Multilayer pH-Responsive Hydrogels Fabricated via Two-Step Ionic Crosslinking: Towards Advanced Wound Dressing Materials
Abstract
1. Introduction
2. Results and Discussion
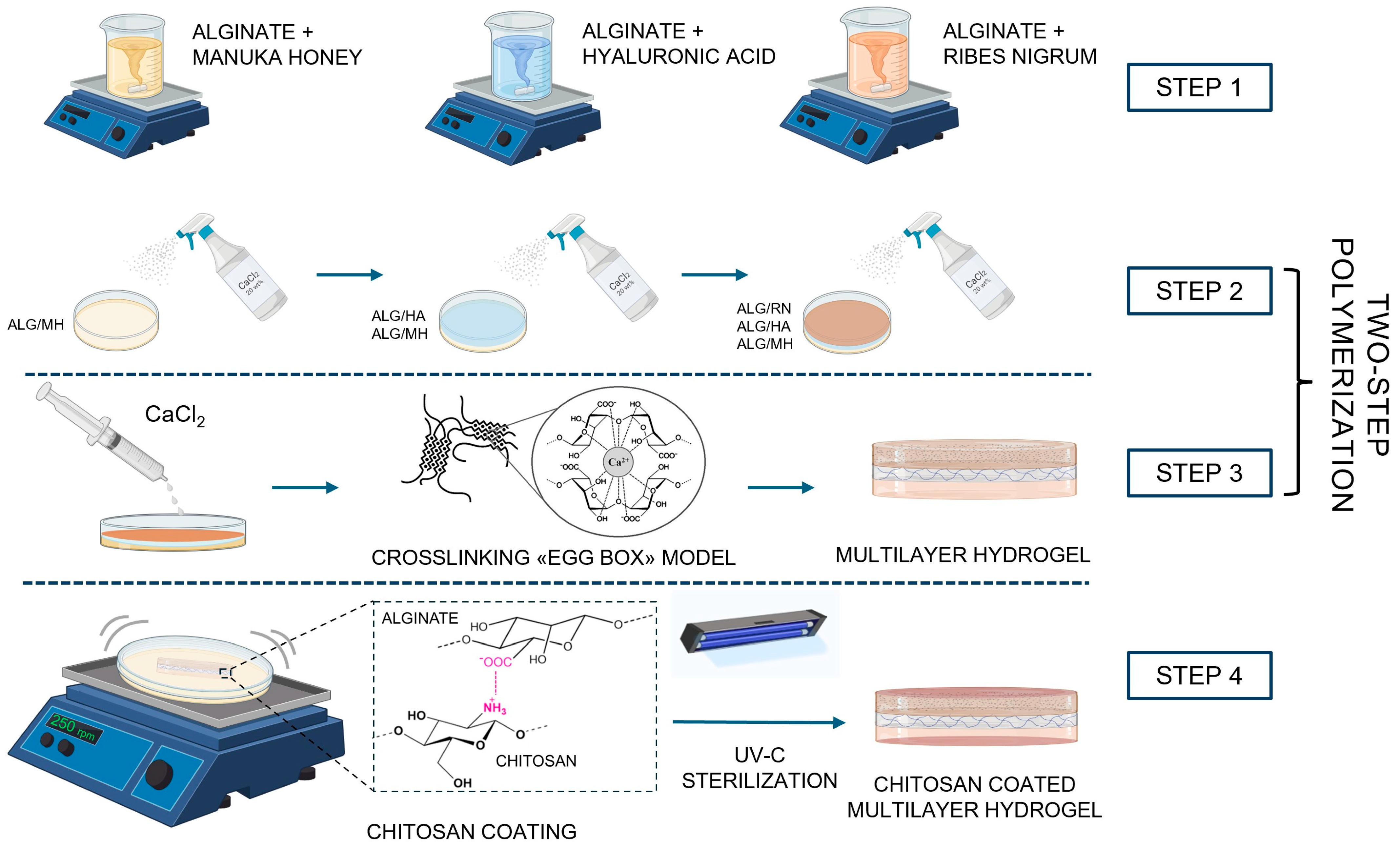
2.1. Hydrogel Fabrication by Two-Step Ionic Crosslinking
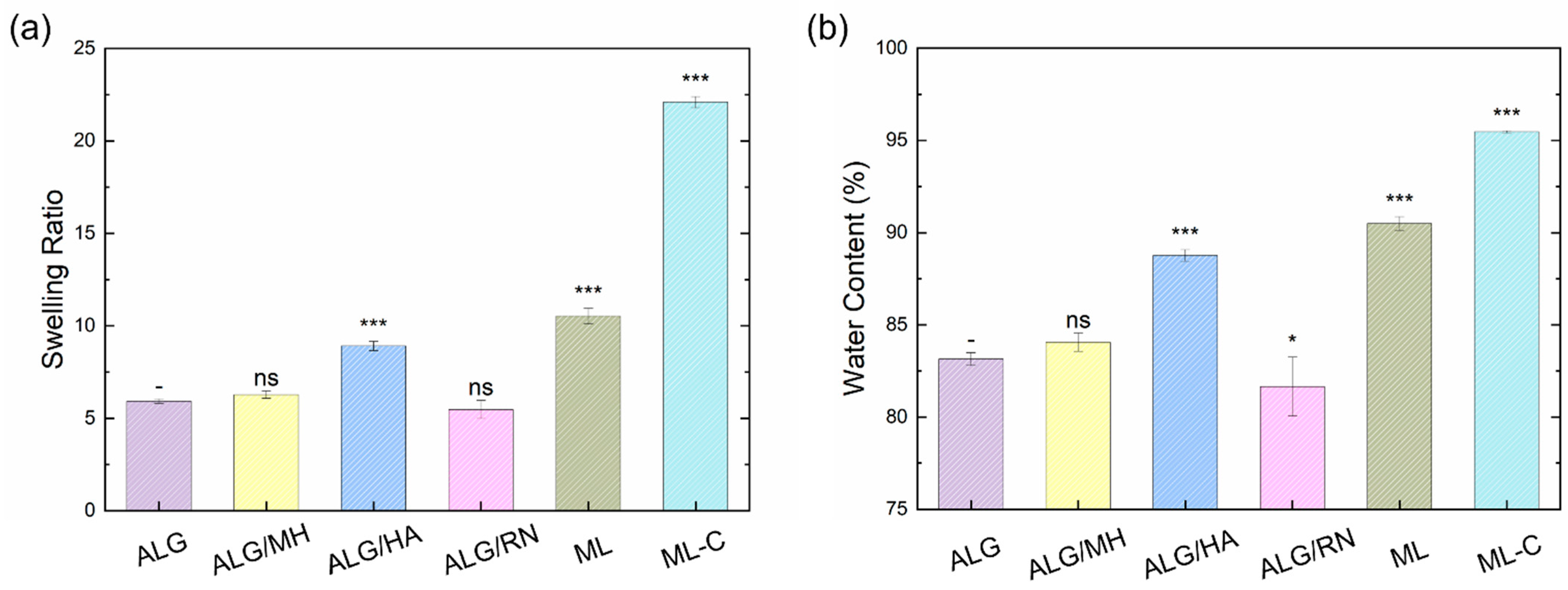
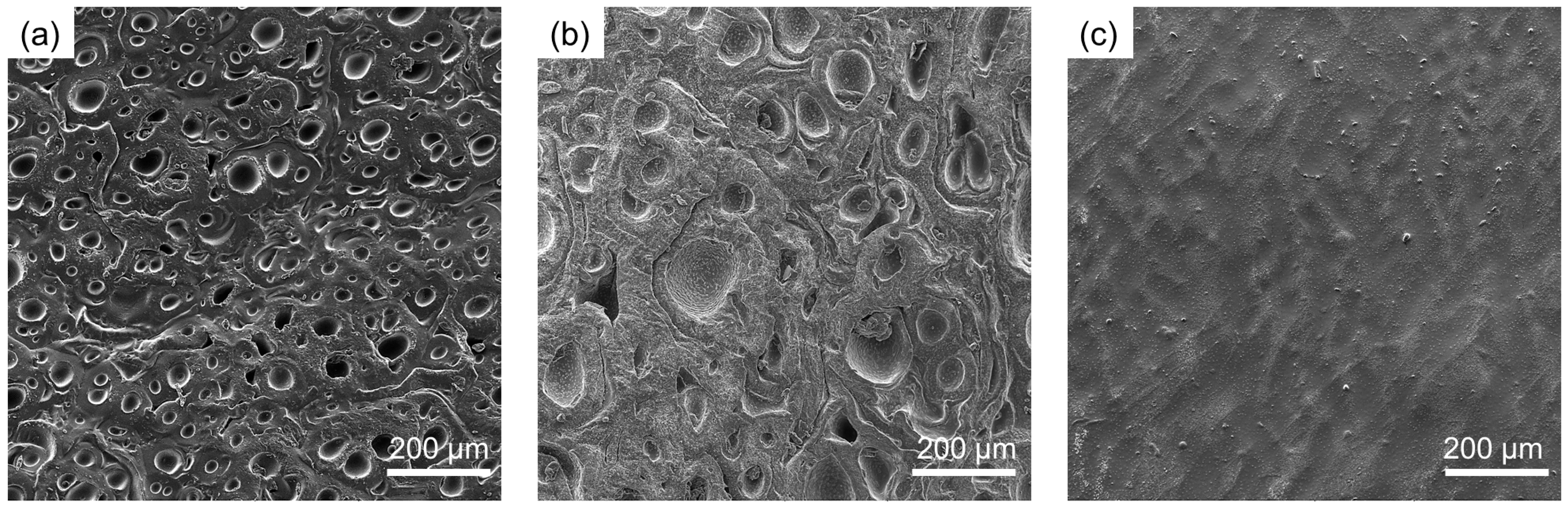
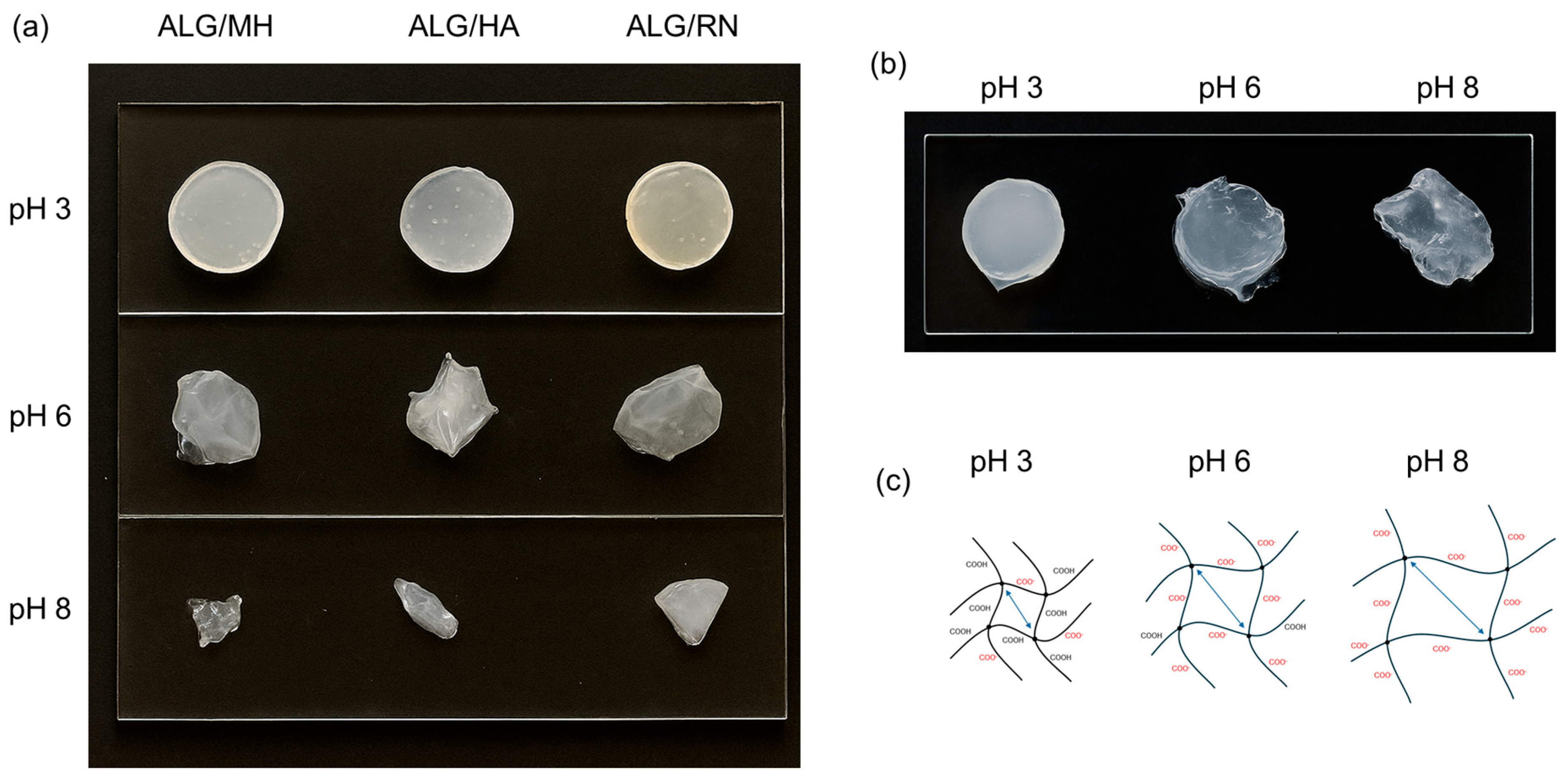
2.2. Swelling Properties and Morphological Characterization
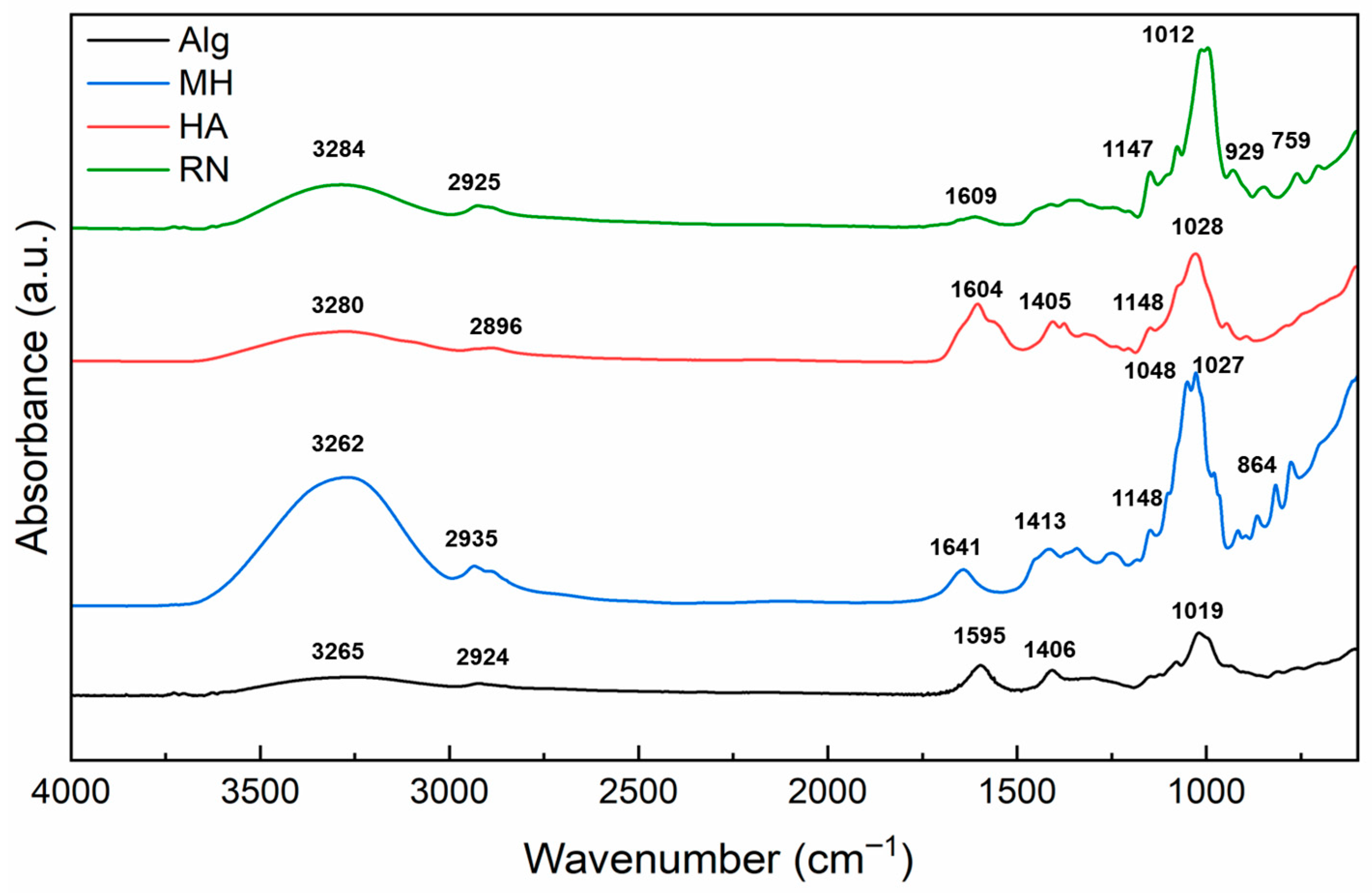
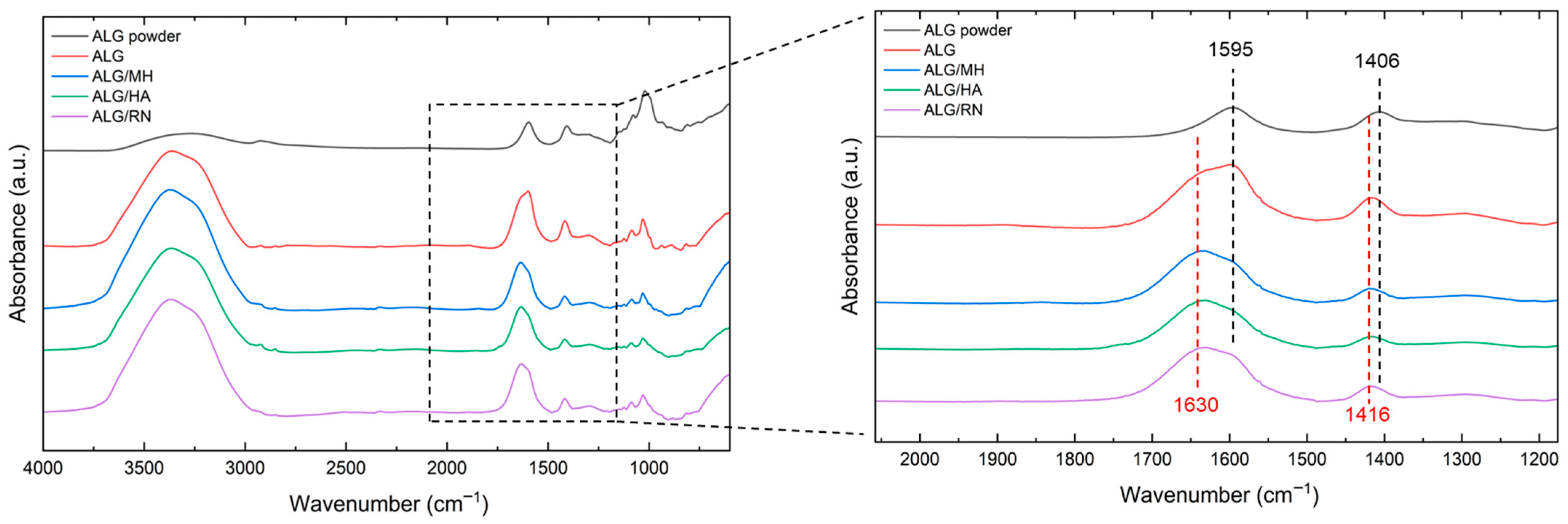
2.3. Chemical Analysis by FTIR Spectroscopy
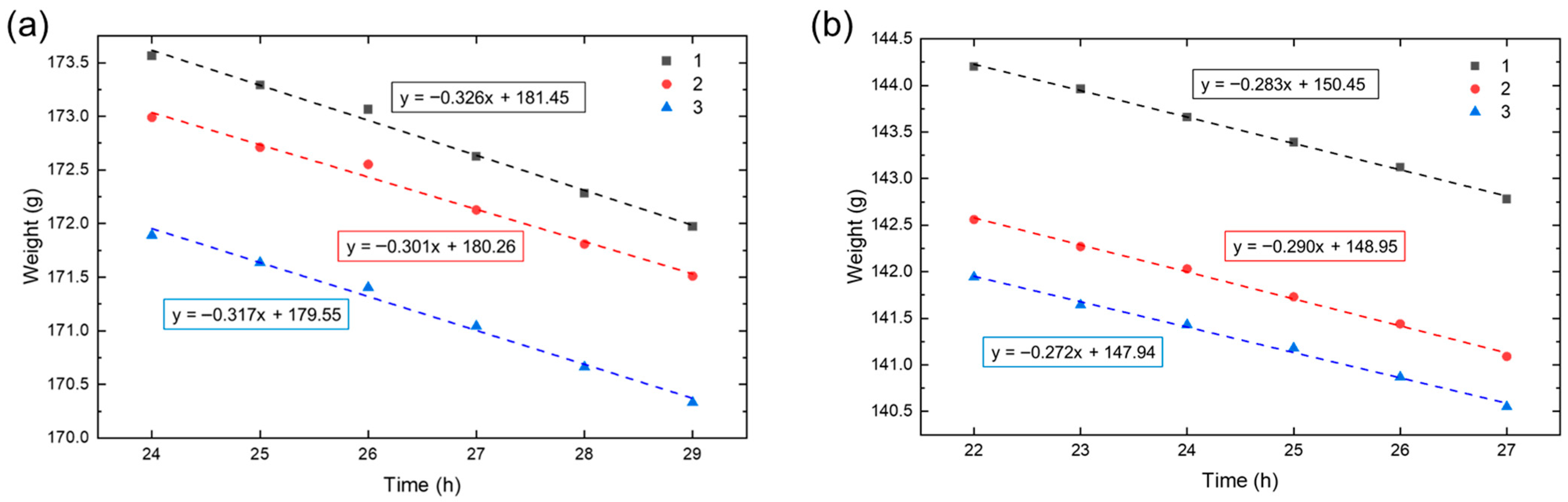
2.4. Analysis of Hydrogel Water Vapor Transmission Rate (WVTR)
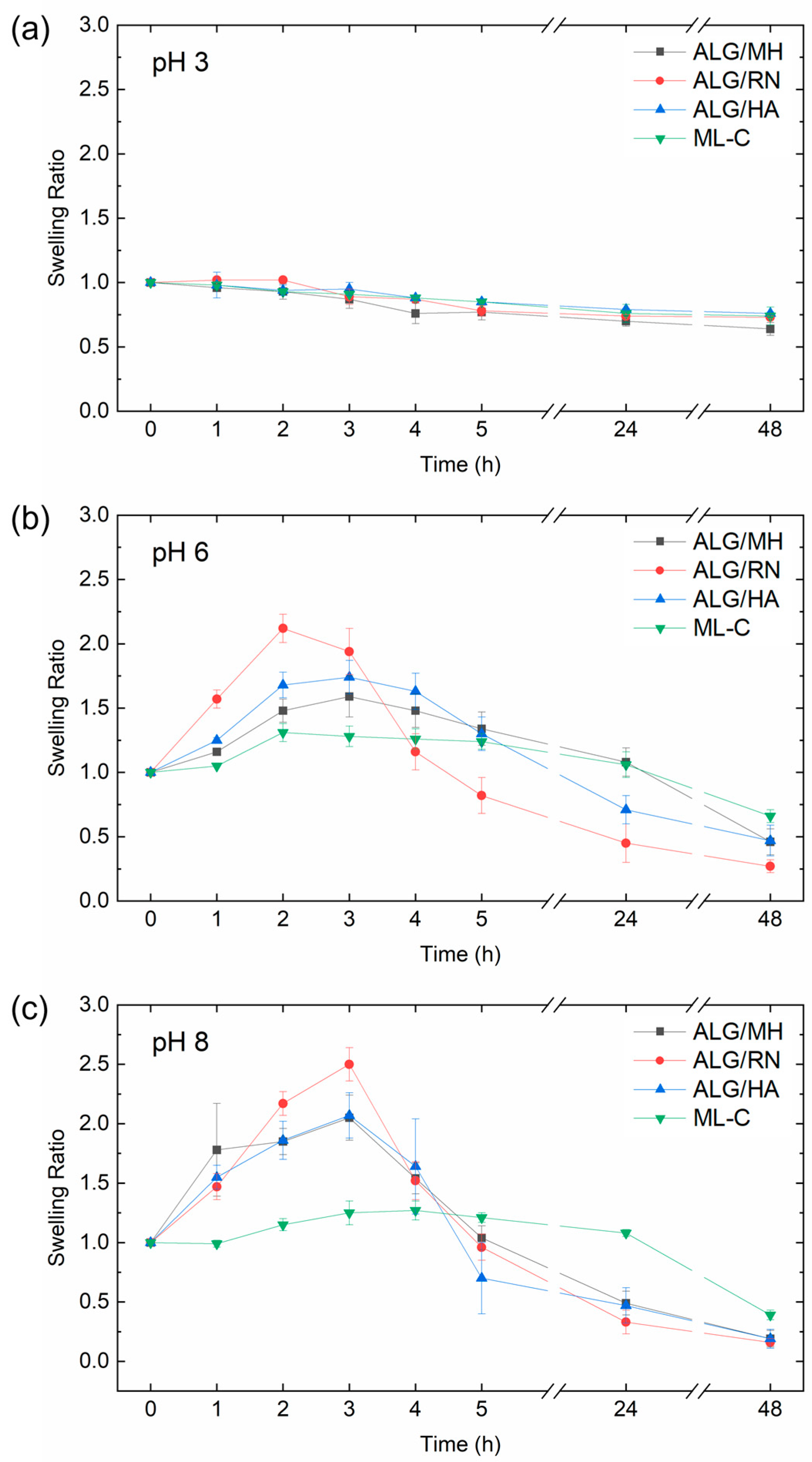
2.5. pH-Responsive Swelling Kinetics
2.6. In Vitro Degradation Tests
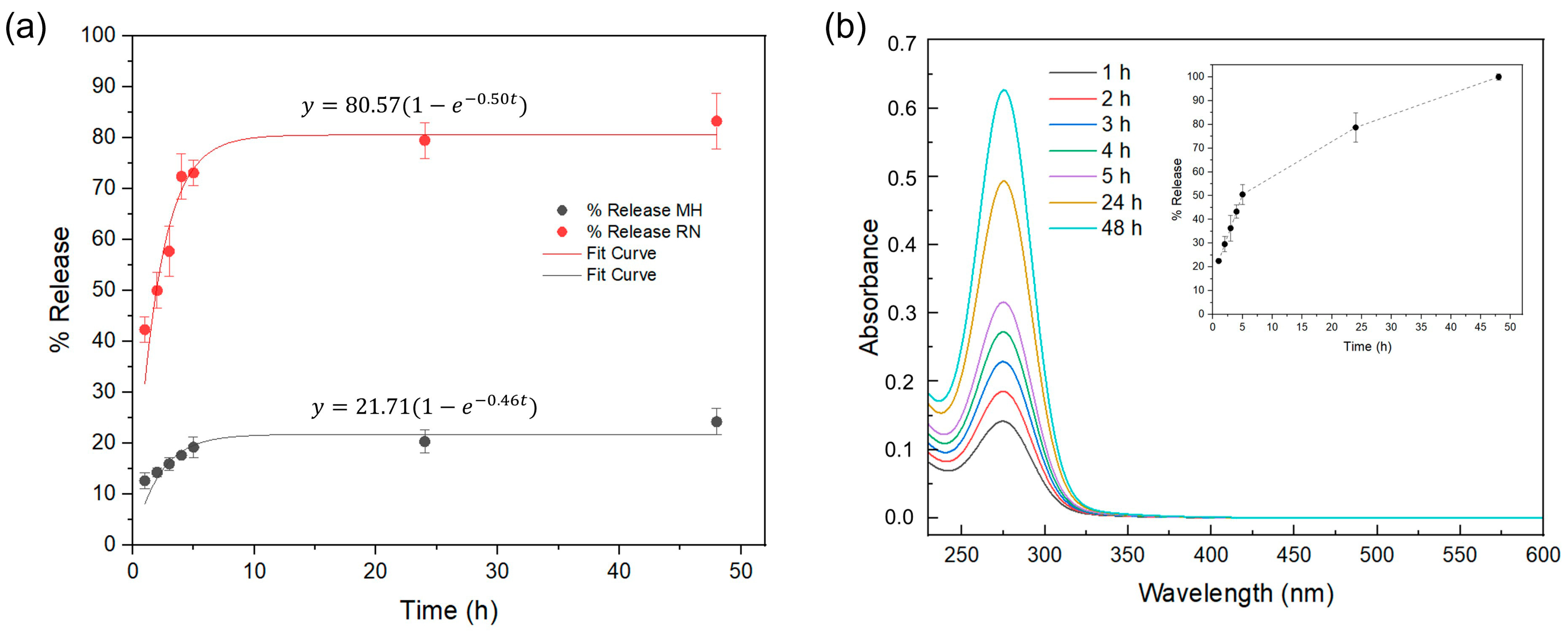
2.7. Kinetic Release Studies of Bioactive Principles
3. Conclusions
4. Materials and Methods
4.1. Fabrication of Multilayered Hydrogel
4.2. Morphological and Swelling Analyses
4.3. Morphological Characterization
4.4. FTIR Analysis
4.5. Water Vapor Transmission Rate (WVTR)
4.6. Swelling Kinetics Under Simulated Wound pH Conditions
4.7. Dissolution Behavior and pH-Responsive Stability
4.8. In Vitro Release Studies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smolle, C.; Cambiaso-Daniel, J.; Forbes, A.A.; Wurzer, P.; Hundeshagen, G.; Branski, L.K.; Huss, F.; Kamolz, L.-P. Recent trends in burn epidemiology worldwide: A systematic review. Burns 2017, 43, 249–257. [Google Scholar] [CrossRef]
- Ferraz, M.P. Wound Dressing Materials: Bridging Material Science and Clinical Practice. Appl. Sci. 2025, 15, 1725. [Google Scholar] [CrossRef]
- Deng, X.; Gould, M.; Ali, M.A. A review of current advancements for wound healing: Biomaterial applications and medical devices. J. Biomed. Mater. Res. 2022, 110, 2542–2573. [Google Scholar] [CrossRef]
- Ciarleglio, G.; Placido, M.; Toto, E.; Santonicola, M.G. Dual-Responsive Alginate/PNIPAM Microspheres Fabricated by Microemulsion-Based Electrospray. Polymers 2024, 16, 2765. [Google Scholar] [CrossRef]
- Çavuşoğlu, S.; Mehmetoğlu Al, A.; Yıldırım, Y. The preparation and in-vitro release studies of the novel chlorhexidine gluconate loaded dual polysaccharide nanogel systems. J. Nanopart. Res. 2025, 27, 182. [Google Scholar] [CrossRef]
- Ciarleglio, G.; Cinti, F.; Toto, E.; Santonicola, M.G. Synthesis and Characterization of Alginate Gel Beads with Embedded Zeolite Structures as Carriers of Hydrophobic Curcumin. Gels 2023, 9, 714. [Google Scholar] [CrossRef] [PubMed]
- Ciarleglio, G.; Vella, S.; Toto, E.; Santonicola, M.G. Emulsion-based multi-responsive microspheres for the delivery of lipophilic Ozoile. Ceram. Int. 2023, 49, 24517–24524. [Google Scholar] [CrossRef]
- Zivari-Ghader, T.; Rashidi, M.-R.; Mehrali, M. Biological macromolecule-based hydrogels with antibacterial and antioxidant activities for wound dressing: A review. Int. J. Biol. Macromol. 2024, 279, 134578. [Google Scholar] [CrossRef]
- Swathe Sriee, A.E.; Shankar, V. Three-dimensional bioprinted materials in alginate-hyaluronic acid complex based hydrogel based bio-ink as absorbents for heavy metal ions removal. Carbohydr. Polym. Technol. Appl. 2024, 8, 100588. [Google Scholar] [CrossRef]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Bergonzi, C.; Bianchera, A.; Remaggi, G.; Ossiprandi, M.C.; Bettini, R.; Elviri, L. 3D Printed Chitosan/Alginate Hydrogels for the Controlled Release of Silver Sulfadiazine in Wound Healing Applications: Design, Characterization and Antimicrobial Activity. Micromachines 2023, 14, 137. [Google Scholar] [CrossRef] [PubMed]
- Ogwu, M.C.; Izah, S.C. Honey as a Natural Antimicrobial. Antibiotics 2025, 14, 255. [Google Scholar] [CrossRef]
- Machado, A.; Toubarro, D.; Baptista, J.; Tejera, E.; Álvarez-Suárez, J.M. Selected honey as a multifaceted antimicrobial agent: Review of compounds, mechanisms, and research challenges. Future Microbiol. 2025, 20, 589–610. [Google Scholar] [CrossRef]
- Sharaf El-Din, M.G.; Farrag, A.F.S.; Wu, L.; Huang, Y.; Wang, K. Health benefits of honey: A critical review on the homology of medicine and food in traditional and modern contexts. J. Tradit. Chin. Med. Sci. 2025, 12, 147–164. [Google Scholar] [CrossRef]
- Yupanqui Mieles, J.; Vyas, C.; Aslan, E.; Humphreys, G.; Diver, C.; Bartolo, P. Honey: An Advanced Antimicrobial and Wound Healing Biomaterial for Tissue Engineering Applications. Pharmaceutics 2022, 14, 1663. [Google Scholar] [CrossRef]
- Privrodski, B.; Jovanović, M.; Delić, N.; Ratajac, R.; Privrodski, V.; Stanojković, A.; Gavlik, B.; Čapo, I. Harnessing Manuka Honey: A Natural Remedy for Accelerated Burn Wound Healing in a Porcine Model. Pharmaceuticals 2025, 18, 296. [Google Scholar] [CrossRef] [PubMed]
- Dovedytis, M.; Liu, Z.J.; Bartlett, S. Hyaluronic acid and its biomedical applications: A review. Eng. Regen. 2020, 1, 102–113. [Google Scholar] [CrossRef]
- Voigt, J.; Driver, V.R. Hyaluronic acid derivatives and their healing effect on burns, epithelial surgical wounds, and chronic wounds: A systematic review and meta-analysis of randomized controlled trials. Wound Repair Regen. 2012, 20, 317–331. [Google Scholar] [CrossRef]
- Ukaegbu, K.; Allen, E.; Svoboda, K.K.H. Reactive Oxygen Species and Antioxidants in Wound Healing: Mechanisms and Therapeutic Potential. Int. Wound J. 2025, 22, e70330. [Google Scholar] [CrossRef]
- Bonarska-Kujawa, D.; Cyboran, S.; Żyłka, R.; Oszmiański, J.; Kleszczyńska, H. Biological Activity of Blackcurrant Extracts (Ribes nigrum L.) in Relation to Erythrocyte Membranes. BioMed Res. Int. 2014, 2014, 783059. [Google Scholar] [CrossRef]
- Cao, L.; Park, Y.; Lee, S.; Kim, D.-O. Extraction, Identification, and Health Benefits of Anthocyanins in Blackcurrants (Ribes nigrum L.). Appl. Sci. 2021, 11, 1863. [Google Scholar] [CrossRef]
- Kumorek, M.; Minisy, I.M.; Krunclová, T.; Voršiláková, M.; Venclíková, K.; Chánová, E.M.; Janoušková, O.; Kubies, D. pH-responsive and antibacterial properties of self-assembled multilayer films based on chitosan and tannic acid. Mater. Sci. Eng. C 2020, 109, 110493. [Google Scholar] [CrossRef]
- Kasprzyk, I.; Depciuch, J.; Grabek-Lejko, D.; Parlinska-Wojtan, M. FTIR-ATR spectroscopy of pollen and honey as a tool for unifloral honey authentication. The case study of rape honey. Food Control. 2018, 84, 33–40. [Google Scholar] [CrossRef]
- Leal, D.; Matsuhiro, B.; Rossi, M.; Caruso, F. FT-IR spectra of alginic acid block fractions in three species of brown seaweeds. Carbohydr. Res. 2008, 343, 308–316. [Google Scholar] [CrossRef]
- Cebi, N.; Bozkurt, F.; Yilmaz, M.T.; Sagdic, O. An evaluation of FTIR spectroscopy for prediction of royal jelly content in hive products. J. Apic. Res. 2020, 59, 146–155. [Google Scholar] [CrossRef]
- Matharu, R.K.; Ahmed, J.; Seo, J.; Karu, K.; Golshan, M.A.; Edirisinghe, M.; Ciric, L. Antibacterial Properties of Honey Nanocomposite Fibrous Meshes. Polymers 2022, 14, 5155. [Google Scholar] [CrossRef]
- Donghui, F.; Beibei, W.; Zheng, X.; Qisheng, G. Determination of hyaluronan by spectroscopic methods. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2006, 21, 32–34. [Google Scholar] [CrossRef]
- Jia, W.; Li, M.; Kang, L.; Gu, G.; Guo, Z.; Chen, Z. Fabrication and Comprehensive Characterization of Biomimetic Extracellular Matrix Electrospun Scaffold for Vascular Tissue Engineering Applications. J. Mater. Sci. 2019, 54, 10871–10883. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, G.; Yu, Z.; Song, X.; Li, X.; Yang, Y.; Wang, L.; Liu, L.; Dai, J. Purification, characterization and antiglycation activity of a novel polysaccharide from black currant. Food Chem. 2016, 199, 694–701. [Google Scholar] [CrossRef]
- Xu, Y.; Niu, X.; Liu, N.; Gao, Y.; Wang, L.; Xu, G.; Li, X.; Yang, Y. Characterization, antioxidant and hypoglycemic activities of degraded polysaccharides from blackcurrant (Ribes nigrum L.) fruits. Food Chem. 2018, 243, 26–35. [Google Scholar] [CrossRef]
- Stehfest, K.; Toepel, J.; Wilhelm, C. The application of micro-FTIR spectroscopy to analyze nutrient stress-related changes in biomass composition of phytoplankton algae. J. Plant Physiol. Biochem. 2005, 43, 717–726. [Google Scholar] [CrossRef]
- Bakr, A.M.; El Nahrawy, A.M.; Mansour, A.M.; Abou Hammad, A.B. Exploring of spectroscopic, dielectric, and bioactivity performance of bioglass/sodium alginate-PVP loaded-Amoxicillin/Clavulanic Acid microspheres for bone tissue engineering. Sci. Rep. 2025, 15, 15395. [Google Scholar] [CrossRef]
- Nastaj, J.; Przewłocka, A.; Rajkowska-Myśliwiec, M. Biosorption of Ni(II), Pb(II) and Zn(II) on calcium alginate beads: Equilibrium, kinetic and mechanism studies. Green Sci. 2016, 18, 81–87. [Google Scholar] [CrossRef]
- Sartori, C.; Finch, D.S.; Ralph, B.; Gilding, K. Determination of the cation content of alginate thin films by FTi.r. spectroscopy. Polymer 1997, 38, 43–51. [Google Scholar] [CrossRef]
- Li, J.; He, J.; Huang, Y.; Li, D.; Chen, X. Improving surface and mechanical properties of alginate films by using ethanol as a co-solvent during external gelation. Carbohydr. Polym. 2015, 123, 208–216. [Google Scholar] [CrossRef]
- ASTM E96/E96M-05; Standard Test Methods for Water Vapor Transmission of Materials. ASTM International: West Conshohocken, PA, USA, 2005.
- EN ISO 2808; Paints and Varnishes—Determination of Film Thickness. International Organization for Standardization (ISO): Geneva, Switzerland, 2019.
- Mi, F.-L.; Shyu, S.-S.; Wu, Y.-B.; Lee, S.-T.; Shyong, J.-Y.; Huang, R.-N. Fabrication and characterization of a sponge-like asymmetric chitosan membrane as a wound dressing. Biomaterials 2001, 22, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Martinović, J.; Lukinac, J.; Jukić, M.; Ambrus, R.; Planinić, M.; Šelo, G.; Perković, G.; Bucić-Kojić, A. The Release of Grape Pomace Phenolics from Alginate-Based Microbeads during Simulated Digestion In Vitro: The Influence of Coatings and Drying Method. Gels 2023, 9, 870. [Google Scholar] [CrossRef] [PubMed]
- El-Kased, R.F.; Amer, R.I.; Attia, D.; Elmazar, M.M. Honey-based hydrogel: In vitro and comparative In vivo evaluation for burn wound healing. Sci. Rep. 2017, 7, 9692. [Google Scholar] [CrossRef] [PubMed]
- Ciarleglio, G.; Toto, E.; Santonicola, M.G. Conductive and Thermo-Responsive Composite Hydrogels with Poly(N-isopropylacrylamide) and Carbon Nanotubes Fabricated by Two-Step Photopolymerization. Polymers 2023, 15, 1022. [Google Scholar] [CrossRef]
- Ciarleglio, G.; Russo, T.; Vella, S.; Toto, E.; Santonicola, M.G. Electrospray Fabrication of pH-Responsive Microspheres for the Delivery of Ozoile. Macromol. Symp. 2024, 413, 2400031. [Google Scholar] [CrossRef]
- Lamke, L.O.; Nilsson, G.E.; Reithner, H.L. The evaporative water loss from burns and the water-vapour permeability of grafts and artificial membranes used in the treatment of burns. Burns 1977, 3, 159–165. [Google Scholar] [CrossRef]
- Buchwalder, S.; Nicolier, C.; Hersberger, M.; Bourgeois, F.; Hogg, A.; Burger, J. Development of a Water Transmission Rate (WTR) Measurement System for Implantable Barrier Coatings. Polymers 2023, 15, 2557. [Google Scholar] [CrossRef]
- Richards, H.; Falder, S. pH of a burn wound. Burns 2018, 44, 2104–2105. [Google Scholar] [CrossRef]
- Po, H.N.; Senozan, N.M. The Henderson-Hasselbalch Equation: Its History and Limitations. J. Chem. Educ. 2001, 78, 1499. [Google Scholar] [CrossRef]
- Wang, X.; Tan, L.-L.; Li, X.; Song, N.; Li, Z.; Hu, J.-N.; Cheng, Y.-M.; Wang, Y.; Yang, Y.-W. Smart mesoporous silica nanoparticles gated by pillararene-modified gold nanoparticles for on-demand cargo release. Chem. Commun. 2016, 52, 13775–13778. [Google Scholar] [CrossRef] [PubMed]
| Sample Type | WVTR (g/m2·Day) |
|---|---|
| Multilayer hydrogel (uncoated) | 3852.29 ± 128.42 |
| Multilayer hydrogel (chitosan-coated) | 3449.18 ± 87.51 |
| Negative control (PBS only) | 5681.63 ± 10.00 |
| Sample | Weight Loss (%) | ||
|---|---|---|---|
| pH 3 | pH 6 | pH 8 | |
| ALG/RN | −26.6 ± 1.2 | −73.2 ± 5.2 | −83.9 ± 3.1 |
| ALG/HA | −24.1 ± 0.8 | −52.9 ± 11.7 | −80.6 ± 7.8 |
| ALG/MH | −36.0 ± 4.7 | −54.2 ± 10.4 | −80.9 ± 6.5 |
| ML-C | −26.4 ± 7.1 | −33.7 ± 5.1 | −41.4 ± 3.8 |
| Sample | pH 3 | pH 6 | pH 8 |
|---|---|---|---|
| ALG/MH | 2.60 ± 0.04 | 4.35 ± 0.13 | 6.92 ± 0.14 |
| ALG/HA | 2.60 ± 0.02 | 4.20 ± 0.05 | 6.55 ± 0.11 |
| ALG/RN | 2.61 ± 0.04 | 4.33 ± 0.10 | 6.90 ± 0.11 |
| ML-C | 2.67 ± 0.02 | 3.92 ± 0.01 | 5.10 ± 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciarleglio, G.; Clarizia, V.; Toto, E.; Santonicola, M.G. Multilayer pH-Responsive Hydrogels Fabricated via Two-Step Ionic Crosslinking: Towards Advanced Wound Dressing Materials. Gels 2025, 11, 840. https://doi.org/10.3390/gels11100840
Ciarleglio G, Clarizia V, Toto E, Santonicola MG. Multilayer pH-Responsive Hydrogels Fabricated via Two-Step Ionic Crosslinking: Towards Advanced Wound Dressing Materials. Gels. 2025; 11(10):840. https://doi.org/10.3390/gels11100840
Chicago/Turabian StyleCiarleglio, Gianluca, Virginia Clarizia, Elisa Toto, and Maria Gabriella Santonicola. 2025. "Multilayer pH-Responsive Hydrogels Fabricated via Two-Step Ionic Crosslinking: Towards Advanced Wound Dressing Materials" Gels 11, no. 10: 840. https://doi.org/10.3390/gels11100840
APA StyleCiarleglio, G., Clarizia, V., Toto, E., & Santonicola, M. G. (2025). Multilayer pH-Responsive Hydrogels Fabricated via Two-Step Ionic Crosslinking: Towards Advanced Wound Dressing Materials. Gels, 11(10), 840. https://doi.org/10.3390/gels11100840











