Porous Silica Gels Doped with Gold Nanoparticles: Preparation, Microstructure, Optical and Textural Properties
Abstract
1. Introduction
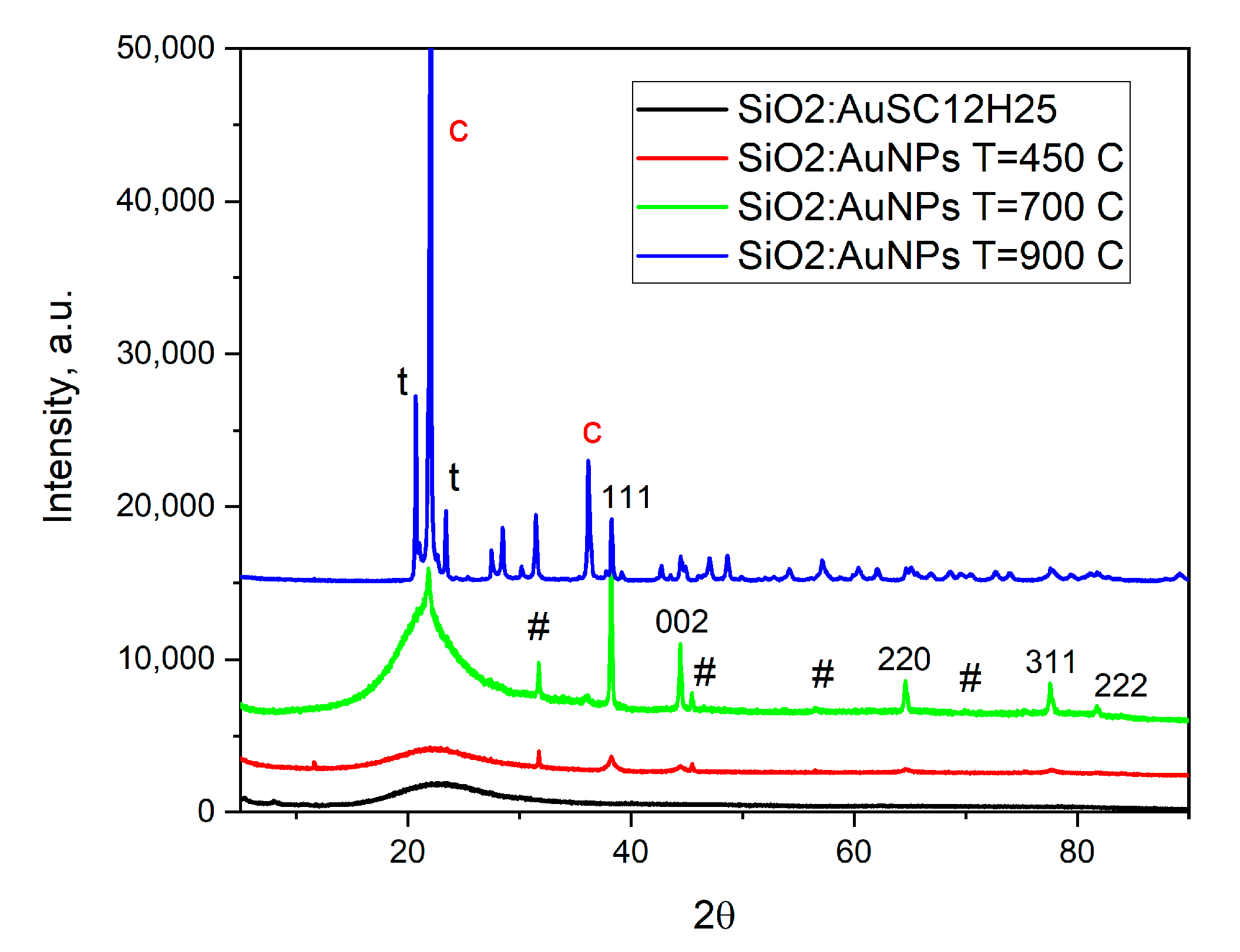
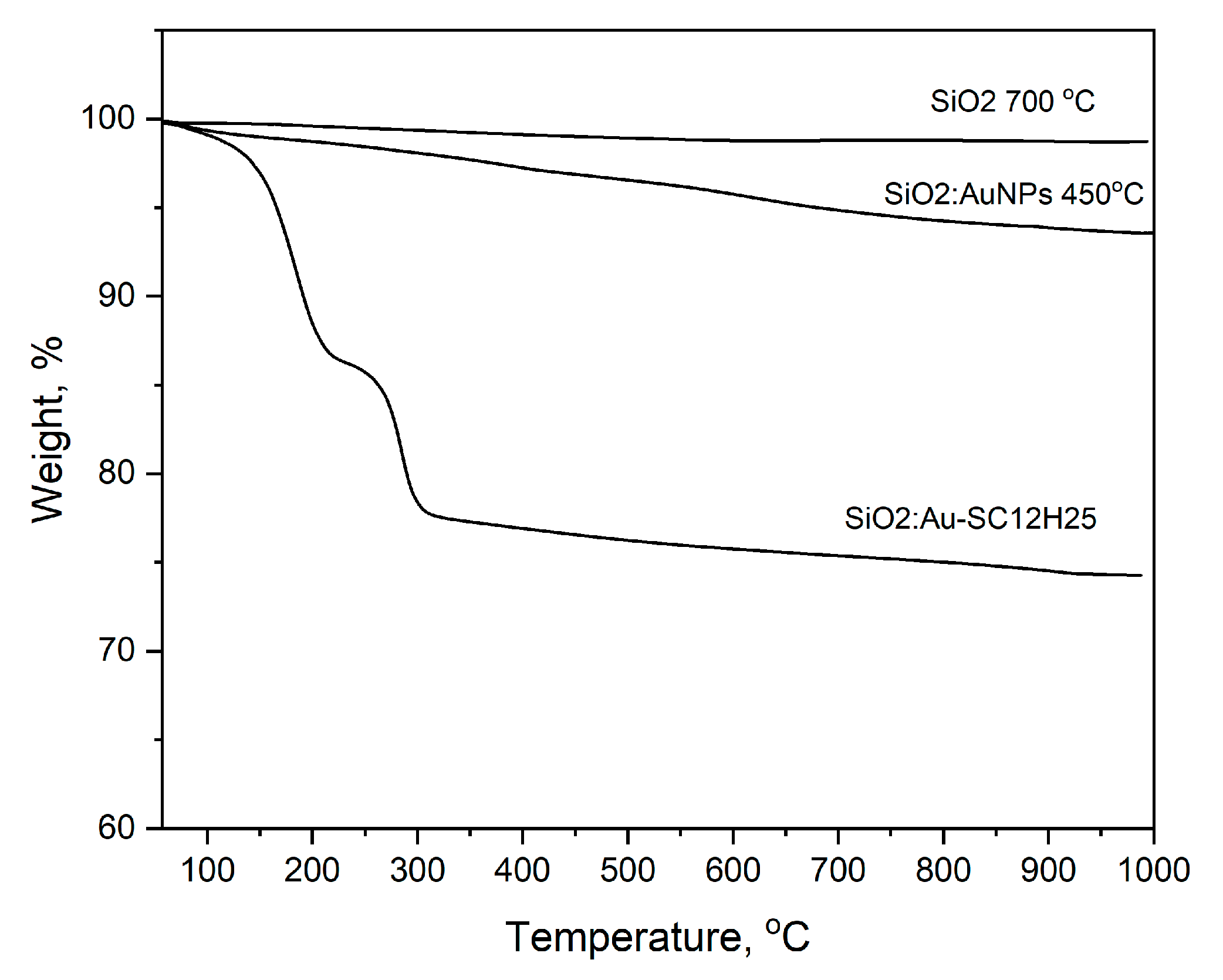
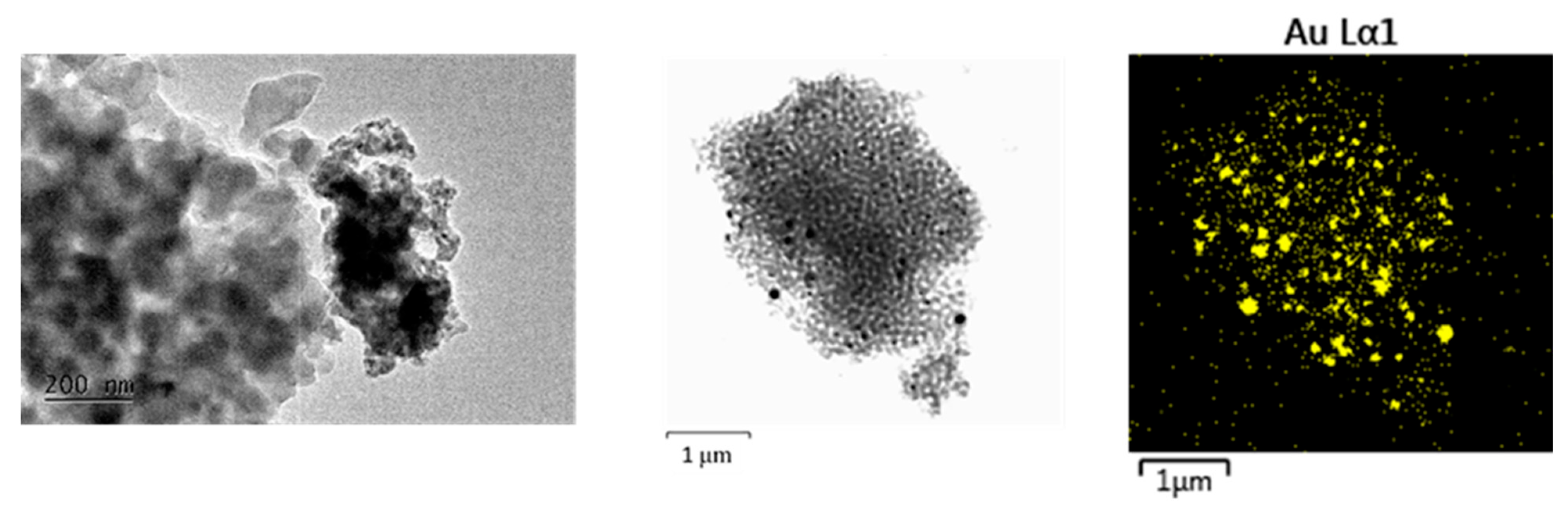
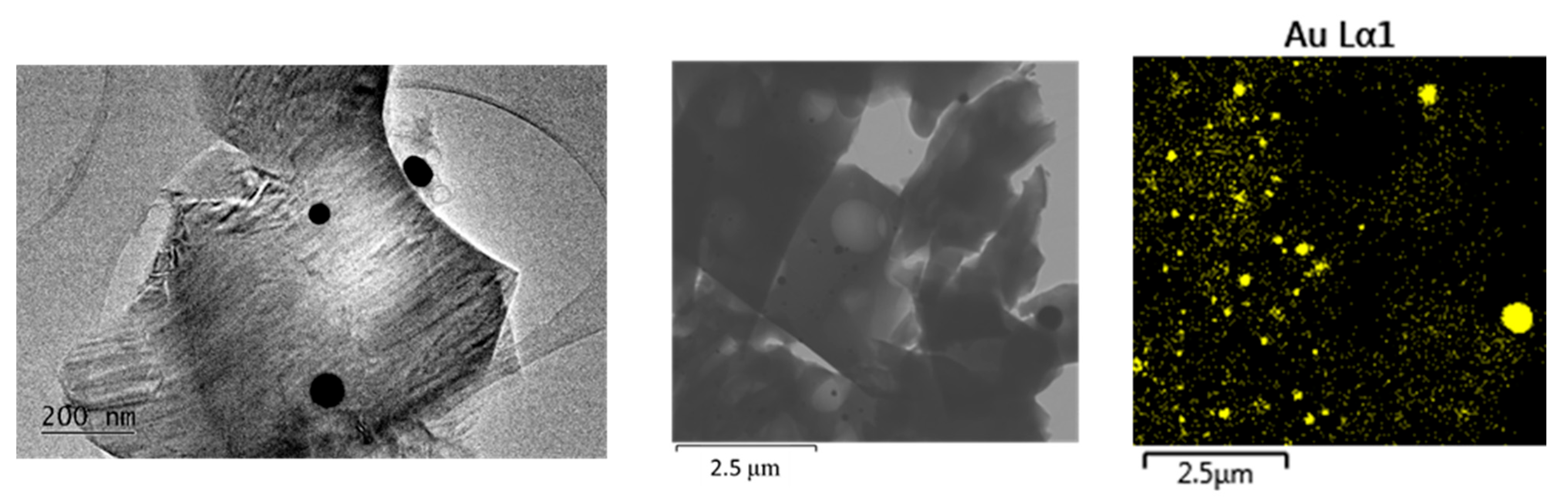
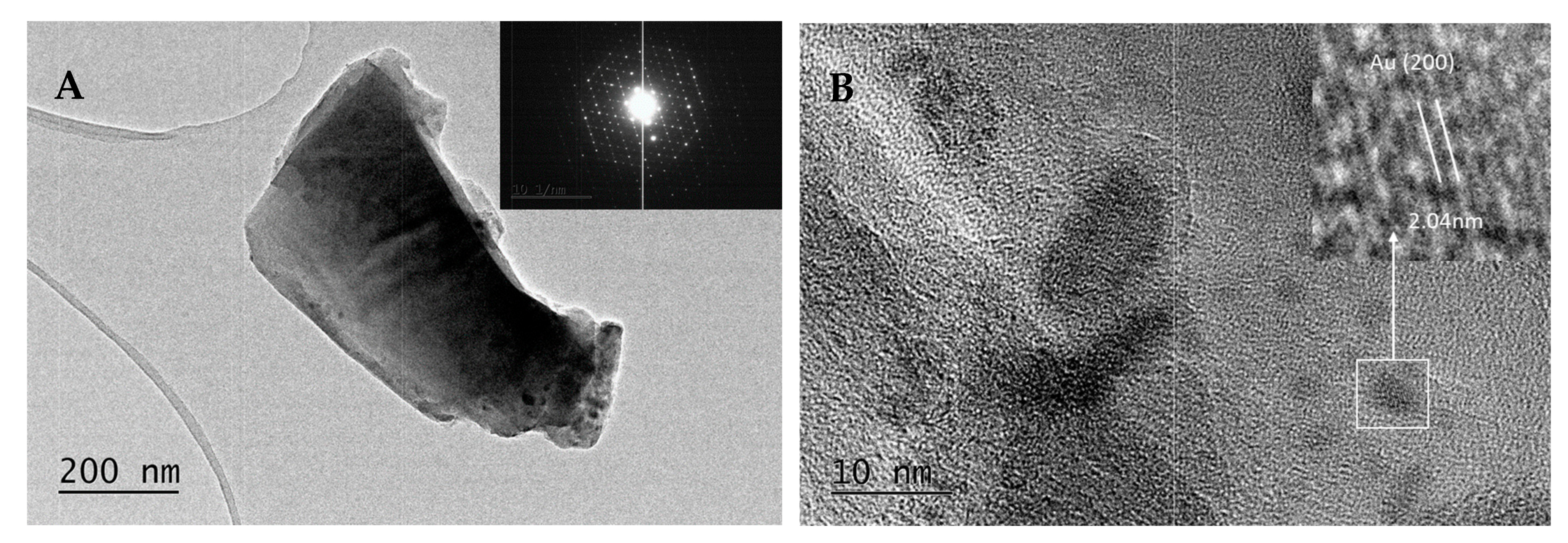
2. Results and Discussion
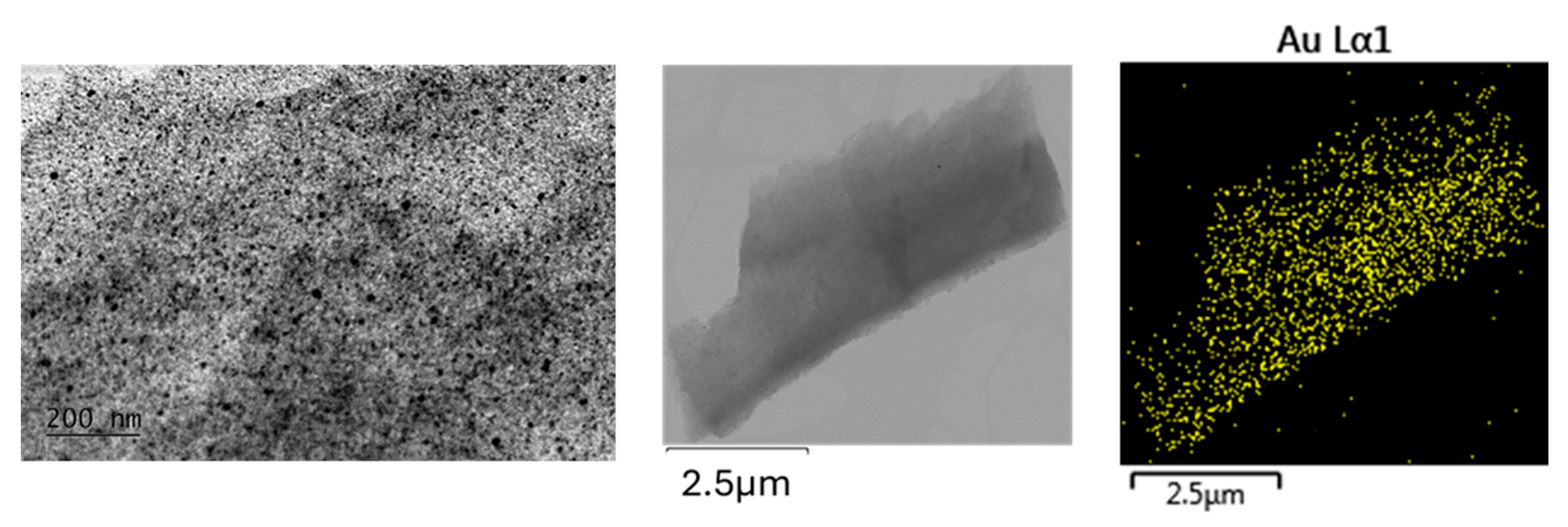
- Fraction A—spherical particles below 40 nm (nanospheres);
- Fraction B—nanoparticles about 100 nm (nanospheres and nanoclusters);
- Fraction C—large, walled nanocrystals above 200 nm (giant nanocrystals).
3. Conclusions
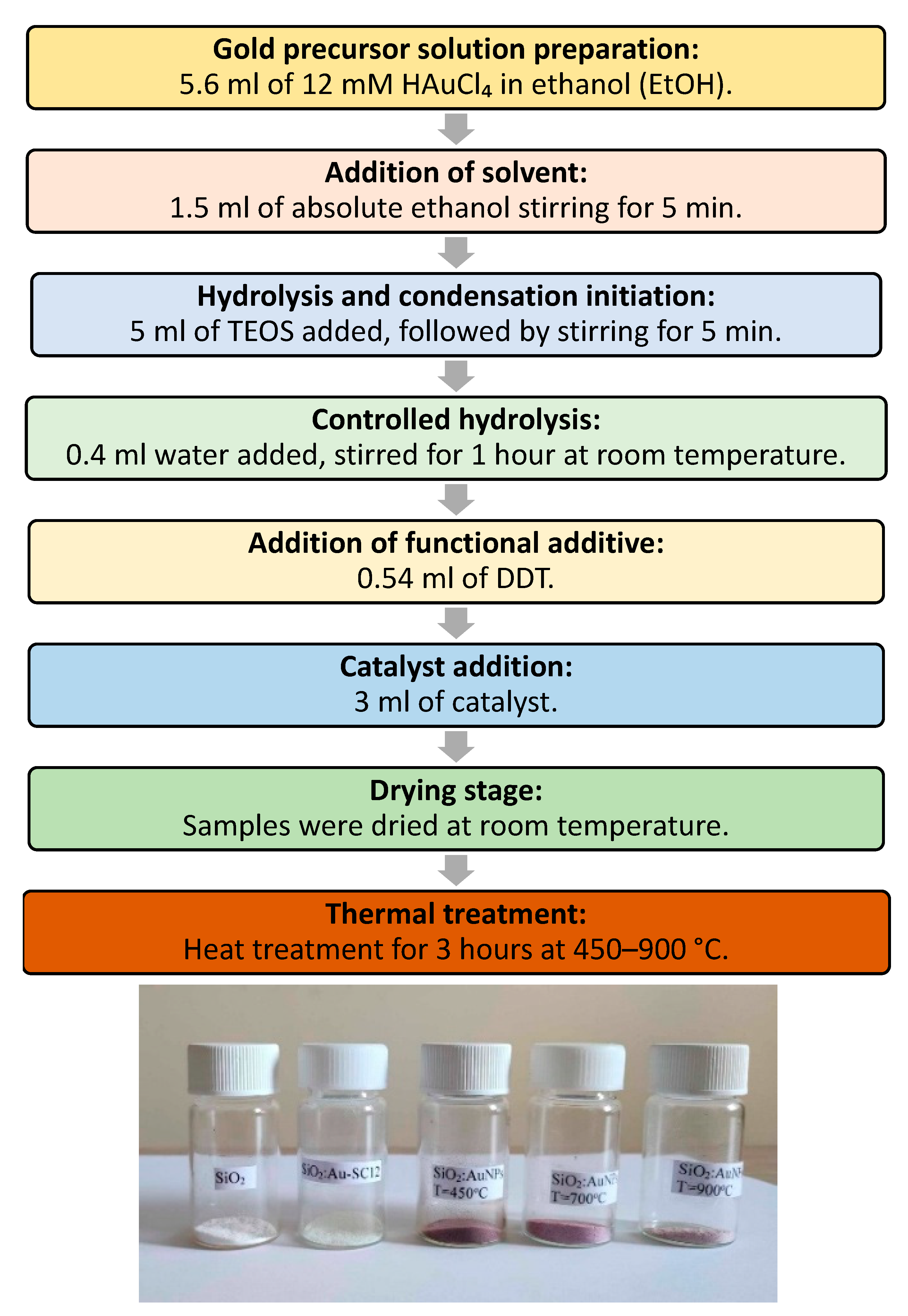
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Habashi, F. Purple of Cassius: Nano Gold or Colloidal Gold? Eur. Chem. Bull. 2016, 5, 416–419. [Google Scholar]
- Shandurkov, D.; Danchova, N.; Spassov, T.; Petrov, V.; Tsekov, R.; Gutzov, S. Silica gels doped with gold nanoparticles: Preparation, structure and optical properties. Gels 2023, 9, 663. [Google Scholar] [CrossRef]
- Danchova, N.; Tsekov, R.; Shandurkov, D.; Gutzov, S.; Lyubenova, L.; Mihaylov, L.; Spassov, T. Silica gels doped with gold nanoparticles and gold thiolate complexes: The effect of heating and preparation conditions. J. Non. Cryst. Solids 2025, 648, 123308. [Google Scholar] [CrossRef]
- Ong, C.; Cha, B.G.; Kim, J. Mesoporous Silica Nanoparticles Doped with Gold Nanoparticles for Combined Cancer Immunotherapy and Photothermal Therapy. ACS Appl. Bio Mater. 2019, 2, 3630–3638. [Google Scholar] [CrossRef] [PubMed]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol derivatised gold nanoparticles in a two phase liquid liquid system. Chem. Soc. Chem. Commun. 1994, 7, 801–802. [Google Scholar] [CrossRef]
- Matsuoka, J.; Mizutani, R.; Nasu, H.; Kamiya, K. Preparation of Au-Doped Silica Glass by Sol-Gel Method. J. Ceram. Soc. Jpn. 1992, 100, 599–601. [Google Scholar] [CrossRef][Green Version]
- Georgiev, P.; Chanachev, A.; Simeonova, S.; Ivanova, T.; Balashev, K. A new method for studying the kinetics of synthesis of gold nanoparticles in hexadecylanilin monolayer at the air/water interface by means of atomic force microscopy. Comptes Rendus L’Academie Bulg. Sci. 2020, 73, 197–202. [Google Scholar]
- Georgiev, P.; Simeonova, S.; Tsekov, R.; Balashev, K. Dependence of Plasmon Spectra of Small Gold Nanoparticles from Their Size: An Atomic Force Microscopy Experimental Approach. Plasmonics 2020, 15, 371–377. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Pham, Q.T.; Ngo, G.L.; Nguyen, X.A.; Nguyen, C.T.; Ledoux-Rak, I.; Lai, N.D. Direct Synthesis of Gold Nanoparticles in Polymer Matrix. Polymers 2023, 15, 16. [Google Scholar] [CrossRef]
- Ozkaraoglu, E.; Tunc, I.; Suzer, S. Preparation of Au and Au–Pt nanoparticles within PMMA matrix using UV and X-ray irradiation. Polymer 2009, 50, 462–466. [Google Scholar] [CrossRef]
- Kudryashov, A.; Baryshnikova, S.; Gusev, S.; Tatarskiy, D.; Lukichev, I.; Agareva, N.; Poddel’sky, A.; Bityurin, N. UV-Induced Gold Nanoparticle Growth in Polystyrene Matrix with Soluble Precursor. Photonics 2022, 9, 776. [Google Scholar] [CrossRef]
- Singh, J.; Soni, R.K. Tunable optical properties of Au nanoparticles encapsulated TiO2 spheres and their improved sunlight mediated photocatalytic activity. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 126011. [Google Scholar] [CrossRef]
- Díaz, C.; Cifuentes-Vaca, O.; Valenzuela, M.L. Matrix Effect of Properties of Au, ZnO and Eu2O3: Silica, Titania and Alumina Matrices. Micro 2023, 3, 699–714. [Google Scholar] [CrossRef]
- Cavaliere-Jaricot, S.; Darbandi, M.; Nann, T. Au–silica nanoparticles by “reverse” synthesis of cores in hollow silica shells. Chem. Commun. 2007, 20, 2031–2033. [Google Scholar] [CrossRef]
- Lu, X.; Mei, T.; Guo, Q.; Zhou, W.; Li, X.; Chen, J.; Zhou, X.; Sun, N.; Fang, Z. Improved performance of lateral flow immunoassays for alpha-fetoprotein and vanillin by using silica shell-stabilized gold nanoparticles. Microchim. Acta 2018, 186, 2. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, C.; Kim, Y. Hollow Au nanoparticles-decorated silica as near infrared-activated heat generating nano pigment. J. Ind. Eng. Chem. 2022, 107, 376–382. [Google Scholar] [CrossRef]
- Zanella, R.; Sandoval, A.; Santiago, P.; Basiuk, V.A.; Saniger, J.M. New Preparation Method of Gold Nanoparticles on SiO2. J. Phys. Chem. B 2006, 110, 8559–8565. [Google Scholar] [CrossRef]
- Ishida, T.; Murayama, T.; Taketoshi, A.; Haruta, M. Importance of Size and Contact Structure of Gold Nanoparticles for the Genesis of Unique Catalytic Processes. Chem. Rev. 2020, 120, 464–525. [Google Scholar] [CrossRef]
- Park, S.; Nguyen, Q.T.; Choi, J.; Park, J.H.; Park, J.R.; Nakate, U.T.; Park, S. Au-nanoparticles-decorated CeO2 electrocatalyst synthesized by direct growth and wet impregnation for enhanced oxygen evolution reaction. Surf. Interfaces 2023, 41, 103206. [Google Scholar] [CrossRef]
- Corma, A.; Garcia, H. Supported gold nanoparticles as catalysts for organic reactions. Chem. Soc. Rev. 2008, 37, 2096–2126. [Google Scholar] [CrossRef]
- Murtaza, A.; Uroos, M.; Sultan, M.; Muazzam, R.; Naz, S. Enhancing catalytic potential of gold nanoparticles by linear and cross-linked polyurethane blending. RSC Adv. 2021, 11, 26635–26643. [Google Scholar] [CrossRef]
- Padikkaparambil, S.; Narayanan, B.; Yaakob, Z.; Viswanathan, S.; Tasirin, S.M. Au/TiO2 Reusable Photocatalysts for Dye Degradation. Int. J. Photoenergy 2013, 2013, 752605. [Google Scholar] [CrossRef]
- Peacor, D.R. High-temperature single-crystal study of the cristobalite inversion. Z. Fuer Krist. 1973, 138, 274–298. [Google Scholar] [CrossRef]
- Kato, K.; Nukui, A. Die Kristallstruktur des monoklinen Tief-Tridymits. Acta Crystallogr. Sect. B 1976, 32, 2486–2491. [Google Scholar] [CrossRef]
- Konon, M.; Polyakova, I.G.; Mazur, A.S.; Saratovskii, A.S.; Danilovich, D.P.; Alikin, M. Crystallization of Cristobalite in Sodium Borosilicate Glass in the Presence of Cr2O3. Materials 2023, 16, 5016. [Google Scholar] [CrossRef]
- Kreibig, U.; Vollmer, M. Optical Properties of Metal Clusters; Springer Berlin Heidelberg: Berlin, Germany, 1995. [Google Scholar]
- Gutzov, S.; Bredol, M. Optical properties of cerium and terbium doped silica xerogels. J. Mat. Sci. 2006, 46, 1835–1837. [Google Scholar] [CrossRef]
- Nesheva, D.; Levi, Z.; Aneva, Z.; Nikolova, V.; Hofmeister, H. Experimental studies on the defect states at the interface between nanocrystalline CdSe and amorphous SiOx. J. Phys. Condens. Matter 2000, 12, 751. [Google Scholar] [CrossRef][Green Version]
- Fan, X.; Zheng, W.; Singh, D.J. Light scattering and surface plasmons on small spherical particles. Light Sci. Appl. 2014, 3, e179. [Google Scholar] [CrossRef]
- Bentea, L.; Watzky, M.A.; Finke, R.G. Sigmoidal Nucleation and Growth Curves Across Nature Fit by the Finke–Watzky Model of Slow Continuous Nucleation and Autocatalytic Growth: Explicit Formulas for the Lag and Growth Times Plus Other Key Insights. J. Phys. Chem. C 2017, 121, 5302–5312. [Google Scholar] [CrossRef]
- Watzky, M.A.; Finke, R.G. Transition Metal Nanocluster Formation Kinetic and Mechanistic Studies. A New Mechanism When Hydrogen Is the Reductant: Slow, Continuous Nucleation and Fast Autocatalytic Surface Growth. J. Am. Chem. Soc. 1997, 119, 10382–10400. [Google Scholar] [CrossRef]
- Buffat, P.; Borel, J.-P. Size effect on the melting temperature of gold particles. Phys. Rev. A 1976, 13, 2287–2298. [Google Scholar] [CrossRef]
- Chegel, V.; Rachkov, O.; Lopatynskyi, A.; Ishihara, S.; Yanchuk, I.; Nemoto, Y.; Hill, J.P.; Ariga, K. Gold Nanoparticles Aggregation: Drastic Effect of Cooperative Functionalities in a Single Molecular Conjugate. J. Phys. Chem. C 2012, 116, 2683–2690. [Google Scholar] [CrossRef]
- Gutzow, I.; Pascova, R.; Jordanov, N.; Gutzov, S.; Petkov, I.; Markovska, I.; Schmelzer, J.W.P.; Ludwig, F.P. Crystalline and Amorphous Modifications of Silica: Structure, Thermodynamic Properties, Solubility and Synthesis in “Glass”; Schmelzer, J.W.P., Ed.; De Gruyter: Berlin, Germany, 2014. [Google Scholar]
- Gutzow, I.S.; Schmelzer, J.W.P. The Vitreous State; Springer: Berlin/Heidelberg, Germany, 1994. [Google Scholar]
- Perrotta, A.J.; Grubbs, D.K.; Martin, E.S.; Dando, N.R.; McKinstry, H.A.; Huarg, C.Y. Chemical Stabilization of β-Cristobalite. J. Am. Ceram. Soc. 1989, 72, 441–447. [Google Scholar] [CrossRef]
- Coppage, R.; Leopold, M.; Allen, G.; Lacy, C. Gold Nanoparticle in Ceramic Glaze 2018. U.S. Patent No. 10,913,856, 23 October 2017. [Google Scholar]
- Deng, Y.; Sha, Z.; Wang, X.; Duan, K.; Xue, W.; Beadham, I.; Xiao, X.; Zhang, C. Exploration of Key Factors in the Preparation of Highly Hydrophobic Silica Aerogel from Rice Husk AshAssisted by Machine Learning. Gels 2025, 11, 74. [Google Scholar] [CrossRef]
- Csupász-Szabó, H.J.; Döncző, B.; Szarka, M.; Daróczi, L.; Lázár, I. Thermal Reverse-Engineered Synthesis and Catalytic Activity of Nanogold-Containing Silica Aerogels. Gels 2025, 11, 87. [Google Scholar] [CrossRef]
- Choi, S.M.; Kang, S.H. Gold Nanoparticle Superlattice Embedded in Porous Silica and Method for Manufacturing Same. U.S. Patent No 11,565,245, 31 January 2023. [Google Scholar]
- Yin, Y.; Gao, C. Templated Synthesis of Metal Nanorods in Silica Nanotubes. U.S. Patent No. 9,937,556, 10 April 2018. [Google Scholar]
- Ostafin, A.E.; Nooney, R.; Maginn, E. Process for Making Mesoporous Silicate Nanoparticle Coatings and Hollow Mesoporous Silica Nano-Shells. U.S. Patent No. 6,913,825, 5 July 2005. [Google Scholar]
- Kang, S.H. Metallic Nanoparticle Catalysts Embedded in Porous Oxide Support, Which Show High Catalytic Activity Even at Low Temperatures. U.S. Patent Application 17/292,986, 20 January 2022. [Google Scholar]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity. Berichte Der Bunsenges. Phys. Chem. 1982, 86, 957. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Shandurkov, D.; Ignatov, P.; Spassova, I.; Gutzov, S. Spectral and Texture Properties of Hydrophobic Aerogel Powders Obtained from Room Temperature Drying. Molecules 2021, 26, 1796. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Williams, R.T. Physisorption Hysteresis Loops and the Characterization of Nanoporous Materials. Adsorpt. Sci. Technol. 2004, 22, 773–782. [Google Scholar] [CrossRef]
- Villarroel-Rocha, J.; Barrera, D.; Blanco, A.A.G.; Jalil, M.E.R.; Sapag, K. Importance of the αs-plot Method in the Characterization of Nanoporous Materials. Adsorpt. Sci. Technol. 2013, 31, 165–183. [Google Scholar] [CrossRef]
- de Boer, J.H.; Lippens, B.C.; Linsen, B.G.; Broekhoff, J.C.P.; van den Heuvel, A.; Osinga, T.J. The T-Curve Of Multimolecular N2-Adsorption. J. Colloid Interface Sci. 1966, 21, 405–414. [Google Scholar] [CrossRef]
- Bustillo, M.A.; Fort, R.; Bustillo, M. Specific surface area and ultramicroporosity in polymorphs of silica. Eur. J. Miner. 1993, 5, 1195–1204. [Google Scholar] [CrossRef]
- Wheatley, K. Measurement of the surface area of tridymite. J. Appl. Chem. 1959, 9, 159–162. [Google Scholar] [CrossRef]
- Bohren, C.F.; Huffman, D.R.; Clothiaux, E.E. Absorption and Scattering of Light by Small Particles; Willey-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Labsphere Spectralon®. Diffuse Reflectance Standards. Available online: https://www.labsphere.com/product/spectralon-diffuse-reflectance-standards/ (accessed on 27 May 2025).
- Powdercell. Available online: http://ccp14.cryst.bbk.ac.uk/ccp/web-mirrors/powdcell/a_v/v_1/powder/e_cell.html (accessed on 27 May 2025).
- ImageJ. Available online: https://imagej.net/ij/ (accessed on 27 May 2025).
- Neimark, A.V.; Ravikovitch, P.I. Capillary condensation in MMS and pore structure characterization. Micropor. Mesopor. Mater. 2001, 44–45, 697–707. [Google Scholar] [CrossRef]
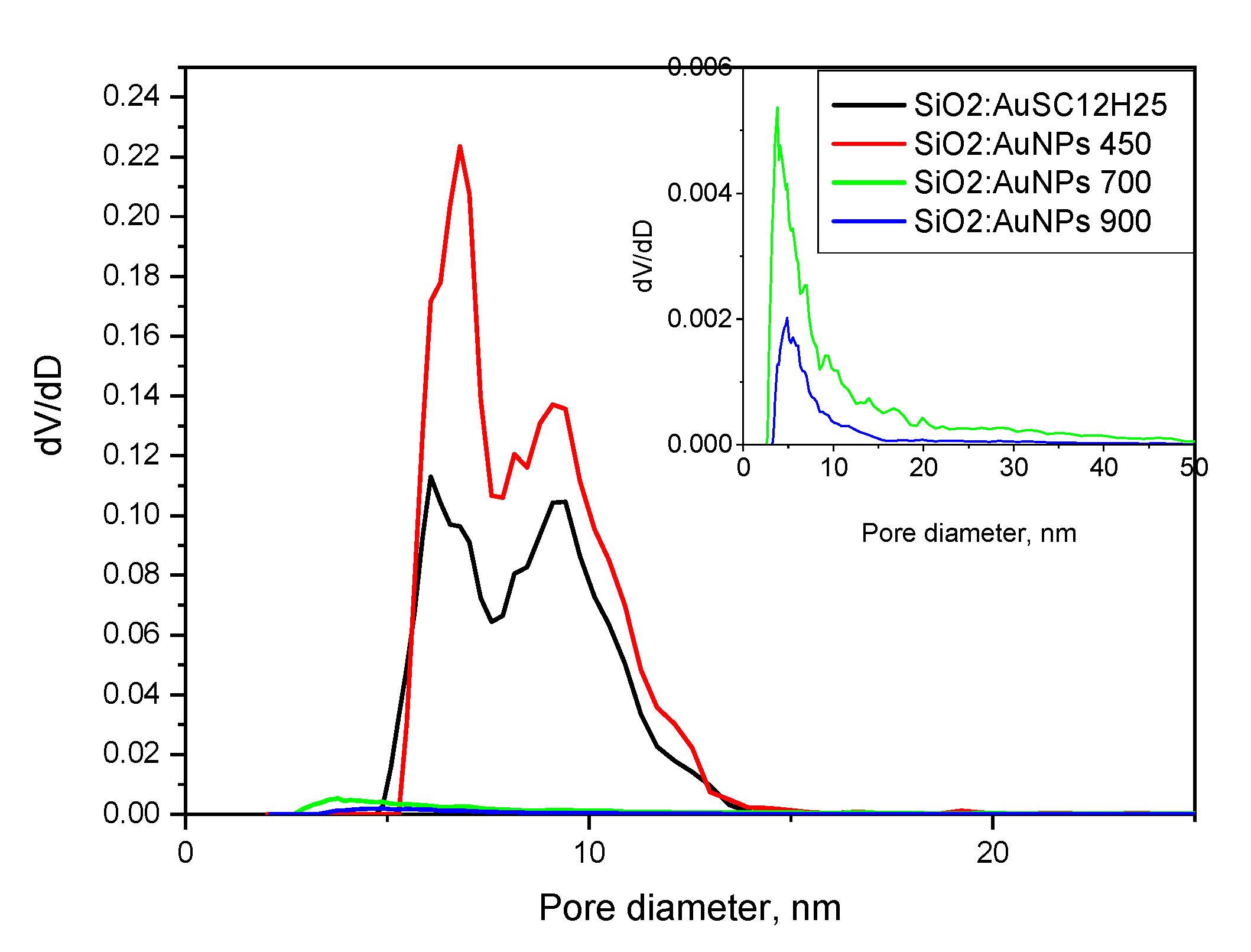
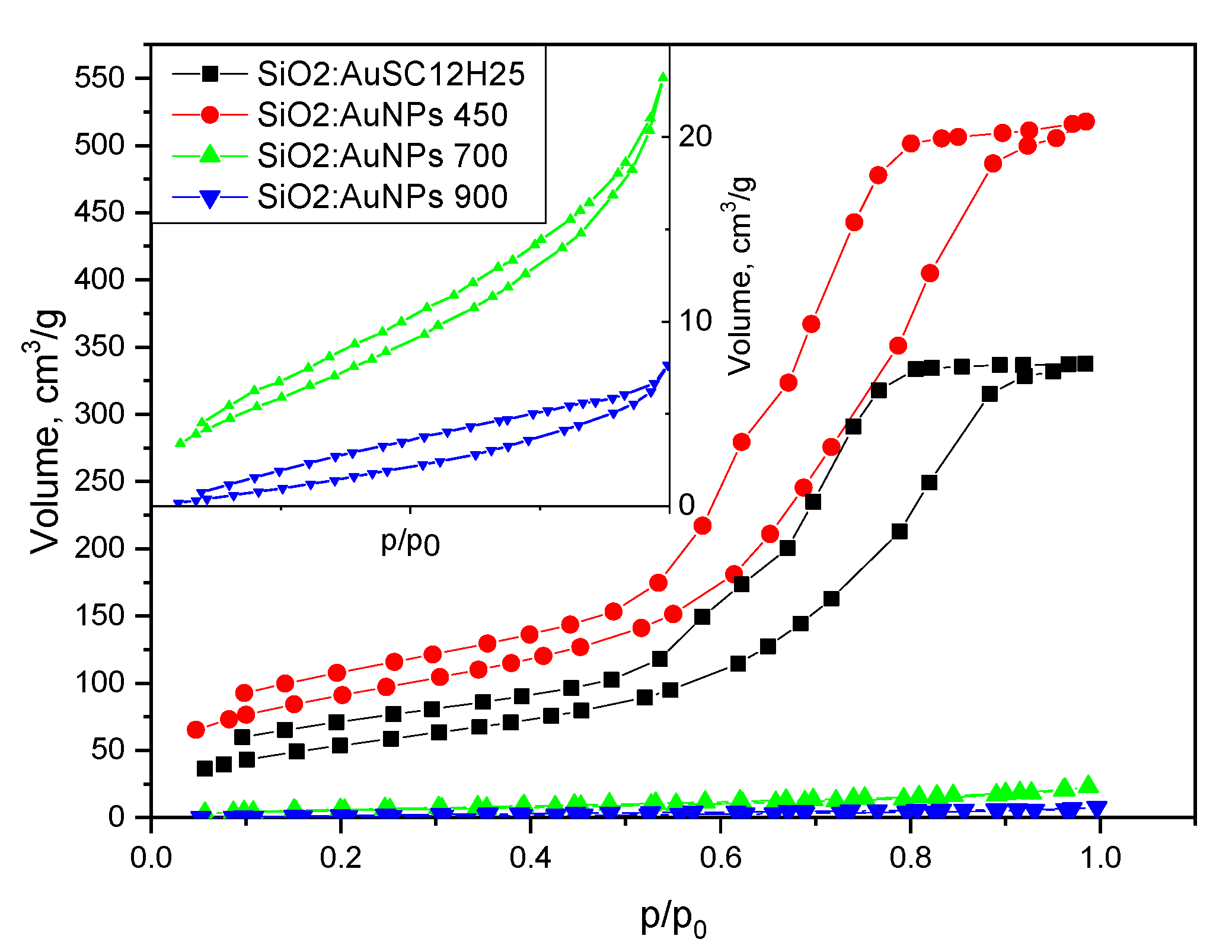
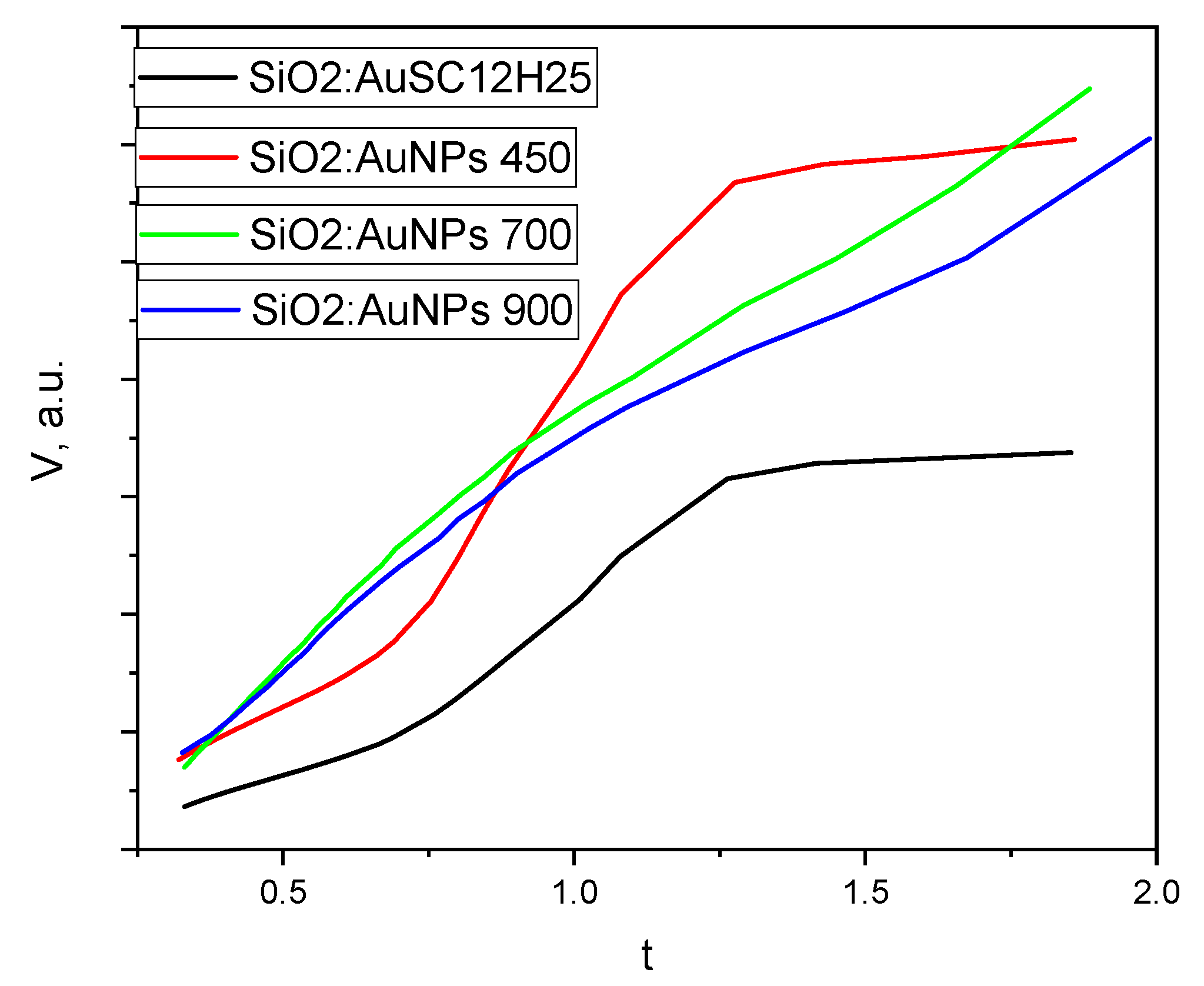
| Sample | SBET, m2/g | Vt, cm3/g | Dav nm | T °C | ρcalc g/cm3 |
|---|---|---|---|---|---|
| SiO2:AuSC12H25 | 204 | 0.52 | 10.0 | - | 1.02 |
| SiO2:AuNPs | 330 | 0.80 | 9.7 | 450 | 0.8 |
| SiO2:AuNPs | 21 | 0.04 | 6.9 | 700 | 2.01 |
| SiO2:AuNPs | 9 | 0.01 | 5.0 | 900 | 2.24 |
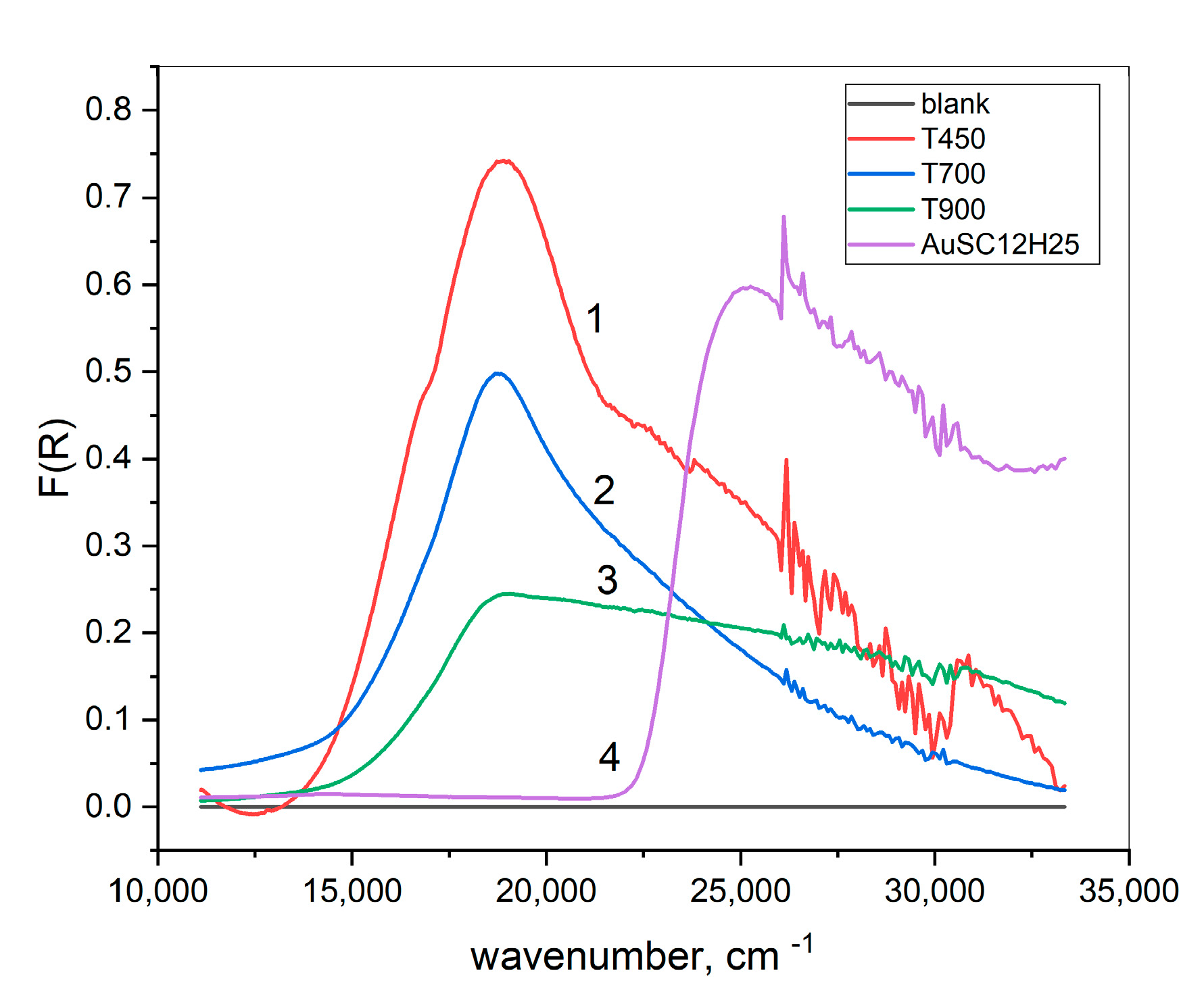
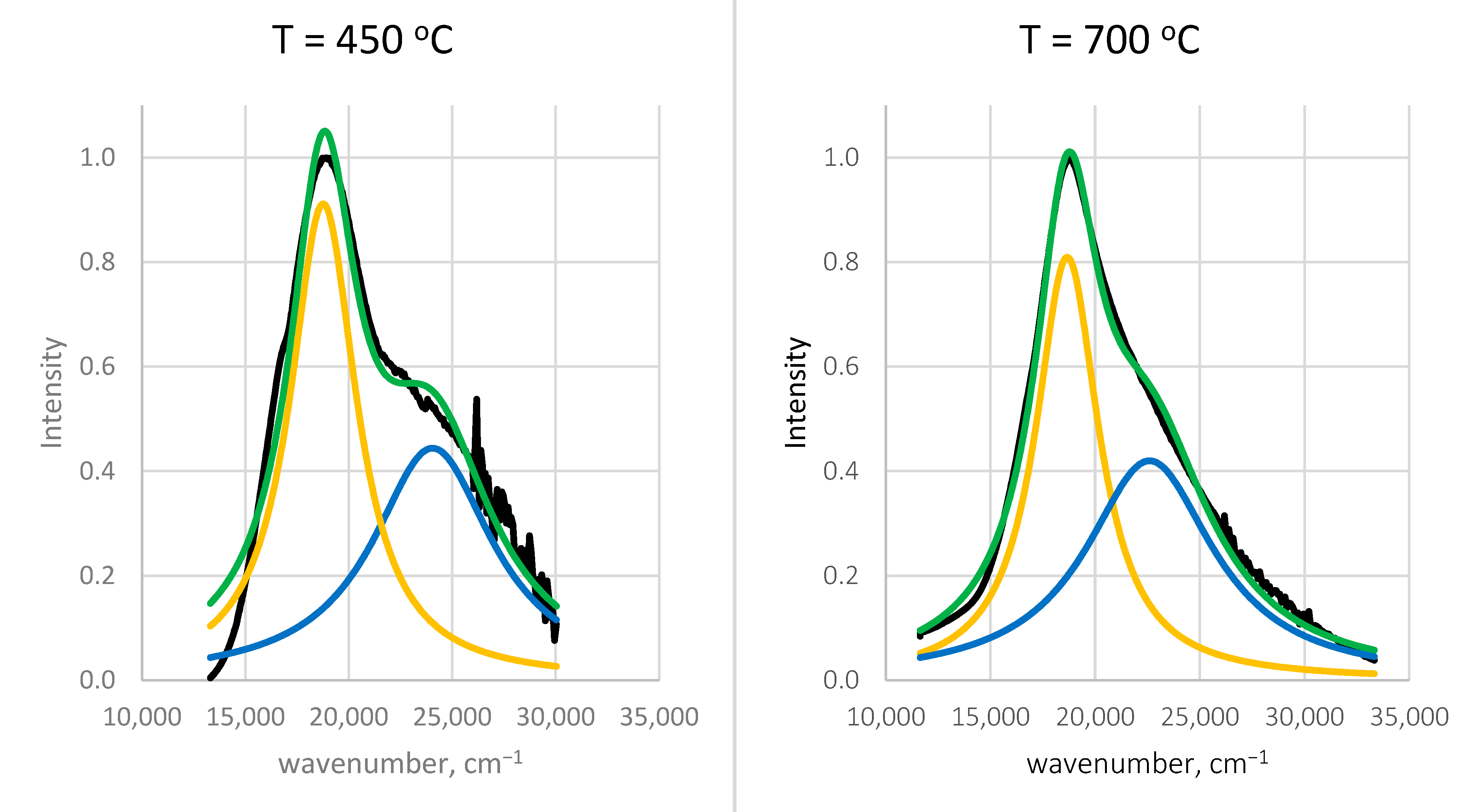
| T [°C] | [cm−1] | [cm−1] | [cm−1] | [cm−1] | ||
|---|---|---|---|---|---|---|
| 450 | 0.91 | 18,761 | 3911 | 0.44 | 24,048 | 7095 |
| 700 | 0.81 | 18,668 | 3666 | 0.42 | 22,599 | 7457 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danchova, N.; Shandurkov, D.; Tsekov, R.; Mihaylov, L.; Spassov, T.; Gutzov, S. Porous Silica Gels Doped with Gold Nanoparticles: Preparation, Microstructure, Optical and Textural Properties. Gels 2025, 11, 454. https://doi.org/10.3390/gels11060454
Danchova N, Shandurkov D, Tsekov R, Mihaylov L, Spassov T, Gutzov S. Porous Silica Gels Doped with Gold Nanoparticles: Preparation, Microstructure, Optical and Textural Properties. Gels. 2025; 11(6):454. https://doi.org/10.3390/gels11060454
Chicago/Turabian StyleDanchova, Nina, Dimitar Shandurkov, Roumen Tsekov, Luben Mihaylov, Tony Spassov, and Stoyan Gutzov. 2025. "Porous Silica Gels Doped with Gold Nanoparticles: Preparation, Microstructure, Optical and Textural Properties" Gels 11, no. 6: 454. https://doi.org/10.3390/gels11060454
APA StyleDanchova, N., Shandurkov, D., Tsekov, R., Mihaylov, L., Spassov, T., & Gutzov, S. (2025). Porous Silica Gels Doped with Gold Nanoparticles: Preparation, Microstructure, Optical and Textural Properties. Gels, 11(6), 454. https://doi.org/10.3390/gels11060454









