Biological Augmentation of Meniscal Repair: A Review with Insights into Injectable Hydrogel Delivery
Abstract
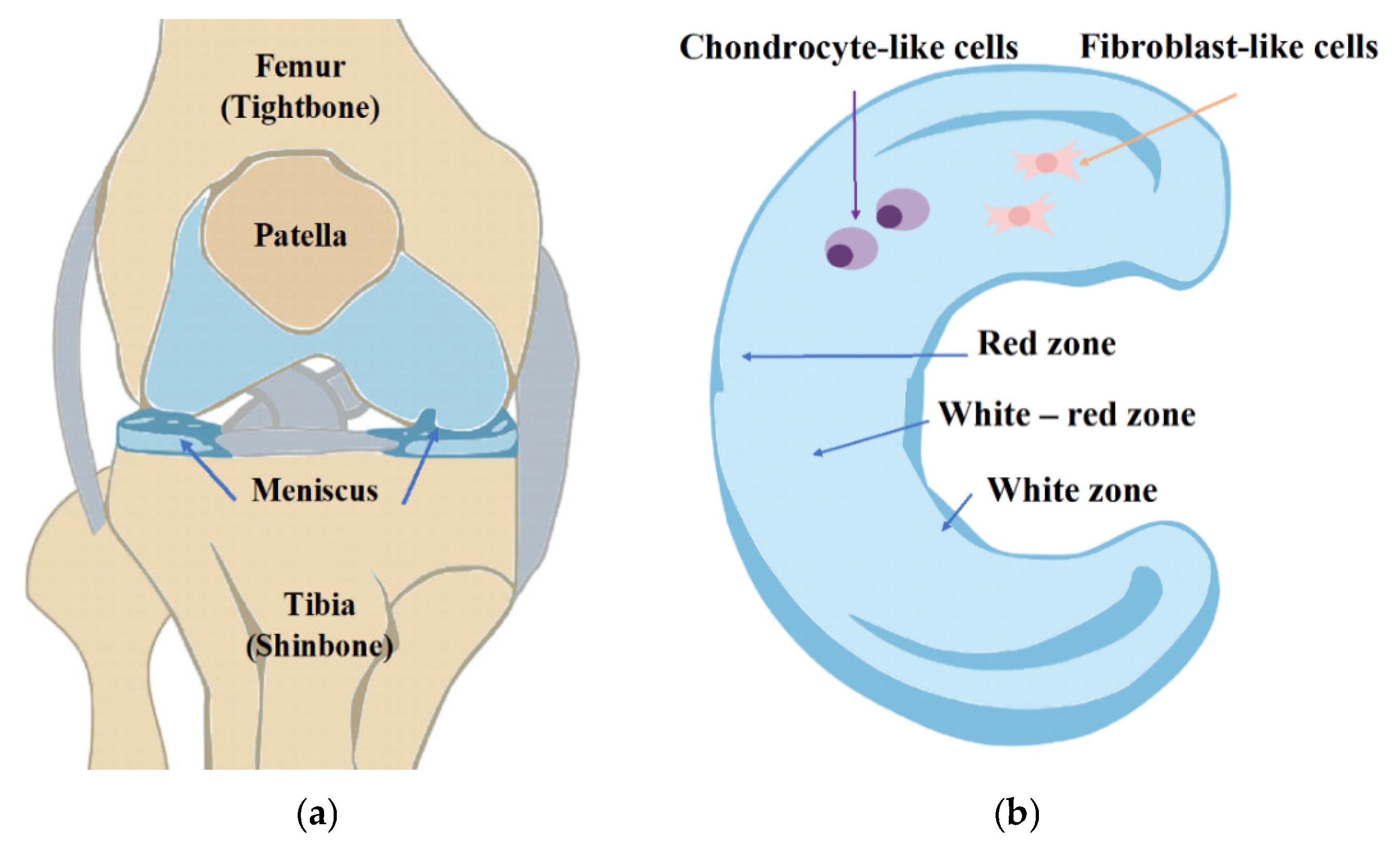
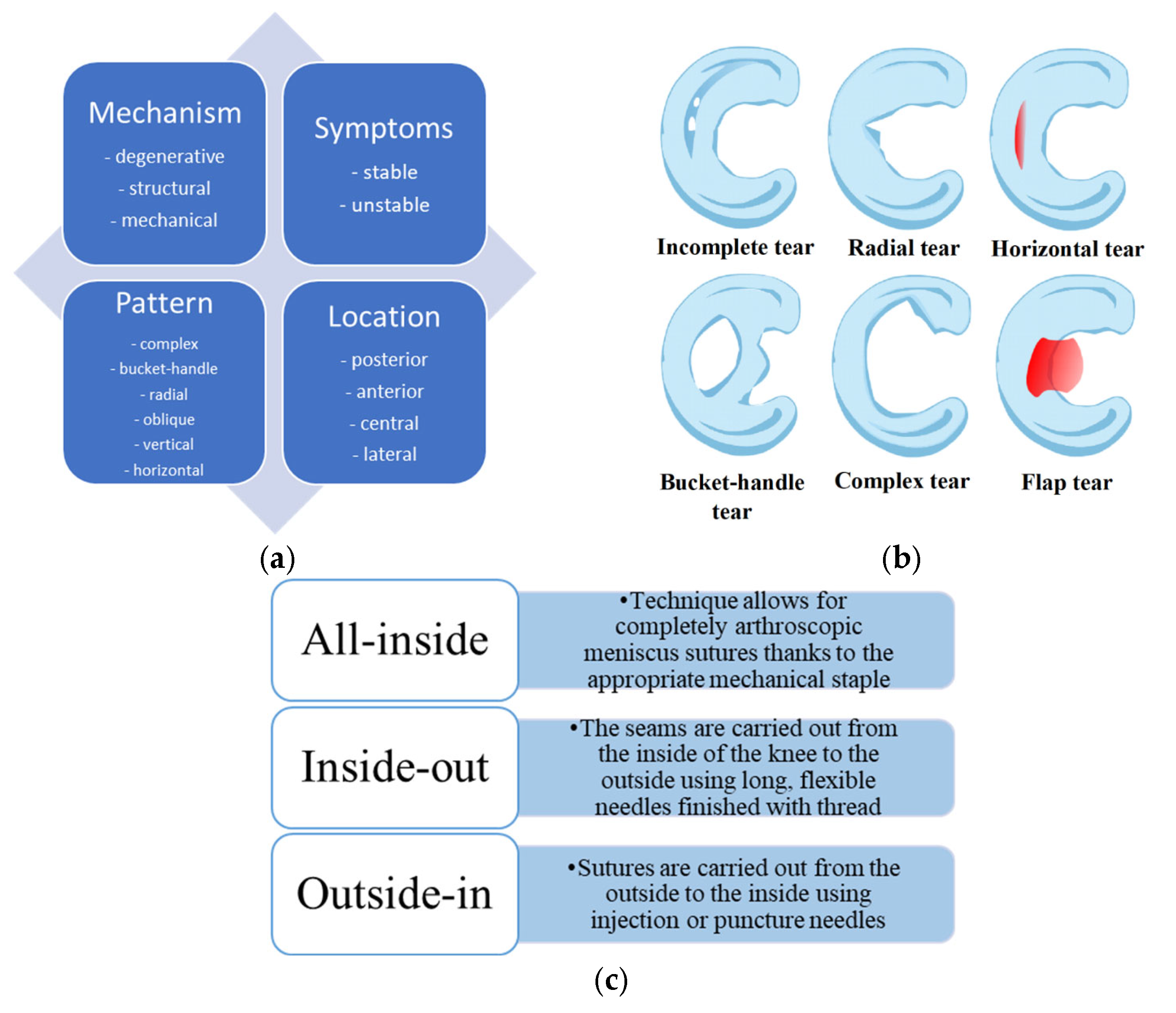
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ozeki, N.; Nakamura, T.; Nakagawa, Y.; Sekiya, I.; Koga, H. Meniscus Repair and Centralization: Preserving Meniscus Function. J. Jt. Surg. Res. 2023, 1, 46–55. [Google Scholar] [CrossRef]
- Lombardo, M.D.M.; Mangiavini, L.; Peretti, G.M. Biomaterials and Meniscal Lesions: Current Concepts and Future Perspectives. Pharmaceutics 2021, 13, 1886. [Google Scholar] [CrossRef]
- Klarmann, G.J.; Gaston, J.; Ho, V.B. A Review of Strategies for Development of Tissue Engineered Meniscal Implants. Biomater. Biosyst. 2021, 4, 100026. [Google Scholar] [CrossRef] [PubMed]
- Avila, A.; Vasavada, K.; Shankar, D.S.; Petrera, M.; Jazrawi, L.M.; Strauss, E.J. Current Controversies in Arthroscopic Partial Meniscectomy. Curr. Rev. Musculoskelet. Med. 2022, 15, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Mine, T.; Ueda, T.; Ihara, K.; Kawamura, H.; Kuriyama, R.; Tominaga, Y. Possibility of Meniscal Repair for Degenerative and Horizontal Tears. Open Orthop. J. 2020, 13, 260–265. [Google Scholar] [CrossRef]
- Posadzy, M.; Joseph, G.B.; McCulloch, C.E.; Nevitt, M.C.; Lynch, J.A.; Lane, N.E.; Link, T.M. Natural History of New Horizontal Meniscal Tears in Individuals at Risk for and with Mild to Moderate Osteoarthritis: Data from Osteoarthritis Initiative. Eur. Radiol. 2020, 30, 5971–5980. [Google Scholar] [CrossRef]
- Shanmugaraj, A.; Tejpal, T.; Ekhtiari, S.; Gohal, C.; Horner, N.; Hanson, B.; Khan, M.; Bhandari, M. The Repair of Horizontal Cleavage Tears Yields Higher Complication Rates Compared to Meniscectomy: A Systematic Review. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 915–925. [Google Scholar] [CrossRef]
- Vadodaria, K.; Kulkarni, A.; Santhini, E.; Vasudevan, P. Materials and Structures Used in Meniscus Repair and Regeneration: A Review. BioMedicine 2019, 9, 11–22. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, H.; Trinh, P.; Heilshorn, S.C.; Yang, F. Elastin-like Protein-Hyaluronic Acid (ELP-HA) Hydrogels with Decoupled Mechanical and Biochemical Cues for Cartilage Regeneration. Biomaterials 2017, 127, 132–140. [Google Scholar] [CrossRef]
- Mancuso, M.E.; Santagostino, E. Platelets: Much More than Bricks in a Breached Wall. Br. J. Haematol. 2017, 178, 209–219. [Google Scholar] [CrossRef]
- Fortier, L.A.; Barker, J.U.; Strauss, E.J.; McCarrel, T.M.; Cole, B.J. The Role of Growth Factors in Cartilage Repair. Clin. Orthop. Relat. Res. 2011, 469, 2706–2715. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Shao, H.; Hu, X. Hybrid Silk Fibers Dry-Spun from Regenerated Silk Fibroin/Graphene Oxide Aqueous Solutions. ACS Appl. Mater. Interfaces 2016, 8, 3349–3358. [Google Scholar] [CrossRef]
- Lang, S.; Loibl, M.; Herrmann, M. Platelet-Rich Plasma in Tissue Engineering: Hype and Hope. Eur. Surg. Res. 2018, 59, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, J.; Wang, W. Progress in the Use of Mesenchymal Stromal Cells for Osteoarthritis Treatment. Cytotherapy 2021, 23, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Ramezanifard, R.; Kabiri, M.; Hanaee Ahvaz, H. Effects of Platelet Rich Plasma and Chondrocyte Co-Culture on MSC Chondrogenesis, Hypertrophy and Pathological Responses. EXCLI J. 2017, 16, 1031–1045. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Q.; Yang, M.; Yang, L.; Wang, W.; Ding, H.; Zhang, D.; Xu, J.; Tang, X.; Ding, H.; et al. Histomorphology and Innate Immunity during the Progression of Osteoarthritis: Does Synovitis Affect Cartilage Degradation? J. Cell. Physiol. 2018, 233, 1342–1358. [Google Scholar] [CrossRef]
- Kinoshita, T.; Hashimoto, Y.; Orita, K.; Iida, K.; Takahashi, S.; Nakamura, H. Bone Marrow–Derived Fibrin Clots Stimulate Healing of a Meniscal Defect in a Rabbit Model. Arthrosc. J. Arthrosc. Relat. Surg. 2023, 39, 1662–1670. [Google Scholar] [CrossRef]
- Kim, G.B.; Seo, M.S.; Park, W.T.; Lee, G.W. Bone Marrow Aspirate Concentrate: Its Uses in Osteoarthritis. Int. J. Mol. Sci. 2020, 21, 3224. [Google Scholar] [CrossRef]
- Massey, P.A.; Zhang, A.; Stairs, C.B.; Hoge, S.; Carroll, T.; Hamby, A.M. Meniscus Repair Outcomes with and without Bone Marrow Aspiration Concentrate. Orthop. J. Sports Med. 2019, 7, 2325967119S0028. [Google Scholar] [CrossRef]
- Sarrigiannidis, S.O.; Rey, J.M.; Dobre, O.; González-García, C.; Dalby, M.J.; Salmeron-Sanchez, M. A Tough Act to Follow: Collagen Hydrogel Modifications to Improve Mechanical and Growth Factor Loading Capabilities. Mater. Today Bio 2021, 10, 100098. [Google Scholar] [CrossRef]
- Pan, D.; Yin, P.; Li, L.; Wu, K.; Tong, C.; Liu, D. Holomycin, a Novel NLRP3 Inhibitor, Attenuates Cartilage Degeneration and Inflammation in Osteoarthritis. Biochem. Biophys. Res. Commun. 2023, 657, 59–68. [Google Scholar] [CrossRef]
- Dong, Q.; Cai, J.; Wang, H.; Chen, S.; Liu, Y.; Yao, J.; Shao, Z.; Chen, X. Artificial Ligament Made from Silk Protein/Laponite Hybrid Fibers. Acta Biomater. 2020, 106, 102–113. [Google Scholar] [CrossRef]
- Ma, D.; Wang, Y.; Dai, W. Silk Fibroin-Based Biomaterials for Musculoskeletal Tissue Engineering. Mater. Sci. Eng. C 2018, 89, 456–469. [Google Scholar] [CrossRef]
- Cao, H.; Deng, S.; Chen, X.; Cui, X.; Yuan, T.; Liang, J.; Zhang, X.; Fan, Y.; Wang, Q. An Injectable Cartilage-Coating Composite with Long-Term Protection, Effective Lubrication and Chondrocyte Nourishment for Osteoarthritis Treatment. Acta Biomater. 2024, 179, 95–105. [Google Scholar] [CrossRef]
- Aulin, C.; Lundbäck, P.; Palmblad, K.; Klareskog, L.; Erlandsson Harris, H. An in Vivo Cross-Linkable Hyaluronan Gel with Inherent Anti-Inflammatory Properties Reduces OA Cartilage Destruction in Female Mice Subjected to Cruciate Ligament Transection. Osteoarthr. Cartil. 2017, 25, 157–165. [Google Scholar] [CrossRef]
- Liu, W.; Ma, M.; Lei, Z.; Xiong, Z.; Tao, T.; Lei, P.; Hu, Y.; Jiang, X.; Xiao, J. Intra-Articular Injectable Hydroxypropyl Chitin/Hyaluronic Acid Hydrogel as Bio-Lubricant to Attenuate Osteoarthritis Progression. Mater. Des. 2022, 217, 110579. [Google Scholar] [CrossRef]
- Hu, W.; Yao, X.; Li, Y.; Li, J.; Zhang, J.; Zou, Z.; Kang, F.; Dong, S. Injectable Hydrogel with Selenium Nanoparticles Delivery for Sustained Glutathione Peroxidase Activation and Enhanced Osteoarthritis Therapeutics. Mater. Today Bio 2023, 23, 100864. [Google Scholar] [CrossRef] [PubMed]
- Abpeikar, Z.; Javdani, M.; Alizadeh, A.; Khosravian, P.; Tayebi, L.; Asadpour, S. Development of Meniscus Cartilage Using Polycaprolactone and Decellularized Meniscus Surface Modified by Gelatin, Hyaluronic Acid Biomacromolecules: A Rabbit Model. Int. J. Biol. Macromol. 2022, 213, 498–515. [Google Scholar] [CrossRef]
- Garcia, J.P.; Stein, J.; Cai, Y.; Riemers, F.; Wexselblatt, E.; Wengel, J.; Tryfonidou, M.; Yayon, A.; Howard, K.A.; Creemers, L.B. Fibrin-Hyaluronic Acid Hydrogel-Based Delivery of Antisense Oligonucleotides for ADAMTS5 Inhibition in Co-Delivered and Resident Joint Cells in Osteoarthritis. J. Control. Release 2019, 294, 247–258. [Google Scholar] [CrossRef]
- Maniwa, S.; Ochi, M.; Motomura, T.; Nishikori, T.; Chen, J.; Naora, H. Effects of Hyaluronic Acid and Basic Fibroblast Growth Factor on Motility of Chondrocytes and Synovial Cells in Culture. Acta Orthop. Scand. 2001, 72, 299–303. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Niu, X.; Lin, Q.; Zhao, B.; Wang, Y.; Zhu, L. An in Situ Photocrosslinkable Platelet Rich Plasma—Complexed Hydrogel Glue with Growth Factor Controlled Release Ability to Promote Cartilage Defect Repair. Acta Biomater. 2017, 62, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z.; Zhang, B.; Zhao, Q.; Liu, Y.; Qi, W. Effect of Activated Platelet-Rich Plasma on Chondrogenic Differentiation of Rabbit Bone Marrow-Derived Mesenchymal Stem Cells. Stem Cells Int. 2021, 2021, 9947187. [Google Scholar] [CrossRef] [PubMed]
- Bendinelli, P.; Matteucci, E.; Dogliotti, G.; Corsi, M.M.; Banfi, G.; Maroni, P.; Desiderio, M.A. Molecular Basis of Anti-Inflammatory Action of Platelet-Rich Plasma on Human Chondrocytes: Mechanisms of NF-ΚB Inhibition via HGF. J. Cell. Physiol. 2010, 225, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, S.; Ren, Y.; Qiang, L.; Liu, Y.; Wang, J.; Dai, K. 3D Printed Hydrogel for Articular Cartilage Regeneration. Compos. B Eng. 2022, 237, 109863. [Google Scholar] [CrossRef]
- Wang, X.; Han, X.; Li, C.; Chen, Z.; Huang, H.; Chen, J.; Wu, C.; Fan, T.; Li, T.; Huang, W.; et al. 2D Materials for Bone Therapy. Adv. Drug Deliv. Rev. 2021, 178, 113970. [Google Scholar] [CrossRef]
- Pan, W.; Dai, C.; Li, Y.; Yin, Y.; Gong, L.; Machuki, J.O.; Yang, Y.; Qiu, S.; Guo, K.; Gao, F. PRP-Chitosan Thermoresponsive Hydrogel Combined with Black Phosphorus Nanosheets as Injectable Biomaterial for Biotherapy and Phototherapy Treatment of Rheumatoid Arthritis. Biomaterials 2020, 239, 119851. [Google Scholar] [CrossRef]
- Olesen, M.L.; Christensen, B.B.; Foldager, C.B.; Hede, K.C.; Bergholt, N.L.; Lind, M. No Effect of Platelet-Rich Plasma as Adjuvant to Bone Marrow Stimulation for the Treatment of Chondral Defects in a Large Animal Model. Arch. Orthop. Trauma. Surg. 2020, 140, 77–84. [Google Scholar] [CrossRef]
- Wilusz, R.E.; Weinberg, J.B.; Guilak, F.; McNulty, A.L. Inhibition of Integrative Repair of the Meniscus Following Acute Exposure to Lnterleukin-1 in Vitro. J. Orthop. Res. 2008, 26, 504–512. [Google Scholar] [CrossRef]
- Lee, K.I.; Olmer, M.; Baek, J.; D’Lima, D.D.; Lotz, M.K. Platelet-Derived Growth Factor-Coated Decellularized Meniscus Scaffold for Integrative Healing of Meniscus Tears. Acta Biomater. 2018, 76, 126–134. [Google Scholar] [CrossRef]
- Wang, Z.; Le, H.; Wang, Y.; Liu, H.; Li, Z.; Yang, X.; Wang, C.; Ding, J.; Chen, X. Instructive Cartilage Regeneration Modalities with Advanced Therapeutic Implantations under Abnormal Conditions. Bioact. Mater. 2022, 11, 317–338. [Google Scholar] [CrossRef]
- Wang, H.; Wu, J.; Yang, L.; Liu, S.; Sui, X.; Guo, Q.; Chen, M.; Xia, Y. Cell-Free Decellularized Skin Matrix Scaffolds: A Promising Approach for Meniscus Regeneration in a Rabbit Meniscectomy Model. Acta Biomater. 2024, 187, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, I.; Koga, H.; Otabe, K.; Nakagawa, Y.; Katano, H.; Ozeki, N.; Mizuno, M.; Horie, M.; Kohno, Y.; Katagiri, K.; et al. Additional Use of Synovial Mesenchymal Stem Cell Transplantation Following Surgical Repair of a Complex Degenerative Tear of the Medial Meniscus of the Knee: A Case Report. Cell Transplant. 2019, 28, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, N.; Kohno, Y.; Kushida, Y.; Watanabe, N.; Mizuno, M.; Katano, H.; Masumoto, J.; Koga, H.; Sekiya, I. Synovial Mesenchymal Stem Cells Promote the Meniscus Repair in a Novel Pig Meniscus Injury Model. J. Orthop. Res. 2021, 39, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, N.; Koga, H.; Sekiya, I. Degenerative Meniscus in Knee Osteoarthritis: From Pathology to Treatment. Life 2022, 12, 603. [Google Scholar] [CrossRef]
- Bozkurt, M. Segmental Medial Meniscus Transplantation in Combination With BMAC (Bone Marrow Aspirate Concentrate) Injection to Improve Healing and Prevent Extrusion. Arthrosc. Tech. 2022, 11, e1997–e2001. [Google Scholar] [CrossRef]
- Zhong, G.; Yao, J.; Huang, X.; Luo, Y.; Wang, M.; Han, J.; Chen, F.; Yu, Y. Injectable ECM Hydrogel for Delivery of BMSCs Enabled Full-Thickness Meniscus Repair in an Orthotopic Rat Model. Bioact. Mater. 2020, 5, 871–879. [Google Scholar] [CrossRef]
- Ying, X.-Z.; Qian, J.-J.; Peng, L.; Zheng, Q.; Zhu, B.; Jin, Y.-H. Silk Fibroin-BMSCs Porous Scaffolds and Repair of Meniscus Injury in Rabbits. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3689–3693. [Google Scholar]
- Tanideh, N.; Dehghani Nazhvani, S.; Mojtahed Jaberi, F.; Mehrabani, D.; Rezazadeh, S.; Pakbaz, S.; Tamadon, A.; Nikahval, B. The Healing Effect of Bioglue in Articular Cartilage Defect of Femoral Condyle in Experimental Rabbit Model. Iran. Red Crescent Med. J. 2011, 13, 629–633. [Google Scholar] [CrossRef]
- Tarafder, S.; Park, G.Y.; Felix, J.; Lee, C.H. Bioadhesives for Musculoskeletal Tissue Regeneration. Acta Biomater. 2020, 117, 77–92. [Google Scholar] [CrossRef]
- Gao, Y.; Kong, W.; Li, B.; Ni, Y.; Yuan, T.; Guo, L.; Lin, H.; Fan, H.; Fan, Y.; Zhang, X. Fabrication and Characterization of Collagen-Based Injectable and Self-Crosslinkable Hydrogels for Cell Encapsulation. Colloids Surf. B Biointerfaces 2018, 167, 448–456. [Google Scholar] [CrossRef]
- Abbadessa, A.; Nuñez Bernal, P.; Buttitta, G.; Ronca, A.; D’Amora, U.; Zihlmann, C.; Stiefel, N.; Ambrosio, L.; Malda, J.; Levato, R.; et al. Biofunctionalization of 3D Printed Collagen with Bevacizumab-Loaded Microparticles Targeting Pathological Angiogenesis. J. Control. Release 2023, 360, 747–758. [Google Scholar] [CrossRef]
- Heo, J.; Koh, R.H.; Shim, W.; Kim, H.D.; Yim, H.G.; Hwang, N.S. Riboflavin-Induced Photo-Crosslinking of Collagen Hydrogel and Its Application in Meniscus Tissue Engineering. Drug Deliv. Transl. Res. 2016, 6, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Chang, P.; Ding, P.; Liu, S.; Rao, Q.; Okoro, O.V.; Wang, L.; Fan, L.; Shavandi, A.; Nie, L. MSCs-Laden Silk Fibroin/GelMA Hydrogels with Incorporation of Platelet-Rich Plasma for Chondrogenic Construct. Heliyon 2023, 9, e14349. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Li, J.; Wang, C.; Su, Z.; Su, H.; Chen, Y.; Yu, B. Injectable Silk Fibroin Peptide Nanofiber Hydrogel Composite Scaffolds for Cartilage Regeneration. Mater. Today Bio 2024, 25, 100962. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, Y.; Liu, J.; Fang, Y.; Guo, W.; Liu, Y.; Li, X.; Li, G.; Wang, X.; Zheng, Z.; et al. Intraarticularly Injectable Silk Hydrogel Microspheres with Enhanced Mechanical and Structural Stability to Attenuate Osteoarthritis. Biomaterials 2022, 286, 121611. [Google Scholar] [CrossRef]
- Yu, S.; Shu, X.; Chen, L.; Wang, C.; Wang, X.; Jing, J.; Yan, G.; Zhang, Y.; Wu, C. Construction of Ultrasonically Treated Collagen/Silk Fibroin Composite Scaffolds to Induce Cartilage Regeneration. Sci. Rep. 2023, 13, 43397. [Google Scholar] [CrossRef]
- Tarafder, S.; Ghataure, J.; Langford, D.; Brooke, R.; Kim, R.; Eyen, S.L.; Bensadoun, J.; Felix, J.T.; Cook, J.L.; Lee, C.H. Advanced Bioactive Glue Tethering Lubricin/PRG4 to Promote Integrated Healing of Avascular Meniscus Tears. Bioact. Mater. 2023, 28, 61–73. [Google Scholar] [CrossRef]
- Xu, Q.; Torres, J.E.; Hakim, M.; Babiak, P.M.; Pal, P.; Battistoni, C.M.; Nguyen, M.; Panitch, A.; Solorio, L.; Liu, J.C. Collagen- and Hyaluronic Acid-Based Hydrogels and Their Biomedical Applications. Mater. Sci. Eng. R Rep. 2021, 146, 100641. [Google Scholar] [CrossRef]
- Yan, W.; Xu, X.; Xu, Q.; Sun, Z.; Jiang, Q.; Shi, D. Platelet-Rich Plasma Combined with Injectable Hyaluronic Acid Hydrogel for Porcine Cartilage Regeneration: A 6-Month Follow-Up. Regen. Biomater. 2019, 7, 77–90. [Google Scholar] [CrossRef]
- Oeding, J.F.; Berlinberg, E.J.; Lu, Y.; Marigi, E.M.; Okoroha, K.R.; Camp, C.L.; Barlow, J.D.; Krych, A.J. Platelet-Rich-Plasma and Marrow Venting May Serve as Cost-Effective Augmentation Techniques for Isolated Meniscal Repair: A Decision-Analytic Markov Model-Based Analysis. Arthrosc. J. Arthrosc. Relat. Surg. 2023, 39, 2058–2068. [Google Scholar] [CrossRef]
- Farkash, U.; Avisar, E.; Volk, I.; Slevin, O.; Shohat, N.; El Haj, M.; Dolev, E.; Ashraf, E.; Luria, S. First Clinical Experience with a New Injectable Recombinant Human Collagen Scaffold Combined with Autologous Platelet-Rich Plasma for the Treatment of Lateral Epicondylar Tendinopathy (Tennis Elbow). J. Shoulder Elb. Surg. 2019, 28, 503–509. [Google Scholar] [CrossRef]
- Zhou, Z.; Cui, J.; Wu, S.; Geng, Z.; Su, J. Silk Fibroin-Based Biomaterials for Cartilage/Osteochondral Repair. Theranostics 2022, 12, 5103–5124. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Yuan, T.; Zhang, Y.; Luo, C.; Zhang, J.; Liu, Y.; Fan, W. Addition of Platelet-Rich Plasma to Silk Fibroin Hydrogel Bioprinting for Cartilage Regeneration. Tissue Eng. Part. A 2020, 26, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.E.; Puskas, B.L.; Mandelbaum, B.R.; Gerhardt, M.B.; Rodeo, S.A. Platelet-Rich Plasma: From Basic Science to Clinical Applications. Am. J. Sports Med. 2009, 37, 2259–2272. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wang, J.; Jin, X.; Wang, X.; Fan, Z.; Zhang, Y.; Sang, X.; Meng, Z. Rapamycin Incorporating Hydrogel Improves the Progression of Osteoarthritis by Inducing Synovial Macrophages Polarization and Reducing Intra-Articular Inflammation. Mater. Des. 2023, 225, 111542. [Google Scholar] [CrossRef]
- Badhe, R.V.; Chatterjee, A.; Bijukumar, D.; Mathew, M.T. Current Advancements in Bio-Ink Technology for Cartilage and Bone Tissue Engineering. Bone 2023, 171, 116746. [Google Scholar] [CrossRef]
- Yang, Z.; Li, H.; Yuan, Z.; Fu, L.; Jiang, S.; Gao, C.; Wang, F.; Zha, K.; Tian, G.; Sun, Z.; et al. Endogenous Cell Recruitment Strategy for Articular Cartilage Regeneration. Acta Biomater. 2020, 114, 31–52. [Google Scholar] [CrossRef]
- Yu, C.; Li, L.; Liang, D.; Wu, A.; Dong, Q.; Jia, S.; Li, Y.; Li, Y.; Guo, X.; Zang, H. Glycosaminoglycan-Based Injectable Hydrogels with Multi-Functions in the Alleviation of Osteoarthritis. Carbohydr. Polym. 2022, 290, 119492. [Google Scholar] [CrossRef]
- An, Y.H.; Kim, J.A.; Yim, H.G.; Han, W.J.; Park, Y.B.; Jin Park, H.; Young Kim, M.; Jang, J.; Koh, R.H.; Kim, S.H.; et al. Meniscus Regeneration with Injectable Pluronic/PMMA-Reinforced Fibrin Hydrogels in a Rabbit Segmental Meniscectomy Model. J. Tissue Eng. 2021, 12, 20417314211050141. [Google Scholar] [CrossRef]
- Narez, G.E.; Wei, F.; Dejardin, L.; Haut, R.C.; Haut Donahue, T.L. A Single Dose of P188 Prevents Cell Death in Meniscal Explants Following Impact Injury. J. Mech. Behav. Biomed. Mater. 2021, 117, 104406. [Google Scholar] [CrossRef]
| Biological Additive | Material | Cells | Animal Model | Effects/Outcomes | Ref. |
|---|---|---|---|---|---|
| Hyaluronic acid | Decellularized cartilage matrix (dECMs) crosslinked by hyaluronan composite | Chondrocytes | Male SD rats | Increased cell viability and ECM deposition, improved joint lubrication | [24] |
| Aldehyde-modified HA (HAA) gel crosslinked by carbamate-modified polyvinyl alcohol (PVAC) | - | C57BL/6 mice | Rapid in situ gelation, reduced inflammation | [25] | |
| Hydroxypropyl chitin (HPCH)/hyaluronic acid hydrogel | Primary chondrocytes, BMSCs | C57BL/6J mice | Sustained release of HA, increased proteoglycan synthesis | [26] | |
| Oxidized hyaluronic acid (OHA) with adipic dihydrazide-grafted HA (HA-ADH) solution and selenium nanoparticles (SeNPs) hydrogel | SW1353 cells | Male SD rats | Antioxidant effect, decrease MMP expression | [27] | |
| Polycaprolactone (PCL) and decellularized meniscus extracellular matrix (DMECM) surface modified by gelatin (G), hyaluronic acid (HU), and selenium (Se) nanoparticles (PCL/DMECM/G/HU/Se) | Chondrocytes and adipose-extracted mesenchymal stem cells (ASCs) obtained from Hoffa’s fat pad | The medial meniscus of the right knee in the rabbit model | Increase mechanical stiffness, enhanced fibrocartilage formation | [28] | |
| Fibrin-hyaluronic acid hydrogel | Human articular chondrocytes | - | Injectable, promotes cell migration | [29] | |
| Hyaluronic acid and basic fibroblast growth factors (bFGF) | Chondrocytes and synovial cells | Japanese white rabbits | Increased cell proliferation, faster defect closure | [30] | |
| Platelet-rich plasma | An in situ photocrosslinkable PRP hydrogel glue (HNPRP) | Human chondrocyte and L929 mouse fibroblast cells | New Zealand rabbits | Rapid gelation, increased matrix production | [31] |
| 10% Calcium chloride-activated PRP | Bone marrow-derived mesenchymal stem cells (BMSCs) | Mature male rabbits | Enhanced osteogenic differentiation | [32,33] | |
| Silk-fibroin with PRP scaffolds (SF-PRP) (50% PRP, v/v) | The rabbit chondrocytes | New Zealand white rabbits | Increased tensile strength, improved healing | [34,35] | |
| Black phosphorus nanosheets (BPNs)- platelet-rich plasma (PRP)-chitosan (BPNs/Chitosan/PRP) | RAW264.7 cells, L929 cells, and MSC cells | DBA1/J mice | Antibacterial and pro-regenerative properties | [36] | |
| Bone marrow stimulation (BMS) with activated PRP | Fibrocartilage cells | Sheep and minipig model | Increased fibrocartilage formation | [37] | |
| Growth factors | Cytokine interleukin-1 (IL-1) in matrix metalloproteinase (MMP) | Meniscal cells | Murine model | Increased cell migration, ECM synthesis | [38] |
| Platelet-derived growth factor (PDGF)-coated decellularized meniscus scaffold (DCM) | Human avascular meniscus cells | - | Increased cell migration, ECM synthesis | [39] | |
| Mesenchymal stem cells | MSCs cultured in PRP | Articular chondrocytes | Rat model | Increased chondrogenic markers | [15,40] |
| Decellularized skin matrix with PFSSTKT (PFS) peptide and mesenchymal stem cells | Murine fibroblasts (L929), rat adipose-derived mesenchymal stem cells (ADSCs) | New Zealand White rabbit | Increased cell infiltration, ECM deposition | [41] | |
| Transplanted autologous synovial MSCs | Synovial MSCs | Human | Clinical improvement, MRI-confirmed repair | [42] | |
| Synovial mesenchymal stem cells | Synovial MSCs | Minipig models | Robust tissue integration | [43,44] | |
| Bone Marrow Aspirate | Bone Marrow Aspirate Concentrate | - | Human | Generation of meniscus-like tissue, integration | [19] |
| Decellularized meniscus extracellular matrix (mECM) hydrogel | Adult mesenchymal stem cells | An SD rat model | Increased fibrocartilage formation | [45] | |
| Silk fibroin-MSCs porous scaffolds | Bone marrow mesenchymal stem cells | New Zealand white rabbits | Mechanical reinforcement and regeneration | [46] | |
| GC/4- arm PEG-CHO hydrogel | Bone mesenchymal stromal cells (BMSCs) | New Zealand White rabbits | Controlled release, improved healing | [47] | |
| Chondroitin sulfate succinimide succinate (CS-NHS) and bone marrow aspirate hydrogels (CS-BM) | Meniscus fibrochondrocytes | The athymic rat model and the rabbit femoral defect model | Increased matrix deposition, reduced OA | [48,49] | |
| Collagen | Human type I collagen combined with autologous platelet-rich plasma (STR/PRP) | Fibroblast | Human | Increased proliferation and matrix production, enhanced healing response | [20] |
| Collagen type I (Col I) and activated chondroitin sulfate hydrogels | Chondrocytes | SD rats | Promoted chondrogenesis, increased ECM synthesis and cartilage-like tissue repair | [50] | |
| Collagen with poly (lactic-co-glycolic acid) (PLGA) microparticles hydrogel | Human umbilical vein endothelial cells (GFP-HUVECs) | - | Supported angiogenesis, increased cell adhesion and viability | [51] | |
| Riboflavin-induced photo-crosslinking of collagen hydrogel | Fibrochondrocyte cells | New Zealand white rabbit | Increased biomechanical strength, improved tissue integration | [52] | |
| Holomycin (HL) | Murine primary chondrocytes | OA mouse model | Anti-inflammatory effects, protected cartilage matrix | [21] | |
| Silk fibroin | Silk fibroin and gelatin methacrylate with encapsulated platelet-rich plasma (PRP) | BMSCs, chondrocytes, | SD rats | Increased chondrogenic differentiation, promoted cartilage repair | [53] |
| Glycidyl methacrylate (GMA)-modified silk fibroin hydrogel | BMSCs | New Zealand rabbits | Enhanced scaffold elasticity, supported cartilage regeneration | [54] | |
| Silk fibroin hydrogel crosslinked by diglycidyl ether (BDDE) | Rat bone mesenchymal stem cells | Male SD rats | Improved mechanical stability, increased cartilage-like ECM deposition | [55] | |
| Silk fibroin/gelatin methacrylate hydrogel | BMSCs | Male SD rats | Supported cell proliferation and chondrogenesis | [53] |
| Biological Additive/Hydrogel | Source/Composition | Delivery Method | Advantages | Limitations | Key Results/Outcomes |
|---|---|---|---|---|---|
| PRP | Autologous platelet concentrate | Direct injection/combined with scaffold | Rich in growth factors, easy preparation | High variability in preparation, short-term activity | Enhanced chondrogenesis, improved short-term healing, and inconsistent long-term results |
| MSCs | Bone marrow/adipose/synovial tissue | Injection/hydrogel encapsulation | Differentiation potential, immunomodulation | Regulatory and safety concerns | Improved healing rates in animal studies, increased matrix deposition |
| Hyaluronic Acid (HA) Hydrogels | Natural polysaccharide | Injectable hydrogel | Biocompatible, lubrication, chondroprotective | Weak mechanical properties | Improved lubrication, reduced inflammation, and early clinical benefits |
| Collagen Hydrogels | Type I/II collagen | Injectable hydrogel/scaffold | Good biocompatibility, a scaffold for cells | Potential immunogenicity | Promoted cell adhesion, enhanced tissue integration |
| Silk Fibroin | Natural protein (silkworms) | Hydrogel/scaffold | Strong mechanical properties, tunable biodegradability | Processing complexity | Provided mechanical reinforcement, supported meniscal regeneration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuszynska, M.; Skopinska-Wisniewska, J.; Bajek, A. Biological Augmentation of Meniscal Repair: A Review with Insights into Injectable Hydrogel Delivery. Gels 2025, 11, 786. https://doi.org/10.3390/gels11100786
Tuszynska M, Skopinska-Wisniewska J, Bajek A. Biological Augmentation of Meniscal Repair: A Review with Insights into Injectable Hydrogel Delivery. Gels. 2025; 11(10):786. https://doi.org/10.3390/gels11100786
Chicago/Turabian StyleTuszynska, Marta, Joanna Skopinska-Wisniewska, and Anna Bajek. 2025. "Biological Augmentation of Meniscal Repair: A Review with Insights into Injectable Hydrogel Delivery" Gels 11, no. 10: 786. https://doi.org/10.3390/gels11100786
APA StyleTuszynska, M., Skopinska-Wisniewska, J., & Bajek, A. (2025). Biological Augmentation of Meniscal Repair: A Review with Insights into Injectable Hydrogel Delivery. Gels, 11(10), 786. https://doi.org/10.3390/gels11100786







