Olive Oil-Based Lipid Coating as a Precursor Organogel for Postharvest Preservation of Lychee: Efficacy Combined with Polyamide/Polyethylene Packaging Under Passive Atmosphere
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Lychee Fruits
2.2. Experimental Design
- -
- Control (CTR): 100 untreated fruits (control samples);
- -
- Organic Coating (OC): 100 fruits were dipped in olive oil.
2.3. Weight Loss
2.4. Epicarp Color
2.5. Firmness Factor
2.6. Total Soluble Solids Content (TSSC) and Maturity Index (MI)
2.7. Daily Pathological Surveys
- No decay (N.D.): This category represents healthy fruits with no visible lesions or spots. It is assigned a contamination level of 0;
- Slight decay (S.D.): Fruits falling into this category have 1 to 4 lesions or spots. They are assigned a contamination level of 1;
- Moderate contamination (M.D.): Includes fruits with 5 to 10 lesions. They are assigned a contamination level of 2;
- High decay (H.D.): Fruits in this category have more than 10 lesions, and their surface is covered with spots. They are assigned the highest contamination level, which is 3.
2.8. Decay Index
2.9. Visual Score and Marketability
2.10. Proximate Analysis
2.11. Microbiological Analysis
2.12. Lychee Fruit Sensory Profile Evaluation
2.13. Statistical Analysis
3. Results and Discussion
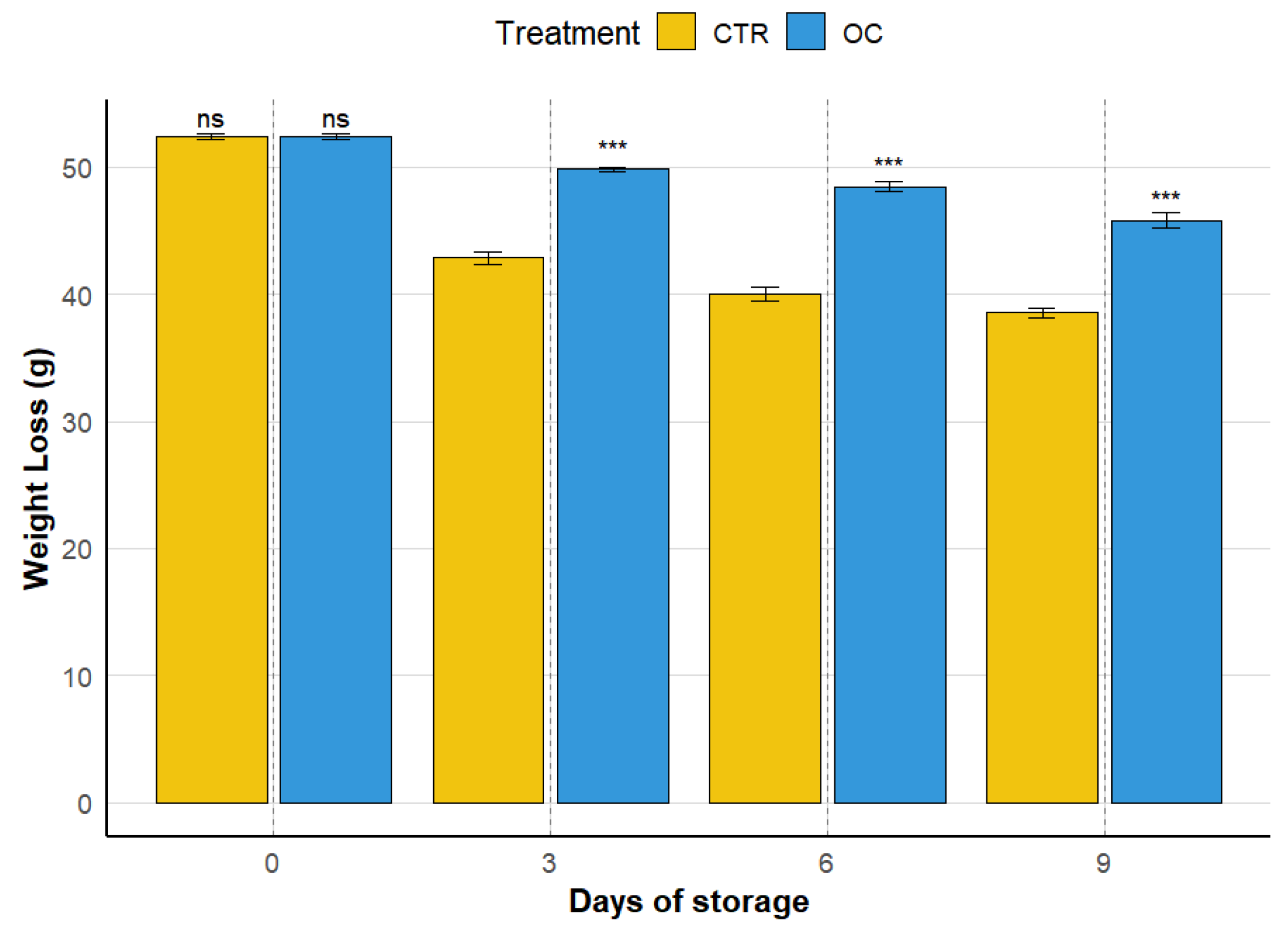
3.1. Weight Loss
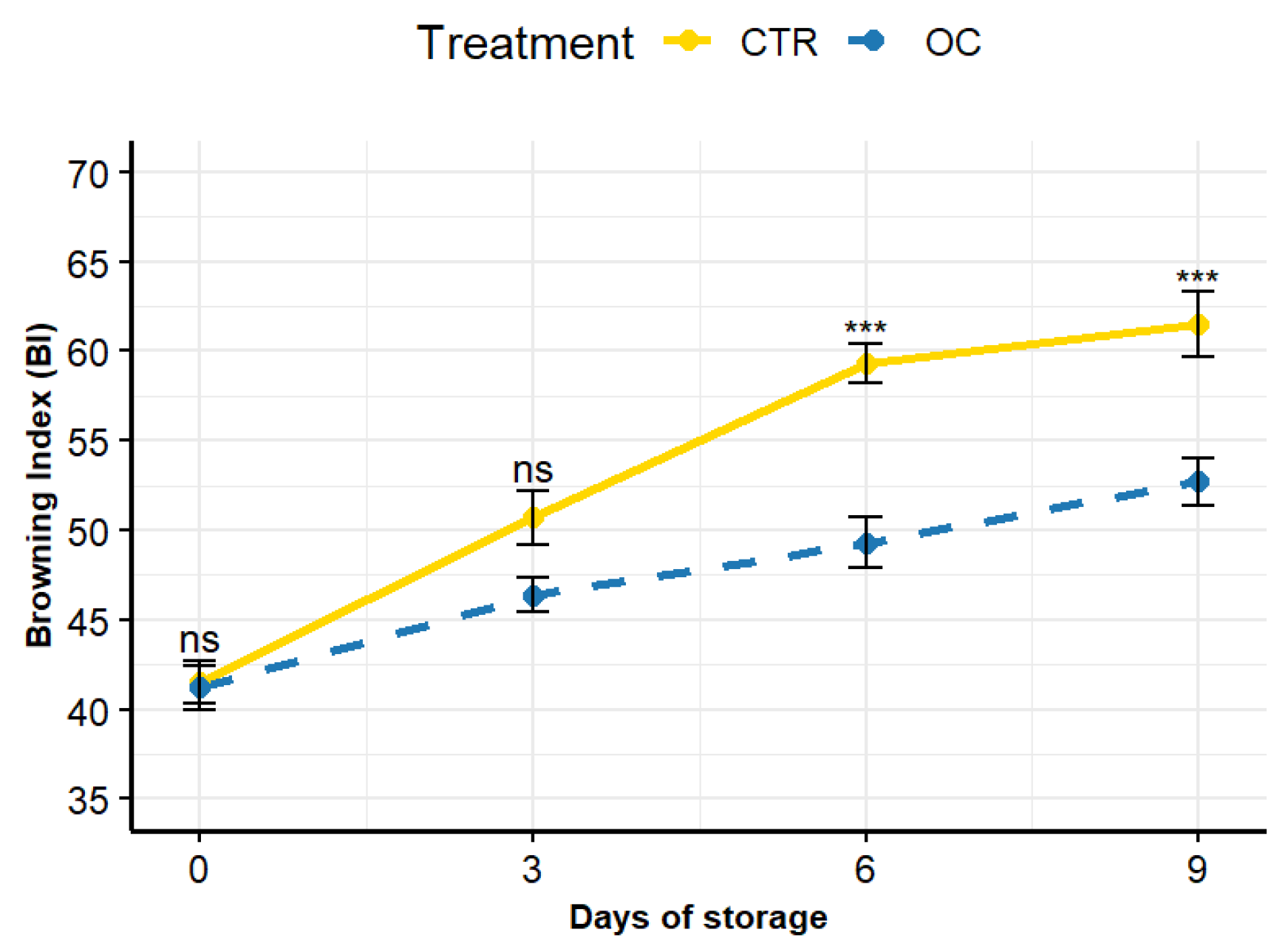
3.2. Epicarp Color
3.3. Firmness Factor
3.4. PCA of Weight Loss (WL), Browning Index (BI), and Firmness Factor (FF)
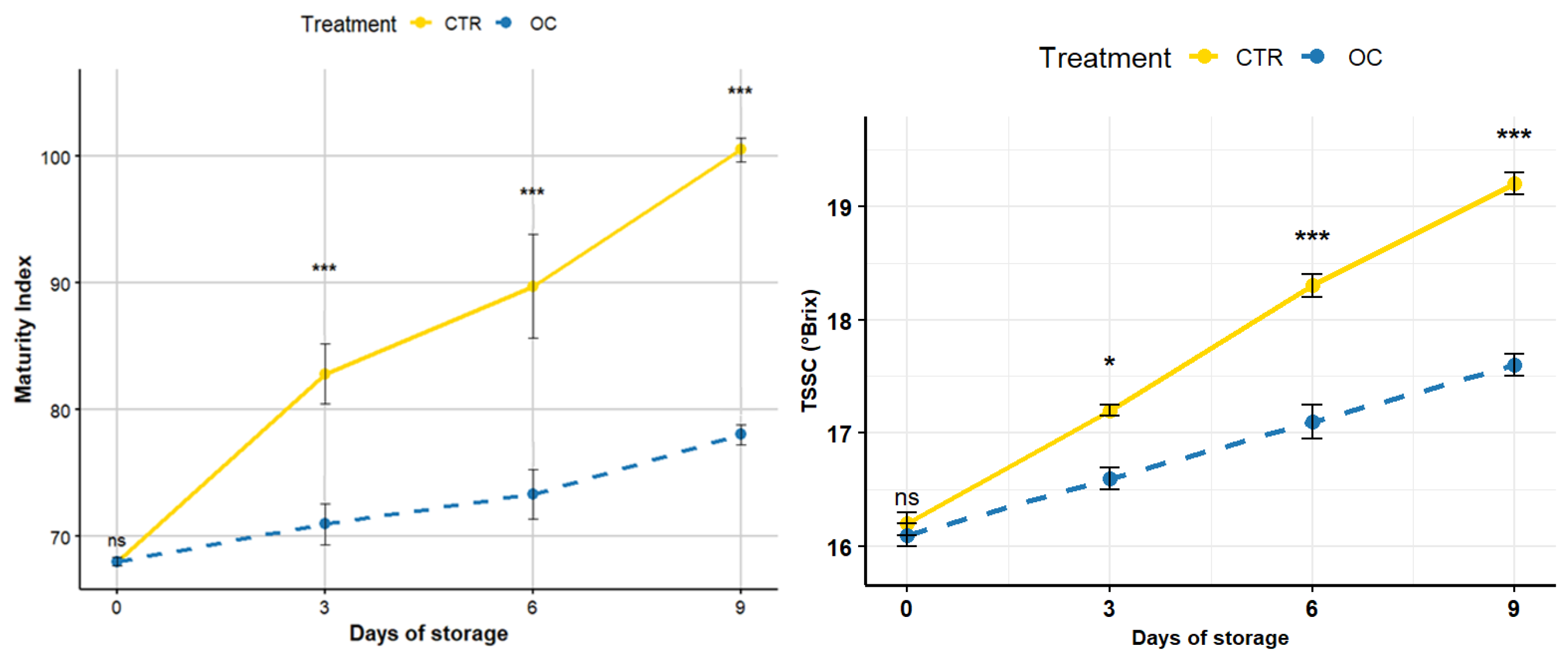
3.5. Total Soluble Solid Content (TSSC) and Maturity Index (MI)
3.6. Daily Pathological Surveys and Decay Index (DI)
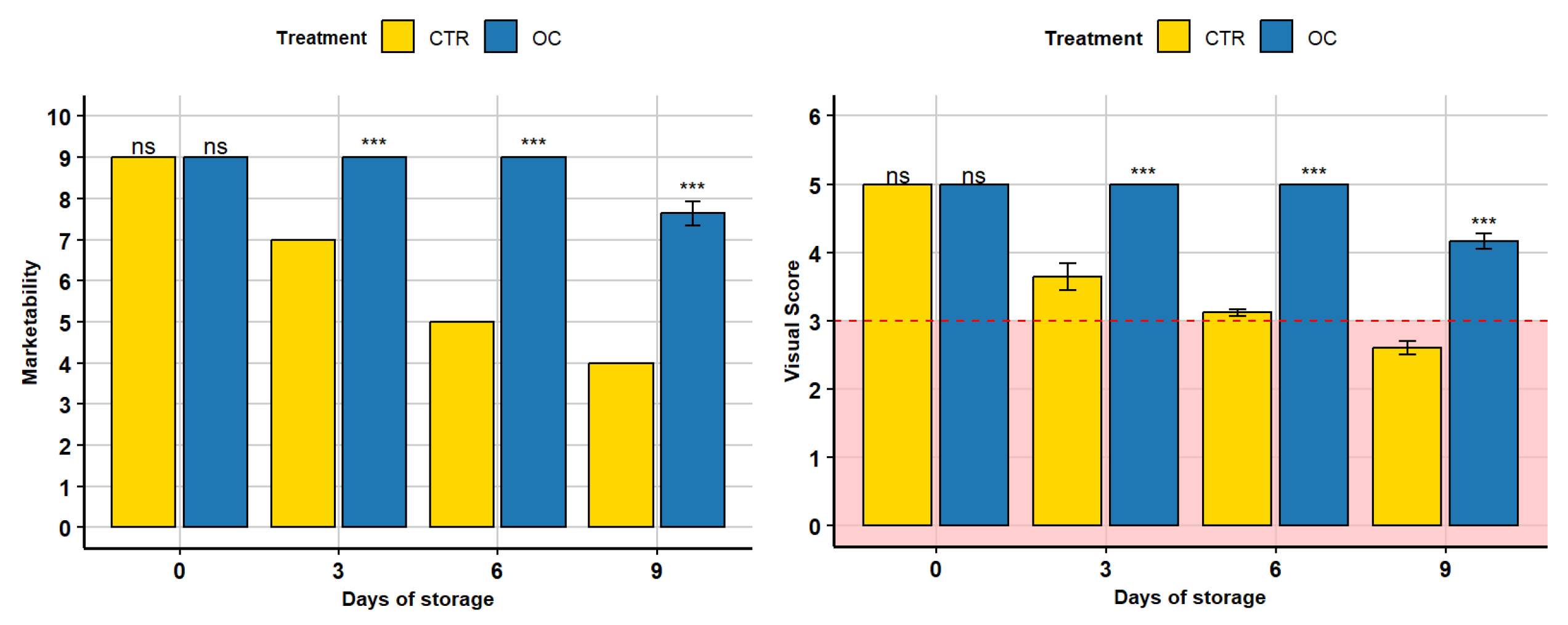
3.7. Visual Score and Marketability
3.8. PCA Visual Score, Marketaability, and Decay Index
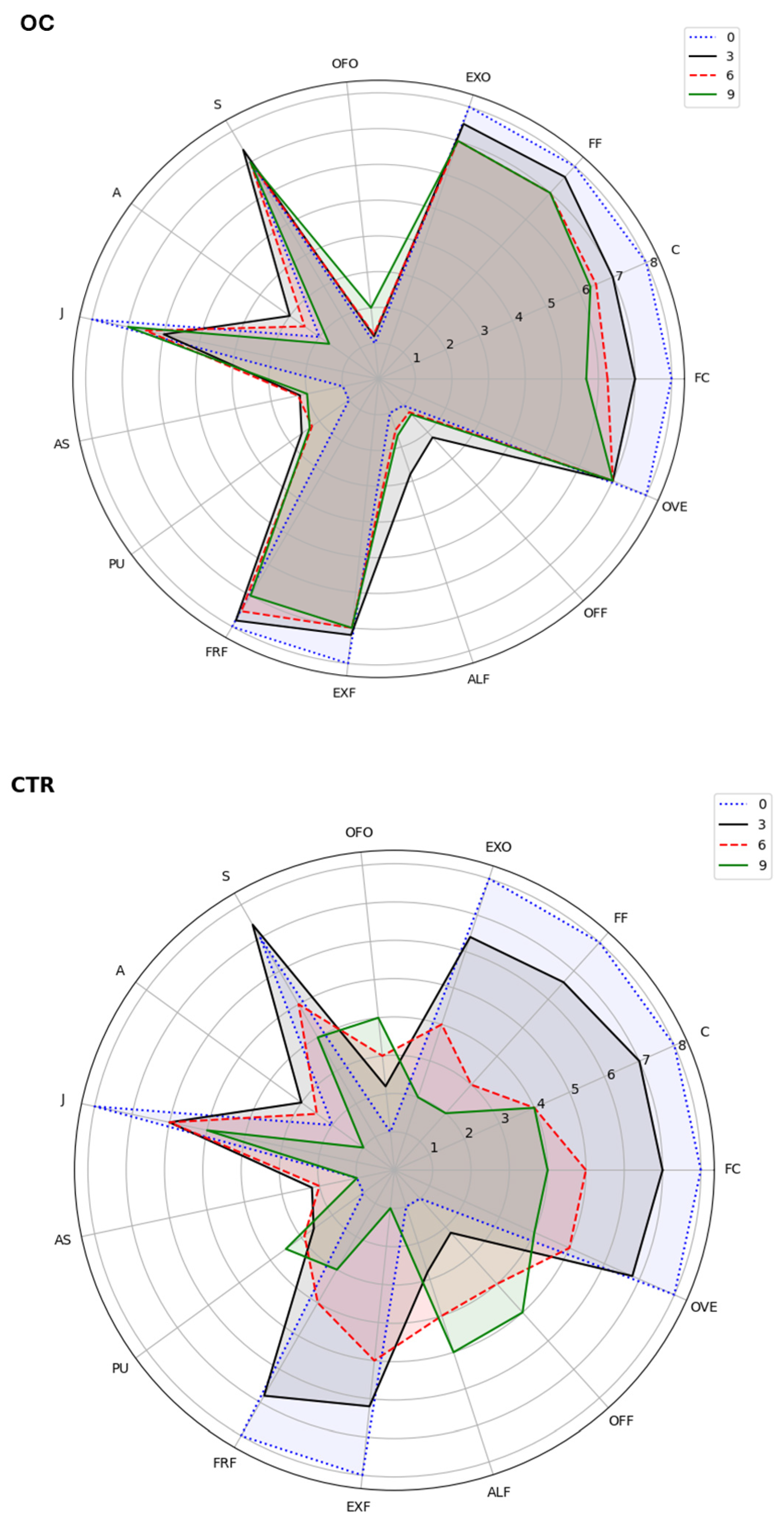
3.9. Heatmap of Quality Parameters
3.10. Proximate Analysis
3.11. Microbiological Analysis
3.12. Lychee Fruit Sensory Profile Evaluation
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farina, V.; Gianguzzi, G.; D’Asaro, A.; Mazzaglia, A.; Palazzolo, E. Fruit Production and Quality Evaluation of Four Litchi Cultivars (Litchi chinensis Sonn.) Grown in Mediterranean Climate. Fruits 2017, 72, 203–211. [Google Scholar] [CrossRef]
- Scuderi, D. Adaptation of Tropical Horticultural Species to a Changing Mediterranean Climate. Ph.D. Thesis, Università degli Studi di Palermo, Palermo, Italy, 2024. Available online: https://tesidottorato.depositolegale.it/bitstream/20.500.14242/81395/1/Tesi%20Dario%20Scuderi%2011%20Dicembre%202023.pdf (accessed on 18 July 2025).
- Kilari, E.K.; Putta, S. Biological and Phytopharmacological Descriptions of Litchi chinensis. Pharmacogn. Rev. 2016, 10, 60–65. [Google Scholar] [CrossRef]
- Surma, S.; Sahebkar, A.; Banach, M. Nutrition, Nutraceuticals and Bioactive Compounds in the Prevention and Fight against Inflammation. Nutrients 2023, 15, 2629. [Google Scholar] [CrossRef] [PubMed]
- Punia, S.; Kumar, M. Litchi (Litchi chinenis) Seed: Nutritional Profile, Bioactivities, and Its Industrial Applications. Trends Food Sci. Technol. 2021, 108, 58–70. [Google Scholar] [CrossRef]
- Bishayee, A.; Kavalakatt, J.; Sunkara, C.; Johnson, O.; Zinzuwadia, S.S.; Collignon, T.E.; Banerjee, S.; Barbalho, S.M. Litchi (Litchi chinensis Sonn.): A Comprehensive and Critical Review on Cancer Prevention and Intervention. Food Chem. 2024, 457, 140142. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Cao, S.; Xu, Y.; Zhang, M.; Xiao, G.; Deng, Q.; Xie, B. Oxidation of (−)-epicatechin is a precursor oflitchi pericarp enzymatic browning. Food Chem. 2010, 118, 508–511. [Google Scholar] [CrossRef]
- Duan, X.; Wu, G.; Jiang, Y. Evaluation of the Antioxidant Properties of Litchi Fruit Phenolics in Relation to Pericarp Browning Prevention. Molecules 2007, 12, 759–771. [Google Scholar] [CrossRef]
- Underhill, S.J.; Simons, D.H. Lychee (Litchi chinensis Sonn.) Pericarp desiccation and the importance of postharvest micro-cracking. Sci. Hortic. 1993, 54, 287–294. [Google Scholar] [CrossRef]
- Bhushan, B.; Pal, A.; Narwal, R.; Meena, V.S.; Sharma, P.C.; Singh, J. Combinatorial Approaches for Controlling Pericarp Browning in Litchi (Litchi chinensis) Fruit. J. Food Sci. Technol. 2015, 52, 5418–5426. [Google Scholar] [CrossRef]
- Meitha, K.; Pramesti, Y.; Suhandono, S. Reactive oxygen species and antioxidants in postharvest vegetables and fruits. Int. J. Food Sci. 2020, 2020, 8817778. [Google Scholar] [CrossRef]
- Zhang, Z.; Pang, X.; Xuewu, D.; Ji, Z.; Jiang, Y. Role of peroxidase in anthocyanin degradation in litchi fruit pericarp. Food Chem. 2005, 90, 47–52. [Google Scholar] [CrossRef]
- Yang, C.; Lee, F.-W.; Cheng, Y.-J.; Chu, Y.-Y.; Chen, C.-N.; Kuan, Y.-C. Chitosan Coating Formulated with Citric Acid and Pomelo Extract Retards Pericarp Browning and Fungal Decay to Extend Shelf Life of Cold-Stored Lychee. Sci. Hortic. 2023, 310, 111735. [Google Scholar] [CrossRef]
- Cao, Y.; Jiang, Y.; Gao, H.; Chen, H.; Fang, X.; Mu, H.; Tao, F. Development of a model for quality evaluation of litchi fruit. Comput. Electron. Agric. 2014, 106, 49–55. [Google Scholar] [CrossRef]
- Bazzano, L.A.; Serdula, M.K.; Liu, S. Dietary intake of fruits and vegetables and risk of cardiovascular disease. Curr. Atheroscler. Rep. 2003, 5, 492–499. [Google Scholar] [CrossRef]
- Cabral, T.A.; de Morais Cardoso, L.; Pinheiro-Sant’Ana, H.M. Chemical composition, vitamins and minerals of a new cultivar of lychee (Litchi chinensis Cv. Tailandes) grown in Brazil. Fruits 2014, 69, 425–434. [Google Scholar] [CrossRef]
- Johnston, J.C.; Welch, R.C.; Hunter, G. Volatile constituents of litchi (Litchi chinensis Sonn.). J. Agric. Food Chem. 1980, 28, 859–861. [Google Scholar] [CrossRef]
- Xiang, X.-H.; Wei, J.; Wang, X.-F.; Xu, Q.; Yu, C.-L.; He, C.-L.; Long, T.; Guo, M.-S.; Chen, X.; Zhou, X.-G. Lychee seed polyphenol ameliorates DR via inhibiting inflammasome/apoptosis and angiogenesis in hRECs and Db/Db Mice. Biomed. Pharmacother. 2023, 167, 115478. [Google Scholar] [CrossRef]
- Kumar, V.; Purbey, S.K.; Anal, A.K.D. Losses in Litchi at Various Stages of Supply Chain and Changes in Fruit Quality Parameters. Crop Prot. 2016, 79, 97–104. [Google Scholar] [CrossRef]
- Kumar, P.; Joshi, A.; Sharma, N.; Lata, S.; Mehmood, S.; Ahlawat, Y.K.; Malik, A.; Moussa, I.M.; Kerketta, A.; Soni, P. Integrative Approaches to Improve Litchi (Litchi chinensis Sonn.) Plant Health Using Bio-Transformations and Entomopathogenic Fungi. BMC Plant Biol. 2024, 24, 902. [Google Scholar] [CrossRef]
- Gabler, F.M.; Mercier, J.; Jiménez, J.; Smilanick, J. Integration of Continuous Biofumigation with Muscodor Albus with Pre-Cooling Fumigation with Ozone or Sulfur Dioxide to Control Postharvest Gray Mold of Table Grapes. Postharvest Biol. Technol. 2010, 55, 78–84. [Google Scholar] [CrossRef]
- Romanazzi, G.; Droby, S. Control Strategies for Postharvest Grey Mould on Fruit Crops. In Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Springer International Publishing: Cham, Switzerland, 2016; pp. 217–228. [Google Scholar] [CrossRef]
- Kathpalia, A.; Arora, S.; Sharma, J.G. Impact of Different Fumigants on the Quality of Perishables. J. Food Saf. Food Qual./Arch. für Lebens. Hyg. 2024, 75, 93–120. [Google Scholar] [CrossRef]
- Sivakumar, D.; Terry, L.A.; Korsten, L. An Overview on Litchi Fruit Quality and Alternative Postharvest Treatments to Replace Sulfur Dioxide Fumigation. Food Rev. Int. 2010, 26, 162–188. [Google Scholar] [CrossRef]
- Kaur, K. Postharvest Treatments and Technologies. Post-Harvest Management and Value Addition of Fruits and Vegetables. p. 57. Available online: https://www.researchgate.net/profile/Komal-Kaur-19/publication/386409968_Post_harvest_Treatments_and_Technologies/links/675024ff3d17281c7df7aa01/Post-harvest-Treatments-and-Technologies.pdf (accessed on 18 July 2025).
- Lemmer, D.; Kruger, F. Identification and Quantification of the Factors Influencing Sulphur Dioxide Residue Levels in South African Export Litchi Fruit. Acta Hortic. 2001, 558, 331–337. [Google Scholar] [CrossRef]
- Yao, Y.; Feng, S.; Li, X.; Liu, T.; Ye, S.; Ma, L.; Man, S. Litchi Procyanidins Inhibit Colon Cancer Proliferation and Metastasis by Triggering Gut-Lung Axis Immunotherapy. Cell Death Dis. 2023, 14, 109. [Google Scholar] [CrossRef]
- Murmu, S.B.; Mishra, H.N. Post-Harvest Shelf-Life of Banana and Guava: Mechanisms of Common Degradation Problems and Emerging Counteracting Strategies. Innov. Food Sci. Emerg. Technol. 2018, 49, 20–30. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Malik, A.U.; Shahid, M. Effect of Controlled Atmosphere Storage on Pericarp Browning, Bioactive Compounds and Antioxidant Enzymes of Litchi Fruits. Food Chem. 2016, 206, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, M.; Mujumdar, A.S.; Ma, Y. Technological Innovations Enhance Postharvest Fresh Food Resilience from a Supply Chain Perspective. Crit. Rev. Food Sci. Nutr. 2024, 64, 11044–11066. [Google Scholar] [CrossRef] [PubMed]
- Moradinezhad, F.; Ranjbar, A. Efficacy of Active and Passive Modified Atmosphere Packaging on Quality Preservation and Storage Life of Pomegranate Fruit and Arils: A Review. Adv. Hortic. Sci. 2024, 38, 83–96. [Google Scholar] [CrossRef]
- Zdulski, J.A.; Rutkowski, K.P.; Konopacka, D. Strategies to Extend the Shelf Life of Fresh and Minimally Processed Fruit and Vegetables with Edible Coatings and Modified Atmosphere Packaging. Appl. Sci. 2024, 14, 11074. [Google Scholar] [CrossRef]
- Passafiume, R.; Roppolo, P.; Tinebra, I.; Pirrone, A.; Gaglio, R.; Palazzolo, E.; Farina, V. Reduction of Pericarp Browning and Microbial Spoilage on Litchi Fruits in Modified Atmosphere Packaging. Horticulturae 2023, 9, 651. [Google Scholar] [CrossRef]
- Xu, C.; Lu, M.; Li, R.; Liu, D.; Guo, C. Super-Atmospheric Oxygen Modified Atmosphere Package of Whole and Fresh-Cut Fruits and Vegetables. Food Bioprocess Technol. 2024, 17, 2499–2518. [Google Scholar] [CrossRef]
- Zuo, Z.; Jiang, P.; Chen, D.; Zhang, C.; Guo, F.; Nie, X.; Wu, D.; Fan, X.; Zhao, H. Improving the Storage Quality and Antioxidant Capacity of Postharvest Winter Jujube by Laser Microporous Modified Atmosphere Packaging. Sci. Hortic. 2024, 337, 113477. [Google Scholar] [CrossRef]
- Pernetti, M.; van Malssen, K.; Kalnin, D.; Flöter, E. Structuring edible oil with lecithin and sorbitan tri-stearate. Food Hydrocoll. 2007, 21, 855–861. [Google Scholar] [CrossRef]
- Chaves, K.F.; Barrera-Arellano, D.; Ribeiro, A.P.B. Potential application of lipid organogels for food industry. Food Res. Int. 2018, 105, 863–872. [Google Scholar] [CrossRef]
- Visioli, F.; Galli, C. Biological Properties of Olive Oil Phytochemicals. Crit. Rev. Food Sci. Nutr. 2002, 42, 209–221. [Google Scholar] [CrossRef]
- Tedesco, F. Use of Chitin and Its Derivatives Obtained from Sustainable Sources as an Alternative to the Use of Chemical Additives in the Food Production. Ph.D. Thesis, Università degli Studi della Basilicata, Potenza, Italy, 2025. Available online: https://tesidottorato.depositolegale.it/handle/20.500.14242/192481 (accessed on 18 July 2025).
- Boghori, P.; Shahidi, F.; Sedaghat, N.; Roshanak, S. Whey Protein Concentrate Coating Incorporated with Modified Atmosphere Packaging for Extending Tangerines Shelf-Life: Physicochemical, Microbiological and Sensory Evaluation Through Refrigerated Storage. J. Packag. Technol. Res. 2024, 8, 51–62. [Google Scholar] [CrossRef]
- Fong-in, S.; Nimitkeatkai, H.; Prommajak, T.; Nowacka, M. Ultrasound-Assisted Osmotic Dehydration of Litchi: Effect of Pretreatment on Mass Transfer and Quality Attributes during Frozen Storage. J. Food Meas. Charact. 2021, 15, 3590–3597. [Google Scholar] [CrossRef]
- Tinebra, I.; Passafiume, R.; Scuderi, D.; Pirrone, A.; Gaglio, R.; Palazzolo, E.; Farina, V. Effects of Tray-Drying on the Physicochemical, Microbiological, Proximate, and Sensory Properties of White-and Red-Fleshed Loquat (Eriobotrya japonica Lindl.) Fruit. Agronomy 2022, 12, 540. [Google Scholar] [CrossRef]
- Passafiume, R.; Tinebra, I.; Gaglio, R.; Settanni, L.; Sortino, G.; Allegra, A.; Farina, V. Fresh-Cut Mangoes: How to Increase Shelf Life by Using Neem Oil Edible Coating. Coatings 2022, 12, 664. [Google Scholar] [CrossRef]
- Ruangchakpet, A.; Sajjaanantakul, T. Effect of Browning on Total Phenolic, Flavonoid Content and Antioxidant Activity in Indian Gooseberry (Phyllanthus emblica Linn.). Kasetsart J. 2007, 41, 331–337. [Google Scholar]
- Peralta-Ruiz, Y.; Tovar, C.G.; Sinning-Mangonez, A.; Bermont, D.; Cordero, A.P.; Paparella, A.; Chaves-López, C. Colletotrichum Gloesporioides Inhibition Using Chitosan-Ruta Graveolens L Essential Oil Coatings: Studies In Vitro and In Situ on Carica papaya Fruit. Int. J. Food Microbiol. 2020, 326, 108649. [Google Scholar] [CrossRef]
- Morcia, C.; Malnati, M.; Terzi, V. In Vitro Antifungal Activity of Terpinen-4-Ol, Eugenol, Carvone, 1, 8-Cineole (Eucalyptol) and Thymol against Mycotoxigenic Plant Pathogens. Food Addit. Contam. Part A 2012, 29, 415–422. [Google Scholar] [CrossRef]
- Barrera Bello, E.; Gil Loaiza, M.; García Pajón, C.M.; Durango Restrepo, D.L.; Gil González, J.H. Empleo de Un Recubrimiento Formulado Con Propóleos Para El Manejo Poscosecha de Frutos de Papaya (Carica papaya L. Cv. Hawaiana). Rev. Fac. Nac. Agron. Medellín 2012, 65, 6497–6506. [Google Scholar][Green Version]
- Perdones, A.; Sánchez-González, L.; Chiralt, A.; Vargas, M. Effect of Chitosan–Lemon Essential Oil Coatings on Storage-Keeping Quality of Strawberry. Postharvest Biol. Technol. 2012, 70, 32–41. [Google Scholar] [CrossRef]
- Petriccione, M.; Pasquariello, M.S.; Mastrobuoni, F.; Zampella, L.; Di Patre, D.; Scortichini, M. Influence of a Chitosan Coating on the Quality and Nutraceutical Traits of Loquat Fruit during Postharvest Life. Sci. Hortic. 2015, 197, 287–296. [Google Scholar] [CrossRef]
- Saidi, L.; Duanis-Assaf, D.; Galsarker, O.; Maurer, D.; Alkan, N.; Poverenov, E. Elicitation of Fruit Defense Response by Active Edible Coatings Embedded with Phenylalanine to Improve Quality and Storability of Avocado Fruit. Postharvest Biol. Technol. 2021, 174, 111442. [Google Scholar] [CrossRef]
- Fogg, D.; Wilkinson, A.N. The Colourimetric Determination of Phosphorus. Analyst 1958, 83, 406–414. [Google Scholar] [CrossRef]
- Palazzolo, E.; Letizia Gargano, M.; Venturella, G. The Nutritional Composition of Selected Wild Edible Mushrooms from Sicily (Southern Italy). Int. J. Food Sci. Nutr. 2012, 63, 79–83. [Google Scholar] [CrossRef]
- Allegra, A.; Inglese, P.; Sortino, G.; Settanni, L.; Todaro, A.; Liguori, G. The Influence of Opuntia Ficus-Indica Mucilage Edible Coating on the Quality of ‘Hayward’Kiwifruit Slices. Postharvest Biol. Technol. 2016, 120, 45–51. [Google Scholar] [CrossRef]
- UNI EN ISO 11290-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp. Part 1: Detection Method. International Organization for Standardization: Geneva, Switzerland, 2017.
- UNI EN ISO 6579-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella. Part 1: Detection of Salmonella spp. International Organization for Standardization: Geneva, Switzerland, 2017.
- Jiang, Y.; Fu, J. Postharvest Browning of Litchi Fruit by Water Loss and Its Prevention by Controlled Atmosphere Storage at High Relative Humidity. LWT-Food Sci. Technol. 1999, 32, 278–283. [Google Scholar] [CrossRef]
- Mangaraj, S.; Goswami, T.; Mahajan, P. Applications of Plastic Films for Modified Atmosphere Packaging of Fruits and Vegetables: A Review. Food Eng. Rev. 2009, 1, 133–158. [Google Scholar] [CrossRef]
- Iñiguez-Moreno, M.; Ragazzo-Sánchez, J.A.; Calderón-Santoyo, M. An Extensive Review of Natural Polymers Used as Coatings for Postharvest Shelf-Life Extension: Trends and Challenges. Polymers 2021, 13, 3271. [Google Scholar] [CrossRef] [PubMed]
- Ramana Rao, T.; Baraiya, N.S.; Vyas, P.B.; Patel, D.M. Composite Coating of Alginate-Olive Oil Enriched with Antioxidants Enhances Postharvest Quality and Shelf Life of Ber Fruit (Ziziphus mauritiana Lamk. Var. Gola). J. Food Sci. Technol. 2016, 53, 748–756. [Google Scholar] [CrossRef]
- Tripoli, E.; Giammanco, M.; Tabacchi, G.; Di Majo, D.; Giammanco, S.; La Guardia, M. The Phenolic Compounds of Olive Oil: Structure, Biological Activity and Beneficial Effects on Human Health. Nutr. Res. Rev. 2005, 18, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, I.; Barakat, H.; El-Mansy, H.A.; Soliman, S.A. Effect of Chitosan–Olive Oil Processing Residues Coatings on Keeping Quality of Cold-storage Strawberry (Fragaria ananassa. Var. Festival). J. Food Qual. 2016, 39, 504–515. [Google Scholar] [CrossRef]
- Nandane, A.; Dave, R.K.; Rao, T.R. Optimization of Edible Coating Formulations for Improving Postharvest Quality and Shelf Life of Pear Fruit Using Response Surface Methodology. J. Food Sci. Technol. 2017, 54, 1–8. [Google Scholar] [CrossRef]
- Debeaufort, F.; Quezada-Gallo, J.-A.; Voilley, A. Edible Films and Coatings: Tomorrow’s Packagings: A Review. Crit. Rev. Food Sci. 1998, 38, 299–313. [Google Scholar] [CrossRef]
- Singh, B.; Suri, K.; Shevkani, K.; Kaur, A.; Kaur, A.; Singh, N. Enzymatic Browning of Fruit and Vegetables: A Review. In Enzymes in Food Technology: Improvements and Innovations; Springer: Singapore, 2018; pp. 63–78. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, M.; Yun, Z.; Wang, J.; Feng, G.; Gao, Z.; Shi, X.; Jiang, Y. Effect of Tea Seed Oil Treatment on Browning of Litchi Fruit in Relation to Energy Status and Metabolism. Postharvest Biol. Technol. 2017, 132, 97–104. [Google Scholar] [CrossRef]
- Saquet, A.; Streif, J.; Bangerth, F. Changes in ATP, ADP and Pyridine Nucleotide Levels Related to the Incidence of Physiological Disorders in ‘Conference’Pears and ‘Jonagold’Apples during Controlled Atmosphere Storage. J. Hortic. Sci. Biotechnol. 2000, 75, 243–249. [Google Scholar] [CrossRef]
- Veltman, R.; Lentheric, I.; Van der Plas, L.; Peppelenbos, H. Internal Browning in Pear Fruit (Pyrus communis L. Cv Conference) May Be a Result of a Limited Availability of Energy and Antioxidants. Postharvest Biol. Technol. 2003, 28, 295–302. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, H.; Jiang, Y.; Zhang, S.; Lin, Y.; Wang, Z. Phomopsis Longanae Chi-Induced Pericarp Browning and Disease Development of Harvested Longan Fruit in Association with Energy Status. Postharvest Biol. Technol. 2014, 93, 24–28. [Google Scholar] [CrossRef]
- Saquet, A.; Streif, J.; Bangerth, F. Energy Metabolism and Membrane Lipid Alterations in Relation to Brown Heart Development in ‘Conference’Pears during Delayed Controlled Atmosphere Storage. Postharvest Biol. Technol. 2003, 30, 123–132. [Google Scholar] [CrossRef]
- Cardarelli, M.; Rouphael, Y.; Pellizzoni, M.; Colla, G.; Lucini, L. Profile of Bioactive Secondary Metabolites and Antioxidant Capacity of Leaf Exudates from Eighteen Aloe Species. Ind. Crops Prod. 2017, 108, 44–51. [Google Scholar] [CrossRef]
- Ding, C.-K.; Chachin, K.; Ueda, Y.; Imahori, Y.; Wang, C.Y. Metabolism of Phenolic Compounds during Loquat Fruit Development. J. Agric. Food Chem. 2001, 49, 2883–2888. [Google Scholar] [CrossRef]
- Liu, G.; Liu, S.; Liu, J.; Xiang, Y.; Zhu, L.; Xu, X.; Zhang, Z. Trehalose Delays Postharvest Browning of Litchi Fruit by Regulating Antioxidant Capacity, Anthocyanin Synthesis and Energy Status. Postharvest Biol. Technol. 2025, 219, 113249. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, V.S.; Junior, M.R.M. Phenolic Compounds: Structure, Classification, and Antioxidant Power. In Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2019; pp. 33–50. [Google Scholar] [CrossRef]
- Sivakumar, D.; Korsten, L. Litchi (Litchi chinensis Sonn.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Elsevier: Amsterdam, The Netherlands, 2011; pp. 361–407,408e–409e. [Google Scholar] [CrossRef]
- Huang, C.C.; Paull, R.E.; Wang, T. Litchi Postharvest Physiology and Handling. Crop Sci. 2024, 64, 2014–2063. [Google Scholar] [CrossRef]
- Vinod, B.; Asrey, R.; Menaka, M.; Ahamad, S.; Meena, N.K.; Bhan, C.; Avinash, G. Synergistic Effect of Aqueous Ozone and Guar Gum Coating on the Quality and Shelf Life of Stored Papaya Fruit. J. Food Meas. Charact. 2024, 18, 4000–4011. [Google Scholar] [CrossRef]
- Matthes, A.; Schmitz-Eiberger, M. Polyphenol Content and Antioxidant Capacity of Apple Fruit: Effect of Cultivar and Storage Conditions. J. Appl. Bot. Food Qual. 2012, 82, 152–157. [Google Scholar]
- Burdon, J.; Clark, C. Effect of Postharvest Water Loss on ‘Hayward’ Kiwifruit Water Status. Postharvest Biol. Technol. 2001, 22, 215–225. [Google Scholar] [CrossRef]
- Jiang, L.; Luo, Z.; Liu, H.; Wang, F.; Li, H.; Gao, H.; Zhang, H. Preparation and Characterization of Chitosan Films Containing Lychee (Litchi chinensis Sonn.) Pericarp Powder and Their Application as Active Food Packaging. Foods 2021, 10, 2834. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, S.; Fu, H.; Lu, H.; Yang, Z. Evaluation of Litchi Impact Damage Degree and Damage Susceptibility. Comput. Electron. Agric. 2020, 173, 105409. [Google Scholar] [CrossRef]
- Wei, J.; Qi, X.; Cheng, Y.; Guan, J. Difference in Activity and Gene Expression of Pectin-Degrading Enzymes during Softening Process in Two Cultivars of Chinese Pear Fruit. Sci. Hortic. 2015, 197, 434–440. [Google Scholar] [CrossRef]
- Atkinson, R.G.; Bolitho, K.M.; Wright, M.A.; Iturriagagoitia-Bueno, T.; Reid, S.J.; Ross, G.S. Apple ACC-Oxidase and Polygalacturonase: Ripening-Specific Gene Expression and Promoter Analysis in Transgenic Tomato. Plant Mol. Biol. 1998, 38, 449–460. [Google Scholar] [CrossRef]
- Nagar, P. Physiological and Biochemical Studies during Fruit Ripening in Litchi (Litchi chinensis Sonn.). Postharvest Biol. Technol. 1994, 4, 225–234. [Google Scholar] [CrossRef]
- Nath, A.; Deka, B.C.; Singh, A.; Patel, R.; Paul, D.; Misra, L.; Ojha, H. Extension of Shelf Life of Pear Fruits Using Different Packaging Materials. J. Food Sci. Technol. 2012, 49, 556–563. [Google Scholar] [CrossRef]
- Kohli, K.; Kumar, A.; Singh, O.; Dey, P. Composite Edible Coatings Can Extend Shelf-Life and Maintain Postharvest Qualities of Guava under Natural Storage. Hortic. Environ. Biotechnol. 2024, 65, 413–431. [Google Scholar] [CrossRef]
- Pesis, E.; Dvir, O.; Feygenberg, O.; Arie, R.B.; Ackerman, M.; Lichter, A. Production of Acetaldehyde and Ethanol during Maturation and Modified Atmosphere Storage of Litchi Fruit. Postharvest Biol. Technol. 2002, 26, 157–165. [Google Scholar] [CrossRef]
- Luo, B.; Xuan, S.; Wang, X.; Ding, K.; Jin, P.; Zheng, Y.; Wu, Z. Liposome/Chitosan Coating Film Bioplastic Packaging for Litchi Fruit Preservation. Food Chem. 2025, 464, 141850. [Google Scholar] [CrossRef]
- Bryant, P. Optimising the Postharvest Management of Lychee (Litchi chinensis Sonn.)—A Study of Mechanical Injury and Desiccation. Ph.D. Thesis, University of Sydney, Sydney, Australia, 2004. Available online: https://core.ac.uk/download/pdf/41229713.pdf (accessed on 18 July 2025).
- Gaur, G.; Bajpai, P. Post Harvest Physiology of Litchi Fruits. I. Progress. Hortic. 1978, 10, 63–77. [Google Scholar]
- Kummu, M.; De Moel, H.; Porkka, M.; Siebert, S.; Varis, O.; Ward, P.J. Lost Food, Wasted Resources: Global Food Supply Chain Losses and Their Impacts on Freshwater, Cropland, and Fertiliser Use. SciTot. Environ. 2012, 438, 477–489. [Google Scholar] [CrossRef]
- Iqbal, J.; Rab, A.; Sajid, M.; Shah, S.H.A.; Bacha, S.A.S.; Gul, G.; Shah, S. Effect of Partial Coating of Olive Oil and Storage Duration on Postharvest Performance of Sweet Orange. Sci. Int. 2017, 29, 731–736. [Google Scholar]
- Sivakumar, D.; Regnier, T.; Demoz, B.; Korsten, L. Effect of Different Post-Harvest Treatments on Overall Quality Retention in Litchi Fruit during Low Temperature Storage. J. Hortic. Sci. Biotechnol. 2005, 80, 32–38. [Google Scholar] [CrossRef]
- Baraiya, N.S.; Rao, T.V.R.; Thakkar, V.R. Enhancement of Storability and Quality Maintenance of Carambola (Averrhoa Carambola L.) Fruit by Using Composite Edible Coating. Fruits 2014, 69, 195–205. [Google Scholar] [CrossRef]
- Passafiume, R.; Gugliuzza, G.; Gaglio, R.; Busetta, G.; Tinebra, I.; Sortino, G.; Farina, V. Aloe-Based Edible Coating to Maintain Quality of Fresh-Cut Italian Pears (Pyrus communis L.) during Cold Storage. Horticulturae 2021, 7, 581. [Google Scholar] [CrossRef]
- Paull, R.E.; Chen, N.J. Effect of Storage Temperature and Wrapping on Quality Characteristics of Litchi Fruit. Sci. Hortic. 1987, 33, 223–236. [Google Scholar] [CrossRef]
- Reichel, M.; Carle, R.; Sruamsiri, P.; Neidhart, S. Influence of Harvest Maturity on Quality and Shelf-Life of Litchi Fruit (Litchi chinensis Sonn.). Postharvest Biol. Technol. 2010, 57, 162–175. [Google Scholar] [CrossRef]
- Cronje, R.B. Effect of Fruit Development, Maturity and Harvesting of Litchi (Litchi chinensis Sonn.) on Postharvest Fruit Quality. Stewart Postharvest Rev. 2008, 4, 1–10. [Google Scholar] [CrossRef]
- Culmone, A.; Mirabile, G.; Tinebra, I.; Michelozzi, M.; Carrubba, A.; Bellardi, M.G.; Farina, V.; Romanazzi, G.; Torta, L. Hydrolate and EO Application to Reduce Decay of Carica papaya during Storage. Horticulturae 2023, 9, 204. [Google Scholar] [CrossRef]
- Lai, D.; Shao, X.; Xiao, W.; Fan, C.; Liu, C.; He, H.; Tian, S.; Kuang, S. Suppression of Fruit Decay and Maintenance of Storage Quality of Litchi by Photorhabdus Luminescens Hb1029 Treatment. Sci. Hortic. 2020, 259, 108836. [Google Scholar] [CrossRef]
- Lever, J.; Krzywinski, M.; Altman, N. Points of Significance: Principal Component Analysis. Nat. Methods 2017, 14, 641–643. [Google Scholar] [CrossRef]
- Hinton, G.E.; Salakhutdinov, R.R. Reducing the Dimensionality of Data with Neural Networks. Science 2006, 313, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Ringnér, M. What Is Principal Component Analysis? Nat. Biotechnol. 2008, 26, 303–304. [Google Scholar] [CrossRef] [PubMed]
- Karamizadeh, S.; Abdullah, S.M.; Manaf, A.A.; Zamani, M.; Hooman, A. An Overview of Principal Component Analysis. J. Signal Inf. Process. 2013, 4, 173–175. [Google Scholar] [CrossRef]
- Rahman, S.; Mele, M.A.; Lee, Y.-T.; Islam, M.Z. Consumer Preference, Quality, and Safety of Organic and Conventional Fresh Fruits, Vegetables, and Cereals. Foods 2021, 10, 105. [Google Scholar] [CrossRef]
- Shen, D.; Zhang, M.; Mujumdar, A.S.; Ma, Y. Consumer-Oriented Smart Dynamic Detection of Fresh Food Quality: Recent Advances and Future Prospects. Crit. Rev. Food Sci. Nutr. 2024, 64, 11281–11301. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y. Towards a New Definition of Quality for Fresh Fruits and Vegetables. Sci. Hortic. 2018, 234, 463–469. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, K.; Wang, K.; Zhu, J.; Hu, Z. Nutrient Components, Health Benefits, and Safety of Litchi (Litchi chinensis Sonn.): A Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2139–2163. [Google Scholar] [CrossRef]
- Roychoudhury, R.; Kabir, J.; Ray, S.; Dhua, R. Effect of Calcium on Fruit Quality of Litchi. Indian J. Hortic. 1992, 49, 27–30. [Google Scholar]
- Liang, Y.S.; Chen, N.L.; Ke, L.S. Influence of Dipping in Sodium Metabisulfite on Pericarp Browning of Litchi Cv. Yu Her Pau (Feizixiao). Postharvest Biol. Technol. 2012, 68, 72–77. [Google Scholar] [CrossRef]
- Mathura, R.; Dey, P.; Gangopadhyay, K.; Das, B.; Nath, V.; Redd, N.; Singi, F.H. Influence of Nitrogen, Phosphorus and Potassium on Growth Parameters, Leaf Nutrient Composition and Yield of Litchi (Litchi chinensis). India J. Agril. Sci 2002, 72, 267–270. [Google Scholar]
- Alegbeleye, O.; Odeyemi, O.A.; Strateva, M.; Stratev, D. Microbial Spoilage of Vegetables, Fruits and Cereals. Appl. Food Res. 2022, 2, 100122. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Van Giau, V.; Vo, T.K. Multiplex PCR for Simultaneous Identification of E. coli O157: H7, Salmonella spp. and L. monocytogenes in Food. 3 Biotech 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Karanth, S.; Feng, S.; Patra, D.; Pradhan, A.K. Linking Microbial Contamination to Food Spoilage and Food Waste: The Role of Smart Packaging, Spoilage Risk Assessments, and Date Labeling. Front. Microbiol. 2023, 14, 1198124. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Leff, J.W.; Adams, B.J.; Nielsen, U.N.; Bates, S.T.; Lauber, C.L.; Owens, S.; Gilbert, J.A.; Wall, D.H.; Caporaso, J.G. Cross-Biome Metagenomic Analyses of Soil Microbial Communities and Their Functional Attributes. Proc. Nat. Acad. Sci. USA 2012, 109, 21390–21395. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, B.; Qadri, O.S. Preservation of Fresh-Cut Fruits and Vegetables by Edible Coatings. In Fresh-Cut Fruits and Vegetables; Elsevier: Amsterdam, The Netherlands, 2020; pp. 225–242. [Google Scholar] [CrossRef]
- Maringgal, B.; Hashim, N.; Tawakkal, I.S.M.A.; Mohamed, M.T.M. Recent Advance in Edible Coating and Its Effect on Fresh/Fresh-Cut Fruits Quality. Trends Food Sci. Technol. 2020, 96, 253–267. [Google Scholar] [CrossRef]
- Barth, M.; Hankinson, T.R.; Zhuang, H.; Breidt, F. Microbiological Spoilage of Fruits and Vegetables. In Compendium of the Microbiological Spoilage of Foods and Beverages; Springer: New York, NY, USA, 2009; pp. 135–183. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Abrams, J.; Alnemri, E.; Baehrecke, E.; Blagosklonny, M.; Dawson, T.; Dawson, V.; El-Deiry, W.; Fulda, S. Molecular Definitions of Cell Death Subroutines: Recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Diff. 2012, 19, 107–120. [Google Scholar] [CrossRef]
- Caldera, L.; Franzetti, L.; Van Coillie, E.; De Vos, P.; Stragier, P.; De Block, J.; Heyndrickx, M. Identification, Enzymatic Spoilage Characterization and Proteolytic Activity Quantification of Pseudomonas spp. Isolated from Different Foods. Food Microbiol. 2016, 54, 142–153. [Google Scholar] [CrossRef]
- Wu, Y.; Pan, Q.; Qu, W.; Duan, C. Comparison of Volatile Profiles of Nine Litchi (Litchi chinensis Sonn.) Cultivars from Southern China. J. Agric. Food Chem. 2009, 57, 9676–9681. [Google Scholar] [CrossRef]
- Feng, S.; Huang, M.; Crane, J.H.; Wang, Y. Characterization of Key Aroma-Active Compounds in Lychee (Litchi chinensis Sonn.). J. Food Drug Anal. 2018, 26, 497–503. [Google Scholar] [CrossRef]
- Chouliara, E.; Badeka, A.; Savvaidis, I.; Kontominas, M.G. Combined Effect of Irradiation and Modified Atmosphere Packaging on Shelf-Life Extension of Chicken Breast Meat: Microbiological, Chemical and Sensory Changes. Eur. Food Res. Technol. 2008, 226, 877–888. [Google Scholar] [CrossRef]
- Qu, P.; Zhang, M.; Fan, K.; Guo, Z. Microporous Modified Atmosphere Packaging to Extend Shelf Life of Fresh Foods: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Chyau, C.-C.; Ko, P.-T.; Chang, C.-H.; Mau, J.-L. Free and Glycosidically Bound Aroma Compounds in Lychee (Litchi chinensis Sonn.). Food Chem. 2003, 80, 387–392. [Google Scholar] [CrossRef]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y. Antioxidant and Biological Activities of Tyrosol, Hydroxytyrosol and Their Esters. Master’s Thesis, Memorial University of Newfoundland, St. John’s, NL, Canada, 2017. Available online: http://research.library.mun.ca/12685/ (accessed on 18 July 2025).
- Nenadis, N.; Pyrka, I.; Tsimidou, M.Z. The Contribution of Theoretical Prediction Studies to the Antioxidant Activity Assessment of the Bioactive Secoiridoids Encountered in Olive Tree Products and By-Products. Molecules 2023, 28, 2267. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y. Effects of Low-Temperature Acclimation on Browning of Litchi Fruit in Relation to Shelf Life. J. Hortic. Sci. Biotechnol. 2003, 78, 437–440. [Google Scholar] [CrossRef]






| Microorganisms | Media | Growth Conditions |
|---|---|---|
| Total mesophilic microorganisms | Plate Count Agar | 3 d at 30 °C |
| Pseudomonadaceae | Pseudomonas Agar Base | 2 d at 25 °C |
| Enterobacteriaceae | Violet Red Bile Glucose Agar | 1 d at 37 °C |
| Coagulase-positive staphylococci | Baird Parker Agar | 1 d at 37 °C |
| Escherichia coli | Chromogenic Medium | 1 d at 37 °C |
| Unicellular fungi (yeasts) | Yeast Peptone Dextrose Agar | 2 d at 30 °C |
| Filamentous fungi (moulds) | Potato Dextrose Agar | 7 d at 25 °C |
| Days of Storage | |||||
|---|---|---|---|---|---|
| Treatments | 0 | 3 | 6 | 9 | |
| K (mg/100 g FW) | CTR | 39.12 ± 0.34 aA | 33.75 ± 0.22 bA | 28.57 ± 0.23 bB | 18.4 5± 0.56 bB |
| OC | 39.12 ± 0.34 aA | 36.45 ± 0.14 aA | 30.11 ± 0.16 aA | 24.36 ± 0.74 aA | |
| Na (mg/100 g FW) | CTR | 10.54 ± 0.27 aA | 7.88 ± 0.22 aA | 7.54 ± 0.48 aA | 7.10 ± 0.65 aA |
| OC | 10.54 ± 0.27 aA | 8.12 ± 0.73 aA | 7.63 ± 0.29 aA | 7.32 ± 0.17 aA | |
| Ca (mg/100 g FW) | CTR | 15.28 ± 0.71 aA | 13.46 ± 0.28 aA | 12.15 ± 0.24 aA | 9.24 ± 0.30 bB |
| OC | 15.28 ± 0.71 aA | 14.22 ± 0.62 aA | 13.37 ± 0.53 aA | 12.89 ± 0.10 aA | |
| P (mg/100 g FW) | CTR | 45.20 ± 0.10 aA | 35.17 ± 0.12 bA | 24.57 ± 0.13 cB | 22.43 ± 0.24 dB |
| OC | 45.20 ± 0.10 aA | 43.28 ± 0.15 aA | 40.28 ± 0.27 aA | 30.28 ± 0.14 aA | |
| Fe (mg/100 g FW) | CTR | 0.30 ± 0.34 aA | 0.12 ± 0.11 bB | 0.09 ± 0.12 bB | 0.09 ± 0.13 bB |
| OC | 0.30 ± 0.34 aA | 0.25 ± 0.26 aA | 0.20 ± 0.16 aA | 0.13 ± 0.17 aA | |
| Storage Time | Samples | Microbial Loads | |
|---|---|---|---|
| TMM | Moulds | ||
| 0 days | CTR | <2 | <2 |
| OC | <2 | <2 | |
| 3 days | CTR | <2 | <2 |
| OC | <2 | <2 | |
| 6 days | CTR | 3.98 ± 0.08 b | 3.80 ± 0.19 b |
| OC | 3.08 ± 0.10 a | 2.98 ± 0.11 a | |
| 9 days | CTR | 5.10 ± 0.12 b | 5.02 ± 0.09 b |
| OC | 4.25 ± 0.12 a | 4.30 ± 0.10 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Culmone, A.; Passafiume, R.; Roppolo, P.; Tinebra, I.; Naselli, V.; Collura, A.; Pirrone, A.; Botta, L.; Carrubba, A.; Francesca, N.; et al. Olive Oil-Based Lipid Coating as a Precursor Organogel for Postharvest Preservation of Lychee: Efficacy Combined with Polyamide/Polyethylene Packaging Under Passive Atmosphere. Gels 2025, 11, 608. https://doi.org/10.3390/gels11080608
Culmone A, Passafiume R, Roppolo P, Tinebra I, Naselli V, Collura A, Pirrone A, Botta L, Carrubba A, Francesca N, et al. Olive Oil-Based Lipid Coating as a Precursor Organogel for Postharvest Preservation of Lychee: Efficacy Combined with Polyamide/Polyethylene Packaging Under Passive Atmosphere. Gels. 2025; 11(8):608. https://doi.org/10.3390/gels11080608
Chicago/Turabian StyleCulmone, Alessandra, Roberta Passafiume, Pasquale Roppolo, Ilenia Tinebra, Vincenzo Naselli, Alfonso Collura, Antonino Pirrone, Luigi Botta, Alessandra Carrubba, Nicola Francesca, and et al. 2025. "Olive Oil-Based Lipid Coating as a Precursor Organogel for Postharvest Preservation of Lychee: Efficacy Combined with Polyamide/Polyethylene Packaging Under Passive Atmosphere" Gels 11, no. 8: 608. https://doi.org/10.3390/gels11080608
APA StyleCulmone, A., Passafiume, R., Roppolo, P., Tinebra, I., Naselli, V., Collura, A., Pirrone, A., Botta, L., Carrubba, A., Francesca, N., Gaglio, R., & Farina, V. (2025). Olive Oil-Based Lipid Coating as a Precursor Organogel for Postharvest Preservation of Lychee: Efficacy Combined with Polyamide/Polyethylene Packaging Under Passive Atmosphere. Gels, 11(8), 608. https://doi.org/10.3390/gels11080608










