Cinnamon-Mediated Silver Nanoparticles and Beta-Carotene Nanocarriers in Alginate Dressings for Wound Healing Applications
Abstract
1. Introduction
2. Results and Discussion
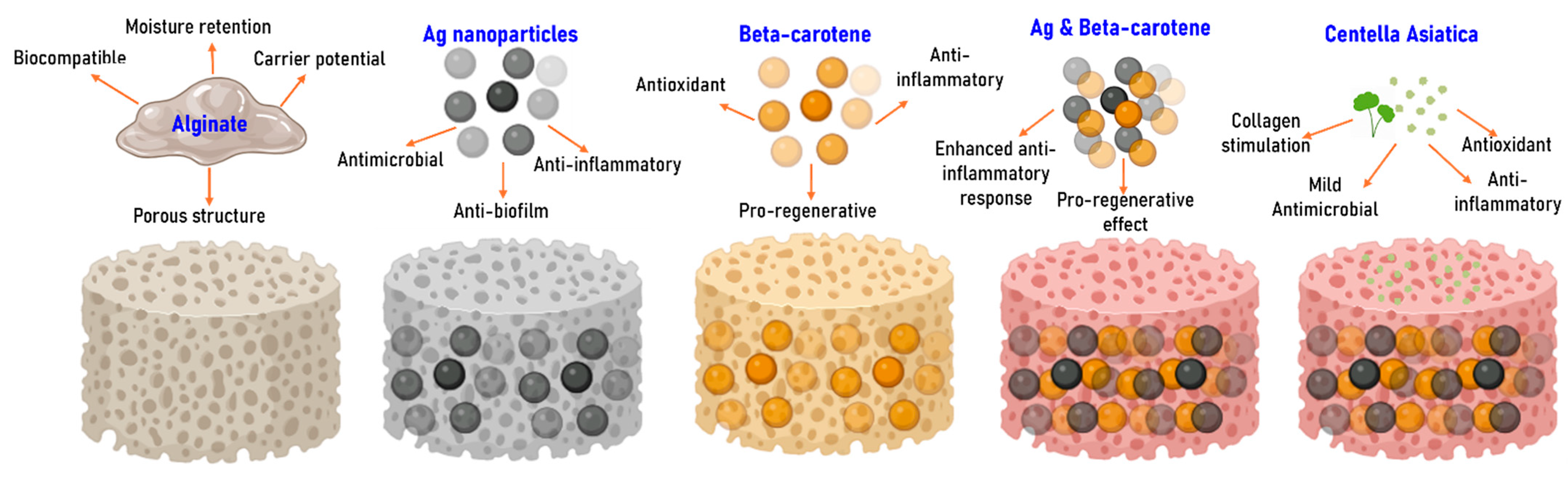
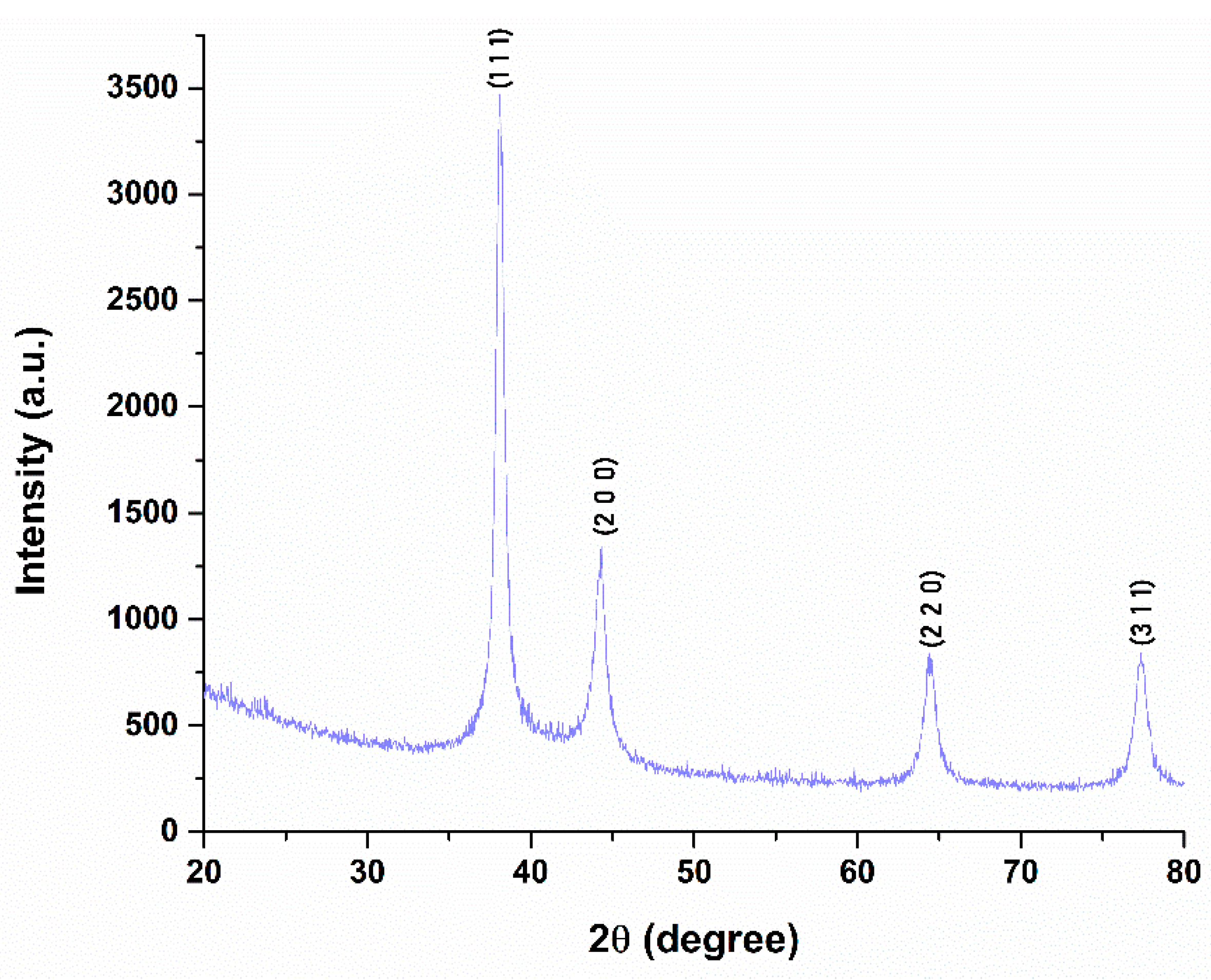
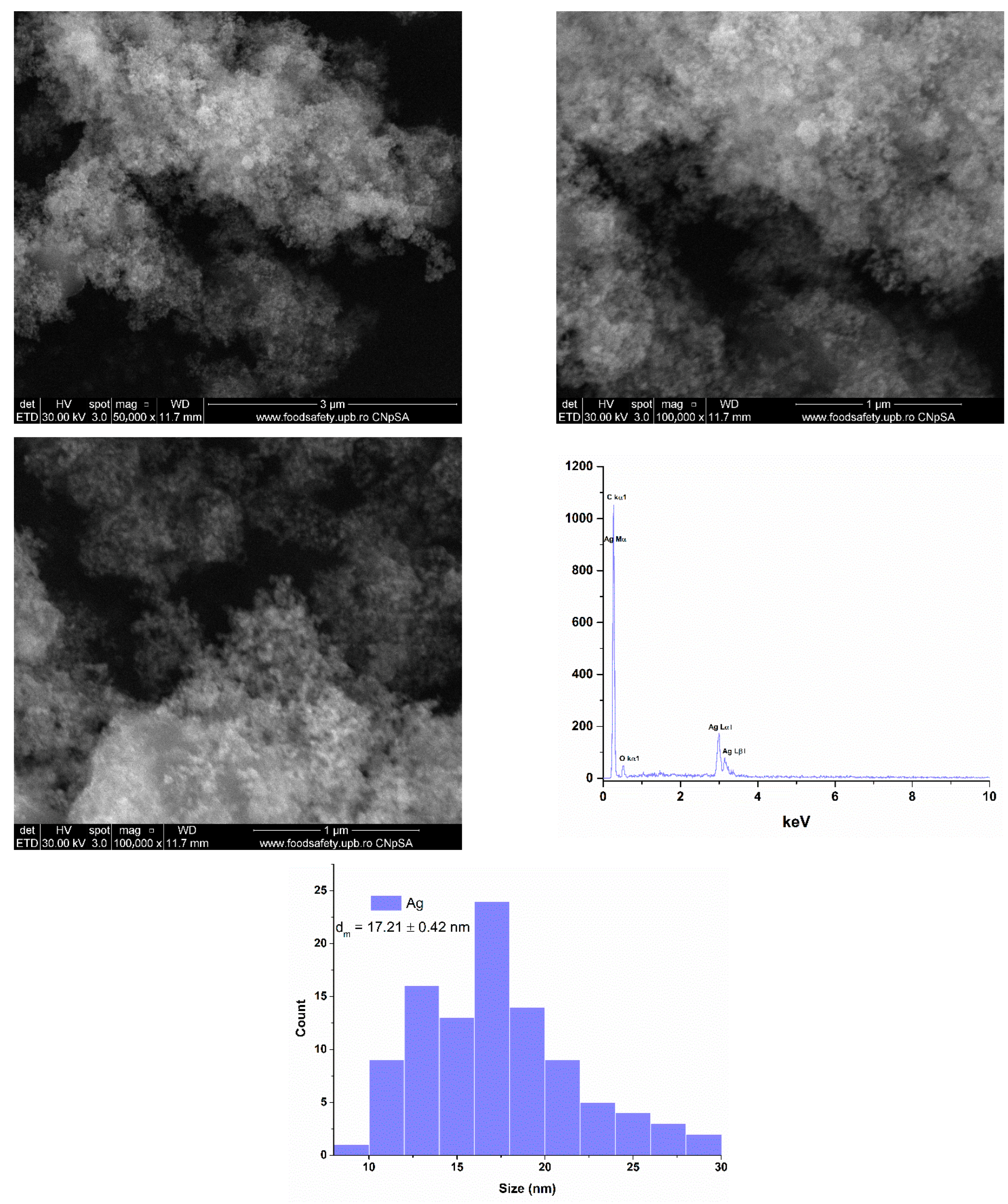
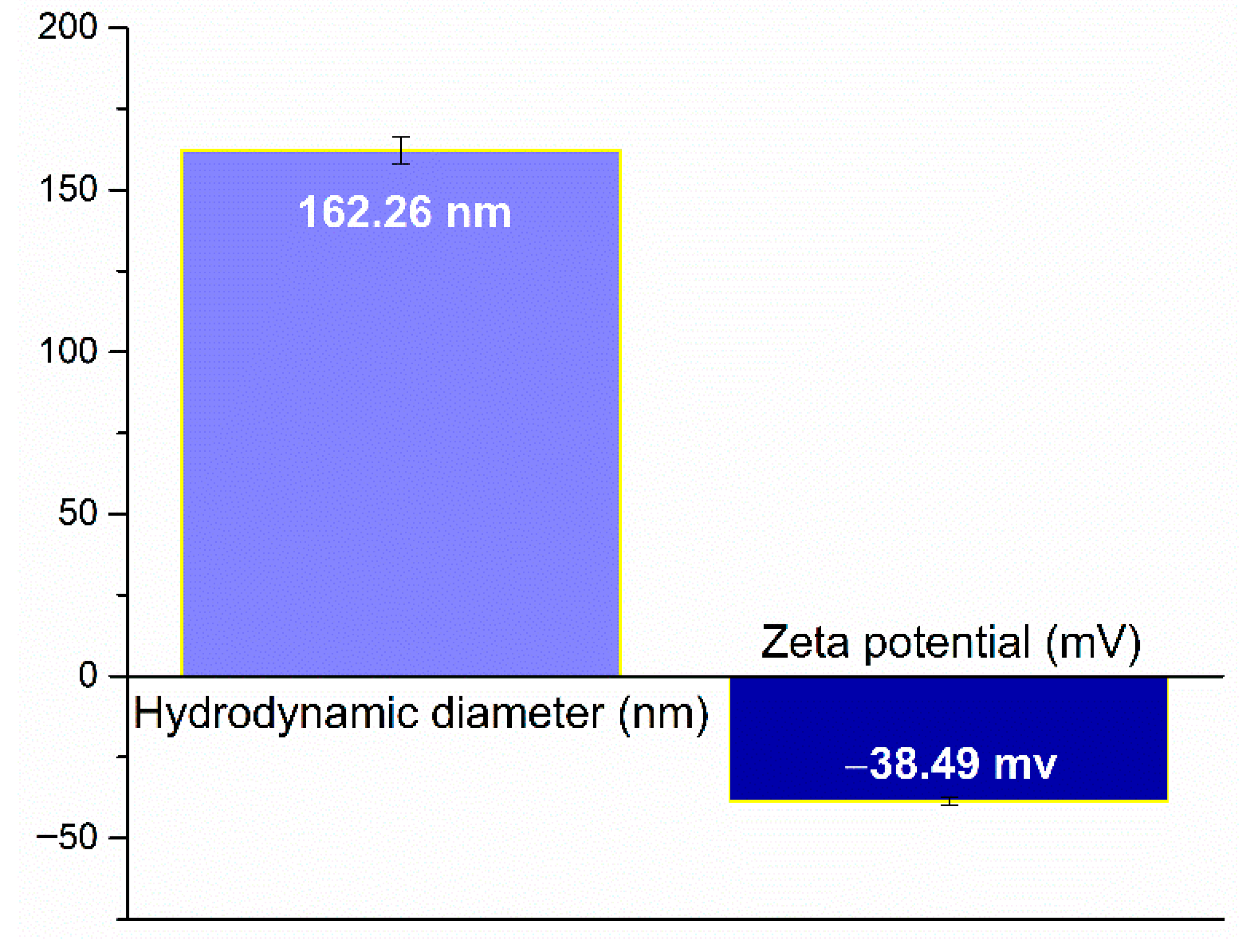
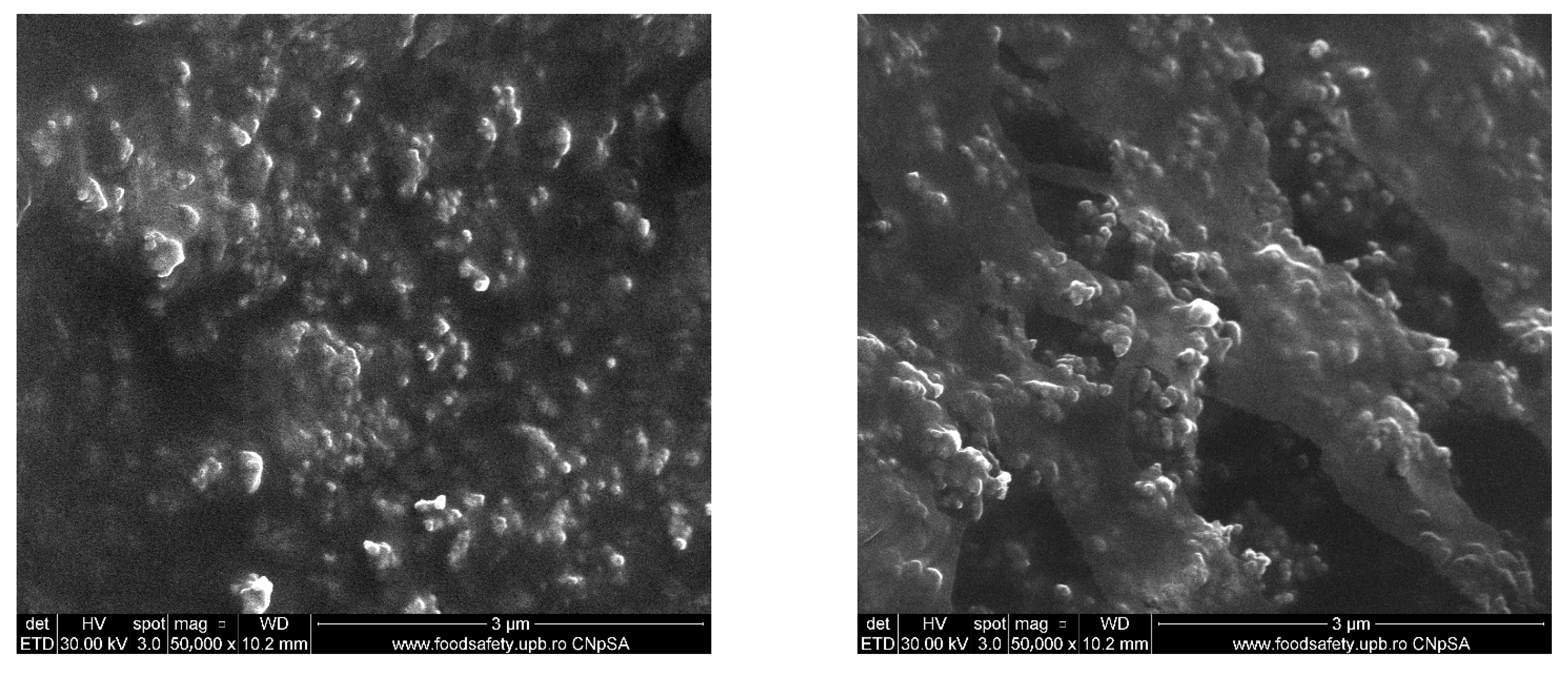
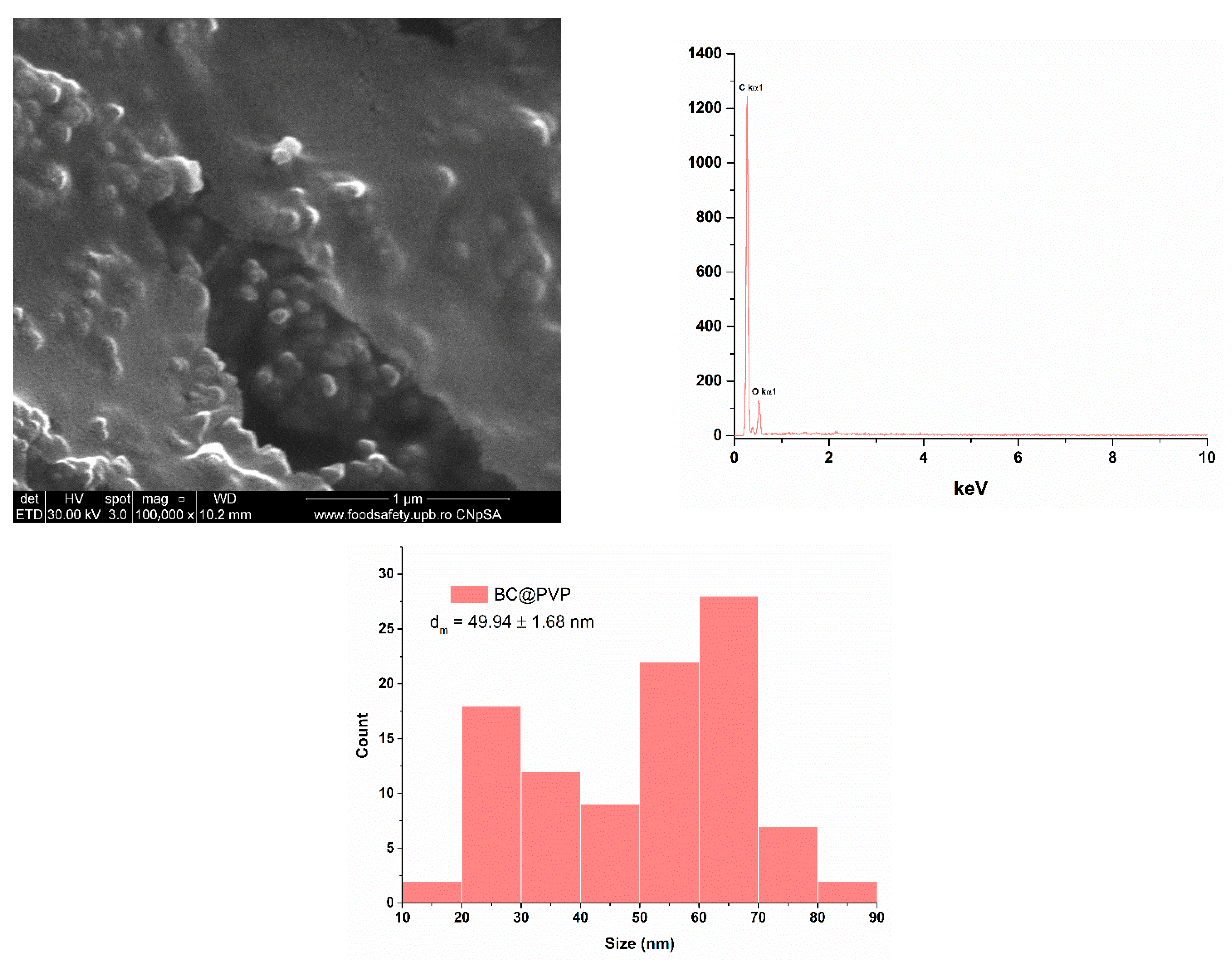
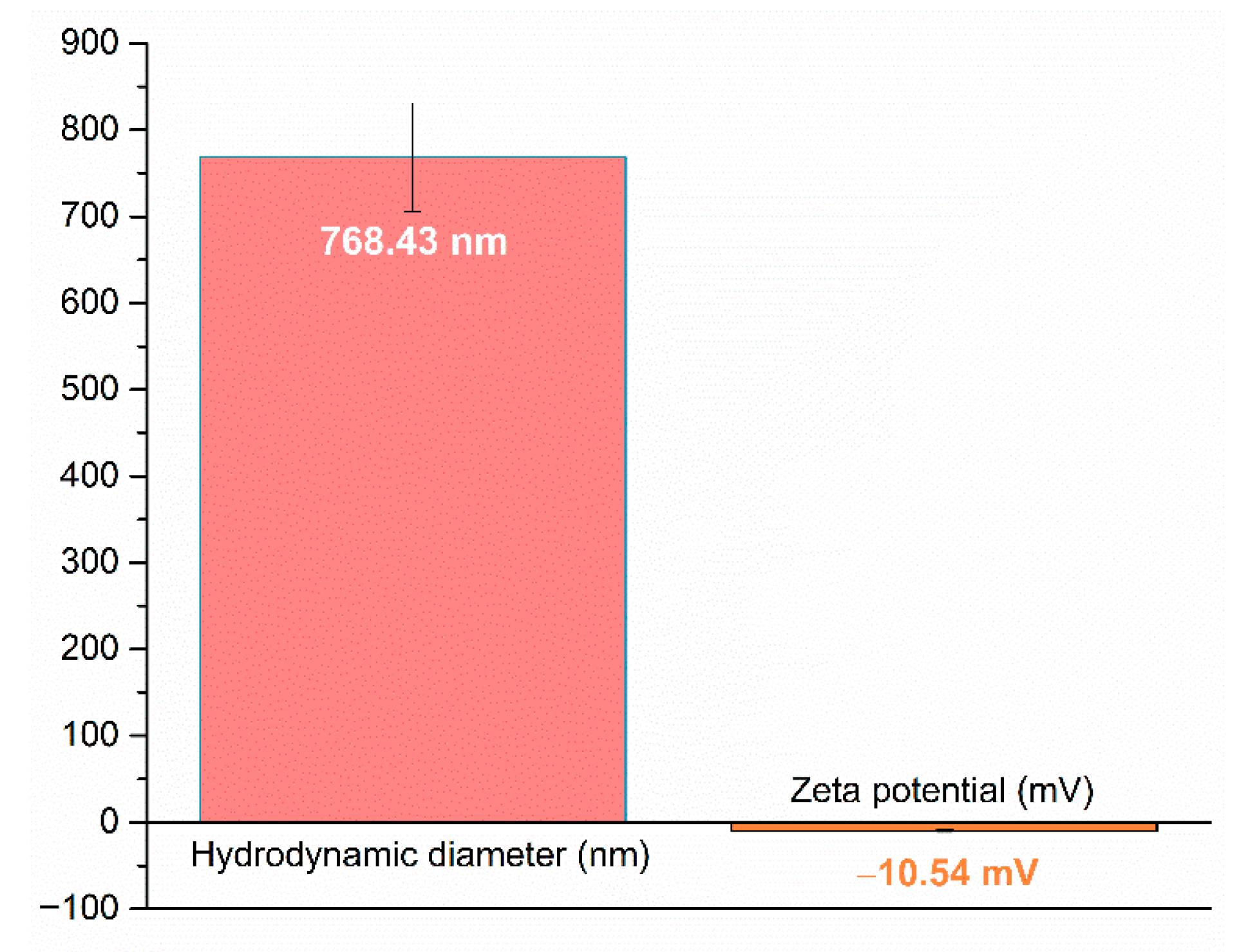
2.1. Silver (Ag) and Polymeric Beta-Carotene (BC@PVP) Nanoparticles Characterization
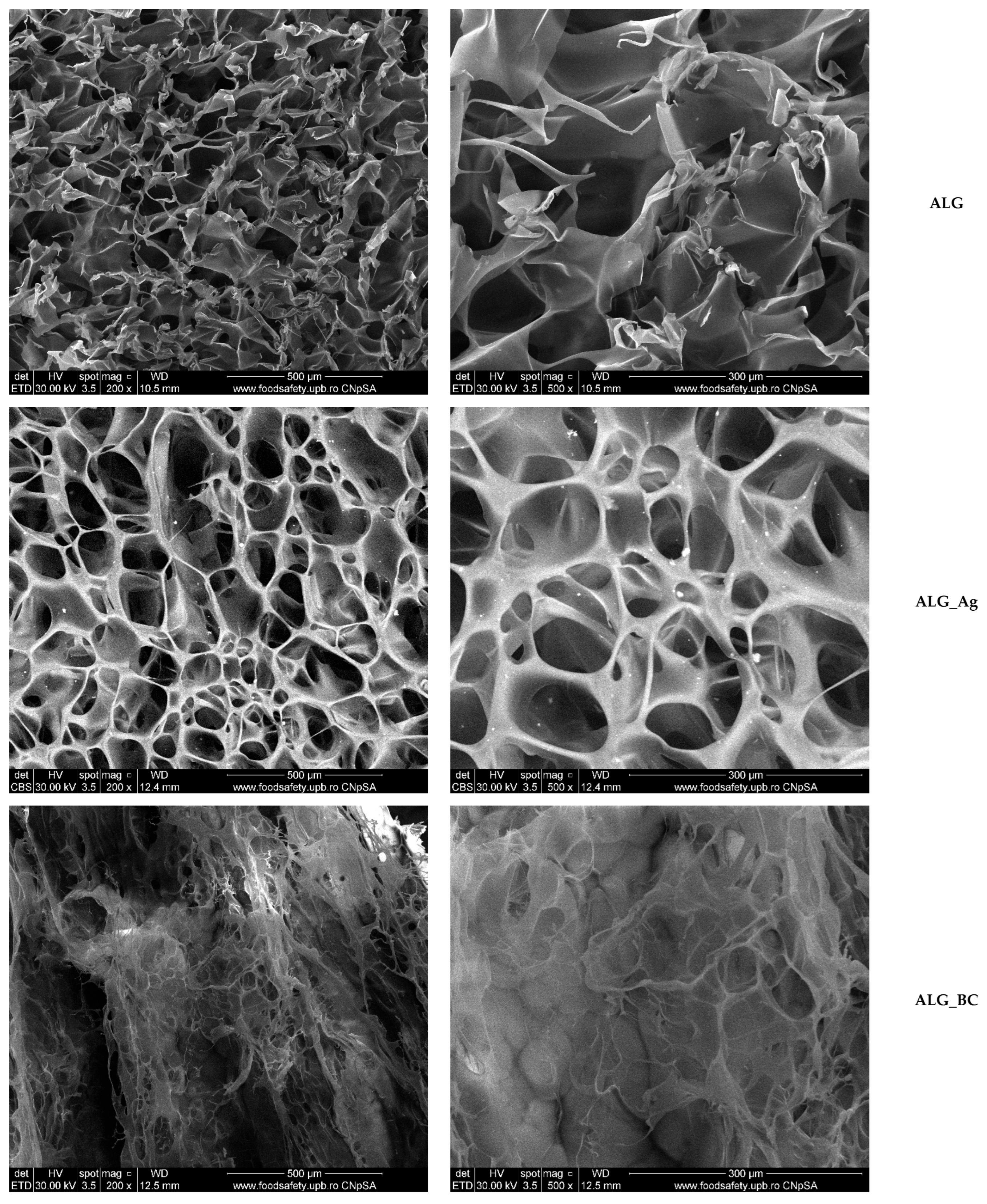
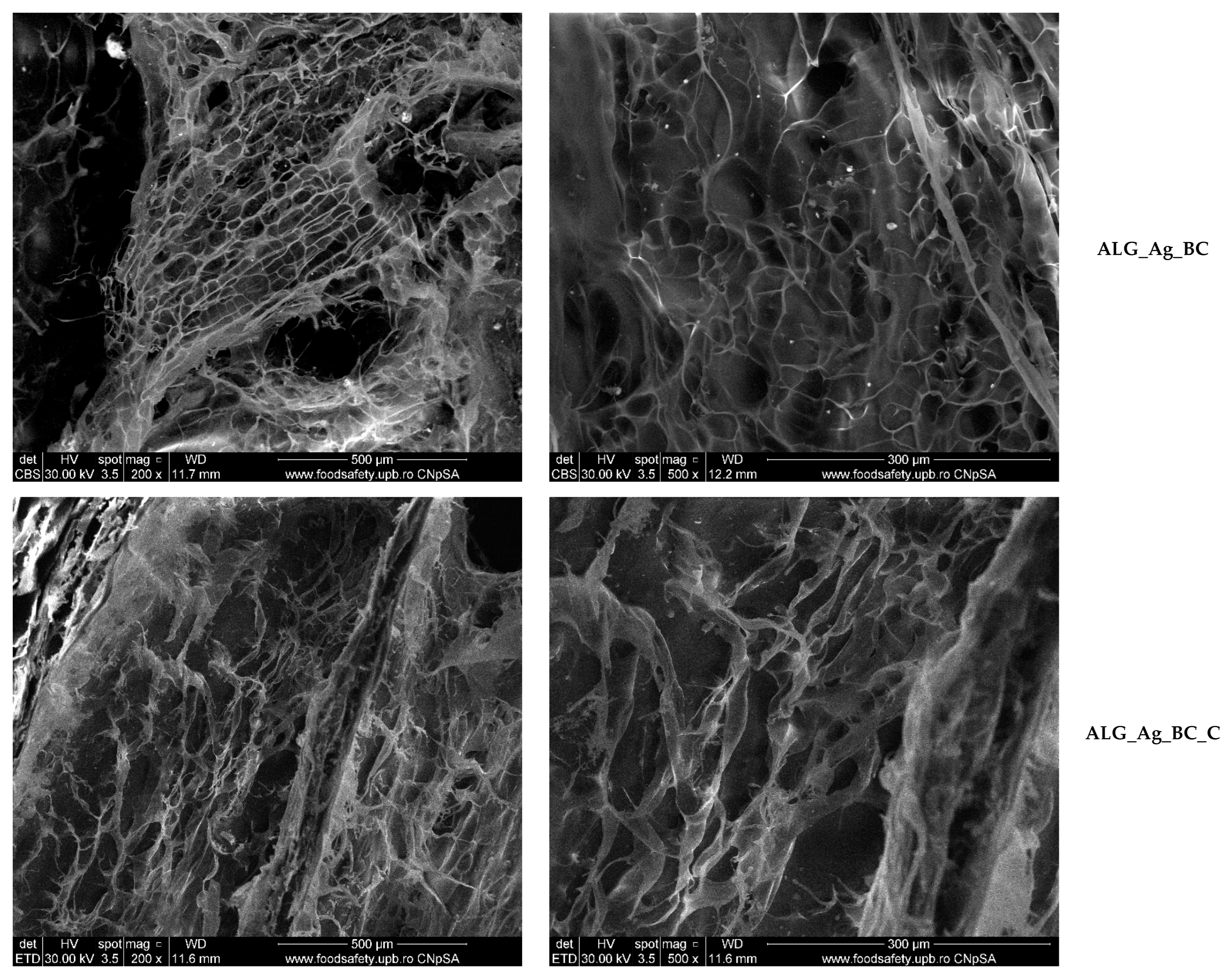
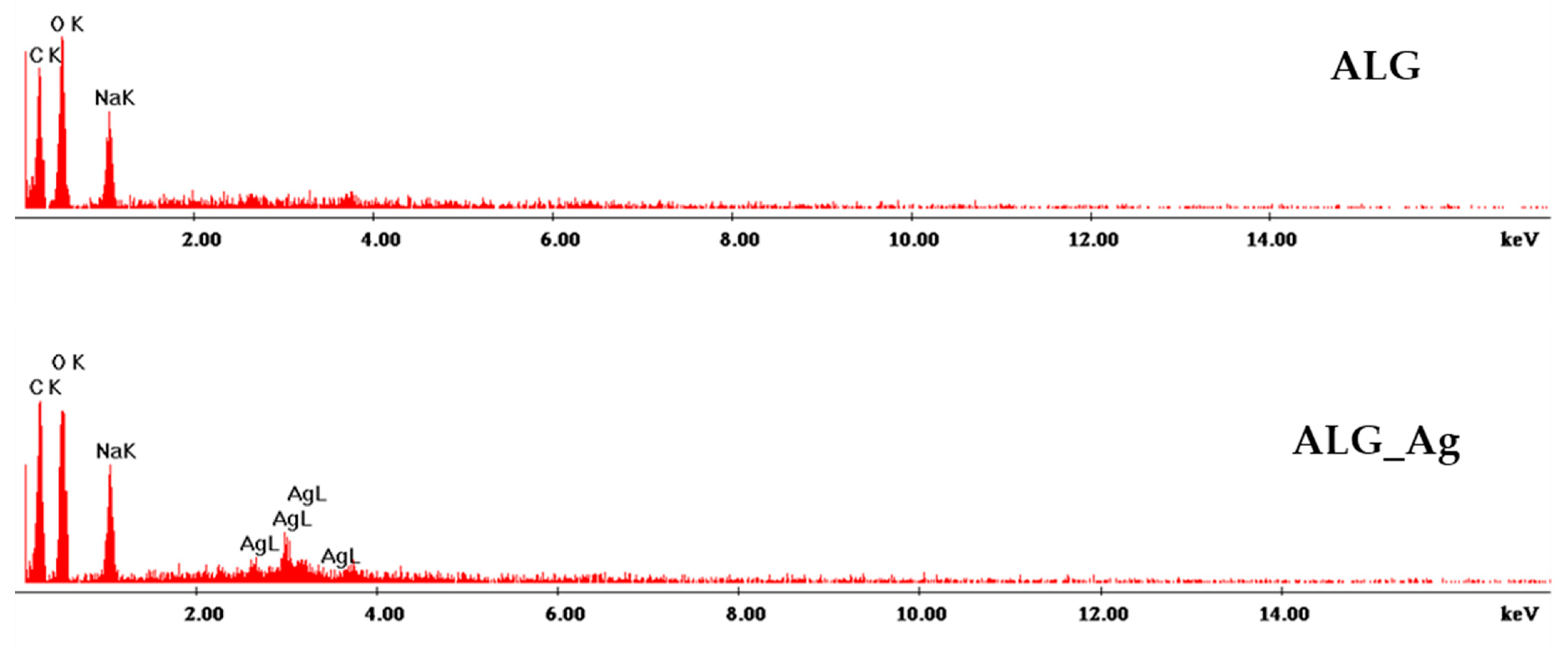
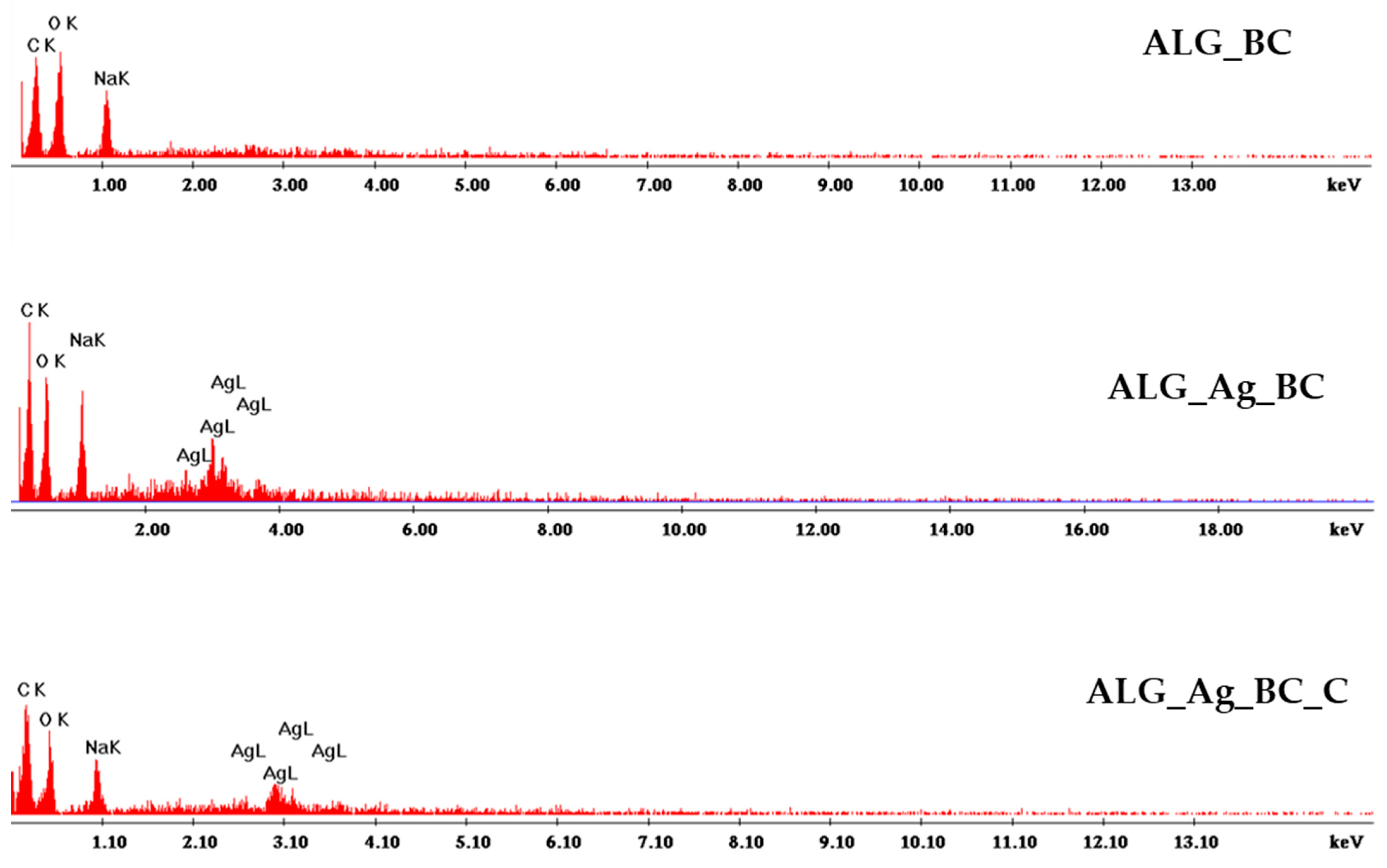
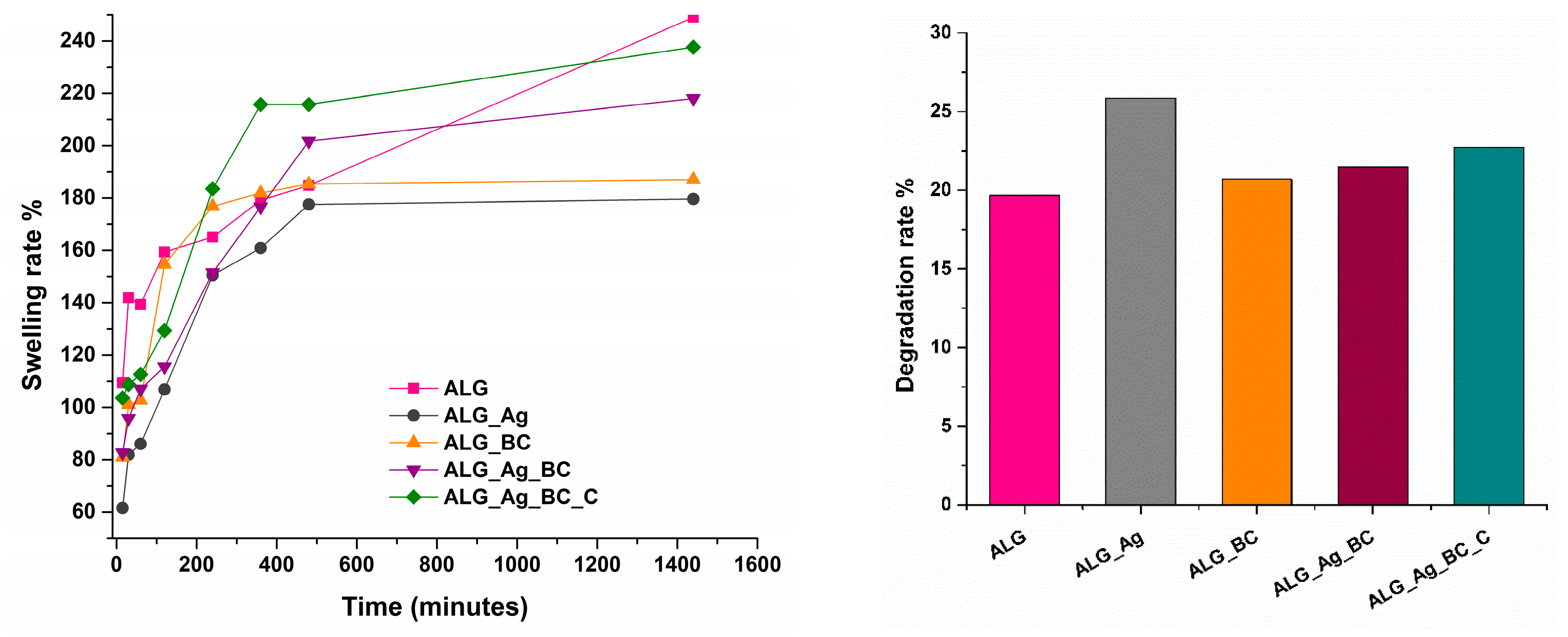
2.2. Alginate-Based Wound Dressings Characterization
2.3. Discussion
3. Conclusions
4. Materials and Methods
4.1. Synthesis of Silver Nanoparticles (Ag)
4.2. Synthesis of Beta-Carotene-Loaded Polymeric Nanoparticles (BC@PVP)
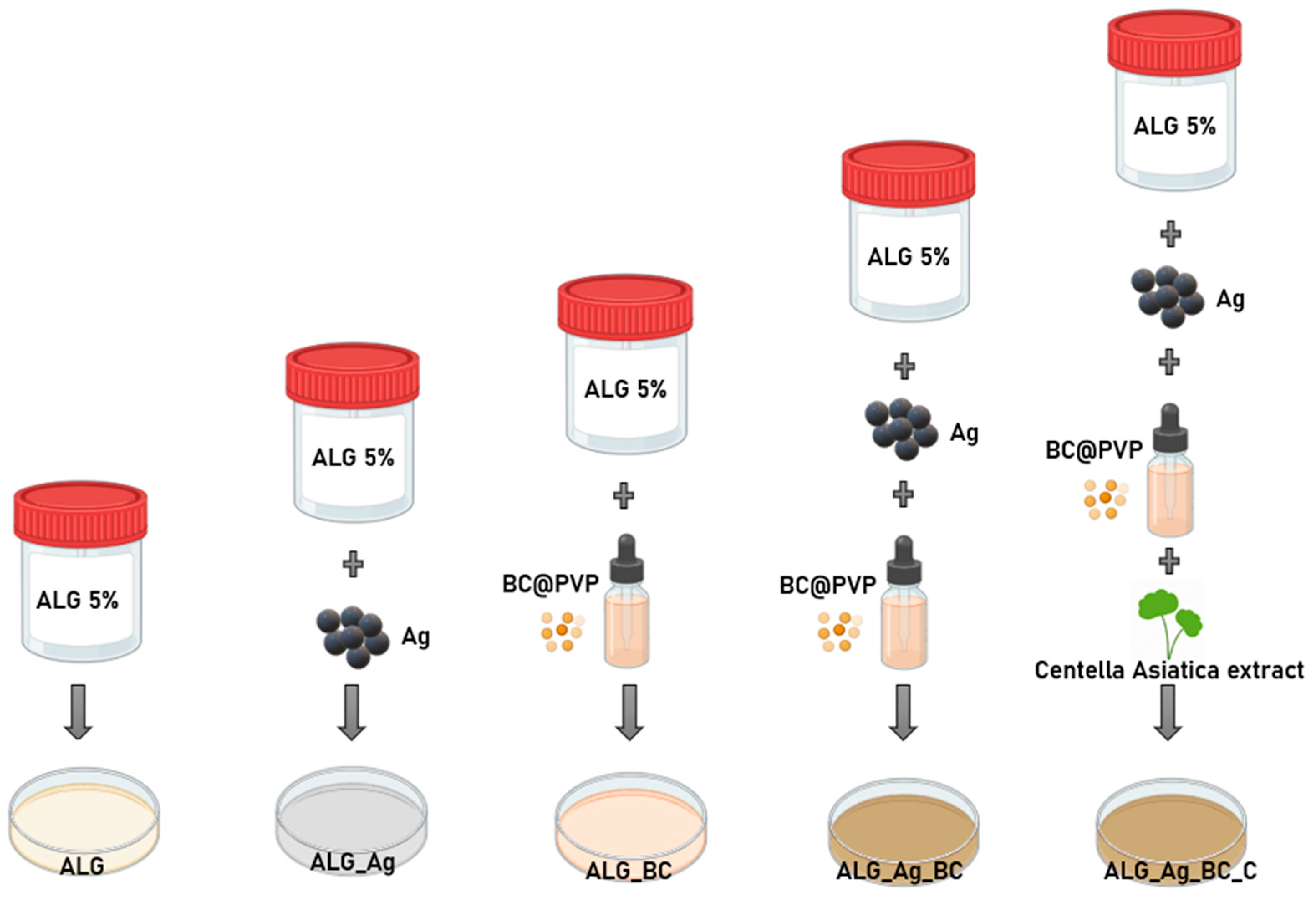
4.3. Formulation of Alginate-Based Hydrogels (ALG, ALG_Ag, ALG_BC, ALG_Ag_BC, ALG_Ag_BC_C)
4.4. Investigation Methods
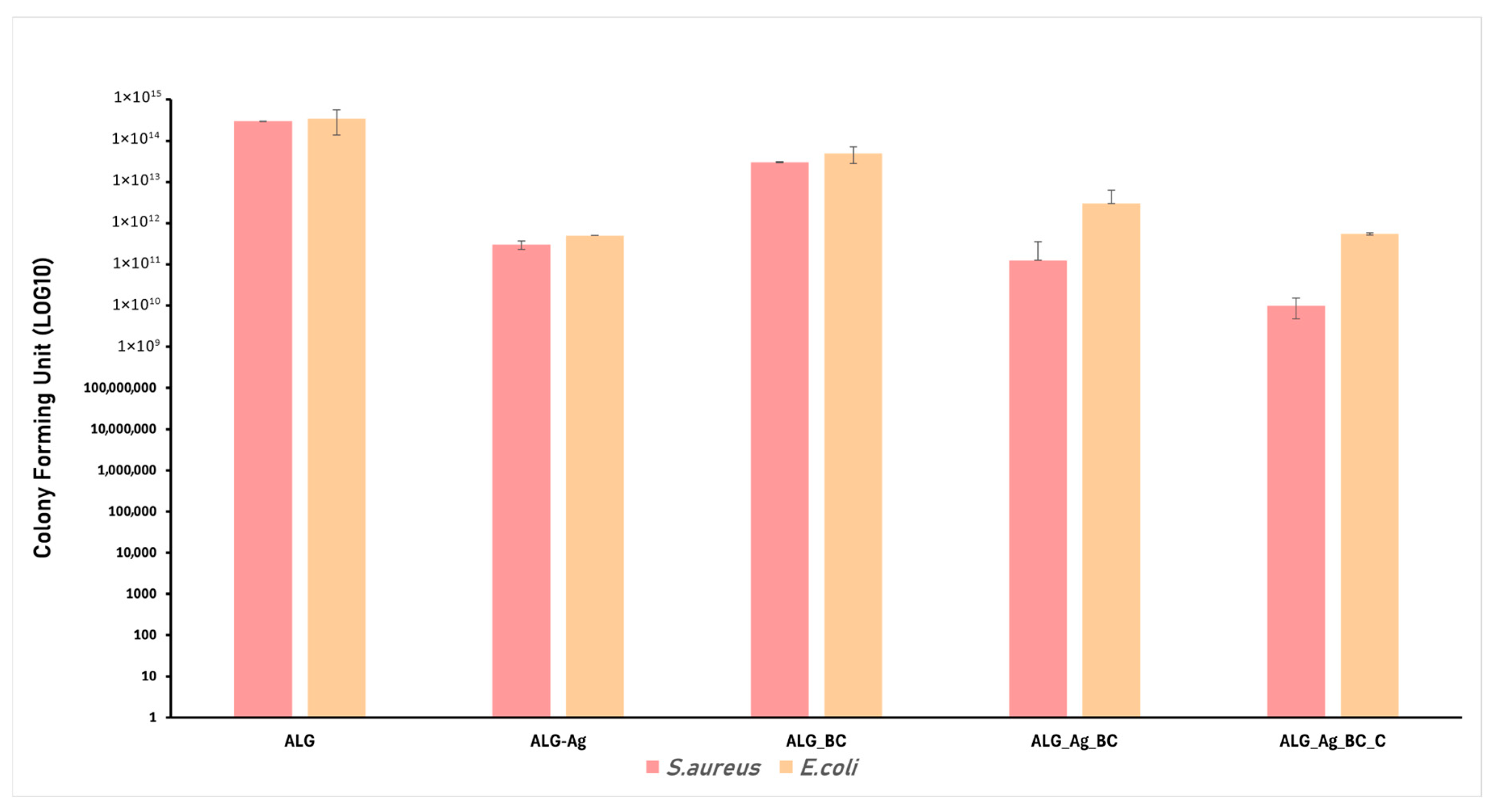
4.5. Antimicrobial Evaluation
4.5.1. Bacterial Strains
4.5.2. Adapted Disc Diffusion Protocol
4.5.3. Monospecific Biofilm Development
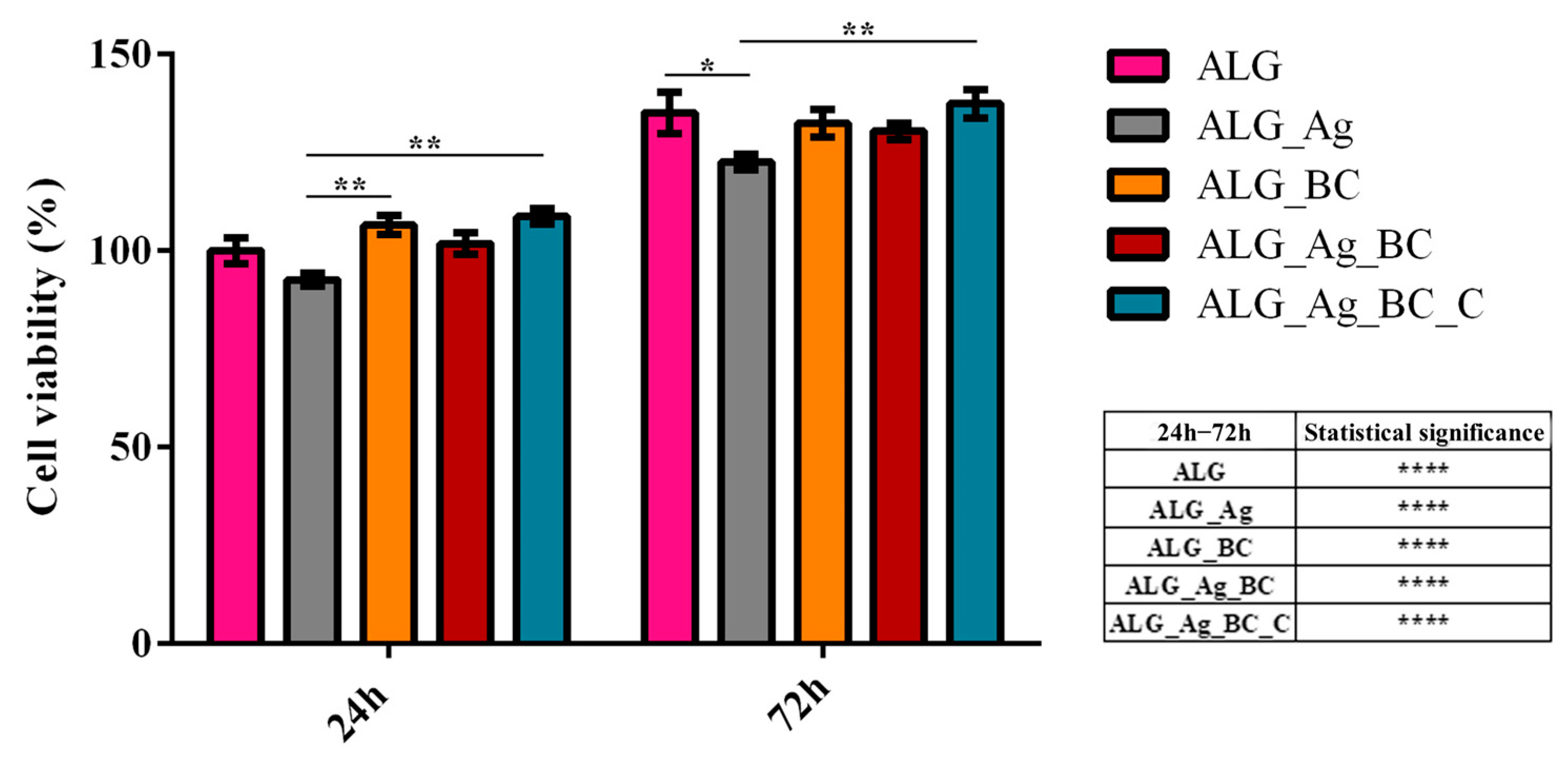
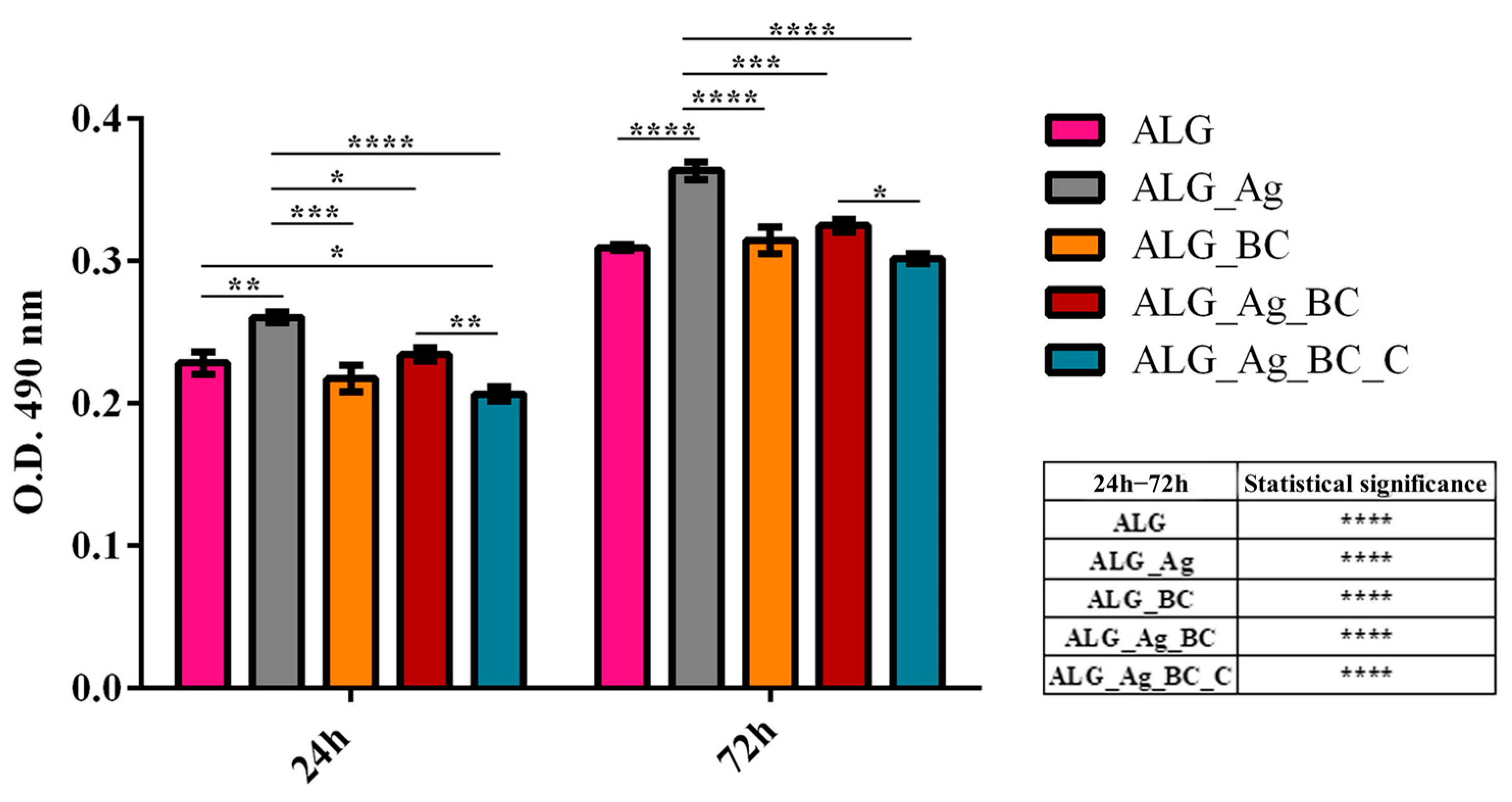
4.6. In Vitro Biological Evaluation
4.6.1. Cell Culture and Experimental Design
4.6.2. Biocompatibility Assessment
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Nouvong, A.; Ambrus, A.M.; Zhang, E.R.; Hultman, L.; Coller, H.A. Reactive oxygen species and bacterial biofilms in diabetic wound healing. Physiol. Genom. 2016, 48, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Khorsandi, K.; Hosseinzadeh, R.; Esfahani, H.; Zandsalimi, K.; Shahidi, F.K.; Abrahamse, H. Accelerating skin regeneration and wound healing by controlled ROS from photodynamic treatment. Inflamm. Regen. 2022, 42, 40. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, H.; Aramideh Khouy, R.; Nosrati, A.; Khodaei, M.; Banitalebi-Dehkordi, M.; Ashrafi-Dehkordi, K.; Sanami, S.; Alizadeh, Z. Nanocomposite scaffolds for accelerating chronic wound healing by enhancing angiogenesis. J. Nanobiotechnol. 2021, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Liu, W.; Hu, C.; Yang, L.; Wang, Y. Construction of multifunctional wound dressings with their application in chronic wound treatment. Biomater. Sci. 2022, 10, 4058–4076. [Google Scholar] [CrossRef]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of Acute and Chronic Wound Healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef]
- Tan, S.; Dosan, R. Lessons From Epithelialization: The Reason Behind Moist Wound Environment. Open Dermatol. J. 2019, 13, 34–40. [Google Scholar] [CrossRef]
- Zarei, F.; Soleimaninejad, M. Role of growth factors and biomaterials in wound healing. Artif. Cells Nanomed. Biotechnol. 2018, 46, 906–911. [Google Scholar] [CrossRef]
- Yamakawa, S.; Hayashida, K. Advances in surgical applications of growth factors for wound healing. Burns Trauma 2019, 7, 10. [Google Scholar] [CrossRef]
- Bazaliński, D.; Przybek-Mita, J.; Pytlak, K.; Kardyś, D.; Bazaliński, A.; Kucharzewski, M.; Więch, P. Larval Wound Therapy: Possibilities and Potential Limitations—A Literature Review. J. Clin. Med. 2023, 12, 6862. [Google Scholar] [CrossRef]
- Gianino, E.; Miller, C.; Gilmore, J. Smart Wound Dressings for Diabetic Chronic Wounds. Bioengineering 2018, 5, 51. [Google Scholar] [CrossRef]
- Cavallo, I.; Sivori, F.; Mastrofrancesco, A.; Abril, E.; Pontone, M.; Di Domenico, E.G.; Pimpinelli, F. Bacterial Biofilm in Chronic Wounds and Possible Therapeutic Approaches. Biology 2024, 13, 109. [Google Scholar] [CrossRef]
- Omar, A.; Wright, J.B.; Schultz, G.; Burrell, R.; Nadworny, P. Microbial Biofilms and Chronic Wounds. Microorganisms 2017, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Long, S.; Wang, H.; Wang, Y. Biofilm therapy for chronic wounds. Int. Wound J. 2024, 21, e14667. [Google Scholar] [CrossRef] [PubMed]
- Pirusca, I.A.; Balaure, P.C.; Grumezescu, V.; Irimiciuc, S.A.; Oprea, O.C.; Birca, A.C.; Vasile, B.; Holban, A.M.; Voinea, I.C.; Stan, M.S.; et al. New Fe3O4-Based Coatings with Enhanced Anti-Biofilm Activity for Medical Devices. Antibiotics 2024, 13, 631. [Google Scholar] [CrossRef]
- Falcone, M.; De Angelis, B.; Pea, F.; Scalise, A.; Stefani, S.; Tasinato, R.; Zanetti, O.; Dalla Paola, L. Challenges in the management of chronic wound infections. J. Glob. Antimicrob. Resist. 2021, 26, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Ngoc Le, T.T.; Nguyen, A.T.; Thien Le, H.N.; Pham, T.T. Biomedical materials for wound dressing: Recent advances and applications. RSC Adv. 2023, 13, 5509–5528. [Google Scholar] [CrossRef]
- Orlińska, K.; Komosinska-Vassev, K.; Olczyk, K.; Glaesel, M.; Olczyk, P. Wound healing—Characteristics of the ideal dressing. Ann. Acad. Medicae Silesiensis 2023, 77, 197–203. [Google Scholar] [CrossRef]
- Borda, L.J.; Macquhae, F.E.; Kirsner, R.S. Wound Dressings: A Comprehensive Review. Curr. Dermatol. Rep. 2016, 5, 287–297. [Google Scholar] [CrossRef]
- Fauzian, F.; Garmana, A.N.; Mauludin, R. Applications of Nanotechnology-Based Drug Delivery System for Delivering Natural Products into Acute and Chronic Wounds: A Review. Biointerface Res. Appl. Chem. 2023, 13, 426. [Google Scholar] [CrossRef]
- Al-Mamari, A.; Shahitha, F.; Al-Sibani, M.; Saadi, A.A.; Harrasi, A.A.; Ahmad, A. Novel Antibacterial Wound Healing Hydrogels Based On HEC/SA/HA Using Green Chemistry Approach. Lett. Appl. NanoBioSci. 2023, 12, 69. [Google Scholar] [CrossRef]
- Nazarnezhada, S.; Abbaszadeh-Goudarzi, G.; Samadian, H.; Khaksari, M.; Ghatar, J.M.; Khastar, H.; Rezaei, N.; Mousavi, S.R.; Shirian, S.; Salehi, M. Alginate hydrogel containing hydrogen sulfide as the functional wound dressing material: In vitro and in vivo study. Int. J. Biol. Macromol. 2020, 164, 3323–3331. [Google Scholar] [CrossRef] [PubMed]
- Bakhsheshi-Rad, H.R.; Hadisi, Z.; Ismail, A.F.; Aziz, M.; Akbari, M.; Berto, F.; Chen, X.B. In vitro and in vivo evaluation of chitosan-alginate/gentamicin wound dressing nanofibrous with high antibacterial performance. Polym. Test. 2020, 82, 106298. [Google Scholar] [CrossRef]
- Zheng, Z.; Qi, J.; Hu, L.; Ouyang, D.; Wang, H.; Sun, Q.; Lin, L.; You, L.; Tang, B. A cannabidiol-containing alginate based hydrogel as novel multifunctional wound dressing for promoting wound healing. Biomater. Adv. 2022, 134, 112560. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef]
- Mazurek, Ł.; Kuś, M.; Jurak, J.; Rybka, M.; Kuczeriszka, M.; Stradczuk-Mazurek, M.; Konop, M. Biomedical potential of alginate wound dressings—From preclinical studies to clinical applications: A review. Int. J. Biol. Macromol. 2025, 309, 142908. [Google Scholar] [CrossRef] [PubMed]
- Brumberg, V.; Astrelina, T.; Malivanova, T.; Samoilov, A. Modern Wound Dressings: Hydrogel Dressings. Biomedicines 2021, 9, 1235. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef]
- Su, L.; Jia, Y.; Fu, L.; Guo, K.; Xie, S. The emerging progress on wound dressings and their application in clinic wound management. Heliyon 2023, 9, e22520. [Google Scholar] [CrossRef]
- Aljghami, M.E.; Saboor, S.; Amini-Nik, S. Emerging Innovative Wound Dressings. Ann. Biomed. Eng. 2019, 47, 659–675. [Google Scholar] [CrossRef]
- Eriksson, E.; Liu, P.Y.; Schultz, G.S.; Martins-Green, M.M.; Tanaka, R.; Weir, D.; Gould, L.J.; Armstrong, D.G.; Gibbons, G.W.; Wolcott, R.; et al. Chronic wounds: Treatment consensus. Wound Repair. Regen. 2022, 30, 156–171. [Google Scholar] [CrossRef]
- Ding, X.; Tang, Q.; Xu, Z.; Xu, Y.; Zhang, H.; Zheng, D.; Wang, S.; Tan, Q.; Maitz, J.; Maitz, P.K.; et al. Challenges and innovations in treating chronic and acute wound infections: From basic science to clinical practice. Burns Trauma 2022, 10, tkac014. [Google Scholar] [CrossRef]
- Singh, M.; Thakur, V.; Kumar, V.; Raj, M.; Gupta, S.; Devi, N.; Upadhyay, S.K.; Macho, M.; Banerjee, A.; Ewe, D.; et al. Silver Nanoparticles and Its Mechanistic Insight for Chronic Wound Healing: Review on Recent Progress. Molecules 2022, 27, 5587. [Google Scholar] [CrossRef]
- Majeed, S.; Zainal Abidin, N.B.; Muthukumarasamy, R.; Danish, M.; Mahmad, A.; Mohamad Ibrahim, M.N.; Alanazi, A.M.; Ansari, M.T.; Sisinthy, S.P. Wound healing and antidiabetic properties of green synthesized silver nanoparticles in 3T3-L1 mouse embryo fibroblast cells through 2-NBDG expression. Inorg. Chem. Commun. 2024, 159, 111692. [Google Scholar] [CrossRef]
- Myronov, P.; Sulaieva, O.; Korniienko, V.; Banasiuk, R.; Vielikov, M.; Husak, Y.; Pernakov, M.; Deineka, V.; Yusupova, A.; Hristova, M.T.; et al. Combination of Chlorhexidine and Silver Nanoparticles: An Efficient Wound Infection and Healing Control System. BioNanoScience 2021, 11, 256–268. [Google Scholar] [CrossRef]
- Nicolae, C.L.; Pîrvulescu, D.C.; Antohi, A.M.; Niculescu, A.G.; Grumezescu, A.M.; Croitoru, G.A. Silica nanoparticles in medicine: Overcoming pathologies through advanced drug delivery, diagnostics, and therapeutic strategies. Rom. J. Morphol. Embryol. 2024, 65, 173–184. [Google Scholar] [CrossRef]
- Swidan, N.S.; Hashem, Y.A.; Elkhatib, W.F.; Yassien, M.A. Antibiofilm activity of green synthesized silver nanoparticles against biofilm associated enterococcal urinary pathogens. Sci. Rep. 2022, 12, 3869. [Google Scholar] [CrossRef] [PubMed]
- Loo, C.-Y.; Rohanizadeh, R.; Young, P.M.; Traini, D.; Cavaliere, R.; Whitchurch, C.B.; Lee, W.-H. Combination of Silver Nanoparticles and Curcumin Nanoparticles for Enhanced Anti-biofilm Activities. J. Agric. Food Chem. 2016, 64, 2513–2522. [Google Scholar] [CrossRef] [PubMed]
- de Lacerda Coriolano, D.; de Souza, J.B.; Bueno, E.V.; Medeiros, S.M.d.F.R.d.S.; Cavalcanti, I.D.L.; Cavalcanti, I.M.F. Antibacterial and antibiofilm potential of silver nanoparticles against antibiotic-sensitive and multidrug-resistant Pseudomonas aeruginosa strains. Braz. J. Microbiol. 2021, 52, 267–278. [Google Scholar] [CrossRef]
- Barabadi, H.; Mojab, F.; Vahidi, H.; Marashi, B.; Talank, N.; Hosseini, O.; Saravanan, M. Green synthesis, characterization, antibacterial and biofilm inhibitory activity of silver nanoparticles compared to commercial silver nanoparticles. Inorg. Chem. Commun. 2021, 129, 108647. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Amin, M.A.; Zayed, G.; Hassan, Y.; El-Mokhtar, M.; Saddik, M.S. In vitro and in vivo synergistic wound healing and anti-methicillin-resistant Staphylococcus aureus (MRSA) evaluation of liquorice-decorated silver nanoparticles. J. Antibiot. 2023, 76, 291–300. [Google Scholar] [CrossRef]
- Liao, S.; Zhang, Y.; Pan, X.; Zhu, F.; Jiang, C.; Liu, Q.; Cheng, Z.; Dai, G.; Wu, G.; Wang, L.; et al. Antibacterial activity and mechanism of silver nanoparticles against multidrug-resistant Pseudomonas aeruginosa. Int. J. Nanomed. 2019, 14, 1469–1487. [Google Scholar] [CrossRef]
- Paladini, F.; Pollini, M. Antimicrobial Silver Nanoparticles for Wound Healing Application: Progress and Future Trends. Materials 2019, 12, 2540. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, P.D.; Banas, D.; Durai, R.D.; Kabanov, D.; Hosnedlova, B.; Kepinska, M.; Fernandez, C.; Ruttkay-Nedecky, B.; Nguyen, H.V.; Farid, A.; et al. Silver Nanomaterials for Wound Dressing Applications. Pharmaceutics 2020, 12, 821. [Google Scholar] [CrossRef]
- Rybka, M.; Mazurek, Ł.; Konop, M. Beneficial Effect of Wound Dressings Containing Silver and Silver Nanoparticles in Wound Healing—From Experimental Studies to Clinical Practice. Life 2023, 13, 69. [Google Scholar] [CrossRef]
- Nqakala, Z.B.; Sibuyi, N.R.S.; Fadaka, A.O.; Meyer, M.; Onani, M.O.; Madiehe, A.M. Advances in Nanotechnology towards Development of Silver Nanoparticle-Based Wound-Healing Agents. Int. J. Mol. Sci. 2021, 22, 11272. [Google Scholar] [CrossRef]
- Kar, A.K.; Singh, A.; Dhiman, N.; Purohit, M.P.; Jagdale, P.; Kamthan, M.; Singh, D.; Kumar, M.; Ghosh, D.; Patnaik, S. Polymer-Assisted In Situ Synthesis of Silver Nanoparticles with Epigallocatechin Gallate (EGCG) Impregnated Wound Patch Potentiate Controlled Inflammatory Responses for Brisk Wound Healing. Int. J. Nanomed. 2019, 14, 9837–9854. [Google Scholar] [CrossRef] [PubMed]
- Srisrimal, D.; Krishnan, S.; Thirunavukkarasu, A. In vitro Virucidal Activity of Silver Nanoparticles against H1N1 Influenza A Virus and Herpes Simplex Virus-1. Lett. Appl. NanoBioSci. 2023, 12, 136. [Google Scholar] [CrossRef]
- Raj, M.; Singh, M.; Kumar, V.; Yadav, M.; Sherawat, N.; Sharma, A.K.; Sharma, V.; Sharma, J. An Updated Overview of Nanostructured Silver as a Novel Class of Biomedical Agent. Lett. Appl. NanoBioSci. 2024, 13, 198. [Google Scholar] [CrossRef]
- Arct, J.; Mieloch, M. β-carotene in skin care. Pol. J. Cosmetol. 2016, 19, 206–221. [Google Scholar]
- Brahma, D.; Dutta, D.; Das, P.; Manvi, C.V. Can Carotenoid Encapsulated Hydrogel Be a Promising Medium for Repairing Skin Damage. Regen. Eng. Transl. Med. 2025, 11, 327–350. [Google Scholar] [CrossRef]
- Semnani, D.; Nasari, M.; Fakhrali, A. PCL nanofibers loaded with beta-carotene: A novel treatment for eczema. Polym. Bull. 2018, 75, 2015–2026. [Google Scholar] [CrossRef]
- Darban, Z.; Singh, H.; Singh, U.; Bhatia, D.; Gaur, R.; Kuddushi, M.; Dhanka, M.; Shahabuddin, S. β-Carotene laden antibacterial and antioxidant gelatin/polyglyceryl stearate nano-hydrogel system for burn wound healing application. Int. J. Biol. Macromol. 2024, 255, 128019. [Google Scholar] [CrossRef]
- Miazek, K.; Beton, K.; Śliwińska, A.; Brożek-Płuska, B. The Effect of β-Carotene, Tocopherols and Ascorbic Acid as Anti-Oxidant Molecules on Human and Animal In Vitro/In Vivo Studies: A Review of Research Design and Analytical Techniques Used. Biomolecules 2022, 12, 1087. [Google Scholar] [CrossRef]
- Thakur, S.; Sharma, B.; Verma, A.; Chaudhary, J.; Tamulevicius, S.; Thakur, V.K. Recent progress in sodium alginate based sustainable hydrogels for environmental applications. J. Clean. Prod. 2018, 198, 143–159. [Google Scholar] [CrossRef]
- Sharma, R.; Malviya, R.; Singh, S.; Prajapati, B. A Critical Review on Classified Excipient Sodium-Alginate-Based Hydrogels: Modification, Characterization, and Application in Soft Tissue Engineering. Gels 2023, 9, 430. [Google Scholar] [CrossRef]
- Wang, H.; Yang, L.; Yang, Y. A review of sodium alginate-based hydrogels: Structure, mechanisms, applications, and perspectives. Int. J. Biol. Macromol. 2025, 292, 139151. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.R.; Biswal, T. Alginate and its application to tissue engineering. SN Appl. Sci. 2021, 3, 30. [Google Scholar] [CrossRef]
- Arribas-López, E.; Zand, N.; Ojo, O.; Snowden, M.J.; Kochhar, T. A Systematic Review of the Effect of Centella asiatica on Wound Healing. Int. J. Environ. Res. Public Health 2022, 19, 3266. [Google Scholar] [CrossRef] [PubMed]
- Witkowska, K.; Paczkowska-Walendowska, M.; Garbiec, E.; Cielecka-Piontek, J. Topical Application of Centella asiatica in Wound Healing: Recent Insights into Mechanisms and Clinical Efficacy. Pharmaceutics 2024, 16, 1252. [Google Scholar] [CrossRef]
- Diniz, L.R.L.; Calado, L.L.; Duarte, A.B.S.; de Sousa, D.P. Centella asiatica and Its Metabolite Asiatic Acid: Wound Healing Effects and Therapeutic Potential. Metabolites 2023, 13, 276. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S. Pharmacological Effects of Centella asiatica on Skin Diseases: Evidence and Possible Mechanisms. Evid.-Based Complement. Altern. Med. 2021, 2021, 5462633. [Google Scholar] [CrossRef]
- Hossain, M.L.; Rahman, M.A.; Siddika, A.; Adnan, M.H.; Rahman, H.; Diba, F.; Hasan, M.Z.; Asaduzzaman, S.M. Burn and Wound Healing Using Radiation Sterilized Human Amniotic Membrane and Centella asiatica Derived Gel: A Review. Regen. Eng. Transl. Med. 2020, 6, 347–357. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef]
- Dhumale, V.; Gangwar, R.; Datar, S.; Sharma, R. Reversible Aggregation Control of Polyvinylpyrrolidone Capped Gold Nanoparticles as a Function of pH. Mater. Express 2012, 2, 311–318. [Google Scholar] [CrossRef]
- Franca, T.; Goncalves, D.; Cena, C. ATR-FTIR spectroscopy combined with machine learning for classification of PVA/PVP blends in low concentration. Vib. Spectrosc. 2022, 120, 103378. [Google Scholar] [CrossRef]
- Quijano-Ortega, N.; Fuenmayor, C.A.; Zuluaga-Dominguez, C.; Diaz-Moreno, C.; Ortiz-Grisales, S.; García-Mahecha, M.; Grassi, S. FTIR-ATR Spectroscopy Combined with Multivariate Regression Modeling as a Preliminary Approach for Carotenoids Determination in Cucurbita spp. Appl. Sci. 2020, 10, 3722. [Google Scholar]
- Saxena, J. Detection of carotenoids in psychrotrophic bacteria by spectroscopic approach. J. Biosci. Biotechnol. 2014, 3, 253–260. [Google Scholar]
- Lorand, T.; Molnár, P.; Deli, J.; Tóth, G. FT-IR study of some seco- and apocarotenoids. J. Biochem. Biophys. Methods 2002, 53, 251–258. [Google Scholar] [CrossRef]
- Bide, Y.; Fashapoyeh, M.A.; Shokrollahzadeh, S. Structural investigation and application of Tween 80-choline chloride self-assemblies as osmotic agent for water desalination. Sci. Rep. 2021, 11, 17068. [Google Scholar] [CrossRef]
- Gühlke, M.; Heiner, Z.; Kneipp, J. Surface-Enhanced Raman and Surface-Enhanced Hyper-Raman Scattering of Thiol-Functionalized Carotene. J. Phys. Chem. C 2016, 120, 20702–20709. [Google Scholar] [CrossRef] [PubMed]
- Sowani, H.; Mohite, P.; Damale, S.; Kulkarni, M.; Zinjarde, S. Carotenoid stabilized gold and silver nanoparticles derived from the Actinomycete Gordonia amicalis HS-11 as effective free radical scavengers. Enzym. Microb. Technol. 2016, 95, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Yonuis, S.; Nawaf, R.N.; Hasan, G. Molecular Identification, Antioxidant Efficacy of Phenolic Compounds, and Antimicrobial Activity of Beta-Carotene Isolated from Fruiting Bodies of Suillus sp. Karbala Int. J. Mod. Sci. 2020, 6, 4. [Google Scholar] [CrossRef]
- Stanescu, C.; Chiscop, I.; Mihalache, D.; Popa, F.; Tamas, C.; Stoleriu, G. Skin Aging and Carotenoids: A Systematic Review of Their Multifaceted Protective Mechanisms. Nutrients 2025, 17, 2596. [Google Scholar] [CrossRef]
- Terao, J. Revisiting carotenoids as dietary antioxidants for human health and disease prevention. Food Funct. 2023, 14, 7799–7824. [Google Scholar] [CrossRef]
- Abed, M.S.; Abed, A.S.; Othman, F.M. Green synthesis of silver nanoparticles from natural compounds: Glucose, eugenol and thymol. J. Adv. Res. Fluid. Mech. Therm. Sci. 2019, 60, 95–111. [Google Scholar]
- Trieu, Q.-A.; Nguyen, N.P.-Y.; Bui, T.H. Cinnamon Essential Oil-Mediated Synthesis of Silver Nanoparticles and Examination of Their Antibacterial Activity. Lett. Appl. NanoBioSci. 2024, 13, 87. [Google Scholar]
- Granja Alvear, A.; Pineda-Aguilar, N.; Lozano, P.; Lárez-Velázquez, C.; Suppan, G.; Galeas, S.; Debut, A.; Vizuete, K.; De Lima, L.; Saucedo-Vázquez, J.P.; et al. Synergistic Antibacterial Properties of Silver Nanoparticles and Its Reducing Agent from Cinnamon Bark Extract. Bioengineering 2024, 11, 517. [Google Scholar] [CrossRef] [PubMed]
- El-Baz, Y.G.; Moustafa, A.; Ali, M.A.; El-Desoky, G.E.; Wabaidur, S.M.; Faisal, M.M. An Analysis of the Toxicity, Antioxidant, and Anti-Cancer Activity of Cinnamon Silver Nanoparticles in Comparison with Extracts and Fractions of Cinnamomum Cassia at Normal and Cancer Cell Levels. Nanomaterials 2023, 13, 945. [Google Scholar] [CrossRef]
- Premkumar, J.; Sudhakar, T.; Dhakal, A.; Shrestha, J.B.; Krishnakumar, S.; Balashanmugam, P. Synthesis of silver nanoparticles (AgNPs) from cinnamon against bacterial pathogens. Biocatal. Agric. Biotechnol. 2018, 15, 311–316. [Google Scholar] [CrossRef]
- Lavelli, V.; Sereikaitė, J. Kinetic Study of Encapsulated β-Carotene Degradation in Dried Systems: A Review. Foods 2022, 11, 437. [Google Scholar] [CrossRef]
- Jain, A.; Sharma, G.; Thakur, K.; Raza, K.; Shivhare, U.; Ghoshal, G.; Katare, O.P. Beta-carotene-encapsulated solid lipid nanoparticles (BC-SLNs) as promising vehicle for cancer: An investigative assessment. AAPS PharmSciTech 2019, 20, 100. [Google Scholar] [CrossRef] [PubMed]
- Reksamunandar, R.P.; Edikresnha, D.; Munir, M.M.; Damayanti, S.; Khairurrijal. Encapsulation of β-carotene in poly(vinylpyrrolidone) (PVP) by Electrospinning Technique. Procedia Eng. 2017, 170, 19–23. [Google Scholar] [CrossRef]
- Liu, W.-Y.; Hsieh, Y.-S.; Wu, Y.-T. Poly (Lactic-Co-Glycolic) Acid–Poly (Vinyl Pyrrolidone) Hybrid Nanoparticles to Improve the Efficiency of Oral Delivery of β-Carotene. Pharmaceutics 2022, 14, 637. [Google Scholar] [CrossRef] [PubMed]
- Sheng, F.; Chow, P.S.; Dong, Y.; Tan, R.B.H. Preparation of β-carotene nanoparticles by antisolvent precipitation under power ultrasound. J. Nanopart. Res. 2014, 16, 2772. [Google Scholar] [CrossRef]
- Sultan, R.; Mahmood, Z.; Azhar, I.; Hasan, M.; Ahmed, S. Pharmacognostic and phytochemical investigation of Aerial Parts of Centella asiatica Linn. Int. J. Phytomed. 2012, 4, 125–133. [Google Scholar]
- Mohapatra, P.; Ray, A.; Jena, S.; Nayak, S.; Mohanty, S. Influence of various drying methods on physicochemical characteristics, antioxidant activity, and bioactive compounds in Centella asiatica L. leaves: A comparative study. Biotechnologia 2022, 103, 235–247. [Google Scholar] [CrossRef]
- Sabaragamuwa, R.; Perera, C.O. Total Triterpenes, Polyphenols, Flavonoids, and Antioxidant Activity of Bioactive Phytochemicals of Centella asiatica by Different Extraction Techniques. Foods 2023, 12, 3972. [Google Scholar] [CrossRef]
- Begum, T.; Follett, P.A.; Mahmud, J.; Moskovchenko, L.; Salmieri, S.; Allahdad, Z.; Lacroix, M. Silver nanoparticles-essential oils combined treatments to enhance the antibacterial and antifungal properties against foodborne pathogens and spoilage microorganisms. Microb. Pathog. 2022, 164, 105411. [Google Scholar] [CrossRef]
- Bouqellah, N.A.; Abdulmajeed, A.M.; Rashed Alharbi, F.K.; Mattar, E.; Al-Sarraj, F.; Abdulfattah, A.M.; Hassan, M.M.; Baazeem, A.; Al-Harthi, H.F.; Musa, A.; et al. Optimizing encapsulation of garlic and cinnamon essential oils in silver nanoparticles for enhanced antifungal activity against Botrytis cinerea pathogenic disease. Physiol. Mol. Plant Pathol. 2025, 136, 102522. [Google Scholar] [CrossRef]
- Abd El-Aziz, N.K.; Ammar, A.M.; El-Naenaeey, E.-s.Y.M.; El Damaty, H.M.; Elazazy, A.A.; Hefny, A.A.; Shaker, A.; Eldesoukey, I.E. Antimicrobial and antibiofilm potentials of cinnamon oil and silver nanoparticles against Streptococcus agalactiae isolated from bovine mastitis: New avenues for countering resistance. BMC Vet. Res. 2021, 17, 136. [Google Scholar] [CrossRef] [PubMed]
- Ghiulai, R.; Roşca, O.J.; Antal, D.S.; Mioc, M.; Mioc, A.; Racoviceanu, R.; Macaşoi, I.; Olariu, T.; Dehelean, C.; Creţu, O.M.; et al. Tetracyclic and Pentacyclic Triterpenes with High Therapeutic Efficiency in Wound Healing Approaches. Molecules 2020, 25, 5557. [Google Scholar] [CrossRef]
- Ebau, F.; Scano, A.; Manca, M.L.; Manconi, M.; Cabras, V.; Pilloni, M.; Ennas, G. Centella asiatica extract-SiO(2) nanocomposite: More than a drug-delivery system for skin protection from oxidative damage. J. Biomed. Mater. Res. A 2023, 111, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Idris, F.N.; Mohd Nadzir, M. Comparative Studies on Different Extraction Methods of Centella asiatica and Extracts Bioactive Compounds Effects on Antimicrobial Activities. Antibiotics 2021, 10, 457. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Mosleh, A.M.; Elghany, A.A.; Shams-Eldin, E.; Abu Serea, E.S.; Ali, S.A.; Shalan, A.E. Coated silver nanoparticles: Synthesis, cytotoxicity, and optical properties. RSC Adv. 2019, 9, 20118–20136. [Google Scholar] [CrossRef]
- Eze, F.N.; Tola, A.J.; Nwabor, O.F.; Jayeoye, T.J. Centella asiatica phenolic extract-mediated bio-fabrication of silver nanoparticles: Characterization, reduction of industrially relevant dyes in water and antimicrobial activities against foodborne pathogens. RSC Adv. 2019, 9, 37957–37970. [Google Scholar] [CrossRef]
- Bozkaya, O.; Bozkaya, E.; Ekici, H.; Alçığır, M.E.; Şahin, Y.; Aytuna Çerçi, N.; Karahan, S.; Yiğitoğlu, M.; Vargel, İ. Evaluation of Burn Wound Healing Efficacy and Biocompatibility of Centella asiatica Mediated Synthesised AgNPs Loaded Hybrid Nanofiber Scaffold: In Vitro and In Vivo Studies. Macromol. Mater. Eng. 2024, 309, 2400186. [Google Scholar] [CrossRef]
- Flieger, J.; Raszewska-Famielec, M.; Radzikowska-Büchner, E.; Flieger, W. Skin Protection by Carotenoid Pigments. Int. J. Mol. Sci. 2024, 25, 1431. [Google Scholar] [CrossRef]
- Lohan, S.B.; Vitt, K.; Scholz, P.; Keck, C.M.; Meinke, M.C. ROS production and glutathione response in keratinocytes after application of β-carotene and VIS/NIR irradiation. Chem.-Biol. Interact. 2018, 280, 1–7. [Google Scholar] [CrossRef]
- Jiravova, J.; Tomankova, K.B.; Harvanova, M.; Malina, L.; Malohlava, J.; Luhova, L.; Panacek, A.; Manisova, B.; Kolarova, H. The effect of silver nanoparticles and silver ions on mammalian and plant cells in vitro. Food Chem. Toxicol. 2016, 96, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Galandáková, A.; Franková, J.; Ambrožová, N.; Habartová, K.; Pivodová, V.; Zálešák, B.; Šafářová, K.; Smékalová, M.; Ulrichová, J. Effects of silver nanoparticles on human dermal fibroblasts and epidermal keratinocytes. Hum. Exp. Toxicol. 2016, 35, 946–957. [Google Scholar] [CrossRef]
- Ahlberg, S.; Rancan, F.; Epple, M.; Loza, K.; Höppe, D.; Lademann, J.; Vogt, A.; Kleuser, B.; Gerecke, C.; Meinke, M.C. Comparison of different methods to study effects of silver nanoparticles on the pro- and antioxidant status of human keratinocytes and fibroblasts. Methods 2016, 109, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, M.; Jiang, S.; Zhang, Y.; Li, R.; Lu, Y.; Liu, L.; Wu, G.; Liu, Y.; Xie, L.; et al. Skin Toxicity Assessment of Silver Nanoparticles in a 3D Epidermal Model Compared to 2D Keratinocytes. Int. J. Nanomed. 2019, 14, 9707–9719. [Google Scholar] [CrossRef] [PubMed]
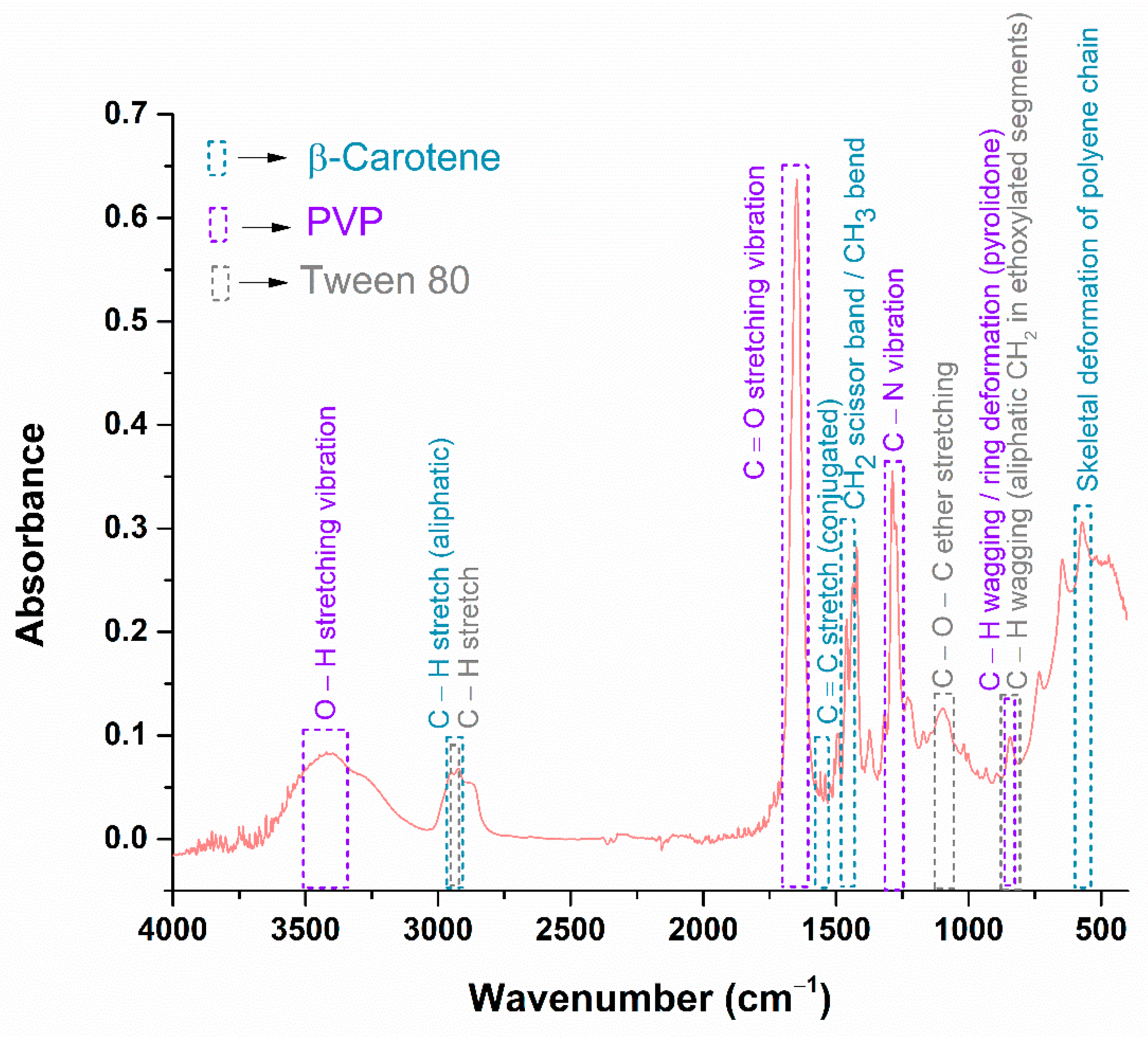




| Functional Group | Wavenumber (cm−1) | References |
|---|---|---|
| (PVP) O–H stretching vibration | 3420 cm−1 | [65,66,67] |
| (β-Carotene) C–H stretch (aliphatic) | 2950 cm−1 | [68,69,70] |
| (Tween 80) C–H stretch | 2923 cm−1 | [71] |
| (PVP) C=O stretching vibration | 1647 cm−1 | [65,66,67] |
| (β-Carotene) C=C stretch conjugated | 1558 cm−1 | [68,69,70] |
| (β-Carotene) CH2 scissor band/CH3 bend | 1436 cm−1 | [68,69,70] |
| (PVP) C–N vibration | 1287 cm−1 | [65,66,67] |
| (Tween 80) C–O–C ether stretching | 1096 cm−1 | [71] |
| (PVP) C–H wagging/ring deformation (pyrrolidone) | 844 cm−1 | [65,66,67] |
| (Tween 80) C–H wagging (aliphatic CH2 in ethoxylated segments) | 844 cm−1 | [71] |
| (β-Carotene) Skeletal deformation of polyene chain | 571 cm−1 | [68,69,70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Țăin, A.E.; Bîrcă, A.C.; Nedelcu, M.S.; Holban, A.M.; Niculescu, A.-G.; Grumezescu, A.M.; Hudiță, A. Cinnamon-Mediated Silver Nanoparticles and Beta-Carotene Nanocarriers in Alginate Dressings for Wound Healing Applications. Gels 2025, 11, 738. https://doi.org/10.3390/gels11090738
Țăin AE, Bîrcă AC, Nedelcu MS, Holban AM, Niculescu A-G, Grumezescu AM, Hudiță A. Cinnamon-Mediated Silver Nanoparticles and Beta-Carotene Nanocarriers in Alginate Dressings for Wound Healing Applications. Gels. 2025; 11(9):738. https://doi.org/10.3390/gels11090738
Chicago/Turabian StyleȚăin (Anastasiu), Anca Elena, Alexandra Cătălina Bîrcă, Monica Sânziana Nedelcu, Alina Maria Holban, Adelina-Gabriela Niculescu, Alexandru Mihai Grumezescu, and Ariana Hudiță. 2025. "Cinnamon-Mediated Silver Nanoparticles and Beta-Carotene Nanocarriers in Alginate Dressings for Wound Healing Applications" Gels 11, no. 9: 738. https://doi.org/10.3390/gels11090738
APA StyleȚăin, A. E., Bîrcă, A. C., Nedelcu, M. S., Holban, A. M., Niculescu, A.-G., Grumezescu, A. M., & Hudiță, A. (2025). Cinnamon-Mediated Silver Nanoparticles and Beta-Carotene Nanocarriers in Alginate Dressings for Wound Healing Applications. Gels, 11(9), 738. https://doi.org/10.3390/gels11090738











