Gel-Type Electrofluorochromic Devices for Advanced Optoelectronic Applications
Abstract
1. Introduction
2. Fundamentals of Electrofluorochromism
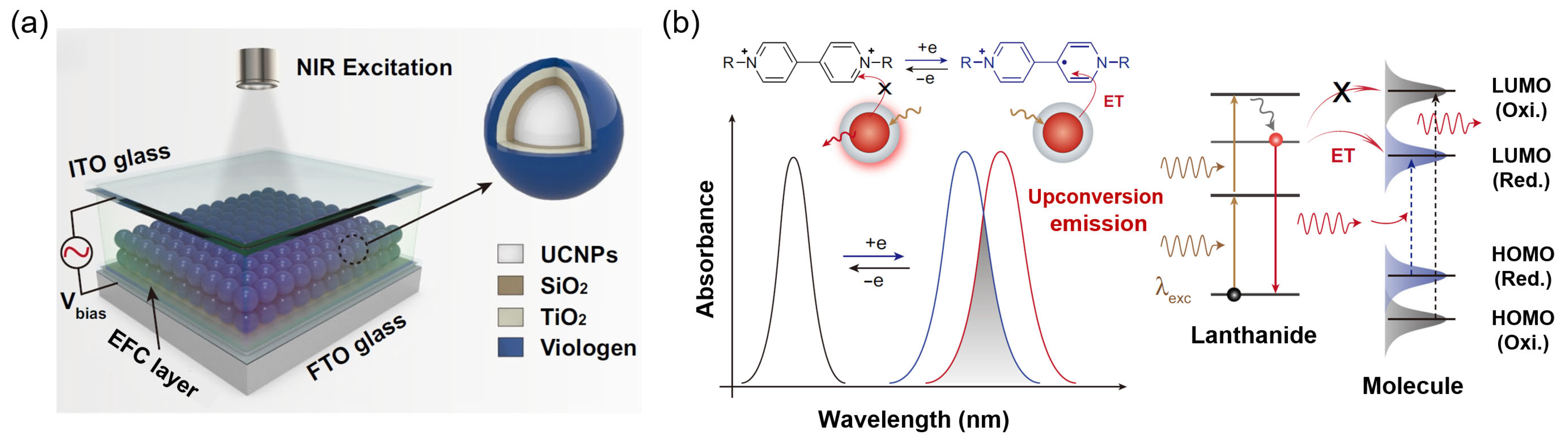
2.1. EFC Mechanism
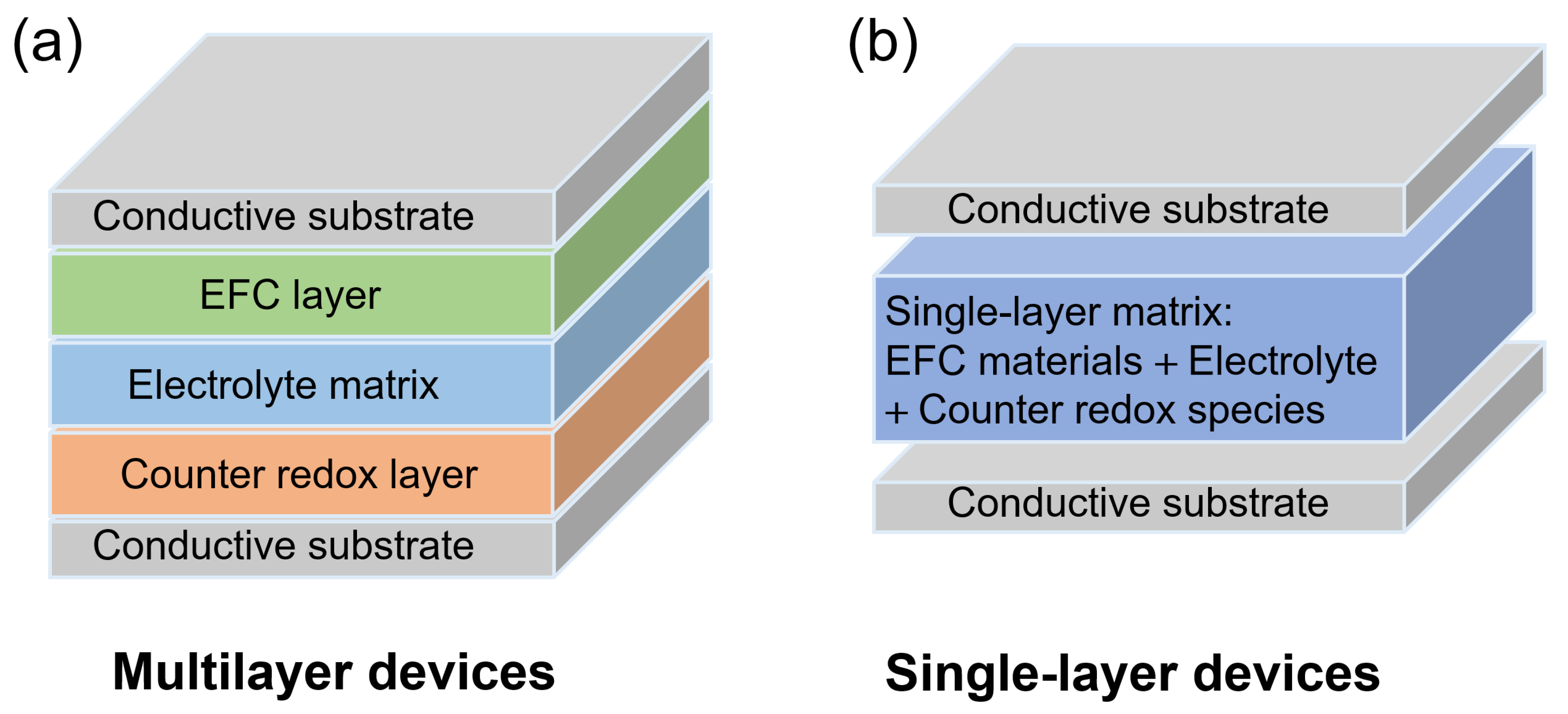
2.2. EFC Devices
2.3. Performance Parameters of EFC Device
3. Gel Matrices for EFC Devices
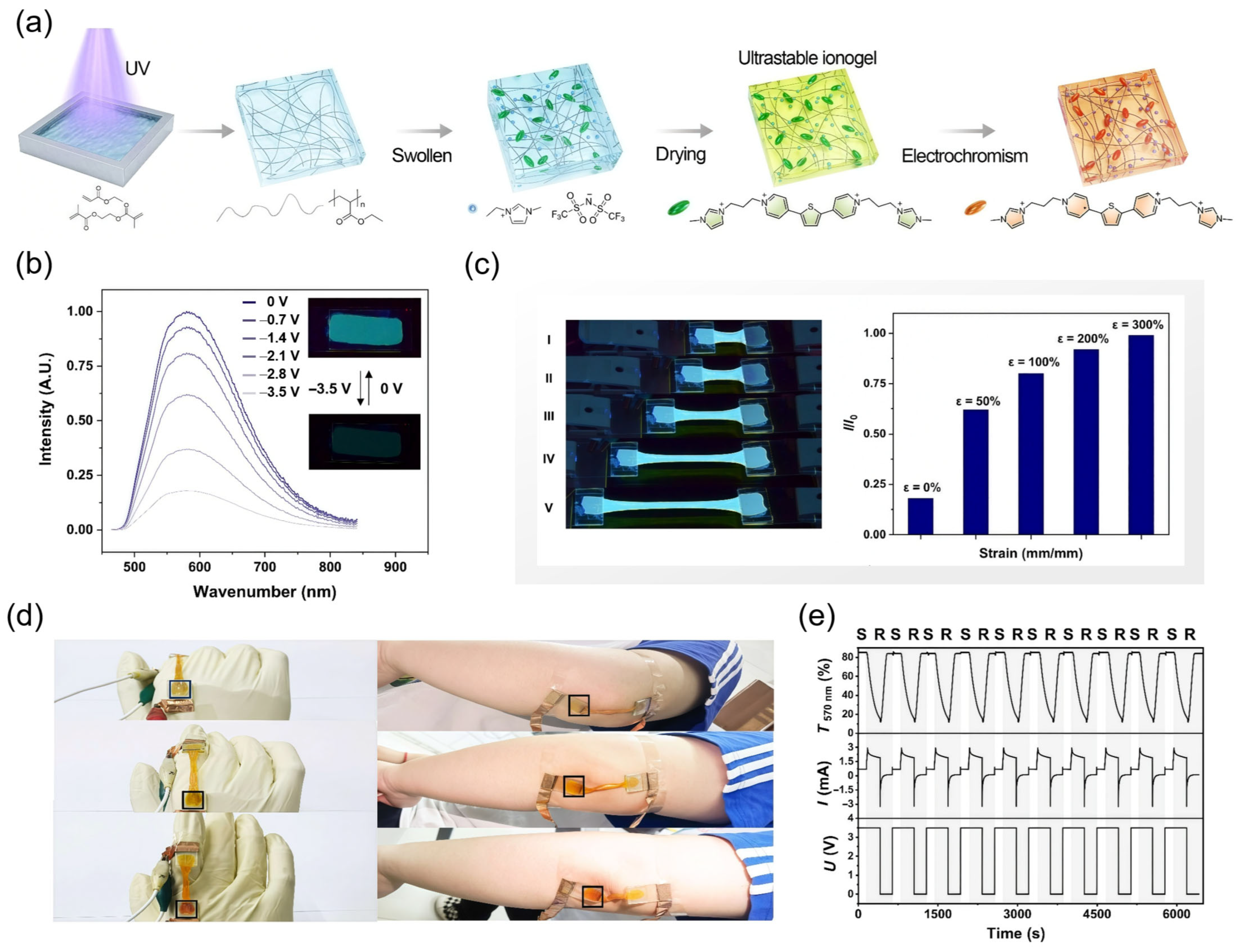
3.1. Ionogels
3.2. Organogels
3.3. Hydrogels
4. Applications of Gel-Type EFC Devices
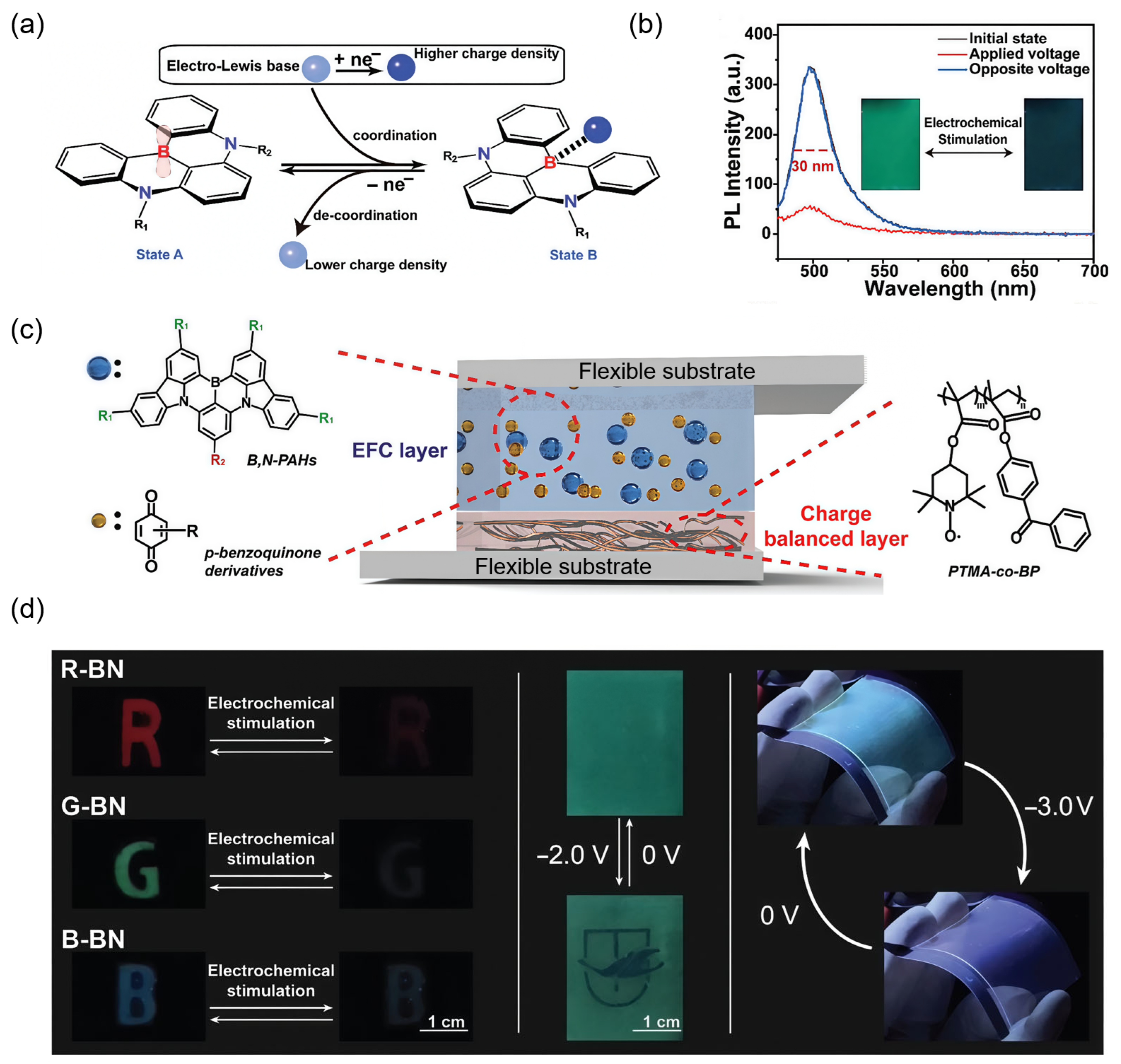
4.1. Displays
| EFC Materials | Gel Type | Application | Ref. |
|---|---|---|---|
| B,N-PAHs/p-BQ | Organogel | Displays | [42] |
| Ir(III) complexes with pyridinium units | Ionogel | Displays | [91,92,93] |
| EFIL-TTM | Organogel | Displays | [94] |
| Fc-TPE | Hydrogel | Displays | [95] |
| Viologen-substituted Ir(III) complexes | Ionogel | Data encryption | [86] |
| 7-Hydroxycoumarin/rhodol/2-(2-(4-hydroxystyryl)-6-methyl-4H-pyran-4-ylidene)malononitrile | Organogel | Data encryption | [96] |
| TPA segments with AIE cores | Organogel | Data encryption | [97] |
| AIE-active 9,10-azaboraphenanthrene (BNP) units | Organogel | Data encryption | [98] |
| Ir(III) complexes with N-H units | Ionogel | Electronic paper | [99,100] |
| Ir(III) complexes with O-H units | Ionogel | Electronic paper | [101] |
| Transition-metal complexes with pyridinium units | Ionogel | Electronic paper | [102] |
| OCBs | Hydrogel | Electronic paper | [103] |
| [ImTV][TFSI] | Ionogel | Sensors | [41] |
| BQ/SPM | Organogel | Sensors | [40] |
| EEIL | Ionogel | Smart windows/Displays | [104] |
| Tetrabenzofluorene derivatives | Organogel | Smart windows/Displays | [105] |
| Thienoviologens | Organogel | Smart windows/Displays | [106] |
| TTz derivatives | Hydrogel | EC/EFC hybrid systems | [90] |
| PolyZn | Organogel | EC/EFC hybrid systems | [107] |
| PPAOF/SRB | Organogel | Thermo-responsive EFC device | [108] |
| Poly(NIPAM-co-TV) | Organogel | Thermo-responsive EFC device | [109] |
| LY-POMs/Rh6G | Organogel | Energy-interactive EFC device | [110] |
| TPA-bpy | Organogel | Energy-interactive EFC device | [111] |
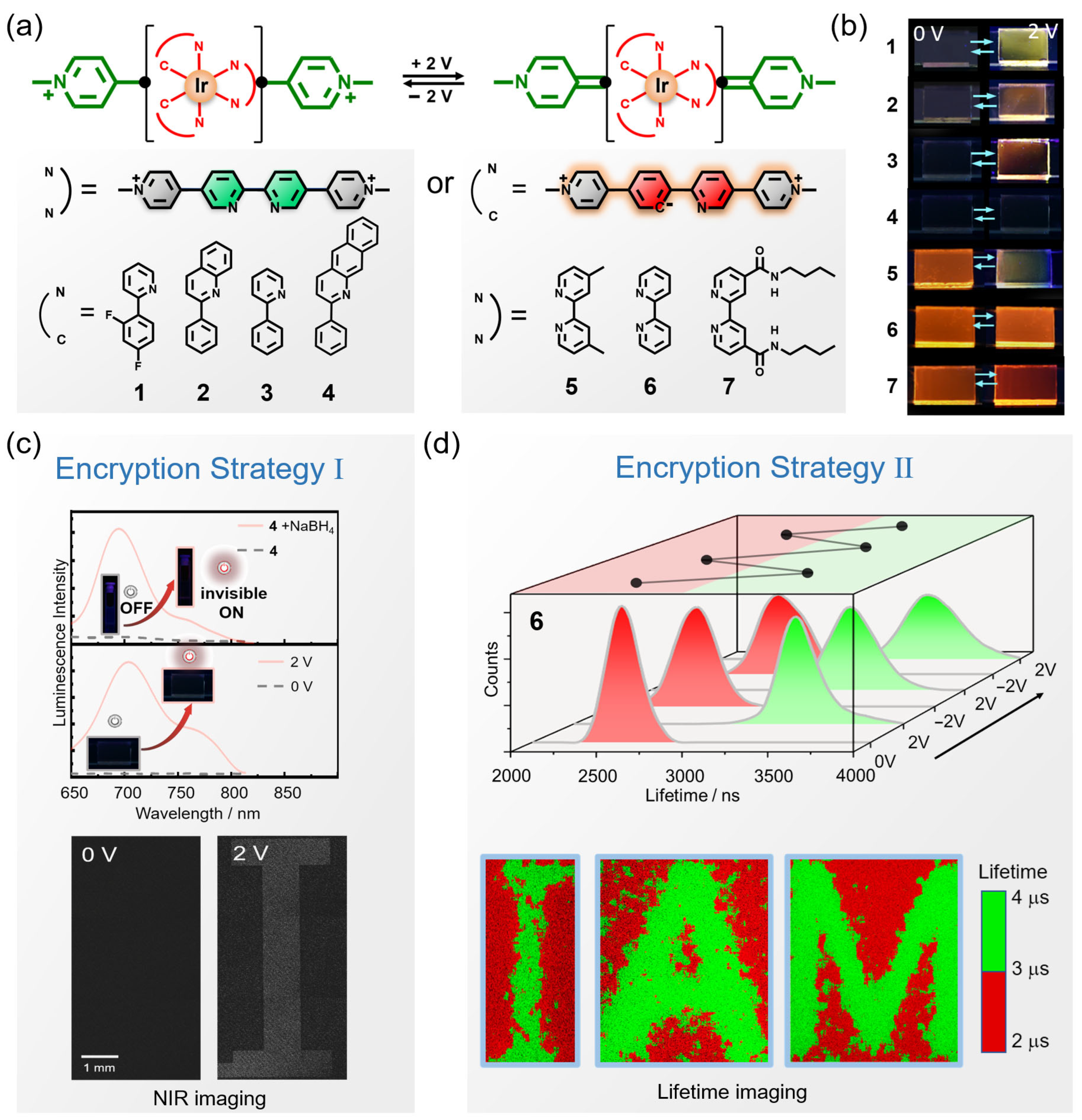
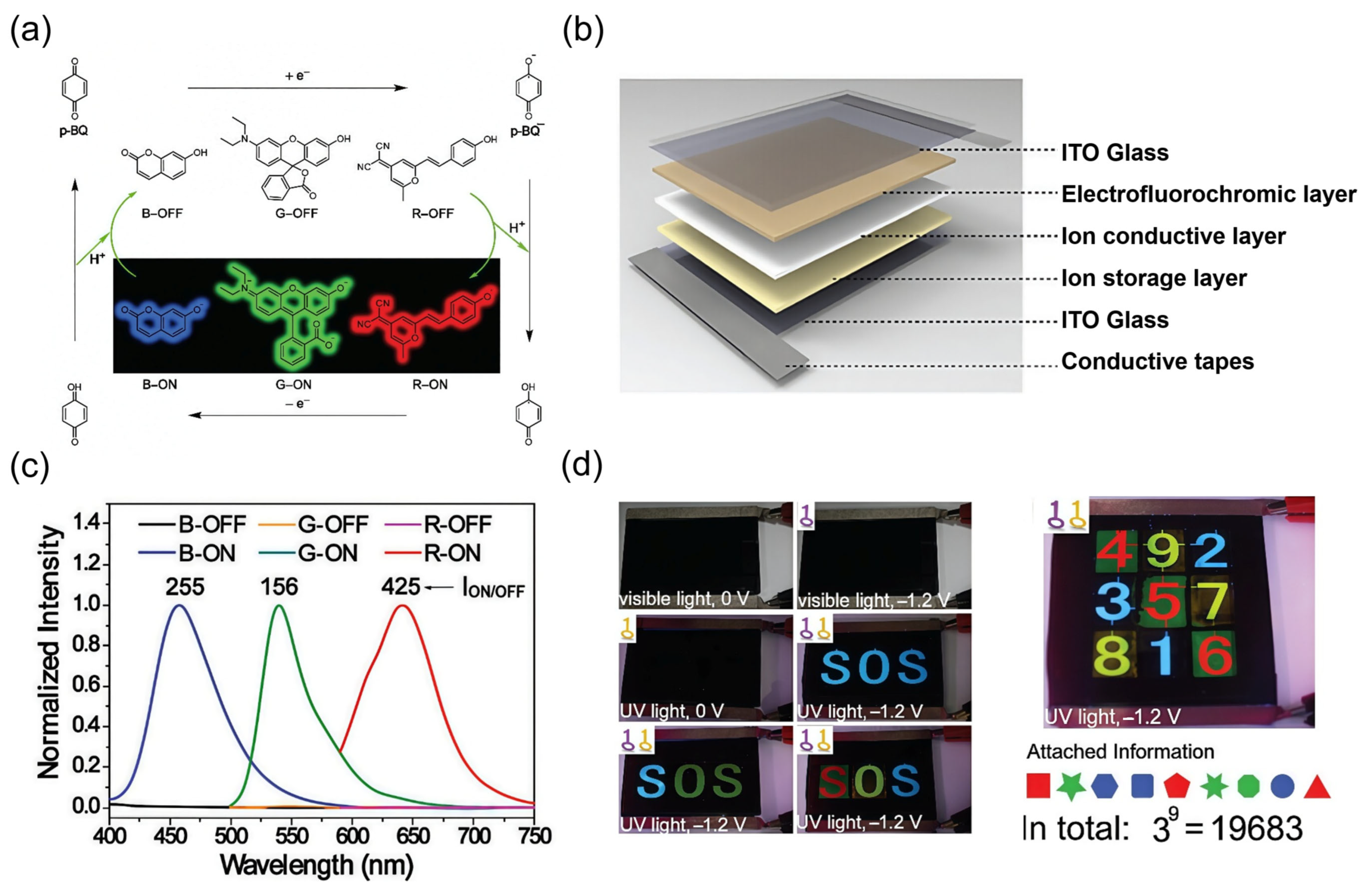
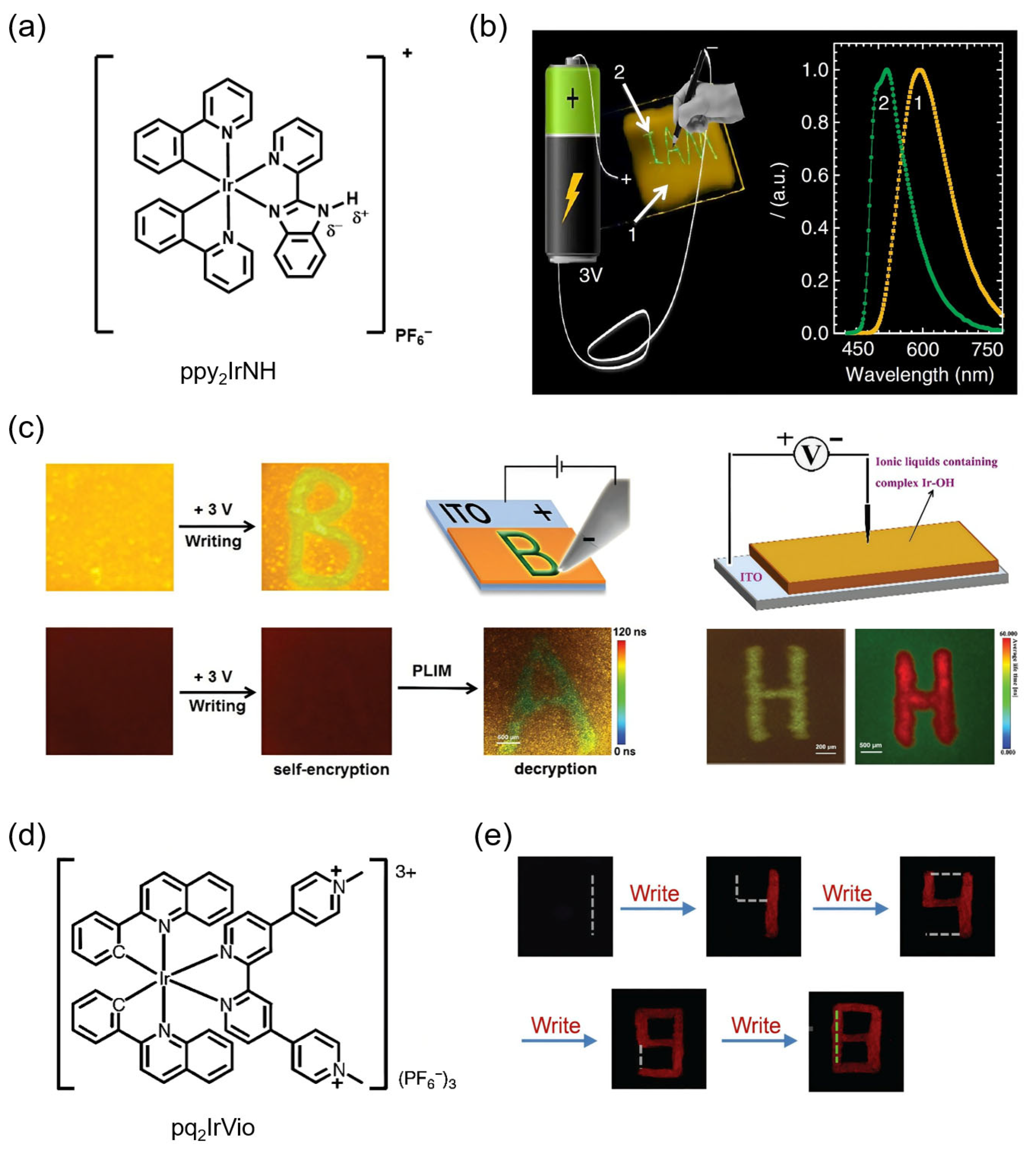
4.2. Data Encryption and Anti-Counterfeiting
4.3. Electronic Paper
4.4. Sensors
4.5. Multifunctional Devices
4.5.1. Dual-Functional EFC Devices for Smart Windows and Displays
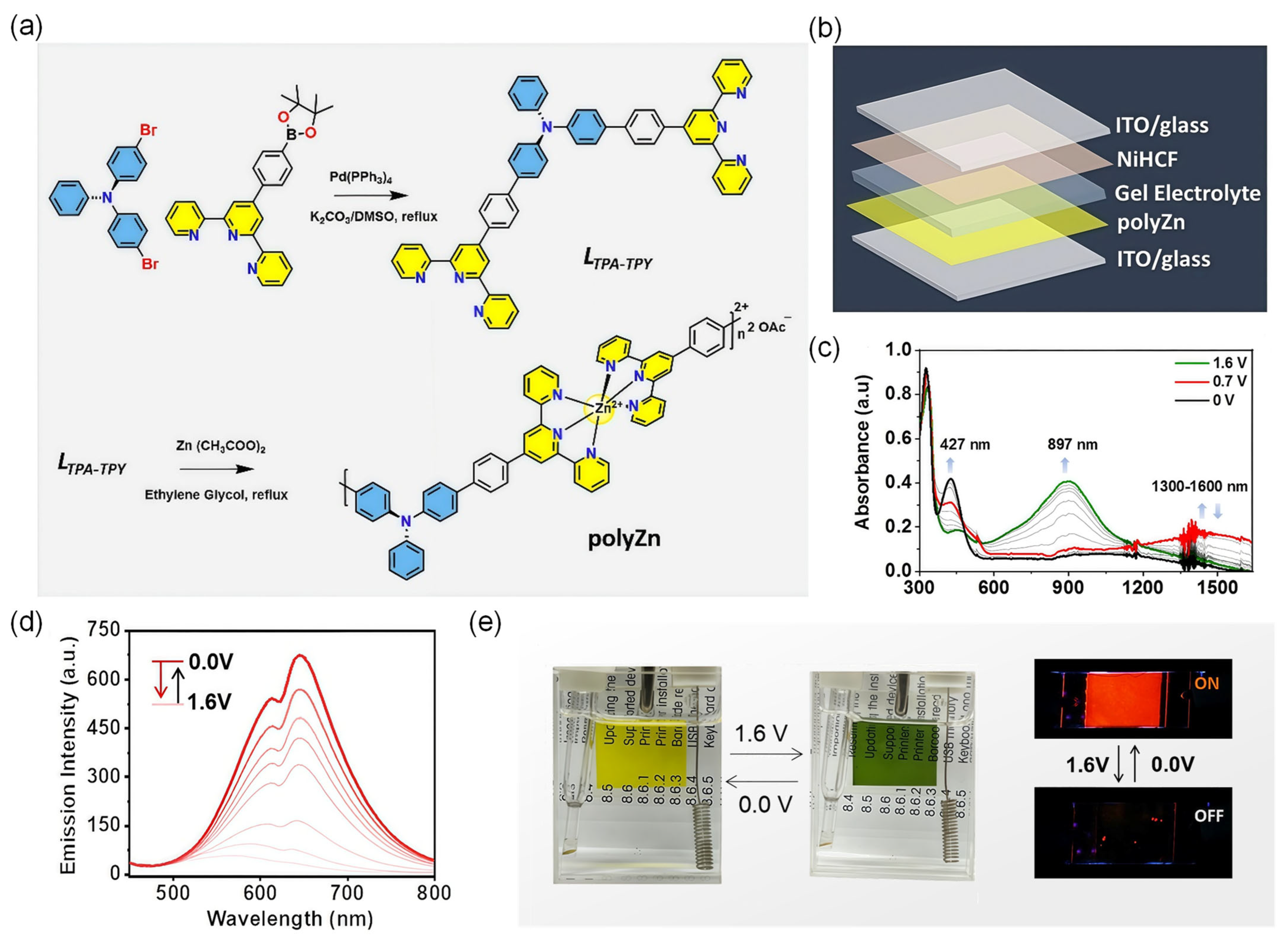
4.5.2. EC/EFC Hybrid Display Systems
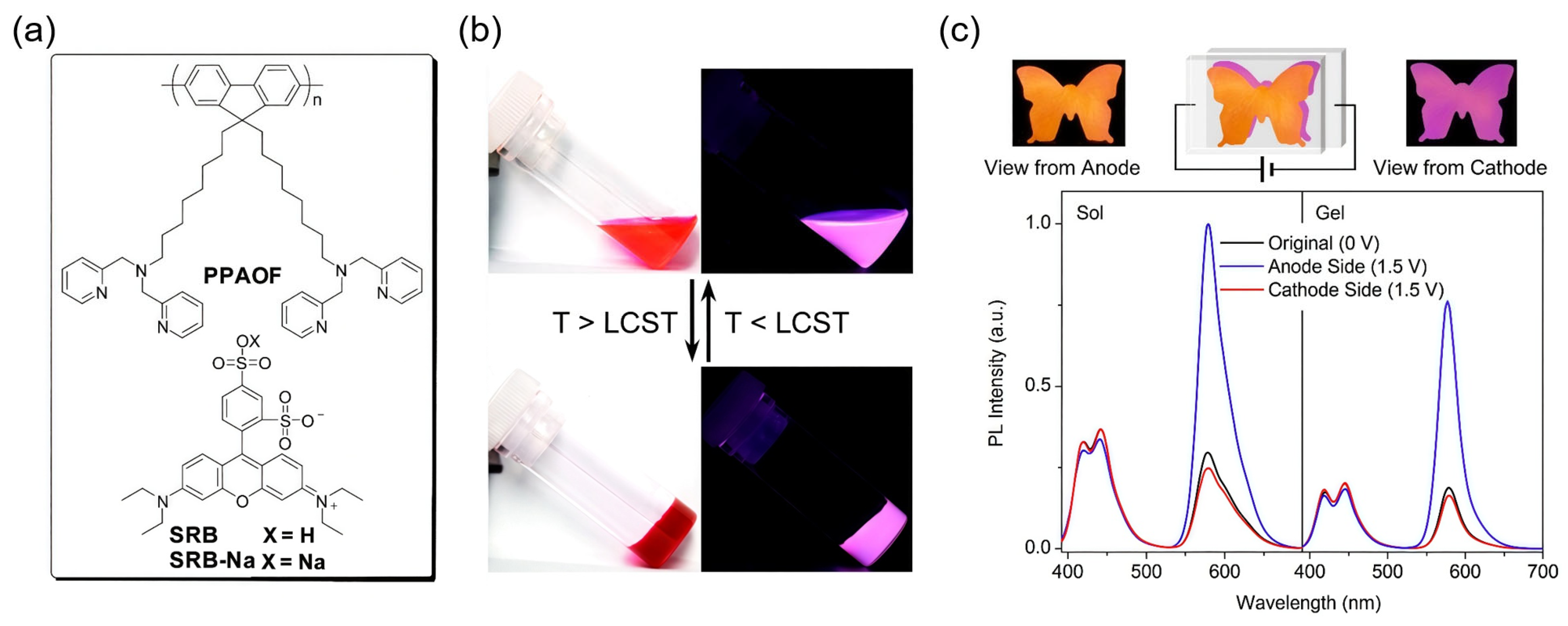
4.5.3. Thermo-Responsive Systems with EFC Display
4.5.4. Energy-Interactive EFC Display Devices
5. Conclusions
6. Perspectives
- (a)
- The molecular design of fluorophores with multi-state switching, environmental responsiveness, and improved fatigue resistance.
- (b)
- The formulation of multifunctional gel matrices that combine ionic conduction, structural support, and protective encapsulation in a single component.
- (c)
- The development of hybrid systems that combine EFC with other functionalities, such as energy storage, biosensing, or thermochromism.
- (d)
- The creation of low-power, fully printed, or self-powered EFC circuits for flexible integration.
- (e)
- The establishment of standardized protocols for evaluating long-term stability, optical contrast, and integration readiness.
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIE | Aggregation-Induced Emission |
| BQ | Benzoquinone |
| CR | Contrast Ratios |
| D-A | Donor-Acceptor |
| DFT | Density Functional Theory |
| EC | Electrochromic |
| EFC | Electrofluorochromic |
| FRET | Förster Resonance Energy Transfer |
| IEFs | Intrinsically Electroactive Fluorophores |
| ILs | Ionic Liquids |
| LCST | Lower Critical Solution Temperature |
| MD | Molecular Dynamics |
| NIR | Near-Infrared |
| PEA | Poly(ethyl acrylate) |
| PVA | Poly(vinyl alcohol) |
| PeT | Photoinduced Electron Transfer |
| PET | Polyethylene Terephthalate |
| PMMA | Poly(methyl methacrylate) |
| POMs | Polyoxometalates |
| TDDFT | Time-Dependent DFT |
| TICT | Twisted Intramolecular Charge-Transfer |
| TTz | Thiazolothiazole |
| TPA | Triphenylamine |
| TPE | Tetraphenylethylene |
References
- Jiang, T.; Zhu, Y.-F.; Zhang, J.-C.; Zhu, J.; Zhang, M.; Qiu, J. Multistimuli-responsive display materials to encrypt differentiated information in bright and dark fields. Adv. Funct. Mater. 2019, 29, 1906068. [Google Scholar] [CrossRef]
- Liu, D.-Y.; Li, H.-Y.; Han, R.-P.; Liu, H.-L.; Zang, S.-Q. Multiple stimuli-responsive luminescent chiral hybrid antimony chlorides for anti-counterfeiting and encryption applications. Angew. Chem. Int. Ed. 2023, 62, e202307875. [Google Scholar] [CrossRef]
- Hu, L.; Yang, Y.; Gao, Y.; Wei, Y.; Zhu, J.; Wu, W. Lanthanide-based stimulus-responsive supramolecular fluorescent materials for information storage and time-dependent information encryption. Chem. Eng. J. 2024, 488, 150965. [Google Scholar] [CrossRef]
- Li, A.; Liu, H.; Song, C.; Geng, Y.; Xu, S.; Zhang, H.; Zhang, H.; Cui, H.; Xu, W. Flexible control of excited state transition under pressure/temperature: Distinct stimuli-responsive behaviours of two ESIPT polymorphs. Mater. Chem. Front. 2019, 3, 2128–2136. [Google Scholar] [CrossRef]
- Fu, H.-Y.; Xu, N.; Pan, Y.-M.; Lu, X.-L.; Xia, M. Emission behaviours of novel V- and X-shaped fluorophores in response to pH and force stimuli. Phys. Chem. Chem. Phys. 2017, 19, 11563–11570. [Google Scholar] [CrossRef]
- Shi, X.; Yan, N.; Niu, G.; Sung, S.H.P.; Liu, Z.; Liu, J.; Kwok, R.T.K.; Lam, J.W.Y.; Wang, W.-X.; Sung, H.H.-Y.; et al. In vivo monitoring of tissue regeneration using a ratiometric lysosomal AIE probe. Chem. Sci. 2020, 11, 3152–3163. [Google Scholar] [CrossRef]
- Seo, S.; Kim, Y.; Zhou, Q.; Clavier, G.; Audebert, P.; Kim, E. White electrofluorescence switching from electrochemically convertible yellow fluorescent dyad. Adv. Funct. Mater. 2012, 22, 3556–3561. [Google Scholar] [CrossRef]
- Woodward, A.N.; Kolesar, J.M.; Hall, S.R.; Saleh, N.-A.; Jones, D.S.; Walter, M.G. Thiazolothiazole fluorophores exhibiting strong fluorescence and viologen-like reversible electrochromism. J. Am. Chem. Soc. 2017, 139, 8467–8473. [Google Scholar] [CrossRef]
- Seddiki, I.; N’Diaye, B.I.; Skene, W.G. Survey of recent advances in molecular fluorophores, unconjugated polymers, and emerging functional materials designed for electrofluorochromic use. Molecules 2023, 28, 3225. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Q.; Sun, T.; Qin, M.; Yue, W.; Gao, S. A perceptual and interactive integration strategy toward telemedicine healthcare based on electroluminescent display and triboelectric sensing 3D stacked device. Adv. Funct. Mater. 2024, 34, 2402356. [Google Scholar] [CrossRef]
- Li, X.-C.; Yao, L.; Song, W.; Liu, F.; Wang, Q.; Chen, J.; Xue, Q.; Lai, W.-Y. Intrinsically stretchable electroluminescent elastomers with self-confinement effect for highly efficient non-blended stretchable OLEDs. Angew. Chem. Int. Ed. 2022, 62, e202213749. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.-M.; Xie, F.; Jin, X.; Li, J.; Yang, G.; Gu, C.; Wang, Y.; Zhang, S.X.-A. Single-pixel RGB device in a colorful alphanumeric electrofluorochromic display. Adv. Mater. 2020, 32, 2003121. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, M.; Rödlmeier, T.; Schlisske, S.; Zimmermann, J.; Romero-Nieto, C.; Hernandez-Sosa, G. Inkjet-printed polymer-based electrochromic and electrofluorochromic dual-mode displays. J. Mater. Chem. C 2019, 7, 7121–7127. [Google Scholar] [CrossRef]
- Yang, C.; Cai, W.; Zhang, X.; Gao, L.; Lu, Q.; Chen, Y.; Zhang, Z.; Zhao, P.; Niu, H.; Wang, W. Multifunctional conjugated oligomers containing novel triarylamine and fluorene units with electrochromic, electrofluorochromic, photoelectron conversion, explosive detection and memory properties. Dye. Pigment. 2019, 160, 99–108. [Google Scholar] [CrossRef]
- Li, D.; Wang, X.; Xia, L.; Luo, Q.; Xu, Y.; Zeng, B.; Luo, W.; Dai, L. Semi aromatic colorless polyimide coatings for dual electrochromic and electrofluorochromic displays and its potential for information encryption. Prog. Org. Coat. 2023, 184, 107867. [Google Scholar] [CrossRef]
- Santra, D.C.; Nad, S.; Malik, S. Electrochemical polymerization of triphenylamine end-capped dendron: Electrochromic and electrofluorochromic switching behaviors. J. Electroanal. Chem. 2018, 823, 203–212. [Google Scholar] [CrossRef]
- Hafed-Khatiri, S.; Quintero-Jaime, A.F.; Salinas-Torres, D.; Montilla, F. Electrofluorochromism of conjugated polymers applied to the development of chemical sensors. ACS Appl. Electron. Mater. 2024, 6, 847–852. [Google Scholar] [CrossRef]
- Yu, T.; Theato, P.; Yao, H.; Liu, H.; Di, Y.; Sun, Z.; Guan, S. Colorless electrochromic/electrofluorochromic dual-functional triphenylamine-based polyimides: Effect of a tetraphenylethylene-based π-bridge on optoelectronic properties. Chem. Eng. J. 2023, 451, 138441. [Google Scholar] [CrossRef]
- Corrente, G.A.; Parisi, F.; Maltese, V.; Cospito, S.; Imbardelli, D.; La Deda, M.; Beneduci, A. Panchromatic fluorescence emission from thienosquaraines dyes: White light electrofluorochromic devices. Molecules 2021, 26, 6818. [Google Scholar] [CrossRef]
- Deng, B.; Zhu, Y.; Ali, M.U.; Li, K.; Liu, X.; Zhang, X.; Ning, J.; Hu, Z.; Chen, H.; He, J.; et al. Pyrrole-based viologen derivatives with high contrast and magenta color for electrochromic-fluorescent devices. Sol. Energy Mater. Sol. Cells 2023, 251, 112149. [Google Scholar] [CrossRef]
- Debnath, S.; Haupa, K.A.; Lebedkin, S.; Strelnikov, D.; Kappes, M.M. Triggering near-infrared luminescence of vanadyl phthalocyanine by charging. Angew. Chem. Int. Ed. 2022, 61, e202201577. [Google Scholar] [CrossRef]
- Nakamura, K.; Yanagawa, N.; Kobayashi, N. Magenta-blue electrofluorochromic device incorporating Eu(III) complex, anthracene derivative, and viologen molecule. Materials 2022, 15, 5202. [Google Scholar] [CrossRef]
- Yabuta, R.; Kobayashi, N.; Nakamura, K. Electrofluorochromism based on the valence change of europium complexes in electrochemical devices with Prussian blue as the counter electrode. Phys. Chem. Chem. Phys. 2024, 26, 28800–28807. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-H.; Liou, G.-S. High-performance electrofluorochromic devices based on electrochromism and photoluminescence-active novel poly(4-cyanotriphenylamine). Adv. Funct. Mater. 2014, 24, 6422–6429. [Google Scholar] [CrossRef]
- Audebert, P.; Miomandre, F. Electrofluorochromism: From molecular systems to set-up and display. Chem. Sci. 2013, 4, 575–584. [Google Scholar] [CrossRef]
- Zhuang, Y.; Li, J.; Zhu, M.; Li, F.; Liu, S.; Zhao, Q. Electrochromism and electrofluorochromism based on viologen derivative with 1,10-phenanthroline moiety for multicolor large-area and patterned display. Adv. Mater. Technol. 2023, 8, 2301092. [Google Scholar] [CrossRef]
- Beneduci, A.; Cospito, S.; La Deda, M.; Veltri, L.; Chidichimo, G. Electrofluorochromism in pi-conjugated ionic liquid crystals. Nat. Commun. 2014, 5, 3105. [Google Scholar] [CrossRef]
- Pal, P.; Paul, B.; Datta, A.; Malik, S. Aryl-substituted buta-1,3-diene and phenothiazine based solid-state emissive copolymers: Photophysical properties and electrochromic behaviors. ACS Appl. Polym. Mater. 2024, 6, 10882–10890. [Google Scholar] [CrossRef]
- Liu, J.; Luo, G.; Mi, S.; Zheng, J.; Xu, C. Trifunctional CdSe quantum dots-polymer composite film with electrochromic, electrofluorescent and light-induced coloration effects. Sol. Energy Mater. Sol. Cells 2018, 177, 82–88. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; El-Demellawi, J.K.; Jiang, Q.; Ge, G.; Liang, H.; Lee, K.; Dong, X.; Alshareef, H.N. MXene hydrogels: Fundamentals and applications. Chem. Soc. Rev. 2020, 49, 7229–7251. [Google Scholar] [CrossRef]
- Khan, S.A.; Ahmad, H.; Zhu, G.; Pang, H.; Zhang, Y. Three-dimensional printing of hydrogels for flexible sensors: A review. Gels 2024, 10, 187. [Google Scholar] [CrossRef]
- Xuan, H.D.; Timothy, B.; Park, H.-Y.; Lam, T.N.; Kim, D.; Go, Y.; Kim, J.; Lee, Y.; Ahn, S.I.; Jin, S.-H.; et al. Super stretchable and durable electroluminescent devices based on double-network ionogels. Adv. Mater. 2021, 33, 2008849. [Google Scholar] [CrossRef]
- Yu, Y.; He, K.; Xu, H.; Xiao, Z.; Chen, L.; Xu, S.; Bai, G. Flexible multi-color electroluminescent devices with a high transmission conducting hydrogel and an organic dielectric. Nanoscale 2023, 15, 9196–9202. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, P.; Shamsi, M.; Thelen, J.L.; Qian, W.; Truong, V.K.; Ma, J.; Hu, J.; Dickey, M.D. Tough and stretchable ionogels by in situ phase separation. Nat. Mater. 2022, 21, 359–365. [Google Scholar] [CrossRef]
- Cai, Y.; Yang, B.; Ji, J.; Sun, F.; Zhao, Y.; Yu, L.; Zhao, C.; Liu, M.; Liu, M.; He, Y.; et al. A universal tandem device of dc-driven electrochromism and ac-driven electroluminescence for multi-functional smart windows. Adv. Mater. Technol. 2023, 8, 2201682. [Google Scholar] [CrossRef]
- Xiong, X.; Chen, Y.; Wang, Z.; Liu, H.; Le, M.; Lin, C.; Wu, G.; Wang, L.; Shi, X.; Jia, Y.-G.; et al. Polymerizable rotaxane hydrogels for three-dimensional printing fabrication of wearable sensors. Nat. Commun. 2023, 14, 1331. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, H.; Yang, H.-B.; Yao, X.; Qin, H.; Cong, H.-P.; Yu, S.-H. Hierarchically aligned heterogeneous core-sheath hydrogels. Nat. Commun. 2025, 16, 400. [Google Scholar] [CrossRef]
- Kuzina, M.A.; Kartsev, D.D.; Stratonovich, A.V.; Levkin, P.A. Organogels versus hydrogels: Advantages, challenges, and applications. Adv. Funct. Mater. 2023, 33, 2301421. [Google Scholar] [CrossRef]
- Lu, P.; Liao, X.; Guo, X.; Cai, C.; Liu, Y.; Chi, M.; Du, G.; Wei, Z.; Meng, X.; Nie, S. Gel-based triboelectric nanogenerators for flexible sensing: Principles, properties, and applications. Nano-Micro Lett. 2024, 16, 206. [Google Scholar] [CrossRef]
- Tian, Z.; Mi, L.; Wu, Y.; Shao, F.; Zou, M.; Zhou, Z.; Liu, S. Visual electrofluorochromic detection of cancer cell surface glycoprotein on a closed bipolar electrode chip. Anal. Chem. 2019, 91, 7902–7910. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, M.; Li, G.; Chen, X.; Liu, Z.; Shao, J.; Huang, Y.; He, G. Ultrastable viologen ionic liquids-based ionogels for visible strain sensor integrated with electrochromism, electrofluorochromism, and strain sensing. CCS Chem. 2023, 5, 1917–1930. [Google Scholar] [CrossRef]
- Yang, B.; Bai, H.; Li, C.; Zhang, Y.-M.; Zhang, S.X.-A. Biomimetic exploration and reflection on switchable coordination and narrow-band electrofluorochromic devices. Adv. Sci. 2024, 11, 2407219. [Google Scholar] [CrossRef] [PubMed]
- Al-Kutubi, H.; Zafarani, H.R.; Rassaei, L.; Mathwig, K. Electrofluorochromic systems: Molecules and materials exhibiting redox-switchable fluorescence. Eur. Polym. J. 2025, 236, 114143. [Google Scholar] [CrossRef]
- Chua, M.H.; Zhu, Q.; Shah, K.W.; Xu, J. Electroluminochromic materials: From molecules to polymers. Polymers 2019, 11, 98. [Google Scholar] [CrossRef]
- Sun, J.; Chen, Y.; Liang, Z. Electroluminochromic materials and devices. Adv. Funct. Mater. 2016, 26, 2783–2799. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, S.; Wu, Y.; Zhang, W.; Song, W. Carbazole-based dual-band electrochromic polymers: The effect of linkage sites on electrochromic performance. ACS Appl. Mater. Interfaces 2024, 16, 58952–58960. [Google Scholar] [CrossRef]
- Madasamy, K.; Velayutham, D.; Suryanarayanan, V.; Kathiresan, M.; Ho, K.-C. Viologen-based electrochromic materials and devices. J. Mater. Chem. C 2019, 7, 4622–4637. [Google Scholar] [CrossRef]
- Goulle, V.; Harriman, A.; Lehn, J.-M. An electro-photoswitch: Redox switching of the luminescence of a bipyridine metal-complex. J. Cem. Soc. Chem. Commun. 1993, 1034–1036. [Google Scholar] [CrossRef]
- Yano, M.; Matsuhira, K.; Tatsumi, M.; Kashiwagi, Y.; Nakamoto, M.; Oyama, M.; Ohkubo, K.; Fukuzumi, S.; Misaki, H.; Tsukube, H. “ON–OFF” switching of europium complex luminescence coupled with a ligand redox process. Chem. Commun. 2012, 48, 4082–4084. [Google Scholar] [CrossRef]
- Miomandre, F.; Pansu, R.B.; Audibert, J.F.; Guerlin, A.; Mayer, C.R. Electrofluorochromism of a ruthenium complex investigated by time resolved TIRF microscopy coupled to an electrochemical cell. Electrochem. Commun. 2012, 20, 83–87. [Google Scholar] [CrossRef]
- Lin, H.-T.; Huang, C.-L.; Liou, G.-S. Design, Synthesis, and electrofluorochromism of new triphenylamine derivatives with aie-active pendent groups. ACS Appl. Mater. Interfaces 2019, 11, 11684–11690. [Google Scholar] [CrossRef] [PubMed]
- Quinton, C.; Alain-Rizzo, V.; Dumas-Verdes, C.; Miomandre, F.; Clavier, G.; Audebert, P. Redox- and protonation-induced fluorescence switch in a new triphenylamine with six stable active or non-active forms. Chem. Eur. J. 2014, 21, 2230–2240. [Google Scholar] [CrossRef]
- Fu, T.; Wei, Y.-L.; Zhang, C.; Li, L.-K.; Liu, X.-F.; Li, H.-Y.; Zang, S.-Q. A viologen-based multifunctional Eu-MOF: Photo/electro-modulated chromism and luminescence. Chem. Commun. 2020, 56, 13093–13096. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, J.; Li, M.; Zheng, J.; Xu, C. Novel electrochromic-fluorescent bi-functional devices based on aromatic viologen derivates. Electrochim. Acta 2018, 285, 415–423. [Google Scholar] [CrossRef]
- Bill, N.L.; Lim, J.M.; Davis, C.M.; Bähring, S.; Jeppesen, J.O.; Kim, D.; Sessler, J.L. π-Extended tetrathiafulvalene BODIPY (ex-TTF-BODIPY): A redox switched “on–off–on” electrochromic system with two near-infrared fluorescent outputs. Chem. Commun. 2014, 50, 6758–6761. [Google Scholar] [CrossRef]
- Gautam, R.; Petritis, S.J.; Tomat, E. Redox-switchable cyan fluorescence of a bodipy analog inspired by propentdyopent pigments. Eur. J. Inorg. Chem. 2019, 2019, 68–72. [Google Scholar] [CrossRef]
- Sun, N.; Zhou, Z.; Meng, S.; Chao, D.; Chu, X.; Zhao, X.; Wang, D.; Zhou, H.; Chen, C. Aggregation-enhanced emission (AEE)-active polyamides with methylsulfonyltriphenylamine units for electrofluorochromic applications. Dye. Pigment. 2017, 141, 356–362. [Google Scholar] [CrossRef]
- Sun, N.; Su, K.; Zhou, Z.; Tian, X.; Jianhua, Z.; Chao, D.; Wang, D.; Lissel, F.; Zhao, X.; Chen, C. High-performance emission/color dual-switchable polymer-bearing pendant tetraphenylethylene (TPE) and triphenylamine (TPA) moieties. Macromolecules 2019, 52, 5131–5139. [Google Scholar] [CrossRef]
- Li, R.-Z.; Hao, Q.; Ren, X.-R.; Wen, C.; Wang, L.; Zhao, Z.-L.; Shao, J.-Y.; Zhong, Y.-W.; Wang, D.; Wan, L.-J. Triphenylamine-based covalent organic framework films for dopamine-responsive electrofluorochromism. ACS Appl. Mater. Interfaces 2024, 16, 49594–49601. [Google Scholar] [CrossRef]
- Martínez, R.; Ratera, I.; Tárraga, A.; Molina, P.; Veciana, J. A simple and robust reversible redox–fluorescence molecular switch based on a 1,4-disubstituted azine with ferrocene and pyrene units. Chem. Commun. 2006, 3809–3811. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Z.; Wu, Y.; Fu, H.; Yao, J. A novel redox-fluorescence switch based on a triad containing ferrocene and perylene diimide units. Org. Lett. 2008, 10, 3065–3068. [Google Scholar] [CrossRef]
- Muras, K.; Kubicki, M.; Wałęsa-Chorab, M. Benzochalcodiazole-based donor-acceptor-donor non-symmetric small molecules as dual-functioning electrochromic and electrofluorochromic materials. Dye. Pigment. 2023, 212, 111098. [Google Scholar] [CrossRef]
- Galangau, O.; Fabre-Francke, I.; Munteanu, S.; Dumas-Verdes, C.; Clavier, G.; Méallet-Renault, R.; Pansu, R.B.; Hartl, F.; Miomandre, F. Electrochromic and electrofluorochromic properties of a new boron dipyrromethene–ferrocene conjugate. Electrochim. Acta 2013, 87, 809–815. [Google Scholar] [CrossRef]
- Pham, J.; Audibert, J.-F.; Rabah, J.; Wright, K.; Marrot, J.; Ha-Thi, M.-H.; Méallet-Renault, R.; Allard, E.; Miomandre, F. Positive electrofluorochromism of bodipy–ferrocene monolayers on ITO. J. Phys. Chem. C 2024, 128, 15162–15170. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, B.; Gao, Y.; Liu, C.; Li, G.; Rao, B.; Chu, D.; Yan, N.; Zhang, M.; He, G. Efficient photoinduced electron transfer from pyrene-o-carborane heterojunction to selenoviologen for enhanced photocatalytic hydrogen evolution and reduction of alkynes. Adv. Sci. 2021, 9, 2101652. [Google Scholar] [CrossRef]
- Yang, J.; Li, M.; Kang, L.; Zhu, W. A luminescence molecular switch via modulation of PET and ICT processes in DCM system. Sci. China Chem. 2017, 60, 607–613. [Google Scholar] [CrossRef]
- Nakamura, K.; Kanazawa, K.; Kobayashi, N. Electrochemically controllable emission and coloration by using Europium(III) complex and viologen derivatives. Chem. Commun. 2011, 47, 10064–10066. [Google Scholar] [CrossRef]
- Agam, G.; Gebhardt, C.; Popara, M.; Mächtel, R.; Folz, J.; Ambrose, B.; Chamachi, N.; Chung, S.Y.; Craggs, T.D.; Boer, M.D.; et al. Reliability and accuracy of single-molecule FRET studies for characterization of structural dynamics and distances in proteins. Nat. Methods 2023, 20, 523–535. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, J.; Qin, X.; Xu, J.; Liu, X. Dynamic upconversion multicolour editing enabled by molecule-assisted opto-electrochemical modulation. Nat. Commun. 2021, 12, 2022. [Google Scholar] [CrossRef]
- Dias, M.; Hudhomme, P.; Levillain, E.; Perrin, L.; Sahin, Y.; Sauvage, F.-X.; Wartelle, C. Electrochemistry coupled to fluorescence spectroscopy: A new versatile approach. Electrochem. Commun. 2004, 6, 325–330. [Google Scholar] [CrossRef]
- Beneduci, A.; Cospito, S.; Deda, M.L.; Chidichimo, G. Highly fluorescent thienoviologen-based polymer gels for single layer electrofluorochromic devices. Adv. Funct. Mater. 2014, 25, 1240–1247. [Google Scholar] [CrossRef]
- Kanazawa, K.; Nakamura, K.; Kobayashi, N. Electroswitchable optical device enabling both luminescence and coloration control consisted of fluoran dye and 1,4-benzoquinone. Sol. Energy Mater. Sol. Cells 2016, 145, 42–53. [Google Scholar] [CrossRef]
- Xiao, G.; Ma, Y.-J.; Qi, Z.H.; Fang, X.; Chen, T.; Yan, D. A flexible ligand and halogen engineering enable one phosphor-based full-color persistent luminescence in hybrid perovskitoids. Chem. Sci. 2024, 15, 3625–3632. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; You, Y. Highly reversible electrofluorochromism from electrochemically decoupled but electronically coupled molecular dyads. Adv. Opt. Mater. 2019, 7, 1900201. [Google Scholar] [CrossRef]
- Guo, S.; Huang, T.; Liu, S.; Zhang, K.Y.; Yang, H.; Han, J.; Zhao, Q.; Huang, W. Luminescent ion pairs with tunable emission colors for light-emitting devices and electrochromic switches. Chem. Sci. 2017, 8, 348–360. [Google Scholar] [CrossRef]
- Yabuta, R.; Kobayashi, N.; Nakamura, K. Electrochemically regulated luminescence of europium complexes with β-diketone in polyether matrices. Phys. Chem. Chem. Phys. 2023, 25, 25979–25984. [Google Scholar] [CrossRef]
- Walesa-Chorab, M.; Skene, W.G. Visible-to-NIR electrochromic device prepared from a thermally polymerizable electroactive organic monomer. ACS Appl. Mater. Interfaces 2017, 9, 21524–21531. [Google Scholar] [CrossRef]
- Zhou, Y.; Berda, E.B.; Ma, Q.; Liu, X.; Wang, C.; Chao, D. Flexible and robust electro-optically responsive films based on novel silica/oligoaniline/carbon dots composite. ChemElectroChem 2019, 6, 5293–5300. [Google Scholar] [CrossRef]
- Zhang, Y.; Berda, E.B.; Jia, X.; Lu, Z.; Zhu, M.; Chao, D. Electrochromic/electrofluorochromic supercapacitor based on a network polysiloxane bearing oligoaniline and cyanophenethylene groups. ACS Appl. Polym. Mater. 2020, 2, 3024–3033. [Google Scholar] [CrossRef]
- Yu, T.; Yao, H.; Liu, H.; Guan, S. High-performance fluorescent/electroactive (A4+B2)-type hyperbranched polyimide with AIE-enhanced electroflourochromic behavior. Dye. Pigment. 2023, 214, 11207. [Google Scholar] [CrossRef]
- Su, K.; Sun, N.; Tian, X.; Guo, S.; Yan, Z.; Wang, D.; Zhou, H.; Zhao, X.; Chen, C. High-performance blue fluorescent/electroactive polyamide bearing p-phenylenediamine and asymmetrical SBF/TPA-based units for electrochromic and electrofluorochromic multifunctional applications. J. Mater. Chem. C 2019, 7, 4644–4652. [Google Scholar] [CrossRef]
- Perez, I. Ab initio methods for the computation of physical properties and performance parameters of electrochemical energy storage devices. Phys. Chem. Chem. Phys. 2023, 25, 1476–1503. [Google Scholar] [CrossRef]
- Tamate, R.; Ueki, T. Adaptive ion-gel: Stimuli-responsive, and self-healing ion gels. Chem. Rec. 2023, 23, e202300043. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, D.; Dong, T.; Das, R.; Pan, D.; Sun, C.; Wu, Z.; Zhang, Q.; Liu, C.; Guo, Z. Overview of ionogels in flexible electronics. Chem. Rec. 2020, 20, 948–967. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Pan, C. Intelligent ion gels: Design, performance, and applications. SmartMat 2023, 5, e1215. [Google Scholar] [CrossRef]
- Wang, X.; Kuang, J.; Wu, P.; Zong, Z.; Li, Z.; Wang, H.; Li, J.; Dai, P.; Zhang, K.Y.; Liu, S.; et al. Manipulating electroluminochromism behavior of viologen-substituted iridium(iii) complexes through ligand engineering for information display and encryption. Adv. Mater. 2022, 34, 2107013. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Chiu, Y.-W.; Liou, G.-S. Substituent effects of AIE-active α-cyanostilbene-containing triphenylamine derivatives on electrofluorochromic behavior. Nanoscale 2019, 11, 8597–8603. [Google Scholar] [CrossRef]
- Corrente, G.A.; González, D.A.; Aktas, E.; Capodilupo, A.L.; Ruighi, F.; Accorsi, G.; Imbardelli, D.; Rodriguez-Seco, C.; Martinez-Ferrero, E.; Palomares, E.; et al. Reversible vis-NIR electrochromic/electrofluorochromic switching in dual-functional devices modulated by different benzothiadiazole-arylamine anodic components. J. Mater. Chem. C 2023, 11, 17115–17127. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, H.; Huang, Y.; Wei, Y.; Chen, J. Naturally sourced hydrogels: Emerging fundamental materials for next-generation healthcare sensing. Chem. Soc. Rev. 2023, 52, 2992–3034. [Google Scholar] [CrossRef]
- Adams, T.J.; Brotherton, A.R.; Molai, J.A.; Parmar, N.; Palmer, J.R.; Sandor, K.A.; Walter, M.G. Obtaining Reversible, High contrast electrochromism, electrofluorochromism, and photochromism in an aqueous hydrogel device using chromogenic thiazolothiazoles. Adv. Funct. Mater. 2021, 31, 2103408. [Google Scholar] [CrossRef]
- Wang, X.; Wu, S.; Wen, Y.; Li, F.; Zhang, K.Y.; Liu, S.; Zhao, Q. Photoluminescent iridium(III) complexes with mono-pyridinium substituted phenylpyridine ligands: Synthesis, electrochemical and photophysical properties, and electroluminochromic performance. Dye. Pigment. 2025, 241, 112891. [Google Scholar] [CrossRef]
- Wang, X.; Wu, S.; Wen, Y.; Yang, J.; Li, F.; Wu, Q.; Wu, P.; Ji, Z.; Zhang, K.Y.; Liu, S.; et al. Triple-state electroluminochromism of iridium(III) complexes through redox-induced geometric transformations for information display and encryption. Sci. China Chem. 2025, 68, 1–10. [Google Scholar] [CrossRef]
- Wang, X.; Chen, K.; Xu, D.; Wu, S.; Wu, P.; Ji, Z.; Kuang, J.; Zhang, K.Y.; Liu, S.; Zhao, Q. Cyclometalated iridium(III) complexes containing viologen-modified phenylpyridine ligands as electroluminochromic active molecules for information display. Small Methods 2024, 8, 2400113. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Jia, X.; Zhou, M.; Xie, Y.; Chao, D. A new electroactive fluorescent ionic liquid enables high-performance reflective/emissive dual-modal display device. Chem. Eng. J. 2023, 459, 141664. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Q.; Liu, H.; Li, X.; Wang, X.; Du, X.; Lv, C.; Wang, Z.; Li, A.; Niu, L. Redox regulation of electrofluorochromic behavior for an AIEgen-doped PVA hydrogel based on a ferrocene derivate. New J. Chem. 2024, 48, 5025–5031. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Li, W.; Gu, C.; Zheng, H.; Wang, Y.; Zhang, Y.-M.; Li, M.; Zhang, S.X.-A. An RGB color-tunable turn-on electrofluorochromic device and its potential for information encryption. Chem. Commun. 2017, 53, 11209–11212. [Google Scholar] [CrossRef]
- Lu, L.; Wang, K.; Wu, H.; Qin, A.; Tang, B.Z. Simultaneously achieving high capacity storage and multilevel anti-counterfeiting using electrochromic and electrofluorochromic dual-functional AIE polymers. Chem. Sci. 2021, 12, 7058–7065. [Google Scholar] [CrossRef]
- Yan, N.; Zhang, W.D.; Li, G.P.; Zhang, S.K.; Yang, X.D.; Zhou, K.; Pei, D.D.; Zhao, Z.J.; He, G. AIE-active 9,10-azaboraphenanthrene-containing viologens for reversible electrochromic and electrofluorochromic applications. Mater. Chem. Front. 2021, 5, 4128–4137. [Google Scholar] [CrossRef]
- Sun, H.; Liu, S.; Lin, W.; Zhang, K.Y.; Lv, W.; Huang, X.; Huo, F.; Yang, H.; Jenkins, G.; Zhao, Q.; et al. Smart responsive phosphorescent materials for data recording and security protection. Nat. Commun. 2014, 5, 3601. [Google Scholar] [CrossRef]
- Lin, W.; Zhao, Q.; Sun, H.; Zhang, K.Y.; Yang, H.; Yu, Q.; Zhou, X.; Guo, S.; Liu, S.; Huang, W. An electrochromic phosphorescent iridium(III) complex for information recording, encryption, and decryption. Adv. Opt. Mater. 2014, 3, 368–375. [Google Scholar] [CrossRef]
- Zhao, Q.; Xu, W.; Sun, H.; Yang, J.; Zhang, K.Y.; Liu, S.; Ma, Y.; Huang, W. Tunable electrochromic luminescence of iridium(III) complexes for information self-encryption and anti-counterfeiting. Adv. Opt. Mater. 2016, 4, 1167–1173. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Chen, X.; Sun, G.; Zhang, T.; Liu, S.; Zhao, Q.; Huang, W. Utilization of electrochromically luminescent transition-metal complexes for erasable information recording and temperature-related information protection. Adv. Mater. 2016, 28, 7137–7142. [Google Scholar] [CrossRef]
- Hong, K.-I.; Choi, S.; Oh, S.; Ahn, H.S.; Jang, W.-D. Electrofluorochromic hydrogels by oligothiophene-based color-tunable fluorescent dye doping. ACS Appl. Mater. Interfaces 2024, 16, 31384–31391. [Google Scholar] [CrossRef]
- Huang, R.; Cao, N.; Gu, G.; Jia, X.; Chao, D. A bifunctional electrochromic/electrofluorochromic ionic liquid enables displayable smart windows with high energy efficiency. Adv. Funct. Mater. 2025, e11224. [Google Scholar] [CrossRef]
- Navya, P.V.; Ganesan, K.; Neyts, E.C.; Sampath, S. Heterocycle- and amine-free electrochromic and electrofluorochromic molecules for energy-saving see-through smart windows and displays. Chem. Eur. J. 2024, 30, e202401647. [Google Scholar] [CrossRef]
- Chang, M.; Chen, W.; Xue, H.; Liang, D.; Lu, X.; Zhou, G. Conjugation-extended viologens with thiophene derivative bridges: Near-infrared electrochromism, electrofluorochromism, and smart window applications. J. Mater. Chem. C 2020, 8, 16129–16142. [Google Scholar] [CrossRef]
- Mondal, S.; Santra, D.C.; Roy, S.; Narayana, Y.; Yoshida, T.; Ninomiya, Y.; Higuchi, M. Reversible electrochromic/electrofluorochromic dual switching in zn(II)-based metallo-supramolecular polymer films. ACS Appl. Mater. Interfaces 2023, 15, 42912–42919. [Google Scholar] [CrossRef]
- Xiang, C.; Wan, H.; Zhu, M.; Chen, Y.; Peng, J.; Zhou, G. Dipicolylamine functionalized polyfluorene based gel with lower critical solution temperature: Preparation, characterization, and application. ACS Appl. Mater. Interfaces 2017, 9, 8872–8879. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, K.; Jiang, H.; Zhang, S.; Zhang, B.; Guo, M.; He, G. Poly(NIPAM-co-thienoviologen) for multi-responsive smart windows and thermo-controlled photodynamic antimicrobial therapy. J. Mater. Chem. A 2021, 9, 18369–18376. [Google Scholar] [CrossRef]
- Sun, J.; Li, Y.; Sun, J.; Zhu, Z.; Zhai, Y.; Dong, S. Reversible self-powered fluorescent electrochromic windows driven by perovskite solar cells. Chem. Commun. 2019, 55, 12060–12063. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Zhao, W.; Wang, L.; Li, F.; Wang, W.; Liu, S.; Huang, W.; Zhao, Q. Soluble triarylamine functionalized symmetric viologen for all-solid-state electrochromic supercapacitors. Sci. China Chem. 2020, 63, 1632–1644. [Google Scholar] [CrossRef]
- Yen, H.-J.; Liou, G.-S. Flexible electrofluorochromic devices with the highest contrast ratio based on aggregation-enhanced emission (AEE)-active cyanotriphenylamine-based polymers. Chem. Commun. 2013, 49, 9797–9799. [Google Scholar] [CrossRef]
- Ma, W.; Xu, L.; Zhang, S.; Li, G.; Ma, T.; Rao, B.; Zhang, M.; He, G. Phosphorescent bismoviologens for electrophosphorochromism and visible light-induced cross-dehydrogenative coupling. J. Am. Chem. Soc. 2021, 143, 1590–1597. [Google Scholar] [CrossRef]
- Bejan, A.E.; Constantin, C.P.; Damaceanu, M.D. Evidence of diimide structure variation on overall performance of electro(fluoro)chromic devices integrating versatile triphenylamine-based polyimides. Mater. Today Chem. 2022, 26, 101100. [Google Scholar] [CrossRef]
- Sun, F.; Zhang, H.; Cai, J.; Su, F.; Tian, Y.; Liu, Y.J. Selenophene, thiophene, and furan functionalized π-extended viologen derivatives for tunable all-in-one ECDs. Sol. Energy Mater. Sol. Cells 2023, 250, 112106. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wen, L.; Ren, J.; Wen, Y.; Li, Y.; Zhang, Y.; Zhang, K.Y. Gel-Type Electrofluorochromic Devices for Advanced Optoelectronic Applications. Gels 2025, 11, 673. https://doi.org/10.3390/gels11080673
Wang X, Wen L, Ren J, Wen Y, Li Y, Zhang Y, Zhang KY. Gel-Type Electrofluorochromic Devices for Advanced Optoelectronic Applications. Gels. 2025; 11(8):673. https://doi.org/10.3390/gels11080673
Chicago/Turabian StyleWang, Xuecheng, Lijing Wen, Jinxia Ren, Yonghen Wen, Yonghua Li, Yizhou Zhang, and Kenneth Yin Zhang. 2025. "Gel-Type Electrofluorochromic Devices for Advanced Optoelectronic Applications" Gels 11, no. 8: 673. https://doi.org/10.3390/gels11080673
APA StyleWang, X., Wen, L., Ren, J., Wen, Y., Li, Y., Zhang, Y., & Zhang, K. Y. (2025). Gel-Type Electrofluorochromic Devices for Advanced Optoelectronic Applications. Gels, 11(8), 673. https://doi.org/10.3390/gels11080673







