Carbon Nitride Gels: Synthesis, Modification, and Water Decontamination Applications
Abstract
1. Introduction
2. Synthesis Strategies of Carbon Nitride-Based Gels
2.1. Precursor Selection
2.1.1. Nitrogen-Rich Cyanamide-Based Precursors
2.1.2. Natural Biomass Precursors
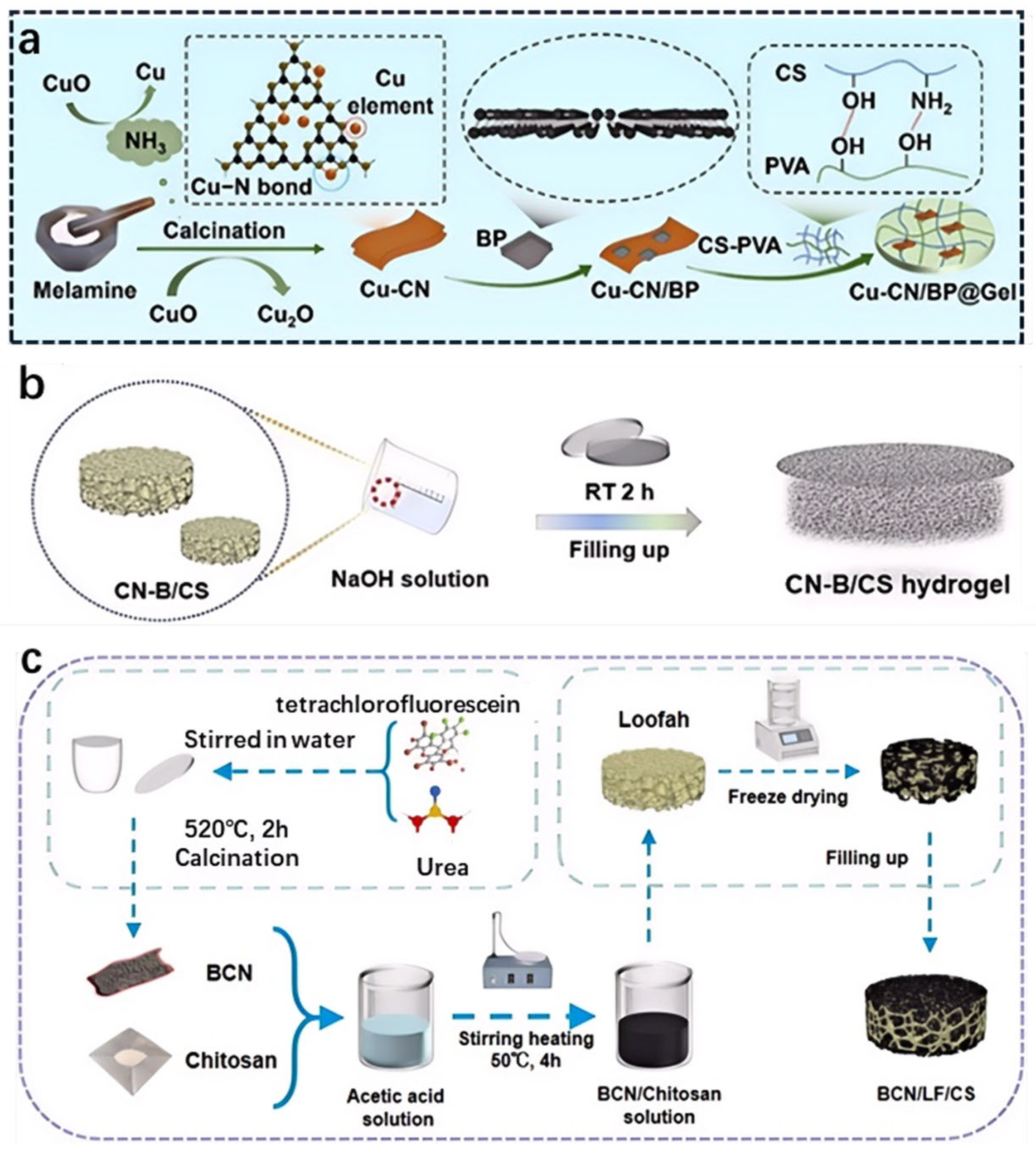
2.2. Hydrogel Synthesis Methods
2.2.1. Physical Crosslinking
2.2.2. Chemical Crosslinking
2.2.3. In Situ Polymerization
2.3. Aerogel Synthesis Methods
2.3.1. Sol–Gel Method with Supercritical Drying/Freeze-Drying
2.3.2. Template Method (Soft/Hard Template for Pore Structure Control)
3. Modification of g-C3N4-Based Gel Composite Photocatalyst
3.1. Chemical Composition Tuning
3.1.1. Elemental Doping
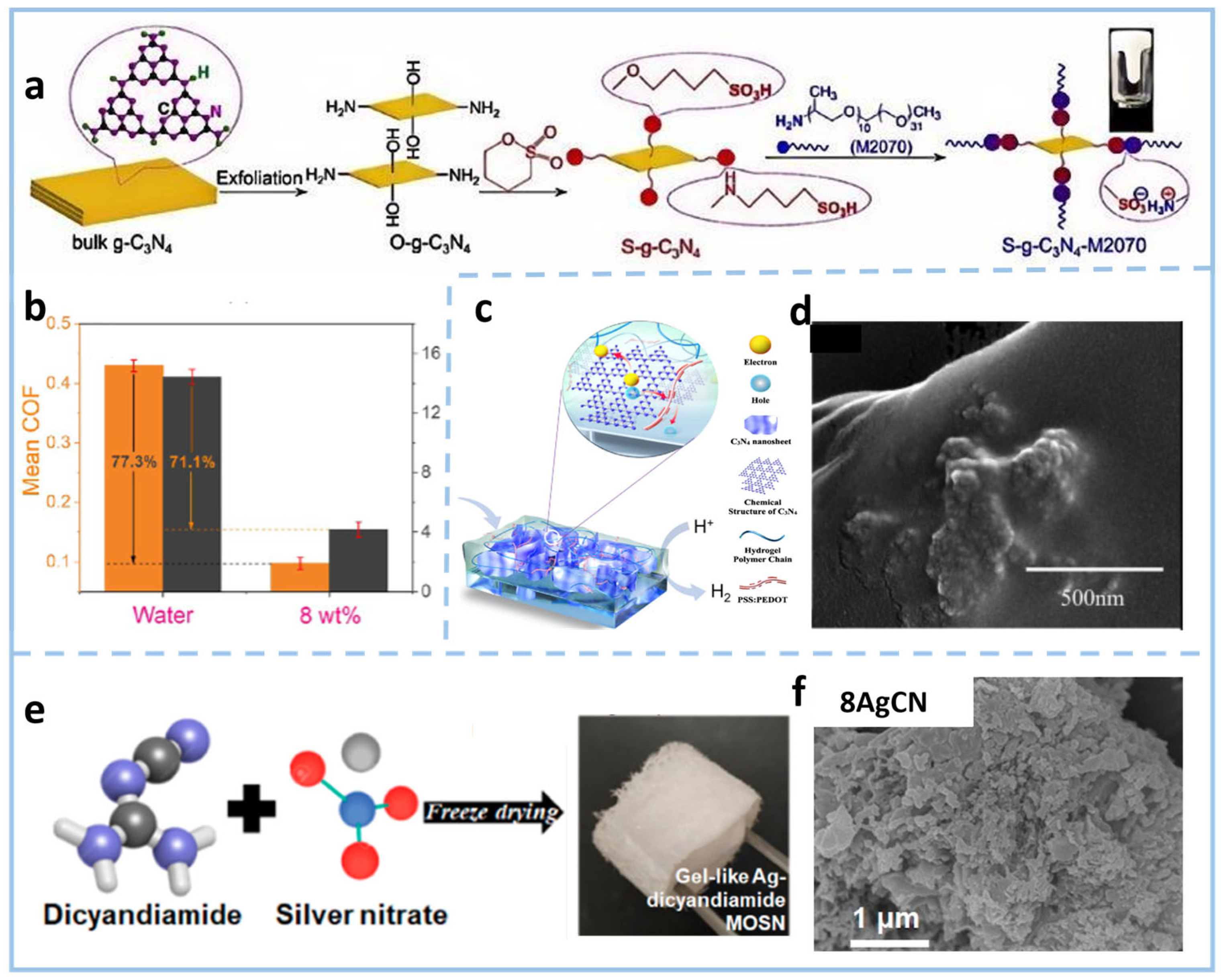
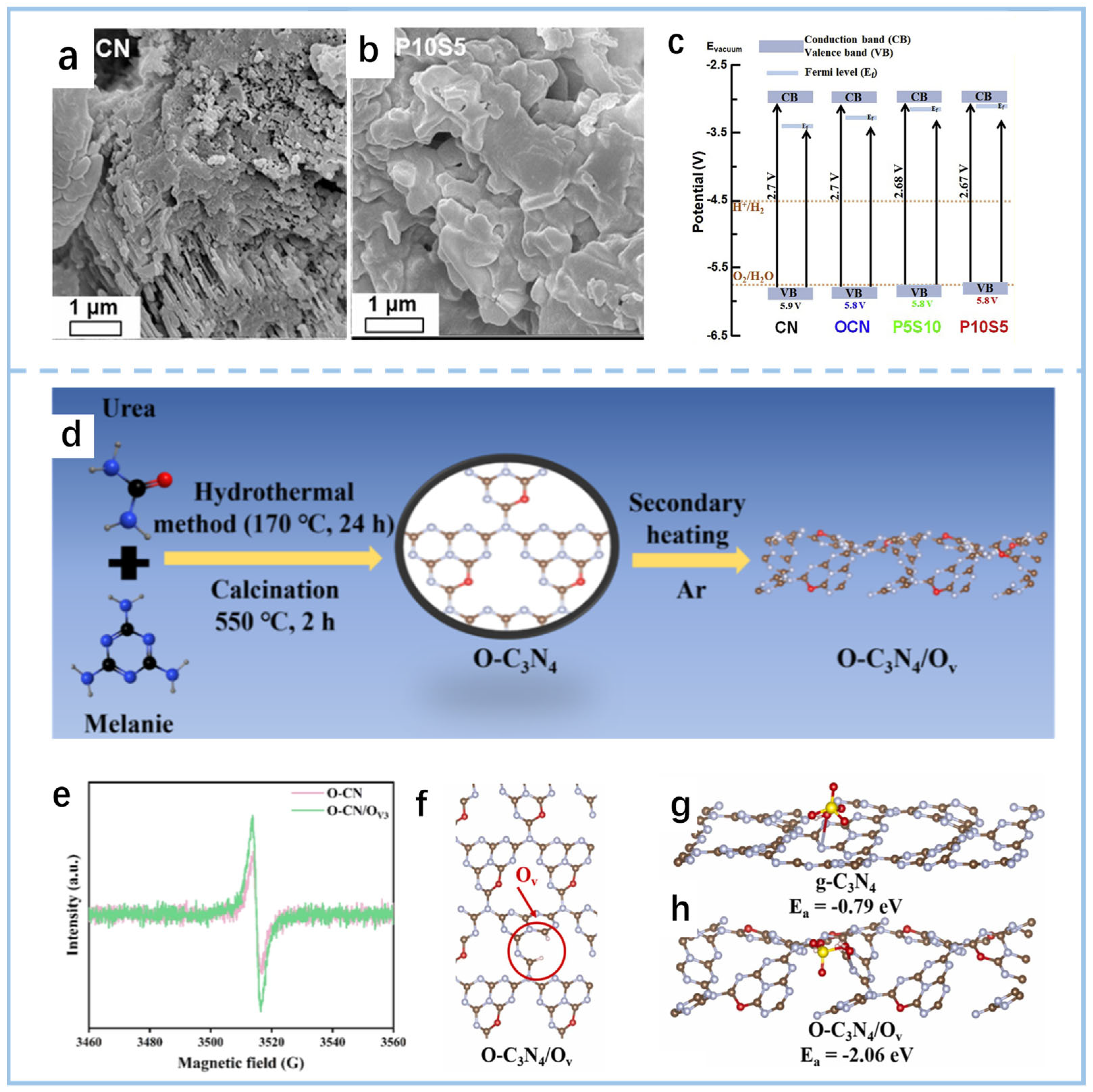
3.1.2. Surface Functional Group Modification
3.2. Structural Engineering
3.2.1. Interfacial Composition Engineering in Heterojunctions
3.2.2. Hierarchical Porosity Engineering
4. Application of Carbon Nitride-Based Gel in Wastewater Purification
4.1. Photocatalytic AOPs for Contaminant Removal
4.1.1. Organic Pollutant Removal
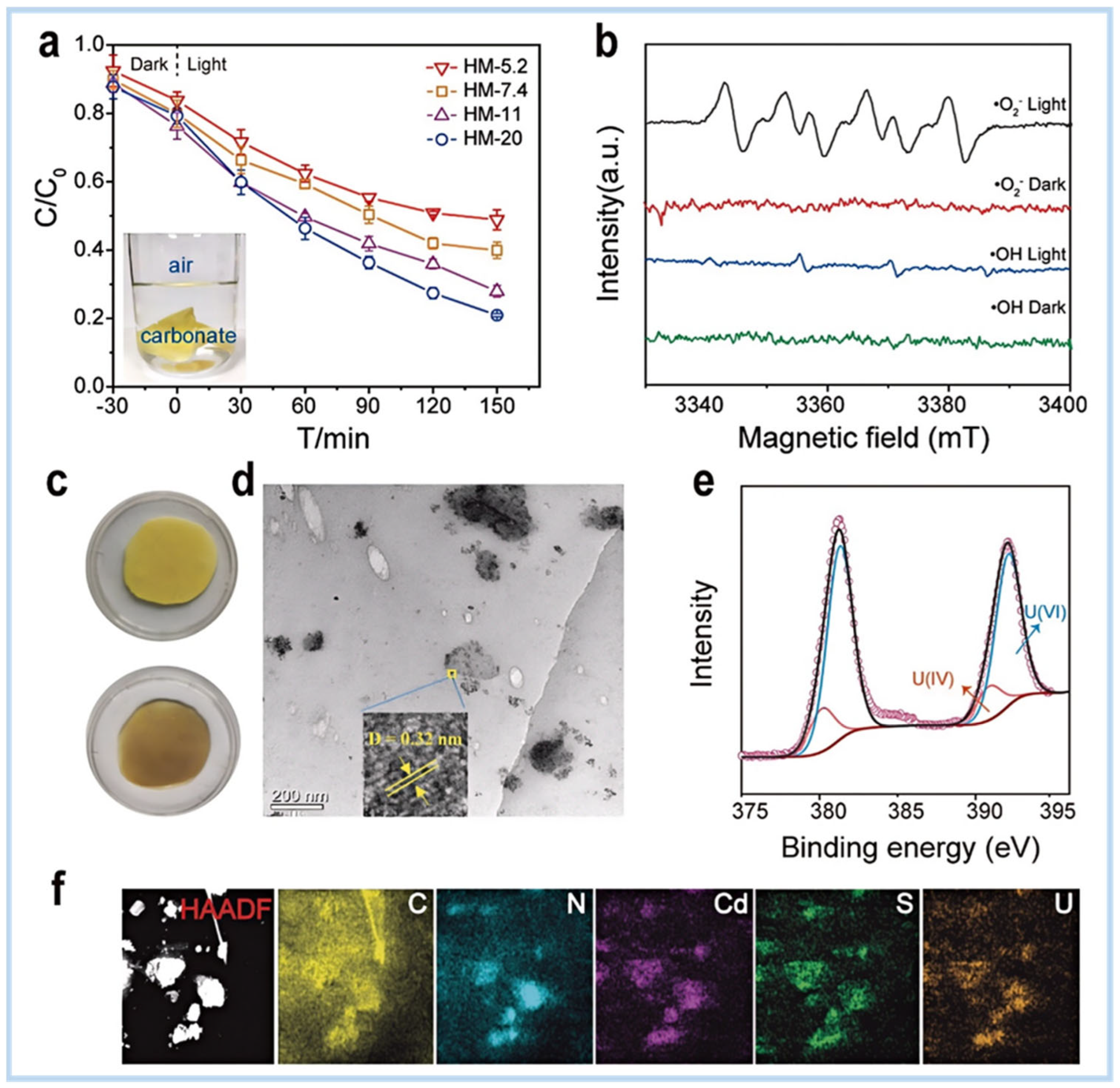
4.1.2. Removal of Metal Ions from Water
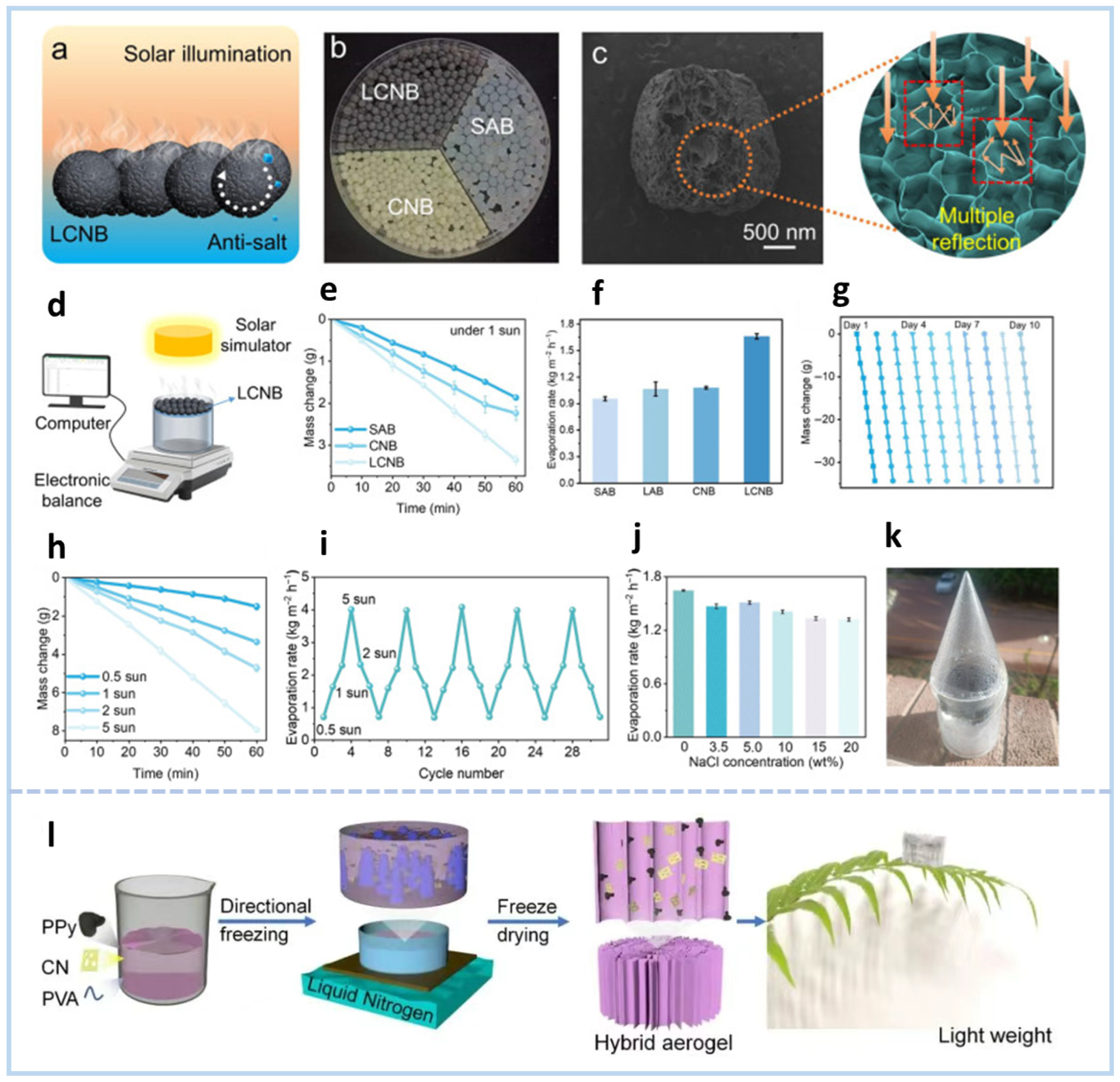
4.2. Synergistic Interfacial Evaporation and Photocatalytic Pollutant Degradation
5. Conclusions and Outlooks
5.1. Conclusions
5.2. Outlooks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boretti, A.; Rosa, L. Reassessing the projections of the World Water Development Report. npj Clean Water 2019, 2, 15. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer To Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C3N4-Based Heterostructured Photocatalysts. Adv. Energy Mater. 2018, 8, 1701503. [Google Scholar] [CrossRef]
- Jiao, X.; Zheng, K.; Liang, L.; Li, X.; Sun, Y.; Xie, Y. Fundamentals and challenges of ultrathin 2D photocatalysts in boosting CO2 photoreduction. Chem. Soc. Rev. 2020, 49, 6592–6604. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Cao, S.; Yu, J. g-C3N4-Based Photocatalysts for Hydrogen Generation. J. Phys. Chem. Lett. 2014, 5, 2101–2107. [Google Scholar] [CrossRef]
- Khan, M.S.; Li, Y.; Li, D.-S.; Qiu, J.; Xu, X.; Yang, H.Y. A review of metal–organic framework (MOF) materials as an effective photocatalyst for degradation of organic pollutants. Nanoscale Adv. 2023, 5, 6318–6348. [Google Scholar] [CrossRef]
- Zhang, C.; Li, N.; An, G. Review of Concentrated Solar Power Technology Applications in Photocatalytic Water Purification and Energy Conversion: Overview, Challenges and Future Directions. Energies 2024, 17, 463. [Google Scholar] [CrossRef]
- Saleem, Z.; Pervaiz, E.; Yousaf, M.U.; Niazi, M.B.K. Two-Dimensional Materials and Composites as Potential Water Splitting Photocatalysts: A Review. Catalysts 2020, 10, 464. [Google Scholar] [CrossRef]
- Yao, B.; Zhang, J.; Fan, X.; He, J.; Li, Y. Surface Engineering of Nanomaterials for Photo-Electrochemical Water Splitting. Small 2019, 15, 1803746. [Google Scholar] [CrossRef] [PubMed]
- Utami, M.; Wang, S.; Musawwa, M.M.; Mafruhah, L.; Fitri, M.; Wijaya, K.; Johnravindar, D.; Abd-Elkader, O.H.; Yadav, K.K.; Ravindran, B.; et al. Photocatalytic degradation of naphthol blue from Batik wastewater using functionalized TiO2-based composites. Chemosphere 2023, 337, 139224. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Raizada, P.; Singh, P.; Saini, R.V.; Saini, A.K.; Hosseini-Bandegharaei, A. Perspective and status of polymeric graphitic carbon nitride based Z-scheme photocatalytic systems for sustainable photocatalytic water purification. Chem. Eng. J. 2020, 391, 123496. [Google Scholar] [CrossRef]
- Zhou, Z.; Xiao, Y.; Tian, J.; Nan, N.; Song, R.; Li, J. Recent advances in metal-free covalent organic frameworks for photocatalytic applications in energy and environmental fields. J. Mater. Chem. A 2023, 11, 3245–3261. [Google Scholar] [CrossRef]
- Hu, C.; Lin, Y.-R.; Yang, H.-C. Recent Developments in Graphitic Carbon Nitride Based Hydrogels as Photocatalysts. ChemSusChem 2019, 12, 1794–1806. [Google Scholar] [CrossRef]
- Han, H.; Fu, M.; Li, Y.; Guan, W.; Lu, P.; Hu, X. In-situ polymerization for PPy/g-C3N4 composites with enhanced visible light photocatalytic performance. Chin. J. Catal. 2018, 39, 831–840. [Google Scholar] [CrossRef]
- Huo, X.; Yi, H.; Fu, Y.; An, Z.; Qin, L.; Liu, X.; Li, B.; Liu, S.; Li, L.; Zhang, M.; et al. Porous graphitic carbon nitride nanomaterials for water treatment. Environ. Sci. Nano 2021, 8, 1835–1862. [Google Scholar] [CrossRef]
- Cheng, Z.; Qi, W.; Pang, C.H.; Thomas, T.; Wu, T.; Liu, S.; Yang, M. Recent Advances in Transition Metal Nitride-Based Materials for Photocatalytic Applications. Adv. Funct. Mater. 2021, 31, 2100553. [Google Scholar] [CrossRef]
- Milošević, K.; Lončarević, D.; Kalagasidis Krušić, M.; Hadnađev-Kostić, M.; Dostanić, J. Eco-Friendly g-C3N4/Carboxymethyl Cellulose/Alginate Composite Hydrogels for Simultaneous Photocatalytic Degradation of Organic Dye Pollutants. Int. J. Mol. Sci. 2024, 25, 7896. [Google Scholar] [CrossRef]
- Thurston, J.H.; Clifford, A.J.; Henderson, B.S.; Smith, T.R.; Quintana, D.; Cudworth, K.F.; Lujan, T.J.; Cornell, K.A. Development of Photoactive g-C3N4/Poly(vinyl alcohol) Composite Hydrogel Films with Antimicrobial and Antibiofilm Activity. ACS Appl. Bio. Mater. 2020, 3, 1681–1689. [Google Scholar] [CrossRef]
- Ruan, X.; Wang, L.; Liang, D.; Shi, Y. Environmental Applications of 3D g-C3N4-Based Hydrogel with Synergistic Effect of Adsorption and Photodegradation. Langmuir 2023, 39, 3371–3379. [Google Scholar] [CrossRef] [PubMed]
- Akaike, K.; Hosokai, A.; Tajima, K.; Akiyama, H.; Nagashima, H. Insights into chemical reactions of graphitic carbon nitride with alkali halides. J. Phys. Energy 2023, 5, 014007. [Google Scholar] [CrossRef]
- Zheng, Q.; Shen, H.; Shuai, D. Emerging investigators series: Advances and challenges of graphitic carbon nitride as a visible-light-responsive photocatalyst for sustainable water purification. Environ. Sci. Water Res. Technol. 2017, 3, 982–1001. [Google Scholar] [CrossRef]
- Zhang, P.; Gao, L.; Song, X.; Sun, J. Micro- and Nanostructures of Photoelectrodes for Solar-Driven Water Splitting. Adv. Mater. 2015, 27, 562–568. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Zeng, G.; Wu, Z.; Liang, J.; Chen, X.; Leng, L.; Wang, H.; Wang, H. Metal-free efficient photocatalyst for stable visible-light photocatalytic degradation of refractory pollutant. Appl. Catal. B Environ. 2018, 221, 715–725. [Google Scholar] [CrossRef]
- Ng, Y.H.; Ikeda, S.; Matsumura, M.; Amal, R. A perspective on fabricating carbon-based nanomaterials by photocatalysis and their applications. Energy Environ. Sci. 2012, 5, 9307–9318. [Google Scholar] [CrossRef]
- Reddy, P.A.K.; Reddy, P.V.L.; Kwon, E.; Kim, K.-H.; Akter, T.; Kalagara, S. Recent advances in photocatalytic treatment of pollutants in aqueous media. Environ. Int. 2016, 91, 94–103. [Google Scholar] [CrossRef]
- Dai, T.; Fang, C.; Liu, T.; Zheng, S.; Lei, G.; Jiang, G. Waste glass powder as a high temperature stabilizer in blended oil well cement pastes: Hydration, microstructure and mechanical properties. Constr. Build. Mater. 2024, 439, 137359. [Google Scholar] [CrossRef]
- Ahtasham Iqbal, M.; Akram, S.; Khalid, S.; Lal, B.; Hassan, S.U.; Ashraf, R.; Kezembayeva, G.; Mushtaq, M.; Chinibayeva, N.; Hosseini-Bandegharaei, A. Advanced photocatalysis as a viable and sustainable wastewater treatment process: A comprehensive review. Environ. Res. 2024, 253, 118947. [Google Scholar] [CrossRef]
- Khandelwal, A.; Maarisetty, D.; Baral, S.S. Fundamentals and application of single-atom photocatalyst in sustainable energy and environmental applications. Renew. Sustain. Energy Rev. 2022, 167, 112693. [Google Scholar] [CrossRef]
- Athanasekou, C.P.; Moustakas, N.G.; Morales-Torres, S.; Pastrana-Martínez, L.M.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M.T.; Dona-Rodriguez, J.M.; Romanos, G.E.; Falaras, P. Ceramic photocatalytic membranes for water filtration under UV and visible light. Appl. Catal. B Environ. 2015, 178, 12–19. [Google Scholar] [CrossRef]
- Zhao, W.; Adeel, M.; Zhang, P.; Zhou, P.; Huang, L.; Zhao, Y.; Ahmad, M.A.; Shakoor, N.; Lou, B.; Jiang, Y.; et al. A critical review on surface-modified nano-catalyst application for the photocatalytic degradation of volatile organic compounds. Environ. Sci. Nano 2022, 9, 61–80. [Google Scholar] [CrossRef]
- Qiu, X.; Zhang, Y.; Zhu, Y.; Long, C.; Su, L.; Liu, S.; Tang, Z. Applications of Nanomaterials in Asymmetric Photocatalysis: Recent Progress, Challenges, and Opportunities. Adv. Mater. 2021, 33, 2001731. [Google Scholar] [CrossRef]
- Chen, Z.; Kuate, L.J.N.; Zhang, H.; Hou, J.; Wen, H.; Lu, C.; Li, C.; Shi, W. Photothermally enabled black g-C3N4 hydrogel with integrated solar-driven evaporation and photo-degradation for efficient water purification. Sep. Purif. Technol. 2025, 355 Pt B, 129751. [Google Scholar] [CrossRef]
- Mishra, K.; Devi, N.; Siwal, S.S.; Gupta, V.K.; Thakur, V.K. Hybrid Semiconductor Photocatalyst Nanomaterials for Energy and Environmental Applications: Fundamentals, Designing, and Prospects. Adv. Sustain. Syst. 2023, 7, 2300095. [Google Scholar] [CrossRef]
- Darkwah, W.K.; Ao, Y. Mini Review on the Structure and Properties (Photocatalysis), and Preparation Techniques of Graphitic Carbon Nitride Nano-Based Particle, and Its Applications. Nanoscale Res. Lett. 2018, 13, 388. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Sun, L.; Hao, P.; Zhang, S.; Shen, Y.; Hou, J.; Guo, F.; Li, C.; Shi, W. Construction of black g-C3N4/loofah/chitosan hydrogel as an efficient solar evaporator for desalination coupled with antibiotic degradation. Sep. Purif. Technol. 2025, 355, 129615. [Google Scholar] [CrossRef]
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent Advances of Photocatalytic Application in Water Treatment: A Review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef]
- Sun, X.; Shi, L.; Huang, H.; Song, X.; Ma, T. Surface engineered 2D materials for photocatalysis. Chem. Commun. 2020, 56, 11000–11013. [Google Scholar] [CrossRef] [PubMed]
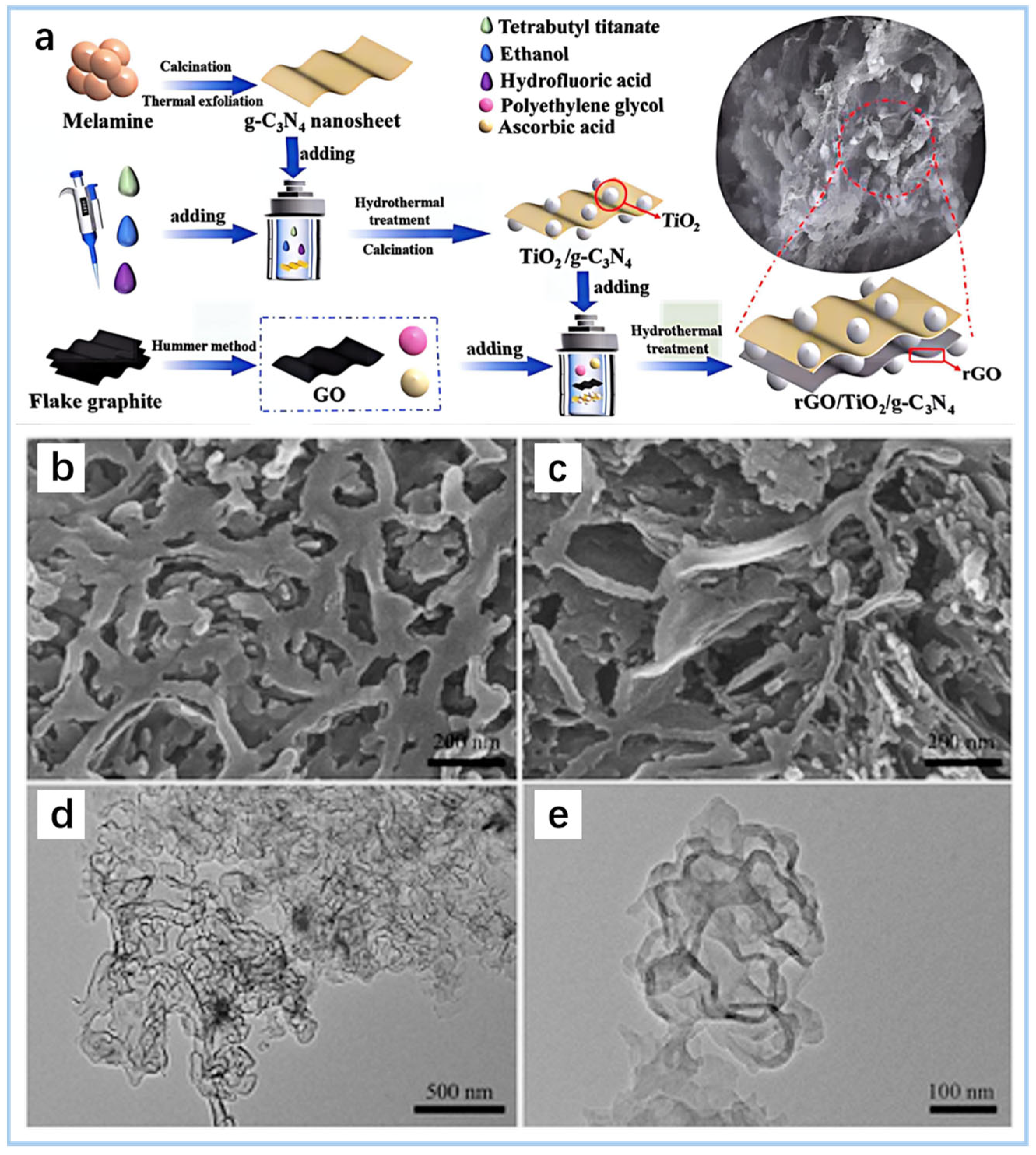
- Yuan, Y.; Niu, Y.; Xu, H.; Fang, C.; Liu, W.; Song, B.; Li, M.; Shao, G.; Lu, H.; Wang, H. High efficiency removal of organic pollutant via the synergistic effect between adsorption and photocatalysis of rGO/TiO2/g-C3N4 composite aerogel. Ceram. Int. 2025, 51, 8965–8979. [Google Scholar] [CrossRef]
- Hu, Z.; Shen, Z.; Yu, J.C. Converting Carbohydrates to Carbon-Based Photocatalysts for Environmental Treatment. Environ. Sci. Technol. 2017, 51, 7076–7083. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Zhao, H.; Pan, F.; Feng, X.; Jung, B.; Abdel-Wahab, A.; Batchelor, B.; Li, Y. Visible-Light-Driven Photocatalytic Degradation of Organic Water Pollutants Promoted by Sulfite Addition. Environ. Sci. Technol. 2017, 51, 13372–13379. [Google Scholar] [CrossRef]
- Pachaiappan, R.; Rajendran, S.; Kumar, P.S.; Vo, D.-V.N.; Hoang, T.K.A.; Cornejo-Ponce, L. Recent advances in carbon nitride-based nanomaterials for hydrogen production and storage. Int. J. Hydrogen Energy 2022, 47, 37490–37516. [Google Scholar] [CrossRef]
- Zuo, X.; Zhou, Y.; Hao, K.; Liu, C.; Yu, R.; Huang, A.; Wu, C.; Yang, Y. 3D Printed All-Natural Hydrogels: Flame-Retardant Materials Toward Attaining Green Sustainability. Adv. Sci. 2024, 11, 2306360. [Google Scholar] [CrossRef]
- Cui, J.; Yu, F.; Zhang, J.; Tang, X.; Liu, Y. Doping mechanism of S, O co-doped in nitrogen vacancy defect rich g-C3N4 nanosheet photocatalyst. Opt. Mater. 2023, 139, 113777. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, Y.; Cai, C.; Lai, S.; Cheng, L.; Zhang, J.; Zhu, W.; Guo, Y.; Hou, M.; Ma, L.; et al. Flash Joule Heating Synthesis of Nitrogen-Rich Defective G-C3N4 for Highly Efficient Photocatalytic Hydrogen Evolution. Small, 2025; early view. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, B.; Luo, M.; Lu, H. Scalable Construction of Gel-like g-C3N4 Nanosheet Hybrids as High-Performance Water-Based Additives. ACS Appl. Nano Mater. 2024, 7, 4162–4172. [Google Scholar] [CrossRef]
- Zha, W.; Ruan, Q.; Kong, L.; Xi, X.; Turgunov, M.A.; Zhang, W.; Chang, K.; Sun, Z. A suspension-mimicking hydrogel-based n-type polymer photocathode for solar-driven water splitting. Cell Rep. Phys. Sci. 2022, 3, 100863. [Google Scholar] [CrossRef]
- Cao, X.-q.; Li, P.; Wei, B.; Zhang, Y.; He, Y.; Chen, K.; Xu, Y.; Zhang, J.; Leung, M.K.H. Dual oxygen and sulfur vacancies trigger double Z-scheme rapid charge transport for efficient photocatalysis with electricity generation. Sep. Purif. Technol. 2025, 373, 133526. [Google Scholar] [CrossRef]
- Catherine, H.N.; Chiu, W.-L.; Chang, L.-L.; Tung, K.-L.; Hu, C. Gel-like Ag-Dicyandiamide Metal–Organic Supramolecular Network-Derived g-C3N4 for Photocatalytic Hydrogen Generation. ACS Sustain. Chem. Eng. 2022, 10, 8360–8369. [Google Scholar] [CrossRef]
- Sun, L.; Jiang, Z.; Yuan, B.; Zhi, S.; Zhang, Y.; Li, J.; Wu, A. Ultralight and superhydrophobic perfluorooctyltrimethoxysilane modified biomass carbonaceous aerogel for oil-spill remediation. Chem. Eng. Res. Des. 2021, 174, 71–78. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, X.; An, W.; Li, Y.; Hu, J.; Cui, W. A g-C3N4@ppy-rGO 3D structure hydrogel for efficient photocatalysis. Appl. Surf. Sci. 2019, 466, 666–672. [Google Scholar] [CrossRef]
- Zheng, M.; Li, W.; Ma, F.; Shao, Y.; Guo, M.; Gao, X.; Du, J. Review on the synergistic effect of adsorption and photocatalytic degradation of patulin by functionalized graphitic carbon nitride nanomaterials and hydrogels. RSC Adv. 2025, 15, 24510–24535. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xue, Y.; Gao, X.; Wang, L.; Liu, X.; Wang, Z.; Shen, S. Cayanamide Group Functionalized Crystalline Carbon Nitride Aerogel for Efficient CO2 Photoreduction. Adv. Funct. Mater. 2023, 34, 2312634. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, Y.; Liu, X.; Guo, L. Phenyl-modified carbon nitride based multifunctional gel for the detection, adsorption, photocatalytic reduction and recycling of chromium(VI) in wastewater. Appl. Surf. Sci. 2024, 652, 159278. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.-J. Recent Advances in TiO2 Films Prepared by Sol-Gel Methods for Photocatalytic Degradation of Organic Pollutants and Antibacterial Activities. Coatings 2019, 9, 613. [Google Scholar] [CrossRef]
- Vijaya Suryaa, K.; Balakrishnan, A.; Chinthala, M.; Bidya Devi, K.; Tripathy, H.; Kumar, A.; Aminabhavi, T.M.; Rtimi, S. Photocatalytic self-Fenton degradation of tetracycline over Z-scheme functionalized g-C3N4/CeO2/Bi2S3 hydrogel beads: Dynamics, mechanism, degradation pathways and toxicity analysis. Chem. Eng. J. 2025, 505, 159470. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, G.; An, Z.; Wang, W.; Hu, X.; Wang, Y.; Du, Z.; Gong, X.; Tan, H.; Guo, F.; et al. Preparation of recyclable g-C3N4/TiO2 heterojunction/alginate hydrogel microbeads and investigation of their adsorption-photocatalytic properties. J. Hazard. Mater. Adv. 2025, 18, 100650. [Google Scholar] [CrossRef]
- Nazir, A.; Huo, P.W.; Rasool, A.T. Recent advances on graphitic carbon nitride-based S-scheme photocatalysts: Synthesis, environmental applications, and challenges. J. Organomet. Chem. 2024, 1004, 122951. [Google Scholar] [CrossRef]
- Mao, J.; Wang, J.; Zhang, J.; Shi, S.; Zhang, Y.; Bai, Y.; Qu, J. Interfacial charge steering in CdS/nitrogen-deficient g-C3N4 heterojunctions boosts solar-driven persulfate activation for PPCPs decontamination. Sep. Purif. Technol. 2025, 377, 134480. [Google Scholar] [CrossRef]
- Liu, J.; Wei, X.; Sun, W.; Guan, X.; Zheng, X.; Li, J. Fabrication of S-scheme CdS-g-C(3)N(4)-graphene aerogel heterojunction for enhanced visible light driven photocatalysis. Env. Res. 2021, 197, 111136. [Google Scholar] [CrossRef]
- Du, C.; Yan, B.; Lin, Z.; Yang, G. Enhanced carrier separation and increased electron density in 2D heavily N-doped ZnIn2S4 for photocatalytic hydrogen production. J. Mater. Chem. A 2020, 8, 207–217. [Google Scholar] [CrossRef]
- Tseng, T.K.; Lin, Y.S.; Chen, Y.J.; Chu, H. A Review of Photocatalysts Prepared by Sol-Gel Method for VOCs Removal. Int. J. Mol. Sci. 2010, 11, 2336–2361. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Tang, Q.; Ran, L.; Xu, Y.; Zhu, Y.; Zhang, Y.; Leung, M.K.H. Built-in Interface electric field microenvironment in covalent organic framework modified heterojunction guiding Electron transfer for effective photocatalytic CO2 reduction. J. Colloid Interface Sci. 2025, 700, 138326. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.F.; Rademann, J. Dynamic template-assisted strategies in fragment-based drug discovery. Trends Biotechnol. 2009, 27, 512–521. [Google Scholar] [CrossRef]
- Xiao, H.; Zhao, X.; Lv, J.; He, X.; Chen, M.; Tan, W.; Yang, W.; Zeng, K.; Hu, J.; Yang, G. Ultrasound-assisted freeze-drying strategy to enhance nanoparticle dispersion in aerogels: A case study on polyimide/silica nanocomposite aerogel. Polym. Eng. Sci. 2023, 63, 3819–3830. [Google Scholar] [CrossRef]
- Zhang, S.; Greenfield, M.A.; Mata, A.; Palmer, L.C.; Bitton, R.; Mantei, J.R.; Aparicio, C.; de la Cruz, M.O.; Stupp, S.I. A self-assembly pathway to aligned monodomain gels. Nat. Mater. 2010, 9, 594–601. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, W.; Ma, W.; Xian, Y.; Zhang, Z.; Pan, Z.; Li, Y.; Huang, L.; Liu, L.; Zheng, Z.; et al. Cartilage-Adaptive Hydrogels via the Synergy Strategy of Protein Templating and Mechanical Training. Adv. Mater. 2025, 37, 2414081. [Google Scholar] [CrossRef]
- Cao, Q.; Barrio, J.; Antonietti, M.; Kumru, B.; Shalom, M.; Schmidt, B.V.K.J. Photoactive Graphitic Carbon Nitride-Based Gel Beads As Recyclable Photocatalysts. ACS Appl. Polym. Mater. 2020, 2, 3346–3354. [Google Scholar] [CrossRef]
- Zhan, X.; Zhang, Z.; Lin, J.; Xu, J.; Wang, X.; Hong, B.; Xia, Y.; Zeng, Y. Surface atom rearrangement enabling graphitic carbon nitride/sodium alginate gel monolith for ultrafast completely photodegrading ciprofloxacin under visible light. Chem. Eng. J. 2024, 489, 151218. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Vijaya Suryaa, K.; Chinthala, M.; Kumar, A. Mechanistic insights of PO43− functionalized carbon nitride homojunction hydrogels in photocatalytic-self-Fenton-peroxymonosulfate system for tetracycline degradation. J. Colloid Interface Sci. 2024, 669, 366–382. [Google Scholar] [CrossRef]
- Liu, S.; Xu, T.; Zhu, L.; Liu, K.; Ji, X.; Yuan, Z.; Si, C. Highly compressible ultra-light 3D cellulose/graphene/carbon nitride aerogel for enhanced photocatalytic activity. Chem. Eng. J. 2025, 503, 158564. [Google Scholar] [CrossRef]
- Cai, X.; Tan, X.; Jiao, D.; Li, H.; Zhang, D.; Wang, Q. Rational construction of 3D recyclable g-C3N4 when encapsulated by cellulose/graphene oxide hybrid aerogels for efficient contaminant removal. Ind. Crop. Prod. 2023, 202, 117096. [Google Scholar] [CrossRef]
- Pan, H.; Gu, J.; Hou, K.; Li, J.; Wang, Y.; Yue, Y. High-efficiency, compressible, and recyclable reduced graphene oxide/chitosan composite aerogels supported g-C3N4/BiOBr photocatalyst for adsorption and degradation of rhodamine B. J. Environ. Chem. Eng. 2022, 10, 107157. [Google Scholar] [CrossRef]
- Du, Y.; Che, H.; Wang, P.; Chen, J.; Ao, Y. Highly efficient removal of organic contaminant with wide concentration range by a novel self-cleaning hydrogel: Mechanism, degradation pathway and DFT calculation. J. Hazard. Mater. 2022, 440, 129738. [Google Scholar] [CrossRef]
- Li, J.; Yang, R.; Luo, Z.; Cheng, X.; Zeng, G.; He, Y.; Zheng, M.; Li, X. The Solar Interfacial Evaporation Hydrogel for the Harmless Treatment of Fracturing Flow-back Wastewater. Sep. Purif. Technol. 2025, 375, 133733. [Google Scholar] [CrossRef]
- Chu, Y.-C.; Lin, T.-J.; Lin, Y.-R.; Chiu, W.-L.; Nguyen, B.-S.; Hu, C. Influence of P,S,O-Doping on g-C3N4 for hydrogel formation and photocatalysis: An experimental and theoretical study. Carbon 2020, 169, 338–348. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Li, H.; Wang, J.; Yu, Y. Photocatalytic activation of peroxymonosulfate by oxygen doped graphitic C3N4 with oxygen vacancy for drug degradation: Theoretical calculation and recyclable products. J. Environ. Chem. Eng. 2025, 13, 115318. [Google Scholar] [CrossRef]
- Anush, S.M.; Naga Raju, S.; Gayathri, B.H.; Ajeya, K.P.; Girish, Y.R.; Darshan, S.; Naveen, Y.P.; Prashantha, K.; Narendra, B.K.; Jayaram, A. Graphitic C3N4 incorporated chitosan-poly(vinyl alcohol) blend nanocomposites for the removal of Cu(II) and Cr(VI) ions from aqueous solutions. Express Polym. Lett. 2024, 18, 102–115. [Google Scholar] [CrossRef]
- Tsouris, C. Uranium extraction: Fuel from seawater. Nat. Energy 2017, 2, 17022. [Google Scholar] [CrossRef]
- Li, P.; He, T.; Wang, J.; Ou, N.; Liang, J.; Wang, G.; Fan, Q. Realizing Direct Uranium Extraction from Seawater Using a Carboxyl-g-C3N4/CdS Hydrogel. Small 2024, 20, 2404417. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, X.; Yang, C.; Qu, F.; Huang, J.; He, J.; Yang, Z.; Guo, W. Photocatalysis assisted solar-driven interfacial water evaporation: Principles, advances and trends. Sep. Purif. Technol. 2025, 360, 130975. [Google Scholar] [CrossRef]
- Song, Y.; Fang, S.; Xu, N.; Zhu, J. Solar-driven interfacial evaporation technologies for food, energy and water. Nat. Rev. Clean Technol. 2025, 1, 55–74. [Google Scholar] [CrossRef]
- Yang, D.; Guo, Y.; Yu, Z.; Jiang, Z.; Xiang, W.; Wu, X.; Wang, J. Surface Oxygen Vacancy Engineering for Enhanced Volatile Organic Compounds Removal in Solar-Interfacial Water Evaporation. Environ. Sci. Technol. 2025, 59, 7117–7128. [Google Scholar] [CrossRef] [PubMed]
- Kuate, L.J.N.; Chen, Z.; Yan, Y.; Lu, J.; Guo, F.; Wen, H.; Shi, W. Construction of 2D/3D black g-C3N4/BiOI S-scheme heterojunction for boosted photothermal-assisted photocatalytic tetracycline degradation in seawater. Mater. Res. Bull. 2024, 175, 112776. [Google Scholar] [CrossRef]
- Li, W.; Wang, G.; Li, T.; Zhang, Z.; Wang, Y.; Xu, Y.; Sui, W.; Xu, T.; Si, C. All-in-one self-cleaning lignin-derived spherical solar evaporator for continuous desalination and synergic water purification. Water Res. 2025, 282, 123932. [Google Scholar] [CrossRef]
- Niu, R.; Ding, Y.; Hao, L.; Ren, J.; Gong, J.; Qu, J. Plant-Mimetic Vertical-Channel Hydrogels for Synergistic Water Purification and Interfacial Water Evaporation. ACS Appl. Mater. Interfaces 2022, 14, 45533–45544. [Google Scholar] [CrossRef]
- Zhong, Z.; Burhan, M.; Ng, K.C.; Cui, X.; Chen, Q. Low-temperature desalination driven by waste heat of nuclear power plants: A thermo-economic analysis. Desalination 2024, 576, 117325. [Google Scholar] [CrossRef]
- Lee, W.H.; Lee, C.W.; Cha, G.D.; Lee, B.-H.; Jeong, J.H.; Park, H.; Heo, J.; Bootharaju, M.S.; Sunwoo, S.-H.; Kim, J.H.; et al. Floatable photocatalytic hydrogel nanocomposites for large-scale solar hydrogen production. Nat. Nanotechnol. 2023, 18, 754–762. [Google Scholar] [CrossRef]
- Kumar, N.; Gusain, R.; Pandey, S.; Ray, S.S. Hydrogel Nanocomposite Adsorbents and Photocatalysts for Sustainable Water Purification. Adv. Mater. Interfaces 2023, 10, 2201375. [Google Scholar] [CrossRef]
- Yang, J.; Liu, D.; Song, X.; Zhao, Y.; Wang, Y.; Rao, L.; Fu, L.; Wang, Z.; Yang, X.; Li, Y.; et al. Recent Progress of Cellulose-Based Hydrogel Photocatalysts and Their Applications. Gels 2022, 8, 270. [Google Scholar] [CrossRef]
- Zhong, Z.; Ding, Y.; Chen, Y.; Liao, P.; Chen, Q. Improving commercial-scale alkaline water electrolysis systems for fluctuating renewable energy: Unsteady-state thermodynamic analysis and optimization. Appl. Energy 2025, 395, 126183. [Google Scholar] [CrossRef]
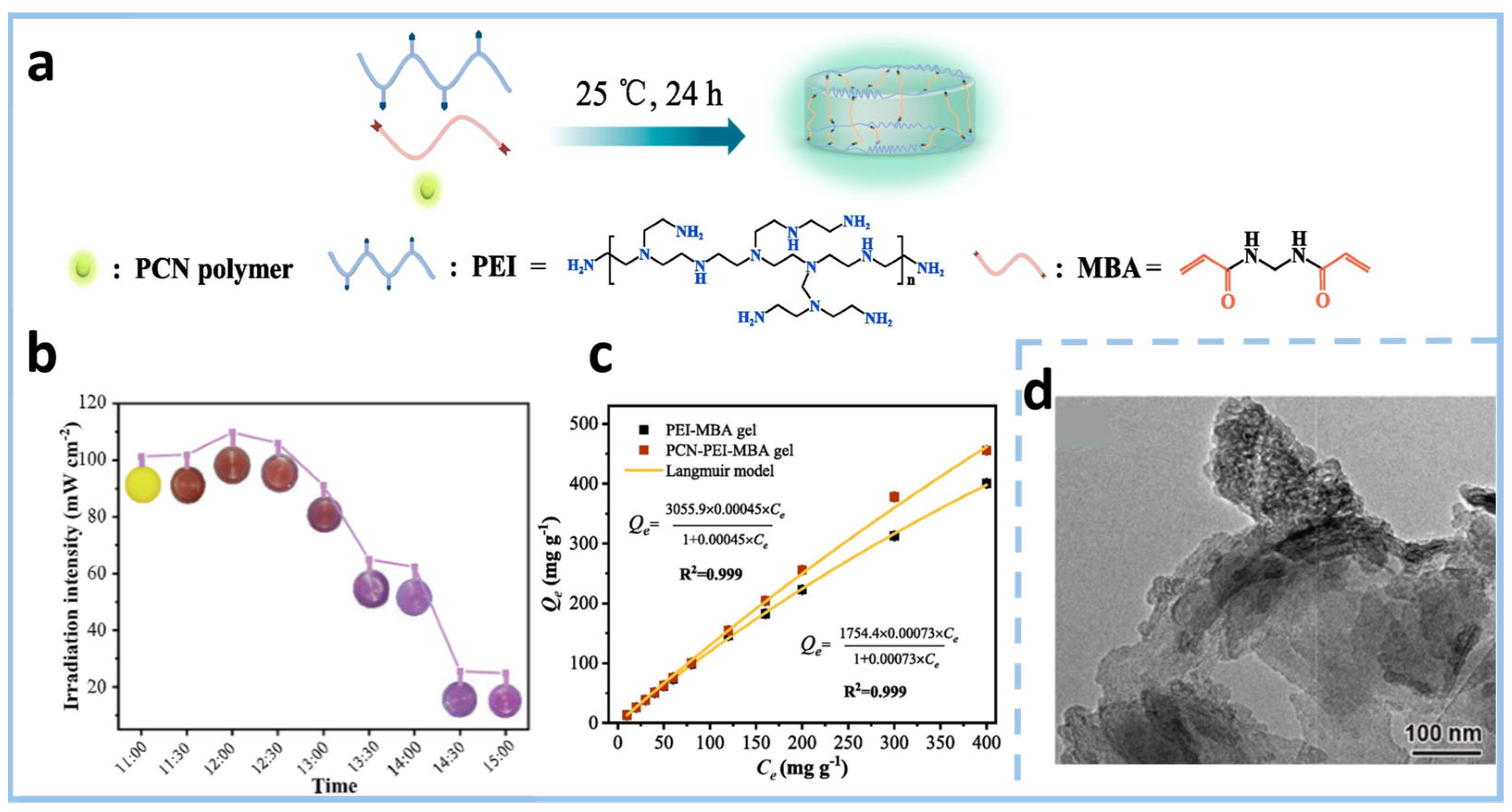
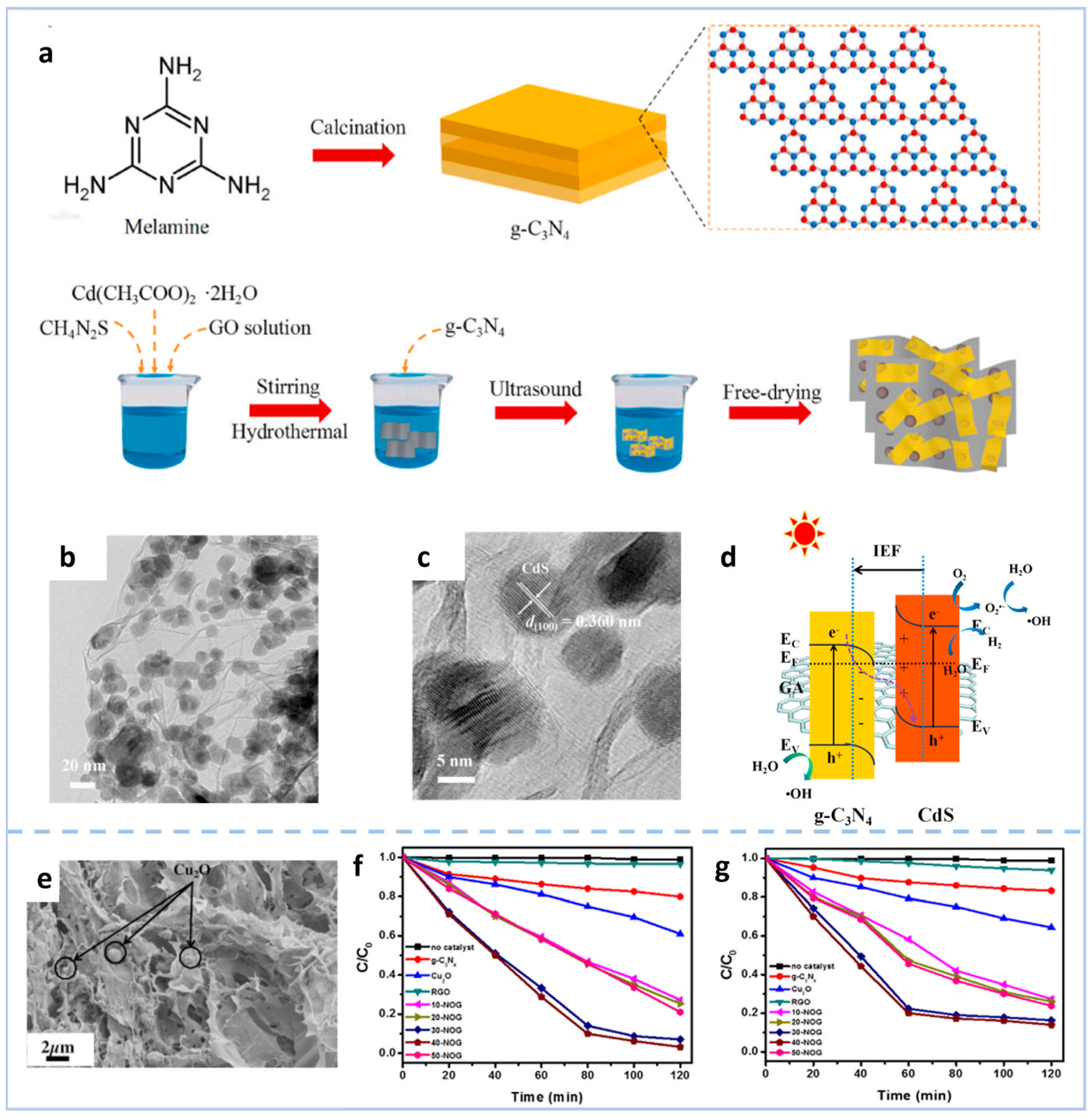
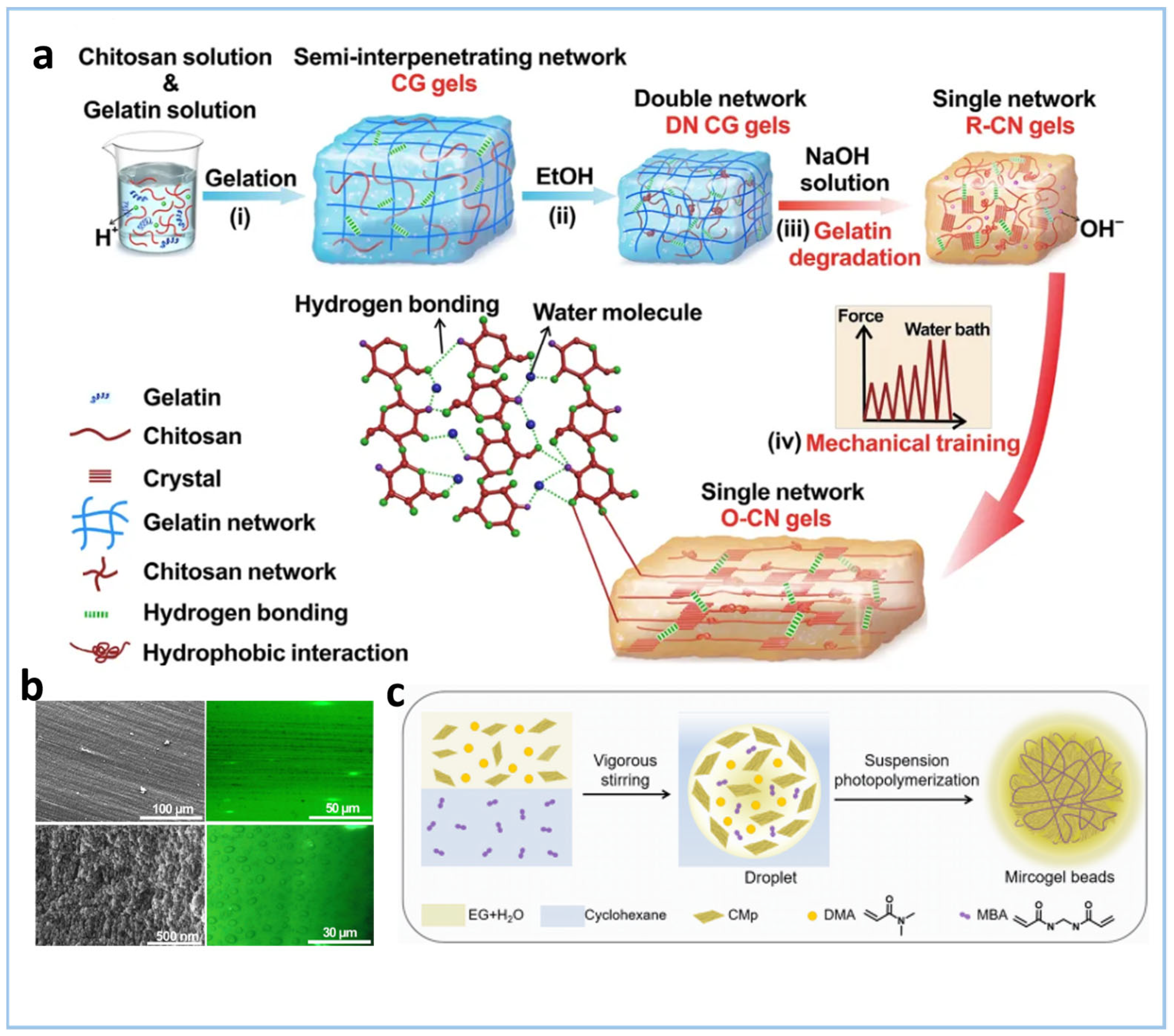
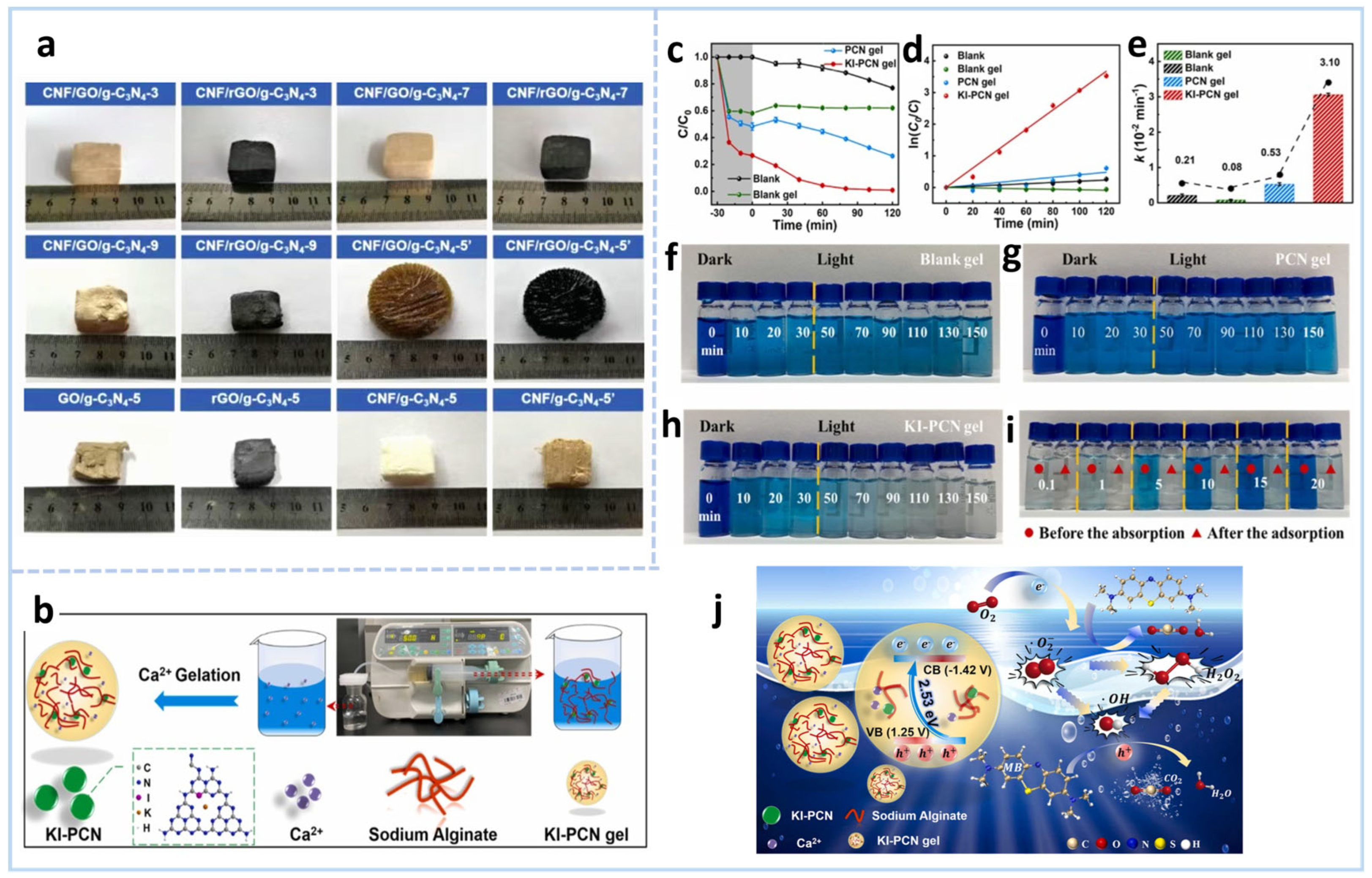

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Q.; Zhang, Z.; Pan, Y.; Leung, M.K.H.; Zhang, Y.; Chen, K. Carbon Nitride Gels: Synthesis, Modification, and Water Decontamination Applications. Gels 2025, 11, 685. https://doi.org/10.3390/gels11090685
Tang Q, Zhang Z, Pan Y, Leung MKH, Zhang Y, Chen K. Carbon Nitride Gels: Synthesis, Modification, and Water Decontamination Applications. Gels. 2025; 11(9):685. https://doi.org/10.3390/gels11090685
Chicago/Turabian StyleTang, Qinglan, Zhen Zhang, Yuwei Pan, Michael K. H. Leung, Yizhen Zhang, and Keda Chen. 2025. "Carbon Nitride Gels: Synthesis, Modification, and Water Decontamination Applications" Gels 11, no. 9: 685. https://doi.org/10.3390/gels11090685
APA StyleTang, Q., Zhang, Z., Pan, Y., Leung, M. K. H., Zhang, Y., & Chen, K. (2025). Carbon Nitride Gels: Synthesis, Modification, and Water Decontamination Applications. Gels, 11(9), 685. https://doi.org/10.3390/gels11090685