Bi-Interfacial Electron Modulation in Co9S8/FeCoS2 Heterostructures Anchored on Bamboo-Derived Carbon Quasi-Aerogel for High-Performance Hydrogen Evolution
Abstract
1. Introduction
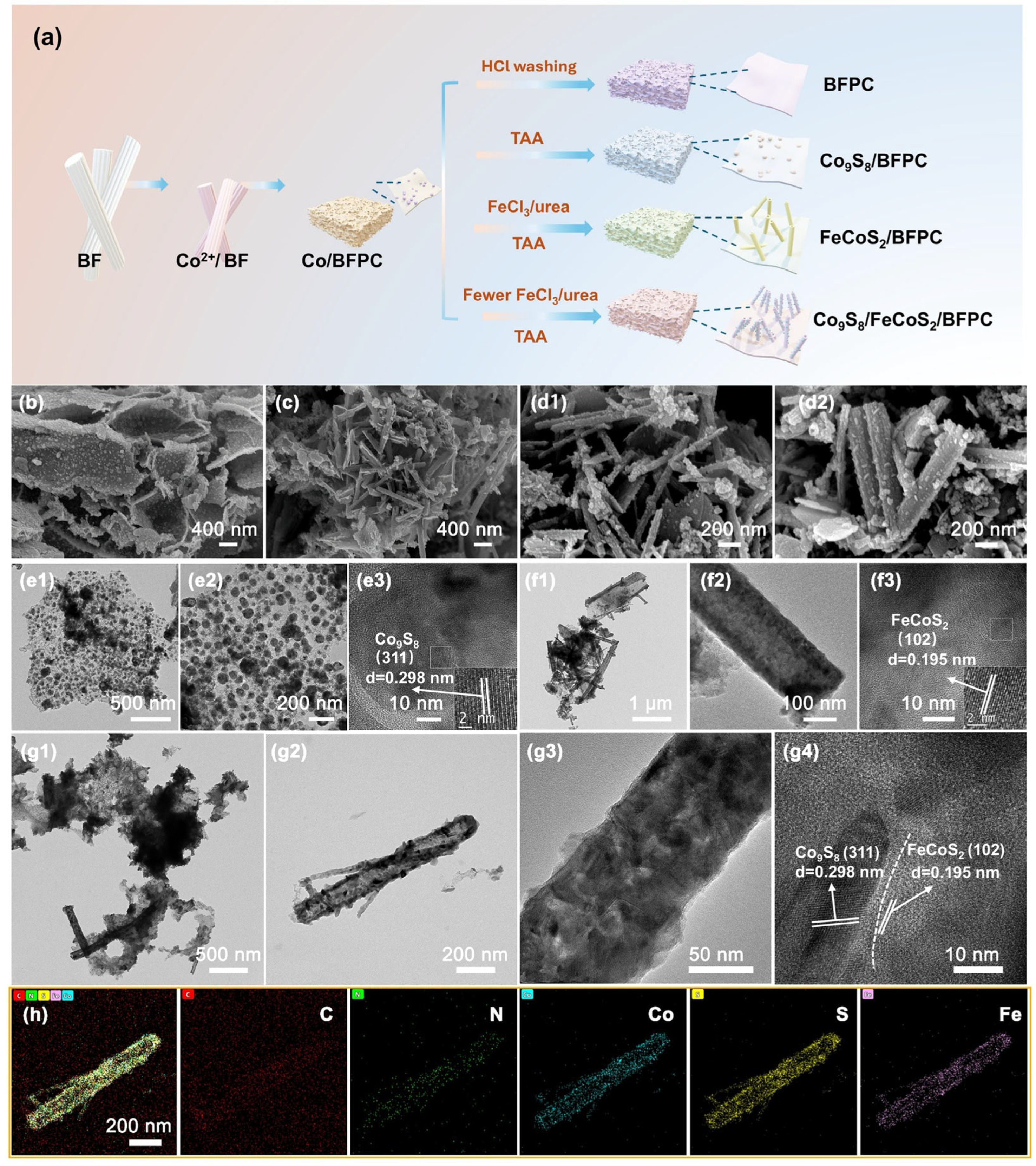
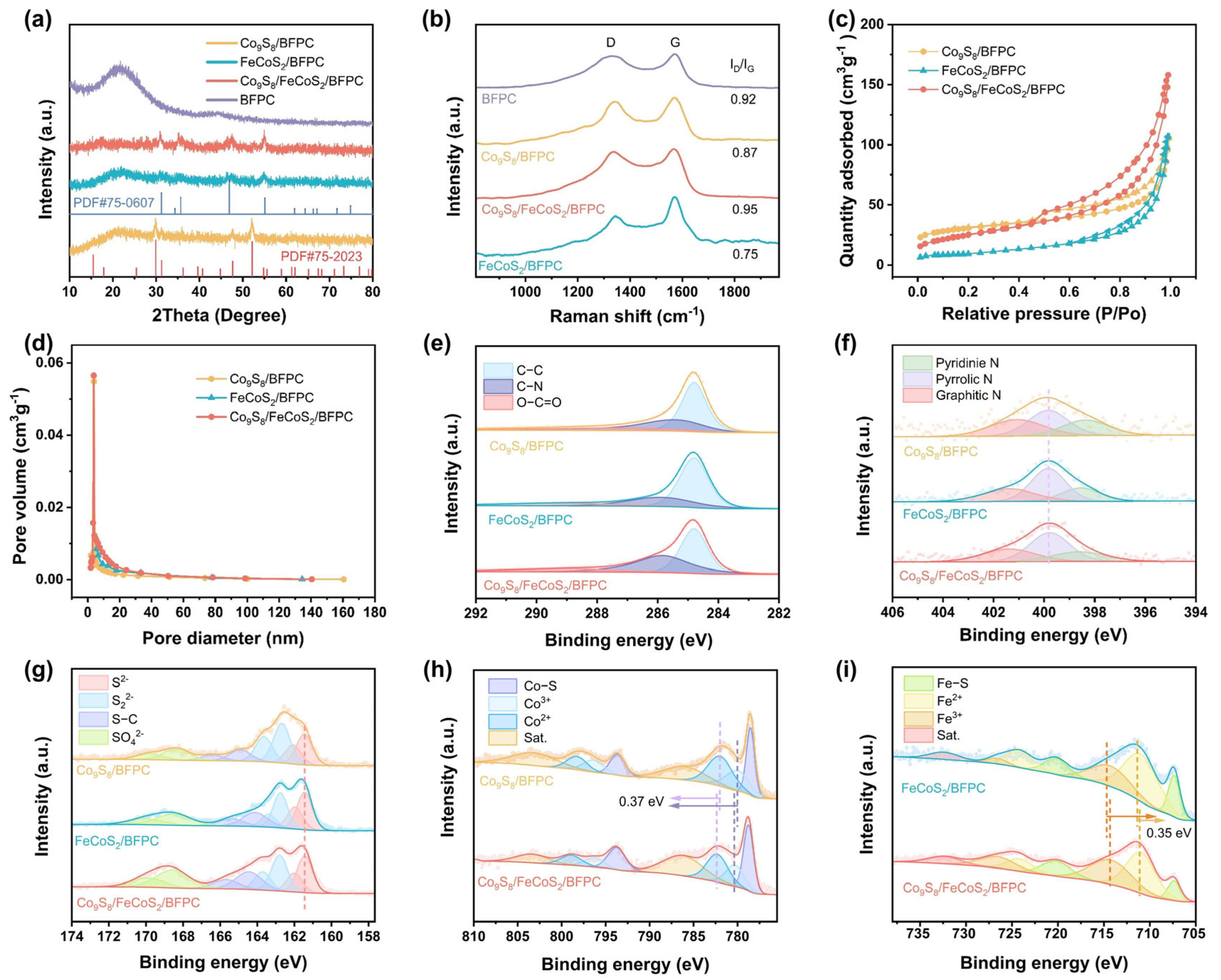
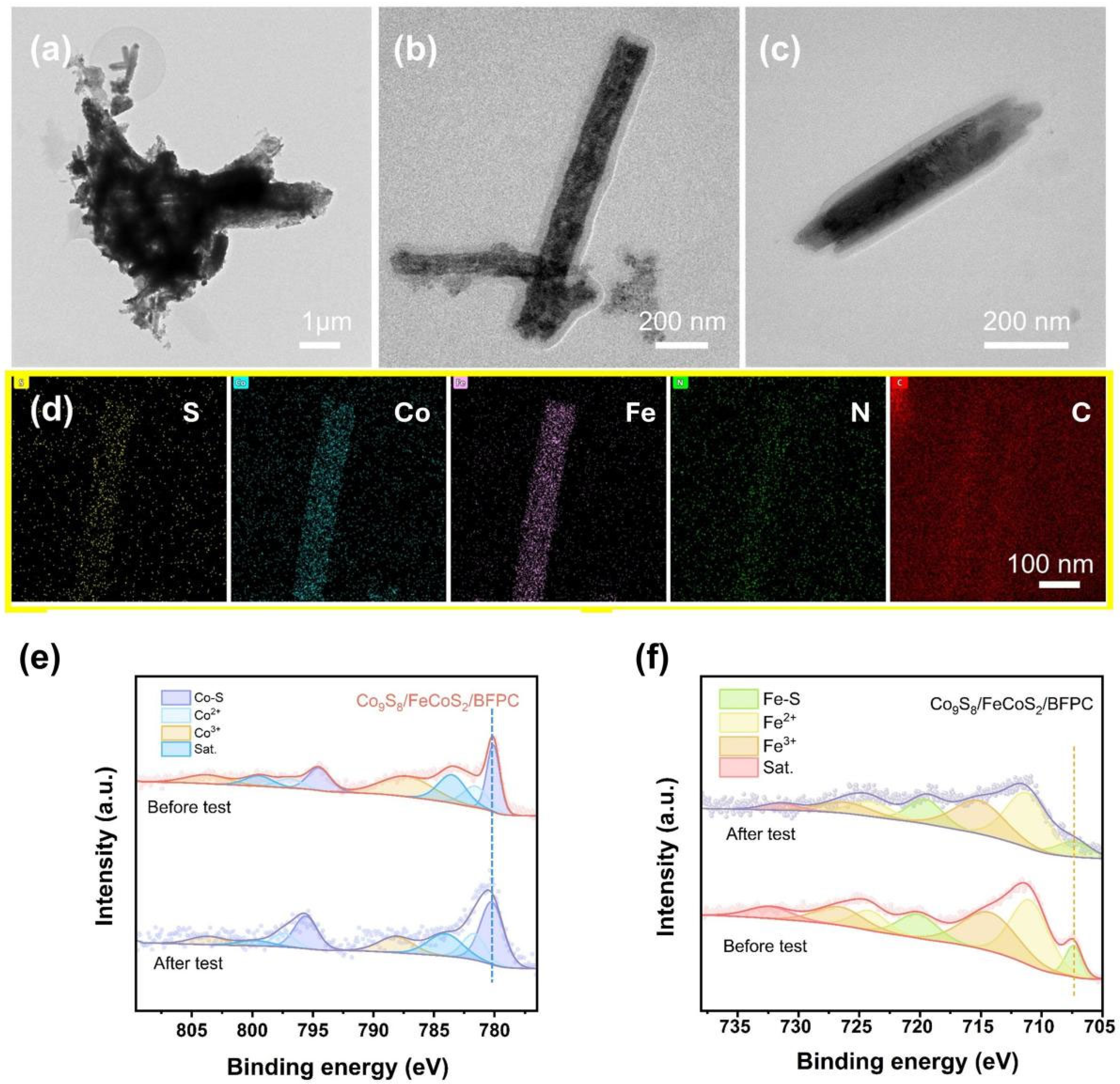
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Co/BFPC, BFC and BFPC
4.3. Synthesis of Co9S8/BFPC, FeCoS2/BFPC and Co9S8/FeCoS2/BFPC
4.4. Electrochemical Measurements
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Y.; Xiao, B.; Liu, K.; Wang, S.; Hou, Y.; Lu, X.F.; Zhang, J. Electrochemical synthesis of high-efficiency water electrolysis catalysts. Electrochem. Energy Rev. 2025, 8, 6. [Google Scholar] [CrossRef]
- Qadeer, M.A.; Zhang, X.X.; Farid, M.A.; Tanveer, M.; Yan, Y.C.; Du, S.F.; Huang, Z.F.; Tahir, M.; Zou, J.J. A review on fundamentals for designing hydrogen evolution electrocatalyst. J. Power Sources 2024, 613, 234856. [Google Scholar] [CrossRef]
- Li, J.; Wu, N.; Zhang, J.; Wu, H.-H.; Pan, K.; Wang, Y.; Liu, G.; Liu, X.; Yao, Z.; Zhang, Q. Machine learning-assisted low-dimensional electrocatalysts design for hydrogen evolution reaction. Nano-Micro Lett. 2023, 15, 227. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, W.Q.; Wang, Y.J.; Liu, R.Y.; Yu, Q.Q.; Hu, R.M.; Jiang, X.C.; Gao, Q.S.; Liu, H.; Yu, J.Y.; et al. Metal-encapsulated nitrogen-doped carbon nanotube arrays electrode for enhancing sulfion oxidation reaction and hydrogen evolution reaction by regulating of intermediate adsorption. Chin. Chem. Lett. 2024, 35, 109166. [Google Scholar] [CrossRef]
- Gultom, N.S.; Li, C.H.; Kuo, D.H.; Silitonga, M.Z. Multiphase Fe-doped Ni3S2/MoOx electrocatalyst prepared by facile one-step hydrothermal for full-cell water splitting: Effect of Mo on physical and electrochemical properties. Appl. Catal. B-Environ. 2024, 353, 124100. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Zhang, J.; Pan, K.; Xu, H.; Chen, H.; Liu, G.; Wu, N.; Yuan, C.; Liu, X. Tailoring supports for enhancing the electrocatalytic hydrogen evolution performance of platinum species: A review. J. Mater. Chem. A 2023, 11, 19812–19844. [Google Scholar] [CrossRef]
- Lei, Z.; Ali, S.; Sathish, C.I.; Ahmed, M.; Qu, J.; Zheng, R.; Xi, S.; Yu, X.; Breese, M.B.H.; Liu, C.; et al. Transition metal carbonitride MXenes anchored with Pt sub-nanometer clusters to achieve high-performance hydrogen evolution reaction at all pH range. Nano-Micro Lett. 2025, 17, 123. [Google Scholar] [CrossRef]
- He, C.; Yang, L.; Wang, J.; Wang, T.; Ju, J.; Lu, Y.; Chen, W. Research progress on electronic and active site engineering of cobalt-based electrocatalysts for oxygen evolution reaction. Carbon Energy 2024, 6, e573. [Google Scholar] [CrossRef]
- Li, S.; Zhang, H.; Li, S.; Wang, J.; Wang, Q.; Cheng, Z. Advances in hierarchically porous materials: Fundamentals, preparation and applications. Renew. Sustain. Energy Rev. 2024, 202, 114641. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Bakandritsos, A.; Zbořil, R. Bimetallic single-atom catalysts for water splitting. Nano-Micro Lett. 2024, 17, 1. [Google Scholar] [CrossRef]
- Alemu, M.A.; Getie, M.Z.; Wassie, H.M.; Alem, M.S.; Assegie, A.A.; Al Afif, R. Biomass-derived metal-free heteroatom doped nanostructured carbon electrocatalysts for high-performance rechargeable lithium–air batteries. Green Chem. 2024, 26, 11427–11443. [Google Scholar] [CrossRef]
- Ma, G.; Gao, S.; Tang, G.; Chen, F.; Lang, X.; Qiu, X.; Song, X. Development of starch-based amorphous CoOx self-supporting carbon aerogel electrocatalyst for hydrogen evolution. Carbohydr. Polym. 2023, 314, 120942. [Google Scholar] [CrossRef]
- Huang, J.; Jiang, Y.; An, T.; Cao, M. Increasing the active sites and intrinsic activity of transition metal chalcogenide electrocatalysts for enhanced water splitting. J. Mater. Chem. A 2020, 8, 25465–25498. [Google Scholar] [CrossRef]
- Hu, X.; Shang, J.; Li, S.; Long, J.; Cheng, S.; Ahmed, S.; Wang, X.; Farooq, U. Atomic catalysis meets heterostructure synergy: Unveiling the trifunctional efficacy of transition Metal@ WS2/ReSe2. Int. J. Hydrogen Energy 2024, 93, 693–703. [Google Scholar] [CrossRef]
- Wang, J.; Ghosh, T.; Ju, Z.; Ng, M.-F.; Wu, G.; Yang, G.; Zhang, X.; Zhang, L.; Handoko, A.D.; Kumar, S.; et al. Heterojunction structure of cobalt sulfide cathodes for high-performance magnesium-ion batteries. Matter 2024, 7, 1833–1847. [Google Scholar] [CrossRef]
- Wang, J.; Seh, Z.W. The Design of Transition Metal Sulfide Cathodes for High-Performance Magnesium-Ion Batteries. Acc. Mater. Res. 2024, 5, 1329–1339. [Google Scholar] [CrossRef]
- Hu, Z.; Jiang, L.; Li, R.; Yuan, D. Interface engineering of MoS2-CoS2 heteronanosheets for enhanced electrochemical hydrogen evolution reaction. Int. J. Hydrogen Energy 2024, 71, 701–708. [Google Scholar] [CrossRef]
- Wu, N.; He, W.; Shi, S.; Yuan, X.; Li, J.; Cao, J.; Yuan, C.; Liu, X. Bamboo fiber-derived carbon support for the immobilization of Pt nanoparticles to enhance hydrogen evolution reaction. J. Colloid Interface Sci. 2025, 684, 658–667. [Google Scholar] [CrossRef]
- Brandiele, R.; Parnigotto, M.; Mazzucato, M.; Dalconi, M.C.; Bertolotti, F.; Rizzi, G.A.; Sasso, G.D.; Durante, C. The interplay between surface area and sulfur doping of carbon support on Pt NPs nucleation and growth: A synergistic enhancement of catalytic activity for oxygen reduction. Appl. Catal. B 2024, 344, 123620. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; Cao, X.; Wu, H.-H.; Pan, K.; Zhang, Q.; Wu, N.; Liu, X. Template-free fabrication of MoP nanoparticles encapsulated in N-doped hollow carbon spheres for efficient alkaline hydrogen evolution. Chem. Eng. J. 2020, 416, 127677. [Google Scholar] [CrossRef]
- Lu, X.; Cai, M.; Zou, Z.; Huang, J.; Xu, C. A novel MoNi@Ni(OH)2 heterostructure with Pt-like and stable electrocatalytic activity for the hydrogen evolution reaction. Chem. Commun. 2020, 56, 1729–1732. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, J.; Shen, J.; Wu, H.; Chen, H.; Yuan, C.; Wu, N.; Liu, G.; Guo, D.; Liu, X. Self-supported electrocatalysts for the hydrogen evolution reaction. Mater. Chem. Front. 2022, 7, 567–606. [Google Scholar] [CrossRef]
- Zhang, Y.; Chao, S.; Wang, X.; Han, H.; Bai, Z.; Yang, L. Hierarchical Co9S8 hollow microspheres as multifunctional electrocatalysts for oxygen reduction, oxygen evolution and hydrogen evolution reactions. Electrochim. Acta 2017, 246, 380–390. [Google Scholar] [CrossRef]
- Zhao, C.; Dai, J.; Zhu, F.; Wu, J.; Cai, Y. FeCoS2 nanoparticles confined in N, S co-doped carbon with reduced polysulfides shuttling for high performance sodium-ion batteries. Appl. Surf. Sci. 2023, 634, 157711. [Google Scholar] [CrossRef]
- Cheng, R.; Wang, Y.; Di, X.; Lu, Z.; Wang, P.; Wu, X. Heterostructure design of MOFs derived Co9S8/FeCoS2/C composite with efficient microwave absorption and waterproof functions. J. Mater. Sci. Technol. 2022, 129, 15–26. [Google Scholar] [CrossRef]
- Luo, H.; Ni, C.; Zhang, C.; Wang, W.; Yang, Y.; Xiong, W.; Cheng, M.; Zhou, C.; Zhou, Y.; Tian, S.; et al. Lignocellulosic biomass derived N-doped and CoO-loaded carbocatalyst used as highly efficient peroxymonosulfate activator for ciprofloxacin degradation. J. Colloid Interface Sci. 2022, 610, 221–233. [Google Scholar] [CrossRef]
- Tan, X.; Chen, S.; Yan, D.; Du, R.; Zhong, Q.; Liao, L.; Tang, Z.; Zeng, F. Recent advances in Ni-based catalysts for the electrochemical oxidation of ethanol. J. Energy Chem. 2024, 98, 588–614. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, J.; Xu, L.; Li, C.; Ning, R.; Ma, J.; Geng, S. Bond engineering: Weakening Ru-O covalency for efficient and stable water oxidation in acidic solutions. J. Energy Chem. 2025, 102, 1–9. [Google Scholar] [CrossRef]
- Wu, N.; Shen, J.; Zhou, X.; Li, S.; Li, J.; Liu, G.; Guo, D.; Deng, W.; Yuan, C.; Liu, X.; et al. Constructing iron vacancies in thiospinel FeIn2S4 to modulate Fe d-band center and accelerate sodiation kinetics enabling high-rate and durable sodium storage. Adv. Energy Mater. 2025, 15, 2405729. [Google Scholar] [CrossRef]
- Yuan, S.; Wu, Y.; Huang, L.; Zhang, Z.; Chen, W.; Wang, Y. Engineering Ni0.85Se/CoSe2 heterojunction for enhanced bifunctional catalysis in urea-assisted hydrogen production. J. Colloid Interface Sci. 2025, 683, 981–994. [Google Scholar] [CrossRef]
- Jiang, T.; Luan, W.; Turyanska, L.; Feng, Q. Cr2O3 nanoparticles boosting Cr-N-C for highly efficient electrocatalysis in acidic oxygen reduction reaction. Int. J. Hydrogen Energy 2021, 46, 18913–18921. [Google Scholar] [CrossRef]
- Abdalla, I.; Elhassan, A.; Ali, S.; Saty, M.Y.H.; Adam, E.; Zou, L.; Ni, Q.; Xu, Z. Impact of defect-rich carbon nanofibers combined with magnetic materials on broadband electromagnetic wave absorption and radar cross-section reduction. Small Struct. 2025, 2400624. [Google Scholar] [CrossRef]
- Saini, R.; Naaz, F.; Bashal, A.H.; Pandit, A.H.; Farooq, U. Recent advances in nitrogen-doped graphene-based heterostructures and composites: Mechanism and active sites for electrochemical ORR and HER. Green Chem. 2024, 26, 57–102. [Google Scholar] [CrossRef]
- Murphy, E.; Liu, Y.; Sun, B.; Chen, Y.-H.; Guo, S.; Atanassov, P. Atomically dispersed metal-nitrogen-carbon catalysts for electrochemical nitrogen transformations to ammonia and beyond. ACS Catal. 2024, 14, 9797–9811. [Google Scholar] [CrossRef]
- Zhao, L.; Li, Y.; Yu, M.; Peng, Y.; Ran, F. Electrolyte-wettability issues and challenges of electrode materials in electrochemical energy storage, energy conversion, and beyond. Adv. Sci. 2023, 10, 2300283. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, S.; Liu, Q.; Yin, J.; Sun, D.; Zhang, M.; Xu, L.; Tang, Y.; Zhang, Y. Immobilization of Ni3Co nanoparticles into N-doped carbon nanotube/nanofiber integrated hierarchically branched architectures toward efficient overall water splitting. Adv. Sci. 2020, 7, 1902371. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Duan, J.; Wang, L.; Xiao, F.-S. Adjustment of molecular sorption equilibrium on catalyst surface for boosting catalysis. Acc. Chem. Res. 2025, 53, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Chang, B.; Ren, Y.; Kong, D.; Tao, H.B.; Zhi, L.; Khan, M.A.; Aleisa, R.; Rueping, M.; Zhang, H. Proton exchange membrane water splitting: Advances in electrode structure and mass-charge transport optimization. Adv. Mater. 2025, 37, 2416012. [Google Scholar] [CrossRef]
- Xu, L.; Peng, Y.; Zheng, B.; Fang, Z. Nitrogen-sulfur co-doped magnetic biochar efficiently activates hydrogen peroxide for the degradation of sulfamethazine. Chem. Eng. J. 2024, 498, 155477. [Google Scholar] [CrossRef]
- Cui, M.; Yuan, Y.; Wu, Y.; Che, Z.; Li, P.; Yang, X.; Chen, Y.; Hu, W.; Wang, J.; Wang, S. Graphdiyne-induced CoN/CoS2 heterojunction: Boosting efficiency for bifunctional oxygen electrochemistry in zinc-air batteries. ChemSusChem 2024, 17, e202400832. [Google Scholar] [CrossRef]
- Zhu, X.; Dong, K.; Nguyen, D.C.; Prabhakaran, S.; Kim, D.H.; Tran, D.T.; Kim, N.H.; Lee, J.H. Inter-atomic electronic interactions enabled by a Rh single atoms/CuCo2S4@MoS2 core-shell heterostructure for high-efficiency solar-assisted water splitting. J. Mater. Chem. A 2024, 12, 25117–25130. [Google Scholar] [CrossRef]
- Wu, N.; Shen, J.; Li, Q.; Li, S.; Guo, D.; Li, J.; Liu, G.; Zhao, J.; Cao, A.; Mi, H.; et al. Synergistic Bimetallic Interaction and Regulated Void Size in Isocubanite CuFe2S3 Enables UltraFast and Durable Sodium Storage. ACS Sustain. Chem. Eng. 2025, 13, 5546–5556. [Google Scholar] [CrossRef]
- Sun, X.; Wang, H.; Wang, W.; Li, Y.; Wang, K.; Zhang, Q.; Zhang, W.; Wang, J. Cobalt-copolymer-derived Co9S8 superstructure as electrode materials for advanced symmetric supercapacitor. J. Energy Storage 2024, 99, 113296. [Google Scholar] [CrossRef]
- Ayyaluri, R.R.; Krishna, B.V.; Kumar, M.; Ankinapalli, O.R.; Yu, J.S. Co3S4/Co9S8 doped nitrogen-enriched carbon polyhedron structures as an efficient catalyst for hydrogen evolution reaction. Int. J. Hydrogen Energy 2024, 70, 686–695. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, S.; Ouyang, M.; Liu, P.; Zhang, J.; Zhang, G.; Chen, K.; Wang, Y.; Wu, L.; Dong, Z. MOF-74 derived FeCoS2/Fe0.95S1.05 composite nano-particles via in-situ sulfurization for highly efficient electrocatalytic nitrate reduction to ammonia. J. Alloys Compd. 2025, 1021, 179637. [Google Scholar] [CrossRef]
- Wang, S.; He, X.; Wang, S.; Huang, X.; Wu, M.; Xiang, D. FeCoS2/Co4S3/N-doped graphene composite as efficient electrocatalysts for overall water splitting. Electrochim. Acta 2023, 441, 141790. [Google Scholar] [CrossRef]
- Kwon, J.; Choi, S.; Park, C.; Han, H.; Song, T. Critical challenges and opportunities for the commercialization of alkaline electrolysis: High current density, stability, and safety. Mater. Chem. Front 2024, 8, 41–81. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, Z.; Yuan, H.; Wang, J.; Hu, H. Rapid hydrogen adsorption-desorption at sulfur sites via an interstitial carbon strategy for efficient HER on MoS2. Appl. Catal. B 2023, 332, 122750. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, J.; Li, F.; Su, X.; Zhang, Q.; Guan, G.; Hu, F.; Zhang, J.; Wang, Q.; Jiang, Y. Modulating hydrogen adsorption via charge transfer at the semiconductor-metal heterointerface for highly efficient hydrogen evolution catalysis. Adv. Mater. 2023, 35, 2207114. [Google Scholar] [CrossRef]
- Yu, X.; Li, Y.; Pei, C.; Lu, Y.; Kim, J.K.; Park, H.S.; Pang, H. Interfacial design of Ti3C2Tx MXene/graphene heterostructures boosted Ru nanoclusters with high activity toward hydrogen evolution reaction. Adv. Sci. 2024, 11, 2310013. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, J.; Zhang, H.; Zhang, L.; Chen, L.; Hu, Y.; Jiang, H.; Li, C. Atomic heterointerface engineering overcomes the activity limitation of electrocatalysts and promises highly-efficient alkaline water splitting. Energy Environ. Sci. 2021, 14, 5228–5259. [Google Scholar] [CrossRef]
- Wu, L.; Guo, P.; Wang, X.; Li, H.; Zhang, X.; Chen, K.; Zhou, P. The synergy of sulfur vacancies and heterostructure on CoS@FeS nanosheets for boosting the peroxymonosulfate activation. Chem. Eng. J. 2022, 446, 136759. [Google Scholar] [CrossRef]
- Liu, J.; Yang, X.; Si, F.; Zhao, B.; Xi, X.; Wang, L.; Zhang, J.; Fu, X.-Z.; Luo, J.-L. Interfacial component coupling effects towards precise heterostructure design for efficient electrocatalytic water splitting. Nano Energy 2022, 103, 107753. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, L.; Li, J.; Doyle-Davis, K.; Li, R.; Wang, Z.; Sun, X. Advanced support materials and interactions for atomically dispersed noble-metal catalysts: From support effects to design strategies. Adv. Energy Mater. 2022, 12, 2102556. [Google Scholar] [CrossRef]
- Feidenhans’l, A.A.; Regmi, Y.N.; Wei, C.; Xia, D.; Kibsgaard, J.; King, L.A. Precious metal free hydrogen evolution catalyst design and application. Chem. Rev. 2024, 124, 5617–5667. [Google Scholar] [CrossRef]
- Han, C.; Zhao, Y.; Yuan, Y.; Guo, Z.; Chen, G.; Yang, J.; Bao, Q.; Guo, L.; Chen, C. Transition metal-based layered double hydroxides and their derivatives for efficient oxygen evolution reaction. Int. J. Hydrogen Energy 2024, 63, 918–936. [Google Scholar] [CrossRef]
- Pei, C.; Chen, S.; Fu, D.; Zhao, Z.-J.; Gong, J. Structured catalysts and catalytic processes: Transport and reaction perspectives. Chem. Rev. 2024, 124, 2955–3012. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, T.; Wang, Q.; Liu, H.; Wu, J.; Sui, Y.; Li, H.; Tang, P.; Wang, Y. Bifunctional PdMoPt trimetallene boosts alcohol-water electrolysis. Chem. Sci. 2024, 15, 16660–16668. [Google Scholar] [CrossRef]
- Zhou, L.; Guo, D.; Wu, L.; Guan, Z.; Zou, C.; Jin, H.; Fang, G.; Chen, X.a.; Wang, S. A restricted dynamic surface self-reconstruction toward high-performance of direct seawater oxidation. Nat. Commun. 2024, 15, 2481. [Google Scholar] [CrossRef]
- Meng, H.; Chen, Z.; Zhu, J.; You, B.; Ma, T.; Wei, W.; Vernuccio, S.; Xu, J.; Ni, B.J. In situ amorphization of electrocatalysts. Adv. Funct. Mater. 2024, 34, 2405270. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, W.; Cao, J.; Zhou, X.; Zhang, N.; Qi, Y.; Li, J.; Wu, N.; Liu, X. Bi-Interfacial Electron Modulation in Co9S8/FeCoS2 Heterostructures Anchored on Bamboo-Derived Carbon Quasi-Aerogel for High-Performance Hydrogen Evolution. Gels 2025, 11, 390. https://doi.org/10.3390/gels11060390
He W, Cao J, Zhou X, Zhang N, Qi Y, Li J, Wu N, Liu X. Bi-Interfacial Electron Modulation in Co9S8/FeCoS2 Heterostructures Anchored on Bamboo-Derived Carbon Quasi-Aerogel for High-Performance Hydrogen Evolution. Gels. 2025; 11(6):390. https://doi.org/10.3390/gels11060390
Chicago/Turabian StyleHe, Wenjing, Jianliang Cao, Xinliang Zhou, Ning Zhang, Yuzhu Qi, Jin Li, Naiteng Wu, and Xianming Liu. 2025. "Bi-Interfacial Electron Modulation in Co9S8/FeCoS2 Heterostructures Anchored on Bamboo-Derived Carbon Quasi-Aerogel for High-Performance Hydrogen Evolution" Gels 11, no. 6: 390. https://doi.org/10.3390/gels11060390
APA StyleHe, W., Cao, J., Zhou, X., Zhang, N., Qi, Y., Li, J., Wu, N., & Liu, X. (2025). Bi-Interfacial Electron Modulation in Co9S8/FeCoS2 Heterostructures Anchored on Bamboo-Derived Carbon Quasi-Aerogel for High-Performance Hydrogen Evolution. Gels, 11(6), 390. https://doi.org/10.3390/gels11060390







