Author Contributions
Conceptualization, B.S. and G.K.; methodology, B.S.; software, B.S.; M.B.-S., and G.K.; validation, G.K. and I.C.; formal analysis, B.S.; investigation, B.S. and M.B.-S.; resources, G.K. and I.C.; data curation, B.S.; writing—original draft preparation, B.S.; M.B.-S., and G.K.; writing—review and editing, I.C.; visualization, B.S. and M.B.-S.; supervision, I.C.; project administration, B.S. and G.K.; funding acquisition, B.S. and I.C. All authors have read and agreed to the published version of the manuscript.
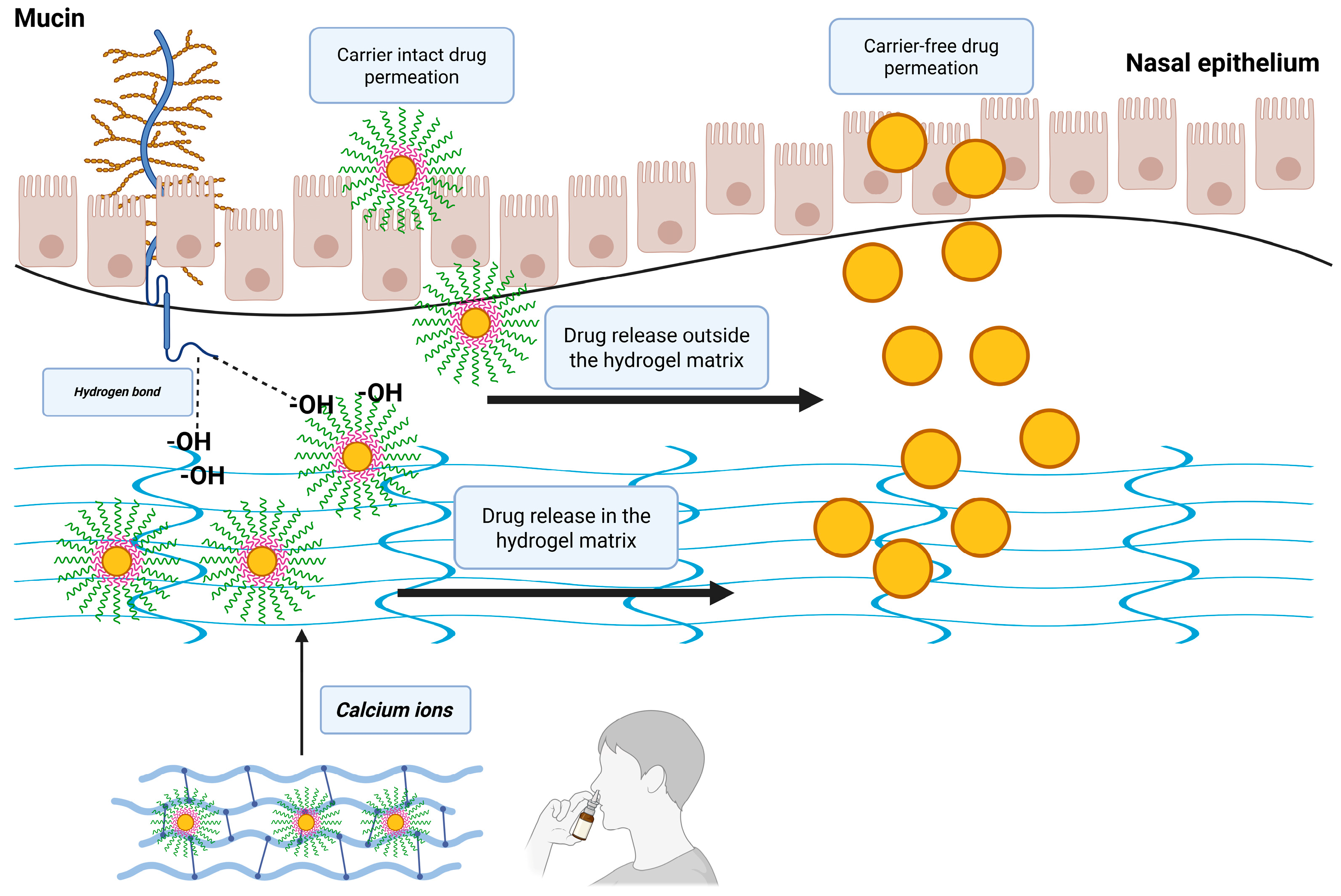
Figure 1.
Schematic representation of the possible nasal administration of gellan gum–cellulose derivative matrices loaded with risperidone-encapsulated polymeric micelles.
Figure 1.
Schematic representation of the possible nasal administration of gellan gum–cellulose derivative matrices loaded with risperidone-encapsulated polymeric micelles.
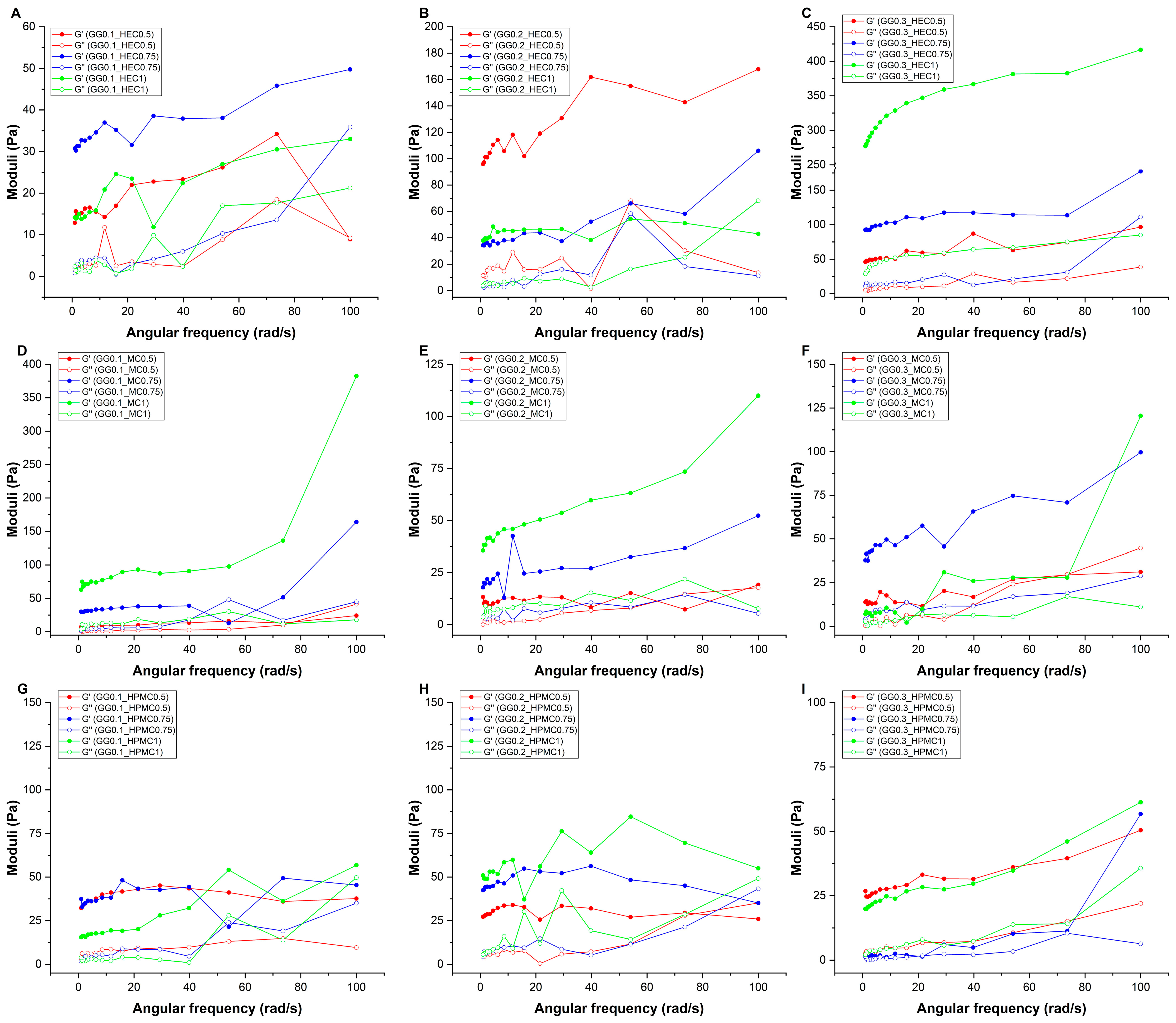
Figure 2.
Elastic (G′) and viscous (G″) moduli curves as a function of the angular frequency in the case of gellan gum-based hydrogels, combined with (A–C) hydroxyethyl cellulose, (D–F) methylcellulose, and (G–I) hydroxypropyl-methylcellulose. Formulations were named via the following system: GG (gellan gum) with its respective concentration (% w/v), followed by the concentration of either HEC (hydroxyethyl cellulose), methylcellulose (MC), or hydroxypropyl-methylcellulose (HPMC), also in the unit of % w/v.
Figure 2.
Elastic (G′) and viscous (G″) moduli curves as a function of the angular frequency in the case of gellan gum-based hydrogels, combined with (A–C) hydroxyethyl cellulose, (D–F) methylcellulose, and (G–I) hydroxypropyl-methylcellulose. Formulations were named via the following system: GG (gellan gum) with its respective concentration (% w/v), followed by the concentration of either HEC (hydroxyethyl cellulose), methylcellulose (MC), or hydroxypropyl-methylcellulose (HPMC), also in the unit of % w/v.
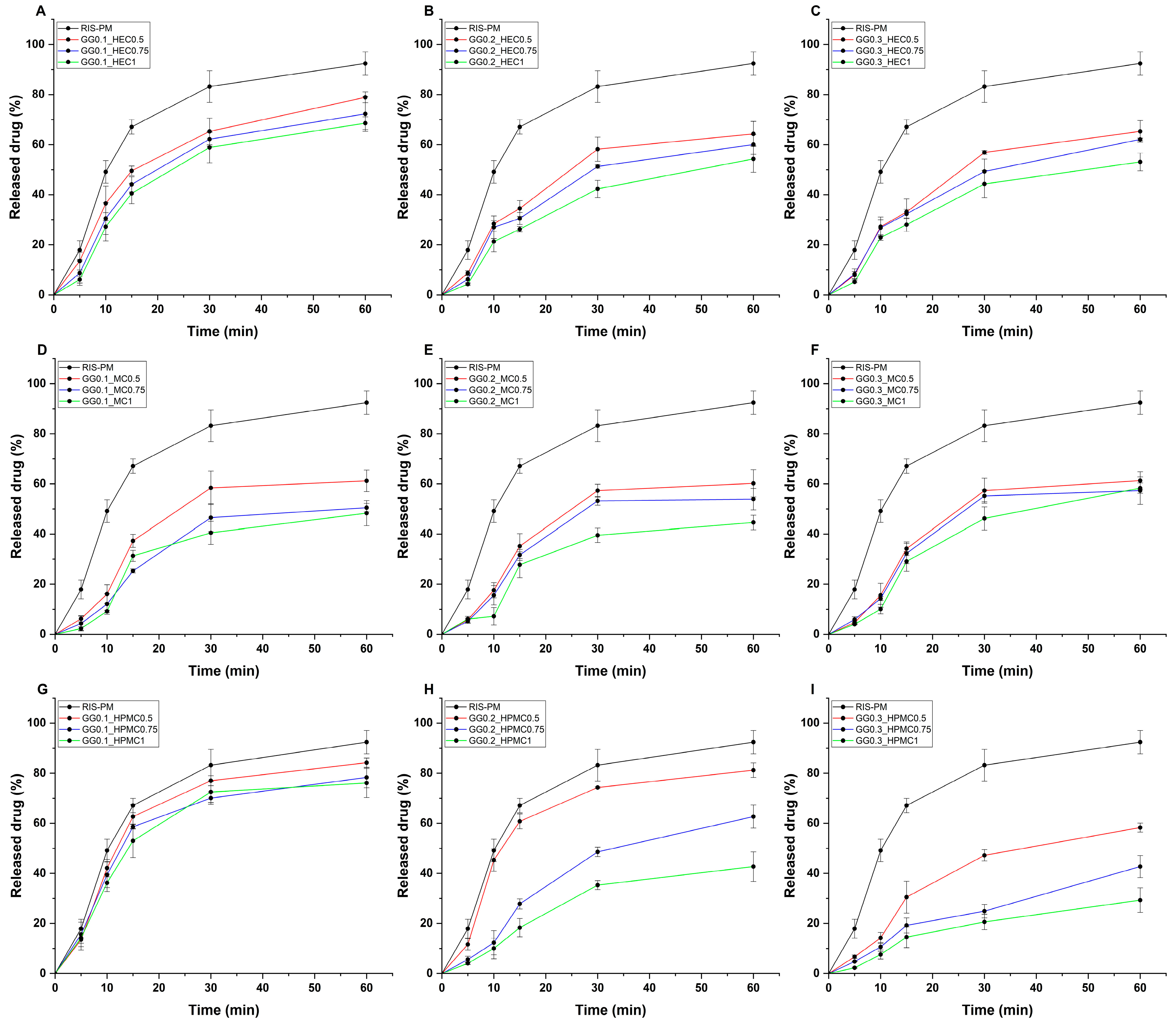
Figure 3.
In vitro drug release profiles of gellan gum-based hydrogels, combined with (A–C) hydroxyethyl cellulose, (D–F) methylcellulose, and (G–I) hydroxypropyl-methylcellulose, compared with gel matrix-free polymeric micelles (RIS-PM). Experiments were conducted at simulated nasal conditions. Data are expressed as average ± SD (n = 3). Formulations were named via the following system: GG (gellan gum) with its respective concentration (% w/v), followed by the concentration of either HEC (hydroxyethyl cellulose), methylcellulose (MC), or hydroxypropyl-methylcellulose (HPMC), also in the unit of % w/v. RIS-PM represents the polymeric micelle formulation dispersed in solely purified water.
Figure 3.
In vitro drug release profiles of gellan gum-based hydrogels, combined with (A–C) hydroxyethyl cellulose, (D–F) methylcellulose, and (G–I) hydroxypropyl-methylcellulose, compared with gel matrix-free polymeric micelles (RIS-PM). Experiments were conducted at simulated nasal conditions. Data are expressed as average ± SD (n = 3). Formulations were named via the following system: GG (gellan gum) with its respective concentration (% w/v), followed by the concentration of either HEC (hydroxyethyl cellulose), methylcellulose (MC), or hydroxypropyl-methylcellulose (HPMC), also in the unit of % w/v. RIS-PM represents the polymeric micelle formulation dispersed in solely purified water.
Table 1.
Gelation of gellan gum–cellulose derivative composites based on their composition in simulated nasal electrolyte solution. “+” means visible gelation at 32 °C, whilst still capable of slipping from the vials. “++” shows a transition between slipping hydrogels and firm hydrogels, and “+++” shows firm hydrogel formation without slipping.
Table 1.
Gelation of gellan gum–cellulose derivative composites based on their composition in simulated nasal electrolyte solution. “+” means visible gelation at 32 °C, whilst still capable of slipping from the vials. “++” shows a transition between slipping hydrogels and firm hydrogels, and “+++” shows firm hydrogel formation without slipping.
| | Gellan Gum Concentration (% w/v) |
|---|
| | 0.1 | 0.2 | 0.3 |
|---|
| HEC 0.5% w/v | + | + | + |
| HEC 0.75% w/v | + | ++ | ++ |
| HEC 1% w/v | ++ | +++ | +++ |
| MC 0.5% w/v | + | + | + |
| MC 0.75% w/v | + | ++ | ++ |
| MC 1% w/v | ++ | ++ | +++ |
| HPMC 0.5% w/v | + | ++ | ++ |
| HPMC 0.75% w/v | ++ | +++ | +++ |
| HPMC 1% w/v | +++ | +++ | +++ |
Table 2.
Clarity, pH, and drug content of the various hydrogel formulations. Data are expressed as average ± SD (n = 3). Formulations were named via the following system: GG (gellan gum) with its respective concentration (% w/v), followed by the concentration of either HEC (hydroxyethyl cellulose), methylcellulose (MC), or hydroxypropyl-methylcellulose (HPMC), also in the unit of % w/v.
Table 2.
Clarity, pH, and drug content of the various hydrogel formulations. Data are expressed as average ± SD (n = 3). Formulations were named via the following system: GG (gellan gum) with its respective concentration (% w/v), followed by the concentration of either HEC (hydroxyethyl cellulose), methylcellulose (MC), or hydroxypropyl-methylcellulose (HPMC), also in the unit of % w/v.
| Formulation | Clarity | pH | Drug Content (%) |
|---|
| GG0.1_HEC0.5 | transparent | 5.47 ± 0.21 | 98.76 ± 1.42 |
| GG0.1_HEC0.75 | transparent | 5.63 ± 0.14 | 95.84 ± 0.67 |
| GG0.1_HEC1 | transparent | 5.52 ± 0.2 | 100.15 ± 1.83 |
| GG0.2_HEC0.5 | transparent | 5.71 ± 0.34 | 99.27 ± 0.91 |
| GG0.2_HEC0.75 | transparent | 5.49 ± 0.11 | 96.43 ± 1.15 |
| GG0.2_HEC1 | transparent | 5.56 ± 0.26 | 97.12 ± 0.44 |
| GG0.3_HEC0.5 | transparent | 5.34 ± 0.19 | 100.98 ± 1.76 |
| GG0.3_HEC0.75 | cloudy | 5.71 ± 0.35 | 96.88 ± 0.58 |
| GG0.3_HEC1 | cloudy | 5.39 ± 0.24 | 99.95 ± 1.27 |
| GG0.1_MC0.5 | cloudy | 5.44 ± 0.15 | 97.59 ± 0.73 |
| GG0.1_MC0.75 | cloudy | 5.34 ± 0.09 | 98.33 ± 1.94 |
| GG0.1_MC1 | cloudy | 5.56 ± 0.18 | 100.41 ± 0.39 |
| GG0.2_MC0.5 | cloudy | 5.48 ± 0.31 | 95.67 ± 1.02 |
| GG0.2_MC0.75 | cloudy | 5.59 ± 0.14 | 99.78 ± 1.68 |
| GG0.2_MC1 | cloudy | 5.50 ± 0.26 | 101.12 ± 0.87 |
| GG0.3_MC0.5 | cloudy | 5.31 ± 0.12 | 98.03 ± 0.6 |
| GG0.3_MC0.75 | cloudy | 5.52 ± 0.27 | 97.45 ± 1.33 |
| GG0.3_MC1 | cloudy | 5.24 ±0.18 | 96.17 ± 1.89 |
| GG0.1_HPMC0.5 | transparent | 5.67 ± 0.33 | 100.64 ± 0.49 |
| GG0.1_HPMC0.75 | transparent | 5.43 ± 0.21 | 95.26 ± 1.11 |
| GG0.1_HPMC1 | transparent | 5.38 ± 0.08 | 99.10 ± 0.85 |
| GG0.2_HPMC0.5 | transparent | 5.59 ± 0.29 | 98.54 ± 1.59 |
| GG0.2_HPMC0.75 | transparent | 5.25 ± 0.31 | 101.3 ± 0.95 |
| GG0.2_HPMC1 | transparent | 5.73 ± 0.15 | 95.93 ± 1.21 |
| GG0.3_HPMC0.5 | transparent | 5.61 ± 0.24 | 97.88 ± 0.36 |
| GG0.3_HPMC0.75 | transparent | 5.46 ± 0.34 | 99.51 ± 1.5 |
| GG0.3_HPMC1 | cloudy | 5.34 ± 0.11 | 96.75 ± 0.78 |
Table 3.
Expansion coefficient (S) and water-holding capacity (WHC) of the various gel formulations. Data are expressed as average ± SD (n = 3). Formulations were named via the following system: GG (gellan gum) with its respective concentration (% w/v), followed by the concentration of either HEC (hydroxyethyl cellulose), methylcellulose (MC), or hydroxypropyl-methylcellulose (HPMC), also in the unit of % w/v. * describes the expansion coefficient as high (>5%) with at least 80% water-holding capacity, suitable for longer-term hydrogel stability.
Table 3.
Expansion coefficient (S) and water-holding capacity (WHC) of the various gel formulations. Data are expressed as average ± SD (n = 3). Formulations were named via the following system: GG (gellan gum) with its respective concentration (% w/v), followed by the concentration of either HEC (hydroxyethyl cellulose), methylcellulose (MC), or hydroxypropyl-methylcellulose (HPMC), also in the unit of % w/v. * describes the expansion coefficient as high (>5%) with at least 80% water-holding capacity, suitable for longer-term hydrogel stability.
| Formulation | S (%) | WHC (%) |
|---|
| GG0.1_HEC0.5 | 1.25 ± 0.09 | 75.07 ± 3.14 |
| GG0.1_HEC0.75 | 1.62 ± 0.42 | 77.14 ± 1.72 |
| GG0.1_HEC1 | 4.18 ± 0.37 | 77.65 ± 4.05 |
| GG0.2_HEC0.5 | 4.77 ± 0.33 | 78.09 ± 2.89 |
| GG0.2_HEC0.75 | 4.43 ± 0.18 | 78.86 ± 0.76 |
| GG0.2_HEC1 | 5.01 ± 0.21 | 79.54 ± 1.38 |
| * GG0.3_HEC0.5 | 7.05 ± 0.28 | 86.44 ± 4.62 |
| * GG0.3_HEC0.75 | 7.31 ± 0.07 | 87.21 ± 2.47 |
| * GG0.3_HEC1 | 7.62 ± 0.41 | 88.03 ± 0.59 |
| GG0.1_MC0.5 | 2.03 ± 0.12 | 75.31 ± 3.91 |
| GG0.1_MC0.75 | 2.47 ± 0.35 | 75.61 ± 4.27 |
| GG0.1_MC1 | 3.31 ± 0.25 | 80.27 ± 2.05 |
| * GG0.2_MC0.5 | 5.29 ± 0.44 | 81.43 ± 1.94 |
| * GG0.2_MC0.75 | 5.52 ± 0.16 | 82.15 ± 0.84 |
| * GG0.2_MC1 | 5.88 ± 0.39 | 82.98 ± 3.53 |
| * GG0.3_MC0.5 | 7.88 ± 0.14 | 88.56 ± 4.11 |
| * GG0.3_MC0.75 | 8.17 ± 0.31 | 89.72 ± 2.71 |
| * GG0.3_MC1 | 8.39 ± 0.24 | 90.35 ± 1.15 |
| GG0.1_HPMC0.5 | 2.88 ± 0.29 | 75.96 ± 4.77 |
| GG0.1_HPMC0.75 | 3.62 ± 0.18 | 76.28 ±0.91 |
| GG0.1_HPMC1 | 3.95 ± 0.43 | 83.74 ± 3.31 |
| GG0.2_HPMC0.5 | 6.11 ± 0.22 | 76.73 ± 2.24 |
| GG0.2_HPMC0.75 | 6.49 ± 0.11 | 84.36 ± 1.59 |
| GG0.2_HPMC1 | 6.78 ± 0.34 | 85.02 ± 3.76 |
| * GG0.3_HPMC0.5 | 8.64 ± 0.27 | 85.67 ± 2.63 |
| * GG0.3_HPMC0.75 | 8.93 ± 0.08 | 90.97 ± 0.68 |
| * GG0.3_HPMC1 | 9.14 ± 0.32 | 91.84 ± 4.39 |
Table 4.
Effect of the various in situ gel compositions on the characteristics of polymeric micelles: micelle size (DH), polydispersity index (PdI), and encapsulation efficiency (EE). Data are expressed as average ± SD (n = 3). Formulations were named via the following system: GG (gellan gum) with its respective concentration (% w/v), followed by the concentration of either HEC (hydroxyethyl cellulose), methylcellulose (MC), or hydroxypropyl-methylcellulose (HPMC), also in the unit of % w/v. RIS-PM represents the polymeric micelle formulation dispersed in solely purified water.
Table 4.
Effect of the various in situ gel compositions on the characteristics of polymeric micelles: micelle size (DH), polydispersity index (PdI), and encapsulation efficiency (EE). Data are expressed as average ± SD (n = 3). Formulations were named via the following system: GG (gellan gum) with its respective concentration (% w/v), followed by the concentration of either HEC (hydroxyethyl cellulose), methylcellulose (MC), or hydroxypropyl-methylcellulose (HPMC), also in the unit of % w/v. RIS-PM represents the polymeric micelle formulation dispersed in solely purified water.
| Formulation | DH (nm) | PdI | EE (%) |
|---|
| RIS-PM | 30.17 ± 2.6 | 0.115 ± 0.012 | 84.15 ± 2.85 |
| GG0.1_HEC0.5 | 39.41 ± 4.32 | 0.183 ± 0.014 | 81.42 ± 3.72 |
| GG0.1_HEC0.75 | 45.19 ± 1.87 | 0.155 ± 0.022 | 79.11 ± 1.84 |
| GG0.1_HEC1 | 39.43 ± 3.45 | 0.260 ± 0.006 | 83.24 ± 5.60 |
| GG0.2_HEC0.5 | 37.12 ± 0.91 | 0.110 ± 0.031 | 78.97 ± 4.15 |
| GG0.2_HEC0.75 | 35.01 ± 2.78 | 0.246 ± 0.017 | 76.55 ± 2.96 |
| GG0.2_HEC1 | 40.14 ± 1.24 | 0.225 ± 0.008 | 81.03 ± 6.03 |
| GG0.3_HEC0.5 | 41.28 ± 4.61 | 0.125 ± 0.025 | 80.15 ± 0.89 |
| GG0.3_HEC0.75 | 40.69 ± 3.08 | 0.211 ± 0.019 | 80.83 ± 5.12 |
| GG0.3_HEC1 | 41.75 ± 0.73 | 0.243 ± 0.012 | 76.12 ± 2.41 |
| GG0.1_MC0.5 | 36.25 ± 2.15 | 0.194 ± 0.029 | 78.95 ± 1.27 |
| GG0.1_MC0.75 | 35.66 ± 4.04 | 0.232 ± 0.007 | 78.52 ± 4.88 |
| GG0.1_MC1 | 39.09 ± 1.39 | 0.145 ± 0.034 | 79.45 ± 6.45 |
| GG0.2_MC0.5 | 36.79 ± 2.52 | 0.120 ± 0.011 | 83.68 ± 0.66 |
| GG0.2_MC0.75 | 37.32 ± 3.91 | 0.158 ± 0.007 | 83.14 ± 3.09 |
| GG0.2_MC1 | 42.10 ± 4.76 | 0.173 ± 0.026 | 84.25 ± 2.18 |
| GG0.3_MC0.5 | 42.84 ± 0.68 | 0.189 ± 0.015 | 83.12 ± 1.96 |
| GG0.3_MC0.75 | 43.29 ± 1.03 | 0.268 ± 0.032 | 82.63 ± 5.81 |
| GG0.3_MC1 | 43.88 ± 2.96 | 0.114 ± 0.009 | 79.02 ± 4.53 |
| GG0.1_HPMC0.5 | 41.05 ± 0.59 | 0.201 ± 0.017 | 85.64 ± 0.58 |
| GG0.1_HPMC0.75 | 37.88 ± 3.66 | 0.214 ± 0.025 | 85.01 ± 6.28 |
| GG0.1_HPMC1 | 38.41 ± 1.75 | 0.267 ± 0.018 | 86.77 ± 3.43 |
| GG0.2_HPMC0.5 | 37.42 ± 4.29 | 0.132 ± 0.003 | 83.67 ± 2.79 |
| GG0.2_HPMC0.75 | 36.11 ± 2.21 | 0.164 ± 0.027 | 79.02 ± 1.09 |
| GG0.2_HPMC1 | 39.05 ± 3.18 | 0.245 ± 0.035 | 81.44 ± 6.67 |
| GG0.3_HPMC0.5 | 39.73 ± 0.84 | 0.223 ± 0.016 | 78.60 ± 0.94 |
| GG0.3_HPMC0.75 | 44.37 ± 4.45 | 0.226 ± 0.013 | 78.53 ± 4.34 |
| GG0.3_HPMC1 | 44.96 ± 2.39 | 0.198 ± 0.030 | 79.33 ± 3.86 |
Table 5.
Mucoadhesive force and work of the in situ gelling formulations compared with the raw polymeric micelle solution. Data are expressed as average ± SD (n = 3). Formulations were named via the following system: GG (gellan gum) with its respective concentration (% w/v), followed by the concentration of either HEC (hydroxyethyl cellulose), methylcellulose (MC), or hydroxypropyl-methylcellulose (HPMC), also in the unit of % w/v. RIS-PM represents the polymeric micelle formulation dispersed in solely purified water.
Table 5.
Mucoadhesive force and work of the in situ gelling formulations compared with the raw polymeric micelle solution. Data are expressed as average ± SD (n = 3). Formulations were named via the following system: GG (gellan gum) with its respective concentration (% w/v), followed by the concentration of either HEC (hydroxyethyl cellulose), methylcellulose (MC), or hydroxypropyl-methylcellulose (HPMC), also in the unit of % w/v. RIS-PM represents the polymeric micelle formulation dispersed in solely purified water.
| Formulation | Mucoadhesive Force (mN) | Mucoadhesive Work (mN × mm) |
|---|
| RIS-PM | 765.98 ± 102.59 | 36.11 ± 3.37 |
| GG0.1_HEC0.5 | 1394.17 ± 211.58 | 66.56 ± 5.12 |
| GG0.1_HEC0.75 | 1662.03 ± 120.37 | 74.71 ± 6.95 |
| GG0.1_HEC1 | 1881.46 ± 208.50 | 81.44 ± 11.71 |
| GG0.2_HEC0.5 | 1924.89 ± 315.07 | 85.28 ± 6.21 |
| GG0.2_HEC0.75 | 1975.33 ± 266.71 | 99.87 ± 7.63 |
| GG0.2_HEC1 | 2308.67 ± 145.98 | 101.81 ± 22.62 |
| GG0.3_HEC0.5 | 1854.01 ± 164.62 | 86.17 ± 5.19 |
| GG0.3_HEC0.75 | 2005.74 ± 134.97 | 92.34 ± 9.78 |
| GG0.3_HEC1 | 2446.29 ± 137.19 | 103.23 ± 12.28 |
| GG0.1_MC0.5 | 1269.91 ± 157.18 | 39.75 ± 4.61 |
| GG0.1_MC0.75 | 1575.59 ± 238.04 | 57.12 ± 8.43 |
| GG0.1_MC1 | 1872.55 ± 326.27 | 75.89 ± 6.88 |
| GG0.2_MC0.5 | 1937.25 ± 194.72 | 83.45 ± 11.85 |
| GG0.2_MC0.75 | 2230.86 ± 270.36 | 91.03 ± 10.43 |
| GG0.2_MC1 | 2513.87 ± 311.94 | 111.58 ± 8.71 |
| GG0.3_MC0.5 | 2130.93 ± 354.26 | 87.85 ± 11.89 |
| GG0.3_MC0.75 | 2312.87 ± 147.63 | 93.69 ± 6.52 |
| GG0.3_MC1 | 2716.81 ± 121.98 | 124.01 ± 9.67 |
| GG0.1_HPMC0.5 | 1405.28 ± 376.45 | 58.29 ± 8.72 |
| GG0.1_HPMC0.75 | 1559.84 ± 244.01 | 75.96 ± 4.63 |
| GG0.1_HPMC1 | 1792.37 ± 175.72 | 85.53 ± 6.95 |
| GG0.2_HPMC0.5 | 1646.12 ± 338.89 | 62.41 ± 5.38 |
| GG0.2_HPMC0.75 | 2004.28 ± 135.54 | 93.67 ± 7.14 |
| GG0.2_HPMC1 | 2285.15 ± 282.33 | 127.35 ± 9.11 |
| GG0.3_HPMC0.5 | 2466.61 ± 362.10 | 104.19 ± 3.89 |
| GG0.3_HPMC0.75 | 2648.04 ± 191.46 | 115.82 ± 10.22 |
| GG0.3_HPMC1 | 2775.43 ± 227.07 | 132.47 ± 6.74 |
Table 6.
Flux (J) and permeability coefficient (Kp) of the investigated formulations in comparison with in situ gelling agent-free risperidone-loaded polymeric micelles (RIS-PM). Data are expressed as average ± SD (n = 3). Formulations were named via the following system: GG (gellan gum) with its respective concentration (% w/v), followed by the concentration of either HEC (hydroxyethyl cellulose), methylcellulose (MC), or hydroxypropyl-methylcellulose (HPMC), also in the unit of % w/v. RIS-PM represents the polymeric micelle formulation dispersed in solely purified water.
Table 6.
Flux (J) and permeability coefficient (Kp) of the investigated formulations in comparison with in situ gelling agent-free risperidone-loaded polymeric micelles (RIS-PM). Data are expressed as average ± SD (n = 3). Formulations were named via the following system: GG (gellan gum) with its respective concentration (% w/v), followed by the concentration of either HEC (hydroxyethyl cellulose), methylcellulose (MC), or hydroxypropyl-methylcellulose (HPMC), also in the unit of % w/v. RIS-PM represents the polymeric micelle formulation dispersed in solely purified water.
| Formulation | J (µg/cm2/h) | Kp (cm/h) |
|---|
| RIS-PM | 317.51 ± 15.86 | 0.5715 ± 0.0142 |
| GG0.1_HEC0.5 | 264.23 ± 13.26 | 0.4756 ± 0.0218 |
| GG0.1_HEC0.75 | 251.27 ± 11.59 | 0.4523 ± 0.0293 |
| GG0.1_HEC1 | 223.17 ± 10.84 | 0.4017 ± 0.0257 |
| GG0.2_HEC0.5 | 198.12 ± 9.95 | 0.3566 ± 0.0159 |
| GG0.2_HEC0.75 | 193.54 ± 15.28 | 0.3484 ± 0.0308 |
| GG0.2_HEC1 | 188.04 ± 10.77 | 0.3385 ± 0.0225 |
| GG0.3_HEC0.5 | 165.28 ± 12.06 | 0.2975 ± 0.0319 |
| GG0.3_HEC0.75 | 148.89 ± 18.05 | 0.2680 ± 0.0123 |
| GG0.3_HEC1 | 147.64 ± 12.39 | 0.2658 ± 0.0276 |
| GG0.1_MC0.5 | 166.07 ± 18.09 | 0.2989 ± 0.0181 |
| GG0.1_MC0.75 | 171.65 ± 13.68 | 0.3090 ± 0.0204 |
| GG0.1_MC1 | 157.38 ± 11.19 | 0.2833 ± 0.0173 |
| GG0.2_MC0.5 | 172.10 ± 16.04 | 0.3098 ± 0.0322 |
| GG0.2_MC0.75 | 160.55 ± 18.62 | 0.2890 ± 0.0136 |
| GG0.2_MC1 | 140.32 ± 11.34 | 0.2526 ± 0.0195 |
| GG0.3_MC0.5 | 151.31 ± 13.70 | 0.2724 ± 0.0249 |
| GG0.3_MC0.75 | 147.18 ± 20.38 | 0.2649 ± 0.0167 |
| GG0.3_MC1 | 153.97 ± 16.00 | 0.2771 ± 0.0260 |
| GG0.1_HPMC0.5 | 283.01 ± 10.81 | 0.5094 ± 0.0285 |
| GG0.1_HPMC0.75 | 289.30 ± 16.53 | 0.5207 ± 0.0115 |
| GG0.1_HPMC1 | 264.12 ± 18.06 | 0.4754 ± 0.0411 |
| GG0.2_HPMC0.5 | 200.39 ± 12.67 | 0.3607 ± 0.0297 |
| GG0.2_HPMC0.75 | 147.68 ± 17.61 | 0.2658 ± 0.0432 |
| GG0.2_HPMC1 | 116.22 ± 20.29 | 0.2092 ± 0.0239 |
| GG0.3_HPMC0.5 | 126.30 ± 19.56 | 0.2273 ± 0.0187 |
| GG0.3_HPMC0.75 | 101.54 ± 11.12 | 0.1828 ± 0.0129 |
| GG0.3_HPMC1 | 97.17 ± 15.99 | 0.1749 ± 0.0301 |
Table 7.
Study design of gellan gum—HEC/MC/HPMC combined in situ gel formulations.
Table 7.
Study design of gellan gum—HEC/MC/HPMC combined in situ gel formulations.
| Gellan Gum Concentration (% w/v) | HEC/MC/HPMC Concentration (% w/v) |
|---|
| 0.1 | 0.5 |
| | 0.75 |
| | 1.0 |
| 0.2 | 0.5 |
| | 0.75 |
| | 1.0 |
| 0.3 | 0.5 |
| | 0.75 |
| | 1.0 |