Columnar Mesophases and Organogels Formed by H-Bound Dimers Based on 3,6-Terminally Difunctionalized Triphenylenes
Abstract
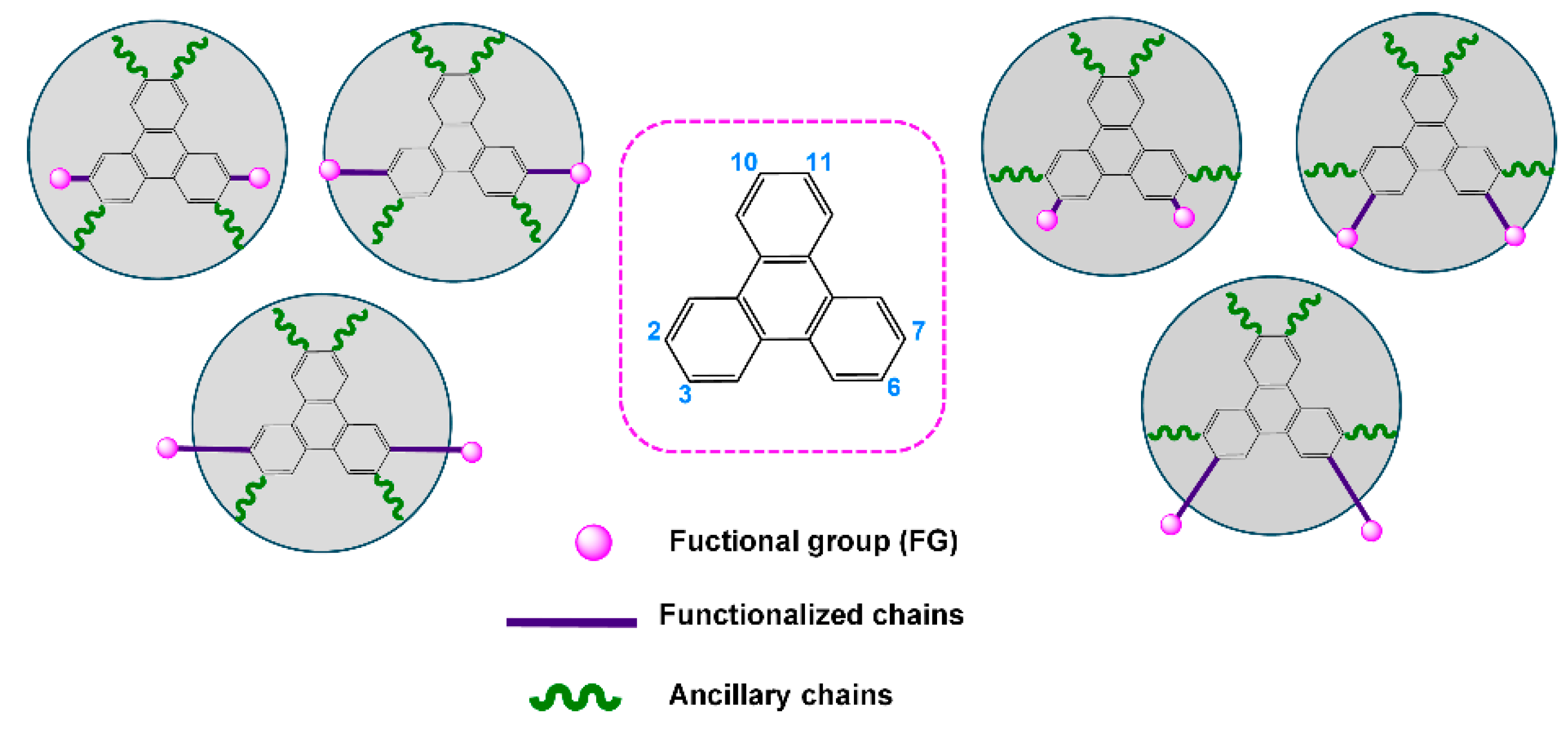
1. Introduction
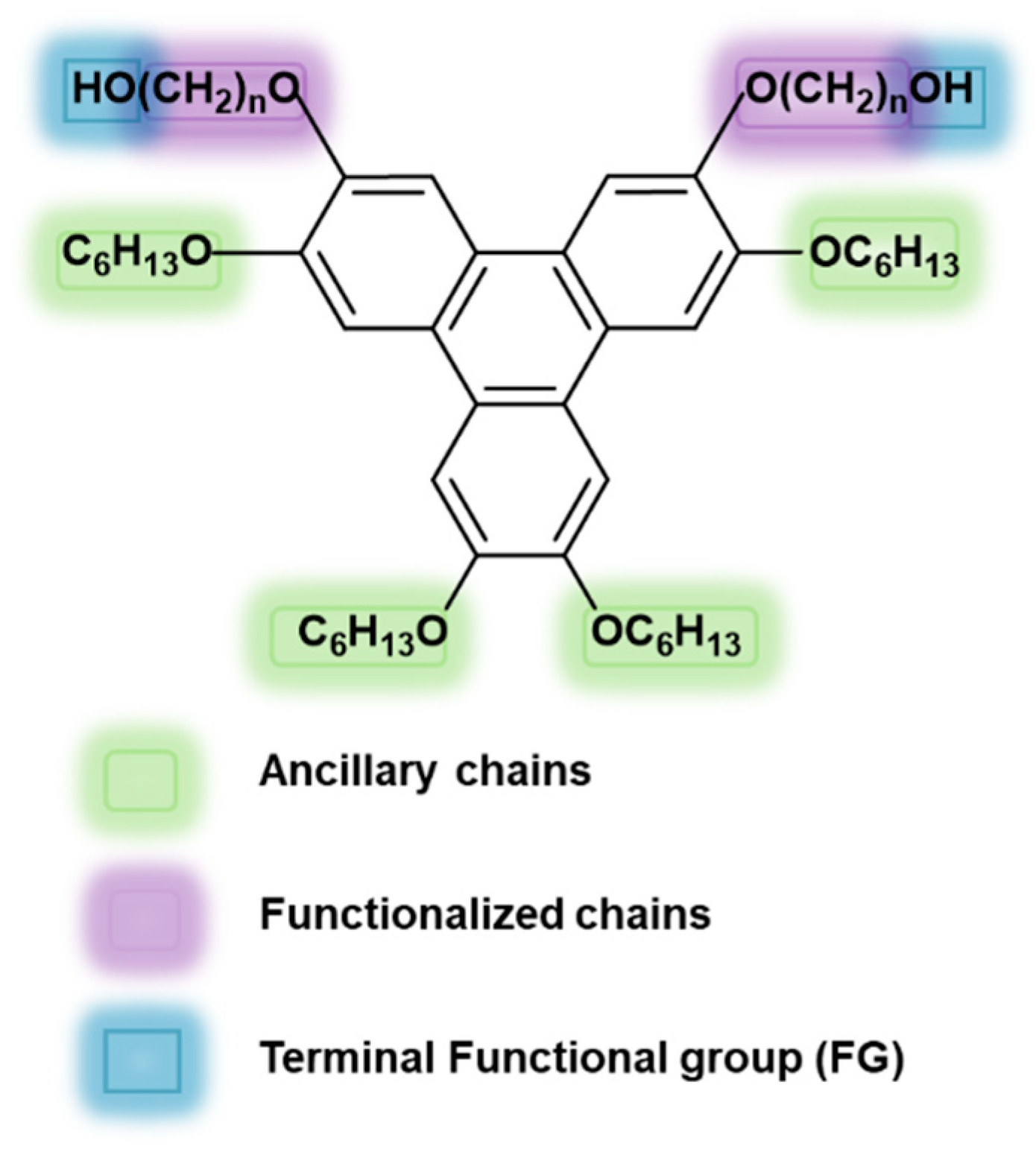
2. Results and Discussion
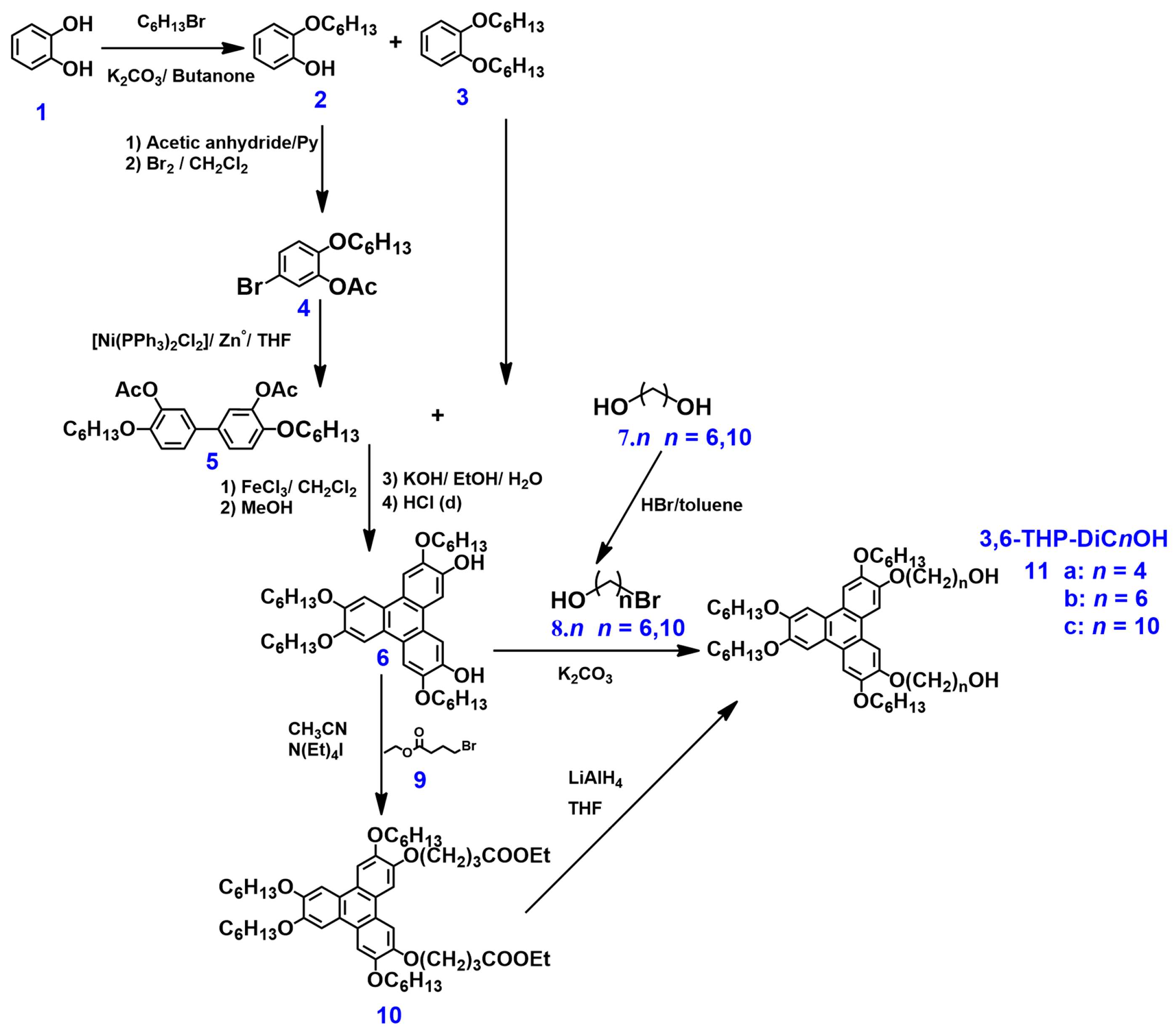
2.1. Synthesis and Characterization
2.2. Mesomorphic Properties
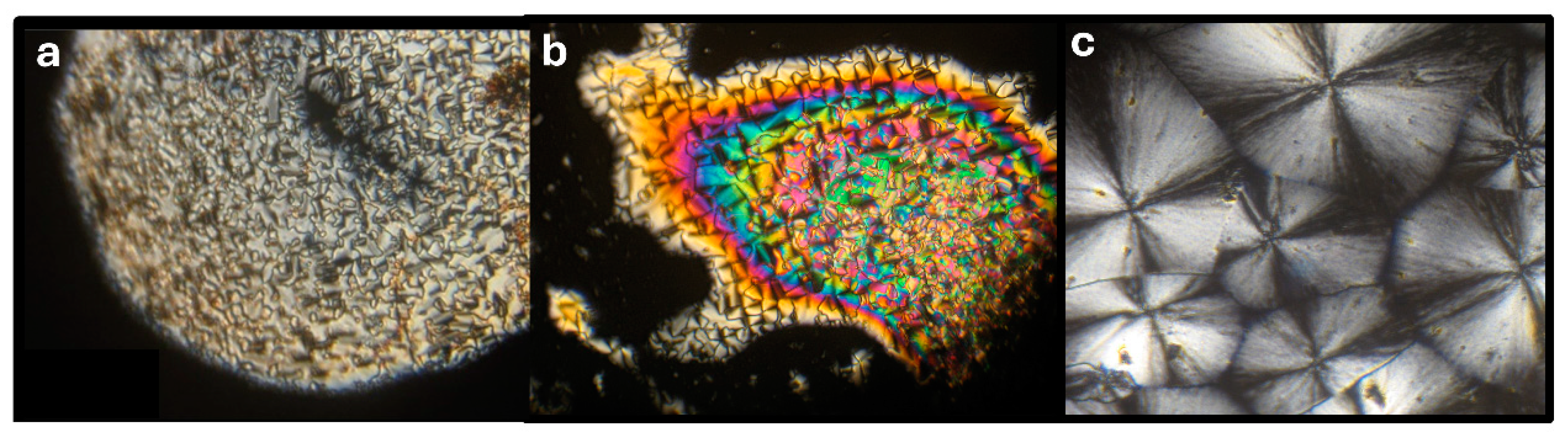
2.3. Organogelating Ability
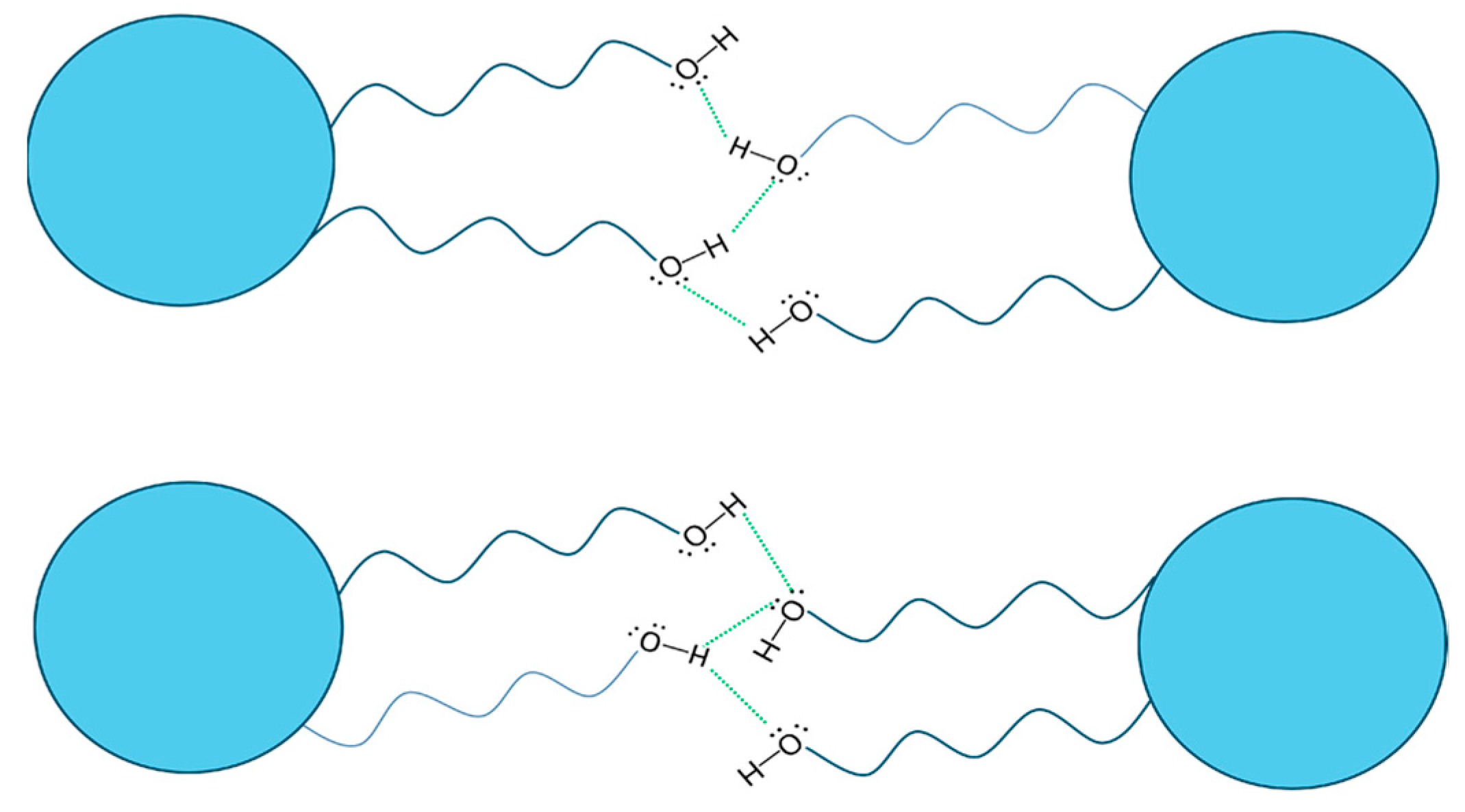
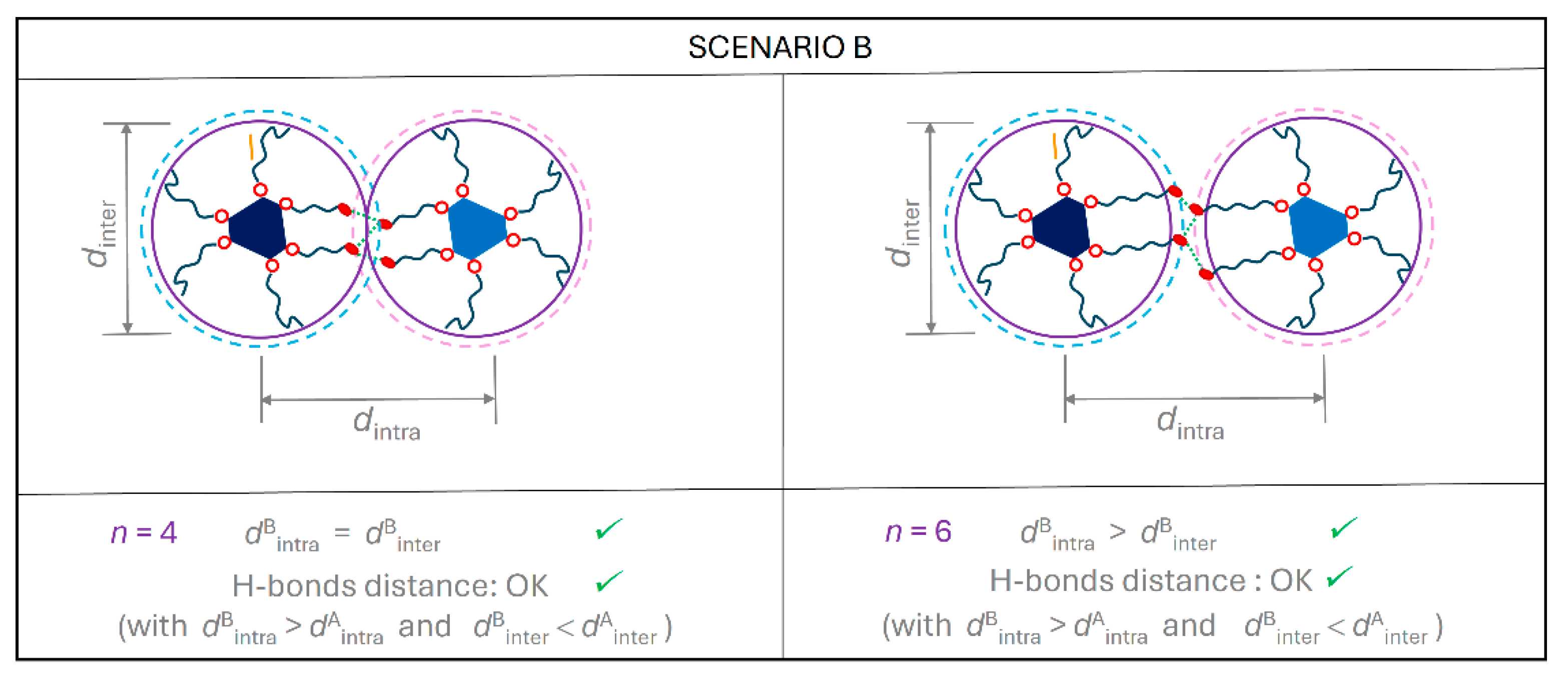
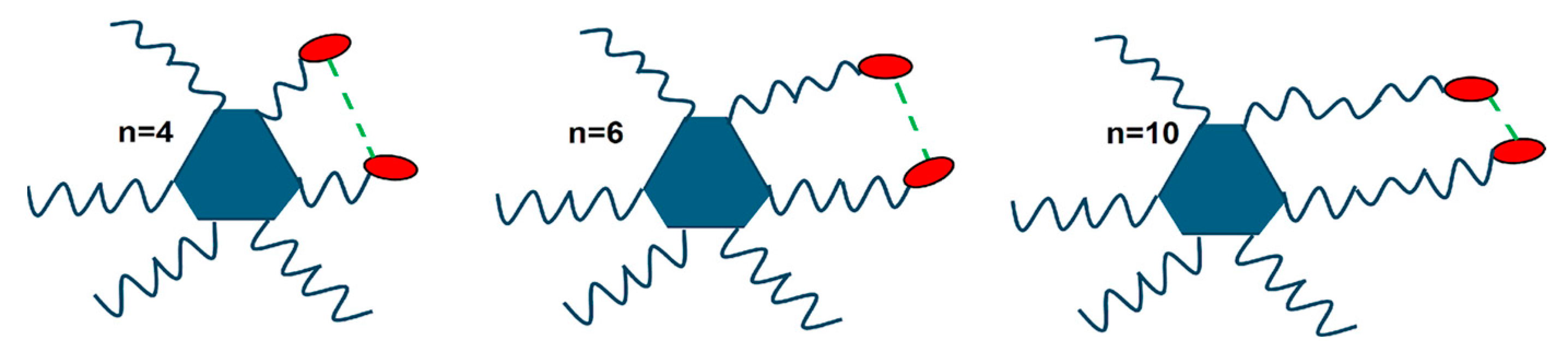
2.4. Supramolecular Models for the Columnar Mesophases and Organogels
2.5. Comparison with Related Compounds: Influence of the Molecular Blocks on Self-Organizing Ability
3. Conclusions
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Handbook of Liquid Crystals, 2nd ed.; Goodby, J.W., Collings, P.J., Kato, T., Tschierske, C., Gleeson, H.F., Raynes, P., Eds.; Wiley-VCH Verlag GmbH & Co.: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Weiss, R.G. The Past, Present and Future of Molecular Gels. What Is the Status of the Fuield, and Where It Is Going? J. Am. Chem. Soc. 2014, 136, 7519–7530. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.J. Personal Perspective on Understanding Low Molecular Weight Gels. J. Am. Chem. Soc. 2022, 144, 11047–11053. [Google Scholar] [CrossRef] [PubMed]
- Kubo, K.; Mori, A.; Ujie, S.; Tschierske, C. Synthesis and Properties of Columnar Liquid Crystals and Organogelators with a Bitropone Core. J. Oleo Sci. 2004, 53, 575–579. [Google Scholar] [CrossRef]
- Kuang, G.-C.; Jia, X.-R.; Teng, M.-J.; Chen, E.-Q.; Li, W.-S.; Ji, Y. Organogels and Liquid Crystalline Properties of Amino Acid-Based Dendrons: A Systematic Study on Structure-Property Relationship. Chem. Mater. 2011, 24, 71–80. [Google Scholar] [CrossRef]
- Kato, T.; Hirai, Y.; Nakaso, S.; Moriyama, M. Liquid-crystalline physical gels. Chem. Soc. Rev. 2007, 36, 1857–1867. [Google Scholar] [CrossRef] [PubMed]
- Iguarbe, V.; Romero, P.; Barberá, J.; Elduque, A.; Giménez, R. Dual liquid Crystalline/Gel behavior with AIE effect promoted by Self-assembly of pyrazole dendrons. J. Mol. Liq. 2022, 365, 120109–120118. [Google Scholar] [CrossRef]
- Ziessel, R.; Pickaert, G.; Camerel, F.; Donnio, B.; Guillon, D.; Cesario, H.; Prangé, T. Tuning Organogels and Mesophases with Phenantroline Ligands and Their Copper Complexes by Inter- to Intromolecular Hydrogen Bonds. J. Am. Chem. Soc. 2004, 126, 12403–12413. [Google Scholar] [CrossRef] [PubMed]
- De, J.; Gupta, S.P.; Swayamprahba, S.S.; Dubey, D.K.; Bala, I.; Sarkar, I.; Dey, G.; Jou, J.H.; Ghosh, S.; Pal, S.K. Blue Luminescent Organic Light Emitting Diode Devices of a New Class of Star-Shaped Clumnar Mesogensn Exhibiting π-π Driven Supergelation. J. Phys. Chem. C 2018, 122, 23659–23674. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Cui, W.; Wang, X.; Li, B.; Zhang, Y.; Yang, J. Novel liquid crystalline organogelators based on terephtalic acid and terephtaldehyde derivatives: Properties and promotion through the formation of halogen bonding. New J. Chem. 2020, 44, 614–625. [Google Scholar] [CrossRef]
- Mac Cormack, A.S.; Busch, V.M.; Japas, M.L.; Giovanetti, L.; Di Salvo, F.; Di Chenna, P.H. The effect of vicinal di-halo substituents on the organogelling properties of aromatic supramolecular gelators and their application as soft templates. New J. Chem. 2020, 44, 8198–8208. [Google Scholar] [CrossRef]
- Kubo, K.; Takahashi, H.; Takechi, H. Liquid Crystals as Organogelators: Liquid Crystals Gelled Organic Liquids. J. Oleo Sci. 2006, 55, 545–549. [Google Scholar] [CrossRef]
- Liu, L.; Li, J.; Zhu, H.; Yang, H.; Feng, X.; Xiao, D.; Yang, Y.; Gao, H. A novel multifunctional C3-symmetric triphenylamine discotic liquid crystal: Synthesis, columnar self-assembly, organogel behavior, selective detection of PA and application in Si solar cells. J. Mater. Chem. C 2024, 12, 6450–6456. [Google Scholar] [CrossRef]
- Iqbal, S.; Khan, A.A. Supramolecular self-assembly and physical-gel formation in disc-like liquid crystals: A scalable predictive model for gelation and an application in photovoltaics. RSC Adv. 2019, 9, 6335–6345. [Google Scholar] [CrossRef]
- Dieterich, S.; Sottmann, T.; Giesselmann, F. Gelation of Lyotropic Liquid-Crystal Phases—The Interplay between Liquid Crystalline Order and Physical Gel Formation. Langmuir 2019, 35, 16793–16802. [Google Scholar] [CrossRef] [PubMed]
- Ilincă, T.A.; Ilis, M.; Micutz, M.; Cîrcu, V. Liquid Crystalline and Gel Properties of Luminescent Cyclometalated Palladium Complexes with Benzoylthiourea Ligands. Gels 2023, 9, 777. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.K.; Setia, S.; Avinash, B.S.; Kumar, S. Triphenylene-Based Discotic Liquid Crystals: Recent Advances. Liq. Cryst. 2013, 40, 1769–1816. [Google Scholar] [CrossRef]
- Wöhrle, T.; Wurzbach, I.; Kirres, J.; Kostidou, A.; Kapernaum, N.; Litterscheidt, J.; Haenle, C.; Staffeld, P.; Baro, A.; Giesselmann, F.; et al. Discotic liquid crystals. Chem. Rev. 2016, 116, 1139–1241. [Google Scholar] [CrossRef]
- Alhunayhina, S.M.N.; Bushby, R.J.; Cammidge, A.N.; Samman, S.S. Triphenylene discotic liquid crystals: Biphenyls, synthesis, and the search fornematic systems. Liq. Cryst. 2024, 51, 1333–1344. [Google Scholar] [CrossRef]
- Muñoz Resta, I.; Manzano, V.; Cecchi, F.; Spagnuolo, C.; Cukiernik, F.; Di Chenna, P. Supramolecular Assembly of PH-Sensitive Triphenylene Derived π-Gelators and Their Application as Molecular Template for the Preparation of Silica Nanotubes. Gels 2016, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Meegan, J.E.; Yang, X.; Rungsirisakun, R.; Cosgrove, S.C.; Bushby, R.J.; Sadeghpour, A.; Rappolt, M.; Brydsonc, R.; Ansell, R.J. Synthesis and organogelating behaviour of amino acid-functionalised triphenylenes. Soft Matter 2017, 13, 5922–5932. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Manjuladevi, V.; Karthik, C.; Choudary, K. Thin films of discotic liquid crystals and their applications. Liq. Cryst. 2016, 43, 2079–2091. [Google Scholar] [CrossRef]
- Kumar, S. Triphenylene-based discotic liquid crystal dimers, oligomers and polymers. Liq. Cryst. 2005, 32, 1089–1113. [Google Scholar] [CrossRef]
- Boden, N.; Bushby, R.J.; Cammidge, A.N. Triphenylene-Based Discotic-Liquid-Crystalline Polymers: A Universal, Rational Synthesis. J. Am. Chem. Soc. 1995, 117, 924–927. [Google Scholar] [CrossRef]
- Vadra, N.; Cecchi, F.; Huck-Iriart, C.; Cukiernik, F.D. Thermal Homeotropic Self-Alignment on a Single Substrate of New Series of Discotic Nematic Triphenylene-Based Main-Chain Polyesters. Eur. Polym. J. 2024, 202, 112608. [Google Scholar] [CrossRef]
- Bai, Y.; Lan, C.; Yu, W.-H.; Li, R.-X.; Li, W.; Zhang, K.; Zhao, K.-Q.; Hu, P.; Wei, Y.; Niu, K. Triphenylene Discotic Pd(II) Metallomesogens Based on Triazole Ligands Derived from the Click Reaction. Cryst. Growth Des. 2024, 24, 4045–4056. [Google Scholar] [CrossRef]
- Tritto, E.; Chico, R.; Ortega, J.; Folcia, C.L.; Etxebarria, J.; Coco, S.; Espinet, P. Synergistic π–π and Pt–Pt interactions in luminescent hybrid inorganic/organic dual columnar liquid crystals. J. Mater. Chem. C 2015, 3, 9385–9392. [Google Scholar] [CrossRef]
- Fang, X.; Guo, H.; Yang, F.; Lin, J. Near-infrared fluorescent and columnar liquid crystal: Synthesis, and photophysical and mesomorphic properties of triphenylene-Bodipy-triphenylene triad. RSC Adv. 2017, 7, 23657–23662. [Google Scholar] [CrossRef]
- Talroze, R.V.; Otmakhova, O.A.; Koval, M.A.; Kuptsov, S.A.; Platé, N.A.; Finkelmann, H. Acrylate based polymers and networks containing triphenylene groups: Synthesis and structures. Macromol. Chem. Phys. 2000, 201, 877–881. [Google Scholar] [CrossRef]
- Xing, C.; Lam, J.W.Y.; Zhao, K.; Tang, B.Z. Synthesis and liquid crystalline properties of poly(1-alkyne)s carrying triphenylene discogens. J. Polym. Sci. A Polym. Chem. 2008, 46, 2960–2974. [Google Scholar] [CrossRef]
- Suburu, M.E.G.; Cecchi, F.; D’Accorso, N.; Cukiernik, F.D. Mesomorphic Properties of Linear and Branched New Triphenylene-based Poly(Ester-ether)s. J. Polym. Sci. 2020, 58, 1163–1176. [Google Scholar] [CrossRef]
- El Mansoury, A.; Bushby, R.G.; Karodia, N. Triphenylene-based discotic liquid crystals: Star-shaped oligomers and branched-chain polymers. Liq. Cryst. 2012, 39, 1222–1230. [Google Scholar] [CrossRef]
- Disch, S.; Finkelmann, H.; Ringsdorf, H.; Schuhmacher, P. Macroscopically Ordered Discotic Columnar Networks. Macromolecules 1995, 28, 2424–2428. [Google Scholar] [CrossRef]
- Henderson, P.; Beyer, D.; Jonas, U.; Karthaus, O.; Ringsdorf, H.; Heiney, P.A.; Maliszewskyj, N.C.; Ghosh, S.S.; Mindyuk, O.Y.; Josefowicz, J.Y. Complex Ordering in Thin Films of Di- and Trifunctionalized Hexaalkoxytriphenylene Derivatives. J. Am. Chem. Soc. 1997, 119, 4740–4748. [Google Scholar] [CrossRef]
- Bai, Y.F.; Zhao, K.Q.; Hu, P.; Wang, B.Q.; Shimizu, Y. Synthesis of Amide Grup Containing Triphenylen Derivatives as Discotic Liquid Crystals and Organic Gelators. Mol. Cryst. Liq. Cryst. 2009, 509, 60–76. [Google Scholar] [CrossRef]
- Zelcer, A.; Cecchi, F.; Alborés, P.; Guillon, D.; Heinrich, B.; Donnio, B.; Cukiernik, F.D. A Convenient Synthesis of a 2,7-Difunctional Tetra(Alkoxy)Triphenylene Involving 4,4′-Diacetoxy-3,3′-Dialkoxybiphenyl as a Key Precursor and Its Conversion to Extended Hybrid Mesogenic Compounds. Liq. Cryst. 2013, 40, 1121–1134. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, C.; Pu, J.; Wang, Y. A convenient synthesis method of 3,6-dihydroxy-2,7,10,11-tetrapentyloxytriphenylene from 4,4′-dihydroxybiphenyl with high yield. Liq. Cryst. 2014, 41, 1173–1178. [Google Scholar] [CrossRef]
- Vadra, N.; Suarez, S.A.; Slep, L.D.; Manzano, V.E.; Halac, E.B.; Baggio, R.F.; Cukiernik, F.D. Synthesis and Crystallographic, Spectroscopic and Computational Characterization of 3,3′,4,4′-Substituted Biphenyls: Effects of OR Substituents on the Intra-Ring Torsion Angle. Acta Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 2020, 76, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Vadra, N.; Huck-Iriart, C.; Giovanetti, L.J.; Di Chenna, P.H.; Cukiernik, F.D. Supramolecular Organogels Based on Mesogenic 2,7-Difunctionalized Triphenylenes as a Simple System for Water Content Assessment in Light Alcohols. New J. Chem. 2020, 44, 2423–2434. [Google Scholar] [CrossRef]
- Cammidge, A.N.; Chausson, C.; Gopee, H.; Li, J.; Hughes, D.L. Probing the structural factors influencing columnar mesophase formation and stability in triphenylene discotics. Chem. Commun. 2009, 47, 7375–7377. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.; Aqad, E.; Peterca, M.; Percec, V. Molecular design principles of helical pyramidal chirality self-organized from achiral hexaki(alyloxy)triphenylene. Giant 2023, 13, 100138. [Google Scholar] [CrossRef]
- Zhang, T.R.; Hu, P.G.; Yu, W.H.; Shi, Y.; Xiang, S.K.; Hu, P.; Zhao, K.Q.; Wang, B.; Gui, Y.Y.; Feng, C. Synthesis, Crystal Structure and Photophysical Properties of Triphenylene 2,3,6,7-Tetracarboxylic Ester-Base Discotic Liquid Crystal. Cryst. Growth Des. 2023, 23, 4424–4434. [Google Scholar] [CrossRef]
- Wan, W.A.; Monobe, H.; Tanaka, T.; Shimizu, Y. Mesomorphic Properties and Hydrogen Bonding Formation of Asymmetrical Triphenylene Discotic Liquid Crystals. Mol. Cryst. Liq. Cryst. 2001, 364, 597–603. [Google Scholar] [CrossRef]
- Wan, W.; Wang, P.Y.; Jiang, H.Z.; Jao, J. Synthesis and Characterization of Triphenylene Derivatives Containing Two Terminal Funtional Groups at the Periphery. Mol. Cryst. Liq. Cryst. 2008, 482, 42–56. [Google Scholar] [CrossRef]
- Imrie, C.; Luckhurst, G.R. Liquid Crystal Dimers and Oligomers. In Handbook of Liquid Crystals, 2nd ed.; Goodby, J.W., Collings, P.J., Kato, T., Tschierske, C., Gleeson, H.F., Raynes, P., Eds.; Wiley-VCH Verlag GmbH & Co.: Hoboken, NJ, USA, 2014; Volume 6. [Google Scholar] [CrossRef]
- Mu, B.; Hao, X.; Chen, J.; Li, O.; Zhang, C.; Chen, D. Discotic columnar liquid crystalline polymer semiconducting materials with high charge carrier mobility via rational macromolecular engineering. Polym. Chem. 2017, 8, 3286–3293. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, C.; Wang, J.; Hong, F.; Hao, X.; Zhang, A.; Wang, Y.; Wu, H.; Zhang, W.; Pu, J. Systematic studies on structure–properties relationship of main chain discotic liquid crystalline polyethers: Effects of the spacer lengths and substitution positions. Polym. Chem. 2016, 7, 3013–3025. [Google Scholar] [CrossRef]
- Tahar-Djebbar, I.; Nekelson, F.; Heinrich, B.; Donnio, B.; Guillon, D.; Kreher, D.; Mathevet, F.; Attias, A.J. Lamello-Columnar Mesophase Formation in a Side-Chain Liquid Crystal π-Conjugated Polymer Architecture. Chem. Mater. 2011, 23, 4653–4656. [Google Scholar] [CrossRef]
- Setoguchi, Y.; Monobe, H.; Wan, W.; Terasaea, N.; Kiyohara, K.; Nakamura, N.; Shimizu, Y. Infrared Spectral Studies of Triphenylene Mesogens Possessing Terminal Functional Groups in the Perypheral Chains for Hydrogen-Bond Interaction. Mol. Cryst. Liq. Cryst. 2004, 412, 9–18. [Google Scholar] [CrossRef]
- Woollins, J.D. Inorganic Experiments, 2nd ed.; Wiley-VCH: Hoboken, NJ, USA, 2010. [Google Scholar]

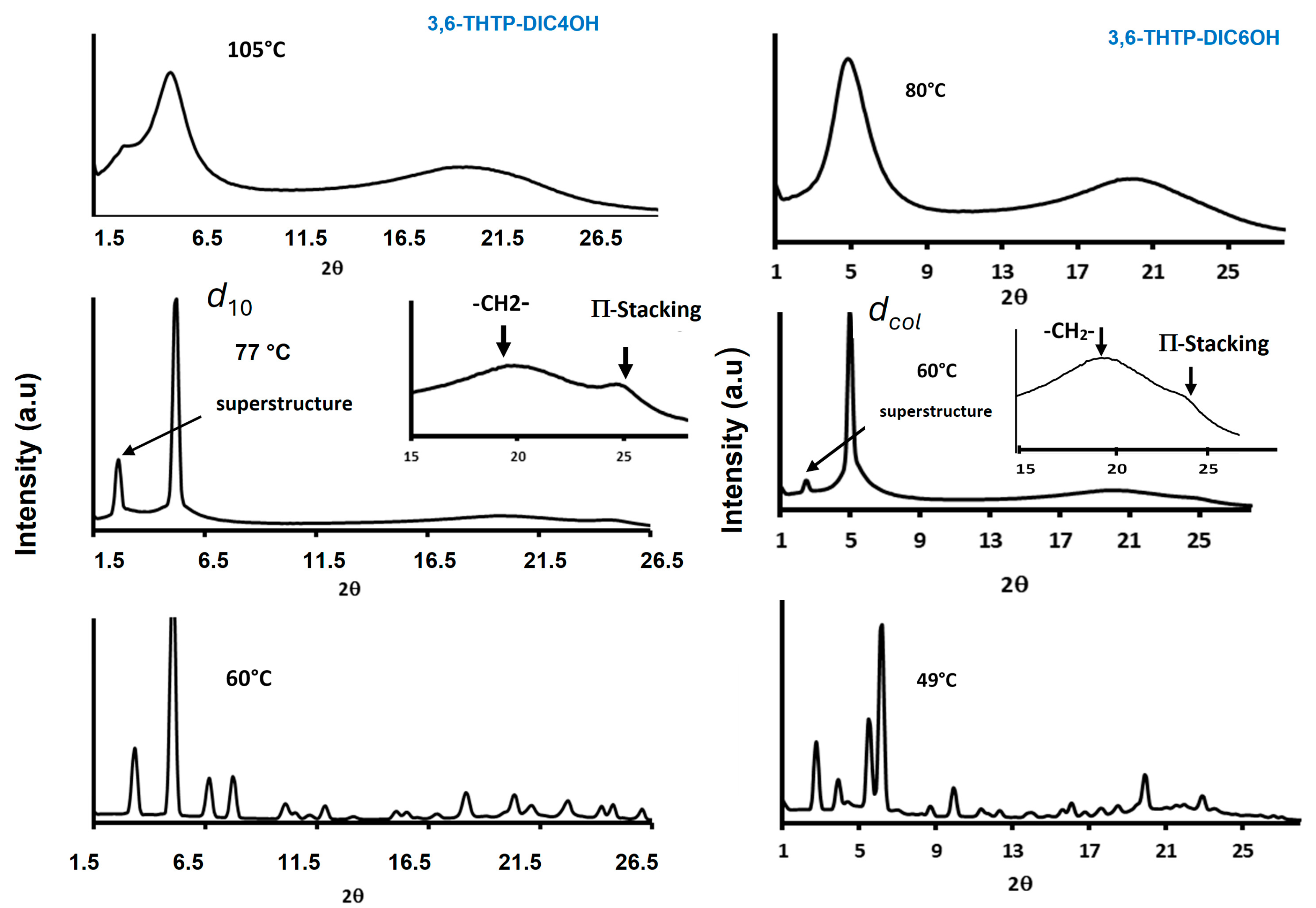
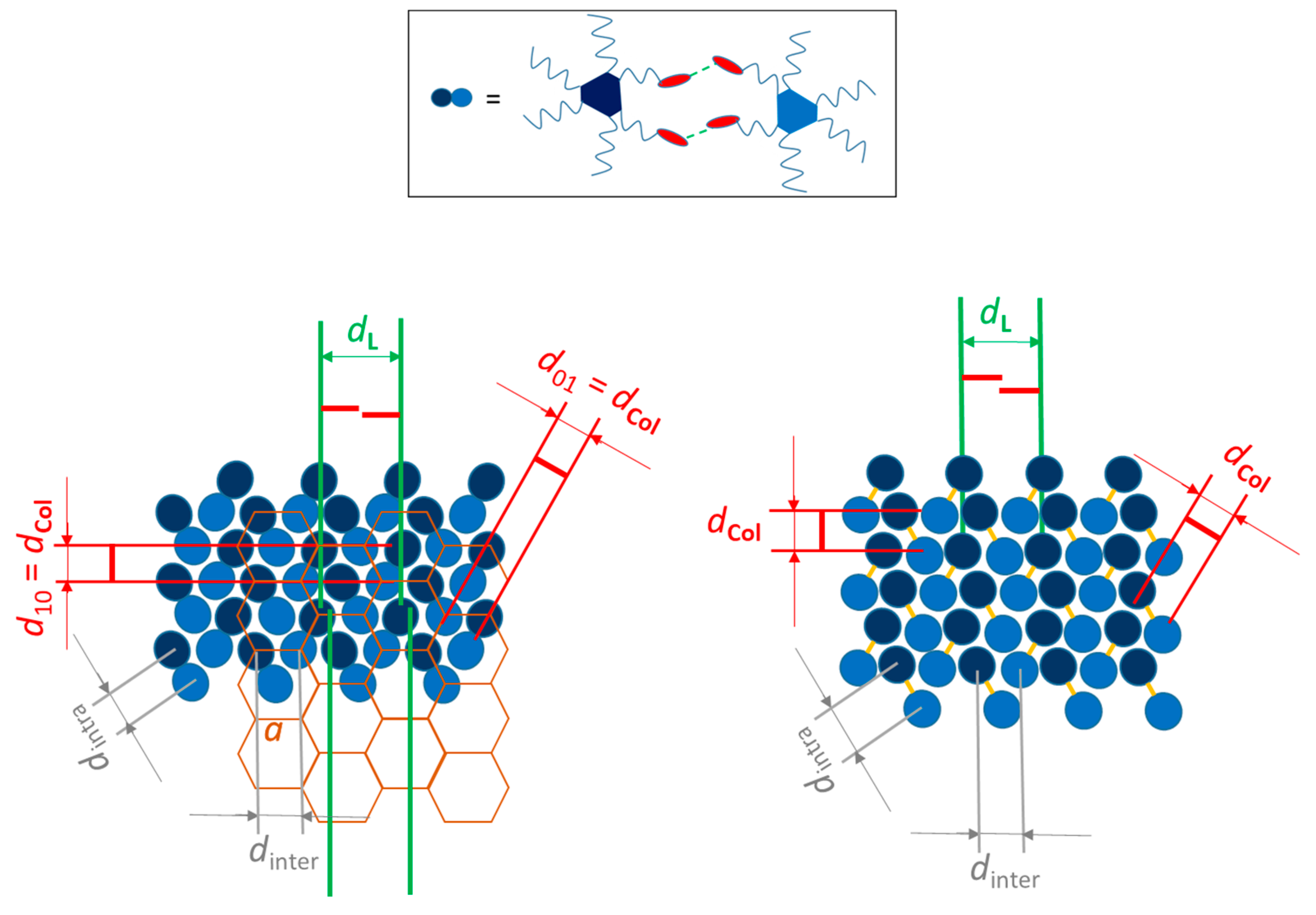

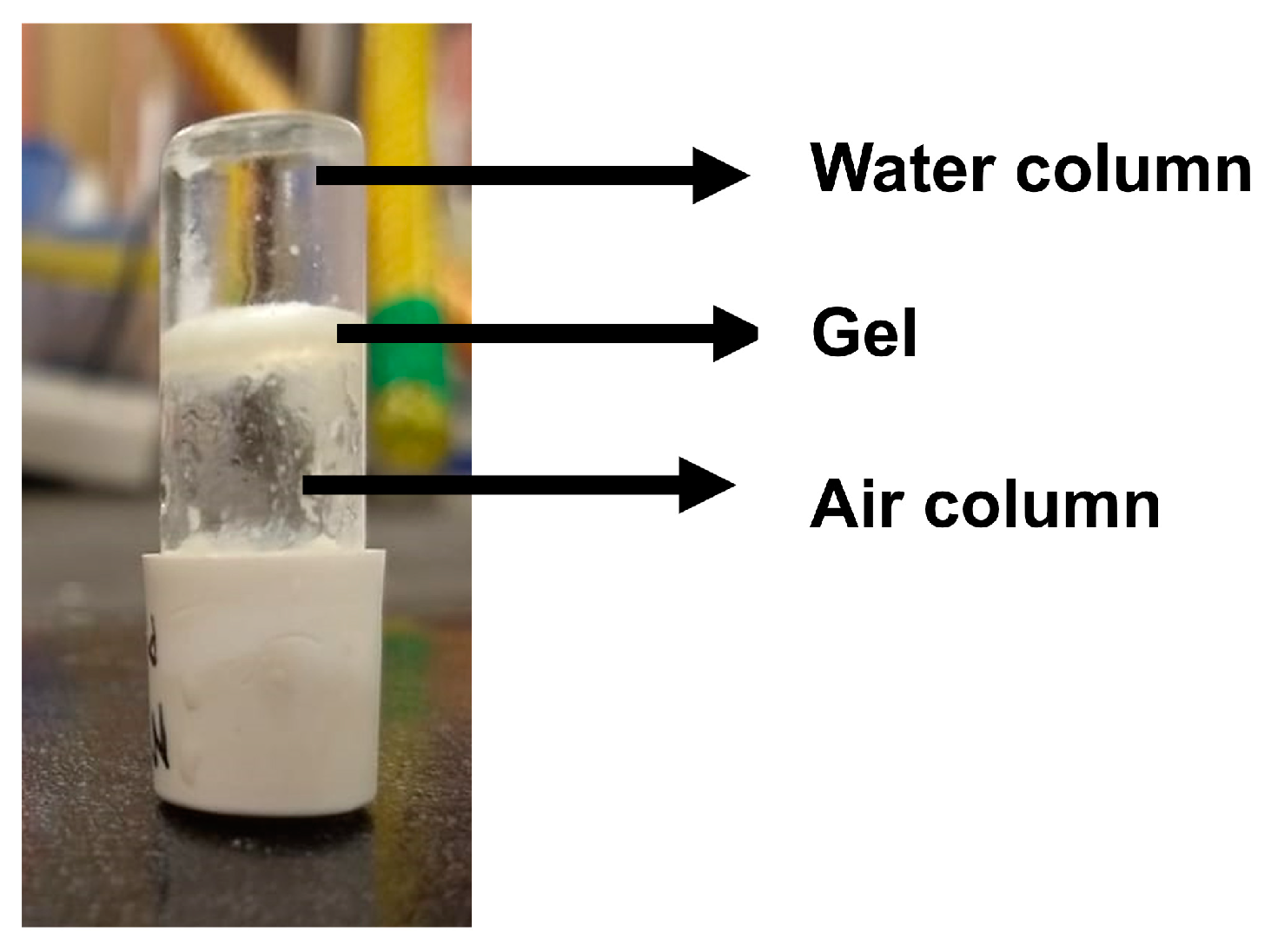
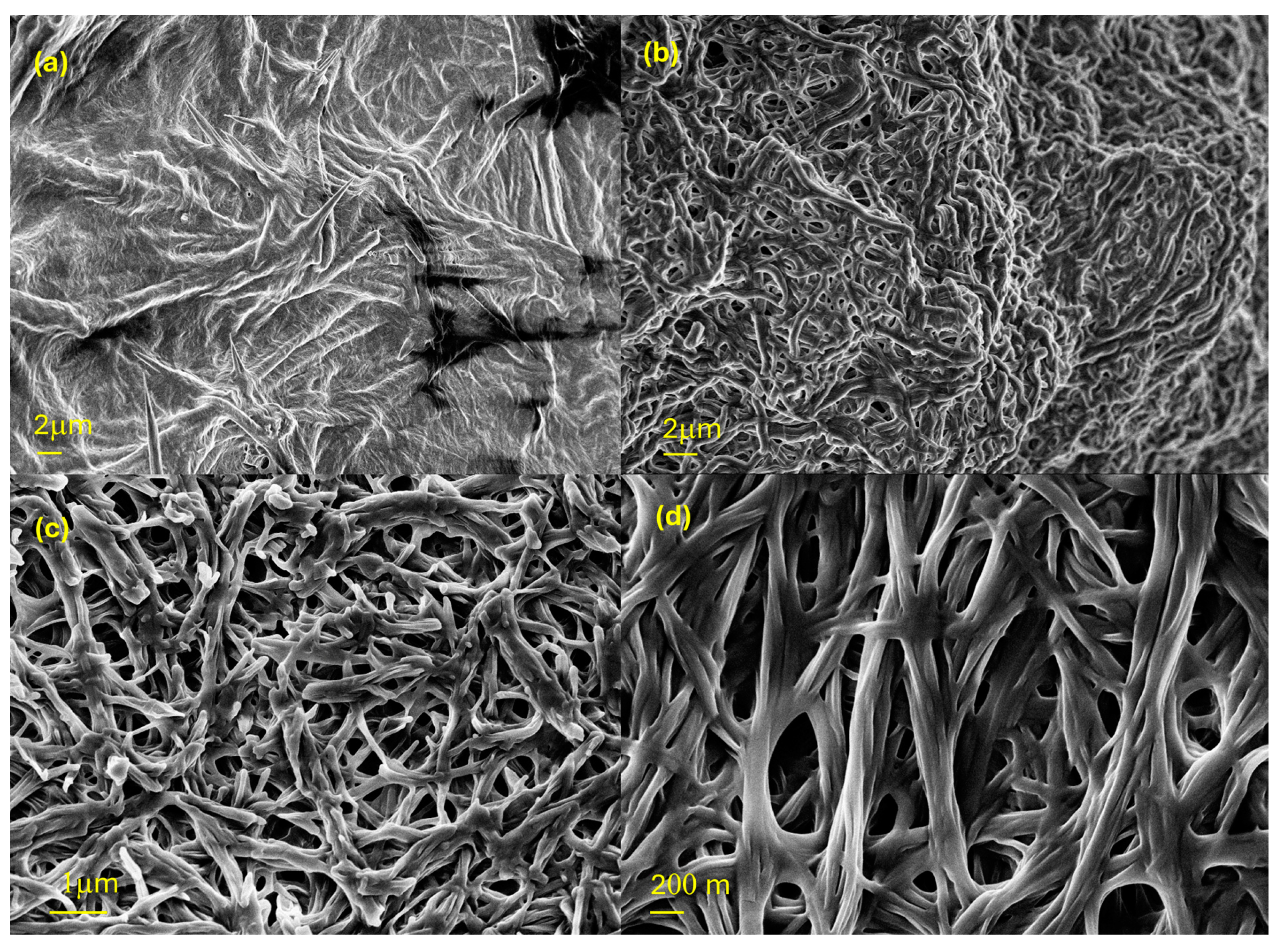


| Compound | Phase Sequence | T (°C) | Dexp. (Å) | dhk | Lattice Parameter (Å) |
|---|---|---|---|---|---|
| 3,6-THTP-DiC4OH | I 81 (4) Colh 66 (40) Cr | 77 | 16.95 33.83 | d10 = dcol dL = 2d10 | 19.57 |
| 3,6-THTP-DiC6OH | I 65 (5) Colh 52 (63) Cr | 60 | 18.02 35.32 | dcol dL < 2dcol | 20.80 |
| 3,6-THTP-DiC10OH | I 45 (83) Cr | - | - | - | - |
| 3,6-THTP-DiCnOH | |||
|---|---|---|---|
| Solvent | n = 4 | n = 6 | n = 10 |
| Methanol | TG (1%) | TG (0.5%) | TG (2%) |
| Ethanol | TG (2%) | S | S |
| 2-Propanol | S | S | S |
| n-Butanol | S | S | I |
| Acetonitrile | S | S | I |
| 1,4-butanediol | I | I | I |
| Pentanol | S | S | S |
| n-hexane | I | TG (2%) | I |
| n-heptane | I | I | I |
| cyclohexane | S | S | S |
| Pentane | I | I | S |
| Dichloromethane | S | S | S |
| Acetone | S | S | S |
| Toluene | S | S | S |
| Ethyl acetate | S | S | S |
| Ethylene glycol | I | I | I |
| 3,6-THTP-DiCnOH | ||
|---|---|---|
| n | Solvent (%wt./V) | Water Detection Limit |
| 4 | Methanol (1) | 1% |
| Ethanol (2) | 1% | |
| 2-Propanol (5) | 1% | |
| 6 | Methanol (0.44) | 1% |
| Ethanol (5) | 1% | |
| 2-Propanol (5) | 1% | |
| 10 | Methanol (2) | 1% |
| Ethanol (5) | 1% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vadra, N.; Giovanetti, L.J.; Di Chenna, P.H.; Cukiernik, F.D. Columnar Mesophases and Organogels Formed by H-Bound Dimers Based on 3,6-Terminally Difunctionalized Triphenylenes. Gels 2025, 11, 9. https://doi.org/10.3390/gels11010009
Vadra N, Giovanetti LJ, Di Chenna PH, Cukiernik FD. Columnar Mesophases and Organogels Formed by H-Bound Dimers Based on 3,6-Terminally Difunctionalized Triphenylenes. Gels. 2025; 11(1):9. https://doi.org/10.3390/gels11010009
Chicago/Turabian StyleVadra, Nahir, Lisandro J. Giovanetti, Pablo H. Di Chenna, and Fabio D. Cukiernik. 2025. "Columnar Mesophases and Organogels Formed by H-Bound Dimers Based on 3,6-Terminally Difunctionalized Triphenylenes" Gels 11, no. 1: 9. https://doi.org/10.3390/gels11010009
APA StyleVadra, N., Giovanetti, L. J., Di Chenna, P. H., & Cukiernik, F. D. (2025). Columnar Mesophases and Organogels Formed by H-Bound Dimers Based on 3,6-Terminally Difunctionalized Triphenylenes. Gels, 11(1), 9. https://doi.org/10.3390/gels11010009








