pH-Responsive Modified Dextran Nanogel for Liver Targeted Doxorubicin Delivery
Abstract
1. Introduction
2. Results and Discussion
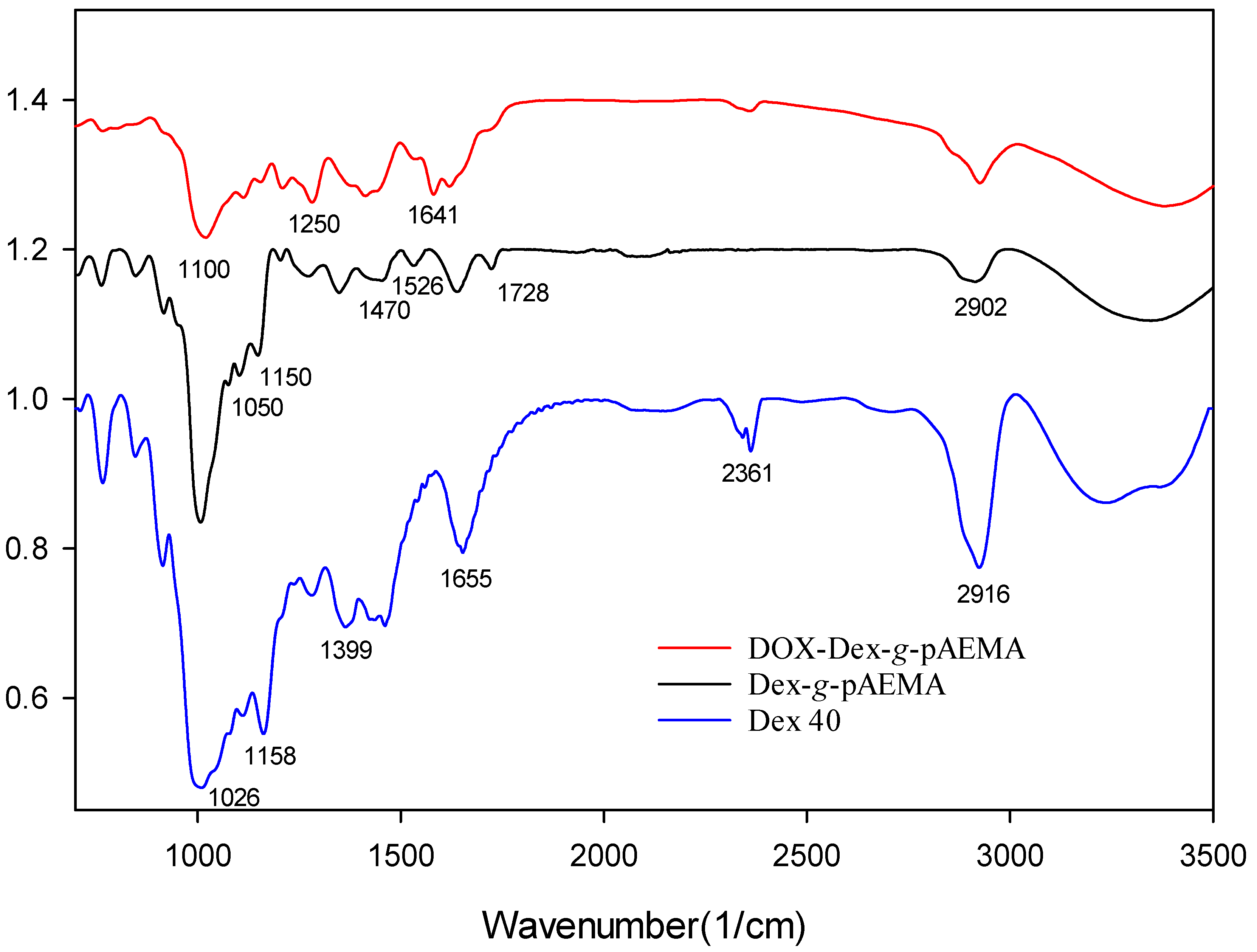
2.1. Synthesis and Characterization of the Polymers
2.2. Gel Permeation Chromatography (GPC)
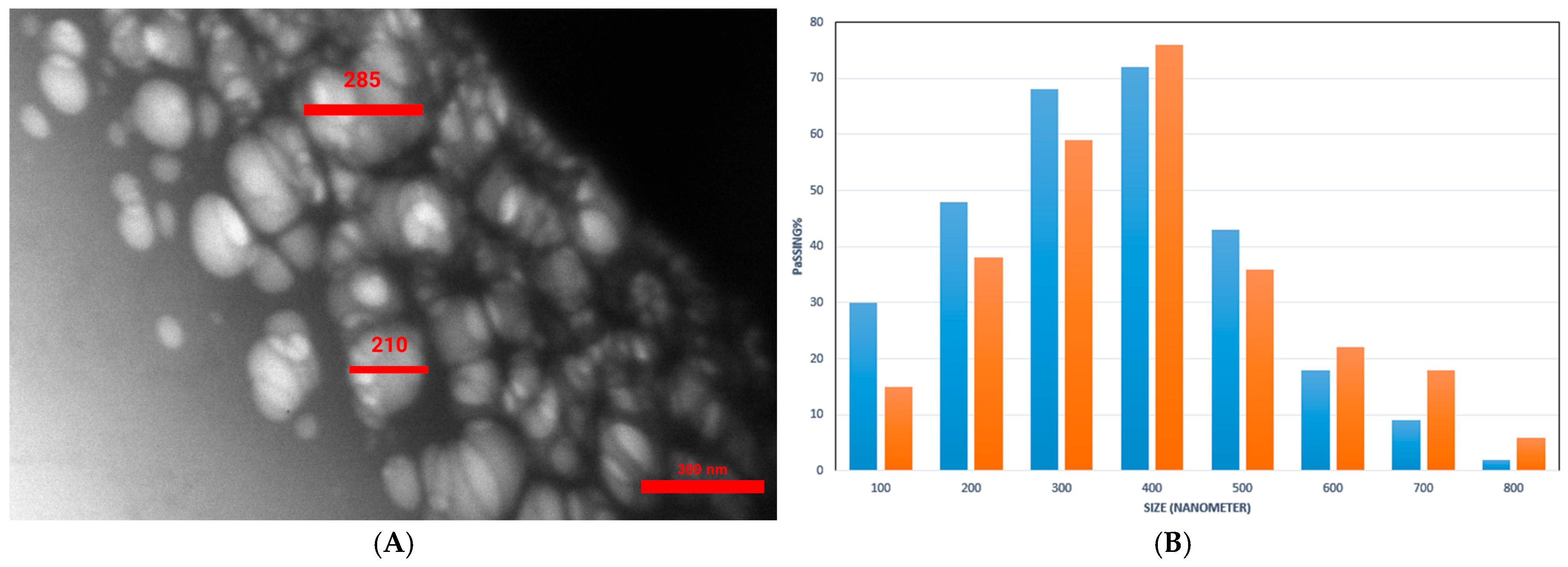
2.3. Morphology and Size Assessment
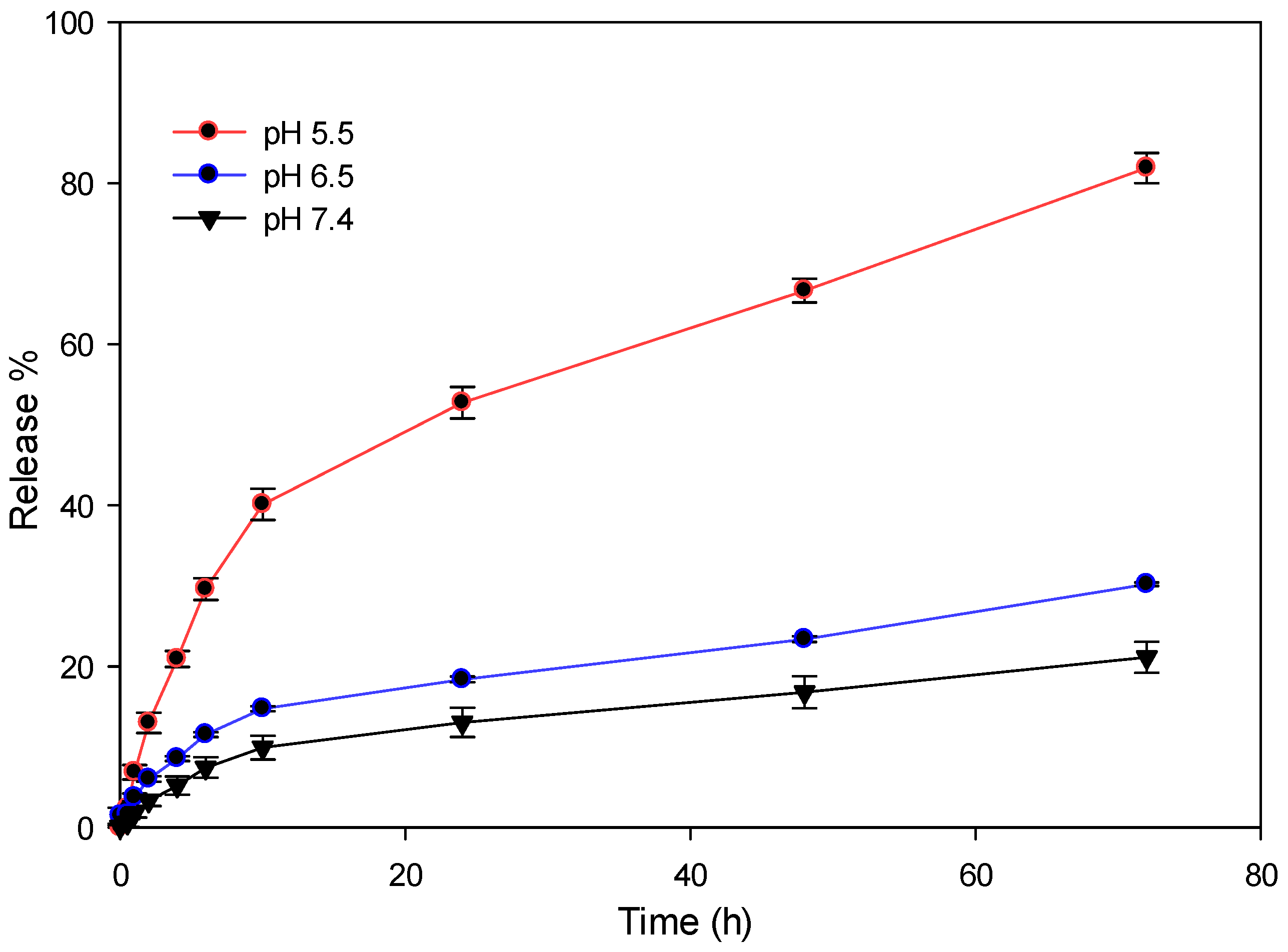
2.4. DOX Conjugation and Release Pattern
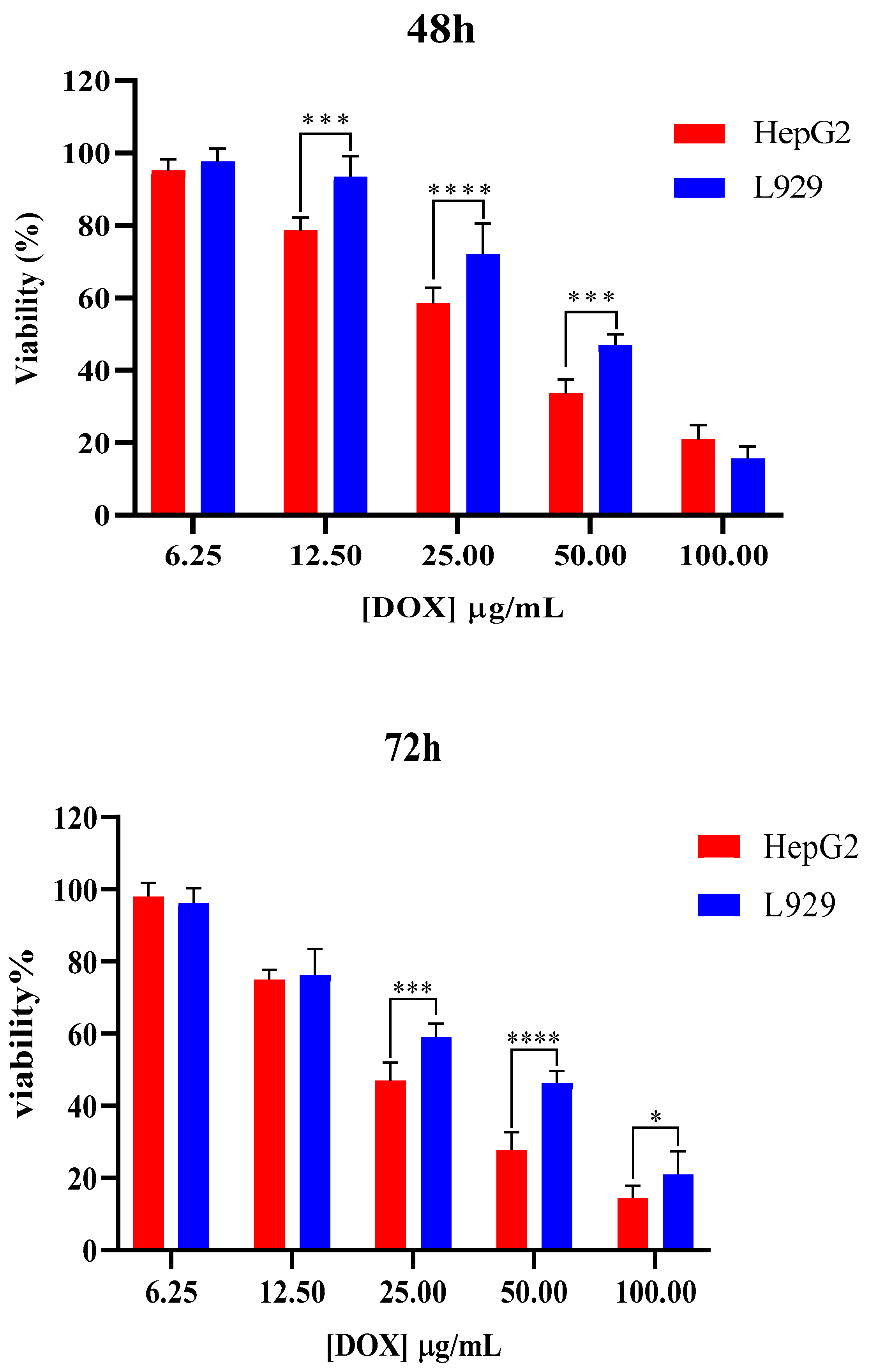
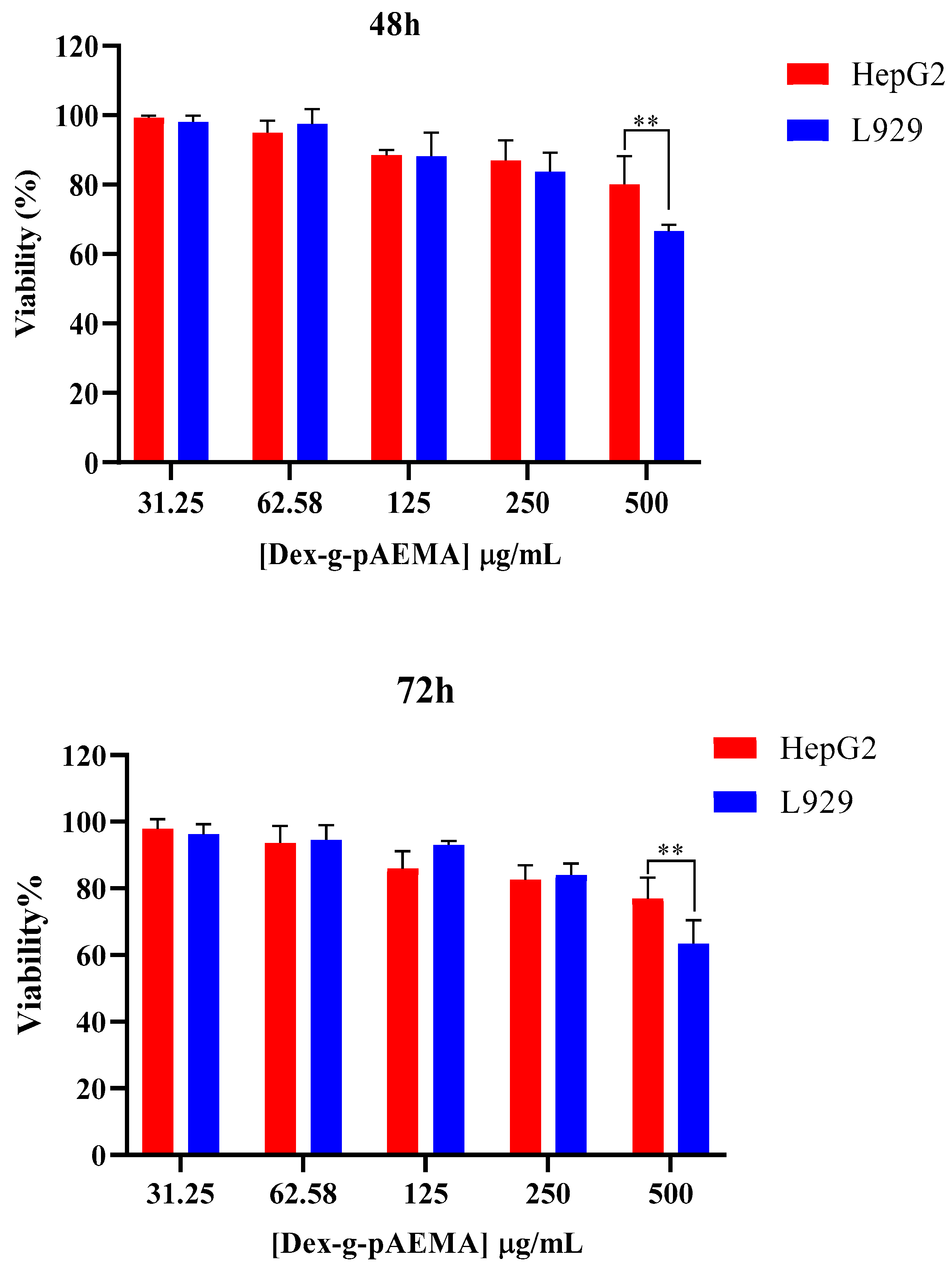
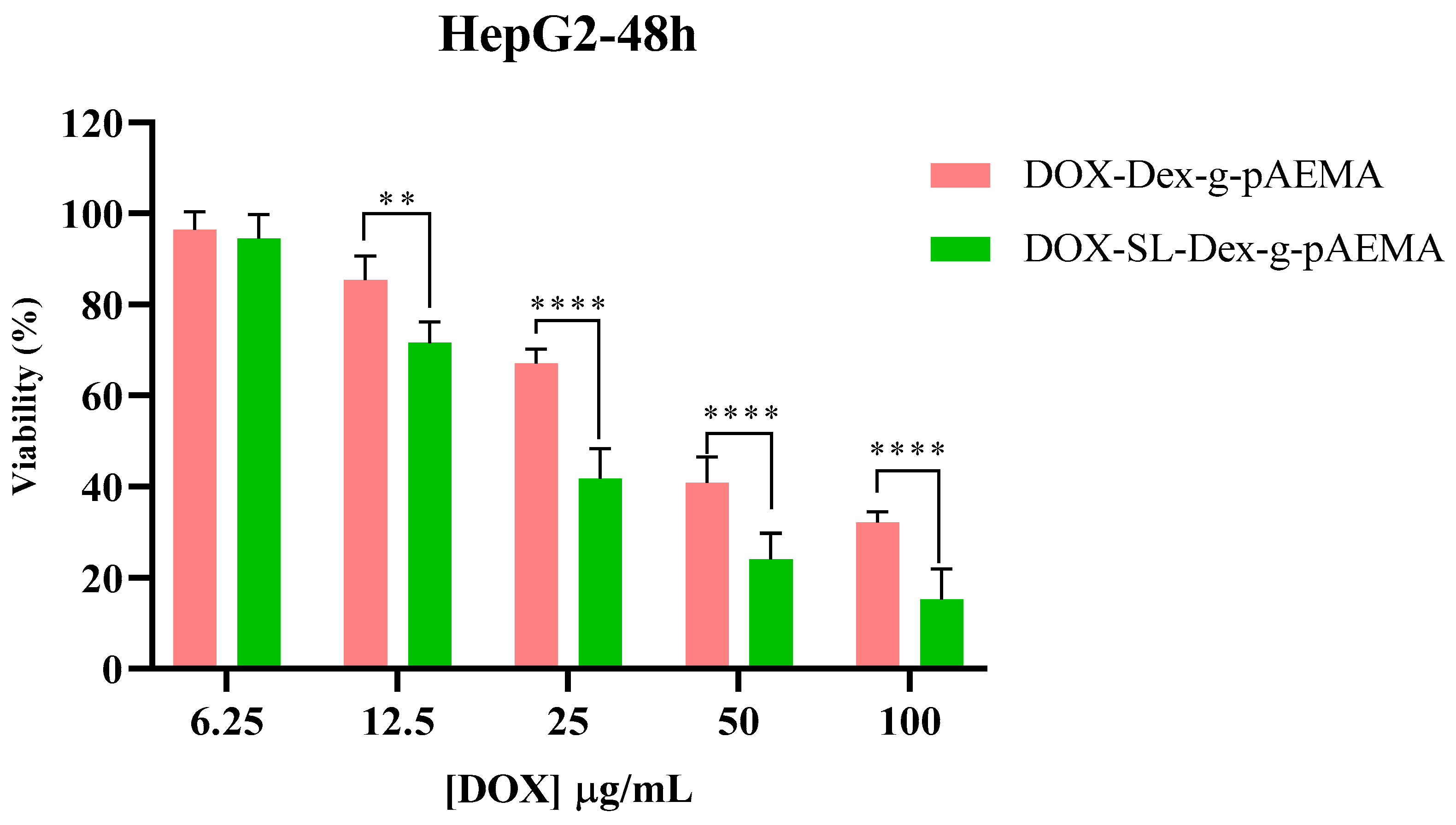
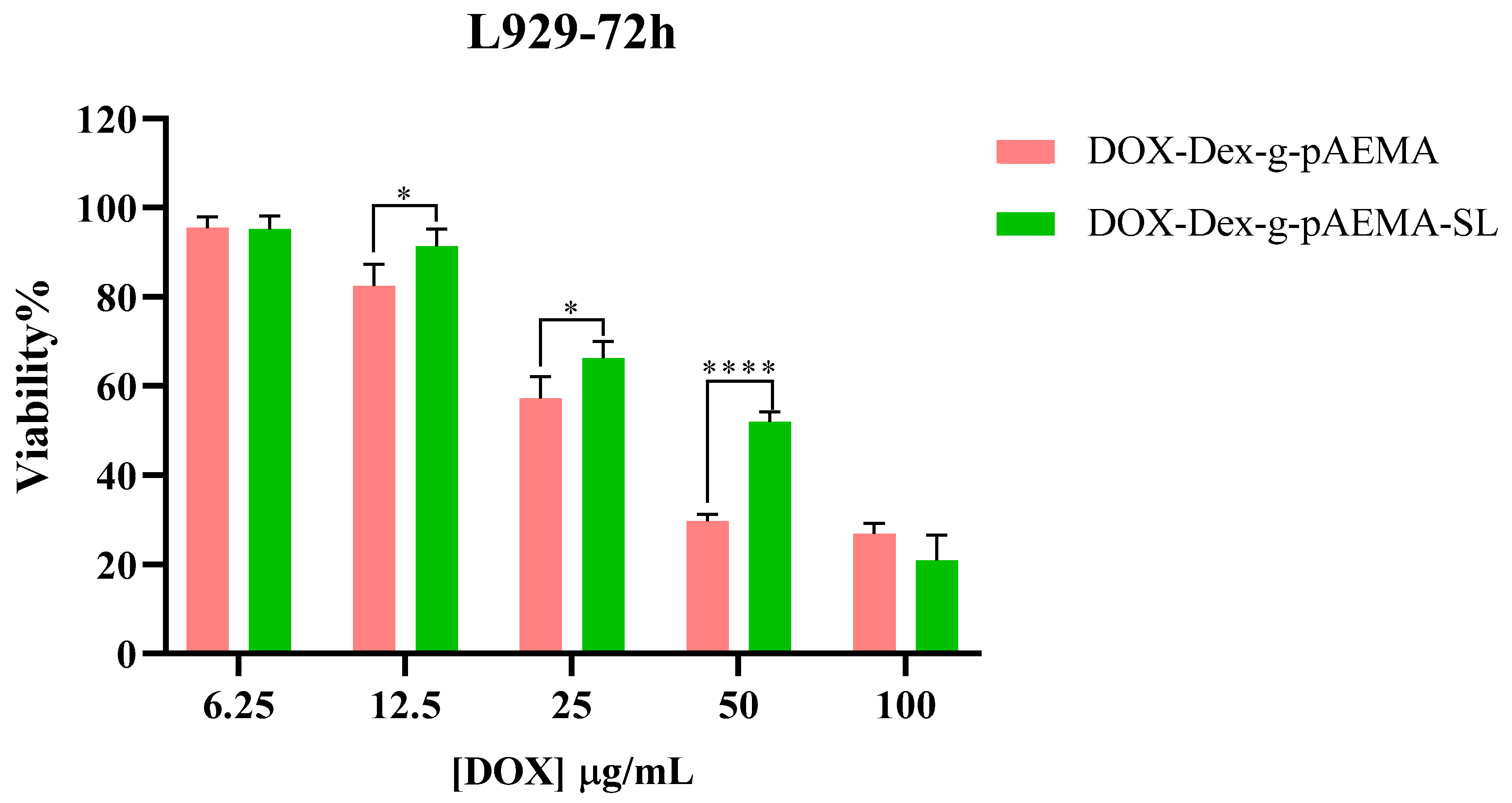
2.5. In Vitro Toxicity Evaluation
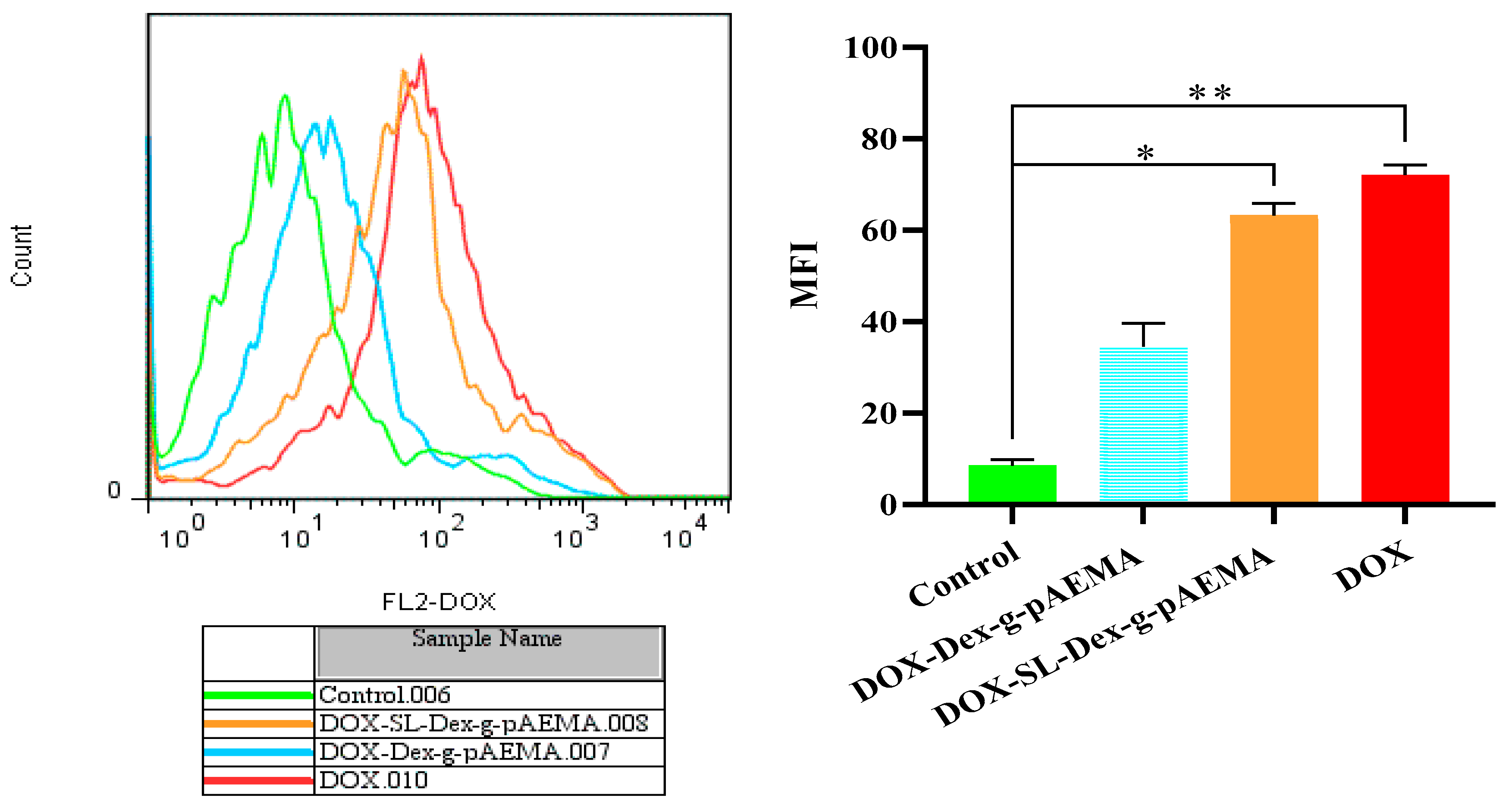
2.6. Cellular Uptake Study
2.7. Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials and Instruments
4.2. Synthesis of NG
4.2.1. Synthesis of Dextran-Grafted Poly(Aminoethyl) Methacrylate (Dex-g-pAEMA)
4.2.2. Conjugation of DOX (DOX-Dex-g-pAEMA)
Preparation of DOX Calibration Standard Curve
4.2.3. Conjugation of Potassium Lactobionate (SL-Dex-g-pAEMA)
4.2.4. Conjugation of DOX (DOX-SL-Dex-g-pAEMA)
4.3. Release Pattern of DOX
4.4. In Vitro Cellular Studies
4.5. Cellular Uptake Study
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rivankar, S. An overview of doxorubicin formulations in cancer therapy. J. Cancer Res. Ther. 2014, 10, 853–858. [Google Scholar] [CrossRef]
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global burden of primary liver cancer in 2020 and predictions to 2040. J. Hepatol. 2022, 77, 1598–1606. [Google Scholar] [CrossRef]
- Cao, C.; Li, Y.; Shi, F.; Jiang, S.; Li, Y.; Yang, L.; Zhou, X.; Gao, Y.; Tang, F.; Li, H. Nano co-delivery of doxorubicin and plumbagin achieves synergistic chemotherapy of hepatocellular carcinoma. Int. J. Pharm. 2024, 661, 124424. [Google Scholar] [CrossRef]
- Carvalho, C.; Santos, R.X.; Cardoso, S.; Correia, S.; Oliveira, P.J.; Santos, M.S.; Moreira, P.I. Doxorubicin: The good, the bad and the ugly effect. Curr. Med. Chem. 2009, 16, 3267–3285. [Google Scholar] [CrossRef]
- D’Angelo, N.A.; Noronha, M.A.; Camara, M.C.; Kurnik, I.S.; Feng, C.; Araujo, V.H.; Santos, J.H.; Feitosa, V.; Molino, J.V.; Rangel-Yagui, C.O. Doxorubicin nanoformulations on therapy against cancer: An overview from the last 10 years. Biomater. Adv. 2022, 133, 112623. [Google Scholar] [CrossRef] [PubMed]
- Kuskov, A.N.; Kukovyakina, E.V. Nanotechnology-Based Drug Delivery Systems, 2nd Edition. Pharmaceutics 2025, 17, 110. [Google Scholar] [CrossRef]
- Emeihe, E.V.; Nwankwo, E.I.; Ajegbile, M.D.; Olaboye, J.A.; Maha, C.C. Revolutionizing drug delivery systems: Nanotechnology-based approaches for targeted therapy. Int. J. Life Sci. Res. Arch. 2024, 7, 40–58. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Fatehbasharzad, P.; Ivanchenko, P.; El Samrout, O.; Morales, J.G. Relevant Properties of Metallic and Non-Metallic Nanomaterials in Biomedical Applications. In Nanomaterials in Healthcare; CRC Press: Boca Raton, FL, USA, 2023; pp. 175–194. [Google Scholar]
- Ghasemi, S.; Ahmadi, L.; Farjadian, F. Thermo-responsive PNIPAAm-b-PLA amphiphilic block copolymer micelle as nanoplatform for docetaxel drug release. J. Mater. Sci. 2022, 57, 17433–17447. [Google Scholar]
- Farjadian, F.; Mirkiani, S.; Ghasemiyeh, P.; Kafshboran, H.R.; Mehdi-Alamdarlou, S.; Raeisi, A.; Esfandiarinejad, R.; Soleymani, S.; Goshtasbi, G.; Firouzabadi, N.; et al. Smart nanogels as promising platform for delivery of drug, gene, and vaccine; therapeutic applications and active targeting mechanism. Eur. Polym. J. 2024, 219, 113400. [Google Scholar] [CrossRef]
- Sasaki, Y.; Akiyoshi, K. Nanogel engineering for new nanobiomaterials: From chaperoning engineering to biomedical applications. Chem. Rec. 2010, 10, 366–376. [Google Scholar] [CrossRef]
- Laftah, W.A.; Hashim, S.; Ibrahim, A.N. Polymer hydrogels: A review. Polym.-Plast. Technol. Eng. 2011, 50, 1475–1486. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Asadi, K.; Samiraninezhad, N.; Akbarizadeh, A.R.; Amini, A.; Gholami, A. Stimuli-responsive hydrogel based on natural polymers for breast cancer. Front. Chem. 2024, 12, 1325204. [Google Scholar] [CrossRef]
- Huang, S.; Huang, G. The dextrans as vehicles for gene and drug delivery. Future Med. Chem. 2019, 11, 1659–1667. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Mao, J.J. Engineering dextran-based scaffolds for drug delivery and tissue repair. Nanomedicine 2012, 7, 1771–1784. [Google Scholar] [CrossRef]
- Grebinyk, A.; Prylutska, S.; Grebinyk, S.; Ponomarenko, S.; Virych, P.; Chumachenko, V.; Kutsevol, N.; Prylutskyy, Y.; Ritter, U.; Frohme, M. Drug delivery with a pH-sensitive star-like dextran-graft polyacrylamide copolymer. Nanoscale Adv. 2022, 4, 5077–5088. [Google Scholar] [CrossRef] [PubMed]
- Prabu, P.; Chaudhari, A.A.; Dharmaraj, N.; Khil, M.; Park, S.; Kim, H. Preparation, characterization, in-vitro drug release and cellular uptake of poly (caprolactone) grafted dextran copolymeric nanoparticles loaded with anticancer drug. J. Biomed. Mater. Res. Part A 2009, 90, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Lorscheider, M.; Gaudin, A.; Nakhlé, J.; Veiman, K.-L.; Richard, J.; Chassaing, C. Challenges and opportunities in the delivery of cancer therapeutics: Update on recent progress. Ther. Deliv. 2021, 12, 55–76. [Google Scholar] [CrossRef]
- Yu, J.; Liu, Y.; Zhang, Y.; Ran, R.; Kong, Z.; Zhao, D.; Liu, M.; Zhao, W.; Cui, Y.; Hua, Y. Smart nanogels for cancer treatment from the perspective of functional groups. Front. Bioeng. Biotechnol. 2024, 11, 1329311. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Nayak, P. pH-responsive polymers for drug delivery: Trends and opportunities. J. Polym. Sci. 2023, 61, 2828–2850. [Google Scholar]
- Li, Z.; Huang, J.; Wu, J. pH-Sensitive nanogels for drug delivery in cancer therapy. Biomater. Sci. 2021, 9, 574–589. [Google Scholar] [CrossRef]
- Ghasemi, S.; Najafi, M.; Doroudian, M.; Rastegari, B.; Behzad-Behbahani, A.; Soltanimehr, H.; Farjadian, F. Temperature-and pH-Responsive Poly (NIPAM-co-HEMA-co-AAm) Nanogel as a Smart Vehicle for Doxorubicin Delivery; Combating Colorectal Cancer. Gels 2025, 11, 227. [Google Scholar] [CrossRef]
- Entezar-Almahdi, E.; Heidari, R.; Ghasemi, S.; Mohammadi-Samani, S.; Farjadian, F. Integrin receptor mediated pH-responsive nano-hydrogel based on histidine-modified poly (aminoethyl methacrylamide) as targeted cisplatin delivery system. J. Drug Deliv. Sci. Technol. 2021, 62, 102402. [Google Scholar] [CrossRef]
- Farzanfar, J.; Farjadian, F.; Roointan, A.; Mohammadi-Samani, S.; Tayebi, L. Assessment of pH responsive delivery of methotrexate based on PHEMA-st-PEG-DA nanohydrogels. Macromol. Res. 2021, 29, 54–61. [Google Scholar] [CrossRef]
- Hashizume, H.; Baluk, P.; Morikawa, S.; McLean, J.W.; Thurston, G.; Roberge, S.; Jain, R.K.; McDonald, D.M. Openings between defective endothelial cells explain tumor vessel leakiness. Am. J. Pathol. 2000, 156, 1363–1380. [Google Scholar] [CrossRef]
- Shreya, A.; Raut, S.Y.; Managuli, R.S.; Udupa, N.; Mutalik, S. Active targeting of drugs and bioactive molecules via oral administration by ligand-conjugated lipidic nanocarriers: Recent advances. AAPS PharmSciTech 2019, 20, 15. [Google Scholar] [CrossRef]
- Wang, X.; Huang, M.; Li, L.; Song, M.; Gong, R. Enhanced cellular uptake and cytotoxicity of doxorubicin by self-assembled lactobionate-phytosterol-alginate nanoparticles. J. Nanosci. Nanotechnol. 2017, 17, 4558–4566. [Google Scholar]
- Li, M.; Zhang, W.; Wang, B.; Gao, Y.; Song, Z.; Zheng, Q.C. Ligand-based targeted therapy: A novel strategy for hepatocellular carcinoma. Int. J. Nanomed. 2016, 2016, 5645–5669. [Google Scholar] [CrossRef]
- Li, Y.; Huang, G.; Diakur, J.; Wiebe, L.I. Targeted delivery of macromolecular drugs: Asialoglycoprotein receptor (ASGPR) expression by selected hepatoma cell lines used in antiviral drug development. Curr. Drug Deliv. 2008, 5, 299–302. [Google Scholar] [CrossRef]
- Wu, T.; Yu, S.; Lin, D.; Wu, Z.; Xu, J.; Zhang, J.; Ding, Z.; Miao, Y.; Liu, T.; Chen, T. Preparation, characterization, and release behavior of doxorubicin hydrochloride from dual cross-linked chitosan/alginate hydrogel beads. ACS Appl. Bio Mater. 2020, 3, 3057–3065. [Google Scholar] [CrossRef]
- Tavassolian, F.; Kamalinia, G.; Rouhani, H.; Amini, M.; Ostad, S.N.; Khoshayand, M.R.; Atyabi, F.; Tehrani, M.R.; Dinarvand, R. Targeted poly (l-γ-glutamyl glutamine) nanoparticles of docetaxel against folate over-expressed breast cancer cells. Int. J. Pharm. 2014, 467, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jin, X.; Li, H.; Zhang, R.-R.; Wu, C.-W. Injectable and body temperature sensitive hydrogels based on chitosan and hyaluronic acid for pH sensitive drug release. Carbohydr. Polym. 2018, 186, 82–90. [Google Scholar] [CrossRef]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Validation of in-vitro bioassay methods: Application in herbal drug research. Profiles Drug Subst. Excip. Relat. Methodol. 2021, 46, 273–307. [Google Scholar]
- Subhan, M.A.; Yalamarty, S.S.K.; Filipczak, N.; Parveen, F.; Torchilin, V.P. Recent advances in tumor targeting via EPR effect for cancer treatment. J. Pers. Med. 2021, 11, 571. [Google Scholar] [CrossRef]
- Wang, S.-N.; Deng, Y.-H.; Xu, H.; Wu, H.-B.; Qiu, Y.-K.; Chen, D.-W. Synthesis of a novel galactosylated lipid and its application to the hepatocyte-selective targeting of liposomal doxorubicin. Eur. J. Pharm. Biopharm. 2006, 62, 32–38. [Google Scholar] [CrossRef]
- Delagustin, M.G.; Goncalves, E.; Carra, S.; Barcellos, T.; Bassani, V.L.; da Silveira, M.M.; Malvessi, E. Sodium, potassium, calcium lactobionates, and lactobionic acid from Zymomonas mobilis: A novel approach about stability and stress tests. J. Pharm. Biomed. Anal. 2019, 174, 104–114. [Google Scholar] [CrossRef]
- Tilsed, C.M.; Fisher, S.A.; Nowak, A.K.; Lake, R.A.; Lesterhuis, W.J. Cancer chemotherapy: Insights into cellular and tumor microenvironmental mechanisms of action. Front. Oncol. 2022, 12, 960317. [Google Scholar] [CrossRef] [PubMed]
- Farjadian, F.; Rezaeifard, S.; Naeimi, M.; Ghasemi, S.; Mohammadi-Samani, S.; Welland, M.E.; Tayebi, L. Temperature and pH-responsive nano-hydrogel drug delivery system based on lysine-modified poly (vinylcaprolactam). Int. J. Nanomed. 2019, 2019, 6901–6915. [Google Scholar] [CrossRef] [PubMed]





| Cell Line | HepG2 | L929 | ||
|---|---|---|---|---|
| Sample (µg/mL) | 48 | 72 | 48 | 72 |
| DOX | 39.27 ± 2.21 | 24.64 ± 1.96 | 50.28 ± 3.24 | 48.98 ± 2.09 |
| SI * | 1.28 | 1.99 | - | - |
| DOX-Dex-pAEMA | 44.99 ± 2.95 | 45.16 ± 2.72 | 44.97 ± 1.69 | 37.38 ± 4.32 |
| SI | 0.99 | 0.83 | - | - |
| DOX-SL-Dex-g-pAEMA | 26.67 ± 1.65 | 22.65 ± 2.56 | 63.91 ± 3.36 | 59.71 ± 3.07 |
| SI | 2.40 | 2.64 | - | - |
| Dex-g-pAEMA | >500 | >500 | >500 | >500 |
| SI | 1.00 | 1.00 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raeisi, A.; Doroudian, M.; Rastegari, B.; Mohammadi-Samani, S.; Behzad-Behbahani, A.; Farjadian, F. pH-Responsive Modified Dextran Nanogel for Liver Targeted Doxorubicin Delivery. Gels 2025, 11, 784. https://doi.org/10.3390/gels11100784
Raeisi A, Doroudian M, Rastegari B, Mohammadi-Samani S, Behzad-Behbahani A, Farjadian F. pH-Responsive Modified Dextran Nanogel for Liver Targeted Doxorubicin Delivery. Gels. 2025; 11(10):784. https://doi.org/10.3390/gels11100784
Chicago/Turabian StyleRaeisi, Amin, Mohammad Doroudian, Banafsheh Rastegari, Soliman Mohammadi-Samani, Abbas Behzad-Behbahani, and Fatemeh Farjadian. 2025. "pH-Responsive Modified Dextran Nanogel for Liver Targeted Doxorubicin Delivery" Gels 11, no. 10: 784. https://doi.org/10.3390/gels11100784
APA StyleRaeisi, A., Doroudian, M., Rastegari, B., Mohammadi-Samani, S., Behzad-Behbahani, A., & Farjadian, F. (2025). pH-Responsive Modified Dextran Nanogel for Liver Targeted Doxorubicin Delivery. Gels, 11(10), 784. https://doi.org/10.3390/gels11100784











