Concave-Octahedral Fe2+-Rich Fe-MOF/FU Nano-Blocks with Enhanced pH-Responsive Nanozyme Activity Toward Stimuli-Responsive Gels for Chemo-Chemodynamic Synergistic Therapy
Abstract
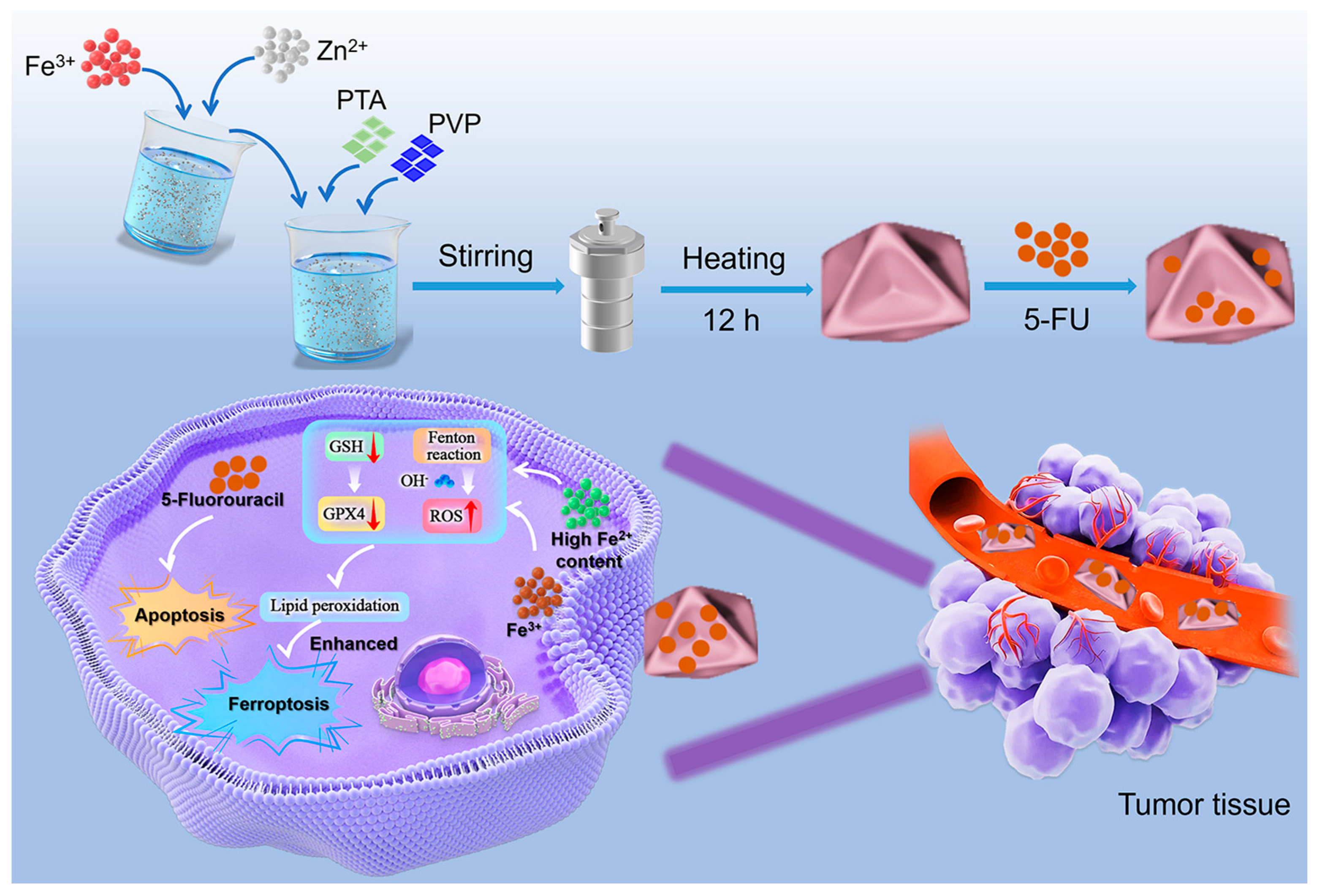
1. Introduction
2. Results and Discussion
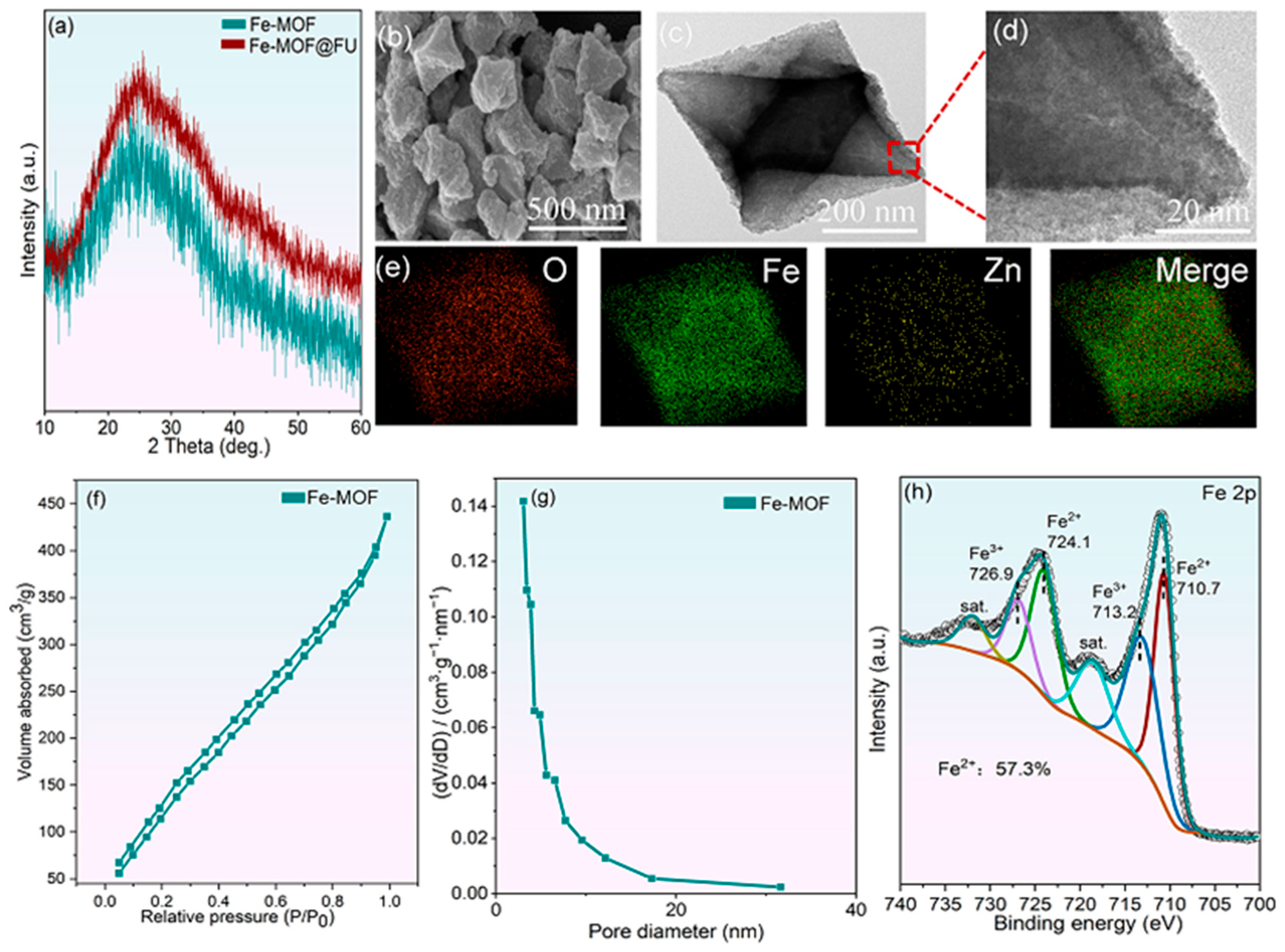
2.1. Characterization of the As-Prepared Fe-MOF Materials
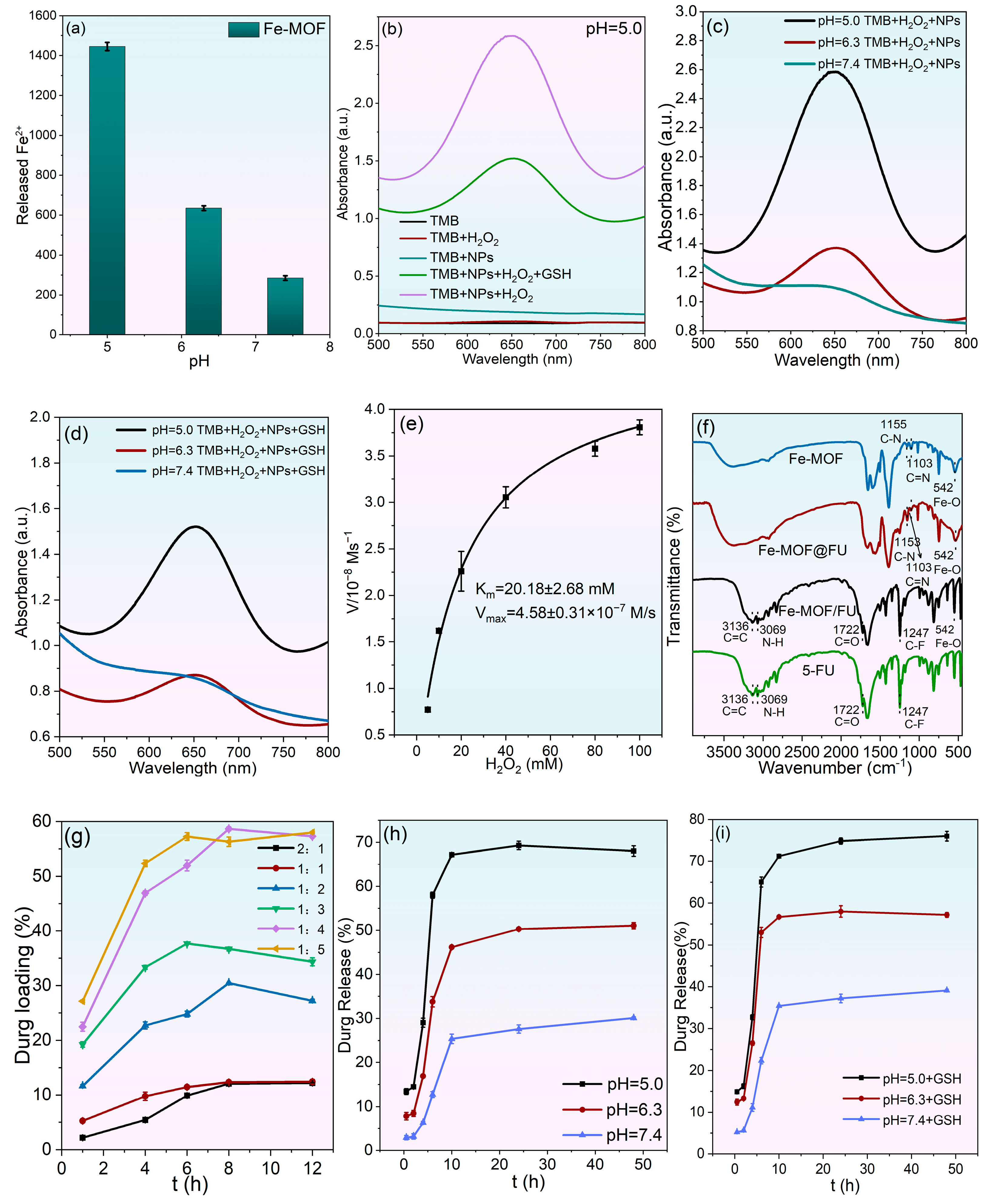
2.2. In Vitro Catalytic Activity and Drug Loading/Release Behavior
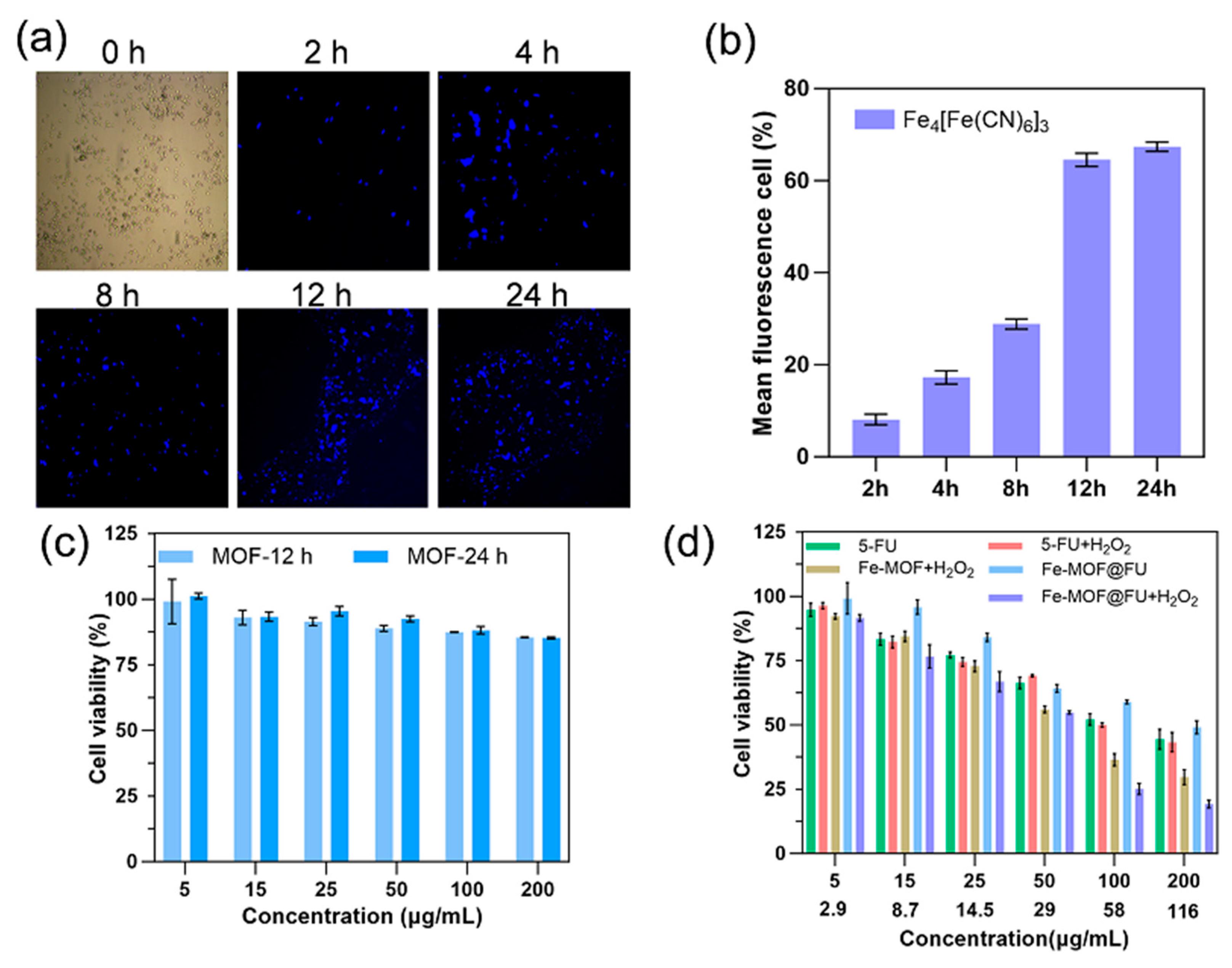
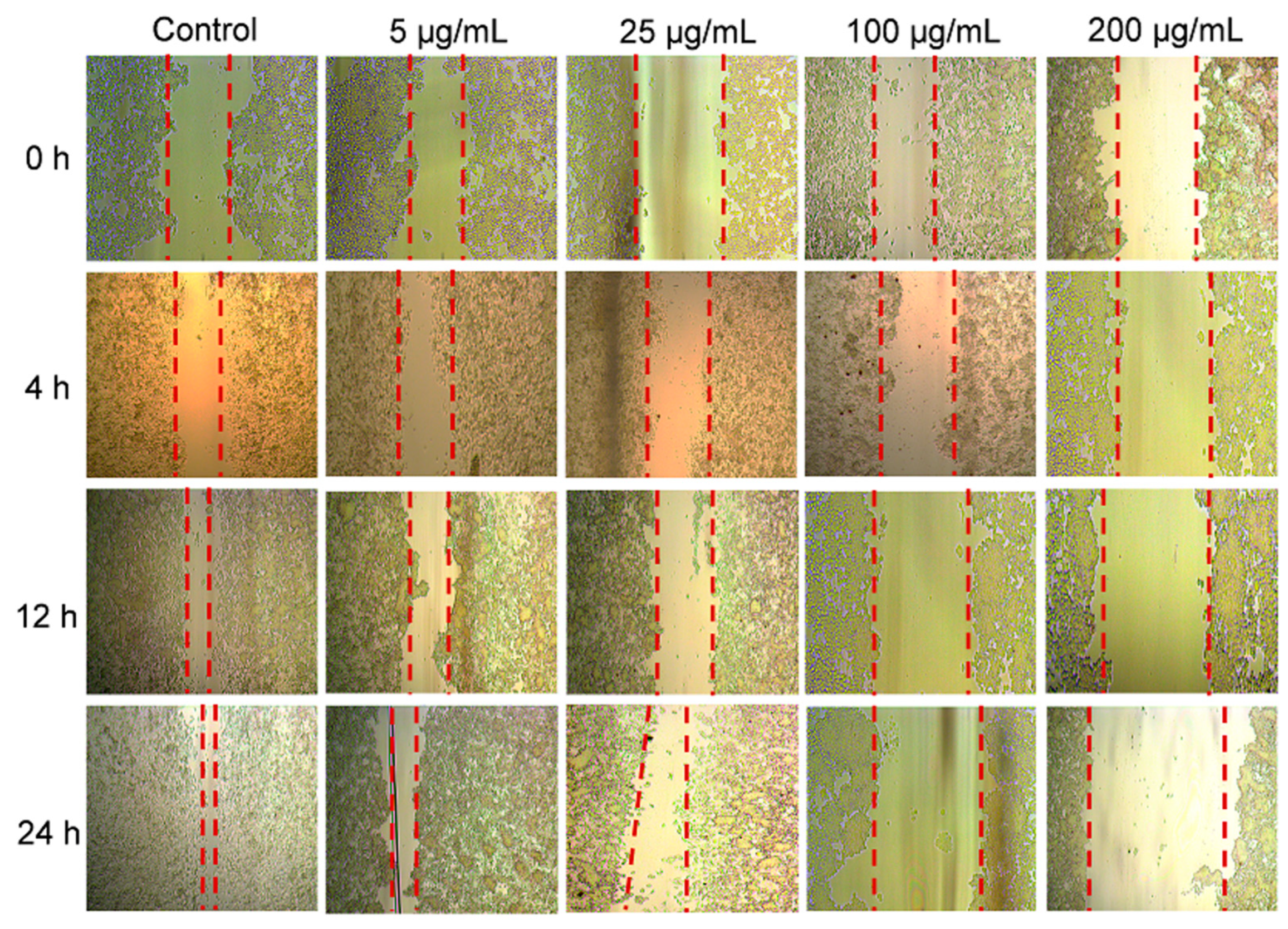
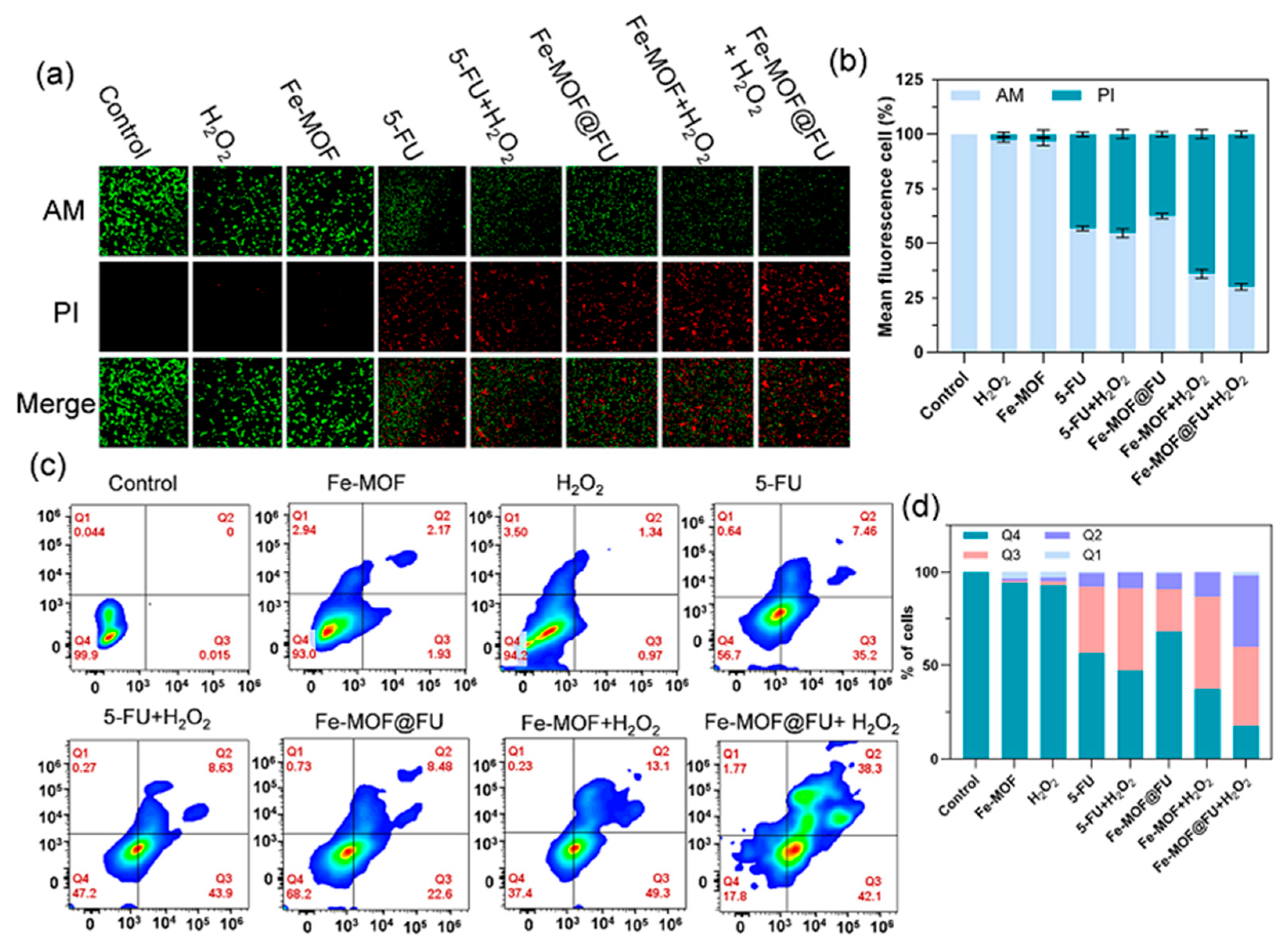
2.3. Cellular Uptake and In Vitro Therapeutic Evaluation of Fe-MOF
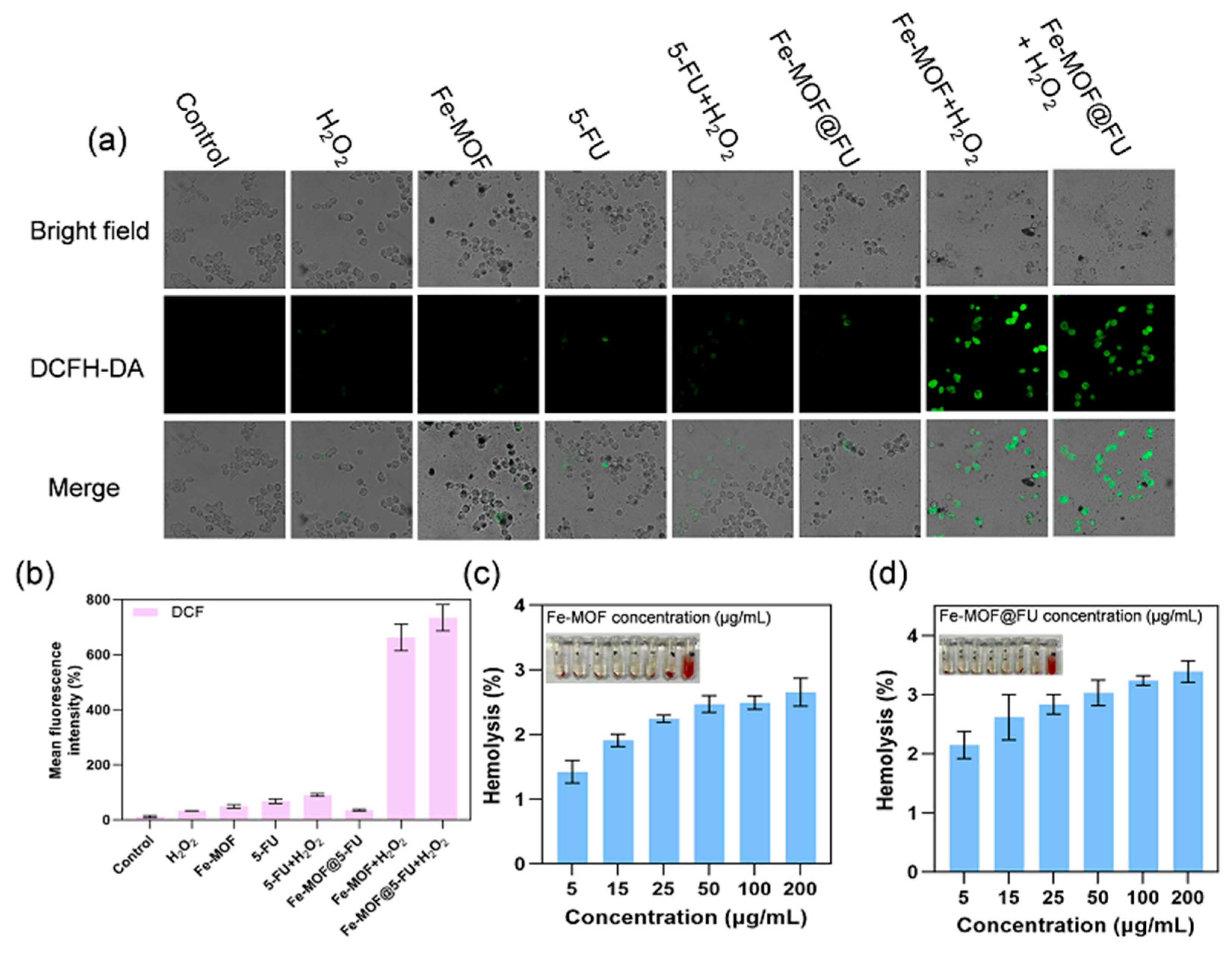
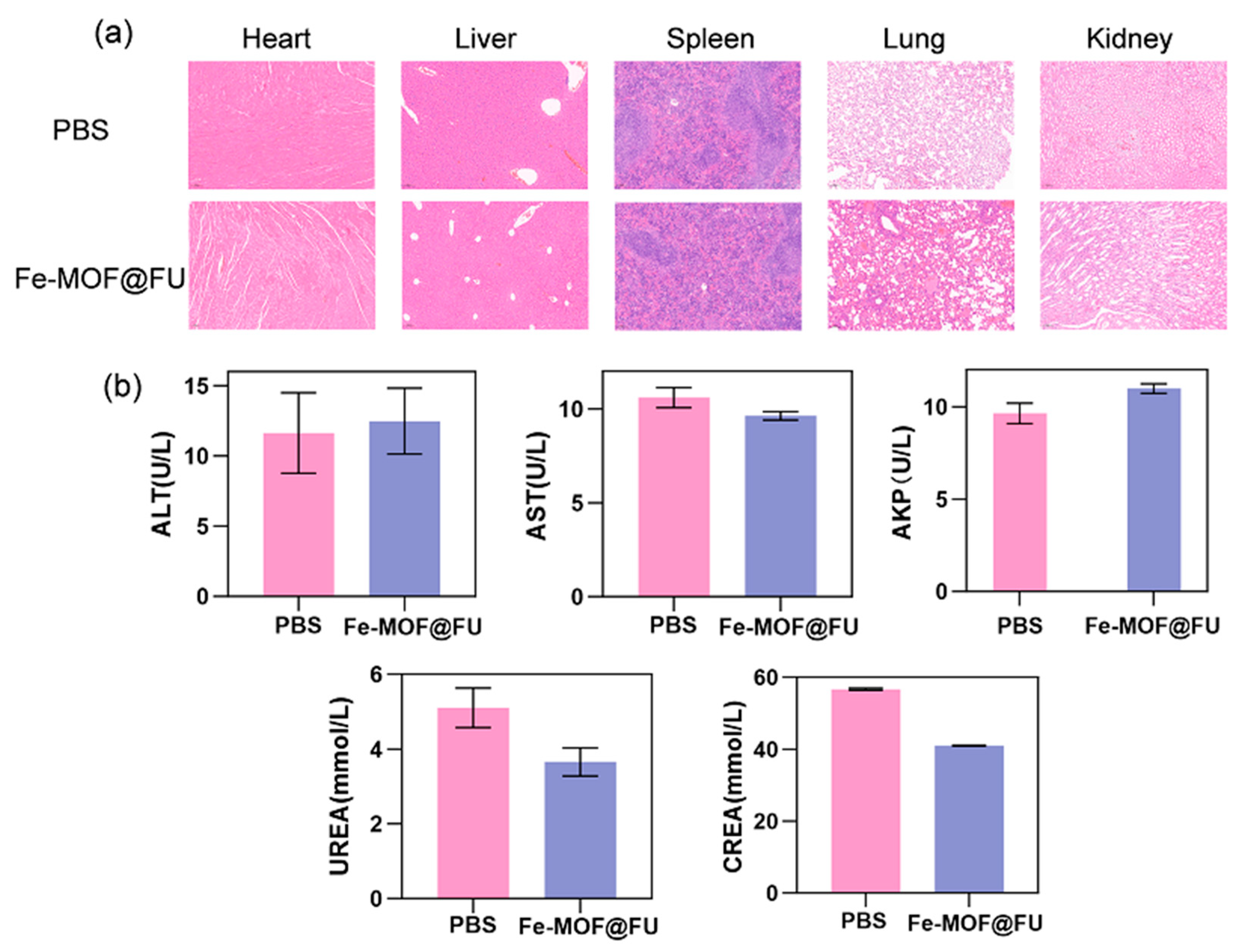
2.4. ROS Generation Capacity and Biocompatibility Assessment
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Concave Octahedral Fe-MOF
4.3. Synthesis of Fe-MOF@FU Materials
4.4. Materials Characterization
4.5. Drug Loading and Release Study
4.6. Fe2+ Release in Fe-MOF Materials Study
4.7. Peroxidase-like Activity and Kinetic Experiments
4.8. Cell Culture
4.9. Cytotoxicity Assessment
4.10. Cellular Uptake of Materials
4.11. Cell Migration Assay
4.12. Calcein-AM/Propidium Iodide (PI) Staining
4.13. Intracellular Reactive Oxygen Species (ROS) Detection
4.14. Flow Cytometric Apoptosis Assay
4.15. Biocompatibility Evaluation
4.15.1. Hemolysis Assay
4.15.2. Mouse Organ Sectioning and Liver/Kidney Function Index Measurement
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chi, Z.; Gu, J.; Li, H.; Wang, Q. Recent progress of metal organic framework based nanozymes with oxidoreductase like activity. Analyst 2024, 149, 1416–1435. [Google Scholar] [CrossRef]
- Ren, G.; Lu, M.; Zhao, Z.; Qin, F.; Li, K.; Chen, W.; Lin, Y. Cobalt single atom nanozyme Co-administration with ascorbic acid enables redox imbalance for tumor catalytic ablation. ACS Biomater. Sci. Eng. 2023, 9, 1066–1076. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Wu, J.; Kang, Y.; Xue, P. Cu2O/CuVO3 nano-heterojunction as a highly active therapeutic catalyst for aggravating redox dyshomeostasis of neoplastic cells. Adv. Mater. 2025, 37, 2502407. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.; Chen, W.; Liu, J.; Huang, S.; Chen, L.; Liu, Q.; Liu, N.; Jin, Q.; Li, Y.; Wang, P.; et al. Macrophage mimic hollow mesoporous Fe-based nanocatalysts for self-amplified chemodynamic therapy and metastasis inhibition via tumor microenvironment remodeling. ACS Appl. Mater. Interfaces 2022, 14, 5053–5065. [Google Scholar] [CrossRef]
- Liang, P.; Huang, X.; Wang, Y.; Chen, D.; Ou, C.; Zhang, Q.; Shao, J.; Huang, W.; Dong, X. Tumor-microenvironment responsive nanoconjugate for synergistic antivascular activity and phototherapy. ACS Nano 2018, 12, 11446–11457. [Google Scholar] [CrossRef]
- Dai, Y.; Xu, C.; Sun, X.; Chen, X. Nanoparticle design strategies for enhanced anticancer therapy by exploiting the tumour microenvironment. Chem. Soc. Rev. 2017, 46, 3830–3852. [Google Scholar] [CrossRef]
- Wu, Z.; Guo, Y.; Jiang, W.; Yang, Y.; Wei, P.; Yi, T. Recent process in organic small molecular fluorescent probes for tracking markers of tumor redox balance. TrAC Trends Anal. Chem. 2024, 170, 117461. [Google Scholar] [CrossRef]
- Yu, H.; Li, Y.; Zhang, Z.; Ren, J.; Zhang, L.; Xu, Z.; Kang, Y.; Xue, P. Silk fibroin capped metal organic framework for tumor specific redox dyshomeostasis treatment synergized by deoxygenation driven chemotherapy. Acta Biomater. 2022, 138, 545–560. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, H.; Lin, L.; Zhao, W.; Zhu, X.; Pang, D.; Liu, A. Redox imbalance triggered intratumoral cascade reaction for tumor “turn on” imaging and synergistic therapy. Small 2023, 19, 2206272. [Google Scholar] [CrossRef]
- Wang, X.; Wan, Z.; Li, C.; Meng, X.; Zhao, L.; Lu, J.; Qian, J.; Liu, X. Defective Cu2-XSe crystals for ultrasound enhanced CDT of tumor therapy. Ceram. Int. 2022, 48, 34564–34570. [Google Scholar] [CrossRef]
- Xu, Q.; Ban, X.; Yang, L.; Chen, Y.; Zhang, F.; Wang, Y.; Cao, P.; Yu, M.; Duan, X. MRI-visualized PTT/CDT for breast cancer ablation and distant metastasis prevention. Appl. Mater. Today 2024, 36, 102059. [Google Scholar] [CrossRef]
- Li, W.; Xiao, Y.; Guo, G.; Peng, J.; Zhu, N.; Chen, Z.; Peng, B.; Jiang, Z.; Li, B.; Yu, G.; et al. Cuprous oxide nanocomposites with photothermal (PTT) and chemical dynamics (CDT) effects induce cuproptosis in breast cancer using the strategy of increasing inflow and reducing outflow. Nano Today 2024, 56, 102223. [Google Scholar] [CrossRef]
- Sui, C.; Tan, R.; Chen, Y.; Yin, G.; Wang, Z.; Xu, W.; Li, X. MOFs derived Fe–N codoped carbon nanoparticles as O2 evolving reactor and ROS generator for CDT/PDT/PTT synergistic treatment of tumors. Bioconjug. Chem. 2021, 32, 318–327. [Google Scholar] [CrossRef]
- Bonet-Aleta, J.; Sancho-Albero, M.; Calzada-Funes, J.; Irusta, S.; Martin-Duque, P.; Hueso, J.L.; Santamaria, J. Glutathione triggered catalytic response of copper iron mixed oxide nanoparticles. Leveraging tumor microenvironment conditions for chemodynamic therapy. J. Colloid Interface Sci. 2022, 617, 704–717. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Liu, P.; Xu, S.; Luo, X. Core shell multifunctional nanomaterial based all-in-one nanoplatform for simultaneous multilayer imaging of dual types of tumor biomarkers and photothermal therapy. Anal. Chem. 2020, 92, 15169–15178. [Google Scholar] [CrossRef]
- Luo, M.; Yukawa, H.; Sato, K.; Tozawa, M.; Tokunaga, M.; Kameyama, T.; Torimoto, T.; Baba, Y. Multifunctional magnetic CuS/Gd2O3 nanoparticles for fluorescence/magnetic resonance bimodal imaging guided photothermal intensified chemodynamic synergetic therapy of targeted tumors. ACS Appl. Mater. Interfaces 2022, 14, 34365–34376. [Google Scholar] [CrossRef]
- Zheng, H.; Huang, L.; An, G.; Guo, L.; Wang, N.; Yang, W.; Zhu, Y. A nanoreactor based on metal organic frameworks with triple synergistic therapy for hepatocellular carcinoma. Adv. Healthc. Mater. 2024, 13, 2401743. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, Q.; Li, C.; Zeng, X.; Zhang, X.-Z. Near infrared light triggered metal ion and photodynamic therapy based on AgNPs/porphyrinic MOFs for tumors and pathogens elimination. Biomaterials 2020, 248, 120029. [Google Scholar] [CrossRef]
- Bao, W.; Liu, M.; Meng, J.; Liu, S.; Wang, S.; Jia, R.; Wang, Y.; Ma, G.; Wei, W.; Tian, Z. MOFs-based nanoagent enables dual mitochondrial damage in synergistic antitumor therapy via oxidative stress and calcium overload. Nat. Commun. 2021, 12, 6399. [Google Scholar] [CrossRef]
- Lu, K.; Aung, T.; Guo, N.; Weichselbaum, R.; Lin, W. Nanoscale metal organic frameworks for therapeutic, imaging, and sensing applications. Adv. Mater. 2018, 30, 1707634. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.; Hu, P.; Xu, Y.; Cheng, T.; Wei, C.; Pan, L.; Shi, J. Simultaneous blood brain barrier crossing and protection for stroke treatment based on edaravone loaded ceria nanoparticles. ACS Nano 2018, 12, 6794–6805. [Google Scholar] [CrossRef]
- Liang, Z.; Li, X.; Chen, X.; Zhou, J.; Li, Y.; Peng, J.; Lin, Z.; Liu, G.; Zeng, X.; Li, C.; et al. Fe/MOF based platform for NIR laser induced efficient PDT/PTT of cancer. Front. Bioeng. Biotechnol. 2023, 11, 1156079. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, D.; Ren, X.; Ren, J.; Meng, X.; Fu, C.; Li, X. Shikonin loaded hollow Fe-MOF nanoparticles for enhanced microwave thermal therapy. ACS Biomater. Sci. Eng. 2023, 9, 5405–5417. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Shi, J.; Wang, L. A multimodal strategy of Fe3O4@ZIF-8/GOx@MnO2 hybrid nanozyme via TME modulation for tumor therapy. Nanoscale 2021, 13, 16571–16588. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tan, W.; Chen, M.; Chen, S.; Tang, K.; Liao, H.; Niu, C. MnO2 coated multi-layer nanoplatform for enhanced sonodynamic therapy and MR imaging of breast cancer. Front. Bioeng. Biotechnol. 2022, 10, 955127. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Yuan, Y.; Zhang, Q.; Chang, F.; Yang, L.; Chen, Z.; Bai, Z. Morphology controlled synthesis of metal-organic frameworks derived lattice plane altered iron oxide for efficient trifunctional electrocatalysts. Nano Energy 2021, 82, 10569. [Google Scholar] [CrossRef]
- Deng, H.; Zhang, J.; Yang, Y.; Yang, J.; Wei, Y.; Ma, S.; Shen, Q. Chemodynamic and photothermal combination therapy based on dual modified metal organic framework for inducing tumor ferroptosis/pyroptosis. ACS Appl. Mater. Interfaces 2022, 14, 24089–24101. [Google Scholar] [CrossRef]
- Wang, J.; Tang, Y.; Yang, Q.; Zhao, K.; Li, X.; Wang, Y.; Zhu, Y.; Zhu, Q.; Yu, F.; Wang, C. Enhanced photocatalytic nitrogen fixation via Pt-induced active hydrogen supply over Pt@NM-101(Fe). Appl. Catal. B Environ. Energy 2025, 373, 125364. [Google Scholar] [CrossRef]
- Gao, X.; Cui, R.; Ji, G.; Liu, Z. Size and surface controllable metal organic frameworks (MOFs) for fluorescence imaging and cancer therapy. Nanoscale 2018, 10, 6205–6211. [Google Scholar] [CrossRef]
- Li, S.; Tan, L.; Meng, X. Nanoscale metal organic frameworks: Synthesis, biocompatibility, imaging applications, and thermal and dynamic therapy of tumors. Adv. Funct. Mater. 2020, 30, 1908924. [Google Scholar] [CrossRef]
- Xu, X.; Chen, Y.; Zhang, Y.; Yao, Y.; Ji, P. Highly stable and biocompatible hyaluronic acid-rehabilitated nanoscale MOF-Fe2+ induced ferroptosis in breast cancer cells. J. Mater. Chem. B 2020, 8, 9129–9138. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Li, X.; Meng, S.; Shi, G.; Li, H.; Du, H.; Dai, L.; Yang, H. Cascade amplification of tumor chemodynamic therapy and starvation with Re-educated TAMs via Fe-MOF based functional nanosystem. J. Nanobiotechnol. 2023, 21, 127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-X.; Hao, Y.-N.; Gao, Y.-R.; Shu, Y.; Wang, J.-H. Mutual benefit between Cu(II) and polydopamine for improving photothermal chemodynamic therapy. ACS Appl. Mater. Interfaces 2021, 13, 38127–38137. [Google Scholar] [CrossRef]
- Feng, K.; Xu, Z.; Wang, Y.; Wu, X.; Xiong, F.; Ruan, Y.; Wu, X.; Ye, L.; Su, D.; Yu, J.; et al. Renal clearable porous hollow copper iron oxide nanoparticles for trimodal chemodynamic photothermal chemo anti-tumor therapy. Nanoscale 2023, 15, 3188–3198. [Google Scholar] [CrossRef]
- Fu, Z.; Zhang, Y.; Chen, X.; Wang, N.; Ma, R.; Luo, X.; Pan, X.; Yang, Y.; Xue, W. A versatile nanoplatform based on metal phenolic networks inhibiting ttumor growth and metastasis by combined starvation/chemodynamic/immunotherapy. Adv. Funct. Mater. 2023, 33, 2211869. [Google Scholar] [CrossRef]
- Gou, K.; Xin, W.; Lv, J.; Ma, Z.; Yang, J.; Zhao, L.; Cheng, Y.; Chen, X.; Zeng, R.; Li, H. A pH-responsive chiral mesoporous silica nanoparticles for delivery of doxorubicin in tumor targeted therapy. Colloids Surf. B Biointerfaces 2023, 221, 113027. [Google Scholar] [CrossRef] [PubMed]
- Zhen, W.; Liu, Y.; Wang, W.; Zhang, M.; Hu, W.; Jia, X.; Wang, C.; Jiang, X. Specific “unlocking” of a nanozyme-based butterfly effect to break the evolutionary fitness of chaotic tumors. Angew. Chem. Int. Ed. 2020, 59, 9491–9497. [Google Scholar] [CrossRef]
- Liang, B.; Zuo, D.; Yu, K.; Cai, X.; Qiao, B.; Deng, R.; Yang, J.; Chu, L.; Deng, Z.; Zheng, Y.; et al. Multifunctional bone cement for synergistic magnetic hyperthermia ablation and chemotherapy of osteosarcoma. Mater. Sci. Eng. C 2020, 108, 110460. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, B.; Sun, Q.; Dong, S.; Kuang, Y.; Dong, Y.; He, F.; Gai, S.; Yang, P. Fusiform like copper(II) based metal organic framework through relief hypoxia and GSH depletion Co-enhanced starvation and chemodynamic synergetic cancer therapy. ACS Appl. Mater. Interfaces 2020, 12, 17254–17267. [Google Scholar] [CrossRef]
- Li, J.; Hu, Z.-E.; We, Y.-J.; Liu, Y.-H.; Wang, N.; Yu, X.-Q. Multifunctional carbon quantum dots as a theranostic nanomedicine for fluorescence imaging guided glutathione depletion to improve chemodynamic therapy. J. Colloid Interface Sci. 2022, 606, 1219–1228. [Google Scholar] [CrossRef]
- Wei, X.; Chen, J.; Ali, M.C.; Munyemana, J.C.; Qiu, H. Cadmium cobaltite nanosheets synthesized in basic deep eutectic solvents with oxidase like, peroxidase-like, and catalase-like activities and application in the colorimetric assay of glucose. Microchim. Acta 2020, 187, 314. [Google Scholar] [CrossRef]
- Li, S.; Shang, L.; Xu, B.; Wang, S.; Gu, K.; Wu, Q.; Sun, Y.; Zhang, Q.; Yang, H.; Zhang, F.; et al. A nanozyme with photo-enhanced dual enzyme like activities for deep pancreatic cancer therapy. Angew. Chem. Int. Ed. 2019, 58, 12624–12631. [Google Scholar] [CrossRef]
- Gong, F.; Yang, N.; Wang, Y.; Zhuo, M.; Zhao, Q.; Wang, S.; Li, Y.; Liu, Z.; Chen, Q.; Cheng, L. Oxygen deficient bimetallic oxide FeWOX nanosheets as peroxidase like nanozyme for sensing cancer via photoacoustic imaging. Small 2020, 16, 2003496. [Google Scholar] [CrossRef]
- Wang, Q.; Li, C.; Wang, X.; Pu, J.; Zhang, S.; Liang, L.; Chen, L.; Liu, R.; Zuo, W.; Zhang, H.; et al. eg Occupancy as a predictive descriptor for spinel oxide nanozymes. Nano Lett. 2022, 22, 10003–10009. [Google Scholar] [CrossRef]
- Li, C.; Hua, C.; Chu, C.; Jiang, M.; Zhang, Q.; Zhang, Y.; Wu, L.; Liu, J.; Yang, H.; Yu, X.-F.; et al. A photothermal responsive multi-enzyme nanoprobe for ROS amplification and glutathione depletion to enhance ferroptosis. Biosens. Bioelectron. 2025, 278, 117384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ning, R.; Wang, W.; Zhou, Y.; Chen, Y. Synthesis of Fe3O4/PDA nanocomposites for osteosarcoma magnetic resonance imaging and photothermal therapy. Front. Bioeng. Biotechnol. 2022, 10, 844540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Fu, J.; Hu, J.; You, Q.; Yao, X.; Hua, D.; Yin, J.; Mao, Y. Evoking and enhancing ferroptosis of cancer stem cells by a liver targeted and metal organic framework based drug delivery system inhibits the growth and lung metastasis of hepatocellular carcinoma. Chem. Eng. J. 2023, 454, 140044. [Google Scholar] [CrossRef]
- Zhao, Q.; Gong, Z.; Li, Z.; Wang, J.; Zhang, J.; Zhao, Z.; Zhang, P.; Zheng, S.; Miron, R.J.; Yuan, Q.; et al. Target reprogramming lysosomes of CD8+ T cells by a mineralized metal organic framework for cancer immunotherapy. Adv. Mater. 2021, 33, 2100616. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, Y.; Wang, J.; Zhou, X.; Kong, X.; Meng, J.; Zhang, X. Dual responsive triple synergistic Fe-MOF for tumor theranostics. ACS Nano 2023, 17, 9003–9013. [Google Scholar] [CrossRef]
- Testa, F.; Pasqua, L.; Crea, F.; Aiello, R.; Lázár, K.; Fejes, P.; Lentz, P.; Nagy, J.B. Synthesis of Fe-MFI zeolites in fluoride containing media. Microporous Mesoporous Mater. 2003, 57, 57–72. [Google Scholar] [CrossRef]
- Cui, R.; Zhao, P.; Yan, Y.; Bao, G.; Damirin, A.; Liu, Z. Outstanding drug loading/release capacity of hollow Fe-metal organic framework based microcapsules: A potential multifunctional drug delivery platform. Inorg. Chem. 2021, 60, 1664–1671. [Google Scholar] [CrossRef]
- Yaghoubian, A.; Setoodehkhah, M.; Parsa, F. Investigation of Pantoprazole Loading and Release from a Magnetic-Coated Chitosan-Modified Zirconium-Based Metal–Organic Framework (MOF) as a Nanocarrier in Targeted Drug Delivery Systems. RSC Adv. 2024, 14, 26091–26102. [Google Scholar] [CrossRef] [PubMed]
- Rananaware, P.; Pandit, P.; Brahmkhatri, V. Gold Nanoparticle Encapsulated Hybrid MOF: Synthesis, Characterization, and Co-Drug Delivery of 5-Fluorouracil and Curcumin. Discov. Nano 2024, 19, 201. [Google Scholar] [CrossRef] [PubMed]
- Moaness, M.; Mabrouk, M.; Ahmed, M.M.; Das, D.B.; Beherei, H.H. Novel Zinc-Silver Nanocages for Drug Delivery and Wound Healing: Preparation, Characterization and Antimicrobial Activities. Int. J. Pharm. 2022, 616, 121559. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Cai, X.; Ding, P.; Lv, H.; Pei, R. Metal–Organic Frameworks with Enhanced Photodynamic Therapy: Synthesis, Erythrocyte Membrane Camouflage, and Aptamer-Targeted Aggregation. ACS Appl. Mater. Interfaces 2020, 12, 23697–23706. [Google Scholar]
- Wang, S.; Liu, X.; Chen, S.; Liu, Z.; Zhang, X.; Liang, X.-J.; Li, L. Regulation of Ca2+ Signaling for Drug-Resistant Breast Cancer Therapy with Mesoporous Silica Nanocapsule Encapsulated Doxorubicin/SiRNA Cocktail. ACS Nano 2019, 13, 274–283. [Google Scholar] [CrossRef]
- Djahaniani, H.; Ghavidel, N.; Kazemian, H. Green and Facile Synthesis of Lignin/HKUST-1 as a Novel Hybrid Biopolymer Metal-Organic-Framework for a PH-Controlled Drug Release System. Int. J. Biol. Macromol. 2023, 242, 124627. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Xu, C.; Wang, L.; Qi, H.; Cheng, R.; Bao, L.; Guo, H.; Wu, S. Concave-Octahedral Fe2+-Rich Fe-MOF/FU Nano-Blocks with Enhanced pH-Responsive Nanozyme Activity Toward Stimuli-Responsive Gels for Chemo-Chemodynamic Synergistic Therapy. Gels 2025, 11, 750. https://doi.org/10.3390/gels11090750
Wang D, Xu C, Wang L, Qi H, Cheng R, Bao L, Guo H, Wu S. Concave-Octahedral Fe2+-Rich Fe-MOF/FU Nano-Blocks with Enhanced pH-Responsive Nanozyme Activity Toward Stimuli-Responsive Gels for Chemo-Chemodynamic Synergistic Therapy. Gels. 2025; 11(9):750. https://doi.org/10.3390/gels11090750
Chicago/Turabian StyleWang, Desheng, Changjin Xu, Laibing Wang, Herima Qi, Riqing Cheng, Liang Bao, Huiqing Guo, and Shikui Wu. 2025. "Concave-Octahedral Fe2+-Rich Fe-MOF/FU Nano-Blocks with Enhanced pH-Responsive Nanozyme Activity Toward Stimuli-Responsive Gels for Chemo-Chemodynamic Synergistic Therapy" Gels 11, no. 9: 750. https://doi.org/10.3390/gels11090750
APA StyleWang, D., Xu, C., Wang, L., Qi, H., Cheng, R., Bao, L., Guo, H., & Wu, S. (2025). Concave-Octahedral Fe2+-Rich Fe-MOF/FU Nano-Blocks with Enhanced pH-Responsive Nanozyme Activity Toward Stimuli-Responsive Gels for Chemo-Chemodynamic Synergistic Therapy. Gels, 11(9), 750. https://doi.org/10.3390/gels11090750







