and Eosin |
| H2O2 | Hydrogen Peroxide |
| HPLC | High-Performance Liquid Chromatography |
| IC50 | Half Maximal Inhibitory Concentration |
| IBD | Inflammatory Bowel Disease |
| ICG | Indocyanine Green |
| IMDQ | Imidazoquinoline |
| IPN | Interpenetrating Polymer Network |
| IVIS | In Vivo Imaging System |
| LCST | Lower Critical Solution Temperature |
| MG | Microgel |
| MHC | Major Histocompatibility Complex |
| miRNA | MicroRNA |
| MIP | Molecularly Imprinted Polymer |
| MPA | 2,2-Bis(hydroxymethyl)propionic acid |
| MPN | Metal–Phenolic Network |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| MTX | Methotrexate |
| MW | Molecular Weight |
| NETs | Neutrophil Extracellular Traps |
| NG | Nanogel |
| NIR | Near-Infrared |
| NIR-II | Second Near-Infrared Window |
| NMR | Nuclear Magnetic Resonance |
| NO | Nitric Oxide |
| NPs | Nanoparticles |
| NTP | Nucleoside Triphosphate |
| PAMAM | Poly(amidoamine) |
| PAAm | Polyacrylamide |
| PAA | Poly(acrylic acid) |
| PBS | Phosphate-Buffered Saline |
| PBA | Phenylboronic Acid |
| PCL | Polycaprolactone |
| PDT | Photodynamic Therapy |
| PEG | Polyethylene Glycol |
| PEG–PLE | Poly(ethylene glycol)-poly(L-glutamic acid) |
| PEI | Polyethylenimine |
| PHEMA | Poly(2-hydroxyethyl methacrylate) |
| PLGA | Poly(lactic-co-glycolic acid) |
| PMMA | Poly(methyl methacrylate) |
| PNIPAM | Poly(N-isopropylacrylamide) |
| PNVCL | Poly(N-vinylcaprolactam) |
| PpIX | Protoporphyrin IX |
| PspA | Pneumococcal Surface Protein A |
| PTT | Photothermal Therapy |
| PVA | Polyvinyl Alcohol |
| PVP | Polyvinylpyrrolidone |
| pDNA | Plasmid DNA |
| PDI | Polydispersity Index |
| RA | Rheumatoid Arthritis |
| RAFT | Reversible Addition–Fragmentation Chain Transfer |
| RAR | Retinoic Acid Receptor |
| RBD | Receptor-Binding Domain |
| RES | Reticuloendothelial System |
| RGD | Arginine-Glycine-Aspartic Acid |
| RNA | Ribonucleic Acid |
| ROS | Reactive Oxygen Species |
| RPM | Revolutions Per Minute |
| RSV | Respiratory Syncytial Virus |
| RT | Room Temperature |
| SCI | Spinal Cord Injury |
| SEM | Scanning Electron Microscopy |
| SiNDs | Silicon Nanodots |
| siRNA | Small Interfering RNA |
| SOD | Superoxide Dismutase |
| TAA | Tumor-Associated Antigen |
| TEM | Transmission Electron Microscopy |
| TGA | Thermogravimetric Analysis |
| TLR | Toll-like Receptor |
| TME | Tumor Microenvironment |
| TMZ | Temozolomide |
| TPZ | Tirapazamine |
| UV-Vis | Ultraviolet–Visible Spectroscopy |
| VPTT | Volume Phase Transition Temperature |
| XRD | X-ray Diffraction |
| NG/MG | Nanogel/Microgel |
References
- Javaid, A.; Malik, N.S.; Tulain, U.R.; Mahmood, A.; Khan, M.F.A.; Akram, S.; Jabeen, A.; Hussain, S. Nanogels as Multifunctional Platforms: From Drug Delivery to Gene Therapy. Polym. Bull. 2025. [Google Scholar] [CrossRef]
- Manimaran, V.; Nivetha, R.P.; Tamilanban, T.; Narayanan, J.; Vetriselvan, S.; Fuloria, N.K.; Chinni, S.V.; Sekar, M.; Fuloria, S.; Wong, L.S.; et al. Nanogels as Novel Drug Nanocarriers for CNS Drug Delivery. Front. Mol. Biosci. 2023, 10, 1232109. [Google Scholar] [CrossRef]
- Fan, M.; Si, J.; Xu, X.; Chen, L.; Chen, J.; Yang, C.; Zhu, J.; Wu, L.; Tian, J.; Chen, X.; et al. A Versatile Chitosan Nanogel Capable of Generating AgNPs In-Situ and Long-Acting Slow-Release of Ag+ for Highly Efficient Antibacterial. Carbohydr. Polym. 2021, 257, 117636. [Google Scholar] [CrossRef] [PubMed]
- Hesan, M.; Gholipour-Kanani, A.; Lotfi, M.; Shafiee, M. The Synthesis and Characterization of Core-Shell Nanogels Based on Alginate and Chitosan for the Controlled Delivery of Mupirocin. Biochem. Eng. J. 2023, 190, 108742. [Google Scholar] [CrossRef]
- Pillarisetti, S.; Vijayan, V.; Rangasamy, J.; Bardhan, R.; Uthaman, S.; Park, I.K. A Multi-Stimuli Responsive Alginate Nanogel for Anticancer Chemo-Photodynamic Therapy. J. Ind. Eng. Chem. 2023, 123, 361–370. [Google Scholar] [CrossRef]
- Byeon, J.H.; Kang, Y.R.; Chang, Y.H. Physicochemical and in Vitro Digestion Properties of Gelatin/Low-Methoxyl Pectin Synbiotic Microgels Co-Encapsulating Lacticaseibacillus casei and Pectic Oligosaccharides via Double-Crosslinking with Transglutaminase and Calcium Ions. Food Hydrocoll. 2023, 142, 108757. [Google Scholar] [CrossRef]
- Fu, Y.; Jang, M.S.; Liu, C.; Lee, J.H.; Li, Y.; Yang, H.Y. Hypoxia-Responsive Hyaluronic Acid Nanogels with Improved Endo/Lysosomal Escape Ability for Tumor-Targeted Cytochrome c Delivery. Eur. Polym. J. 2022, 173, 111259. [Google Scholar] [CrossRef]
- Peters, J.T.; Hutchinson, S.S.; Lizana, N.; Verma, I.; Peppas, N.A. Synthesis and Characterization of Poly(N-Isopropyl Methacrylamide) Core/Shell Nanogels for Controlled Release of Chemotherapeutics. Chem. Eng. J. 2018, 340, 58–65. [Google Scholar] [CrossRef]
- Basak, S.; Khare, H.A.; Roursgaard, M.; Kempen, P.J.; Lee, J.H.; Bazban-Shotorbani, S.; Kræmer, M.; Chernyy, S.; Andresen, T.L.; Almdal, K.; et al. Simultaneous Cross-Linking and Cross-Polymerization of Enzyme Responsive Polyethylene Glycol Nanogels in Confined Aqueous Droplets for Reduction of Low-Density Lipoprotein Oxidation. Biomacromolecules 2021, 22, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Feng, S.; Wang, J.; Cao, K.; Shi, Z.; Men, C.; Yu, T.; Wang, S.; Huang, Y. Collagen-Based Micro/Nanogel Delivery Systems: Manufacturing, Release Mechanisms, and Biomedical Applications. Chin. Med. J. 2025, 138, 1135–1152. [Google Scholar] [CrossRef]
- Silverstein, M.S. Interpenetrating Polymer Networks: So Happy Together? Polymer 2020, 207, 122929. [Google Scholar] [CrossRef]
- Chandra, S.S.; Kim, J.; Ahmed, F.; Yang, H.; Yoon, S.; Kim, W.; Ryu, T.; Lee, S.; Kim, K. Synthesis of Sulfonation Poly(N-Propylsulfonicacid Isatin Biphenylene) for Polymer Electrolyte Membrane Fuel Cell Containing SiO2 Nanocomposite Membrane. J. Nanosci. Nanotechnol. 2018, 19, 1562–1566. [Google Scholar] [CrossRef]
- Sharma, B.K.; Singh, M.; Lokhandwala, S.; Wagh, S.; Chowdhury, S.R.; Ray, S. Radiation Processed Emerging Materials for Biomedical Applications. In Applications of High Energy Radiations: Synthesis and Processing of Polymeric Materials; Springer: Berlin/Heidelberg, Germany, 2023; pp. 185–218. [Google Scholar] [CrossRef]
- Katopodi, T.; Petanidis, S.; Floros, G.; Porpodis, K.; Kosmidis, C. Hybrid Nanogel Drug Delivery Systems: Transforming the Tumor Microenvironment through Tumor Tissue Editing. Cells 2024, 13, 908. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, X.Y.; Xu, J.R. Self-Assembled Supramolecular Hydrogels Formed by Biodegradable PLA/CS Diblock Copolymers and β-Cyclodextrin for Controlled Dual Drug Delivery. Int. J. Biol. Macromol. 2018, 108, 18–23. [Google Scholar] [CrossRef]
- Yapar, E.A.; Inal, Ö. Poly(Ethylene Oxide)-Poly(Propylene Oxide)-Based Copolymers for Transdermal Drug Delivery: An Overview. Trop. J. Pharm. Res. 2012, 11, 855–866. [Google Scholar] [CrossRef]
- Chen, M.; Cui, Y.; Wang, Y.; Chang, C. Triple Physically Cross-Linked Hydrogel Artificial Muscles with High-Stroke and High-Work Capacity. Chem. Eng. J. 2023, 453, 139893. [Google Scholar] [CrossRef]
- Dong, X.; Yao, F.; Jiang, L.; Liang, L.; Sun, H.; He, S.; Shi, M.; Guo, Z.; Yu, Q.; Yao, M.; et al. Facile Preparation of a Thermosensitive and Antibiofouling Physically Crosslinked Hydrogel/Powder for Wound Healing. J. Mater. Chem. B 2022, 10, 2215–2229. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Manna, K.; Pal, S. Recent Advances in Various Stimuli-Responsive Hydrogels: From Synthetic Designs to Emerging Healthcare Applications. Mater. Chem. Front. 2022, 6, 2338–2385. [Google Scholar] [CrossRef]
- Cuggino, J.C.; Blanco, E.R.O.; Gugliotta, L.M.; Alvarez Igarzabal, C.I.; Calderón, M. Crossing Biological Barriers with Nanogels to Improve Drug Delivery Performance. J. Control. Release 2019, 307, 221–246. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Zhu, C.; Wang, Y.; Gu, Q.; Shao, Y.; Jin, A.; Zhang, X.; Lei, L.; Li, Y. Stimulus-Responsive Cellulose Hydrogels in Biomedical Applications and Challenges. Mater. Today Bio 2025, 32, 101814. [Google Scholar] [CrossRef]
- She, D.; Huang, H.; Li, J.; Peng, S.; Wang, H.; Yu, X. Hypoxia-Degradable Zwitterionic Phosphorylcholine Drug Nanogel for Enhanced Drug Delivery to Glioblastoma. Chem. Eng. J. 2021, 408, 127359. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, H.; Zong, X.; Wu, J.; Ji, X.; Liu, W.; Yuan, P.; Chen, X.; Yang, C.; Li, X.; et al. Artificial M2 Macrophages for Disease-Modifying Osteoarthritis Therapeutics. Biomaterials 2021, 274, 120865. [Google Scholar] [CrossRef]
- Obuobi, S.; Julin, K.; Fredheim, E.G.A.; Johannessen, M.; Škalko-Basnet, N. Liposomal Delivery of Antibiotic Loaded Nucleic Acid Nanogels with Enhanced Drug Loading and Synergistic Anti-Inflammatory Activity against S. Aureus Intracellular Infections. J. Control. Release 2020, 324, 620–632. [Google Scholar] [CrossRef]
- Zhu, Q.; Chen, X.; Xu, X.; Zhang, Y.; Zhang, C.; Mo, R. Tumor-Specific Self-Degradable Nanogels as Potential Carriers for Systemic Delivery of Anticancer Proteins. Adv. Funct. Mater. 2018, 28, 1707371. [Google Scholar] [CrossRef]
- Yavvari, P.S.; Verma, P.; Mustfa, S.A.; Pal, S.; Kumar, S.; Awasthi, A.K.; Ahuja, V.; Srikanth, C.V.; Srivastava, A.; Bajaj, A. A Nanogel Based Oral Gene Delivery System Targeting SUMOylation Machinery to Combat Gut Inflammation. Nanoscale 2019, 11, 4970–4986. [Google Scholar] [CrossRef]
- Xue, H.; Ding, F.; Zhang, J.; Guo, Y.; Gao, X.; Feng, J.; Zhu, X.; Zhang, C. DNA Tetrahedron-Based Nanogels for SiRNA Delivery and Gene Silencing. Chem. Commun. 2019, 55, 4222–4225. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhang, X.; Wang, S.; Zhang, J.; Chen, J.; Lu, J.; Yao, L.; Jin, W.; Li, N.; Li, Q. Functional Monomers Equipped Microgel System for Managing Parkinson’s Disease by Intervening Chemokine Axis-mediated Nerve Cell Communications. Adv. Sci. 2025, 12, 2410070. [Google Scholar] [CrossRef]
- Berger, M.; Cüppers, H.J.; Hegner, H.; Jörgens, V.; Berchtold, P. Absorption Kinetics and Biologic Effects of Subcutaneously Injected Insulin Preparations. Diabetes Care 1982, 5, 77–91. [Google Scholar] [CrossRef]
- Wiita, E.G.; Toprakcioglu, Z.; Jayaram, A.K.; Knowles, T.P.J. Selenium-Silk Microgels as Antifungal and Antibacterial Agents. Nanoscale Horiz. 2024, 9, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Kittel, Y.; Kuehne, A.J.C.; De Laporte, L. Translating Therapeutic Microgels into Clinical Applications. Adv. Healthc. Mater. 2022, 11, 2101989. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Lin, Z.I.; Chen, J.A.; Xu, Z.; Gu, J.; Law, W.C.; Yang, J.H.C.; Chen, C.K. Organic/Inorganic Self-Assembled Hybrid Nano-Architectures for Cancer Therapy Applications. Macromol. Biosci. 2022, 22, 2100349. [Google Scholar] [CrossRef]
- Lye, F.S.N.; Loo, Y.S.; Mat Azmi, I.D.; Lee, C.S.; Zahid, N.I.; Madheswaran, T. Microfluidic-Enabled Nanomedicine: A Comprehensive Review of Recent Advances and Translational Potential. Microfluid. Nanofluidics 2025, 29, 51. [Google Scholar] [CrossRef]
- Urifa, J.; Shah, K.W. Developmental Framework and Umbrella Review of AI-Driven Strategies for Hydrogel Microneedles. Preprints 2025. [Google Scholar] [CrossRef]
- Kausar, N.; Chowdhry, B.Z.; Snowden, M. Microgels from Smart Polymers. In Smart Polymers: Applications in Biotechnology and Biomedicine; CRC Press: Boca Raton, FL, USA, 2007; pp. 138–169. [Google Scholar] [CrossRef]
- Pinyakit, Y.; Hoven, V.P. Microgels and Nanogels. In Natural and Synthetic Hydrogels: Rational Design, Synthesis and Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2025; pp. 313–349. [Google Scholar] [CrossRef]
- Plamper, F.A.; Richtering, W. Functional Microgels and Microgel Systems. Acc. Chem. Res. 2017, 50, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.P.; Oo, M.N.N.L.; Deen, G.R.; Li, Z.; Loh, X.J. Nano-Star-Shaped Polymers for Drug Delivery Applications. Macromol. Rapid Commun. 2017, 38, 1700410. [Google Scholar] [CrossRef] [PubMed]
- Chelu, M.; Musuc, A.M. Polymer Gels: Classification and Recent Developments in Biomedical Applications. Gels 2023, 9, 161. [Google Scholar] [CrossRef]
- Mauri, E.; Giannitelli, S.M.; Trombetta, M.; Rainer, A. Synthesis of Nanogels: Current Trends and Future Outlook. Gels 2021, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Nasution, H.; Harahap, H.; Dalimunthe, N.F.; Ginting, M.H.S.; Jaafar, M.; Tan, O.O.H.; Aruan, H.K.; Herfananda, A.L. Hydrogel and Effects of Crosslinking Agent on Cellulose-Based Hydrogels: A Review. Gels 2022, 8, 568. [Google Scholar] [CrossRef]
- Zhao, W.; Jin, X.; Cong, Y.; Liu, Y.; Fu, J. Degradable Natural Polymer Hydrogels for Articular Cartilage Tissue Engineering. J. Chem. Technol. Biotechnol. 2013, 88, 327–339. [Google Scholar] [CrossRef]
- Mano, J.F.; Silva, G.A.; Azevedo, H.S.; Malafaya, P.B.; Sousa, R.A.; Silva, S.S.; Boesel, L.F.; Oliveira, J.M.; Santos, T.C.; Marques, A.P.; et al. Natural Origin Biodegradable Systems in Tissue Engineering and Regenerative Medicine: Present Status and Some Moving Trends. J. R. Soc. Interface 2007, 4, 999–1030. [Google Scholar] [CrossRef]
- Chelu, M.; Calderon Moreno, J.; Atkinson, I.; Pandele Cusu, J.; Rusu, A.; Bratan, V.; Aricov, L.; Anastasescu, M.; Seciu-Grama, A.M.; Musuc, A.M. Green Synthesis of Bioinspired Chitosan-ZnO-Based Polysaccharide Gums Hydrogels with Propolis Extract as Novel Functional Natural Biomaterials. Int. J. Biol. Macromol. 2022, 211, 410–424. [Google Scholar] [CrossRef]
- Zerbinati, N.; Capillo, M.C.; Sommatis, S.; Maccario, C.; Alonci, G.; Rauso, R.; Galadari, H.; Guida, S.; Mocchi, R. Rheological Investigation as Tool to Assess Physicochemical Stability of a Hyaluronic Acid Dermal Filler Cross-Linked with Polyethylene Glycol Diglycidyl Ether and Containing Calcium Hydroxyapatite, Glycine and L-Proline. Gels 2022, 8, 264. [Google Scholar] [CrossRef]
- Balaci, T.; Ozon, E.; Baconi, D.; Nițulescu, G.; Velescu, B.; Bălălău, C.; Păunică, I.; Fița, C. Study on the Formulation and Characterization of a Photoprotective Cream Containing a New Synthetized Compound. J. Mind Med. Sci. 2020, 7, 193–200. [Google Scholar] [CrossRef]
- Shi, Y.; Ma, C.; Peng, L.; Yu, G. Conductive “Smart” Hybrid Hydrogels with PNIPAM and Nanostructured Conductive Polymers. Adv. Funct. Mater. 2015, 25, 1219–1225. [Google Scholar] [CrossRef]
- Chafran, L.; Carfagno, A.; Altalhi, A.; Bishop, B. Green Hydrogel Synthesis: Emphasis on Proteomics and Polymer Particle-Protein Interaction. Polymers 2022, 14, 4755. [Google Scholar] [CrossRef]
- Ahmad, Z.; Salman, S.; Khan, S.A.; Amin, A.; Rahman, Z.U.; Al-Ghamdi, Y.O.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Versatility of Hydrogels: From Synthetic Strategies, Classification, and Properties to Biomedical Applications. Gels 2022, 8, 167. [Google Scholar] [CrossRef]
- Guilherme, M.R.; Silva, R.; Girotto, E.M.; Rubira, A.F.; Muniz, E.C. Hydrogels Based on PAAm Network with PNIPAAm Included: Hydrophilic–Hydrophobic Transition Measured by the Partition of Orange II and Methylene Blue in Water. Polymer 2003, 44, 4213–4219. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, D.; Chu, C.C. Synthesis and Characterization of Partially Biodegradable, Temperature and PH Sensitive Dex–MA/PNIPAAm Hydrogels. Biomaterials 2004, 25, 4719–4730. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Anseth, K.S. PEG Hydrogels for the Controlled Release of Biomolecules in Regenerative Medicine. Pharm. Res. 2009, 26, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A. Turbidimetric Studies of Aqueous Poly(Vinyl Alcohol) Solutions. Die Makromol. Chem. 1975, 176, 3433–3440. [Google Scholar] [CrossRef]
- Schacht, E.H. Polymer Chemistry and Hydrogel Systems. J. Phys. Conf. Ser. 2004, 3, 22. [Google Scholar] [CrossRef]
- Das, N. Preparation Methods and Properties of Hydrogel: A Review. Int. J. Pharm. Pharm. Sci. 2013, 5, 112–117. [Google Scholar]
- Kumar, A.; Srivastava, A.; Galaev, I.Y.; Mattiasson, B. Smart Polymers: Physical Forms and Bioengineering Applications. Prog. Polym. Sci. 2007, 32, 1205–1237. [Google Scholar] [CrossRef]
- Piras, C.C.; Slavik, P.; Smith, D.K. Self-Assembling Supramolecular Hybrid Hydrogel Beads. Angew. Chem.-Int. Ed. 2020, 59, 853–859. [Google Scholar] [CrossRef]
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An Overview of Properties, Biomedical Applications and Obstacles to Clinical Translation. J. Control. Release 2016, 240, 109–126. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Liu, D.; Lyu, X.; Liu, Y.; Liu, Y.; Yang, W.; Shen, Z.; Fan, X. Ultra-Stretchable Ion Gels Based on Physically Cross-Linked Polymer Networks. J. Mater. Chem. C 2022, 10, 10926–10934. [Google Scholar] [CrossRef]
- Mahmood, S.; Khan, N.R.; Razaque, G.; Shah, S.U.; Shahid, M.G.; Albarqi, H.A.; Alqahtani, A.A.; Alasiri, A.; Basit, H.M. Microwave-Treated Physically Cross-Linked Sodium Alginate and Sodium Carboxymethyl Cellulose Blend Polymer Film for Open Incision Wound Healing in Diabetic Animals—A Novel Perspective for Skin Tissue Regeneration Application. Pharmaceutics 2023, 15, 418. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wu, M.; Li, R.; Cai, Z.; Zhang, H. Thermostable Physically Crosslinked Cryogel from Carboxymethylated Konjac Glucomannan Fabricated by Freeze-Thawing. Food Hydrocoll. 2022, 122, 107103. [Google Scholar] [CrossRef]
- Wu, M.; Chen, X.; Xu, J.; Zhang, H. Freeze-Thaw and Solvent-Exchange Strategy to Generate Physically Cross-Linked Organogels and Hydrogels of Curdlan with Tunable Mechanical Properties. Carbohydr. Polym. 2022, 278, 119003. [Google Scholar] [CrossRef]
- Sarmah, D.; Karak, N. Physically Cross-Linked Starch/Hydrophobically-Associated Poly(Acrylamide) Self-Healing Mechanically Strong Hydrogel. Carbohydr. Polym. 2022, 289, 119428. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical Applications of Hydrogels: A Review of Patents and Commercial Products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Horkay, F.; Douglas, J.F. Polymer Gels: Basics, Challenges, and Perspectives. In Gels and Other Soft Amorphous Solids; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2018; Volume 1296, pp. 1–13. [Google Scholar] [CrossRef]
- Zhou, Y.; Chu, R.; Fan, L.; Meng, X.; Zhao, J.; Wu, G.; Li, X.; Jiang, X.; Sun, F.C. Study on the Mechanism and Performance of Polymer Gels by TE and PVA Chemical Cross-Linking. J. Appl. Polym. Sci. 2022, 139, 52043. [Google Scholar] [CrossRef]
- Khan, R.; Zaman, M.; Salawi, A.; Khan, M.A.; Iqbal, M.O.; Riaz, R.; Ahmed, M.M.; Butt, M.H.; Alvi, M.N.; Almoshari, Y.; et al. Synthesis of Chemically Cross-Linked PH-Sensitive Hydrogels for the Sustained Delivery of Ezetimibe. Gels 2022, 8, 281. [Google Scholar] [CrossRef]
- Barbero, C.A.; Martínez, M.V.; Acevedo, D.F.; Molina, M.A.; Rivarola, C.R. Cross-Linked Polymeric Gels and Nanocomposites: New Materials and Phenomena Enabling Technological Applications. Macromol 2022, 2, 440–475. [Google Scholar] [CrossRef]
- Wang, P.; Meng, X.; Wang, R.; Yang, W.; Yang, L.; Wang, J.; Wang, D.A.; Fan, C. Biomaterial Scaffolds Made of Chemically Cross-Linked Gelatin Microsphere Aggregates (C-GMSs) Promote Vascularized Bone Regeneration. Adv. Healthc. Mater. 2022, 11, 2102818. [Google Scholar] [CrossRef] [PubMed]
- Sen-Britain, S.; Hicks, W.L.; Hard, R.; Gardella, J.A. Differential Orientation and Conformation of Surface-Bound Keratinocyte Growth Factor on (Hydroxyethyl)Methacrylate, (Hydroxyethyl)Methacrylate/Methyl Methacrylate, and (Hydroxyethyl)Methacrylate/Methacrylic Acid Hydrogel Copolymers. Biointerphases 2018, 13, 06E406. [Google Scholar] [CrossRef] [PubMed]
- Lanzalaco, S.; Armelin, E. Poly(N-Isopropylacrylamide) and Copolymers: A Review on Recent Progresses in Biomedical Applications. Gels 2017, 3, 36. [Google Scholar] [CrossRef]
- Mohite, P.B.; Adhav, S.S. A Hydrogels: Methods of Preparation and Applications. Int. J. Adv. Pharm. 2017, 6, 79–85. [Google Scholar]
- Singhal, R.; Gupta, K. A Review: Tailor-Made Hydrogel Structures (Classifications and Synthesis Parameters). Polym.-Plast. Technol. Eng. 2016, 55, 54–70. [Google Scholar] [CrossRef]
- Thakur, S.; Thakur, V.K.; Arotiba, O.A. History, Classification, Properties and Application of Hydrogels: An Overview. In Hydrogels: Gels Horizons—From Science to Smart Materials; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Zha, L.; Banik, B.; Alexis, F. Stimulus Responsive Nanogels for Drug Delivery. Soft Matter 2011, 7, 5908–5916. [Google Scholar] [CrossRef]
- Karimi, M.; Ghasemi, A.; Sahandi Zangabad, P.; Rahighi, R.; Moosavi Basri, S.M.; Mirshekari, H.; Amiri, M.; Shafaei Pishabad, Z.; Aslani, A.; Bozorgomid, M.; et al. Smart Micro/Nanoparticles in Stimulus-Responsive Drug/Gene Delivery Systems. Chem. Soc. Rev. 2016, 45, 1457–1501. [Google Scholar] [CrossRef]
- Balcerak-Woźniak, A.; Dzwonkowska-Zarzycka, M.; Kabatc-Borcz, J. A Comprehensive Review of Stimuli-Responsive Smart Polymer Materials—Recent Advances and Future Perspectives. Materials 2024, 17, 4255. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yi, J.; Mukherjee, S.; Banerjee, P.; Zhou, S. Magnetic/NIR-Thermally Responsive Hybrid Nanogels for Optical Temperature Sensing, Tumor Cell Imaging and Triggered Drug Release. Nanoscale 2014, 6, 13001–13011. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Sahandi Zangabad, P.; Ghasemi, A.; Amiri, M.; Bahrami, M.; Malekzad, H.; Ghahramanzadeh Asl, H.; Mahdieh, Z.; Bozorgomid, M.; Ghasemi, A.; et al. Temperature-Responsive Smart Nanocarriers for Delivery of Therapeutic Agents: Applications and Recent Advances. ACS Appl. Mater. Interfaces 2016, 8, 21107–21133. [Google Scholar] [CrossRef]
- Uchiyama, S.; Tsuji, T.; Kawamoto, K.; Okano, K.; Fukatsu, E.; Noro, T.; Ikado, K.; Yamada, S.; Shibata, Y.; Hayashi, T.; et al. A Cell-Targeted Non-Cytotoxic Fluorescent Nanogel Thermometer Created with an Imidazolium-Containing Cationic Radical Initiator. Angew. Chem.-Int. Ed. 2018, 57, 5413–5417. [Google Scholar] [CrossRef]
- Theune, L.E.; Buchmann, J.; Wedepohl, S.; Molina, M.; Laufer, J.; Calderón, M. NIR- and Thermo-Responsive Semi-Interpenetrated Polypyrrole Nanogels for Imaging Guided Combinational Photothermal and Chemotherapy. J. Control. Release 2019, 311–312, 147–161. [Google Scholar] [CrossRef]
- Gao, F.; Wu, X.; Wu, D.; Yu, J.; Yao, J.; Qi, Q.; Cao, Z.; Cui, Q.; Mi, Y. Preparation of Degradable Magnetic Temperature- and Redox-Responsive Polymeric/Fe3O4 Nanocomposite Nanogels in Inverse Miniemulsions for Loading and Release of 5-Fluorouracil. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 587, 124363. [Google Scholar] [CrossRef]
- Zuo, Y.; Jiao, Z.; Ma, L.; Song, P.; Wang, R.; Xiong, Y. Hydrogen Bonding Induced UCST Phase Transition of Poly(Ionic Liquid)-Based Nanogels. Polymer 2016, 98, 287–293. [Google Scholar] [CrossRef]
- Elluru, M.; Ma, H.; Hadjiargyrou, M.; Hsiao, B.S.; Chu, B. Synthesis and Characterization of Biocompatible Hydrogel Using Pluronics-Based Block Copolymers. Polymer 2013, 54, 2088–2095. [Google Scholar] [CrossRef]
- Seo, S.; Lee, C.S.; Jung, Y.S.; Na, K. Thermo-Sensitivity and Triggered Drug Release of Polysaccharide Nanogels Derived from Pullulan-g-Poly(l-Lactide) Copolymers. Carbohydr. Polym. 2012, 87, 1105–1111. [Google Scholar] [CrossRef]
- Ko, D.Y.; Moon, H.J.; Jeong, B. Temperature-Sensitive Polypeptide Nanogels for Intracellular Delivery of a Biomacromolecular Drug. J. Mater. Chem. B 2015, 3, 3525–3530. [Google Scholar] [CrossRef]
- Peng, S.; Wang, H.; Zhao, W.; Xin, Y.; Liu, Y.; Yu, X.; Zhan, M.; Shen, S.; Lu, L. Zwitterionic Polysulfamide Drug Nanogels with Microwave Augmented Tumor Accumulation and On-Demand Drug Release for Enhanced Cancer Therapy. Adv. Funct. Mater. 2020, 30, 2001832. [Google Scholar] [CrossRef]
- Unger, E.C.; Mccreery, T.P.; Sweitzer, R.H. Ultrasound Enhances Gene Expression of Liposomal Transfection. Investig. Radiol. 1997, 32, 723–727. [Google Scholar] [CrossRef]
- Teng, Y.; Jin, H.; Nan, D.; Li, M.; Fan, C.; Liu, Y.; Lv, P.; Cui, W.; Sun, Y.; Hao, H.; et al. In Vivo Evaluation of Urokinase-Loaded Hollow Nanogels for Sonothrombolysis on Suture Embolization-Induced Acute Ischemic Stroke Rat Model. Bioact. Mater. 2018, 3, 102–109. [Google Scholar] [CrossRef]
- Obeso-Vera, C.; Cornejo-Bravo, J.M.; Serrano-Medina, A.; Licea-Claverie, A. Effect of Crosslinkers on Size and Temperature Sensitivity of Poly(N-Isopropylacrylamide) Microgels. Polym. Bull. 2013, 70, 653–664. [Google Scholar] [CrossRef]
- Halliwell, M. A Tutorial on Ultrasonic Physics and Imaging Techniques. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2010, 224, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Cazares-Cortes, E.; Espinosa, A.; Guigner, J.M.; Michel, A.; Griffete, N.; Wilhelm, C.; Ménager, C. Doxorubicin Intracellular Remote Release from Biocompatible Oligo(Ethylene Glycol) Methyl Ether Methacrylate-Based Magnetic Nanogels Triggered by Magnetic Hyperthermia. ACS Appl. Mater. Interfaces 2017, 9, 25775–25788. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.; Maji, S.; Panja, S.; Bajpai, O.P.; Maiti, T.K.; Chattopadhyay, S. Magnetic Particle Ornamented Dual Stimuli Responsive Nanogel for Controlled Anticancer Drug Delivery. New J. Chem. 2019, 43, 3026–3037. [Google Scholar] [CrossRef]
- Ekanger, L.A.; Allen, M.J. Overcoming the Concentration-Dependence of Responsive Probes for Magnetic Resonance Imaging. Metallomics 2015, 7, 405–421. [Google Scholar] [CrossRef]
- Ying, X.; Wang, Y.; Liang, J.; Yue, J.; Xu, C.; Lu, L.; Xu, Z.; Gao, J.; Du, Y.; Chen, Z. Angiopep-Conjugated Electro-Responsive Hydrogel Nanoparticles: Therapeutic Potential for Epilepsy. Angew. Chem. 2014, 126, 12644–12648. [Google Scholar] [CrossRef]
- Mehrali, M.; Thakur, A.; Pennisi, C.P.; Talebian, S.; Arpanaei, A.; Nikkhah, M.; Dolatshahi-Pirouz, A. Nanoreinforced Hydrogels for Tissue Engineering: Biomaterials That Are Compatible with Load-Bearing and Electroactive Tissues. Adv. Mater. 2016, 8, 1603612. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.L.; Larosa, J.M.; Luo, X.; Cui, X.T. Electrically Controlled Drug Delivery from Graphene Oxide Nanocomposite Films. ACS Nano 2014, 8, 1834–1843. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Park, K. Environment-Sensitive Hydrogels for Drug Delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef] [PubMed]
- Hosseinifar, T.; Sheybani, S.; Abdouss, M.; Hassani Najafabadi, S.A.; Shafiee Ardestani, M. Pressure Responsive Nanogel Base on Alginate-Cyclodextrin with Enhanced Apoptosis Mechanism for Colon Cancer Delivery. J. Biomed. Mater. Res.-Part A 2018, 106, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Kanamala, M.; Wilson, W.R.; Yang, M.; Palmer, B.D.; Wu, Z. Mechanisms and Biomaterials in PH-Responsive Tumour Targeted Drug Delivery: A Review. Biomaterials 2016, 85, 152–167. [Google Scholar] [CrossRef]
- Li, D.; Xu, W.; Liu, H. Fabrication of Chitosan Functionalized Dual Stimuli-Responsive Injectable Nanogel to Control Delivery of Doxorubicin. Colloid Polym. Sci. 2023, 301, 879–891. [Google Scholar] [CrossRef]
- Lee, E.S.; Oh, K.T.; Kim, D.; Youn, Y.S.; Bae, Y.H. Tumor PH-Responsive Flower-like Micelles of Poly(l-Lactic Acid)-b-Poly(Ethylene Glycol)-b-Poly(l-Histidine). J. Control. Release 2007, 123, 19–26. [Google Scholar] [CrossRef]
- Cheng, R.; Feng, F.; Meng, F.; Deng, C.; Feijen, J.; Zhong, Z. Glutathione-Responsive Nano-Vehicles as a Promising Platform for Targeted Intracellular Drug and Gene Delivery. J. Control. Release 2011, 152, 2–12. [Google Scholar] [CrossRef]
- Nakahata, M.; Mori, S.; Takashima, Y.; Hashidzume, A.; Yamaguchi, H.; Harada, A. PH- and Sugar-Responsive Gel Assemblies Based on Boronate-Catechol Interactions. ACS Macro Lett. 2014, 3, 337–340. [Google Scholar] [CrossRef]
- Mu, M.; Luo, X.; Wang, W.; Yin, H.; Feng, Y. CO2 Switchable Hollow Nanospheres. J. Colloid Interface Sci. 2018, 522, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Ghadiali, J.E.; Stevens, M.M. Enzyme-Responsive Nanoparticle Systems. Adv. Mater. 2008, 20, 4359–4363. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Y.; Zhao, Q.; Wang, Z.; Xu, Z.; Jia, X. An Enzyme-Responsive Nanogel Carrier Based on PAMAM Dendrimers for Drug Delivery. ACS Appl. Mater. Interfaces 2016, 8, 19899–19906. [Google Scholar] [CrossRef]
- DeVinney, R.; Steele-Mortimer, O.; Finlay, B.B. Phosphatases and Kinases Delivered to the Host Cell by Bacterial Pathogens. Trends Microbiol. 2000, 8, 29–33. [Google Scholar] [CrossRef]
- Padmavathy, N.; Samantaray, P.K.; Ghosh, L.D.; Madras, G.; Bose, S. Selective Cleavage of the Polyphosphoester in Crosslinked Copper Based Nanogels: Enhanced Antibacterial Performance through Controlled Release of Copper. Nanoscale 2017, 9, 12664–12676. [Google Scholar] [CrossRef] [PubMed]
- Haddar, H.O.; Zaghloul, T.I.; Saeed, H.M. Biodegradation of Native Feather Keratin by Bacillus Subtilis Recombinant Strains. Biodegradation 2009, 20, 687–694. [Google Scholar] [CrossRef]
- Chai, P.J.; Li, Y.S.; Tan, C.X. An Efficient and Convenient Method for Preparation of Disulfides from Thiols Using Air as Oxidant Catalyzed by Co-Salophen. Chin. Chem. Lett. 2011, 22, 1403–1406. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, Y.; Yuan, S.; Xu, X.; Wu, Z.; Wu, Z.; Qi, X. ATP Responsive DNA Nanogels Grown on Biocompatible Branches for Anticancer Drug Delivery. Soft Matter 2019, 15, 3655–3658. [Google Scholar] [CrossRef]
- Kashyap, N.; Viswanad, B.; Sharma, G.; Bhardwaj, V.; Ramarao, P.; Ravi Kumar, M.N.V. Design and Evaluation of Biodegradable, Biosensitive in Situ Gelling System for Pulsatile Delivery of Insulin. Biomaterials 2007, 28, 2051–2060. [Google Scholar] [CrossRef]
- Saravanakumar, G.; Kim, J.; Kim, W.J. Reactive-Oxygen-Species-Responsive Drug Delivery Systems: Promises and Challenges. Adv. Sci. 2017, 4, 1600124. [Google Scholar] [CrossRef]
- Lee, S.H.; Gupta, M.K.; Bang, J.B.; Bae, H.; Sung, H.J. Current Progress in Reactive Oxygen Species (ROS)-Responsive Materials for Biomedical Applications. Adv. Healthc. Mater. 2013, 2, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, W.; Wu, D.; Zeng, H.; Hall, D.G.; Narain, R. Multiresponsive and Self-Healing Hydrogel via Formation of Polymer-Nanogel Interfacial Dynamic Benzoxaborole Esters at Physiological PH. ACS Appl. Mater. Interfaces 2019, 11, 44742–44750. [Google Scholar] [CrossRef]
- Biglione, C.; Bergueiro, J.; Wedepohl, S.; Klemke, B.; Strumia, M.C.; Calderón, M. Revealing the NIR-Triggered Chemotherapy Therapeutic Window of Magnetic and Thermoresponsive Nanogels. Nanoscale 2020, 12, 21635–21646. [Google Scholar] [CrossRef]
- Ferguson, C.T.J.; Huber, N.; Landfester, K.; Zhang, K.A.I. Dual-Responsive Photocatalytic Polymer Nanogels. Angew. Chem.-Int. Ed. 2019, 58, 10567–10571. [Google Scholar] [CrossRef]
- Zhou, N.; Cao, X.; Du, X.; Wang, H.; Wang, M.; Liu, S.; Nguyen, K.; Schmidt-Rohr, K.; Xu, Q.; Liang, G.; et al. Hyper-Crosslinkers Lead to Temperature- and PH-Responsive Polymeric Nanogels with Unusual Volume Change. Angew. Chem.-Int. Ed. 2017, 56, 2623–2627. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Meng, F.; Deng, C.; Klok, H.A.; Zhong, Z. Dual and Multi-Stimuli Responsive Polymeric Nanoparticles for Programmed Site-Specific Drug Delivery. Biomaterials 2013, 34, 3647–3657. [Google Scholar] [CrossRef]
- Gao, J.; Frisken, B.J. Cross-Linker-Free N-Isopropylacrylamide Gel Nanospheres. Langmuir 2003, 19, 5212–5216. [Google Scholar] [CrossRef]
- Virtanen, O.L.J.; Mourran, A.; Pinard, P.T.; Richtering, W. Persulfate Initiated Ultra-Low Cross-Linked Poly (N-Isopropylacrylamide) Microgels Possess an Unusual Inverted Cross-Linking Structure. Soft Matter 2016, 12, 3919–3928. [Google Scholar] [CrossRef]
- Nayak, S.; Gan, D.; Serpe, M.J.; Lyon, L.A. Hollow Thermoresponsive Microgels. Small 2005, 1, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guan, Y.; Zhou, S. Permeability Control of Glucose-Sensitive Nanoshells. Biomacromolecules 2007, 8, 3842–3847. [Google Scholar] [CrossRef]
- Brossault, D.F.F.; McCoy, T.M.; Routh, A.F. Preparation of Multicore Colloidosomes: Nanoparticle-Assembled Capsules with Adjustable Size, Internal Structure, and Functionalities for Oil Encapsulation. ACS Appl. Mater. Interfaces 2021, 13, 51495–51503. [Google Scholar] [CrossRef]
- Eqbal, M.D.; Gundabala, V. Controlled Fabrication of Multi-Core Alginate Microcapsules. J. Colloid Interface Sci. 2017, 507, 27–34. [Google Scholar] [CrossRef]
- Trongsatitkul, T.; Budhlall, B.M. Multicore–Shell PNIPAm-Co-PEGMa Microcapsules for Cell Encapsulation. Langmuir 2011, 27, 13468–13480. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, J.S.; Park, K.M.; Bae, J.W.; Lee, Y.; Park, K.D. Multi-Layered Nanogels with MMP-Sheddable PEG Masks: Preparation and Promotion of Tumor Cell Uptake by Controlling Surface Characteristics. Colloids Surfaces B Biointerfaces 2017, 156, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Yoo, C.Y.; Park, S.N. Improved Stability and Skin Permeability of Sodium Hyaluronate-Chitosan Multilayered Liposomes by Layer-by-Layer Electrostatic Deposition for Quercetin Delivery. Colloids Surfaces B Biointerfaces 2015, 129, 7–14. [Google Scholar] [CrossRef]
- Seyfoori, A.; Koshkaki, K.; Majidzadeh, A. Nanohybrid Stimuli-Responsive Microgels: A New Approach in Cancer Therapy. In Nanoarchitectonics for Smart Delivery and Drug Targeting; Holban, A.M., Ed.; William Andrew Norwich: New York, NY, USA, 2016; pp. 715–742. [Google Scholar]
- Das, M.; Sanson, N.; Fava, D.; Kumacheva, E. Microgels Loaded with Gold Nanorods: Photothermally Triggered Volume Transitions under Physiological Conditions. Langmuir 2007, 23, 196–201. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Khutoryanskiy, V.V.; Mun, G.A.; Nurkeeva, Z.S.; Dubolazov, A.V. PH and Salt Effects on Interpolymer Complexation via Hydrogen Bonding in Aqueous Solutions. Polym. Int. 2004, 53, 1382–1387. [Google Scholar] [CrossRef]
- Sing, C.E. Development of the Modern Theory of Polymeric Complex Coacervation. Adv. Colloid Interface Sci. 2017, 239, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Kadlubowski, S. Radiation-Induced Synthesis of Nanogels Based on Poly(N-Vinyl-2-Pyrrolidone)—A Review. Radiat. Phys. Chem. 2014, 102, 29–39. [Google Scholar] [CrossRef]
- Neamtu, I.; Rusu, A.G.; Diaconu, A.; Nita, L.E.; Chiriac, A.P. Basic Concepts and Recent Advances in Nanogels as Carriers for Medical Applications. Drug Deliv. 2017, 24, 539–557. [Google Scholar] [CrossRef]
- Zhang, H.; Zhai, Y.; Wang, J.; Zhai, G. New Progress and Prospects: The Application of Nanogel in Drug Delivery. Mater. Sci. Eng. C 2016, 60, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Jawaid, M.; Mohammad, F. Nanocellulose and Nanohydrogel Matrices: Biotechnological and Biomedical Applications; John Wiley & Sons: Hoboken, NJ, USA, 2017; ISBN 3527803823. [Google Scholar]
- Chacko, R.T.; Ventura, J.; Zhuang, J.; Thayumanavan, S. Polymer Nanogels: A Versatile Nanoscopic Drug Delivery Platform. Adv. Drug Deliv. Rev. 2012, 64, 836–851. [Google Scholar] [CrossRef]
- Duygu Sütekin, S.; Güven, O. Application of Radiation for the Synthesis of Poly(n-Vinyl Pyrrolidone) Nanogels with Controlled Sizes from Aqueous Solutions. Appl. Radiat. Isot. 2019, 145, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Lanzalaco, S.; Sirés, I.; Sabatino, M.A.; Dispenza, C.; Scialdone, O.; Galia, A. Synthesis of Polymer Nanogels by Electro-Fenton Process: Investigation of the Effect of Main Operation Parameters. Electrochim. Acta 2017, 246, 812–822. [Google Scholar] [CrossRef]
- An, J.C.; Weaver, A.; Kim, B.; Barkatt, A.; Poster, D.; Vreeland, W.N.; Silverman, J.; Al-Sheikhly, M. Radiation-Induced Synthesis of Poly(Vinylpyrrolidone) Nanogel. Polymer 2011, 52, 5746–5755. [Google Scholar] [CrossRef]
- de Lima, C.S.A.; Balogh, T.S.; Varca, J.P.R.O.; Varca, G.H.C.; Lugão, A.B.; Camacho-Cruz, L.A.; Bucio, E.; Kadlubowski, S.S. An Updated Review of Macro, Micro, and Nanostructured Hydrogels for Biomedical and Pharmaceutical Applications. Pharmaceutics 2020, 12, 970. [Google Scholar] [CrossRef]
- Fernandez-Nieves, A.; Wyss, H.M.; Mattsson, J.; Weitz, D.A. Microgel Suspensions: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Sung, M.R.; Xiao, H.; Decker, E.A.; McClements, D.J. Fabrication, Characterization and Properties of Filled Hydrogel Particles Formed by the Emulsion-Template Method. J. Food Eng. 2015, 155, 16–21. [Google Scholar] [CrossRef]
- McClements, D.J. Designing Biopolymer Microgels to Encapsulate, Protect and Deliver Bioactive Components: Physicochemical Aspects. Adv. Colloid Interface Sci. 2017, 240, 31–59. [Google Scholar] [CrossRef]
- Oh, J.K.; Drumright, R.; Siegwart, D.J.; Matyjaszewski, K. The Development of Microgels/Nanogels for Drug Delivery Applications. Prog. Polym. Sci. 2008, 33, 448–477. [Google Scholar] [CrossRef]
- Makuuchi, K. Critical Review of Radiation Processing of Hydrogel and Polysaccharide. Radiat. Phys. Chem. 2010, 79, 267–271. [Google Scholar] [CrossRef]
- Pino-Ramos, V.H.; Ramos-Ballesteros, A.; López-Saucedo, F.; López-Barriguete, J.E.; Varca, G.C.; Bucio, E. Radiation Grafting for the Functionalization and Development of Smart Polymeric Materials. Top. Curr. Chem. 2016, 374, 63. [Google Scholar] [CrossRef]
- Farjami, T.; Madadlou, A. Fabrication Methods of Biopolymeric Microgels and Microgel-Based Hydrogels. Food Hydrocoll. 2017, 62, 262–272. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, S. Hybrid Micro-/Nanogels for Optical Sensing and Intracellular Imaging. Nano Rev. 2010, 1, 5730. [Google Scholar] [CrossRef] [PubMed]
- Kendre, P.N.; Satav, T.S. Current Trends and Concepts in the Design and Development of Nanogel Carrier Systems. Polym. Bull. 2019, 76, 1595–1617. [Google Scholar] [CrossRef]
- Yong, C.P.; Gan, L.M. Microemulsion Polymerizations and Reactions. Polym. Part. -/- 2005, 175, 257–298. [Google Scholar]
- Capek, I. The Inverse Mini-Emulsion Polymerization of Acrylamide. Des. Monomers Polym. 2003, 6, 399–409. [Google Scholar] [CrossRef]
- Guo, Q.; Yin, L.; Wang, X.; Yuan, J.; Zhang, Q. An Environmentally Friendly Inverse Microemulsion Method to Synthesize Polyacrylamide. Materials 2022, 15, 5927. [Google Scholar] [CrossRef]
- Murphy, A.C.; Oldenkamp, H.F.; Peppas, N.A. A Highly Tuneable Inverse Emulsion Polymerization for the Synthesis of Stimuli-Responsive Nanoparticles for Biomedical Applications. Biomater. Sci. 2024, 12, 1707–1715. [Google Scholar] [CrossRef]
- Mastella, P.; Todaro, B.; Luin, S. Nanogels: Recent Advances in Synthesis and Biomedical Applications. Nanomaterials 2024, 14, 1300. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Islam, M.; Hasan, M.K.; Nam, K.-W. A Comprehensive Review of Radiation-Induced Hydrogels: Synthesis, Properties, and Multidimensional Applications. Gels 2024, 10, 381. [Google Scholar] [CrossRef]
- Sütekin, S.D.; Güven, O.; Şahiner, N. Nanogel Synthesis by Irradiation of Aqueous Polymer Solutions. In Emerging Technologies for Nanoparticle Manufacturing; Springer: Cham, Switzerland, 2021; pp. 167–202. [Google Scholar] [CrossRef]
- Ashfaq, A.; Clochard, M.C.; Coqueret, X.; Dispenza, C.; Driscoll, M.S.; Ulański, P.; Al-Sheikhly, M. Polymerization Reactions and Modifications of Polymers by Ionizing Radiation. Polymers 2020, 12, 2877. [Google Scholar] [CrossRef]
- El-Adl, K.; Ghobashy, M.M.; Ismail, A.F.M.; El-Morsy, A.; Shoman, N.A. Radiation-Induced Nanogel Engineering Based on Pectin for PH-Responsive Rutin Delivery for Cancer Treatment. Naunyn. Schmiedebergs. Arch. Pharmacol. 2025, 398, 5249–5271. [Google Scholar] [CrossRef]
- Calvillo-Muñoz, E.Y.; Vega-Paz, A.; Guzman-Lucero, D.; Lijanova, I.V.; Olivares-Xometl, O.; Likhanova, N. V Synthesis of Water-Soluble Ionic Terpolymers by Inverse Microemulsion and Solution Polymerization Methods. RSC Adv. 2022, 12, 12273–12282. [Google Scholar] [CrossRef]
- Chanda, M. Direct Synthesis of Nano-Size Polymers by Microemulsion Polymerization. In Nano-Size Polymers: Preparation, Properties, Applications; Fakirov, S., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 49–86. ISBN 978-3-319-39715-3. [Google Scholar]
- Arif, M.; Raza, H.; Haroon, S.M.; Naseem, K.; Majeed, H.; Tahir, F.; Fatima, U.; Ibrahim, S.M.; Ul Mahmood, S. Copper (II) Ions Extraction by Poly(N-Vinylcaprolactam-Mathacrylic Acid) Microgels for in Situ Reduction Formation of Copper Nanoparticles to Reduce Pollutants. J. Mol. Liq. 2023, 392, 123541. [Google Scholar] [CrossRef]
- Begum, R.; Farooqi, Z.H.; Aboo, A.H.; Ahmed, E.; Sharif, A.; Xiao, J. Reduction of Nitroarenes Catalyzed by Microgel-Stabilized Silver Nanoparticles. J. Hazard. Mater. 2019, 377, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Colazo, M.G.; Serpe, M.J. Poly(N-Isopropylacrylamide) Microgel-Based Sensor for Progesterone in Aqueous Samples. Colloid Polym. Sci. 2016, 294, 1733–1741. [Google Scholar] [CrossRef]
- Kawano, S.; Kida, T.; Akashi, M.; Sato, H.; Shizuma, M.; Ono, D. Preparation of Pickering Emulsions through Interfacial Adsorption by Soft Cyclodextrin Nanogels. Beilstein J. Org. Chem. 2015, 11, 2355–2364. [Google Scholar] [CrossRef]
- Wu, W.; Yao, W.; Wang, X.; Xie, C.; Zhang, J.; Jiang, X. Bioreducible Heparin-Based Nanogel Drug Delivery System. Biomaterials 2015, 39, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Van Zee, N.J.; Bates, F.S.; Lodge, T.P. Polymer Nanogels as Reservoirs to Inhibit Hydrophobic Drug Crystallization. ACS Nano 2019, 13, 1232–1243. [Google Scholar] [CrossRef]
- Chaudhary, G.; Ghosh, A.; Kang, J.G.; Braun, P.V.; Ewoldt, R.H.; Schweizer, K.S. Linear and Nonlinear Viscoelasticity of Concentrated Thermoresponsive Microgel Suspensions. J. Colloid Interface Sci. 2021, 601, 886–898. [Google Scholar] [CrossRef]
- Highley, C.B.; Song, K.H.; Daly, A.C.; Burdick, J.A. Jammed Microgel Inks for 3D Printing Applications. Adv. Sci. 2019, 6, 1801076. [Google Scholar] [CrossRef]
- Lee, S.; Choi, G.; Yang, Y.J.; Joo, K.I.; Cha, H.J. Visible Light-Crosslinkable Tyramine-Conjugated Alginate-Based Microgel Bioink for Multiple Cell-Laden 3D Artificial Organ. Carbohydr. Polym. 2023, 313, 120895. [Google Scholar] [CrossRef]
- MacCuspie, R. Comparison of Nanoparticle Sizing Techniques: TEM vs. DLS vs. AFM. Delong Am. 2011, 1–4. [Google Scholar]
- Fissan, H.; Ristig, S.; Kaminski, H.; Asbach, C.; Epple, M. Comparison of Different Characterization Methods for Nanoparticle Dispersions before and after Aerosolization. Anal. Methods 2014, 6, 7324–7334. [Google Scholar] [CrossRef]
- Dufrêne, Y.F.; Ando, T.; Garcia, R.; Alsteens, D.; Martinez-Martin, D.; Engel, A.; Gerber, C.; Müller, D.J. Imaging Modes of Atomic Force Microscopy for Application in Molecular and Cell Biology. Nat. Nanotechnol. 2017, 12, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Balash, M.; Boucetta, H.; He, W. Factors Affecting Drug Delivery System Translation: A Focus on Advanced Technologies, Biological Barriers, and Regulatory Challenges. J. Control. Release 2025, 387, 114167. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y.C. Doxil®—The First FDA-Approved Nano-Drug: Lessons Learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Gillies, E.R.; Frechet, J.M.J. Dendrimers and Dendritic Polymers in Drug Delivery. Drug Discov. Today 2005, 10, 35–43. [Google Scholar] [CrossRef]
- Stiriba, S.; Frey, H.; Haag, R. Dendritic Polymers in Biomedical Applications: From Potential to Clinical Use in Diagnostics and Therapy. Angew. Chem. Int. Ed. 2002, 41, 1329–1334. [Google Scholar] [CrossRef]
- Bosman, A.W.; Janssen, H.M.; Meijer, E.W. About Dendrimers: Structure, Physical Properties, and Applications. Chem. Rev. 1999, 99, 1665–1688. [Google Scholar] [CrossRef] [PubMed]
- Ballarín-González, B.; Howard, K.A. Polycation-Based Nanoparticle Delivery of RNAi Therapeutics: Adverse Effects and Solutions. Adv. Drug Deliv. Rev. 2012, 64, 1717–1729. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, B.; Jiang, H.; Wang, B.; Ma, B. Cationic Lipids and Polymers Mediated Vectors for Delivery of SiRNA. J. Control. Release 2007, 123, 1–10. [Google Scholar] [CrossRef]
- Cavazzana-Calvo, M.; Thrasher, A.; Mavilio, F. The Future of Gene Therapy. Nature 2004, 427, 779–781. [Google Scholar] [CrossRef]
- Waehler, R.; Russell, S.J.; Curiel, D.T. Engineering Targeted Viral Vectors for Gene Therapy. Nat. Rev. Genet. 2007, 8, 573–587. [Google Scholar] [CrossRef]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M. Emerging Applications of Stimuli-Responsive Polymer Materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef]
- Cai, K.; He, X.; Song, Z.; Yin, Q.; Zhang, Y.; Uckun, F.M.; Jiang, C.; Cheng, J. Dimeric Drug Polymeric Nanoparticles with Exceptionally High Drug Loading and Quantitative Loading Efficiency. J. Am. Chem. Soc. 2015, 137, 3458–3461. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, R.; Sasaki, Y.; Katagiri, K.; Mukai, S.; Sawada, S.; Akiyoshi, K. Magnetically Guided Protein Transduction by Hybrid Nanogel Chaperones with Iron Oxide Nanoparticles. Angew. Chem. 2016, 128, 11549–11553. [Google Scholar] [CrossRef]
- Singh, S.; Topuz, F.; Hahn, K.; Albrecht, K.; Groll, J. Embedding of Active Proteins and Living Cells in Redox-Sensitive Hydrogels and Nanogels through Enzymatic Cross-Linking. Angew. Chem. Int. Ed. Engl. 2013, 52, 3000. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Wang, H.Y.; Jia, H.R.; Wu, F.G. Supramolecular Nanogel-Based Universal Drug Carriers Formed by “Soft–Hard” Co-Assembly: Accurate Cancer Diagnosis and Hypoxia-Activated Cancer Therapy. Adv. Ther. 2019, 2, 1800140. [Google Scholar] [CrossRef]
- Zhang, Y.; Andrén, O.C.J.; Nordström, R.; Fan, Y.; Malmsten, M.; Mongkhontreerat, S.; Malkoch, M. Off-Stoichiometric Thiol-Ene Chemistry to Dendritic Nanogel Therapeutics. Adv. Funct. Mater. 2019, 29, 1806693. [Google Scholar] [CrossRef]
- Mi, P. Stimuli-Responsive Nanocarriers for Drug Delivery, Tumor Imaging, Therapy and Theranostics. Theranostics 2020, 10, 4557–4588. [Google Scholar] [CrossRef]
- Chen, X.; Wu, D.; Chen, Z. Biomedical Applications of Stimuli-Responsive Nanomaterials. MedComm 2024, 5, e643. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, P.; Chen, X.; Zhao, X.; Cui, L. Harnessing Nanoprodrugs to Enhance Cancer Immunotherapy: Overcoming Barriers to Precision Treatment. Mater. Today Bio 2025, 32, 101933. [Google Scholar] [CrossRef]
- Blagojevic, L.; Kamaly, N. Nanogels: A Chemically Versatile Drug Delivery Platform. Nano Today 2025, 61, 102645. [Google Scholar] [CrossRef]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in Drug Delivery Systems, Challenges and Future Directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, S.; Wu, F.; Li, D.; Zhang, X.; Chen, W.; Xing, B. Rational Design of Nanogels for Overcoming the Biological Barriers in Various Administration Routes. Angew. Chem.-Int. Ed. 2021, 60, 14760–14778. [Google Scholar] [CrossRef]
- Zhang, D.; Tian, S.; Liu, Y.; Zheng, M.; Yang, X.; Zou, Y.; Shi, B.; Luo, L. Near Infrared-Activatable Biomimetic Nanogels Enabling Deep Tumor Drug Penetration Inhibit Orthotopic Glioblastoma. Nat. Commun. 2022, 13, 6835. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Torres-Luna, C.; Azadi, M.; Domszy, R.; Hu, N.; Yang, A.; David, A.E. Evaluation of Commercial Soft Contact Lenses for Ocular Drug Delivery: A Review. Acta Biomater. 2020, 115, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, X.; Zhang, X.; Sheng, R.; Lin, Q.; Song, W.; Hao, L. Novel Contact Lenses Embedded with Drug-loaded Zwitterionic Nanogels for Extended Ophthalmic Drug Delivery. Nanomaterials 2021, 11, 2328. [Google Scholar] [CrossRef]
- Kuno, N.; Fujii, S. Recent Advances in Ocular Drug Delivery Systems. Polymers 2011, 3, 193–221. [Google Scholar] [CrossRef]
- Lu, Y.; Jia, D.; Ma, X.; Liang, M.; Hou, S.; Qiu, W.; Gao, Y.; Xue, P.; Kang, Y.; Xu, Z. Reduction-Responsive Chemo-Capsule-Based Prodrug Nanogel for Synergistic Treatment of Tumor Chemotherapy. ACS Appl. Mater. Interfaces 2021, 13, 8940–8951. [Google Scholar] [CrossRef]
- Li, X.; Ouyang, Z.; Li, H.; Hu, C.; Saha, P.; Xing, L.; Shi, X.; Pich, A. Dendrimer-Decorated Nanogels: Efficient Nanocarriers for Biodistribution in Vivo and Chemotherapy of Ovarian Carcinoma. Bioact. Mater. 2021, 6, 3244–3253. [Google Scholar] [CrossRef]
- Wei, P.; Gangapurwala, G.; Pretzel, D.; Wang, L.; Schubert, S.; Brendel, J.C.; Schubert, U.S. Tunable Nanogels by Host-Guest Interaction with Carboxylate Pillar[5]Arene for Controlled Encapsulation and Release of Doxorubicin. Nanoscale 2020, 12, 13595–13605. [Google Scholar] [CrossRef]
- Zhang, Y.; Dosta, P.; Conde, J.; Oliva, N.; Wang, M.; Artzi, N. Prolonged Local In Vivo Delivery of Stimuli-Responsive Nanogels That Rapidly Release Doxorubicin in Triple-Negative Breast Cancer Cells. Adv. Healthc. Mater. 2020, 9, e1901101. [Google Scholar] [CrossRef]
- Borah, P.K.; Das, A.S.; Mukhopadhyay, R.; Sarkar, A.; Duary, R.K. Macromolecular Design of Folic Acid Functionalized Amylopectin–Albumin Core–Shell Nanogels for Improved Physiological Stability and Colon Cancer Cell Targeted Delivery of Curcumin. J. Colloid Interface Sci. 2020, 580, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; He, L.; Fan, Z.; Tang, R.; Du, J. Effective Treatment of Drug-Resistant Lung Cancer via a Nanogel Capable of Reactivating Cisplatin and Enhancing Early Apoptosis. Biomaterials 2020, 257, 120252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tung, C.H. Redox-Responsive Cisplatin Nanogels for Anticancer Drug Delivery. Chem. Commun. 2018, 54, 8367–8370. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Zhao, G.; Hu, J.; Ren, Q.; Yang, K.; Wan, C.; Huang, A.; Li, P.; Feng, J.P.; Chen, J.; et al. Melittin-Containing Hybrid Peptide Hydrogels for Enhanced Photothermal Therapy of Glioblastoma. ACS Appl. Mater. Interfaces 2017, 9, 25755–25766. [Google Scholar] [CrossRef]
- Jing, T.; Fu, L.; Liu, L.; Yan, L. A Reduction-Responsive Polypeptide Nanogel Encapsulating NIR Photosensitizer for Imaging Guided Photodynamic Therapy. Polym. Chem. 2016, 7, 951–957. [Google Scholar] [CrossRef]
- Nuhn, L.; De Koker, S.; Van Lint, S.; Zhong, Z.; Catani, J.P.; Combes, F.; Deswarte, K.; Li, Y.; Lambrecht, B.N.; Lienenklaus, S.; et al. Nanoparticle-Conjugate TLR7/8 Agonist Localized Immunotherapy Provokes Safe Antitumoral Responses. Adv. Mater. 2018, 30, e1803397. [Google Scholar] [CrossRef]
- Singh, S.; Drude, N.; Blank, L.; Desai, P.B.; Königs, H.; Rütten, S.; Langen, K.J.; Möller, M.; Mottaghy, F.M.; Morgenroth, A. Protease Responsive Nanogels for Transcytosis across the Blood−Brain Barrier and Intracellular Delivery of Radiopharmaceuticals to Brain Tumor Cells. Adv. Healthc. Mater. 2021, 10, 2100812. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zheng, Y.; Shi, H.; Xie, H.; Yang, Y.; Zhu, F.; Ke, L.; Chen, H.; Gao, Y. Convenient Tuning of the Elasticity of Self-Assembled Nano-Sized Triterpenoids to Regulate Their Biological Activities. ACS Appl. Mater. Interfaces 2021, 13, 44065–44078. [Google Scholar] [CrossRef]
- Li, S.; Zhang, T.; Xu, W.; Ding, J.; Yin, F.; Xu, J.; Sun, W.; Wang, H.; Sun, M.; Cai, Z.; et al. Sarcoma-Targeting Peptide-Decorated Polypeptide Nanogel Intracellularly Delivers Shikonin for Upregulated Osteosarcoma Necroptosis and Diminished Pulmonary Metastasis. Theranostics 2018, 8, 1361–1375. [Google Scholar] [CrossRef]
- Gao, C.; Lin, Z.; Jurado-Sánchez, B.; Lin, X.; Wu, Z.; He, Q. Stem Cell Membrane-Coated Nanogels for Highly Efficient In Vivo Tumor Targeted Drug Delivery. Small 2016, 12, 4056–4062. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Guo, Y.; Jia, H.R.; Jiang, Y.W.; Wu, F.G. Endosome/Lysosome-Detained Supramolecular Nanogels as an Efflux Retarder and Autophagy Inhibitor for Repeated Photodynamic Therapy of Multidrug-Resistant Cancer. Nanoscale Horiz. 2020, 5, 481–487. [Google Scholar] [CrossRef]
- Liu, D.; Ma, L.; An, Y.; Li, Y.; Liu, Y.; Wang, L.; Guo, J.; Wang, J.; Zhou, J. Thermoresponsive Nanogel-Encapsulated PEDOT and HSP70 Inhibitor for Improving the Depth of the Photothermal Therapeutic Effect. Adv. Funct. Mater. 2016, 26, 4749–4759. [Google Scholar] [CrossRef]
- Guo, H.; Li, F.; Xu, W.; Chen, J.; Hou, Y.; Wang, C.; Ding, J.; Chen, X. Mucoadhesive Cationic Polypeptide Nanogel with Enhanced Penetration for Efficient Intravesical Chemotherapy of Bladder Cancer. Adv. Sci. 2018, 5, 1800004. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Li, F.; Qiu, H.; Xu, W.; Li, P.; Hou, Y.; Ding, J.; Chen, X. Synergistically Enhanced Mucoadhesive and Penetrable Polypeptide Nanogel for Efficient Drug Delivery to Orthotopic Bladder Cancer. Research 2020, 2020, 8970135. [Google Scholar] [CrossRef]
- De Araújo Pereira, R.R.; Bruschi, M.L. Vaginal Mucoadhesive Drug Delivery Systems. Drug Dev. Ind. Pharm. 2012, 38, 643–652. [Google Scholar] [CrossRef]
- Di Colo, G.; Zambito, Y.; Zaino, C. Polymeric Enhancers of Mucosal Epithelia Permeability: Synthesis, Transepithelial Penetration-Enhancing Properties, Mechanism of Action, Safety Issues. J. Pharm. Sci. 2008, 97, 1652–1680. [Google Scholar] [CrossRef]
- Salatin, S.; Barar, J.; Barzegar-Jalali, M.; Adibkia, K.; Milani, M.A.; Jelvehgari, M. Hydrogel Nanoparticles and Nanocomposites for Nasal Drug/Vaccine Delivery. Arch. Pharm. Res. 2016, 39, 1181–1192. [Google Scholar] [CrossRef]
- Al-Eisawi, Z.; Beale, P.; Chan, C.; Yu, J.Q.; Huq, F. Carboplatin and Oxaliplatin in Sequenced Combination with Bortezomib in Ovarian Tumour Models. J. Ovarian Res. 2013, 6, 78. [Google Scholar] [CrossRef]
- Grunberg, S.M.; Dugan, M.; Muss, H.; Wood, M.; Burdette-Radoux, S.; Weisberg, T.; Siebel, M. Effectiveness of a Single-Day Three-Drug Regimen of Dexamethasone, Palonosetron, and Aprepitant for the Prevention of Acute and Delayed Nausea and Vomiting Caused by Moderately Emetogenic Chemotherapy. Support. Care Cancer 2009, 17, 589–594. [Google Scholar] [CrossRef]
- Gu, Z.; Biswas, A.; Zhao, M.; Tang, Y. Tailoring Nanocarriers for Intracellular Protein Delivery. Chem. Soc. Rev. 2011, 40, 3638–3655. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zou, Y.; Deng, C.; Meng, F.; Zhang, J.; Zhong, Z. Multifunctional Click Hyaluronic Acid Nanogels for Targeted Protein Delivery and Effective Cancer Treatment in Vivo. Chem. Mater. 2016, 28, 8792–8799. [Google Scholar] [CrossRef]
- Kawasaki, R.; Sasaki, Y.; Nishimura, T.; Katagiri, K.; Morita, K.; Sekine, Y.; Sawada, S.I.; Mukai, S.A.; Akiyoshi, K. Magnetically Navigated Protein Transduction In Vivo Using Iron Oxide-Nanogel Chaperone Hybrid. Adv. Healthc. Mater. 2021, 10, 2001988. [Google Scholar] [CrossRef] [PubMed]
- Canakci, M.; Canakci, M.; Canakci, M.; Singh, K.; Singh, K.; Munkhbat, O.; Shanthalingam, S.; Mitra, A.; Gordon, M.; Osborne, B.A.; et al. Targeting CD4+Cells with Anti-CD4 Conjugated Mertansine-Loaded Nanogels. Biomacromolecules 2020, 21, 2473–2481. [Google Scholar] [CrossRef]
- Wu, Q.; He, Z.; Wang, X.; Zhang, Q.; Wei, Q.; Ma, S.; Ma, C.; Li, J.; Wang, Q. Cascade Enzymes within Self-Assembled Hybrid Nanogel Mimicked Neutrophil Lysosomes for Singlet Oxygen Elevated Cancer Therapy. Nat. Commun. 2019, 10, 240. [Google Scholar] [CrossRef]
- Chen, J.; Ouyang, J.; Chen, Q.; Deng, C.; Meng, F.; Zhang, J.; Cheng, R.; Lan, Q.; Zhong, Z. EGFR and CD44 Dual-Targeted Multifunctional Hyaluronic Acid Nanogels Boost Protein Delivery to Ovarian and Breast Cancers in Vitro and in Vivo. ACS Appl. Mater. Interfaces 2017, 9, 24140–24147. [Google Scholar] [CrossRef]
- Zhao, W.; Hu, J.; Gao, W. Glucose Oxidase-Polymer Nanogels for Synergistic Cancer-Starving and Oxidation Therapy. ACS Appl. Mater. Interfaces 2017, 9, 23528–23535. [Google Scholar] [CrossRef]
- Li, D.; van Nostrum, C.F.; Mastrobattista, E.; Vermonden, T.; Hennink, W.E. Nanogels for Intracellular Delivery of Biotherapeutics. J. Control. Release 2017, 259, 16–28. [Google Scholar] [CrossRef]
- Liang, K.; Ng, S.; Lee, F.; Lim, J.; Chung, J.E.; Lee, S.S.; Kurisawa, M. Targeted Intracellular Protein Delivery Based on Hyaluronic Acid-Green Tea Catechin Nanogels. Acta Biomater. 2016, 33, 142–152. [Google Scholar] [CrossRef]
- Li, Y.; Maciel, D.; Rodrigues, J.; Shi, X.; Tomás, H. Biodegradable Polymer Nanogels for Drug/Nucleic Acid Delivery. Chem. Rev. 2015, 115, 8564–8608. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Gao, Y.; Yang, C.; Guo, R.; Shi, X.; Cao, X. Low-Molecular-Weight Poly(Ethylenimine) Nanogels Loaded with Ultrasmall Iron Oxide Nanoparticles for T1-Weighted MR Imaging-Guided Gene Therapy of Sarcoma. ACS Appl. Mater. Interfaces 2021, 13, 27806–27813. [Google Scholar] [CrossRef]
- Dykxhoorn, D.M.; Palliser, D.; Lieberman, J. The Silent Treatment: SiRNAs as Small Molecule Drugs. Gene Ther. 2006, 13, 541–552. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, H.; Mou, Q.; Yan, D.; Zhu, X.; Zhang, C. Floxuridine-Containing Nucleic Acid Nanogels for Anticancer Drug Delivery. Nanoscale 2018, 10, 8367–8371. [Google Scholar] [CrossRef]
- Gao, X.; Li, S.; Ding, F.; Liu, X.; Wu, Y.; Li, J.; Feng, J.; Zhu, X.; Zhang, C. A Virus-Mimicking Nucleic Acid Nanogel Reprograms Microglia and Macrophages for Glioblastoma Therapy. Adv. Mater. 2021, 33, e2006116. [Google Scholar] [CrossRef] [PubMed]
- Mou, Q.; Ma, Y.; Pan, G.; Xue, B.; Yan, D.; Zhang, C.; Zhu, X. DNA Trojan Horses: Self-Assembled Floxuridine-Containing DNA Polyhedra for Cancer Therapy. Angew. Chem.-Int. Ed. 2017, 56, 12528–12532. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Rana, T.M. Molecular Mechanisms of RNA-Triggered Gene Silencing Machineries. Acc. Chem. Res. 2012, 45, 1122–1131. [Google Scholar] [CrossRef]
- Shen, Y.; Qiu, L. Effective Oral Delivery of Gp100 Plasmid Vaccine against Metastatic Melanoma through Multi-Faceted Blending-by-Blending Nanogels. Nanomed. Nanotechnol. Biol. Med. 2019, 22, 102114. [Google Scholar] [CrossRef]
- Li, R.Q.; Wu, W.; Song, H.Q.; Ren, Y.; Yang, M.; Li, J.; Xu, F.J. Well-Defined Reducible Cationic Nanogels Based on Functionalized Low-Molecular-Weight PGMA for Effective PDNA and SiRNA Delivery. Acta Biomater. 2016, 41, 282–292. [Google Scholar] [CrossRef]
- Ding, F.; Mou, Q.; Ma, Y.; Pan, G.; Guo, Y.; Tong, G.; Choi, C.H.J.; Zhu, X.; Zhang, C. A Crosslinked Nucleic Acid Nanogel for Effective SiRNA Delivery and Antitumor Therapy. Angew. Chem.-Int. Ed. 2018, 57, 3064–3068. [Google Scholar] [CrossRef]
- Li, H.; Qian, K.; Zhang, H.; Li, L.; Yan, L.; Geng, S.; Zhao, H.; Zhang, H.; Xiong, B.; Li, Z.; et al. Pickering Gel Emulsion of Lipiodol Stabilized by Hairy Nanogels for Intra-Artery Embolization Antitumor Therapy. Chem. Eng. J. 2021, 418, 129534. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.; Gao, C.; Fan, X.; Pang, Y.; Li, T.; Wu, Z.; Xie, H.; He, Q. Dual-Responsive Biohybrid Neutrobots for Active Target Delivery. Sci. Robot. 2020, 6, eaaz9519. [Google Scholar] [CrossRef]
- Fan, M.; Jia, L.; Pang, M.; Yang, X.; Yang, Y.; Kamel Elyzayati, S.; Liao, Y.; Wang, H.; Zhu, Y.; Wang, Q. Injectable Adhesive Hydrogel as Photothermal-Derived Antigen Reservoir for Enhanced Anti-Tumor Immunity. Adv. Funct. Mater. 2021, 31, 2010587. [Google Scholar] [CrossRef]
- Qin, X.; Wu, C.; Niu, D.; Qin, L.; Wang, X.; Wang, Q.; Li, Y. Peroxisome Inspired Hybrid Enzyme Nanogels for Chemodynamic and Photodynamic Therapy. Nat. Commun. 2021, 12, 5243. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, G.; Wang, B.; Cao, G.; Li, D.; Wang, Y.; Zhang, Y.; Geng, J.; Li, H.; Li, Y. Reinforcing the Combinational Immuno-Oncotherapy of Switching “Cold” Tumor to “Hot” by Responsive Penetrating Nanogels. ACS Appl. Mater. Interfaces 2021, 13, 36824–36838. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liang, S.; Jiang, D.; Gao, T.; Fang, Y.; Fu, S.; Guan, L.; Zhang, Z.; Mu, W.; Chu, Q.; et al. Manipulation of TAMs Functions to Facilitate the Immune Therapy Effects of Immune Checkpoint Antibodies. J. Control. Release 2021, 336, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Li, T.; Shi, X.; Wang, Y.; Fang, S.; Wang, H. A General Prodrug Nanohydrogel Platform for Reduction-Triggered Drug Activation and Treatment of Taxane-Resistant Malignancies. Acta Biomater. 2021, 130, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, F.; Wang, S.; Liu, L.; Liu, K.; Ye, Y.; Wang, Z.; Wang, H.; Chen, B.; Jiang, J.; et al. Hyperthermia-Triggered On-Demand Biomimetic Nanocarriers for Synergetic Photothermal and Chemotherapy. Adv. Sci. 2020, 7, 1903642. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, F.; Chen, Y.; Liu, J.; Wang, X.; Chen, A.T.; Deng, G.; Zhang, H.; Liu, J.; Hong, Z.; et al. Targeted Delivery of CRISPR/Cas9-Mediated Cancer Gene Therapy via Liposome-Templated Hydrogel Nanoparticles. Adv. Funct. Mater. 2017, 27, 1703036. [Google Scholar] [CrossRef]
- Fiorica, C.; Mauro, N.; Pitarresi, G.; Scialabba, C.; Palumbo, F.S.; Giammona, G. Double-Network-Structured Graphene Oxide-Containing Nanogels as Photothermal Agents for the Treatment of Colorectal Cancer. Biomacromolecules 2017, 18, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, X.; Guo, Y.; Zhu, Y.X.; Liu, X.; Chen, Z.; Wu, F.G. Smart Supramolecular “Trojan Horse”-Inspired Nanogels for Realizing Light-Triggered Nuclear Drug Influx in Drug-Resistant Cancer Cells. Adv. Funct. Mater. 2019, 29, 1807772. [Google Scholar] [CrossRef]
- Pu, X.Q.; Ju, X.J.; Zhang, L.; Cai, Q.W.; Liu, Y.Q.; Peng, H.Y.; Xie, R.; Wang, W.; Liu, Z.; Chu, L.Y. Novel Multifunctional Stimuli-Responsive Nanoparticles for Synergetic Chemo-Photothermal Therapy of Tumors. ACS Appl. Mater. Interfaces 2021, 13, 28802–28817. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Xu, W.; Liu, J.; Li, D.; Li, G.; Ding, J.; Chen, X. Polypeptide Nanoformulation-Induced Immunogenic Cell Death and Remission of Immunosuppression for Enhanced Chemoimmunotherapy. Sci. Bull. 2021, 66, 362–373. [Google Scholar] [CrossRef]
- Ding, F.; Gao, X.; Huang, X.; Ge, H.; Xie, M.; Qian, J.; Song, J.; Li, Y.; Zhu, X.; Zhang, C. Polydopamine-Coated Nucleic Acid Nanogel for SiRNA-Mediated Low-Temperature Photothermal Therapy. Biomaterials 2020, 245, 119976. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Guo, Y.; Gao, G.; Wang, D.; Wu, Y.; Liu, J.; Liang, G.; Zhao, Y.; Wu, F.G. Dual Gate-Controlled Therapeutics for Overcoming Bacterium-Induced Drug Resistance and Potentiating Cancer Immunotherapy. Angew. Chem.-Int. Ed. 2021, 60, 14013–14021. [Google Scholar] [CrossRef]
- Wang, J.; Xu, W.; Zhang, N.; Yang, C.; Xu, H.; Wang, Z.; Li, B.; Ding, J.; Chen, X. X-Ray-Responsive Polypeptide Nanogel for Concurrent Chemoradiotherapy. J. Control. Release 2021, 332, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, H.; Li, H.; Hu, C.; Luo, Y.; Shi, X.; Pich, A. Multi-Responsive Biodegradable Cationic Nanogels for Highly Efficient Treatment of Tumors. Adv. Funct. Mater. 2021, 31, 2100227. [Google Scholar] [CrossRef]
- Zhang, H.; Ren, Y.; Hou, L.; Chang, J.; Zhang, Z.; Zhang, H. Positioning Remodeling Nanogels Mediated Codelivery of Antivascular Drug and Autophagy Inhibitor for Cooperative Tumor Therapy. ACS Appl. Mater. Interfaces 2020, 12, 6978–6990. [Google Scholar] [CrossRef]
- Martin, S.J.; Henry, C.M.; Cullen, S.P. A Perspective on Mammalian Caspases as Positive and Negative Regulators of Inflammation. Mol. Cell 2012, 46, 387–397. [Google Scholar] [CrossRef]
- Bonfil, R.D.; Bustuoabad, O.D.; Ruggiero, R.A.; Meiss, R.P.; Pasqualini, C.D. Tumor Necrosis Can Facilitate the Appearance of Metastases. Clin. Exp. Metastasis 1988, 6, 121–129. [Google Scholar] [CrossRef]
- Huang, H.C.; Sung, Y.C.; Li, C.P.; Wan, D.; Chao, P.H.; Tseng, Y.T.; Liao, B.W.; Cheng, H.T.; Hsu, F.F.; Huang, C.C.; et al. Reversal of Pancreatic Desmoplasia by a Tumour Stroma-Targeted Nitric Oxide Nanogel Overcomes TRAIL Resistance in Pancreatic Tumours. Gut 2022, 71, 1843–1855. [Google Scholar] [CrossRef]
- Fisher, J.W.; Sarkar, S.; Buchanan, C.F.; Szot, C.S.; Whitney, J.; Hatcher, H.C.; Torti, S.V.; Rylander, C.G.; Rylander, M.N. Photothermal Response of Human and Murine Cancer Cells to Multiwalled Carbon Nanotubes after Laser Irradiation. Cancer Res. 2010, 70, 9855–9864. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug Resistance in Cancer: Role of ATP-Dependent Transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, E.; Cui, Y.; Huang, Y. Nanotechnology-Based Combination Therapy for Overcoming Multidrug-Resistant Cancer. Cancer Biol. Med. 2017, 14, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, B.; Liu, Y.; Gao, R.; Zhou, J.; Fu, L.M.; Wang, J. A Spontaneous Membrane-Adsorption Approach to Enhancing Second Near-Infrared Deep-Imaging-Guided Intracranial Tumor Therapy. ACS Nano 2021, 15, 4518–4533. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Li, C.; Liu, A.; Zhen, X.; Gao, J.; Wu, W.; Cai, W.; Jiang, X. Responsive Hyaluronic Acid-Gold Cluster Hybrid Nanogel Theranostic Systems. Biomater. Sci. 2021, 9, 1363–1373. [Google Scholar] [CrossRef]
- Wang, Y.; Zu, M.; Ma, X.; Jia, D.; Lu, Y.; Zhang, T.; Xue, P.; Kang, Y.; Xu, Z. Glutathione-Responsive Multifunctional “Trojan Horse” Nanogel as a Nanotheranostic for Combined Chemotherapy and Photodynamic Anticancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 50896–50908. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhu, J.; Lin, L.; Zhang, C.; Sun, W.; Fan, Y.; Yin, F.; van Hest, J.C.M.; Wang, H.; Du, L.; et al. Multifunctional PVCL Nanogels with Redox-Responsiveness Enable Enhanced MR Imaging and Ultrasound-Promoted Tumor Chemotherapy. Theranostics 2020, 10, 4349–4358. [Google Scholar] [CrossRef] [PubMed]
- Peng, N.; Ding, X.; Wang, Z.; Cheng, Y.; Gong, Z.; Xu, X.; Gao, X.; Cai, Q.; Huang, S.; Liu, Y. Novel Dual Responsive Alginate-Based Magnetic Nanogels for Onco-Theranostics. Carbohydr. Polym. 2019, 204, 32–41. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, X.; Chen, J.; Zhang, J.; Meng, F.; Deng, C.; Cheng, R.; Feijen, J.; Zhong, Z. Bioresponsive and Fluorescent Hyaluronic Acid-Iodixanol Nanogels for Targeted X-Ray Computed Tomography Imaging and Chemotherapy of Breast Tumors. J. Control. Release 2016, 244, 229–239. [Google Scholar] [CrossRef]
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining Imaging and Therapy. Bioconjug. Chem. 2011, 22, 1879–1903. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Sun, S.; Li, S.; Lv, A.; Chen, Q.; Jiang, K.; Jiang, Z.; Li, Z.; Wu, A.; Lin, H. Programmed Stimuli-Responsive Carbon Dot-Nanogel Hybrids for Imaging-Guided Enhanced Tumor Phototherapy. ACS Appl. Mater. Interfaces 2022, 14, 10142–10153. [Google Scholar] [CrossRef]
- Pan, Y.T.; Ding, Y.F.; Han, Z.H.; Yuwen, L.; Ye, Z.; Mok, G.S.P.; Li, S.; Wang, L.H. Hyaluronic Acid-Based Nanogels Derived from Multicomponent Self-Assembly for Imaging-Guided Chemo-Photodynamic Cancer Therapy. Carbohydr. Polym. 2021, 268, 118257. [Google Scholar] [CrossRef]
- Duan, Q.Y.; Zhu, Y.X.; Jia, H.R.; Guo, Y.; Zhang, X.; Gu, R.; Li, C.; Wu, F.G. Platinum-Coordinated Dual-Responsive Nanogels for Universal Drug Delivery and Combination Cancer Therapy. Small 2022, 18, e2203260. [Google Scholar] [CrossRef]
- Clegg, J.R.; Irani, A.S.; Ander, E.W.; Ludolph, C.M.; Venkataraman, A.K.; Zhong, J.X.; Peppas, N.A. Synthetic Networks with Tunable Responsiveness, Biodegradation, and Molecular Recognition for Precision Medicine Applications. Sci. Adv. 2019, 5, eaax7946. [Google Scholar] [CrossRef]
- Miura, R.; Sawada, S.I.; Mukai, S.A.; Sasaki, Y.; Akiyoshi, K. Antigen Delivery to Antigen-Presenting Cells for Adaptive Immune Response by Self-Assembled Anionic Polysaccharide Nanogel Vaccines. Biomacromolecules 2020, 21, 621–629. [Google Scholar] [CrossRef]
- Hilf, N.; Kuttruff-Coqui, S.; Frenzel, K.; Bukur, V.; Stevanović, S.; Gouttefangeas, C.; Platten, M.; Tabatabai, G.; Dutoit, V.; van der Burg, S.H.; et al. Actively Personalized Vaccination Trial for Newly Diagnosed Glioblastoma. Nature 2019, 565, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Mosquera, M.J.; Kim, S.; Zhou, H.; Jing, T.T.; Luna, M.; Guss, J.D.; Reddy, P.; Lai, K.; Leifer, C.A.; Brito, I.L.; et al. Immunomodulatory Nanogels Overcome Restricted Immunity in a Murine Model of Gut Microbiome–Mediated Metabolic Syndrome. Sci. Adv. 2019, 5, eaav9788. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Wei, W.; Gu, Z.; Ni, D.; Luo, N.; Yang, Z.; Zhao, L.; Garate, J.A.; Zhou, R.; Su, Z.; et al. Exploration of Graphene Oxide as an Intelligent Platform for Cancer Vaccines. Nanoscale 2015, 7, 19949–19957. [Google Scholar] [CrossRef]
- Shao, K.; Singha, S.; Clemente-Casares, X.; Tsai, S.; Yang, Y.; Santamaria, P. Nanoparticle-Based Immunotherapy for Cancer. ACS Nano 2015, 9, 16–30. [Google Scholar] [CrossRef]
- Li, D.; Sun, F.; Bourajjaj, M.; Chen, Y.; Pieters, E.H.; Chen, J.; Van Den Dikkenberg, J.B.; Lou, B.; Camps, M.G.M.; Ossendorp, F.; et al. Strong: In Vivo Antitumor Responses Induced by an Antigen Immobilized in Nanogels via Reducible Bonds. Nanoscale 2016, 8, 19592–19604. [Google Scholar] [CrossRef]
- Mehta, N.K.; Moynihan, K.D.; Irvine, D.J. Engineering New Approaches to Cancer Vaccines. Cancer Immunol. Res. 2015, 3, 836–843. [Google Scholar] [CrossRef]
- Warnatsch, A.; Bergann, T.; Krüger, E. Oxidation Matters: The Ubiquitin Proteasome System Connects Innate Immune Mechanisms with MHC Class I Antigen Presentation. Mol. Immunol. 2013, 55, 106–109. [Google Scholar] [CrossRef]
- Palucka, K.; Banchereau, J. Cancer Immunotherapy via Dendritic Cells. Nat. Rev. Cancer 2012, 12, 265–277. [Google Scholar] [CrossRef]
- Wang, C.; Li, P.; Liu, L.; Pan, H.; Li, H.; Cai, L.; Ma, Y. Self-Adjuvanted Nanovaccine for Cancer Immunotherapy: Role of Lysosomal Rupture-Induced ROS in MHC Class I Antigen Presentation. Biomaterials 2016, 79, 88–100. [Google Scholar] [CrossRef]
- Mier, A.; Maffucci, I.; Merlier, F.; Prost, E.; Montagna, V.; Ruiz-Esparza, G.U.; Bonventre, J.V.; Dhal, P.K.; Tse Sum Bui, B.; Sakhaii, P.; et al. Molecularly Imprinted Polymer Nanogels for Protein Recognition: Direct Proof of Specific Binding Sites by Solution STD and WaterLOGSY NMR Spectroscopies. Angew. Chem.-Int. Ed. 2021, 60, 20849–20857. [Google Scholar] [CrossRef] [PubMed]
- Vlatakis, G.; Andersson, L.I.; Müller, R.; Mosbach, K. Drug Assay Using Antibody Mimics Made by Molecular Imprinting. Nature 1993, 361, 645–647. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Kitayama, Y.; Sasao, R.; Yamada, T.; Toh, K.; Matsumoto, Y.; Kataoka, K. Molecularly Imprinted Nanogels Acquire Stealth In Situ by Cloaking Themselves with Native Dysopsonic Proteins. Angew. Chem.-Int. Ed. 2017, 56, 7088–7092. [Google Scholar] [CrossRef]
- Montanari, E.; Mancini, P.; Galli, F.; Varani, M.; Santino, I.; Coviello, T.; Mosca, L.; Matricardi, P.; Rancan, F.; Di Meo, C. Biodistribution and Intracellular Localization of Hyaluronan and Its Nanogels. A Strategy to Target Intracellular S. Aureus in Persistent Skin Infections. J. Control. Release 2020, 326, 1–12. [Google Scholar] [CrossRef]
- Weldrick, P.J.; Hardman, M.J.; Paunov, V.N. Enhanced Clearing of Wound-Related Pathogenic Bacterial Biofilms Using Protease-Functionalized Antibiotic Nanocarriers. ACS Appl. Mater. Interfaces 2019, 11, 43902–43919. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Wang, S.W.; Mao, J.Y.; Chang, H.T.; Harroun, S.G.; Lin, H.J.; Huang, C.C.; Lai, J.Y. Carbonized Nanogels for Simultaneous Antibacterial and Antioxidant Treatment of Bacterial Keratitis. Chem. Eng. J. 2021, 411, 128469. [Google Scholar] [CrossRef]
- Li, B.; Xie, J.; Yuan, Z.; Jain, P.; Lin, X.; Wu, K.; Jiang, S. Mitigation of Inflammatory Immune Responses with Hydrophilic Nanoparticles. Angew. Chem.-Int. Ed. 2018, 57, 4527–4531. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, S.; Lu, Y.; Lai, R.; Liu, Z.; Luo, W.; Xu, Y. Chitosan/Hyaluronan Nanogels Co-Delivering Methotrexate and 5-Aminolevulinic Acid: A Combined Chemo-Photodynamic Therapy for Psoriasis. Carbohydr. Polym. 2022, 277, 118819. [Google Scholar] [CrossRef]
- Choi, Y.; Arron, J.R.; Townsend, M.J. Promising Bone-Related Therapeutic Targets for Rheumatoid Arthritis. Nat. Rev. Rheumatol. 2009, 5, 543–548. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Vismara, I.; Papa, S.; Veneruso, V.; Mauri, E.; Mariani, A.; De Paola, M.; Affatato, R.; Rossetti, A.; Sponchioni, M.; Moscatelli, D.; et al. Selective Modulation of A1 Astrocytes by Drug-Loaded Nano-Structured Gel in Spinal Cord Injury. ACS Nano 2020, 14, 360–371. [Google Scholar] [CrossRef]
- Knipe, J.M.; Strong, L.E.; Peppas, N.A. Enzyme- and PH-Responsive Microencapsulated Nanogels for Oral Delivery of SiRNA to Induce TNF-α Knockdown in the Intestine. Biomacromolecules 2016, 17, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.; Lee, Y.M.; Lee, J.; Park, D.; Kim, K.; Kim, J.; Park, J.; Kim, W.J. Nitric Oxide-Scavenging Nanogel for Treating Rheumatoid Arthritis. Nano Lett. 2019, 19, 6716–6724. [Google Scholar] [CrossRef]
- Nagy, G.; Koncz, A.; Telarico, T.; Fernandez, D.; Érsek, B.; Buzás, E.; Perl, A. Central Role of Nitric Oxide in the Pathogenesis of Rheumatoid Arthritis and Sysemic Lupus Erythematosus. Arthritis Res. Ther. 2010, 12, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Kolios, G.; Valatas, V.; Ward, S.G. Nitric Oxide in Inflammatory Bowel Disease: A Universal Messenger in an Unsolved Puzzle. Immunology 2004, 113, 427–437. [Google Scholar] [CrossRef]
- Vincent, J.L.; Zhang, H.; Szabo, C.; Preiser, J.C. Effects of Nitric Oxide in Septic Shock. Am. J. Respir. Crit. Care Med. 2000, 161, 1781–1785. [Google Scholar] [CrossRef]
- Li, L.; Fu, L.; Ai, X.; Zhang, J.; Zhou, J. Design and Fabrication of Temperature-Sensitive Nanogels with Controlled Drug Release Properties for Enhanced Photothermal Sterilization. Chem.—A Eur. J. 2017, 23, 18180–18186. [Google Scholar] [CrossRef]
- Chen, Y.F.; Chiou, Y.H.; Chen, Y.C.; Jiang, Y.S.; Lee, T.Y.; Jan, J.S. ZnO-Loaded DNA Nanogels as Neutrophil Extracellular Trap-like Structures in the Treatment of Mouse Peritonitis. Mater. Sci. Eng. C 2021, 131, 112484. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Tian, J.; Zhu, J.; Chen, J.; Li, L.; Yang, C.; Chen, J.; Chen, D. Photodynamic and Photothermal Co-Driven CO-Enhanced Multi-Mode Synergistic Antibacterial Nanoplatform to Effectively Fight against Biofilm Infections. Chem. Eng. J. 2021, 426, 131919. [Google Scholar] [CrossRef]
- Weldrick, P.J.; Iveson, S.; Hardman, M.J.; Paunov, V.N. Breathing New Life into Old Antibiotics: Overcoming Antibacterial Resistance by Antibiotic-Loaded Nanogel Carriers with Cationic Surface Functionality. Nanoscale 2019, 11, 10472–10485. [Google Scholar] [CrossRef]
- Zhu, J.; Li, F.; Wang, X.; Yu, J.; Wu, D. Hyaluronic Acid and Polyethylene Glycol Hybrid Hydrogel Encapsulating Nanogel with Hemostasis and Sustainable Antibacterial Property for Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 13304–13316. [Google Scholar] [CrossRef]
- Al-Awady, M.J.; Fauchet, A.; Greenway, G.M.; Paunov, V.N. Enhanced Antimicrobial Effect of Berberine in Nanogel Carriers with Cationic Surface Functionality. J. Mater. Chem. B 2017, 5, 7885–7897. [Google Scholar] [CrossRef]
- Li, N.; Han, H.; Li, M.; Qiu, W.; Wang, Q.; Qi, X.; He, Y.; Wang, X.; Liu, L.; Yu, J.; et al. Eco-Friendly and Intrinsic Nanogels for Durable Flame Retardant and Antibacterial Properties. Chem. Eng. J. 2021, 415, 129008. [Google Scholar] [CrossRef]
- Han, H.; Zhu, J.; Wu, D.Q.; Li, F.X.; Wang, X.L.; Yu, J.Y.; Qin, X.H. Inherent Guanidine Nanogels with Durable Antibacterial and Bacterially Antiadhesive Properties. Adv. Funct. Mater. 2019, 29, 1806594. [Google Scholar] [CrossRef]
- Panja, S.; Bharti, R.; Dey, G.; Lynd, N.A.; Chattopadhyay, S. Coordination-Assisted Self-Assembled Polypeptide Nanogels to Selectively Combat Bacterial Infection. ACS Appl. Mater. Interfaces 2019, 11, 33599–33611. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial Biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, B.; Rashidipour, M.; Marzban, A.; Soroush, S.; Azadpour, M.; Delfani, S.; Ramak, P. Mentha Piperita Essential Oils Loaded in a Chitosan Nanogel with Inhibitory Effect on Biofilm Formation against S. Mutans on the Dental Surface. Carbohydr. Polym. 2019, 212, 142–149. [Google Scholar] [CrossRef]
- Wang, A.; Weldrick, P.J.; Madden, L.A.; Paunov, V.N. Biofilm-Infected Human Clusteroid Three-Dimensional Coculture Platform to Replace Animal Models in Testing Antimicrobial Nanotechnologies. ACS Appl. Mater. Interfaces 2021, 13, 22182–22194. [Google Scholar] [CrossRef]
- Horvat, S.; Yu, Y.; Böjte, S.; Teßmer, I.; Lowman, D.W.; Ma, Z.; Williams, D.L.; Beilhack, A.; Albrecht, K.; Groll, J. Engineering Nanogels for Drug Delivery to Pathogenic Fungi Aspergillus Fumigatus by Tuning Polymer Amphiphilicity. Biomacromolecules 2020, 21, 3112–3121. [Google Scholar] [CrossRef]
- Kłodzińska, S.N.; Wan, F.; Jumaa, H.; Sternberg, C.; Rades, T.; Nielsen, H.M. Utilizing Nanoparticles for Improving Anti-Biofilm Effects of Azithromycin: A Head-to-Head Comparison of Modified Hyaluronic Acid Nanogels and Coated Poly (Lactic-Co-Glycolic Acid) Nanoparticles. J. Colloid Interface Sci. 2019, 555, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, X.; Liu, Y.; Huang, F.; Wu, G.; Liu, Y.; Zhang, Z.; Ding, Y.; Lv, J.; Ma, R.; et al. Glucose and H2O2 Dual-Sensitive Nanogels for Enhanced Glucose-Responsive Insulin Delivery. Nanoscale 2019, 11, 9163–9175. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.S.; Larsson, M.; Hsiao, M.H.; Chen, Y.C.; Röding, M.; Nydén, M.; Liu, D.M. Injectable Insulin-Lysozyme-Loaded Nanogels with Enzymatically-Controlled Degradation and Release for Basal Insulin Treatment: In Vitro Characterization and in Vivo Observation. J. Control. Release 2016, 224, 33–42. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, D.; Liu, L.; Li, X. Development of Poly(Hydroxyethyl Methacrylate) Nanogel for Effective Oral Insulin Delivery. Pharm. Dev. Technol. 2018, 23, 351–357. [Google Scholar] [CrossRef]
- Li, C.; Wu, G.; Ma, R.; Liu, Y.; Liu, Y.; Lv, J.; An, Y.; Shi, L. Nitrilotriacetic Acid (NTA) and Phenylboronic Acid (PBA) Functionalized Nanogels for Efficient Encapsulation and Controlled Release of Insulin. ACS Biomater. Sci. Eng. 2018, 4, 2007–2017. [Google Scholar] [CrossRef]
- Hejaz, H.A. Palestinian Strategies, Guidelines, and Challenges in the Treatment and Management of Coronavirus Disease-2019 (COVID-19). Avicenna J. Med. 2020, 10, 135–162. [Google Scholar] [CrossRef]
- Sharp, P.M.; Hahn, B.H. Origins of HIV and the AIDS Pandemic. Cold Spring Harb. Perspect. Med. 2011, 1, a006841. [Google Scholar] [CrossRef]
- Dick, O.B.; San Martín, J.L.; Montoya, R.H.; Del Diego, J.; Zambrano, B.; Dayan, G.H. Review: The History of Dengue Outbreaks in the Americas. Am. J. Trop. Med. Hyg. 2012, 87, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Chowell, G.; Echevarría-Zuno, S.; Viboud, C.; Simonsen, L.; Tamerius, J.; Miller, M.A.; Borja-Aburto, V.H. Characterizing the Epidemiology of the 2009 Influenza A/H1N1 Pandemic in Mexico. PLoS Med. 2011, 8, e1000436. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Thapa, M.; Hua, D.H.; Chang, K.O. Biodegradable Nanogels for Oral Delivery of Interferon for Norovirus Infection. Antivir. Res. 2011, 89, 165–173. [Google Scholar] [CrossRef]
- Warren, G.; Makarov, E.; Lu, Y.; Senanayake, T.; Rivera, K.; Gorantla, S.; Poluektova, L.Y.; Vinogradov, S.V. Amphiphilic Cationic Nanogels as Brain-Targeted Carriers for Activated Nucleoside Reverse Transcriptase Inhibitors. J. Neuroimmune Pharmacol. 2015, 10, 88–101. [Google Scholar] [CrossRef]
- Jang, H.; Sutradhar, S.C.; Ryu, T.; Choi, K.; Yoon, S.; Lee, S.; Kim, W. Preparation and Characterization of Anti-Scratch Polycarbonate Containing Acrylate Group. J. Nanosci. Nanotechnol. 2017, 17, 7454–7459. [Google Scholar] [CrossRef]
- Hossain, M.A.; Jang, H.; Sutradhar, S.C.; Ha, J.; Yoo, J.; Lee, C.; Lee, S.; Kim, W. Novel Hydroxide Conducting Sulfonium-Based Anion Exchange Membrane for Alkaline Fuel Cell Applications. Int. J. Hydrogen Energy 2016, 41, 10458–10465. [Google Scholar] [CrossRef]
- Lopa, N.S.; Rahman, M.M.; Jang, H.; Sutradhar, S.C.; Ahmed, F.; Ryu, T.; Kim, W. A Glassy Carbon Electrode Modified with Poly(2,4-Dinitrophenylhydrazine) for Simultaneous Detection of Dihydroxybenzene Isomers. Microchim. Acta 2018, 185, 23. [Google Scholar] [CrossRef]
- Cai, G.; Yang, J.; Wang, L.; Chen, C.; Cai, C.; Gong, H. A Point-to-Point “Cap” Strategy to Construct a Highly Selective Dual-Function Molecularly-Imprinted Sensor for the Simultaneous Detection of HAV and HBV. Biosens. Bioelectron. 2023, 219, 114794. [Google Scholar] [CrossRef]
- Bhatia, S.; Hilsch, M.; Cuellar-Camacho, J.L.; Ludwig, K.; Nie, C.; Parshad, B.; Wallert, M.; Block, S.; Lauster, D.; Böttcher, C.; et al. Adaptive Flexible Sialylated Nanogels as Highly Potent Influenza A Virus Inhibitors. Angew. Chem.-Int. Ed. 2020, 59, 12417–12422. [Google Scholar] [CrossRef]
- Nagatomo, D.; Taniai, M.; Ariyasu, H.; Taniguchi, M.; Aga, M.; Ariyasu, T.; Ohta, T.; Fukuda, S. Cholesteryl Pullulan Encapsulated TNF-α Nanoparticles Are an Effective Mucosal Vaccine Adjuvant against Influenza Virus. Biomed Res. Int. 2015, 2015, 471468. [Google Scholar] [CrossRef]
- Nuhn, L.; Van Hoecke, L.; Deswarte, K.; Schepens, B.; Li, Y.; Lambrecht, B.N.; De Koker, S.; David, S.A.; Saelens, X.; De Geest, B.G. Potent Anti-Viral Vaccine Adjuvant Based on PH-Degradable Nanogels with Covalently Linked Small Molecule Imidazoquinoline TLR7/8 Agonist. Biomaterials 2018, 178, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, B.; Sun, P.; Wang, W.; Luo, S.; Zhang, W.; Yang, Y.; Wang, Z.; Lin, J.; Chen, P.R. Severe Acute Respiratory Syndrome Coronavirus-2 Spike Protein Nanogel as a Pro-Antigen Strategy with Enhanced Protective Immune Responses. Small 2020, 16, e2004237. [Google Scholar] [CrossRef]
- Wibowo, D.; Jorritsma, S.H.T.; Gonzaga, Z.J.; Evert, B.; Chen, S.; Rehm, B.H.A. Polymeric Nanoparticle Vaccines to Combat Emerging and Pandemic Threats. Biomaterials 2021, 268, 120597. [Google Scholar] [CrossRef] [PubMed]
- Vasilakos, J.P.; Tomai, M.A. The Use of Toll-like Receptor 7/8 Agonists as Vaccine Adjuvants. Expert Rev. Vaccines 2013, 12, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Bergmann, T.; Cuellar-Camacho, J.L.; Ehrmann, S.; Chowdhury, M.S.; Zhang, M.; Dahmani, I.; Haag, R.; Azab, W. Multivalent Flexible Nanogels Exhibit Broad-Spectrum Antiviral Activity by Blocking Virus Entry. ACS Nano 2018, 12, 6429–6442. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Nguyen, T.N.; Nguyen, T.T.T.; Ivanov, I.A.; Nguyen, K.C.; Tran, Q.N.; Hoang, A.N.; Utkin, Y.N. Nanoencapsulation Enhances Anticoagulant Activity of Adenosine and Dipeptide IleTrp. Nanomaterials 2019, 9, 1191. [Google Scholar] [CrossRef]
- Xu, X.; Huang, X.; Zhang, Y.; Shen, S.; Feng, Z.; Dong, H.; Zhang, C.; Mo, R. Self-Regulated Hirudin Delivery for Anticoagulant Therapy. Sci. Adv. 2020, 6, eabc0382. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, H.; Zhou, Z.; Maitz, M.F.; Liu, K.; Zhang, B.; Yang, L.; Luo, R.; Wang, Y. A Thrombin-Triggered Self-Regulating Anticoagulant Strategy Combined with Anti-Inflammatory Capacity for Blood-Contacting Implants. Sci. Adv. 2022, 8, eabm3378. [Google Scholar] [CrossRef] [PubMed]
- Nan, D.; Jin, H.; Yang, D.; Yu, W.; Jia, J.; Yu, Z.; Tan, H.; Sun, Y.; Hao, H.; Qu, X.; et al. Combination of Polyethylene Glycol-Conjugated Urokinase Nanogels and Urokinase for Acute Ischemic Stroke Therapeutic Implications. Transl. Stroke Res. 2021, 12, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, H.; Joung, Y.K.; Jung, K.H.; Choi, J.H.; Lee, D.H.; Park, K.D.; Hong, S.S. The Use of Low Molecular Weight Heparin-Pluronic Nanogels to Impede Liver Fibrosis by Inhibition the TGF-β/Smad Signaling Pathway. Biomaterials 2011, 32, 1438–1445. [Google Scholar] [CrossRef]
- Eskandari, S.K.; Sulkaj, I.; Melo, M.B.; Li, N.; Allos, H.; Alhaddad, J.B.; Kollar, B.; Borges, T.J.; Eskandari, A.S.; Zinter, M.A.; et al. Regulatory T Cells Engineered with TCR Signaling-Responsive IL-2 Nanogels Suppress Alloimmunity in Sites of Antigen Encounter. Sci. Transl. Med. 2020, 12, eaaw4744. [Google Scholar] [CrossRef]
- Ashrafi, H.; Azadi, A.; Mohammadi-Samani, S.; Hamidi, M. New Candidate Delivery System for Alzheimer’s Disease: Deferoxamine Nanogels. Biointerface Res. Appl. Chem. 2020, 10, 7106–7119. [Google Scholar] [CrossRef]
- Prieto, M.; Usón, L.; Garcia-Salinas, S.; Yus, C.; Landa, G.; Alejo, T.; Lujan, L.; Perez, M.; Irusta, S.; Sebastian, V.; et al. Light Activated Pulsatile Drug Delivery for Prolonged Peripheral Nerve Block. Biomaterials 2022, 283, 121453. [Google Scholar] [CrossRef]
- Van Hoeck, J.; Van de Vyver, T.; Harizaj, A.; Goetgeluk, G.; Merckx, P.; Liu, J.; Wels, M.; Sauvage, F.; De Keersmaecker, H.; Vanhove, C.; et al. Hydrogel-Induced Cell Membrane Disruptions Enable Direct Cytosolic Delivery of Membrane-Impermeable Cargo. Adv. Mater. 2021, 33, 2008054. [Google Scholar] [CrossRef]
- Ussar, S.; Griffin, N.W.; Bezy, O.; Fujisaka, S.; Vienberg, S.; Softic, S.; Deng, L.; Bry, L.; Gordon, J.I.; Kahn, C.R. Interactions between Gut Microbiota, Host Genetics and Diet Modulate the Predisposition to Obesity and Metabolic Syndrome. Cell Metab. 2015, 22, 516–530. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Hajifathalian, K.; Ezzati, M.; Woodward, M.; Rimm, E.B.; Danaei, G.; Selmer, R.; Strand, B.H.; Dobson, A.; Hozawa, A.; et al. Metabolic Mediators of the Effects of Body-Mass Index, Overweight, and Obesity on Coronary Heart Disease and Stroke: A Pooled Analysis of 97 Prospective Cohorts with 1.8 Million Participants. Lancet 2014, 383, 970–983. [Google Scholar] [CrossRef] [PubMed]
- Lusis, A.J.; Attie, A.D.; Reue, K. Metabolic Syndrome: From Epidemiology to Systems Biology. Nat. Rev. Genet. 2008, 9, 819–830. [Google Scholar] [CrossRef]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human Nutrition, the Gut Microbiome and the Immune System. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef]
- Sheridan, P.A.; Paich, H.A.; Handy, J.; Karlsson, E.A.; Hudgens, M.G.; Sammon, A.B.; Holland, L.A.; Weir, S.; Noah, T.L.; Beck, M.A. Obesity Is Associated with Impaired Immune Response to Influenza Vaccination in Humans. Int. J. Obes. 2012, 36, 1072–1077. [Google Scholar] [CrossRef]
- Azegami, T.; Yuki, Y.; Sawada, S.; Mejima, M.; Ishige, K.; Akiyoshi, K.; Itoh, H.; Kiyono, H. Nanogel-Based Nasal Ghrelin Vaccine Prevents Obesity. Mucosal Immunol. 2017, 10, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Liu, H.; Huang, C.; Shen, X. Blood Group Antigen Shielding Facilitated by Selective Cell Surface Engineering. ACS Appl. Mater. Interfaces 2020, 12, 22426–22432. [Google Scholar] [CrossRef]
- Gupta, J.; Sharma, G. Nanogel: A Versatile Drug Delivery System for the Treatment of Various Diseases and Their Future Perspective. Drug Deliv. Transl. Res. 2025, 15, 455–482. [Google Scholar] [CrossRef]
- Mao, W.; Jia, S.; Chen, P. Research Progress on Tumor Whole-Cell Vaccines Prepared with Nanoparticles for Tumor Immunotherapy. J. Nanoparticle Res. 2023, 25, 135. [Google Scholar] [CrossRef]
- Rosales-Mendoza, S.; González-Ortega, O. (Eds.) Nanogels-Based Mucosal Vaccines. In Nanovaccines: An Innovative Technology to Fight Human and Animal Diseases; Springer International Publishing: Cham, Switzerland, 2019; pp. 131–157. ISBN 978-3-030-31668-6. [Google Scholar]
- Wagner, A.M.; Lanier, O.L.; Savk, A.; Peppas, N.A. Polybasic Nanogels for Intracellular Co-Delivery of Paclitaxel and Carboplatin: A Novel Approach to Ovarian Cancer Therapy. RSC Pharm. 2025, 2, 553–569. [Google Scholar] [CrossRef]
- Mohammadi, M.; Arabi, L.; Alibolandi, M. Doxorubicin-Loaded Composite Nanogels for Cancer Treatment. J. Control. Release 2020, 328, 171–191. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.; Almutairi, A. Nanogels as Imaging Agents for Modalities Spanning the Electromagnetic Spectrum. Mater. Horiz. 2016, 3, 21–40. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Okur, N.Ü.; Karantas, I.D.; Okur, M.E.; Gündoğdu, E.A. Current Update on Nanoplatforms as Therapeutic and Diagnostic Tools: A Review for the Materials Used as Nanotheranostics and Imaging Modalities. Asian J. Pharm. Sci. 2021, 16, 24–46. [Google Scholar] [CrossRef] [PubMed]
- Altinbasak, I.; Alp, Y.; Sanyal, R.; Sanyal, A. Theranostic Nanogels: Multifunctional Agents for Simultaneous Therapeutic Delivery and Diagnostic Imaging. Nanoscale 2024, 16, 14033. [Google Scholar] [CrossRef] [PubMed]
- Ghosh Majumdar, A.; Pany, B.; Parua, S.S.; Mukherjee, D.; Panda, A.; Mohanty, M.; Das, B.; Si, S.; Mohanty, P.S. Stimuli-Responsive Nanogel/Microgel Hybrids as Targeted Drug Delivery Systems: A Comprehensive Review. Bionanoscience 2024, 14, 3496–3521. [Google Scholar] [CrossRef]
- Zhou, Z.; Lu, Z.-R. Gadolinium-Based Contrast Agents for Magnetic Resonance Cancer Imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 190. [Google Scholar] [CrossRef]
- Priyanka; Abusalah, M.A.H.; Chopra, H.; Sharma, A.; Mustafa, S.A.; Choudhary, O.P.; Sharma, M.; Dhawan, M.; Khosla, R.; Loshali, A.; et al. Nanovaccines: A Game Changing Approach in the Fight against Infectious Diseases. Biomed. Pharmacother. 2023, 167, 115597. [Google Scholar] [CrossRef]
- Esporrín-Ubieto, D.; Huck-Iriart, C.; Picco, A.S.; Beloqui, A.; Calderón, M. Hybrid Nanogel-wrapped Anisotropic Gold Nanoparticles Feature Enhanced Photothermal Stability. Small 2024, 20, 2404097. [Google Scholar] [CrossRef]
- Yanar, F.; Carugo, D.; Zhang, X. Hybrid Nanoplatforms Comprising Organic Nanocompartments Encapsulating Inorganic Nanoparticles for Enhanced Drug Delivery and Bioimaging Applications. Molecules 2023, 28, 5694. [Google Scholar] [CrossRef]
- Badir, A.; Refki, S.; Sekkat, Z. Utilizing Gold Nanoparticles in Plasmonic Photothermal Therapy for Cancer Treatment. Heliyon 2025, 11, e42738. [Google Scholar] [CrossRef]
- Rahman, M. Magnetic Resonance Imaging and Iron-Oxide Nanoparticles in the Era of Personalized Medicine. Nanotheranostics 2023, 7, 424–449. [Google Scholar] [CrossRef]
- Zhang, N.; Xiong, G.; Liu, Z. Toxicity of Metal-Based Nanoparticles: Challenges in the Nano Era. Front. Bioeng. Biotechnol. 2022, 10, 1001572. [Google Scholar] [CrossRef]
- Khan, S.M.; Saleemi, S.; Mannan, H.A. Toxicology, Stability, and Recycling of Organic–Inorganic Nanohybrids. In Hybrid Nanomaterials: Biomedical, Environmental and Energy Applications; Rizwan, K., Bilal, M., Rasheed, T., Nguyen, T.A., Eds.; Springer Nature: Singapore, 2022; pp. 485–497. ISBN 978-981-19-4538-0. [Google Scholar]
- Li, Y.; Vulpe, C.; Lammers, T.; Pallares, R.M. Assessing Inorganic Nanoparticle Toxicity through Omics Approaches. Nanoscale 2024, 16, 15928–15945. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liu, Y.; Zhang, Y.; Ran, R.; Kong, Z.; Zhao, D.; Liu, M.; Zhao, W.; Cui, Y.; Hua, Y.; et al. Smart Nanogels for Cancer Treatment from the Perspective of Functional Groups. Front. Bioeng. Biotechnol. 2024, 11, 1329311. [Google Scholar] [CrossRef]
- Wang, H.; Gao, L.; Fan, T.; Zhang, C.; Zhang, B.; Al-Hartomy, O.A.; Al-Ghamdi, A.; Wageh, S.; Qiu, M.; Zhang, H. Strategic Design of Intelligent-Responsive Nanogel Carriers for Cancer Therapy. ACS Appl. Mater. Interfaces 2021, 13, 54621–54647. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Carneiro, J.; Campos, J.B.L.M.; Miranda, J.M. Production of Hydrogel Microparticles in Microfluidic Devices: A Review. Microfluid. Nanofluidics 2021, 25, 10. [Google Scholar] [CrossRef]
- Yu, M.; Mathew, A.; Liu, D.; Chen, Y.; Wu, J.; Zhang, Y.; Zhang, N. Microfluidics for Formulation and Scale-Up Production of Nanoparticles for Biopharma Industry. In Microfluidics in Pharmaceutical Sciences: Formulation, Drug Delivery, Screening, and Diagnostics; Lamprou, D.A., Weaver, E., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 395–420. ISBN 978-3-031-60717-2. [Google Scholar]
- Agha, A.; Waheed, W.; Stiharu, I.; Nerguizian, V.; Destgeer, G.; Abu-Nada, E.; Alazzam, A. A Review on Microfluidic-Assisted Nanoparticle Synthesis, and Their Applications Using Multiscale Simulation Methods. Discov. Nano 2023, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.E.; Alharthi, S.; Alavi, S.F.; Alavi, S.Z.; Zahra, G.E.; Raza, A.; Ebrahimi Shahmabadi, H. Microfluidics for Personalized Drug Delivery. Drug Discov. Today 2024, 29, 103936. [Google Scholar] [CrossRef] [PubMed]
- Ejeta, F. Recent Advances of Microfluidic Platforms for Controlled Drug Delivery in Nanomedicine. Drug Des. Devel. Ther. 2021, 15, 3881–3891. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Yang, M.; Wu, H.; Li, D. Modular Microfluidics: Current Status and Future Prospects. Micromachines 2022, 13, 1363. [Google Scholar] [CrossRef]
- Nunziata, G.; Borroni, A.; Rossi, F. Advanced Microfluidic Strategies for Core-Shell Nanoparticles: The next-Generation of Polymeric and Lipid-Based Drug Nanocarriers. Chem. Eng. J. Adv. 2025, 22, 100759. [Google Scholar] [CrossRef]
- Bi, Y.; Xie, S.; Li, Z.; Dong, S.; Teng, L. Precise Nanoscale Fabrication Technologies, the “Last Mile” of Medicinal Development. Acta Pharm. Sin. B 2025, 15, 2372–2401. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Dash, S.K.; Dhawan, D.; Sahoo, P.K.; Jindal, A.; Gugulothu, D. Freeze-Drying Revolution: Unleashing the Potential of Lyophilization in Advancing Drug Delivery Systems. Drug Deliv. Transl. Res. 2024, 14, 1111–1153. [Google Scholar] [CrossRef]
- Lee, S.-K.; Ha, E.-S.; Park, H.; Jeong, J.-S.; Shin, C.Y.; Kim, J.-S.; Jeong, S.H.; Kim, M.-S. Lyophilization of Pharmaceuticals: An Overview and Practical Approaches for Developing the Lyophilization Cycle. J. Pharm. Investig. 2025, 1–25. [Google Scholar] [CrossRef]
- Seong, Y.-J.; Lin, G.; Kim, B.J.; Kim, H.-E.; Kim, S.; Jeong, S.-H. Hyaluronic Acid-Based Hybrid Hydrogel Microspheres with Enhanced Structural Stability and High Injectability. ACS Omega 2019, 4, 13834–13844. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).